Abstract
It is widely accepted that traditional models of clinical investigation are becoming unsustainable in oncology and that trials must become more efficient in matching effective treatments to the patients most likely to benefit. In 2008, gastrointestinal oncologists from many countries began a collaboration to improve the design and conduct of clinical trials in their field, through the auspices of a French/U.S. charitable foundation, ARCAD. Whether this model of academic collaboration will be judged a success will depend on the quality of its scientific output during the next few years and whether this output, alongside that of other scientists, groups, and institutions, ultimately leads to more efficient trials and improved treatment options for patients.
Keywords: Gastrointestinal neoplasms, Clinical trials, Drugs, Investigational, Database, Data interpretation, Statistical
It is widely accepted that traditional models of clinical investigation are becoming unsustainable in oncology and that trials must become more efficient in matching effective treatments to the patients most likely to benefit. The need for innovation has prompted initiatives by the U.S. Food and Drug Administration (FDA) [1, 2], National Institutes of Health [3], American Society for Clinical Oncology [4], and Biomarkers Consortium [5], among others, as well as numerous innovative studies by individual research groups. It was against this backdrop that, in 2008, gastrointestinal oncologists from many countries began a collaboration to improve the design and conduct of clinical trials in their field, through the auspices of a French/U.S. charitable foundation, ARCAD (Aide et Recherche en Cancérologie Digestive) [6]. Here, we describe the progress of the ARCAD Clinical Trials Program to date and discuss its potential as a template for academic collaboration in oncology. We also invite colleagues to join us in collaborative studies using a large patient-level database newly constructed by ARCAD from advanced colorectal cancer trials.
The ARCAD Clinical Trials Program has an informal membership comprising a gradually expanding network of over 50 senior oncologists, scientists, and statisticians from North and South America, Europe, Asia, and Australia [7]. It is organized by an operational office based in Paris, France, with assistance from colleagues in the United Kingdom and the United States. The program is funded by grants and philanthropic donations to the ARCAD Foundation, including grants from industry. However, neither industry nor other donors play any role in the group's discussions or research activities, which solely involve the academic membership. The group meets twice yearly during congresses in the United States and Europe and maintains a dialogue throughout the year through e-mails, ongoing research, and publications development. There is also a secured access website for members. At present, the main focus of the group is on advanced colorectal cancer, although other gastrointestinal malignancies have also been considered. Specifically, following the success of the colorectal cancer database, the ARCAD group has recently decided to launch a similar program in pancreatic cancer.
ARCAD has adopted a multitrack approach to the challenge of developing more efficient clinical trials. First, it is generating discussion papers drawing on the expertise of its members [8, 9]. Second, groups of ARCAD members are engaged in technical studies of specific problems, using a combination of theoretical analysis and research. Finally, the group is engaged in a large research undertaking, the ARCAD Advanced Colorectal Cancer Database Project. These activities will in due course lead to recommendations on clinical trials design and endpoint selection for discussion with academic colleagues and regulatory authorities.
At the group's twice yearly meetings, selected members deliver presentations on topics in clinical trials design or their own research, with roundtable discussions following each presentation. Subjects addressed to date have included the following: technical challenges of assessing biomarkers; statistical aspects of biomarker qualification; study endpoints; phase I, II, and III trial design; drug codevelopment; drug approval criteria; and tumor biology and its therapeutic implications. The group has published discussion papers on the statistical validation of biomarkers and endpoints and their integration into clinical colorectal cancer trials [8, 9], as well as an analysis of endpoints in multiline trials [10]. Further publications are in development on progression-free survival and associated endpoints in advanced colorectal cancer trials, as well as signals for launching phase III trials in the adjuvant setting.
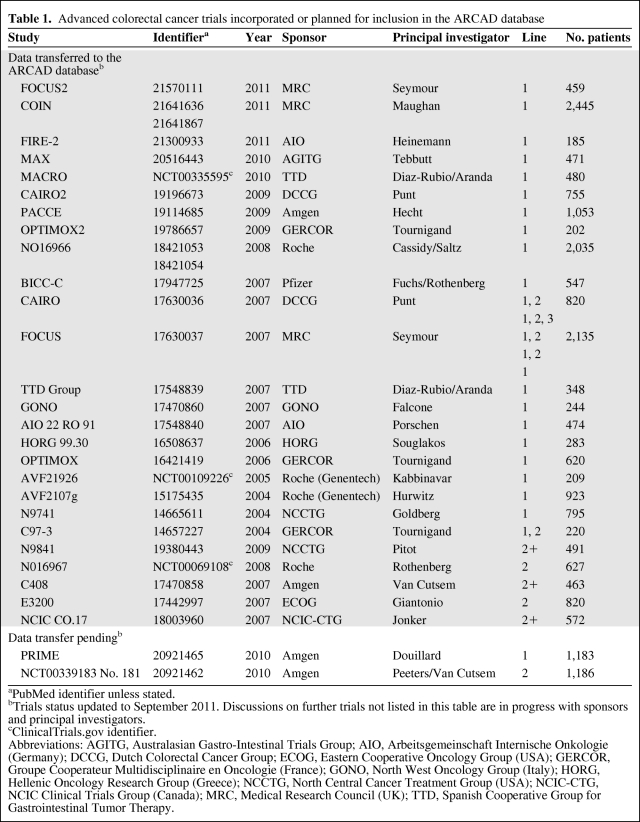
The centerpiece of the group's research activities has been the construction during the past 2 years of the ARCAD Advanced Colorectal Cancer Database. The database is located at the Mayo Clinic (Rochester, MN, USA) and will be mirrored at the International Drug Development Institute, Louvain-la-Neuve, Belgium, and GERCOR, Paris. The database brings together individual patient-level data from most of the recent prospective clinical trials in advanced colorectal cancer, including both industry and academic trials across all lines of therapy. At present, data from 26 trials comprising over 18,000 patients have been incorporated into the database, with data on over 5,000 patients from further trials pending or under discussion with sponsors and principal investigators (Table 1). The data recorded include baseline demographic, clinical and laboratory assessments (including relevant biomarkers), treatments, tumor measurements over time, and outcomes. The database will shortly be locked to allow the first analyses to be conducted, but data from future trials will be incorporated periodically.
Table 1.
Advanced colorectal cancer trials incorporated or planned for inclusion in the ARCAD database
aPubMed identifier unless stated.
bTrials status updated to September 2011. Discussions on further trials not listed in this table are in progress with sponsors and principal investigators.
cClinicalTrials.gov identifier.
Abbreviations: AGITG, Australasian Gastro-Intestinal Trials Group; AIO, Arbeitsgemeinschaft Internische Onkologie (Germany); DCCG, Dutch Colorectal Cancer Group; ECOG, Eastern Cooperative Oncology Group (USA); GERCOR, Groupe Cooperateur Multidisciplinaire en Oncologie (France); GONO, North West Oncology Group (Italy); HORG, Hellenic Oncology Research Group (Greece); NCCTG, North Central Cancer Treatment Group (USA); NCIC-CTG, NCIC Clinical Trials Group (Canada); MRC, Medical Research Council (UK); TTD, Spanish Cooperative Group for Gastrointestinal Tumor Therapy.
As with the similar GASTRIC and ACCENT databases in gastric and adjuvant colorectal cancer [11, 12], the patient-level approach adopted in the ARCAD database provides a more informative and statistically robust basis for research than could be achieved through meta-analyses based on published literature or summary statistics. Compared with these methodologies, the individual-patient approach reduces publication and reporting bias, gives access to the most recent data, permits data checking, and, crucially, affords greater scope for novel analyses [13, 14]. By adopting this paradigm, the ACCENT project established the validity of 3-year disease-free survival (DFS) as a surrogate endpoint for overall survival in the adjuvant treatment of colorectal cancer, leading to a change in FDA drug approvals criteria and shorter clinical trials [15]. Subsequent studies using the ACCENT database have clarified a variety of further issues including the influence of disease stage on the prognostic utility of DFS, time-dependent patterns of failure, survival after recurrence, and the prognostic impact of race [15]. The initial studies using the ARCAD database will also focus on endpoints, with analyses of the relationship between progression-free and overall survival; the utility of fixed time-point and continuous tumor measurements; and the potential of on-treatment markers as surrogate endpoints. Further studies using the ARCAD database will examine the prognostic significance of baseline parameters including age, gender, performance status, laboratory values, body mass index, and site of disease. In addition to these studies, Response Evaluation Criteria In Solid Tumors (RECIST) criteria [16] will be examined in detail, and a “trial simulator” will be constructed to assist future trial design and endpoint selection, particularly in the multiline setting. It is hoped these studies will in due course support the development of improved endpoints and patient selection criteria in advanced colorectal cancer.
The ARCAD database has been constructed by the group as a resource for the wider academic community, and both ARCAD and non-ARCAD members are invited to propose further studies with a view to collaborative projects. It is anticipated that academic groups whose trial data is incorporated into the database will be important collaborators, but unsolicited proposals from groups not currently connected with the ARCAD program are also welcomed. Proposals for projects should be communicated to the authors or directly to the Fondation ARCAD office (mdebausset@fondationarcad.org) and will be considered by a panel consisting of the principal investigators and ARCAD colleagues.
The ARCAD database is to our knowledge one of the largest of its kind yet constructed in gastrointestinal oncology, and we are grateful to our colleagues in academia and industry for their participation. As with the GASTRIC and ACCENT databases, use of the database is subject to strict safeguards. In particular, all data contributors are consulted before every analysis and have the freedom to withhold their trial data from any analysis. These measures have been adopted to respect the interests of data providers; moreover, to ensure patient confidentiality is upheld, names and personal details of patients are not recorded in the database.
It is useful at this stage in the ARCAD Program's development to evaluate the experience to date and consider whether its model is transferable to other areas of oncology. First, it should be reiterated that the collaboration was not formed to conduct a specific research project but in response to a general problem for oncology—the need for more efficient models of clinical development. The ARCAD Program's goal is to analyze this problem and develop solutions in the specific setting of gastrointestinal oncology, where its members have expertise. We believe this approach is useful and replicable in other fields. Second, the ARCAD Program is distinctive in that, despite a substantial membership, central administration, and significant external funding, it retains informal features characteristic of smaller academic collaborations. Thus, particularly active members supported by the secretariat are responsible for day-to-day decision-making, whereas the broader academic membership steers the overall direction of the project and determines its outputs. ARCAD's informal, collaborative features have permitted rapid progress based on consensual decision-making but rely in part on the fact that the membership consists of a community of peers, most of whom have known and worked with each other over many years.
The ARCAD Clinical Trials Program has generated considerable interest, both from the many academics who have chosen to participate and from the institutions and companies who have contributed trials data to the database project. Whether this model of academic collaboration will be judged a success will depend on the quality of its scientific output during the next few years and whether this output, alongside that of other scientists, groups, and institutions, ultimately leads to more efficient trials and improved treatment options for patients. We look forward to these challenges in the next phase of the ARCAD Program's evolution.
Acknowledgments
We acknowledge the exceptional contributions of Mariella de Bausset, Secretaty General of Fondation ARCAD, and our colleagues at the Mayo Clinic, Qian Shi, Ph.D., Erin Green, and Jeff Meyers, for their work in the construction of the ARCAD database. No external funding or support was used in the preparation of this article. D.J.S., M.B., and A.d.G. are principal investigators, ARCAD Advanced Colorectal Cancer Database Project.
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Daniel J. Sargent, Marc Buyse, Alastair Matheson, Richard M. Goldberg, Aimery de Gramont
Collection and/or assembly of data: Daniel J. Sargent, Alastair Matheson
Data analysis and interpretation: Marc Buyse, Richard M. Goldberg
Manuscript writing: Daniel J. Sargent, Marc Buyse, Alastair Matheson, Richard M. Goldberg, Aimery de Gramont
Final approval of manuscript: Daniel J. Sargent, Marc Buyse, Alastair Matheson, Richard M. Goldberg, Aimery de Gramont
References
- 1.United States Food and Drug Administration. Critical Path Initiative. [Accessed August 22, 2011]. Available at http://www.fda.gov/ScienceResearch/SpecialTopics/CriticalPathInitiative/default.htm.
- 2.Office of the Chief Scientist. U.S. Food and Drug Administration. Advancing Regulatory Science for Public Health: A Framework for FDA's Regulatory Science Initiative. 2010. Oct, [Accessed August 22, 2011]. Available at http://www.fda.gov/ScienceResearch/SpecialTopics/RegulatoryScience/ucm228131.htm.
- 3.United States National Institutes of Health. The NIH Common Fund. Regulatory Science. [Accessed August 22, 2011]. Available at http://commonfund.nih.gov/regulatoryscience.
- 4.American Society for Clinical Oncology. Research Activities. [Accessed December 12, 2011]. Available at http://www.asco.org/ASCOv2/Research+Resources/Research+Activities.
- 5.The Biomarkers Consortium. [Accessed August 22, 2011]. Available at http://biomarkersconsortium.org.
- 6.Fondation ARCAD (Aide et Recherche en Cancérologie Digestive) [Accessed August 22, 2011]. Available at http://www.fondationarcad.org.
- 7.de Gramont A, Haller DG, Sargent DJ, et al. Toward efficient trials in colorectal cancer: The ARCAD Clinical Trials Program. J Clin Oncol. 2010;28:527–530. doi: 10.1200/JCO.2009.25.2544. [DOI] [PubMed] [Google Scholar]
- 8.Buyse M, Sargent DJ, Grothey A, et al. Biomarkers and surrogate end points–The challenge of statistical validation. Nat Rev Clin Oncol. 2010;7:309–317. doi: 10.1038/nrclinonc.2010.43. [DOI] [PubMed] [Google Scholar]
- 9.Buyse M, Michiels S, Sargent DJ, et al. Integrating biomarkers in clinical trials. Expert Rev Mol Diagn. 2011;11:171–182. doi: 10.1586/erm.10.120. [DOI] [PubMed] [Google Scholar]
- 10.Chibaudel B, Bonnetain F, Shi Q, et al. Alternative end points to evaluate a therapeutic strategy in advanced colorectal cancer: evaluation of progression-free survival, duration of disease control, and time to failure of strategy–An Aide et Recherche en Cancerologie Digestive Group Study. J Clin Oncol. 2011;29:4199–4204. doi: 10.1200/JCO.2011.35.5867. [DOI] [PubMed] [Google Scholar]
- 11.Paoletti X, Oba K, Burzykowski T, et al. GASTRIC Group. Benefit of adjuvant chemotherapy for resectable gastric cancer: A meta-analysis. JAMA. 2010;303:1729–1737. doi: 10.1001/jama.2010.534. [DOI] [PubMed] [Google Scholar]
- 12.Sargent DJ, Patiyil S, Yothers G, et al. ACCENT Group. End points for colon cancer adjuvant trials: observations and recommendations based on individual patient data from 20,898 patients enrolled onto 18 randomized trials from the ACCENT Group. J Clin Oncol. 2007;25:4569–4574. doi: 10.1200/JCO.2006.10.4323. [DOI] [PubMed] [Google Scholar]
- 13.Buyse M. Contributions of meta-analyses based on individual patient data to therapeutic progress in colorectal cancer. Int J Clin Oncol. 2009;14:95–101. doi: 10.1007/s10147-009-0879-2. [DOI] [PubMed] [Google Scholar]
- 14.Buyse M. Use of meta-analysis for the validation of surrogate endpoints and biomarkers in cancer trials. Cancer J. 2009;15:421–425. doi: 10.1097/PPO.0b013e3181b9c602. [DOI] [PubMed] [Google Scholar]
- 15.Gill S, Sargent DJ. Individual data pooled analyses to improve understanding of adjuvant therapy in colon cancer: review of the ACCENT collaborative group. Curr Colorectal Cancer Rep. 2008;4:155–159. [Google Scholar]
- 16.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]