Abstract
The findings of the National Institutes of Health State-of-the-Science Conference on the role of active surveillance in the management of men with localized prostate cancer are examined.
In the opening statement of Herman Melville's Moby Dick, the narrator unambiguously declares his identity with the words, “Call me Ishmael” [1]. It would serve us well to remember this injunction as we ponder the ambiguous status of prostate cancer. Consensus panels have offered us important advice about the management of prostate cancer in recent weeks, with the latest coming from a National Institutes of Health (NIH) panel convened to consider whether or not active surveillance is appropriate for low-grade (Gleason score ≤ 6), low-volume (prostate-specific antigen [PSA] <10 ng/mL) disease [2]. Their conclusion, that active surveillance is an acceptable strategy for many patients in this category, is eminently reasonable advice, particularly for elderly men with significant comorbidity and limited life expectancy. The majority of men currently diagnosed with prostate cancer have low-grade and low-volume disease, and many are advanced in age and will never experience morbidity or mortality as a result of this disease. However, the panel does advise us that a diagnosis of prostate cancer in any patient entails, at a minimum, active surveillance: careful follow-up with monitoring of PSA on an annual basis, or more frequently, and potentially, repeat biopsies, depending on the clinical setting. For the younger patient, active surveillance entails accepting some risk for later disease-related morbidity or mortality. This depends on the accuracy of the Gleason score (subject, of course, to sampling error and reader interpretation) and on patient compliance with the required surveillance. The advantages of active surveillance are obvious: it postpones or avoids costly, morbid, and potentially unnecessary treatment. A number of urologists and radiation oncologists participated in this panel, and one would have a hard time arguing with their conclusion.
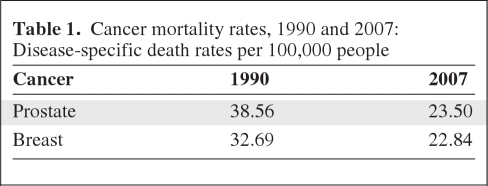
A more controversial aspect of the NIH panel's report was the statement that it may be a mistake to call low-grade low-volume prostate cancer a “cancer” in these patients. The panel's opinion is that the term causes unnecessary anxiety, and may prompt patients to take unnecessary action. Although we agree that the connotation of lethality for some patients is unfortunate, the diagnosis of cancer depends on pathological findings, specifically, pathological appearance and invasive behavior. Indolent cancers occur as part of the spectrum of breast cancers, lymphoma, and other forms of malignancy, and those will continue to be called cancer. Prostate cancer, in all its manifestations, is a real cancer. It is the second leading killer of men in the U.S., with incidence and mortality rates little different from those of breast cancer (Table 1). Approximately 15% of patients diagnosed with prostate cancer die from it. Although the PSA level and Gleason score are useful predictors for clinical behavior, curability, and survival, they are imperfect. Estimation of noncancer mortality, a critical step in assessing competing risks, is also notoriously unreliable. What is clear is that prostate cancer in patients with a favorable presentation usually progresses slowly and, for elderly men, a period of active surveillance may not place them in danger of eventual death resulting from cancer. The same can be said for small, estrogen receptor–positive breast cancers in elderly women, a presentation that may not require aggressive therapy [2]. However, for patients aged <70 years, with a life expectancy >15 years, the outcome of active surveillance is less certain. Much will depend on the judgment of the individual oncologist following the patient as to when to rebiopsy and when to intervene with treatment. The actual benefits of active surveillance, compared with aggressive intervention, in this younger age group remain to be proven by a clinical trial. An unknown number, perhaps 10%–20%, of such younger men with favorable findings at presentation if untreated will live long enough to develop metastatic disease, and some will die as a result of its dissemination. Because of this uncertainty, many younger patients will choose to proceed with treatment.
Table 1.
Cancer mortality rates, 1990 and 2007: Disease-specific death rates per 100,000 people
A further ambiguity is posed by the lack of consistency of these recommendations with those of the U.S. Preventive Services Task Force, which, after reviewing available prospective trials, recently pronounced PSA measurement as having unproven value as a screening test for men aged <75 years and of no proven value for men aged ≥75 years [3]. Most oncologists agree that PSA screening is of unproven value for men aged ≥75 years. However, for younger men, without a PSA test, how will doctors detect the higher-grade prostate cancers, and how will physicians proceed with active surveillance for low-grade tumors? The active surveillance strategy itself depends on identifying patients early in the course of their disease and tailoring treatment decisions to the biopsy results. Urologists, radiation oncologists, and medical oncologists know that the digital rectal exam is not a tool for early detection of disease and is able to detect locally advanced disease, which in most cases is no longer curable. Some will argue that low-grade disease does not ever require treatment whereas advanced disease is unaffected by treatment, but if this is so, why undertake active surveillance at all? The fact is that prostate cancer treatment is effective in eradicating disease and delaying its progression, and early treatment is very likely contributing to the steady 4% yearly decline in mortality in the U.S. since the introduction of widespread PSA screening [4]. It is true that there is limited evidence from prospective clinical trials to prove that the decline in mortality is directly related to screening and early institution of therapy. The European screening trial did show a significantly higher prostate cancer-specific survival rate for men aged 55–69 years with regular PSA screening, but the Prostate, Lung, Colorectal, and Ovarian Cancer trial did not show this advantage [5, 6]. Both trials have been criticized for methodological flaws, and further prospective trials are warranted.
Meanwhile, the oncologist (surgeon, radiation oncologist, medical oncologist) and primary care physician must sort out these conflicting messages and provide sensible advice for men at risk for prostate cancer. Prostate cancer is cancer. It has the potential to kill, and decisions to screen or not to screen, to treat or not to treat, may well affect an individual's survival and quality of life. Patients deserve to know this uncertainty, and to make informed decisions. Ignoring the fact that it is cancer, or renaming it something else, does not help the discussion.
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor or patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Bruce A. Chabner, Matthew Smith
Manuscript writing: Bruce A. Chabner, Matthew Smith
Final approval of manuscript: Bruce A. Chabner, Matthew Smith
References
- 1.Melville H. Moby-Dick: or, The Whale. New York, NY: Penguin Classics; 2001. [Google Scholar]
- 2.Ganz PA, Barry JM, Burke W, et al. National Institutes of Health State-of-the-Science Conference: Role of Active Surveillance in the Management of Men with Localized Prostate Cancer. [accessed December 11, 2011]. Available at http://consensus.nih.gov/2011/docs/prostate/ASPC Final Draft Statement.pdf. [DOI] [PMC free article] [PubMed]
- 3.U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:185–191. doi: 10.7326/0003-4819-149-3-200808050-00008. [DOI] [PubMed] [Google Scholar]
- 4.Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011: The impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 5.Schröder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 6.Andriole GL, Crawford ED, Grubb RL, 3rd, et al. Mortality results from a randomized prostate cancer screening trial. N Engl J Med. 2009;360:1310–1319. Erratum in: N Engl J Med. 2009;360:1797. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]