Abstract
Cowden syndrome is a rare autosomal dominant disorder that is characterized by multiple hamartomas in a variety of tissues and this is associated with germline mutations in the phosphatase and tensin homologue (PTEN) gene, which is the tumor suppressor gene located on chromosome 10q23.3. It is characterized by multiple hamartomatous neoplasms of the skin, oral mucosa, gastrointestinal (GI) tract, bones, central nervous system, eyes, and genitourinary tract. Cowden syndrome does not have increased risk of GI malignancy; however, it has an increased risk of breast, thyroid and endometrial cancer development. Here the authors report a rare case of Cowden syndrome incidentally diagnosed from multiple gastric polyposis. A 29-year-old woman presented with multiple gastric polyps. The laboratory results were normal except for mild anemia, with a hemoglobin level of 11.9 g/dL. Esophagogastroduodenoscopy revealed multiple gastric, duodenal polyps and esophageal acanthosis. Colonoscopy revealed possible hamartomatous polyps in the rectum. Under the suspicion of Cowden syndrome, sonography of the thyroid and breasts was carried out, which revealed multiple thyroid masses. Subsequent fine-needle aspiration biopsy revealed the presence of clusters of follicular epithelial cells, and due to the possibility of malignancy, the patient underwent total thyroidectomy. The pathology was reported as invasive follicular carcinoma. A gene study by direct sequencing showed the presence of a PTEN mutation (c.633C > A /p.Cys211*).
Keywords: Cowden syndrome, Gastric polyposis, Pho-sphatase and tensin homologue mutation, Esophageal acanthosis, Thyroid cancer
INTRODUCTION
Cowden syndrome is a rare autosomal dominant disorder that is characterized by multiple hamartomas in a variety of tissues, and is associated with germline mutations in the phosphatase and tensin homologue (PTEN) gene, which is a tumor suppressor gene located on chromosome 10q23.3[1,2]. It is characterized by multiple hamartomatous neoplasms of the skin, oral mucosa, gastrointestinal (GI) tract, bones, central nervous system, eyes and genitourinary tract. Cowden syndrome does not have an increased risk of GI malignancy; however, it has an increased risk of developing breast, thyroid and endometrial cancer[3]. Here we present a case of Cowden syndrome which was confirmed through genetic testing of the PTEN gene in a 29-year-old female patient with multiple gastric polyps on esophagogastroduodenoscopy (EGD), and thyroid cancer.
CASE REPORT
A 29-year-old woman presented with multiple gastric polyps, which were detected at a private clinic. On presentation, she complained of no symptoms other than dyspepsia; her vital signs were stable and the laboratory results were normal except for mild anemia, with a hemoglobin level of 11.9 g/dL.
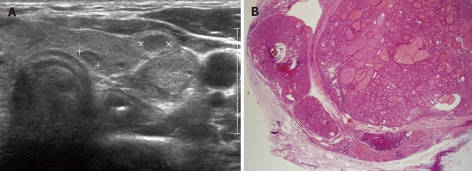
Multiple gastric, duodenal polyps and esophageal acanthosis were observed on EGD, and colonoscopy revealed possible hamartomatous polyps in the rectum and oral mucosal papillomatosis (Figure 1). The polyps, observed through EGD and colonoscopy, were shown upon pathologic analysis to be hamartomatous polyps accompanied by mild inflammation and fibrosis (Figure 2). She also had papillomatous papules around her lips and oral mucosal papillomatosis. Because of a high index of suspicion of Cowden syndrome, ultrasonography (USG) of the thyroid and breasts was carried out, which revealed multiple thyroid masses (Figure 3A). Fine-needle aspiration biopsy revealed the presence of clusters of follicular epithelial cells. Although benign lesions were expected on USG, because of the possibility of malignancy, left lobectomy and right enucleation with ipsilateral central node dissection were carried out. Pathology showed the lesions to be invasive follicular carcinoma, and right lobectomy with ipsilateral central node dissection (completion total thyroidectomy) was carried out (Figure 3B).
Figure 1.
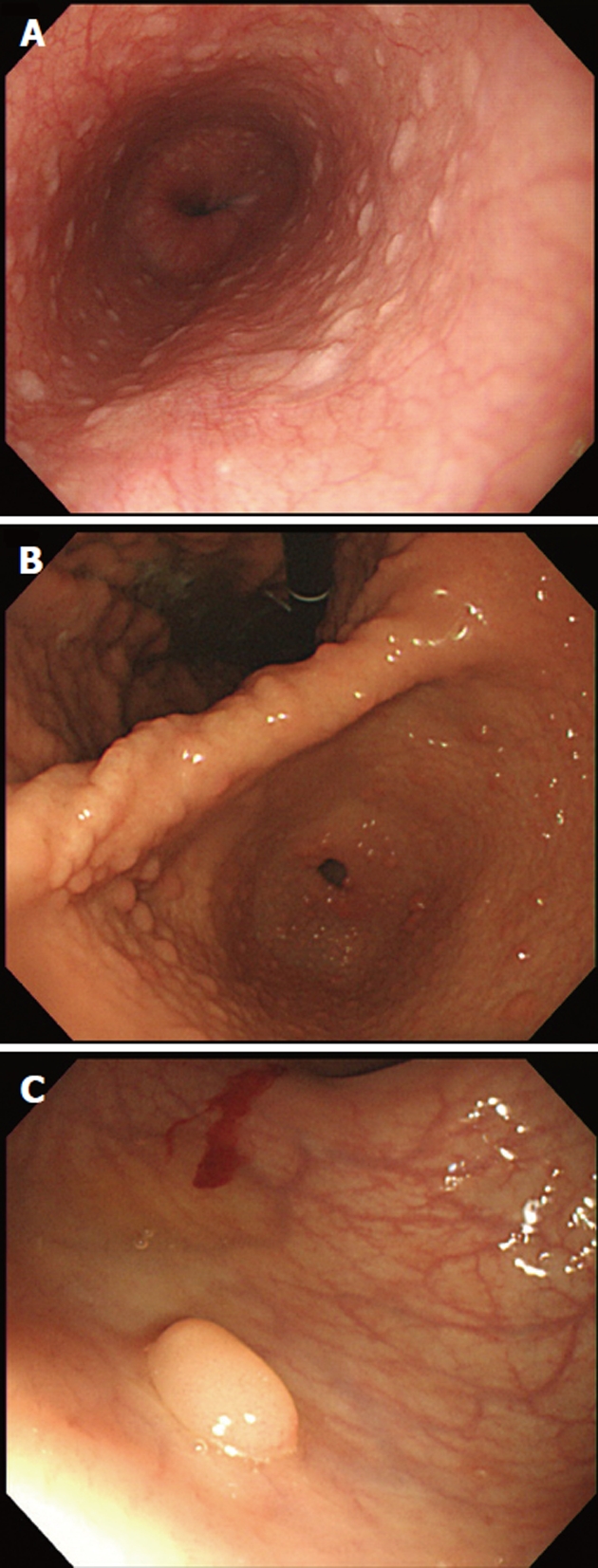
Endoscopic examination. A: Esophageal acanthosis was noted; B: Multiple gastric polyps were noted on entire stomach; C: Several polyps were observed at the rectum.
Figure 2.
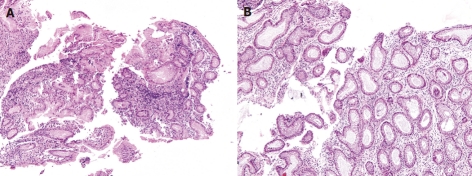
Microscopic examination (hematoxylin and eosin stain, × 100). A: Gastric polyp shows hyperplastic foveolar epithelium; B: Colon polyp exhibits dilated and branched crypts with slightly fibrotic stroma.
Figure 3.
Thyroid masses. A: Multiple thyroid masses in thyroid ultrasonography; B: Postoperative thyroid pathology showed invasive follicular carcinoma (hematoxylin and eosin stain, × 12.5).
Genetic analysis of the PTEN genes was performed. Genomic DNA was extracted from the patient’s whole blood. Polymerase chain reaction direct sequencing with primers targeting all the coding regions and flanking introns was performed. Sequencing analysis of the PTEN gene revealed a heterozygous transition of C to A at nucleotide 633 in exon 6 (NM_000314.4 c.633C > A), which is a nonsense mutation, making a stop codon (p.Cys211*) (Figure 4). This mutation has been previously reported and seems to be the cause of the disorder in this patient[4]. During the course of the evaluation of the patient, the authors investigated other members of the patient’s family, and a history of endometrial cancer was identified in the patient’s mother. However, she refused further evaluation related to Cowden syndrome, and thus further examination could not be carried out.
Figure 4.
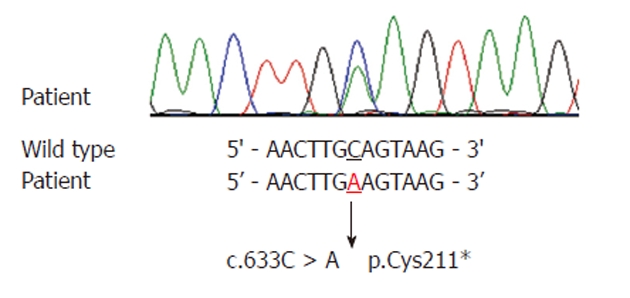
Sequencing result of the phosphatase and tensin homologue mutation. A heterozygous transition of C to A at nucleotide 633 in exon 6 (c.633C > A), making a stop codon (p.Cys211*).
DISCUSSION
Cowden syndrome was first described by Lloyd and Dennis in 1963 in a case report on a patient named Rachel Cowden[5]. Later, in 1972, Weary et al[6] confirmed the existence of this new syndrome through examination of five additional patients with similar clinical findings. The hamartomatous lesions observed in Cowden syndrome can arise in any of the three embryonic germ cell layers and thus it may be ectodermal, mesodermal or endodermal in origin[7]. These lesions can arise in the derivatives of any of the three embryonic germ cell layers[3,7]. The cardinal manifestations of Cowden syndrome include facial trichilemmomas, which are hamartomas of the infundibulum of the hair follicle, acral keratoses and mucocutaneous papillomatous papules[8-10]. The incidence of Cowden syndrome was estimated to be 1:1 000 000[1]. However, after identification of the relevant gene, the estimate for the prevalence of Cowden syndrome has been increased to between 1 in 200 000 and 1 in 250 000[11,12]. The diagnosis of Cowden syndrome is mainly based on clinical criteria. Nowadays the criteria of the International Cowden Consortium are commonly used for making the diagnosis[7]. The first set of clinical criteria were proposed by Salem et al[8], and have subsequently been revised, most recently in the United States by the National Comprehensive Cancer Network, which published the Cowden syndrome testing criteria based on pathognomonic criteria along with major and minor diagnostic criteria. In 1996, the International Cowden Consortium identified germline PTEN mutations as being the cause of Cowden syndrome[1,2]. Cowden syndrome is now well recognized as a highly variable, autosomal-dominant hereditary cancer susceptibility syndrome that is characterized by multiple hamartomas and an increased risk of developing malignant transformations.
Cowden syndrome is an autosomal dominant inherited disorder with incomplete penetrance and variable expressivity. Since the first case report of this disorder in Korea in 1997[2], 20 such cases have been reported, but most of these cases involved breast cancer and gynecological cancers, with few involving colon polyps[13]. In this current case study, Cowden syndrome was identified in a patient with multiple gastric polyps, as was reported in 2010, and in addition, thyroid cancer was diagnosed through screening and surgically resecting the tumor, and the PTEN mutation was confirmed through genetic testing (c.633C > A/p.Cys211*)[14].
Considering the worldwide prevalence of Cowden syndrome, the number of cases reported in Korea appears to be disproportionately small. Although this difference must be examined in the context of ethnicity, it may also imply that some cases have gone undetected. However, since Cowden syndrome, as aforementioned, may involve malignancies in several organs, aggressive diagnostic evaluation should be carried out in suspected cases, and screening should be performed in light of the autosomal dominant inheritance of this disorder.
Finally, as was the case with this patient, adequate screening for the organs known to develop malignancies in Cowden syndrome should be carried out in suspected patients. Even when benign lesions are suspected, the potential for malignant lesions must be kept in mind, and further pathologic examination and surgical resection should be aggressively performed without reservation.
In conclusion, this case is a reminder of the importance of early screening for patients suspected of having Cowden syndrome, and female patients should be evaluated for lesions in the breasts and thyroid. Even if benign lesions are suspected, there have been published reports on cases diagnosed with cancer postoperatively; consequently, the possibility of malignancy should always be considered.
Footnotes
Peer reviewer: Maha Maher Shehata, Professor, Internal Medicine, Department of Gastroenterology and Hepatology Unit, Medical Specialized Hospital, Mansoura, 35516, Egypt
S- Editor Shi ZF L- Editor Cant MR E- Editor Li JY
References
- 1.Nelen MR, Padberg GW, Peeters EA, Lin AY, van den Helm B, Frants RR, Coulon V, Goldstein AM, van Reen MM, Easton DF, et al. Localization of the gene for Cowden disease to chromosome 10q22-23. Nat Genet. 1996;13:114–116. doi: 10.1038/ng0596-114. [DOI] [PubMed] [Google Scholar]
- 2.Liaw D, Marsh DJ, Li J, Dahia PL, Wang SI, Zheng Z, Bose S, Call KM, Tsou HC, Peacocke M, et al. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet. 1997;16:64–67. doi: 10.1038/ng0597-64. [DOI] [PubMed] [Google Scholar]
- 3.Eng C. PTEN: one gene, many syndromes. Hum Mutat. 2003;22:183–198. doi: 10.1002/humu.10257. [DOI] [PubMed] [Google Scholar]
- 4.Lee SA, Kim EH, Lee YM, Lee W, Min WK, Lee YJ, Huh JR, Lee WJ. A novel mutation of the succinate dehydrogenase B gene in a Korean family with pheochromocytoma. Fam Cancer. 2010;9:643–646. doi: 10.1007/s10689-010-9359-0. [DOI] [PubMed] [Google Scholar]
- 5.Lloyd KM, Dennis M. Cowden’s disease. A possible new symptom complex with multiple system involvement. Ann Intern Med. 1963;58:136–142. doi: 10.7326/0003-4819-58-1-136. [DOI] [PubMed] [Google Scholar]
- 6.Weary PE, Gorlin RJ, Gentry WC, Comer JE, Greer KE. Multiple hamartoma syndrome (Cowden’s disease) Arch Dermatol. 1972;106:682–690. [PubMed] [Google Scholar]
- 7.Eng C. Will the real Cowden syndrome please stand up: revised diagnostic criteria. J Med Genet. 2000;37:828–830. doi: 10.1136/jmg.37.11.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salem OS, Steck WD. Cowden’s disease (multiple hamartoma and neoplasia syndrome). A case report and review of the English literature. J Am Acad Dermatol. 1983;8:686–696. doi: 10.1016/s0190-9622(83)70081-2. [DOI] [PubMed] [Google Scholar]
- 9.Kovich O, Cohen D. Cowden’s syndrome. Dermatol Online J. 2004;10:3. [PubMed] [Google Scholar]
- 10.Jornayvaz FR, Philippe J. Mucocutaneous papillomatous papules in Cowden’s syndrome. Clin Exp Dermatol. 2008;33:151–153. doi: 10.1111/j.1365-2230.2007.02602.x. [DOI] [PubMed] [Google Scholar]
- 11.Li FP, Abramson DH, Tarone RE, Kleinerman RA, Fraumeni JF, Boice JD. Hereditary retinoblastoma, lipoma, and second primary cancers. J Natl Cancer Inst. 1997;89:83–84. doi: 10.1093/jnci/89.1.83. [DOI] [PubMed] [Google Scholar]
- 12.Nelen MR, Kremer H, Konings IB, Schoute F, van Essen AJ, Koch R, Woods CG, Fryns JP, Hamel B, Hoefsloot LH, et al. Novel PTEN mutations in patients with Cowden disease: absence of clear genotype-phenotype correlations. Eur J Hum Genet. 1999;7:267–273. doi: 10.1038/sj.ejhg.5200289. [DOI] [PubMed] [Google Scholar]
- 13.Lee HR, Moon YS, Yeom CH, Kim KW, Chun JY, Kim HK, Choi HS, Kim DK, Chung TS. Cowden’s disease--a report on the first case in Korea and literature review. J Korean Med Sci. 1997;12:570–575. doi: 10.3346/jkms.1997.12.6.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yi JE, Cho DH, Woo JG, Kwon OU, Jung KW, Jung CW, Yoo GJ, Sim SG. Cowden’s Disease Detected by Gastric Polyposis during Endoscopy in a Routine Check Up: A Case Report. Korean J Gastrointest Endosc. 2010;40:361–365. [Google Scholar]