Among women with surgically removed, high-risk HER-2/neu-positive breast cancer, trastuzumab has demonstrated significant improvements in disease-free and overall survival. The objective of this study is to evaluate the cost-effectiveness of the currently recommended 12-month adjuvant protocol of trastuzumab using a Markov modeling approach and real-world cost data. Over the long term, treatment of HER-2/neu mutation positive breast cancer with a 12-month protocol of trastuzumab in the adjuvant setting is predicted to be cost-effective in a Canadian context.
Keywords: Cost-benefit analysis, Drug costs, Breast neoplasms, Antibodies, Monoclonal
Abstract
Background.
Among women with surgically removed, high-risk HER-2/neu-positive breast cancer, trastuzumab has demonstrated significant improvements in disease-free and overall survival. The objective of this study is to evaluate the cost-effectiveness of the currently recommended 12-month adjuvant protocol of trastuzumab using a Markov modeling approach and real-world cost data.
Methods.
A 10-health-state Markov model tracked patients' quarterly transitions between health states in the local and advanced states of breast cancer. Clinical data were obtained from the joint analysis of the National Surgical Adjuvant Breast and Bowel Project and North Central Cancer Treatment Group, as well as from the metastatic study conducted by Norum et al. Clinical outcomes were adjusted for quality of life using utility estimates published in a systematic review. Real cost data were obtained from the British Columbia Cancer Agency and were evaluated from a payer perspective. Costs and utilities were discounted at 5% per year, respectively, for a 28-year time horizon.
Results.
In the base case analysis, treatment with a 12-month adjuvant trastuzumab regimen resulted in a gain of 1.38 quality-adjusted life years or 1.17 life years gained at a cost of $18,133 per patient. Thus, the cost per QALY gained for the base case is $13,095. Cost per LYG is $15,492.
Conclusions.
Over the long term, treatment of HER-2/neu mutation positive breast cancer with a 12-month protocol of trastuzumab in the adjuvant setting is predicted to be cost-effective in a Canadian context.
Introduction
HER-2/neu proto-oncogene amplification is found in 20%–30% of breast cancers and is associated with reduced disease-free and overall survival [1]. Trastuzumab (Herceptin; Genentech, San Francisco), given in combination with chemotherapy, lengthens median survival time by 5–8 months among HER-2/neu-positive metastatic disease patients [2–4]. Given during or following adjuvant anthracycline-based chemotherapy, trastuzumab also confers longer disease-free and overall survival in early HER-2/neu-positive breast cancer patients than chemotherapy alone [2, 5, 6]. Analysis by the National Surgical Adjuvant Breast and Bowel Project (NSABP) and the North Central Cancer Treatment Group (NCCTG) demonstrated that the addition of trastuzumab to standard adjuvant chemotherapy regimens resulted in a 52% relative reduction in the risk of recurrence (p < .0001) and a 33% relative increase in overall survival (p = .015) in patients with early and operable HER-2/neu-positive breast cancer [6].
Cardiac dysfunction can be associated with the use of trastuzumab, necessitating the use of cardiac monitoring prior to treatment initiation and at 3-month intervals during therapy. In the NSABP/NCCTG analysis, the incidence of New York Heart Association class III or IV congestive heart failure or death from cardiac causes was higher in the trastuzumab arm at a 4.1% incidence rate than the standard chemotherapy-only arms at a 0.8% incidence rate [6].
The use of trastuzumab in the adjuvant setting was approved by the British Columbia Cancer Agency (BCCA) in July 2005. Since that time, trastuzumab has represented approximately 15% of BCCA's $100 million drug budget. Although there is increasing evidence of a substantial clinical benefit of trastuzumab in the adjuvant setting, the high cost of this therapy necessitates an analysis of its cost-effectiveness. To date, no published studies have assessed the cost-effectiveness of this drug from a Canadian perspective, or using real-world data, nor have they accounted for patients who may receive trastuzumab in both the adjuvant and metastatic settings [7–9]. The aim of this study was to develop a Markov model to estimate the incremental cost-effectiveness of adjuvant trastuzumab for operable, HER-2/neu-positive early breast cancer, accounting for the differences in costs and health outcomes associated with trastuzumab and standard of care treatments in the adjuvant and metastatic settings.
Materials and Methods
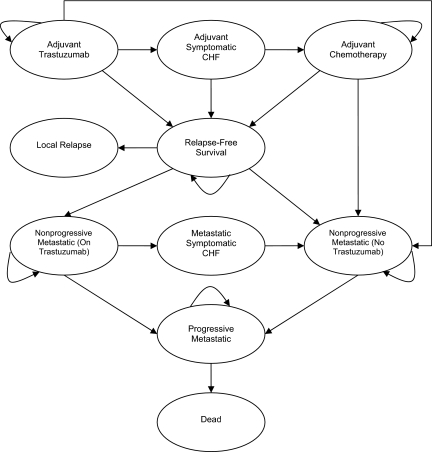
With use of R-Graphical User Interface (R-Gui), a decision-analysis model for HER-2/neu-positive breast cancers was developed to evaluate the cost-effectiveness of a 12-month adjuvant trastuzumab protocol (presented in Figure 1) compared with that of standard chemotherapy. Selected model parameters are presented in Table 1 and complete data in the Supplemental Online Appendix. The model used a Markov transition process with 10 health states, was created with a 3-month cycle length, and was run on a 28-year time horizon. Costs and quality-of-life values assigned to each state were derived from pre-existing literature and BCCA databases. Results are presented as incremental cost-effectiveness ratios (ICERs) in cost per quality-adjusted life year (QALY) gained and cost per life year gained (LYG). (The term LYG refers to the cost of prolonging a patient's life for a single year, attributable to a particular intervention. QALYs gained can then be computed as the duration of survival multiplied by a utility weight that represents the quality of health experienced during that time period.) The model is notably more complex than those previously published, to account for the possibility of the use of trastuzumab in both the adjuvant and metastatic settings [7–9].
Figure 1.
Markov model. Note: Arrows indicate transitions. Arrows that curve back to the same state represent patients who remained in the health state in question for more the one 3-month cycle.
Abbreviation: CHF, congestive heart failure.
Table 1.
Key model parameters
The model was applied to a hypothetical patient population similar to those used in recently published phase III clinical trials [5, 6]: one thousand 50-year-old women with early HER-2/neu-positive breast cancer, who had successfully completed a surgical resection of disease. Patients entered the model in the postsurgical with trastuzumab or postsurgical without trastuzumab states, depending on the presence or absence of pre-existing low left ventricular ejection fraction (LVEF) (95% and 5%, respectively, based on expert opinion), which would preclude the use of trastuzumab.
Patients could incur a local or metastatic relapse while still receiving treatment or following treatment completion. Conforming to BCCA policy, patients who suffered any relapse before 1 year following the completion of adjuvant trastuzumab would be ineligible to receive the drug a second time, either in the adjuvant or metastatic setting. Patients who incur a distant relapse are funneled to either a trastuzumab-based or standard chemotherapy-treated, nonprogressive metastatic disease state. Patients who experience a local relapse and are treated with adjuvant trastuzumab a second time are considered ineligible for metastatic trastuzumab.
From a nonprogressive metastatic disease state, patients could experience symptomatic cardiac complications, or could move to a state of progressive disease, eventually leading to death. All patients who experience symptomatic cardiac complications from a nonprogressive metastatic state will have trastuzumab-based therapy halted, and will resume standard chemotherapy, as per trial protocols.
Clinical Data
Data collected from the clinical trials on the use of trastuzumab in both the adjuvant and metastatic settings were used to derive transition probabilities (Table 1, and Supplemental Online Appendix, Table 1A) [6, 10]. The rate of reappearance of local disease was 25% of the rate of reappearance of metastatic disease in the usual care arm, and 23% of the rate of reappearance of metastatic disease in the adjuvant trastuzumab arm [6]. The transition from local to metastatic relapse was three times the rate of reappearance of metastatic disease, reflecting a higher risk of the development of metastases in patients who have already suffered a local recurrence [11].
Estimates of progression-free and overall survival in the metastatic setting (maximum follow-up, 5 years) were derived from a phase II trial assessing the efficacy and safety of combined trastuzumab and docetaxel therapy in HER-2/neu-positive metastatic breast cancer [3]. Long-term outcomes for the usual care model arm were obtained from a 30-year survival analysis of women who were treated with adjuvant cyclophosphamide, methotrexate, and fluorouracil [12]. However, long-term outcomes for women who received adjuvant trastuzumab therapy are not yet available. A range of hazard ratios—from no improvement post-5-year survival to 50% improvement post-5-year survival in the trastuzumab group relative to the non-trastuzumab group—were examined to account for uncertainty in this area. The base case assumed no benefit from trastuzumab post-5-year survival. National mortality rates for females, adjusted for patient age, were used for all Markov states.
Costs
The costs associated with trastuzumab-based treatment of breast cancer were estimated from the perspective of the health system (Table 1, and Supplemental Online Appendix, Table 2A). Chemotherapy and radiotherapy costs were based on real-word utilization data: cost per unit of treatment (which has been negotiated with various pharmaceutical companies) is multiplied to account for the number of units per patient, over their complete treatment duration. Costs for diagnostics, inpatient services, and treatment of cardiac dysfunction were based on previously published literature [13, 18]. For the adjuvant arms, quarterly chemotherapy costs were calculated using utilization data from women receiving adjuvant treatment for stage I–III breast cancer in British Columbia from 2002 to 2003. The additional average quarterly cost associated with the use of trastuzumab was calculated using BCCA pharmacy data from a random sample of women who were treated with the drug between 2005 and 2006 (N = 350). The same sample of women was used to calculate radiotherapy cost. Seventy-one percent of women underwent radiotherapy in the adjuvant setting, at a cost of $5,291.56 per protocol, which accounts for dropouts.
The costs of hormone treatment were determined using the same 2002–2003 sample as for the chemotherapy costs, and were included in the relapse-free survival states in both model arms. These costs were adjusted for the proportion of patients expected to be receiving therapy at any given point in follow-up time.
Costs associated with detection and treatment of cardiac complications were estimated from data from the BC Linked Health Database and the New York Heart Association [13]; these are attributed as one-time costs.
The average quarterly cost of chemotherapy for women with metastatic disease was calculated using BCCA Pharmacy Data. Records used included women on metastatic chemotherapy protocols between 2003 and 2007. Protocols that did not include trastuzumab were used to calculate an average cost for non-trastuzumab-based chemotherapy treatment. The cost of trastuzumab was calculated separately, consistent with the methods used in the adjuvant model. Radiotherapy cost was generated via expert opinion, and was included for one third of women in each model arm in both the nonprogressive and progressive disease states, accounting for the proportion of women who would be expected to receive the therapy (and accounting for dropouts and continuations). Costs were adjusted for inflation.
Quality of Life
Utilities for each Markov state were derived from a systematic review of health utilities in cancer [14] (Table 1, and Supplemental Online Appendix, Table 3A). Utility values—a value between 0 and 1 that indicates values a health state relative to full health—were converted to standard gamble equivalents where necessary [14], and were discounted at a rate of 1.25% per Markov cycle (5% per year).
The utility value associated with symptomatic cardiac complications occurring during adjuvant therapy was obtained from a New York Heart Association study [15]. There are no data available on the utilities associated with cardiac complications combined with the effects of metastatic disease. In line with previously published cost-effectiveness studies [8], it was assumed that the appropriate value would fall into the range of progressive-terminal disease reported by Hutton et al. [16]. The range of utility values associated with the local relapse states was calculated as the average of the calculated scores of first (0.94), second (0.80), and third recurrence, respectively (0.56).
Analyses
A Bayesian approach characterized by a Dirichlet distribution was applied to all transition probabilities to generate a fully probabilistic Markov transition matrix [17]. Gamma and beta distributions were applied to cost and utility parameters, respectively. Probabilistic sensitivity analysis characterized by a second-order, 10,000-simulation Monte Carlo method was used on the transition matrix [27]. The rate of relapse post-5-year survival in the trastuzumab arm was also modeled as a varying hazard ratio (HR) relative to that of the standard chemotherapy arm. HRs of 1.0, 0.9, 0.8, 0.7, and 0.5 were tested, representing improvements in survival ranging from 0% to 50%.
Additional one-way sensitivity analyses were performed on key parameters including the rate of relapse prior to 5-year survival in the trastuzumab group, and the cost of trastuzumab-based chemotherapy in both the adjuvant and metastatic settings. Costs were varied ±1 SD, or according to the 95% confidence intervals reported in the literature [18, 19].
Results
Base Case
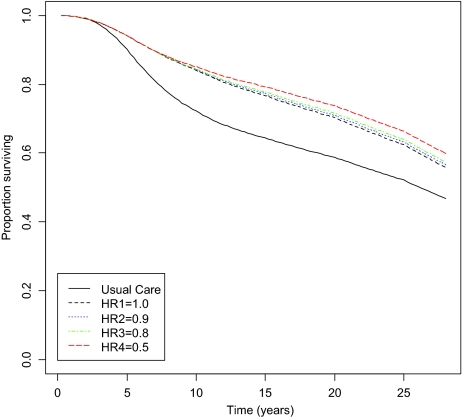
Treatment with a 12-month adjuvant trastuzumab regimen resulted in a gain of 1.38 QALYs or 1.17 LYG at a cost of $18,133 per patient. Thus, the cost per QALY gained for the base case is $13,095. Cost per LYG is $15,492. Assuming no further benefit of trastuzumab post-5-year survival, overall survival improved from 45% in the no trastuzumab arm compared with 54% in the trastuzumab arm (Figure 2) and disease-free survival improved from 47% to 56%, respectively. Five-year survival rates and survival projections from the Markov model are in line with previously published clinical trial data [5, 6].
Figure 2.
Overall survival: adjuvant trastuzumab versus usual care. The rate of relapse post-5-year survival in trastuzumab arm was modeled as a varying HR relative to the standard chemotherapy arm. HRs of 1.0, 0.9, 0.8, 0.7, and 0.5 were tested, representing improvements in survival ranging from 0% to 50%. Abbreviation: HR, hazard ratio.
Sensitivity Analyses
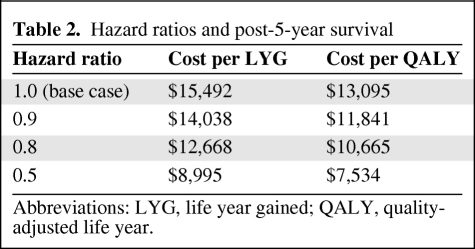
The cost per LYG and cost per QALY results from the four long-term survival hazard scenarios are presented in Table 2 and survival curves are presented in Figure 2. Predictably, reducing the HR for post-5-year survival improved the cost-effectiveness profile for adjuvant trastuzumab (from $13,095 for a HR of 1.0 to $7,531 for a HR of 0.5).
Table 2.
Hazard ratios and post-5-year survival
Abbreviations: LYG, life year gained; QALY, quality-adjusted life year.
Additional one- and two-way sensitivity analyses are presented in Table 3. (In one-way sensitivity analyses, one model parameter is varied to demonstrate its impact on overall cost-effectiveness. In two-way sensitivity analyses, two parameters are varied simultaneously.) The model was sensitive to changes in the discount rate for cost and outcomes; a 0% discount rate improved the base-case ratio to $5,165 per QALY. A 3% discount rate for both costs and outcomes, which is commonly utilized in cost-effectiveness studies done in the United States, produced an ICER of $8,479.
Table 3.
One- and two-way sensitivity analyses on base case
aHigher value corresponds to reducing cost of metastatic trastuzumab by 2 SD.
Abbreviations: LVEF, left ventricular ejection fraction; LYG, life year gained; QALY, quality-adjusted life year.
The model was not particularly sensitive to changes in the costs of adjuvant or metastatic trastuzumab, or to changes in the risk of relapse before 5-year survival is reached. The costs of adjuvant and metastatic trastuzumab—varied individually or simultaneously by ±1 SD—produced a range from $11,936–$14,255 per QALY. Varying pre-5-year survival relapse rates in the trastuzumab arm had a similarly limited impact on the ICER: a ±25% relapse rate resulted in a $12,175–$14,109 per QALY range. The model was also minimally sensitive to trastuzumab-related cardiotoxicity.
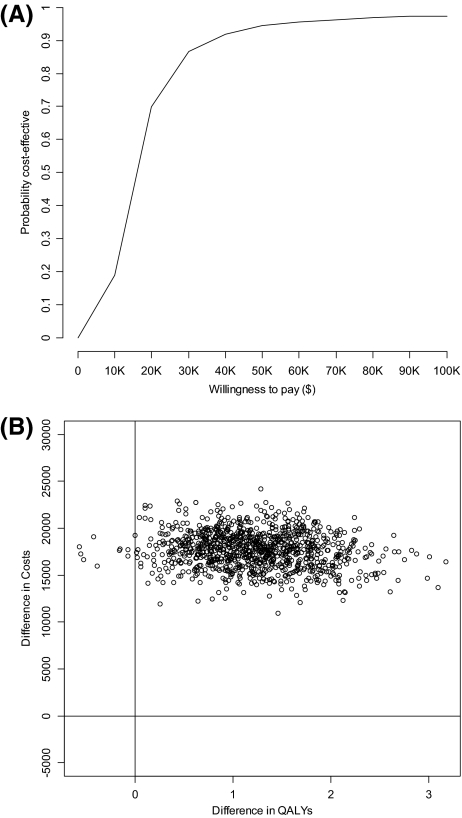
Probabilistic sensitivity analyses (Figs. 3A, 3B) produced an interquartile range of $10,900–$32,030 per QALY. The majority of the 10,000 Monte Carlo simulations resulted in ICERs appearing in the upper right-hand quadrant of the cost-effectiveness plane, indicating that treatment with adjuvant trastuzumab resulted in an improvement in survival at an increased cost. The limited vertical variation indicates limited variability associated with the costs of treatment.
Figure 3.
(A): Cost-effectiveness acceptability curve; (B): Cost-effectiveness plane.
Abbreviation: QALY, quality-adjusted life year.
Discussion
Trastuzumab has been approved for use in Canada as an adjuvant therapy for early HER-2/neu-positive breast cancer and is covered under the umbrella of publicly funded services in all 10 Canadian provinces. However, to our knowledge this is the first study to (a) assess the cost-effectiveness of trastuzumab from a Canadian perspective, (b) examine cost-effectiveness in the “real world” using postmarketing data, and (c) estimate the cost-effectiveness of trastuzumab in both the adjuvant and metastatic breast cancer settings. With use of local costing data combined with probabilities derived from clinical trials and utility values from quality of life studies, this study has assessed the cost per QALY gained associated with a 12-month adjuvant trastuzumab protocol using a Markov modeling approach.
The use of trastuzumab resulted in a 9% absolute improvement in overall survival over the 28-year time horizon, for a $18,133 increase in cost per patient. The cost-effectiveness ratio for the model base case is $13,095 per QALY or $15,492 per LYG. Whether an additional survival benefit associated with a 12-month protocol of adjuvant trastuzumab is conferred after 5-year survival remains to be seen. To account for this uncertainty, five HR scenarios ranging from no additional benefit to a 50% improvement in survival were modeled, resulting in an ICER range of $7,934–$13,095 per QALY. The small difference in incremental cost-effectiveness between each HR scenario suggests that the benefit accrued from trastuzumab in the first 5 years has a much greater impact on cost-effectiveness than benefits accrued in later years.
The model proved to be relatively robust, showing minimal sensitivity to changes in rates of relapse and cardiotoxiciy, and cost of therapy. The model was sensitive to one- and two-way changes in discount rates for costs and outcomes.
A significant strength of this study was the use of real patient treatment utilization data to calculate the costs of trastuzumab, anthracycline-based chemotherapy, and radiation therapies, accounting for both reductions in cost associated with failure to complete a full treatment protocol and changes in cost associated with changing therapies during treatment.
Potential limitations relate to the reliance on clinical trial data to generate some model probabilities. Some of these data rely on surrogate endpoints, such as progression- or disease-free survival as measures of overall survival, which operate on the underlying assumption that once disease progresses, survival time would not be differential between the two study arms.
Our results demonstrate a lower cost-effectiveness ratio than those published in earlier studies [8, 9]. For example, a commonly cited study published in 2007 reported a cost-effectiveness ratio of $18,970 (U.S. dollars) per QALY. There are two main reasons for this difference. Our work uses survival data from a more recent clinical trial in which participants had a longer median survival than had been previously reported [6]. In accordance with BCCA policy, our study assumes that patients who are treated with trastuzumab in the adjuvant setting are also eligible to receive the drug as first-line treatment for a relapse, providing that relapse occurs later than 1 year following completion of initial treatment.
Additionally, the acquisition cost of trastuzumab, and indeed other chemotherapy drugs, is likely to be lower in Canada as often Canadian purchasers negotiate discounts when contracting with pharmaceutical companies. Indeed, this is a common practice in other countries as well [26]. It is difficult to estimate what impact these discounts have on cost-effectiveness since discounts and contracts are determined in confidence. And since discounting frequently involves volumes, not simply prices, even if the discounts were in the realm of the public domain, the impact on cost-effectiveness would still be difficult to interpret.
Over the long-term, treatment of HER-2/neu mutation positive breast cancer with a 12-month protocol of trastuzumab in the adjuvant setting is predicted to be cost-effective in a Canadian context. There is, however, no accepted cost per QALY threshold in Canada, or in most developed nations. An examination of the Canadian Common Drug Review's recommendations for the funding of new drugs does demonstrate that treatments whose cost-effectiveness ratio are around or under $50,000 per QALY tend to be listed, whereas those significantly above that figure tend not to be, regardless of the treatment's intended use [26]. Additional relevant factors, such as comparative effectiveness (and uncertainty therein), budgetary impact, ability to negotiate drug price, and others, also impact the decision of whether or not to list a drug at the BCCA, or other agencies [26].
Conclusions
Twelve-month adjuvant trastuzumab-based chemotherapy for women with HER-2/neu mutation positive breast cancer appears to be cost-effective from a Canadian standpoint, suggesting that continuing to make this treatment available in Canada is warranted. However, the overall budgetary impact of a full 12-month course of trastuzumab is significant. At the time of writing, there has been limited interest in examining a shortened 9-week regimen; however, a cost-effectiveness analysis of this shortened protocol may be warranted. It would also be worthwhile to revisit this work once more long-term survival data become available.
Supplemental Online Appendix
Complete model parameters can be found in Supplemental Online Tables A1, A2, and A3.
See the accompanying commentary on pages 157–159 of this issue.
Supplementary Material
Acknowledgments
Work on this manuscript was completed at the British Columbia Cancer Agency. Funding for this project was provided by Canadian Institutes of Health Research Grant No. 162964. The Canadian Centre for Applied Research in Cancer Control (ARCC) is funded by the Canadian Cancer Society.
Author Contributions
Conception/Design: Stuart Peacock, Lindsay Hedden, Caroline Lohrisch, Susan O'Reilly, Stephen Chia, Caroline Speers, Laurel Kovacic, Suzanne Taylor
Provision of study material or patients: Stuart Peacock, Lindsay Hedden, Susan O'Reilly, Stephen Chia
Collection and/or assembly of data: Stuart Peacock, Lindsay Hedden, Caroline Lohrisch, Susan O'Reilly, Caroline Speers, Laurel Kovacic, Suzanne Taylor
Data analysis and interpretation: Stuart Peacock, Lindsay Hedden, Caroline Lohrisch, Stephen Chia, Caroline Speers, Laurel Kovacic, Suzanne Taylor
Manuscript writing: Stuart Peacock, Lindsay Hedden
Final approval of manuscript: Stuart Peacock, Lindsay Hedden, Caroline Lohrisch, Susan O'Reilly, Stephen Chia, Caroline Speers, Laurel Kovacic, Suzanne Taylor
References
- 1.Ross JS, Fletcher JA. The HER-2/neu oncogene in breast cancer: prognostic factor, predictive factor, and target for therapy. Stem Cells. 1998;16:413–428. doi: 10.1002/stem.160413. [DOI] [PubMed] [Google Scholar]
- 2.Nahta R, Esteva FJ. Trastuzumab: triumphs and tribulations. Oncogene. 2007;26:3637–3643. doi: 10.1038/sj.onc.1210379. [DOI] [PubMed] [Google Scholar]
- 3.Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol. 2005;23:4265–4274. doi: 10.1200/JCO.2005.04.173. [DOI] [PubMed] [Google Scholar]
- 4.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 5.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 6.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 7.Kurian AW, Thompson RN, Gaw AF, et al. A cost-effectiveness analysis of adjuvant trastuzumab regimens in early HER2/neu-positive breast cancer. J Clin Oncol. 2007;25:634–641. doi: 10.1200/JCO.2006.06.3081. [DOI] [PubMed] [Google Scholar]
- 8.Liberato NL, Marchetti M, Barosi G. Cost effectiveness of adjuvant trastuzumab in human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2007;25:625–633. doi: 10.1200/JCO.2006.06.4220. [DOI] [PubMed] [Google Scholar]
- 9.Norum J, Olsen JA, Wist EA, et al. Trastuzumab in adjuvant breast cancer therapy. A model based cost-effectiveness analysis. Acta Oncol. 2007;46:153–164. doi: 10.1080/02841860601096841. [DOI] [PubMed] [Google Scholar]
- 10.Tan-Chiu E, Yothers G, Romond E, et al. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J Clin Oncol. 2005;23:7811–7819. doi: 10.1200/JCO.2005.02.4091. [DOI] [PubMed] [Google Scholar]
- 11.Wapnir IL, Anderson SJ, Mamounas EP, et al. Prognosis after ipsilateral breast tumor recurrence and locoregional recurrences in five national surgical adjuvant breast and bowel project node-positive adjuvant breast cancer trials. J Clin Oncol. 2006;24:2028–2037. doi: 10.1200/JCO.2005.04.3273. [DOI] [PubMed] [Google Scholar]
- 12.Bonadonna G, Moliterni A, Zambetti M, et al. 30 years' follow up of randomised studies of adjuvant CMF in operable breast cancer: cohort study. BMJ. 2005;330:217. doi: 10.1136/bmj.38314.622095.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cowper PA, DeLong ER, Whellan DJ, et al. Economic effects of beta-blocker therapy in patients with heart failure. Am J Med. 2004;116:104–111. doi: 10.1016/j.amjmed.2003.07.016. [DOI] [PubMed] [Google Scholar]
- 14.Earle CC, Chapman RH, Baker CS, et al. Systematic overview of cost-utility assessments in oncology. J Clin Oncol. 2000;18:3302–3317. doi: 10.1200/JCO.2000.18.18.3302. [DOI] [PubMed] [Google Scholar]
- 15.Lewis EF, Johnson PA, Johnson W, et al. Preferences for quality of life or survival expressed by patients with heart failure. J Heart Lung Transplant. 2001;20:1016–1024. doi: 10.1016/s1053-2498(01)00298-4. [DOI] [PubMed] [Google Scholar]
- 16.Hutton J, Brown R, Borowitz M, et al. A new decision model for cost-utility comparisons of chemotherapy in recurrent metastatic breast cancer. Pharmacoeconomics. 1996;9(Suppl 2):8–22. doi: 10.2165/00019053-199600092-00004. [DOI] [PubMed] [Google Scholar]
- 17.Briggs AH, Ades AE, Price MJ. Probabilistic sensitivity analysis for decision trees with multiple branches: use of the Dirichlet distribution in a Bayesian framework. Med Decis Making. 2003;23:341–350. doi: 10.1177/0272989X03255922. [DOI] [PubMed] [Google Scholar]
- 18.Wai ES, Trevisan CH, Malfair Taylor SC, et al. Health system costs of metastatic breast cancer. Breast Cancer Res Treat. 2001;65:233–240. doi: 10.1023/a:1010686118469. [DOI] [PubMed] [Google Scholar]
- 19.Will BP, Petit CL, Berthelot JM, et al. Diagnostic and therapeutic approaches for nonmetastatic breast cancer in Canada, and their associated costs. Br J Cancer. 1999;79:1428–1436. doi: 10.1038/sj.bjc.6690228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hristova L, Hakama M. Effect of screening for cancer in the Nordic countries on deaths, cost and quality of life up to the year 2017. Acta Oncol. 1997;36(Suppl 9):1–60. [PubMed] [Google Scholar]
- 21.Hillner BE, Smith TJ. Efficacy and cost effectiveness of adjuvant chemotherapy in women with node-negative breast cancer. A decision-analysis model. N Engl J Med. 1991;324:160–168. doi: 10.1056/NEJM199101173240305. [DOI] [PubMed] [Google Scholar]
- 22.Smith TJ, Hillner BE. The efficacy and cost-effectiveness of adjuvant therapy of early breast cancer in premenopausal women. J Clin Oncol. 1993;11:771–776. doi: 10.1200/JCO.1993.11.4.771. [DOI] [PubMed] [Google Scholar]
- 23.Lidgren M, Wilking N, Jönsson B, et al. Health related quality of life in different states of breast cancer. Qual Life Res. 2007;16:1073–1081. doi: 10.1007/s11136-007-9202-8. [DOI] [PubMed] [Google Scholar]
- 24.Launois R, Reboul-Matry J, Henry B. A cost-utility analysis of second-line chemotherapy in metastatic breast cancer: docetaxel versus paclitaxel versus vinorelbine. Pharmacoeconomics. 1996;10:504–521. doi: 10.2165/00019053-199610050-00008. [DOI] [PubMed] [Google Scholar]
- 25.Hillner BE, Smith TJ, Desch CE. Efficacy and cost-effectiveness of autologous bone marrow transplantation in metastatic breast cancer. Estimates using decision analysis while awaiting clinical trial results. JAMA. 1992;267:2055–2061. [PubMed] [Google Scholar]
- 26.Clement FM, Harris A, Li JJ, et al. Using effectiveness and cost-effectiveness to make drug coverage decisions: a comparison of Britain, Australia and Canada. JAMA. 2009;302:1437–1443. doi: 10.1001/jama.2009.1409. [DOI] [PubMed] [Google Scholar]
- 27.Claxton K, Schulpher M, McCabe C, et al. Probabilistic sensitivity analysis for NICE technology assessment: not an optional extra. Health Econ. 2005;14:339–347. doi: 10.1002/hec.985. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.