Abstract
AIM: To characterize the efficacy of rifaximin in the management of hepatic encephalopathy (HE) as several randomized controlled studies have shown contradictory results on its effectiveness in comparison to other oral agents.
METHODS: We performed a systematic review and random effects meta-analysis of all eligible trials identified through electronic and manual searches. Twelve randomized controlled trials met the inclusion criteria with a total of 565 patients.
RESULTS: The clinical effectiveness of rifaximin was equivalent to disaccharides or other oral antibiotics [odds ratio (OR) 0.96; 95% CI: 0.94-4.08] but with a better safety profile (OR 0.27; 95% CI: 0.12-0.59). At the completion of treatment protocols, patients receiving rifaximin showed lower serum ammonia levels [weighted mean difference (WMD) = -10.65; 95% CI: -23.4-2.1; P = 0.10], better mental status (WMD = -0.24; 95% CI: -0.57-0.08; P = 0.15) and less asterixis (WMD -0.1; 95% CI -0.26-0.07; P = 0.25) without reaching statistical significance. On the other hand, other psychometric outcomes such as electroencephalographic response and grades of portosystemic encephalopathy were superior in patients treated with rifaximin in comparison to the control group (WMD = 0.21, 95% CI: -0.33-0.09, P = 0.0004; and WMD = -2.33, 95% CI: -2.68-1.98, P = 0.00001, respectively). Subgroup and sensitivity analysis did not show any significant difference in the above findings.
CONCLUSION: Rifaximin appears to be at least as effective as other conventional oral agents for the treatment of HE with a better safety profile.
Keywords: Hepatic encephalopathy, Lactulose, Neomycin, Non-absorbable disaccharides, Rifaximin
INTRODUCTION
Hepatic encephalopathy (HE) is a reversible neuropsychiatric and functional syndrome occurring in 50%-70% of patients with advanced liver disease[1]. The pathophysiology of HE is complex and it manifests with progressive deterioration of the superior neurological functions. HE occurs in the presence of insufficient hepatic clearance of toxins absorbed from the intestine resulting in neurochemical abnormalities across the blood brain barrier[2]. The clinical manifestations of HE range from altered mental status to deep coma[3]. Elevated serum ammonia level is the best described cause of HE and is detected in 60%-80% of affected patients[4,5]. Current treatment strategies[6] are aimed at reducing the serum level of ammonia. This is done by introducing agents that reduce or inhibit production of intestinal ammonia or minimize its absorption from the gastrointestinal tract as well as correcting precipitating factors such as gastrointestinal hemorrhage, electrolyte imbalances and constipation[7].
For both acute and chronic HE, the mainstay treatment has been the use of non-absorbable disaccharides[3] since they decrease the absorption of ammonia through cathartic effects and by altering the colonic pH[6]. Several oral antibiotics such as neomycin, paromomycin, metronidazole, vancomycin and rifaximin have shown some degree of effectiveness in lowering serum ammonia concentration by reducing the intestinal flora responsible for its production[8]. With the exception of rifaximin, all the other antibiotics have been associated with some side effects such as ototoxicity and nephrotoxicity (neomycin)[9,10] and neurotoxicity (metronidazole)[11,12]. Vancomycin may be a safer option, however, its use has been associated with the development of bacterial resistance[13]. On the other hand, rifaximin is a poorly-absorbed broad spectrum antibiotic with very few systemic side effects and at low risk of inducing bacterial resistance[14,15]. These properties make rifaximin an ideal antibiotic for the treatment of patients with HE as several studies have shown a significant decrease in plasma ammonia levels[16-18] with minimal impact on the normal gastrointestinal flora[13].
Several small randomized controlled trials (RCT) comparing rifaximin with oral disaccharides or with other antibiotics have found that rifaximin is effective and safe. Nevertheless, these trials were insufficiently powered. A meta-analysis of randomized controlled trials comparing disaccharides vs antibiotics for the treatment of HE has shown superior outcomes with the use of antibiotic therapy[19]. Sub-group analysis of five studies comparing rifaximin to disaccharides favored the use of rifaximin (P = 0.04)[19]. On the other hand, a more recent and larger meta-analysis including seven studies comparing rifaximin with non-absorbable disaccharides showed no significant difference between the two interventions, although rifaximin had fewer side effects[20]. In light of these limitations, we conducted a systematic review of the literature to identify, appraise and collectively analyze all RCTs comparing rifaximin with conventional oral therapies for the treatment of patients with HE.
MATERIALS AND METHODS
Data sources and study selection
Randomized controlled trials comparing oral rifaximin to non-absorbable disaccharides and other antibiotics used for the treatment of HE were searched in PubMed, Excerpta Medica Database, Scopus, Web of Science, Cochrane central register of controlled trials, and hepatobiliary group in the Cochrane library, EMBASE, CINAHL through December 4th 2010 without restriction on the publication status or language. Database specific search terms for rifaximin (rifaximin, rifamycins), disaccharides (disaccharides, lactulose, lactitol, sugar alcohols) and antibiotics (anti-bacterial-agents, antibiotics) were combined and all reference sections of eligible studies and review articles on the topic were hand-searched for additional potential studies. Two reviewers (Eltawil KM and Molinari M) independently assessed the eligibility of all potential abstracts and titles. When in disagreement or in the presence of insufficient information, the full text of the potential paper was reviewed for eligibility. Authors of all potential trials were also contacted by electronic mail for additional information if the published data were insufficiently described.
Inclusion and exclusion criteria
We included all RCTs that reported the effect of rifaximin vs non-adsorbable disaccharides or other antibiotics on the grade of HE according to Conn’s modification of Parsons Smith classification[21], irrespective of language and publication status. Exclusion criteria were: studies conducted on pediatric patients, studies that compared the use of rifaximin vs placebo, non-controlled clinical trials, studies that assessed the efficacy of rifaximin in preventing HE, trials including patients with psychiatric illness, with undercurrent infections, with hypersensitivity to rifaximin and other antibiotics and/or intolerance to non-absorbable disaccharides, trials that included individuals affected by gastrointestinal bleeding and studies reporting results of the same population published more than once.
Outcomes
The primary outcomes of this study were the effectiveness and the safety of the use of rifaximin for the treatment of patients with at least one episode of HE. Secondary outcomes were reduction of serum ammonia levels and changes in psychometric parameters [mental status, asterixis, electroencephalographic characteristics and portosystemic encephalopathy (PSE) sum] measured at the end of the treatment.
Definitions
The study population was defined as patients older than 18 years of age with a diagnosis of reversible neurological decline secondary to end-stage liver disease. Effectiveness was calculated by the proportion of patients who had resolution or clinical improvement of HE during the treatment. Partial neurological response was measured by mental status scores according to Conn’s classification[21] as follows: 0, no personality or behavioral abnormality; 1, trivial lack of awareness, euphoria or anxiety, shortened attention span, or impairment of ability to add or subtract; 2, lethargy, disorientation with respect to time, obvious personality change, or inappropriate behavior; 3, somnolence or semi-stupor, responsiveness to stimuli, confusion, gross disorientation, or bizarre behavior; and 4, coma.
Side effects of rifaximin and other oral therapies assessed in this study were: severe diarrhea, episodes of intense abdominal pain and at least one of the following symptoms: nausea, anorexia and weight loss.
Serum ammonia levels were assessed at the end of the treatment and expressed in mg/dL.
The severity of asterixis was graded according to established criteria as follows: 0, no tremors; 1, few flapping motions; 2, occasional flapping motions; 3, frequent flapping motions; and 4, almost continuous flapping motions[22,23].
Electroencephalogram (EEG) abnormalities recorded in patients with HE were scored according to criteria previously published in the medical literature[24]: 0, well-structured EEG with stable and symmetrical posterior basic rhythm (8 Hz-13 Hz) dominant in the posterior regions medium amplitude without slow activities or epileptic pattern; 1, unstable or suppressed alpha rhythm frequently replaced by high prevalence of diffuse beta rhythm (normal-limit EEG); 2, low frequency alpha rhythm (8 Hz) disturbed by random waves in the theta range over both hemispheres (mild signs of encephalopathy); 3, background activity in the theta range, diffused over both hemispheres, random appearance of high waves in the delta range (distinctive features of encephalopathy); and 4, severe disorganization of EEG activity without any normal element (signs of severe encephalopathy).
Grades of PSE were calculated as the sum of the degree of mental status abnormality scores, the severity of asterixis, level of serum ammonia elevation and the degree of EEG abnormality[25].
Data extraction
Two independent reviewers extracted publication variables and clinical data for each study. The following variables were collected: the name of the primary author, journal and year of publication, country where the study was carried out, number of patients randomized in each arm, daily dosage of oral therapy, duration of the treatment, allocation sequence generation, allocation concealment, power calculation, study design, methods used to deal with missing data, appropriate description of attrition and drop-outs.
Clinical variables extracted were: the proportion of patients that experienced improvement of their neurological function (effectiveness), common side effects, serum ammonia level and psychometric parameters.
Assessment of study quality
The quality of included studies was scored using the Cochrane Collaboration risk assessment tool[26]. The methodological quality was essentially based on the attention that each study design paid to control potential bias based on the available description reported in the methodology of each paper. When in doubt, authors were contacted by digital letters. The randomization methods were classified as the primary way to control bias and the randomization process was evaluated by the allocation sequence generation and allocation concealment. The randomization methods were considered adequate if based on a table of random numbers, computer-generated random numbers or based on similar techniques. Allocation concealment was classified as adequate if based on central randomization, if using identically appearing coded drugs, if serially numbered opaque sealed envelopes were employed or when other equivalent methods were used. Blinding was extracted and appraised for caregivers, patients and assessors. Studies were classified as single-blinded if the patients did not have any opportunity to know the nature of their therapy and double-blinded if the authors described in their methods how they prevented patients and caregivers or assessors knowing the nature of the treatments. In addition, we appraised the risk of attrition bias by assessing the number and reason for dropouts and withdrawals and whether all patients were accounted for in the report and analysis of the study. Quality of the included studies also assessed the way the authors described sample size calculation to power the trial and if the sample size was achieved, whether there was a clear definition of primary outcomes and if they were reported and whether a crossover design was used.
Statistical analysis
All statistical analyses were performed using RevMan Version 5.0.5 software[27] (Nordic Cochrane Centre, Copenhagen, Denmark). The meta-analysis was performed using the random effects model of DerSirmonian and Laird[28] due to expected clinical heterogeneity. The results are reported as pooled odds ratios (OR) for binary and weighted mean differences (WMD) for continuous outcomes, both with 95% CI. Pooled OR and WMD were calculated using the general inverse variance (IV) with random effect model. Measure of the degree of inter-trial heterogeneity was explored with the I2 test[29]. Heterogeneity was evaluated with a χ2-based Q statistic of OR and defined at a P value less than 0.1 and potential reasons for heterogeneity were explored. Data on all patients randomized were extracted to allow intention-to-treat analysis. For patients with missing data, carry-forward of the last observed response was used. Only data from the first period of cross-over trials were included. For the primary outcome measure, we performed subgroup analyses of trials stratified by the treatment regimen and methodological quality. The preferred reporting items for systematic reviews and meta-analysis recommendations were used for study reporting[30,31].
RESULTS
Ascertainment of the studies
After initial screening, a total of 220 potentially relevant trials were identified through the electronic searches as summarized in Figure 1. After subsequent evaluation for eligibility, we retained 12 published RCTs that assessed the effectiveness of rifaximin for the treatment of patients with HE. One study was available only in abstract form[32]. The remaining studies were excluded because they were prospective cohort studies or randomized controlled trials assessing the effectiveness of rifaximin for the prevention rather than for the treatment of HE.
Figure 1.
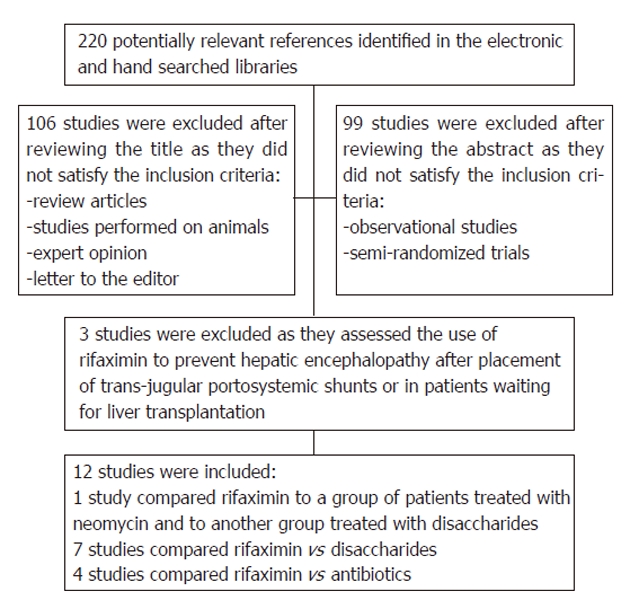
Flowchart of the included studies.
Study characteristics
Table 1 lists the characteristics of the included studies. All trials were single-center studies except one which was multicentric[17] with the total number of patients per study ranging from 14 to 136 and with a minimum therapeutic interval of 7 d to a maximum of 6 mo. The majority of studies treated one arm of patients with rifaximin at a dose of 1200 mg/d divided in three doses, although some used 1100 mg in two divided doses. The comparison arm of patients received non-absorbable oral disaccharides (lactulose or lactitol) at doses ranging from 45 to 120 mL/d for lactulose and 60 g/d for lactitol or antibiotic therapy with neomycin or paromomycin. One study[33] was designed as a double cross study where patients received rifaximin in addition to oral disaccharides for one week and were switched to neomycin in combination with oral disaccharides on the third week of therapy. Festi et al[17] carried on a randomized controlled trial that included 4 arms of patients with HE: in one group the effect of rifaximin was compared to non-absorbable disaccharides and in the other group rifaximin was compared to neomycin.
Table 1.
Characteristics of included studies
| Authors | Country | Rifaximin (n) | Control (n) | Rifaximin dose(mg/d) | Comparative agent | Duration of treatment | Outcomes |
| Bucci et al[35] | Italy | 30 | 28 | 1200 | Lactulose 45 mL/d | 15 d | Mental status, asterixis, cancellation test, reitan test, EEG, serum ammonia, degree and severity of HE |
| Di Piazza et al[33] | Italy | 8 | 6 | 1200 | Neomycin 4500 mg/d | 21 d | Bradylalia, flapping tremor, performance, visual evoked potentials and the trial making test |
| Fera et al[37] | Italy | 20 | 20 | 1200 | Lactulose 120 mL/d | 90 d | Mental status, asterixis, cancellation test, reitan test, EEG, PSE severity |
| Festi et al[17] | Italy | 20 | 15 | 1200 | Neomycin 3000 mg/d | 21 d | Asterixis, EEG, blood ammonia |
| Festi et al[17] | Italy | 9 | 12 | 1200 | Lactulose 60 mL/d | 21 d | Asterixis, EEG, blood ammonia |
| Loguercio et al[39] | Italy | 14 | 13 | 1200 | Lactulose 90 mL/d | 3 mo | Mental status, asterixis, NCT, blood ammonia |
| Mas et al[36] | Spain | 50 | 53 | 1200 | Lactitol 60 g/d | 5-10 d | HE grade, mental status, asterixis, NCT, EEG, PSE index, blood ammonia |
| Massa et al[18] | Italy | 20 | 20 | 1200 | Lactulose 90 mL/d | 15 d | Mental status, asterixis, cancellation test, EEG, trail making test, PSE index, blood ammonia |
| Miglio et al[38] | Italy | 25 | 24 | 1200 | Neomycin 3000 mg/d | 6 mo | HE grade, blood ammonia, neuropsychiatric signs |
| Paik et al[21] | South Korea | 32 | 22 | 1200 | Lactulose 90 mL/d | 7 d | Mental status, flapping tremors, NCT, blood ammonia, HE index |
| Parini et al[34] | Italy | 15 | 15 | 1200 | Paromomycin 1500 mg/d | 10 d | Blood ammonia, state of consciousness, behavior, intellectual functions, neurologic symptoms |
| Pedretti et al[25] | Italy | 15 | 15 | 1200 | Neomycin 3000 mg/d | 21 d | PSE index, blood ammonia, EEG, NCT, asterixis, trail making test, mental status |
| Song et al[32] | South Korea | 39 | 25 | 1200 | Lactulose 90 mL/d | 7 d | Blood ammonia, mental status, flapping tremors, NCT, HE index |
EEG: Electroencephalogram; HE: Hepatic encephalopathy; PSE: Portosystemic encephalopathy; NCT: Number connection test.
The majority of control patients who received neomycin were treated with a total of 3000 mg/d divided in three doses except for participants in Di Piazza’s study[33] who were treated with a total daily dose of 4500 mg divided in three administrations. Paromomycin was used in only one study[2] and administered at a total dose of 1500 mg/d divided in three doses of 500 mg each.
The majority of trials that used non-absorbable oral disaccharides aimed at inducing several soft bowel movements per day although none of the included studies reported how they monitored their participants.
Risk of bias
We assessed the risk of bias for all the included studies based on published reports and information provided by the authors (Table 2). The allocation system was described in five trials[21,25,34-36] and allocation concealment was clearly defined in eight trials[18,21,25,34-38]. The majority of trials were blinded to patients; however, blinding of observers was described only in six studies[18,25,33,36-38]. Methods for handling missing data, description of drop-outs and possible causes of attrition and power calculations were not adequately described in any of the included studies. One study was available only in abstract form and therefore the risk of bias appraisal was not satisfactory.
Table 2.
Risk of bias in the published controlled trials
| Author | Allocation system described | Allocation concealment | Blinding | Handling of missing data | Power calculation for number of patients to be treated | ||
| Patient | Personnel | Assessor | |||||
| Bucci et al[35] | Yes | Yes | Yes | Yes | NA | Unclear | No |
| Di Piazza et al[33] | No | No | Yes | Yes | NA | Unclear | No |
| Fera et al[37] | No | Yes | Yes | No | NA | Unclear | No |
| Festi et al[17] | No | No | No | No | NA | Unclear | No |
| Loguercio et al[39] | No | No | Yes | No | NA | Unclear | No |
| Mas et al[36] | Yes | Yes | Yes | Yes | NA | Unclear | No |
| Massa et al[18] | No | Yes | Yes | Yes | NA | Unclear | No |
| Miglio et al[38] | No | Yes | Yes | Yes | NA | Unclear | No |
| Paik et al[21] | Yes | Yes | Yes | No | NA | Unclear | No |
| Parini et al[34] | Yes | Yes | Yes | No | No | Unclear | No |
| Pedretti et al[25] | Yes | Yes | Yes | Yes | NA | Unclear | No |
| Song et al[32] | NA | NA | NA | NA | NA | NA | NA |
NA: Not available.
Primary outcomes
Effectiveness: First, the effectiveness of therapy was assessed by comparing rifaximin to non-adsorbable disaccharides (lactulose or lactitiol) and then to other oral antibiotics (neomycin or paromomycin). After that, the overall effectiveness of rifaximin vs other conventional oral therapies was assessed by combining the two subgroups.
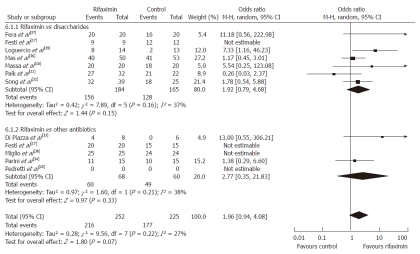
Using the random-effect model, the pooled analysis of 7 studies[17,18,21,32,36,37,39] that investigated the efficacy of rifaximin (n = 184) vs non-absorbable disaccharides (n = 165) revealed that both groups experienced either full resolution of HE or clinical improvement that was considered significant by the primary investigators without reaching statistical significance (OR = 1.92, 95% CI: 0.79-4.68, P = 0.15).
Similar findings were observed when the data of all 5 RCTs comparing the efficacy of rifaximin vs other antibiotics[17,25,33,34,38] were pooled. This confirmed that rifaximin (n = 68) had similar effectiveness to neomycin or paromomycin (n = 60, neomycin or paromomycin) (OR = 2.77, 95% CI: 0.35-21.83, P = 0.21).
The results of the combined analysis with both groups of patients receiving antibiotics or disaccharides showed a trend that favored the use of rifaximin without statistical significance (OR = 1.96, 95% CI: 0.94-4.08, P = 0.07) (Figure 2).
Figure 2.
Efficacy of rifaximin vs oral disaccharides, rifaximin vs other oral antibiotics and rifaximin vs control therapy which included the combination of oral disaccharides and other antibiotics. M-H: Mantel Haenszel.
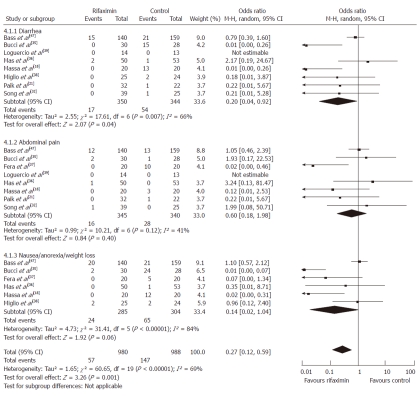
Adverse events: The following were side effects used in our analysis: severe diarrhea beyond the expected cathartic effect of disaccharides, abdominal pain, and the combination of nausea, anorexia and weight loss. First, each of the adverse events experienced by patients was compared separately. Second, all the adverse events were pooled and compared between the group of patients who received rifaximin (n = 980) and the control group (n = 988).
Participants who received rifaximin had less risk of suffering from diarrhea (OR = 0.20, 95% CI: 0.04-0.92, P = 0.04) although the rate of abdominal pain nausea/anorexia/weight loss was similar between the two groups (P = 0.40, P = 0.06, respectively). Yet, combined analysis of all the adverse events favored the use of rifaximin as it was associated with fewer side effects (OR = 0.27, 95% CI: 0.12-0.59, P = 0.001) (Figure 3).
Figure 3.
Adverse events experienced by patients treated with rifaximin vs control therapy. M-H: Mantel Haenszel.
Secondary outcomes
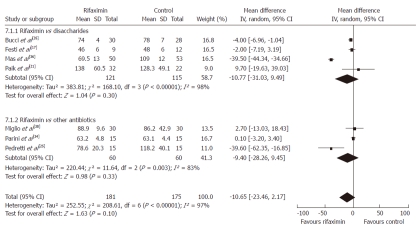
Serum ammonia level: At the end of 7 RCTs[17,21,25,34-36,38], a significant reduction in serum ammonia level was observed in both treatment arms; rifaximin vs non-adsorbable disaccharides and rifaximin vs neomycin or paromomycin. Participants who received rifaximin (n = 138) had lower serum ammonia levels in comparison to patients who received non-adsorbable disaccharides (n = 128) although the difference did not reach statistical significance (P = 0.30). Similar results were observed when comparing rifaximin (n = 60) vs other oral antibiotics (n = 60) (P = 0.33) although the differences were not statistically significant. When compared to all the controls (n = 176), patients treated with rifaximin (n = 181) had an overall lower mean serum ammonia level but this, too, was not statistically significant (WMD = -10.65, 95% CI: -23.46-2.17, P = 0.10) (Figure 4).
Figure 4.
Serum ammonia levels at the end of the treatment protocols: rifaximin vs oral disaccharides, rifaximin vs other oral antibiotics and rifaximin vs control therapy which included the combination of oral disaccharides and other antibiotics. IV: Inverse variance.
Psychometric parameters: Improvement in mental status and degree of asterixis after rifaximin therapy were compared to controls in seven trials with no statistically significant results (P = 0.15 and P = 0.25, respectively) (Table 3).
Table 3.
Summary of the meta-analysis on psychometric outcomes measured at the end of each randomized controlled trial
| Variable | Rifaximin | Control | Mean difference | ||||
| Mean | SD | Total | Mean | SD | Total | IV, random, 95% CI | |
| Mental status | |||||||
| Bucci et al[35] | 0.8 | 0.5 | 30 | 1.2 | 0.3 | 28 | -0.40 [-0.61, -0.19] |
| Loguercio et al[39] | 0.42 | 0.67 | 14 | 0.9 | 0.74 | 13 | -0.48 [-1.01, 0.05] |
| Massa et al[18] | 0.6 | 0.2 | 20 | 1.2 | 0.3 | 20 | -0.60 [-0.76, -0.44] |
| Paik et al[21] | 0.5 | 0.7 | 32 | 0.3 | 0.4 | 22 | 0.20 [-0.09, 0.49] |
| Parini et al[34] | 0.22 | 0.39 | 15 | 0.16 | 0.34 | 15 | 0.06 [-0.20, 0.32] |
| Subtotal (95% CI) | 111 | 98 | -0.24 [-0.57, 0.08] | ||||
| Heterogeneity: Tau² = 0.11; χ² = 32.85, df = 4 (P < 0.00001); I² = 88% | |||||||
| Test for overall effect: Z = 1.45 (P = 0.15) | |||||||
| Asterixis | |||||||
| Bucci et al[35] | 0.5 | 0.3 | 30 | 0.9 | 0.5 | 28 | -0.40 [-0.61, -0.19] |
| Mas et al[36] | 0 | 0.5 | 50 | 0 | 0.5 | 53 | 0.00 [-0.19, 0.19] |
| Massa et al[18] | 0.1 | 0.2 | 20 | 0.1 | 0.2 | 20 | 0.00 [-0.12, 0.12] |
| Paik et al[21] | 0.3 | 0.7 | 32 | 0.4 | 0.6 | 22 | -0.10 [-0.45, 0.25] |
| Parini et al[34] | 0.28 | 0.5 | 15 | 0.16 | 0.04 | 15 | 0.12 [-0.13, 0.37] |
| Pedretti et al[25] | 1.6 | 0.7 | 15 | 2 | 0.8 | 15 | -0.40 [-0.94, 0.14] |
| Subtotal (95% CI) | 162 | 153 | -0.10 [-0.26, 0.07] | ||||
| Heterogeneity: Tau² = 0.02; χ² = 14.47, df = 5 (P = 0.01); I² = 65% | |||||||
| Test for overall effect: Z = 1.15 (P = 0.25) | |||||||
| EEG | |||||||
| Bucci et al[35] | 0.4 | 0.2 | 30 | 0.6 | 0.3 | 28 | -0.20 [-0.33, -0.07] |
| Mas et al[36] | 0.6 | 0.9 | 50 | 0.9 | 0.9 | 53 | -0.30 [-0.65, 0.05] |
| Pedretti et al[25] | 0.4 | 0.5 | 15 | 0.6 | 0.6 | 15 | -0.20 [-0.60, 0.20] |
| Subtotal (95% CI) | 95 | 96 | -0.21 [-0.33, -0.09] | ||||
| Heterogeneity: Tau² = 0.00; χ² = 0.28, df = 2 (P = 0.87); I² = 0% | |||||||
| Test for overall effect: Z = 3.52 (P = 0.0004) | |||||||
| PSE sum | |||||||
| Mas et al[36] | 4 | 0.1 | 50 | 6 | 2 | 53 | -2.00 [-2.54, -1.46] |
| Massa et al[18] | 3 | 0.5 | 20 | 5.5 | 0.5 | 20 | -2.50 [-2.81, -2.19] |
| Pedretti et al[25] | 7.1 | 2.4 | 15 | 9.3 | 2.7 | 15 | -2.20 [-4.03, -0.37] |
| Subtotal (95% CI) | 85 | 88 | -2.33 [-2.68, -1.98] | ||||
| Heterogeneity: Tau² = 0.02; χ² = 2.52, df = 2 (P = 0.28); I² = 21% | |||||||
| Test for overall effect: Z = 13.11 (P < 0.00001) | |||||||
IV: Inverse variance; EEG: Electroencephalogram; PSE: Portosystemic encephalopathy.
The changes in EEG patterns and PSE sum were studied in 3 trials (rifaximin vs control)[18,25,36]. For both parameters, the meta-analysis showed a statistically significant improvement favoring the use of rifaximin (WMD = 0.21, 95% CI: -0.33-0.09, P = 0.0004, and WMD = -2.33, 95% CI: -2.68-1.98, P = 0.00001, respectively) (Table 3). The overall improvement in psychometric parameters measured between the two drug groups was statistically significant favoring the use of rifaximin (P = 0.005) as summarized in Table 3.
Sensitivity analysis
A sensitivity analysis for both primary and secondary outcomes was conducted to explore heterogeneity on the basis of the quality of study design. By excluding studies considered at higher risk of bias according to the Cochrane Collaboration risk assessment tool, we identified consistency of findings and no statistically significant changes were noted for all the comparisons performed.
DISCUSSION
Hepatic encephalopathy represents a challenging clinical complication of liver insufficiency and presents with a wide spectrum of neuropsychiatric symptoms that range from mild disturbances in cognitive function to coma to even death[13,40]. The pathogenesis of this complex syndrome is thought to be multifactorial, but a key role is played by circulating gut-derived toxins such as ammonia[2,40]. With appropriate medical treatment most of the clinical manifestations of HE are reversible when precipitating factors are corrected[2]. The most common known conditions responsible for HE include: gastrointestinal bleeding, infections or systemic inflammation, renal and electrolyte abnormalities, dehydration, use of narcotics and other psychoactive medications, constipation and an excess protein intake[6].
Traditionally, non-absorbable disaccharides have been used as the first-line therapy for patients with HE[13] even if their effectiveness in comparison to placebo has not been proven[19]. Although safe, the need to adjust disaccharide doses to achieve two to three loose bowel movements per day often leads to frequent nausea, vomiting, and flatulence and affects compliance[3]. Poorly absorbed oral antibiotics such as neomycin, vancomycin or paromomycin seem to be more effective than disaccharides[19] with fewer side effects, although ototoxicity[41], nephrotoxicity[42], neurotoxicity and bacterial resistance have been described[20,42]. This significant risk of severe toxicity is the reason why most of these agents are seldom used in modern practice.
On the other hand, rifaximin is an agent that appears to be effective in the treatment of HE without carrying the risk of severe side effects. It has the advantage of being well tolerated and has minimal risk of causing bacterial resistance[43]. It was initially introduced in Italy in 1987[44] and recently approved in the United States for the treatment[45] and prevention[46] of HE. The purported advantages of rifaximin over other oral agents make it a very attractive choice for treatment of HE, although at considerably greater expense[13]. A recent mathematical model has shown that initial disaccharide monotherapy followed by rifaximin as a second-line therapy would be the most cost-effective strategy[47]. So far, the evidence for use of rifaximin as the first-line therapy for HE has been supported only by underpowered randomized controlled trials with conflicting results. A previous meta-analysis of seven randomized controlled trials concluded that rifaximin was not superior to non-absorbable disaccharides, except that it was better tolerated[20]. One of the limitations of that study was that rifaximin was not compared to other established oral therapies and that psychometric functional outcomes were not included in the final analysis.
In this study, we incorporated 12 randomized controlled trials published in the last 20 years assessing the efficacy and adverse events of rifaximin vs other oral therapies such as disaccharides and antibiotics. In addition to the effectiveness and safety profile, we also analyzed the effects of these treatments on serum ammonia levels and several other psychometric outcomes. Statistical assessment of patients’ compliance was not possible as none of the included trials measured attrition or reported any drop-outs as all the included participants appeared to be able to complete the treatment protocols.
The results of this study confirm that rifaximin has similar effectiveness to other oral therapies but with fewer side effects. As such, it is the first new effective treatment for HE in a long time and its impact on patients’ quality of life and survival has yet to be fully realized.
With regard to other secondary outcomes measured at completion of treatment protocols, patients receiving rifaximin had lower serum ammonia levels and superior mental status and asterixis profiles in comparison to the control group without reaching statistical significance. On the other hand, the grade of electroencephalographic abnormalities and PSE sums showed better profiles for participants treated with rifaximin when compared to their controls. These findings are of some importance as HE is a syndrome with a wide spectrum of neuropsychiatric abnormalities and it is important to be able to quantify subtle clinical changes during the course of therapy.
All trials in the present review excluded patients with uncorrected precipitating factors causing HE. Nevertheless, we could not obtain individual patient data to determine if response to the treatments varied according to the grade or etiology of liver disease.
One of the major limitations of this study was that the trials that satisfied the inclusion criteria had been performed during a relatively long period of time (1991-2005), the lengths of the treatment protocols varied significantly (5 d to 6 mo) and there was a lack of data on the severity of liver disease or other co-morbidities for each population. These important aspects were most likely responsible for the heterogeneity observed among the pooled studies. Minor imputations were required for some studies specially when considering standard deviations. In addition, pooling may not have been appropriate in all cases because of the heterogeneity between trials, but we attributed heterogeneity to statistical rather than clinical reasons.
On the other hand, this study also has several stren-gths. An extensive literature search was performed and provided the most up-to-date information on the effects of rifaximin in the treatment of HE. Using two reviewers, the inclusion or exclusion of studies and data extraction were performed independently and therefore more accurately. In addition, we did not exclude potential studies due to language, publication status or year of publication, and the fact that included trials were performed in several countries and in different settings increases the external validity of our results. Another strength of this study is that we assessed not only the efficacy and side effects of the treatments, but we also included other important clinical outcomes that could be measured objectively such as serum ammonia levels, asterixis and electroencephalographic features. Because we used a rigorous search strategy to reduce the introduction of potential publication bias, a funnel plot was not included as the number of trials was moderate and it would not have added any significant information.
In summary, this study has shown that rifaximin is comparable to other oral agents in regard to clinical efficacy for HE and is associated with fewer side effects. These results did not change during sensitivity analysis based on the quality of the trials, and the effect of rifaximin was more favorable in improving psychometric parameters and serum ammonia level measured at the end of the protocols. Given its safety profile, rifaximin should be considered as second-line in the treatment of HE patients who fail disaccharide therapy and as first-line in those intolerant of disaccharides.
COMMENTS
Background
Hepatic encephalopathy (HE) represents a debilitating and even potentially deadly complication occurring in patients with advanced liver disease. The clinical manifestations of encephalopathy range from altered mental status to deep coma. Rifaximin is an oral broad spectrum, non-absorbable antibiotic with very few systemic side effects and is used to treat or prevent hepatic encephalopathy in cirrhotic patients.
Research frontiers
The authors performed a systematic review of the literature and meta-analysis of the effectiveness and safety of rifaximin compared to other conventional oral agents such as non-absorbable disaccharides (lactulose) and other antibiotics (e.g., neomycin, metronidazole). Conventional therapies are known to cause local side effects such as diarrhea and abdominal pain in the case of Lactulose and systemic side effects such as nephrotoxicity and neurotoxicity with neomycin and metronidazole, respectively.
Innovations and breakthroughs
Several studies have shown contradictory results on the effectiveness of rifaximin in comparison to other oral agents.
Applications
The analysis has shown rifaximin to be as effective as non-absorbable disaccharides concerning improvement in the clinical symptoms of patients with hepatic encephalopathy with a lower incidence of side effects such as diarrhea, abdominal pain and nausea.
Terminology
HE: HE is a worsening of brain function that occurs when the liver is no longer able to remove toxic substances in the blood; Systematic review: A literature review focused on a research question that tries to identify, appraise, select and synthesize all high quality research evidence relevant to that question; Meta-analysis: A combination of the results of several studies that address a set of related research hypotheses.
Peer review
Rifaximin is a novel antimicrobial agent with a wide spectrum of activity that has shown promise as an alternative antimicrobial treatment option for HE. Rifaximin appears to be at least as effective as other conventional drug therapy and has been associated with fewer adverse events due to its limited systemic absorption.
Footnotes
Peer reviewer: Vezali Elena, MD, Department of Hepatology, “Hygeia” Diagnostic and Therapeutic Center of Athens, Eruthrou Staurou 4, Marousi 15123, Greece
S- Editor Sun H L- Editor Webster JR E- Editor Li JY
References
- 1.Schafer DF, Jones EA. Hepatic encephalopathy. In: Zakim D, Boyer TD, editors. Hepatology. A textbook of liver disease. Philadelphia: W. B. Saunders; 1990. pp. 447–460. [Google Scholar]
- 2.Abou-Assi S, Vlahcevic ZR. Hepatic encephalopathy. Metabolic consequence of cirrhosis often is reversible. Postgrad Med. 2001;109:52–54, 57-60, 63-65, passim. doi: 10.3810/pgm.2001.02.850. [DOI] [PubMed] [Google Scholar]
- 3.de Melo RT, Charneski L, Hilas O. Rifaximin for the treatment of hepatic encephalopathy. Am J Health Syst Pharm. 2008;65:818–822. doi: 10.2146/ajhp070298. [DOI] [PubMed] [Google Scholar]
- 4.Fitz G. Hepatic encephalopathy, hepatopulmonary syndrome, coagulopathy and other complications of chronic liver disease. In: Feldman M, Friedman LS, Sleisenger MH, editors. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. 7th ed. Philadelphia: Saunders; 2002. [Google Scholar]
- 5.Schiano TD. Complications of chronic liver disease. In: Friedman S, Grendell J, McQuaid K, editors. Current Diagnosis and Treatment in Gastroenterology. 2nd ed. Lange Current Series; 2002. [Google Scholar]
- 6.Blei AT, Córdoba J. Hepatic Encephalopathy. Am J Gastroenterol. 2001;96:1968–1976. doi: 10.1111/j.1572-0241.2001.03964.x. [DOI] [PubMed] [Google Scholar]
- 7.Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716–721. doi: 10.1053/jhep.2002.31250. [DOI] [PubMed] [Google Scholar]
- 8.Zeneroli ML, Avallone R, Corsi L, Venturini I, Baraldi C, Baraldi M. Management of hepatic encephalopathy: role of rifaximin. Chemotherapy. 2005;51 Suppl 1:90–95. doi: 10.1159/000081994. [DOI] [PubMed] [Google Scholar]
- 9.Conn HO, Leevy CM, Vlahcevic ZR, Rodgers JB, Maddrey WC, Seeff L, Levy LL. Comparison of lactulose and neomycin in the treatment of chronic portal-systemic encephalopathy. A double blind controlled trial. Gastroenterology. 1977;72:573–583. [PubMed] [Google Scholar]
- 10.Mullen KD, Dasarathy S. Hepatic encephalopathy. In: Schiff ER, Sorrell MF, Maddrey WC, editors. Schiff’s diseases of the liver. 8th ed. New York: Lippincott-Raven; 1999. [Google Scholar]
- 11.Tarao K, Ikeda T, Hayashi K, Sakurai A, Okada T, Ito T, Karube H, Nomoto T, Mizuno T, Shindo K. Successful use of vancomycin hydrochloride in the treatment of lactulose resistant chronic hepatic encephalopathy. Gut. 1990;31:702–706. doi: 10.1136/gut.31.6.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morgan MH, Read AE, Speller DC. Treatment of hepatic encephalopathy with metronidazole. Gut. 1982;23:1–7. doi: 10.1136/gut.23.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Phongsamran PV, Kim JW, Cupo Abbott J, Rosenblatt A. Pharmacotherapy for hepatic encephalopathy. Drugs. 2010;70:1131–1148. doi: 10.2165/10898630-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 14.Debbia EA, Maioli E, Roveta S, Marchese A. Effects of rifaximin on bacterial virulence mechanisms at supra- and sub-inhibitory concentrations. J Chemother. 2008;20:186–194. doi: 10.1179/joc.2008.20.2.186. [DOI] [PubMed] [Google Scholar]
- 15.Gerard L, Garey KW, DuPont HL. Rifaximin: a nonabsorbable rifamycin antibiotic for use in nonsystemic gastrointestinal infections. Expert Rev Anti Infect Ther. 2005;3:201–211. doi: 10.1586/14787210.3.2.201. [DOI] [PubMed] [Google Scholar]
- 16.Leevy CB, Phillips JA. Hospitalizations during the use of rifaximin versus lactulose for the treatment of hepatic encephalopathy. Dig Dis Sci. 2007;52:737–741. doi: 10.1007/s10620-006-9442-4. [DOI] [PubMed] [Google Scholar]
- 17.Festi D, Mazzella G, Orsini M, Sottili S, Sangermano A, Li Bassi S, Parinin P, Ferrieri A, Falcucci M, Grossi L, et al. Rifaximin in the treatment of chronic hepatic encephalopathy; results of a multicenter study of efficacy and safety. Current Therapeuitic Research. 1993;54:598–609. [Google Scholar]
- 18.Massa P, Vallerino F, Dodero M. Treatment of hepatic encephalopathy with rifaximin: Double-blind, double-dummy study versus lactulose. Eur J Clin Res. 1993;4:7–18. [Google Scholar]
- 19.Als-Nielsen B, Gluud LL, Gluud C. Non-absorbable disaccharides for hepatic encephalopathy: systematic review of randomised trials. BMJ. 2004;328:1046. doi: 10.1136/bmj.38048.506134.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang Q, Jiang XH, Zheng MH, Jiang LM, Chen YP, Wang L. Rifaximin versus nonabsorbable disaccharides in the management of hepatic encephalopathy: a meta-analysis. Eur J Gastroenterol Hepatol. 2008;20:1064–1070. doi: 10.1097/MEG.0b013e328302f470. [DOI] [PubMed] [Google Scholar]
- 21.Paik YH, Lee KS, Han KH, Song KH, Kim MH, Moon BS, Ahn SH, Lee SJ, Park HJ, Lee DK, et al. Comparison of rifaximin and lactulose for the treatment of hepatic encephalopathy: a prospective randomized study. Yonsei Med J. 2005;46:399–407. doi: 10.3349/ymj.2005.46.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams R, James OF, Warnes TW, Morgan MY. Evaluation of the efficacy and safety of rifaximin in the treatment of hepatic encephalopathy: a double-blind, randomized, dose-finding multi-centre study. Eur J Gastroenterol Hepatol. 2000;12:203–208. doi: 10.1097/00042737-200012020-00012. [DOI] [PubMed] [Google Scholar]
- 23.Conn Ho. Asterixis in non-hepatic disorders. Am J Med. 1960;29:647–661. doi: 10.1016/0002-9343(60)90098-x. [DOI] [PubMed] [Google Scholar]
- 24.Pellegrini A, Ubiali E, Orsato R, Schiff S, Gatta A, Castellaro A, Casagrande A, Amodio P. Electroencephalographic staging of hepatic encephalopathy by an artificial neural network and an expert system. Neurophysiol Clin. 2005;35:162–167. doi: 10.1016/j.neucli.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Pedretti G, Calzetti C, Missale G, Fiaccadori F. Rifaximin versus neomycin on hyperammoniemia in chronic portal systemic encephalopathy of cirrhotics. A double-blind, randomized trial. Ital J Gastroenterol. 1991;23:175–178. [PubMed] [Google Scholar]
- 26.Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions, version 5.0.2. Available from: http: //www.cochrane-handbook.org.
- 27.Review Manager (RevMan). Computer program Version 5.0. The Cochrane Collaboration; 2008. [Google Scholar]
- 28.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 29.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 31.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Song H, Lee KS, Kim MH, Paik YH, Moon BS, Yoon SH. The clinical efficacy of rifaximin in the treatment of hepatic encephalopathy (comparison with lactulose) [abstract] Hepatology. 2000;32:407. [Google Scholar]
- 33.Di Piazza S, Gabriella Filippazzo M, Valenza LM, Morello S, Pastore L, Conti A, Cottone S, Pagliaro L. Rifaximine versus neomycin in the treatment of portosystemic encephalopathy. Ital J Gastroenterol. 1991;23:403–407. [PubMed] [Google Scholar]
- 34.Parini P, Cipolla A, Ronchi M, Salzetta A, Mazzella G, Roda E. Effect of rifaximin and paromomycin in the treatment of porto-systemic encephalopathy. Current Therapeuitic Research. 1992;52:34–39. [Google Scholar]
- 35.Bucci L, Palmieri GC. Double-blind, double-dummy comparison between treatment with rifaximin and lactulose in patients with medium to severe degree hepatic encephalopathy. Curr Med Res Opin. 1993;13:109–118. doi: 10.1185/03007999309111539. [DOI] [PubMed] [Google Scholar]
- 36.Mas A, Rodés J, Sunyer L, Rodrigo L, Planas R, Vargas V, Castells L, Rodríguez-Martínez D, Fernández-Rodríguez C, Coll I, et al. Comparison of rifaximin and lactitol in the treatment of acute hepatic encephalopathy: results of a randomized, double-blind, double-dummy, controlled clinical trial. J Hepatol. 2003;38:51–58. doi: 10.1016/s0168-8278(02)00350-1. [DOI] [PubMed] [Google Scholar]
- 37.Fera G, Agostinachio F, Nigro M, Schiraldi O, Ferrieri A. Rifaximin in the treatment of hepatic encephalopathy. European J Clinical Res. 1993;4:57–63. [Google Scholar]
- 38.Miglio F, Valpiani D, Rossellini SR, Ferrieri A. Rifaximin, a non-absorbable rifamycin, for the treatment of hepatic encephalopathy. A double-blind, randomised trial. Curr Med Res Opin. 1997;13:593–601. doi: 10.1185/03007999709113333. [DOI] [PubMed] [Google Scholar]
- 39.Loguercio C, Federico A, De Girolamo V, Ferrieri A, Del Vecchio Blanco C. Cyclic treatment of chronic hepatic encephalopathy with rifaximin. Results of a double-blind clinical study. Minerva Gastroenterol Dietol. 2003;49:53–62. [PubMed] [Google Scholar]
- 40.Riordan SM, Williams R. Treatment of hepatic encephalopathy. N Engl J Med. 1997;337:473–479. doi: 10.1056/NEJM199708143370707. [DOI] [PubMed] [Google Scholar]
- 41.Berk DP, Chalmers T. Deafness complicating antibiotic therapy of hepatic encephalopathy. Ann Intern Med. 1970;73:393–396. doi: 10.7326/0003-4819-73-3-393. [DOI] [PubMed] [Google Scholar]
- 42.Greenberg LH, Momary H. Audiotoxicity and nephrotoxicity due to orally administered neomycin. JAMA. 1965;194:827–828. [PubMed] [Google Scholar]
- 43.Brigidi P, Swennen E, Rizzello F, Bozzolasco M, Matteuzzi D. Effects of rifaximin administration on the intestinal microbiota in patients with ulcerative colitis. J Chemother. 2002;14:290–295. doi: 10.1179/joc.2002.14.3.290. [DOI] [PubMed] [Google Scholar]
- 44.Koo HL, DuPont HL. Rifaximin: a unique gastrointestinal-selective antibiotic for enteric diseases. Curr Opin Gastroenterol. 2010;26:17–25. doi: 10.1097/MOG.0b013e328333dc8d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mechcatie E. Rifaximin Approved for Hepatic Encephalopathy. Gastroenterology. 2010:18–19. [Google Scholar]
- 46.Bass NM, Mullen KD, Sanyal A, Poordad F, Neff G, Leevy CB, Sigal S, Sheikh MY, Beavers K, Frederick T, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071–1081. doi: 10.1056/NEJMoa0907893. [DOI] [PubMed] [Google Scholar]
- 47.Huang E, Esrailian E, Spiegel BM. The cost-effectiveness and budget impact of competing therapies in hepatic encephalopathy - a decision analysis. Aliment Pharmacol Ther. 2007;26:1147–1161. doi: 10.1111/j.1365-2036.2007.03464.x. [DOI] [PubMed] [Google Scholar]