Abstract
Cerebral venous thrombosis is increasing common disease in daily practice with sharing clinical nonspecific symptoms. This disorder is potentially lethal but treatable, oftenly it was overlooked in both clinical and radiologic in routine practice. Whenever, clinical suspected, prompt investigation by noninvasive imaging Magnetic resonance (MR) or advanced modilities such as cerebral venous thrombosis (CVT), MRV (MR Venography) will helpful in prompt diagnosis and treatment. These imaging modalities may reveal either direct sign (visualization of intraluminal clot) and indirect signs (paranchymatous change, intracranial hemorrhage). By using of effective treatment will improve the prognosis of the patient. This review summarizes insights into etiology, incidence, imaging modalities and current of the treatment.
Key words: cerebral venous thrombosis, computed tomography, magnetic resonance.
Introduction
Clinical manifestations of cerebral venous thrombosis (CVT) are often nonspecific,1 thus making difficulty in definite diagnosis.2 The presentation of venous thrombosis is usually subacute, with symptoms evolving over days to weeks.3 Common presentation includes headache, focal neurologic deficits, seizures, and altered consciousness.1 The other symptoms and signs include obscuration of vision, alteration of consciousness, nausea, vomiting, papilledema, cranial nerve palsies and coma.4 Most patients with deep venous thrombosis presented with symptoms of elevated intracranial pressure.5
There was no association between localization of headache and site of sinus thrombosis except sigmoid sinus thrombosis. About 61% of patients with involvement of sigmoid sinus alone or in combination with transverse sinus had pain in the occipital and neck regions.6
Focal neurological deficit comprises hemiparesis usually with facial sparing and may occur depending on the area involved.6,7 In some patients, contralateral cortical veins may be involved resulting in paresis or paralysis of the opposite lower limb.7 Cranial nerve syndromes are seen with venous sinus thrombosis.6 Sinus thrombosis may occur as part of the intracranial hypertension syndrome (also known as pseudotumor cerebri) and CVT should be excluded with imaging in all patients with such manifestation.8
Isolated subarachnoid hemorrhage is a rare presentation of CVT1,4,9 and should be considered in the workup, especially when the basilar cisterns are not involved.6 Intracerebral hemorrhages (ICH) occur in approximately one-third of patients with CVT, and are usually associated with a more severe clinical presentation at onset and a worse outcome.10 There are four statistically significant predictors influencing outcome of CVST. They were underlying malignancy, low GCS, presence of hemorrhagic infarction (for poor outcome), and involvement of lateral sinus (for good outcome).11
Materials and Methods
Etiology
The etiology of CVT is multifactorial1,3 and may involve one or more mechanisms.1,6 No cause is identified in as many as 25%.8 Changes in the vessel walls blood flow or coagulability of blood can develop cerebral venous thrombosis.7 Causal factors may be classified as local (related to intrinsic or mechanical conditions of the cerebral veins and dural sinuses) or systemic (related to clinical conditions that promote thrombosis).4,8 The local conditions that alter the venous blood flow, including brain and skull damage, intracranial and local regional infection may potentiate the development of thrombosis.4,8 The systemic causes include hormonal (pregnancy or puerperium, estroprogestative and steroid therapy), surgery, immobilization, hematologic and hyper-coagulable disorders, connective tissue disease, malignancy, systemic infection and dehydration.4,8 The frequency of these etiologic factors depends on age. In neonates, acute systemic illness, such as shock or dehydration, may be the cause. Frequent causes in older children include local infection, such as otitis media, mastoiditis, and coagulopathy. In adults, intrinsic or acquired coagulopathies become the most important factors, contributing to as many as 70% of cases. Infection contributes to less than 10% of cases in adults. In women of childbearing age, oral contraceptive use and pregnancy are strong risk factors.1 The main risk factors for deep venous cerebral thrombosis include oral anticonception, post partum, trauma, pregnancies, Crohn disease, sickle cell disease, tumor, protein S deficiency, inflammation, paroxysmal nocturnal haemoglobinuria, diabetes mellitus, and nephrotic syndrome.5
Incidence
Cerebral venous thrombosis (CVT) is responsible for 1–2% of all strokes in adults12 and affect all age groups.13 They estimated annual incidence of 3–4 cases per million people and a mortality rate of 8%.12 Isolated CVT (ICoVT) (ie, without sinus involvement) appears extremely rare and has been mainly reported as isolated case reports or in small series.14 Multiple locations of thrombosis, particularly in the contiguous transverse and sigmoid sinuses, are found in as many as 90% of patients.8
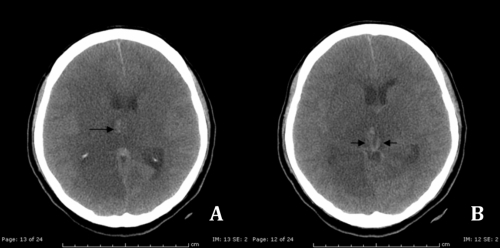
The deep cerebral veins (Figure 1) [ie, the internal cerebral veins (ICVs), the basal veins of Rosenthal (BVR) and their tributaries] are involved in approximately 10% of patients.15 Cortical venous involvement is seen in 6% of patients but is likely to be underreported when the dominant imaging finding is dural sinus involvement.8
Figure 1.
Computed-tomography scan demonstrates a) hyperdense of intraluminal blood clot in deep venous system (internal cerebral veins) (arrows); b) diffuse hypodensity areas in bilateral thalami and basal ganglia represent cerebral venous infarction.
Investigation
Neuroimaging is usually contributing a key role in the diagnosis of CVT. Radiologist often plays an important role in establishing the diagnosis of CVT. Though angiography is still considered to be the gold standard.2 Magnetic resonance (MR) imaging, un-enhanced computed tomography (CT), unenhanced time-of-flight MR venography, and contrast material-enhanced MR venography and CT venography are particularly useful techniques for detecting cerebral venous and brain parenchymal changes that may be related to thrombosis.9.
The pathophysiology of brain parenchymal involvement in venous occlusion differs from that in arterial occlusion.9 Mechanism for venous infarction is the obstruction of venous drainage with increasing venous pressure in the affected region of brain. CVT progresses to cerebral venous infarction in approximately 50% of cases.4 Parenchymal changes may be secondary to cytotoxic edema, vasogenic edema, or intracranial hemorrhage.9
The radiological signs of CVT are usually classified as direct and indirect. The direct signs consist of demonstration of the thrombus on imaging or indirect, as when there are ischemic or vascular changes related to the venous outflow disturbance.3,4 Indirect signs are not specific, but they should draw attention and prompt searching for direct visualization of a cortical or sinus thrombus.9 The indirect signs are such as parenchyma change by brain swelling, white matter edema, cortical sulci effacement, loss of gray-white matter differentiation, hemorrhagic infarction as hemorrhagic spot in white matter edema and yet Kozic et al.16 found subtle curvilinear hyperdensities within the left posterior cerebral hemisphere associated with typical signs of developing venous infarction from dural venous thrombosis.
Computed tomography
CT is the initial modality of investigation of choice for most neurological conditions. Its widespread availability, comparatively shorter scan times and lower cost.2 It is reported that in the acute phase of CVT, almost 38% of CT scans are normal.5 Thrombus is visible on non-enhanced CT as a high-attenuation lesion in the venous channel, producing dense triangle or cord sign represents an intravascular acute blood clot.3,4 This sign is reported in 20% of patients and takes approximately 1–2 weeks to disappear. However, similarly increased attenuation of the cerebral venous sinuses may also represent polycythemia, dehydration, a subjacent subarachnoid or subdural hemorrhage and nonmyelinated brain in neonates makes sinuses appear unusually attenuating.4,8,9 Increased attenuation in the sinus (Figure 2A, Figure 3, Figure 4A,B) may be the only finding suggestive of sinus thrombosis on unenhanced CT images, and patients with this sign should be further evaluated with contrast-enhanced CT, MR imaging, or both, in the proper clinical scenario.8
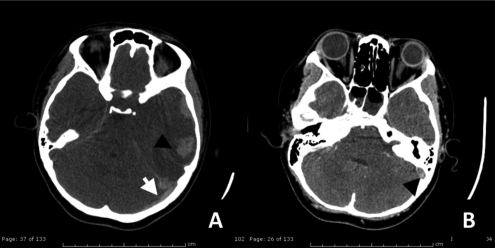
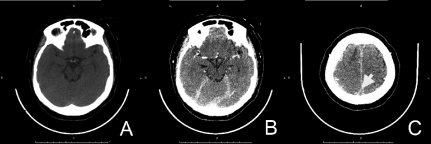
Figure 2.
(A) Axial computed-tomography noncontrast study) demonstrated hyperdensity of acute thrombus in left transverse sigmoid sinus (white arrow) with mixed hypo-hyperdensity lesion at left parieto-temporal region, measured about 4.9×2.8 cm in size (arrowhead). (B) Contrast enhanced computed-tomography scan shows filling defect in left transverse-sigmoid sinus (black arrow). All of these findings are suggestive of left sigmoid and transeverse sinovenous sinus thromboses with acute intracerebral hemorrhage.
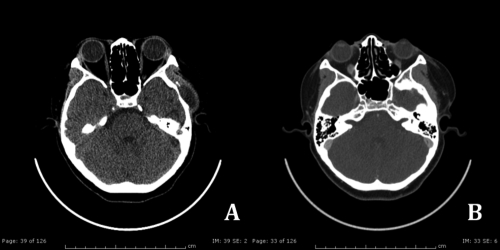
Figure 3.
S/P fibrinolytic agent treatment for 6 months. Follow up revealed A) further resolving of hematoma and B) almost disappearance of intraluminal clot (black arrow).
Figure 4.
Axial compted-tomography noncontrast study revealed a) hyperdensity cord sign and intraluminal clot sign at anterior of superior sagittal sinus (arrow head) and right cortical vein (arrows) . Contrast enhanced CT scan demonstrated b) empty delta sign (arrowhead).
Early changes in CVT are often subtle, with brain edema and swelling of the gyri. Venous infarction demonstrates as a low-attenuation lesion with or without subcortical hemorrhage (Figure 2A and Figure 4A,B). Brain lesions are related to a venous distribution (e.g. bilateral parasagittal hemispheric lesions-superior sagittal sinus thrombosis, ipsilateral temporooccipital and cerebellar lobe lesions-transverse sinus thrombosis, bilateral thalamic lesions-deep cerebral venous thrombosis). In Crombe D et al. study, the most frequently encountered sign is a hypodensity of the thalami and internal capsule (76%) and hemorrhagic infarction (19%). The extensive edema of the thalami and basal ganglia (Figure 1) may cause hydrocephalus due to compression of the foramen of Monro or the third ventricle.5 Thrombosis of a deep cerebral vein can very rarely occurring unilaterally.4,9 On contrast administration, non visualization of the thrombosed sinus is seen as an empty delta sign (Figure 2B, 4B, 5A–C).3 However, this direct evidence may be available in only 20–30% of cases of sinus thrombosis, and rarely in isolated cortical vein thrombosis.3 Transcortical medullary veins can become sources of collateral blood drainage. Subsequent dilatation of these vessels causes them to be visible at contrast-enhanced CT. Tentorial enhancement is caused by prominence of dural collaterals. Small subdural hematomas or effusion are seen occasionally.4,9 Because of volume averaging, conventional axial CT scans can fail to demonstrate a hyperattenuating sinus or an empty delta sign in the horizontal segment of the superior sagittal sinus or transverse sinus (Figure 6A–C).4 The empty delta sign can disappear in chronic stages with enhancement of organized clot.4
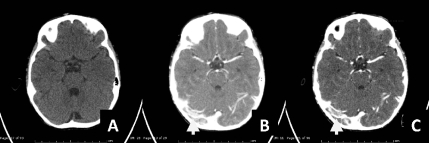
Figure 5.
A,B,C. Computed-tomography brain scan in post contrast study revealed filling defect in superior sagittal sinus could be superior sagittal sinus thrombosis.
Figure 6.
Axial computed-tomography noncontrast study revealed A) isodensity lesion at right transverse sinus in non contrast image which showing as filling defect along right transverse sinus, right sigmoid sinus and right jugular bulb in post contrast image B,C), suggestive of partial venous sinus thrombosis.
Variant anatomy of the torcular herophili is common and may lead to diagnostic error, particularly in the interpretation of CT images. A high or asymmetric bifurcation may resemble an intrasinus thrombus, produced a pseudo empty delta sign.8
CT venography
CTV (Figure 7A–D) has proved to be a reliable method to investigate the structure of the cerebral veins, with a reported sensitivity of 95% with multiplanar reformatted (MPR) images when compared with digital subtraction angiography (DSA) as the standard of reference.4,9 CTV is a promising for better delineation of venous anatomy, and equally sensitive for demonstrating thrombosis.3 However, common variants of the sinovenous system should not be mistaken for sinus thrombosis.9 CT venography is standardized and involves the following steps:
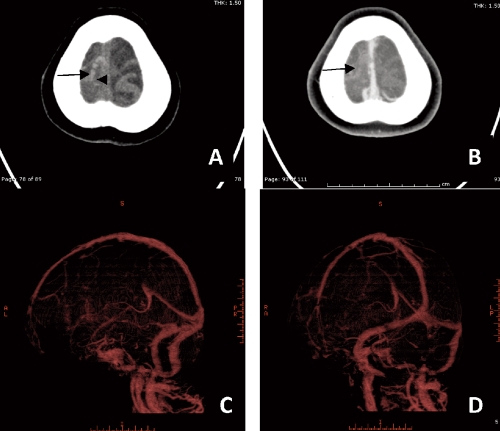
Figure 7.
Axial computed-tomography scan demonstrates A,B) intraluminal hyperdensity of clot at right sided of superior sagittal sinus wall, cortical veins of right frontal regions (cord sign, arrow). CT venography showed C,D) no contrast filled in thrombosed cortical vein in right frontal region. The dural venous sinus is well opacity by contrast medium.
First, two-dimensional (2D) MPR images are used to visualize dural venous sinuses and cerebral veins, with adequate window level and width. Second, 2D maximum intensity projection (MIP) series are created and saved. Optional reformations include 3D MIP and volume rendering display algorithms.4 CT and CTV remains a rapid screening modality for early diagnosis of CVT in the emergency setting.3 In patients with unenhanced CT findings suggestive of venous thrombosis, Mutidetector Computed Tomography (MDCT) venography can be performed without delay to confirm the diagnosis and to start appropriate therapy immediately.9
Magnetic resonance imaging
Magnetic resonance imaging (MRI) with MR venography (Figure 8A–8H) has become the investigation of choice in diagnosis of CVT. MRI is more sensitive in picking up the thrombus, and demonstrates age dependant signal characteristics.3 One of the most important findings on MRI is abnormal signal intensity within the venous structure, indicating altered flow and thrombus formation. Gradient recalled-echo (GRE) T2*WI is becoming more widely used as a standard MR pulse sequence because of its sensitivity to acute intracranial hemorrhage. The signal intensity of CVT and normal flow states can both be variable, leading to diagnostic confusion.12 Acute stage thrombus (within the first 7 days after clinical symptoms) has been shown to exhibit very subtle signal intensity alterations on noncontrast MR that can be mimic a normal flow void on T2WI.4,12 One of the major reasons for the variable appearances of venous thrombus is thought to be alterations in hemoglobin oxygenation and iron oxidation state within trapped red-blood cells or extracellularly within the thrombus itself.12 In the acute stage, thrombus is usually isointense on T1WI and hypointense on T2WI (Figure 8A,B) and relates to paramagnetic deoxyhemoglobin within trapped red blood cells within the thrombus. However, by using of T2W_FLAIR as conventional sequence is also helpful in detection of acute thrombus. This will be high signal intensity in T2W_FLAIR sequence.17 In the subacute phase (7–14 days) divided into early and late subacute phase. Thrombus signal intensity of early subacute phase appeared hypersignal intensity on T1WI, and hyposignal intensity on T2WI, relating to intracellular methemoglobin. A late subacute phase can occur relating to extracellular methemoglobin in the evolving hyalinizing thrombus, where there is hypersignal intensity on both T1WI and T2WI. In the chronic stage (greater than 15 days), signal intensity is typically isointense on T1WI and hyperintense on T2WI and is probably related to the vascularized connective tissue of chronic thrombus.11 There has been recent interest in evaluating the appearance of intraluminal venous thrombi on DWI. Signal hyperintensity in thrombosed sinuses on diffusion weighted images, with corresponding diminishment in the mean apparent diffusion coefficient (ADC) values, has been described in 41% of patients with sinus thrombosis. The duration of clinical symptoms was longer and complete recanalization was less frequent in patients with restricted diffusion in the thrombus.8
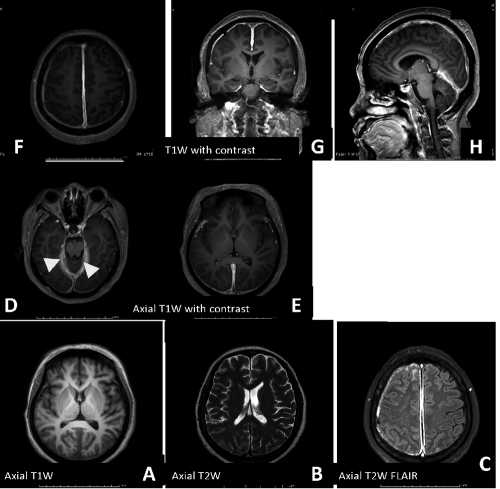
Figure 8.
Axial T1W, T2W and FLAIR demonstrate A,B,C) intermediate signal intensity T1W and hyposignal intensity T2W (acute thrombus) and high signal intensity of thrombus in FLAIR. Post gadolinium contrast study revealed D,E,F,G,H) empty delta sign (arrow) and tentorial dural enhancement (arrowhead).
Parenchymal abnormalities are better depicted and more commonly identified on MRI than CT.8 Diffused weighted MRI techniques allow subclassfication of parenchymal abnormalities as either primarily vasogenic edema (with increased ADC values presumably related to venous congestion) or primarily cytotoxic edema The hyposignal on ADC mapping can confirm this and make the (with decreased ADC values related to cellular energy disruption).8 In contrast with arterial ischemic states, many parenchymal abnormalities secondary to venous occlusion are reversible.8 The ADC mapping can make distinction between cytotoxic edema and venous congestion. An increased ADC values presumably related to venous congestion but decreased ADC values represent cytotoxic edema that related to cellular energy disruption.5,8 On the T1 contrast enhanced images, the venous congestion causes a marked enhancement of the subependymal plexus and the medullary veins that run perpendicular to the wall of the lateral ventricles.5 Hemorrhagic infarction can sometimes be present, cause susceptibility artefacts on T2*WI.5
Thalamic edema is the imaging hallmark of deep venous occlusion (e.g. internal cerebral vein, vein of Galen, or straight sinus), demonstrated hypersignal intensity on the T2 and FLAIR weighted images.5,8 It may extend into the caudate regions and deep white matter. Hemorrhage is noted in 19% of patients and typically is located in the thalami. Unilateral thalamic edema may occur but is rare.8 The lack of flow-void in the deep venous system is very suggestive for a venous thrombosis. At present, MR has become the method of choice for the diagnosis and follow-up of deep venous thrombosis because of its sensitivity to blood flow, its ability to visualise thrombus, and its non-invasiveness.5
Isolated cortical venous thrombosis is a relatively rare entity.8 It is particularly difficult to diagnose by using only T1WI, T2WI, and MRV imaging for different reasons (e.g. cortical veins are extremely variable in number, size, and location; occluded small veins at the cortical level are difficult to identify by using these MRI; only the occlusion of the largest veins is detectable on MRV).3 Typical parenchymal findings are areas of focal cortical edema or hemorrhage, which may be nonspecfic.8 The hyperintense vein sign is seen on MRI. Blooming artifacts within the thrombosed veins can be a very useful adjunct finding on GRE images.8
Pitfalls of MRI in the diagnosis of CVTinclude flow-related enhancement and refocusing of slow flow, which may mimic intraluminal thrombus, or paramagnetic blood products (intracellular deoxyhemoglobin or methemoglobin) mimicking a normal signal void on long repetition time images.4 The low T2 signal intensity at the early stage can be interpreted as a flow-void.5 The susceptibility effect of T2 * -weighted imaging does not always indicate intravascular thrombosis or blood products, since arterial flow voids, calcifications, and the bone surfaces of the skull commonly result in susceptibility artifacts.4 Variants of normal venous anatomy may mimic sinus thrombosis. These can be subdivided into venous anatomic variants that mimic occlusion (sinus atresia or hypoplasia), asymmetric or variant sinus drainage (occipital sinuses, sinus duplication), and normal sinus filling defects (arachnoid granulations, intrasinus septa). Arachnoid granulations typically have signal intensity similar to those of cerebrospinal fluid and appear as focal rounded filling defects with a characteristic anatomic distribution. If they are large and appear in the dominant sinus or only in the transverse sinus, they could cause venous obstruction and lead to symptoms of venous hypertension.8
MR venography
MRI combined (Figure 9) with MR venography (MRV) has largely replaced invasive cerebral angiography and conventional CT.2 CT is entirely normal in 10–20% of cases with proven CVT. MRV in conjunction with conventional MRI can accurately diagnose CVT and is reliable as the sole examination for this condition. MRV is currently considered to be the noninvasive test of choice for evaluation of the dural sinus. However, flow-related and susceptibility artifacts can impair the evaluation of the venous structures. The more invasive arterial DSA is still the standard of reference.2 MRV may be performed without the use of a contrast agent using the time-of-flight (TOF) technique or the phase contrast technique. Because these techniques use MR flow phenomena for contrast generation, they are subject to flow-related image artifacts. Similar to CTV, contrast-enhanced MRV (Figure 8D,E,F,G,H) takes advantage of luminal filling by contrast material rather than relying on the MR flow phenomena as in TOF or phase contrast MRV. Therefore, contrast-enhanced MRV is less likely to be affected by complex flow. Recently, gadolinium-enhanced MRV has been shown to be superior to TOF MRV and may offer the best evaluation using MRI.12 In Meckel et al study, compare with MR and MRV sequences for the diagnostic accuracy of a combined dynamic and static contrast-enhanced MRV in CVT, found that combo-4D MRV demonstrated the highest overall sensitivity (binary test) among the 4 assessed techniques, T2w, GRE, TOF, 4D-MRV.18 In dural venous sinus thrombosis alone, it showed the highest sensitivity (97%) and specificity (99%).18
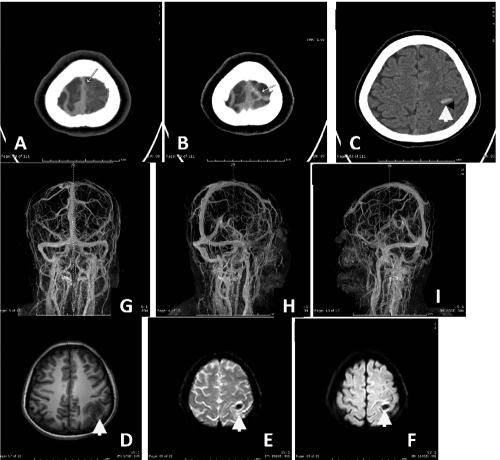
Figure 9.
Axial noncontrast computed-tomography scan revealed A,B,C) hyperdense of cord sign in left cortical vein with noncontrast filling in post contrast enhanced study. Hemorrhagic spot in left parietal lobe is noted (arrow). Magnetic resonance imaging shows D,E,F) hyposignal T1W, T2W_FFE (shows blooming susceptibility effct (arrow) of hemorrhage in left parietal lobe. Post contrast enhanced MR venography showed G,H,I) a small filling defect at superior sagittal sinus (seen on 3D_T1W/Gd) and lacking of cortical vein of left high parietal region corresponding with CT imaging, compatible with cerebral venous thrombosis.
The combined analysis of dynamic contrast-enhancement patterns enabled the identification of chronic sinus obliteration by its delayed enhancement characteristics on the dynamic part of combo-4D MRV.18 A major drawback of static MRV approaches is that the imaging appearance of dural sinus thrombosis, in particular chronic thrombosis, can overlap with that of dural arteriovenous fistulas. This may lead to false diagnosis, as the latter entity frequently occurs as a sequelae of chronic CVT and is clinically initially often undiagnosed.18 An irregular appearance of the sinus, with multiple intrasinus channels and dural collateral vessels, may be seen on MRV images and is characteristic of incomplete recanalization.8
The artifacts on MRV can mimic with cerebral venous thrombosis by absence of normal flow signal in sinus or a vein. In 2D time-of-flight imaging, flow gaps result from slow intravascular blood flow, in plane flow, and complex blood flow patterns. Most of the artifactual loss of vascular signal seen with the use of 2D MRV occurs in nondominant transverse sinuses. Depiction of flowing blood with time-of-flight imaging is limited when short T1 substances such as methemoglobin are present.4 In 2D phase-contrast imaging, if blood flow is sufficiently slow, the motion-induced phase shifts may be inadequate to distinguish the flow from stationary tissue. Postcontrast 3D magnetization-prepared rapid gradientecho T1-weighted images are not affected by the angle between vessels and imaging slab or flow velocity.4
Cerebral angiography
With the advent of CTV and MRI (Figure 10A–J), digital subtraction angiography (DSA) is now rarely required for diagnosis4 because its invasive procedure. However, in patients with subarachnoid hemorrhage and cerebral venous thrombosis, DSA should be considered to rule out other causes of rebleeding, such as distal aneurysm and dural arteriovenous fistula, before anticoagulant therapy is administered.4 The techniques include standard arterial catheter angiography, thrombosis within the venous system was diagnosed from the venous phase of a cerebral angiogram.15 This may directly show the non-filling of the larger veins when involved, appeared as partial or complete lack of filling of veins or sinuses.3,4 The otherwise show increased tortuous collaterals around the thrombosed vein, the pseudophlebitic pattern.3 The DSA is usually required for diagnostic confirmation in most cases of isolated cortical venous thrombosis to show indirect signs such as collateral venous pathways, tortuous veins, or delayed local venous drainage.13 In addition, to evaluate the associated condition such as dural AVM in patient with venous sinus thrombosis, angiography plays role in definite diagnosis and for proper treatment planning.
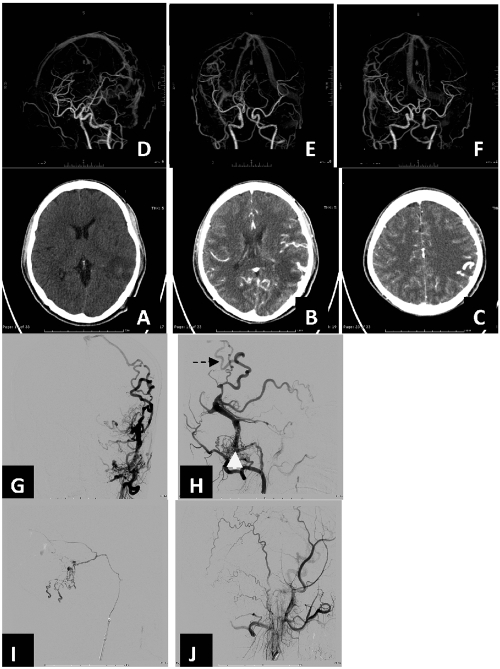
Figure 10.
Computed-tomography (CT) noncontrast study demonstrated A) ill-defined hypodensity lesion at left parieto-occipital region with small internal slightly hyperdensity (HU∼45) area. Effacement of left cerebral convexity is noted. After contrast medium administration in CT angiography there are B,C,D,E,F) multiple dilated, tortuous cortical vein of left temporal region. Selective arteriography of left occipital artery demonstrates G,H) dural arteriovenous shunt at left transverse –sigmoid sinus with thrombosed blinded pouch of left sigmoid sinus (white arrow) with retrograde leptomeningeal venous drainage (arrow) corresponding to CTA. Completed obliteration of malignant dural AVM at left transverse sigmoid sinus status I,J)post acrylic glue embolization.
Compare modalities
CTV has several advantages over DSA. It is less invasive and less expensive, and the shorter time to diagnosis in the initial work-up.4 MPR images from CTV were superior to DSA images in showing the cavernous sinus, inferior sagittal sinus, and basal vein of Rosenthal.4
Reported advantages of CTV compared with MRI techniques are rapid image acquisition (reduction of motion-related artifacts), no contraindication to pacemaker and ferromagnetic devices, increased imaging resolution, and fewer equivocal imaging findings. No flow related artifacts have been reported with use of a contrast material bolus and acquisition in the venous phase.4 High-resolution CT has been reported to be comparable or superior to MRV (2D-TOF or phase-contrast).9 Comparing the sensitivity of MRI and CT, MRI seems superior to CT.5 CTV more commonly shows sinuses or smaller cerebral veins with low flow as compared with MRV (2D phase contrast, 3D phase contrast, and 2D time of flight). Limitations of CTV include exposure to ionizing radiation, adverse reactions to iodinated contrast medium, and limited visualization of skull base structures in 3D display.4
Treatment
Medical treatment for this condition includes anticoagulation or local thrombolysis, which raises concerns about the associated high risk of intracerebral hemorrhage.19 Systemic anticoagulation is the first-line treatment for cerebral venous thrombosis because of its efficacy, safety, and feasibility.4 In CVT hemorrhage, use of heparin had generated enough evidence in treatment and thus it has emerged as a useful drug in the management of CVT. Heparin reduces both mortality and morbidity in CVT.7 Yamini et al. reported on a patient with extensive cranial sinus thrombosis that did not improve after a 25 mg bolus of rt-PA. A continuous infusion of rt-PA was administered at 1 mg/h for 19 hours19 Low molecular weight heparin has also been found to be equally effective. Thrombolytic and fibrinolytic drugs like urokinase, streptokinase and oral anticoagulants, e.g., warfarin have also been tried and found to be effective in some cases (Figure 3).7
Surgical management may include decompressive craniectomy, thrombectomy and evacuation of inter-cerebral haematoma need still further evaluation before it is recommended as a safe procedure.7
Endovascular intervention with locally infused rt-PA, or mechanical thrombolysis using microwires or available commercial devices.16 The adjunction of local thrombolysis is indicated in the rare cases of worsening despite adequate anticoagulation and optimal symptomatic and etiologic treatments.4 The role of endovascular reopening of the dural sinuses with angioplasty and stent placement in the presence of severe intracranial venous hypertension is anecdotal, and long-term results are not known.4
Prognosis
Early clinical diagnosis and early institution of therapy specially heparin or thrombolytic therapy followed by oral anticoagulants have improved the prognosis of CVT. Srinivasan observed that mortality has been reduced from 50.6% to 10% in the last three decade.20 The early convulso-paralytic state, impairment of consciousness, and presence of hemorrhagic infarcts are the factors adversely affecting prognosis.7 Patient with intracerebral hemorrhage whose are older, men, having a thrombosis of the deep cerebral venous system or of the right lateral sinus, having a motor deficit, and having a right lateral sinus thrombosis are predictors of poor outcome.7 The central nervous system infection is also increased risk of bad outcome.4 The prognosis for return of function is believed to be somewhat better than for arterial stroke.2
Conclusions
The clinical manifestations of CVT are often non-specific and are easily mistaken for those caused by other neurological disease processes. Imaging plays a primary role in diagnosis. A variety of different imaging techniques are available for the diagnosis of the CVT. Daily routine practical work for diagnosis this condition include CT, CTV, MRI and MRV (TOF or contrast-enhanced MRV). CT scan include plain and dynamic sequences as CT venography are the simple, effective method to diagnosis of cerebral venous thrombosis and coule be the first screening method. Compare among those imaging modalities, MR is necessary in many situations without radiation exposure to confirm or exclude thrombosis such as clot identification, parenchyma changes to the most subtle condition as cortical cortical vein thrombosis by 3D or 4D MR venography.
References
- 1.Poon CS, Chang JK, Swarnkar A, et al. Radiologic diagnosis of cerebral venous thrombosis: pictorial review. Am J Radiol. 2007;189:S64–S75. doi: 10.2214/AJR.07.7015. [DOI] [PubMed] [Google Scholar]
- 2.Karthikeyan D, Vijay S, Kumar T, et al. Cerebral venous thrombosis-spectrum of CT findings. Neuroradiology. 2004;14:129–37. [Google Scholar]
- 3.Aliasgar V, Moiyadi M Ch, Indira Devi B. Posttraumatic non-sinus cerebral venous thrombosis. Indian J of Neurotrauma. 2006;3:143–6. [Google Scholar]
- 4.Rodallec MH, Krainik A, Feydy A, et al. Cerebral venous thrombosis and multidetector CT angiography: tips and tricks. RadioGraphics. 2006;26:S5–S18. doi: 10.1148/rg.26si065505. [DOI] [PubMed] [Google Scholar]
- 5.Crombe D, Haven Fr, Gille M. Isolated deep cerebral venous thrombosis diagnosed on CT and MR imaging. a case study and review literature review. JBR–BTR. 2003;86:257–61. [PubMed] [Google Scholar]
- 6.Wasay M, Kojan S, Dai AI, et al. Headache in cerebral venous thrombosis: incidence, pattern and location in 200 consecutive patients. J Headache Pain. 2010;11:137–9. doi: 10.1007/s10194-010-0186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prakash C, Bansal BC. Cerebral venous thrombosis. J India Aca Clin Med. 2000;5:55–61. [Google Scholar]
- 8.Leach JL, Fortuna RB, Jones BV, et al. Imaging of cerebral venous thrombosis: current techniques, spectrum of findings, and diagnostic pitfalls. Radiographic. 2006;26:S19–S43. doi: 10.1148/rg.26si055174. [DOI] [PubMed] [Google Scholar]
- 9.Mahmoud MAL, Elbeblawy MMS. The role of multidetector CT venography in diagnosis of cerebral venous sinus thrombosis. Research J Med Med Sc. 2009;4:284–9. [Google Scholar]
- 10.Girot M, Ferro JM, Canhao P, et al. Predictors of outcome in patients with cerebral venous thrombosis and intracerebral hemorrhage. Stroke. 2007;38:337–42. doi: 10.1161/01.STR.0000254579.16319.35. [DOI] [PubMed] [Google Scholar]
- 11.Poungvarin N, Prayoonwiwat N, Ratanakorn D, et al. Thai venous stroke prognostic score: TV-SPSS. J Med Assoc Thai. 2009;92:1413–22. [PubMed] [Google Scholar]
- 12.Leach JL, Strub WM, Gaskill-Shipley MF. Cerebral venous thrombus signal intensity and susceptibility effects on gradient recalled-echo MR imaging. Am J Neuroradiol. 2007;28:940–5. [PMC free article] [PubMed] [Google Scholar]
- 13.Linn J, Pfefferkorn T, Ivanicova K, et al. Noncontrast CT in deep cerebral venous thrombosis and sinus thrombosis: comparison of its diagnostic value for both entities. Am J Neuroradiol. 2009:A1451–A1451. doi: 10.3174/ajnr.A1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boukobza M, Crassard I, Bousser MGH, et al. MR Imaging features of isolated cortical vein thrombosis: diagnosis and follow-up. Am J Neuroradiol. 2009;30:344–8. doi: 10.3174/ajnr.A1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Teasdale E. Cerebral venous thrombosis: making the most of imaging. J R Soc Med. 2000;93:234–37. doi: 10.1177/014107680009300505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kozic D, Zarkov M, Semnic RR, et al. Overlooked early CT signs of cerebral venous thrombosis with lethal outcome. Acta Neurol Belg. 2010;110:345–8. [PubMed] [Google Scholar]
- 17.Latchaw RE, Alberts MJ, Lev MH, et al. Recommendations for Imaging of Acute Ischemia Stroke: A Scientific Statement From the American Heart Association. Stroke. 2009;40:3646–78. doi: 10.1161/STROKEAHA.108.192616. [DOI] [PubMed] [Google Scholar]
- 18.Meckel S, Reisinger C, Bremerich J, et al. Cerebral venous thrombosis: diagnostic accuracy of combined, dynamic and static, contrast-enhanced 4D MR venography. Am J Neuroradiol. 2010;31:527–35. doi: 10.3174/ajnr.A1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan S-NH, Adeoye O, Abruzzo TA, et al. Intracranial dural sinus thrombosis: novel use of a mechanical thrombectomy catheter and review of management strategies. CM&R. 2009;7:157–65. doi: 10.3121/cmr.2009.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srinivasan K. Cerebral venous and arterial thrombosis in pregnancy and puerperium. Angiology. 1983;134:737–46. doi: 10.1177/000331978303401107. [DOI] [PubMed] [Google Scholar]