Pancreatic cancer is the fourth most common cancer-related death in men and women in the United States, and the 5-year survival rate for patients with pancreatic cancer remains <5%. This article describes the rationales of neoadjuvant approaches and reviews important clinical trials of neoadjuvant therapies in patients with resectable pancreatic cancer. At this point in time, the major benefit of the neoadjuvant approach, once more effective regimens are available, is that it will allow downstaging of borderline resectable disease, and perhaps a small subset of locally advanced pancreatic cancer cases, to improve R0 resection and survival.
Keywords: Pancreatic cancer, Neoadjuvant, Borderline resectable chemotherapy, Borderline resectable, Chemoradiation
After completing this course, the reader will be able to:
Discuss the current literature on the neoadjuvant therapies in patients with potentially resectable and borderline resectable pancreatic cancer.
Cite the definition of borderline resectable pancreatic cancer as determined by the 2008 AHPBA consensus guidelines.
This article is available for continuing medical education credit at CME.TheOncologist.com
Abstract
Pancreatic cancer remains one of the deadliest cancers due to difficulty in early diagnosis and its high resistance to chemotherapy and radiation. It is now clear that even patients with potentially resectable disease require multimodality treatment including chemotherapy and/or radiation to improve resectability and reduce recurrence. Tremendous efforts are currently being invested in refining preoperative staging to identify optimal surgical candidates, and also in developing various neoadjuvant or adjuvant regimens to improve surgical outcome. Although at present no studies have been done to directly compare the benefit of neoadjuvant versus adjuvant approaches, accumulating evidence suggests that the neoadjuvant approach is probably beneficial for a subset of the patient population, particularly those with borderline resectable disease in which complete surgical resection is almost certainly unachievable. In this article, we review the literature and rationales of neoadjuvant chemotherapy and chemoradiation, as well as their potential limitations and caveats. We also review the pathological findings following neoadjuvant therapies, and potential surgical complications that may be associated with neoadjuvant therapies.
Introduction
Pancreatic cancer is the fourth most common cancer-related death in men and women in the United States. The 5-year survival rate for patients with pancreatic cancer remains <5% [1]. To date, the only chance for cure exists for patients with local disease undergoing surgical resection of the primary tumor and regional lymph nodes. Unfortunately, only 15%–20% of patients with pancreatic cancer are deemed resectable at the time of presentation, and the majority of those who receive curative surgical resection will eventually succumb to locoregional recurrence or distant metastasis [2, 3].
At least two major factors contribute to the poor outcome after surgical resection of pancreatic cancer: (a) the inability of current radiographic techniques to distinguish patients with truly localized disease from those with micrometastasis prior to surgery and (b) the lack of therapeutic agents that are effective against micrometastatic disease. In fact, studies have shown that the length of survival for patients with an incomplete resection is no different from that of patients with locally advanced, surgically unresectable disease treated with chemoradiation. Increasing attention is now focused on neoadjuvant chemotherapy and/or radiation to improve resectability and long-term survival. This article describes the rationales of neoadjuvant approaches and reviews important clinical trials of neoadjuvant therapies in patients with resectable pancreatic cancer. The pathologic findings after neoadjuvant therapy and the surgical concerns associated with the neoadjuvant approach are also discussed.
Rationales for Neoadjuvant Therapy
The rationale of neoadjuvant therapy was born out of the adjuvant approach to cancer therapy. Over the last few years, three large randomized phase III trials in pancreatic cancer have reported the benefit of adjuvant therapy either with chemotherapy or chemoradiation. In the Charite Onkologie Clinical Studies in GI Cancer (CONKO)-001 trial, 368 patients after R0 or R1 surgical resection were randomized to strict observation or administration of six cycles of gemcitabine [4]. During a median follow-up of 53 months, patients in the gemcitabine arm were found to have superior median disease-free survival (DFS) compared with the observation arm (13.4 months versus 6.9 months; p < .001), but the median overall survival (OS) between the two groups was not different (22.1 months with gemcitabine versus 20.2 months of observation, p = .06). The authors attributed the lack of difference in median OS to the fact that almost all patients in the observation arm received gemcitabine or other chemotherapy upon relapse. The final results of the CONKO-001 trial were presented at the American Society of Clinical Oncology annual meeting in 2008. By intention-to-treat analysis, patients who received adjuvant gemcitabine for 6 months (n = 179) had superior DFS (13.4 months versus 6.9 months, p < .001), median survival (22.8 months versus 20.2 months, p = .006), 3-year survival (36.5% versus 19.5%), and 5-year survival (21% versus 9%) compared to patients in the observation arm (n = 175) [5]. These results support the role of gemcitabine as adjuvant therapy in pancreatic cancer.
In a prospective randomized trial conducted by the Radiation Therapy Oncology Group-9704, 451 patients underwent surgical resection, but importantly, those without radiographic evidence of persistent disease or relapse were randomized to receive either adjuvant infusional fluorouracil or gemcitabine for 3 weeks followed by fluorouracil-based chemoradiation an additional 12 weeks after chemoradiation. No statistically significant difference in survival was observed between the two arms (gemcitabine versus fluorouracil; median survival 20.5 months versus 16.9 months; p = .9) [6].
Lastly, in the European Study Group for Pancreatic Cancer (ESPAC)-3 trial, 1,088 patients who underwent pancreatic resection were randomized to receive either adjuvant fluorouracil plus folinic acid (n = 551) or gemcitabine (n = 537) for six cycles. At the 2-year follow-up, adjuvant gemcitabine or fluorouracil plus folinic acid showed similar median OS (23.6 months versus 23.0 months, p = .39) and median progression-free survival (14.3 months versus 14.1 months), although gemcitabine was associated with significantly less adverse side effects [7]. The ESPAC-3 trial suggested that fluorouracil plus folinic acid can be used as an alternative to gemcitabine in the adjuvant setting.
In patients who completed surgical resection and adjuvant chemotherapy and/or radiation, the risk for systemic recurrence can be as high as 77% [6]. Furthermore, the survival outcomes in these three studies are similar, reflecting the fact that the benefit of the adjuvant approach, at least based on these regimens, might have reached a plateau. Unquestionably, various other combinations of chemotherapeutic and targeted agents are currently being investigated and could potentially improve surgical outcomes in the future. In parallel, several rationales have been proposed to advocate use of chemotherapy and/or chemoradiation before curative resection:
The initiation of adjuvant therapy is frequently delayed due to inadequate recovery from surgery, especially in patients receiving the Whipple procedure to resect a tumor in the head of pancreas. Such a delay can be easily avoided by administering therapies before surgery. Additionally, the chance of delivering full-dose chemotherapy and/or radiation is much higher when given prior to surgery. For instance, 90%–100% of patients were able to complete all planned neoadjuvant gemcitabine-based regimens prior to surgery [8–10], as opposed to only 62% of patients in the CONKO-001 trial who received adjuvant gemcitabine as planned due to poor wound healing or other postoperative complications [4].
Neoadjuvant therapies provide a window to spare patients who progress or develop distant metastasis during treatment from undergoing a major surgery which would not be curative anyway.
Neoadjuvant therapies could potentially downstage borderline resectable disease and enhance the rate of R0 resection.
Neoadjuvant Radiation
The neoadjuvant approach has been validated in other tumor types, and it was initially evaluated in pancreatic cancer three decades ago. In the 1980s, Ishikawa et al. reported a retrospective study comparing 23 patients with radiographically resectable pancreatic cancer who received neoadjuvant radiotherapy (totally 50 Gy) and 31 patients who only underwent resection. The curative pancreaticoduodenectomy rate was similar for both groups (74% versus 61%). However, patients who received preoperative radiation had a better one-year survival rate (75% versus 43%), primarily due to lower locoregional recurrence, but a similar 3- to 5-year survival rate (8% versus 32% and 22% versus 26%, respectively) due to the higher incidence of liver metastases [11]. Despite several limitations, a retrospective analysis of the surveillance, epidemiology, and end results (SEER) database from 1988 to 2003 showed a survival benefit in patients who received radiation before surgery [12].
Neoadjuvant Chemotherapy and Chemoradiation
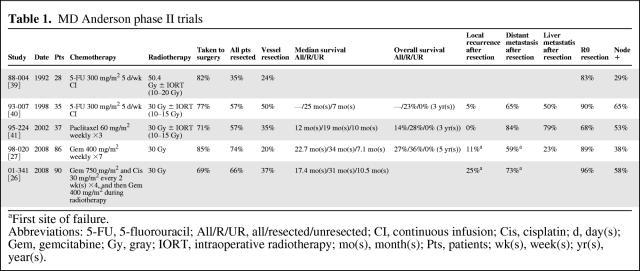
Evidence to support neoadjuvant chemoradiation was established from several phase II trials conducted in the past two decades. The series of studies with perhaps the most impact were from the MD Anderson Cancer Center (MDACC) (Table 1). In the six published phase II trials, the definition of “resectable” tumors was defined and remained constant, as have the operative techniques employed. This series of trials provides a unique framework to understand the context and incremental changes of each successive trial. Overall, these studies showed that patients who completed neoadjuvant chemoradiation and did not progress at restaging had a higher chance of achieving R0 resection when compared to historical data. Importantly, this was translated into a higher median and overall survival compared to patients who did not receive surgical resection. However, a note should be made about the large number of vascular resections performed at MDACC. In a review of 132 consecutively treated patients with neoadjuvant chemoradiation, 43% required a vascular resection and reconstruction at the time of pancreaticoduodenectomy [13]. The authors acknowledge that what may be considered “resectable” at MDACC might not be considered resectable in many other centers.
Table 1.
MD Anderson phase II trials
aFirst site of failure.
Abbreviations: 5-FU, 5-fluorouracil; All/R/UR, all/resected/unresected; CI, continuous infusion; Cis, cisplatin; d, day(s); Gem, gemcitabine; Gy, gray; IORT, intraoperative radiotherapy; mo(s), month(s); Pts, patients; wk(s), week(s); yr(s), year(s).
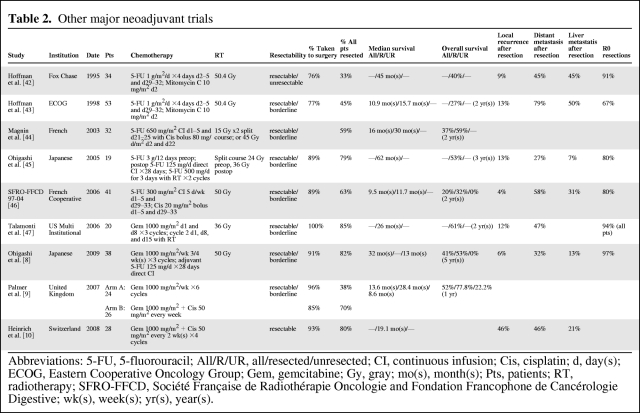
Although combined chemoradiation has been shown to improve local control, it does not effectively decrease distant metastasis, and this could be due to patients having a lower tolerance for cytotoxic agents when also receiving radiation. Accordingly, neoadjuvant chemotherapy alone may allow delivery of higher doses of cytotoxic agents, which could theoretically lead to more effective elimination of distant micrometastasis and potentially improve long-term outcome. To date, no comparison has been done between neoadjuvant chemotherapy versus combined chemoradiation. Several other studies by various institutions have also shown improved resectability and survival with neoadjuvant chemotherapy with or without radiation (Table 2).
Table 2.
Other major neoadjuvant trials
Abbreviations: 5-FU, 5-fluorouracil; All/R/UR, all/resected/unresected; CI, continuous infusion; Cis, cisplatin; d, day(s); ECOG, Eastern Cooperative Oncology Group; Gem, gemcitabine; Gy, gray; mo(s), month(s); Pts, patients; RT, radiotherapy; SFRO-FFCD, Société Française de Radiothérapie Oncologie and Fondation Francophone de Cancérologie Digestive; wk(s), week(s); yr(s), year(s).
At least two caveats must be acknowledged while interpreting data from these neoadjuvant trials. First, the definition of resectability used in most literature is inconsistent and often difficult to interpret, which leads to difficulty in comparing results from different trials. Additionally, what may be considered “resectable” with a venous resection in one institution may be considered “borderline” resectable or even unresectable in another. A second caveat is that our ability to correctly stage pancreatic cancer has changed over time, which has led to potential stage migration. In a number of earlier trials, one method for obtaining a tissue diagnosis and determining resectability included an exploratory laparotomy [14, 15]. Over time, diagnostic and staging laparotomies have decreased, and the utilization of endoscopic ultrasounds and computer tomography scans (CTs) has increased. With improved CTs, magnetic resonance imaging scans, and positron emission tomography (PET)-CTs, investigators are more equipped to determine whether a tumor is resectable, locally advanced, or even metastatic. Any survival benefit seen with improved treatment regimens and newer chemotherapies may simply be a result of stage migration. For instance, even within the MDACC phase II trials, the trend toward improvement in median survival over time might be a result of better selection of patients.
Surgical Definition of Resectable, Borderline Resectable, and Locally Advanced Disease
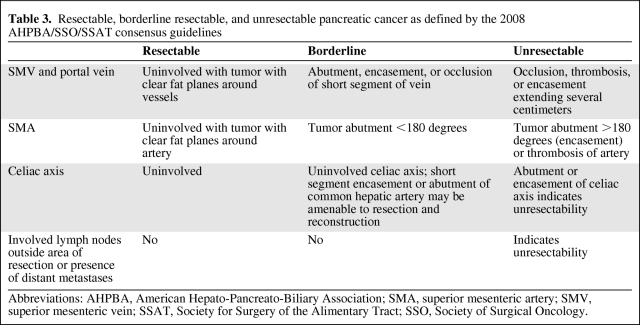
Multidetector CT with three-dimensional (3-D) reconstruction is currently the modality of choice to preoperatively stage pancreatic cancer. Preoperative CTs for pancreatic cancer have been shown to have a high predictive value for unresectability (90%–100%) with a slightly lower predictive value of resectability (76%–90%). A thorough radiographic assessment includes noting the presence or absence of distant metastatic disease and the relationship of the tumor to superior mesenteric vein and portal vein and their tributaries, superior mesenteric artery, celiac axis, hepatic artery, and gastroduodenal artery. On the basis of the radiographic findings, pancreatic cancers are classified as resectable, borderline resectable, or unresectable (locally advanced or metastatic). However, the ambiguity of these terms has generated a lot of controversy in recent years, and it is likely that undefined discrepancies exist between trials reported in the literature, making it difficult to compare data between trials. In January of 2008, the American Hepato-Pancreato-Biliary Association (AHPBA) convened a Consensus Conference on Resectable and Borderline Resectable Pancreatic cancer with the goal of clearly defining resectable and borderline resectable pancreatic cancer and reviewing the indications and contraindications for surgery, neoadjuvant therapy, and adjuvant therapy for these lesions (Table 3) [16].
Table 3.
Resectable, borderline resectable, and unresectable pancreatic cancer as defined by the 2008 AHPBA/SSO/SSAT consensus guidelines
Abbreviations: AHPBA, American Hepato-Pancreato-Biliary Association; SMA, superior mesenteric artery; SMV, superior mesenteric vein; SSAT, Society for Surgery of the Alimentary Tract; SSO, Society of Surgical Oncology.
Up to one third of the patients with apparent resectable pancreatic cancer are found to have metastatic disease at the time of operation. This is attributed to the low sensitivity of CTs to visualize small-volume metastatic disease. This has led surgeons to perform laparoscopy as part of preoperative staging in selected patients with the goal of avoiding the morbidity associated with an unnecessary laparotomy. A number of predictors have been identified that optimize the yield of performing a staging laparoscopy for apparently resectable pancreatic cancer: (a) tumors >3 cm at the head of the pancreas; (b) tumors of the pancreatic body and tail; (c) equivocal findings of resectability based on the above-mentioned parameters on CTs; and (d) CA19–9 levels >100 U/mL. A staging laparoscopy may also be used in patients with locally advanced unresectable pancreatic cancer to rule out undetected metastatic disease to optimize treatment selection.
Neoadjuvant Therapy for Borderline Resectable Pancreatic Cancer
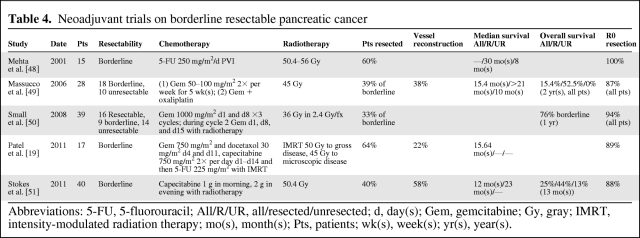
First defined by Varadhachary et al. in 2006 and later adopted in the National Comprehensive Cancer Network (NCCN) guidelines in 2008, borderline resectable pancreatic cancer represents a distinct disease subset defined by CT findings that straddle the definitions of resectable and locally advanced disease [17, 18]. Patients with borderline resectable disease pose a therapeutic dilemma because of their low chance of R0 resection if immediate surgery is preferred but otherwise bleak chance of cure with nonsurgical therapies. One often-cited advantage of neoadjuvant therapy has been the potential to downstage patients with borderline resectable pancreatic cancer and thereby increases their chance for R0 resections. The veracity of this claim is difficult to ascertain given the inconsistent inclusion of patients with both resectable and borderline resectable tumors in published clinical trials. A review of the prospective neoadjuvant trials for resectable pancreatic cancers is summarized in Table 4.
Table 4.
Neoadjuvant trials on borderline resectable pancreatic cancer
Abbreviations: 5-FU, 5-fluorouracil; All/R/UR, all/resected/unresected; d, day(s); Gem, gemcitabine; Gy, gray; IMRT, intensity-modulated radiation therapy; mo(s), month(s); Pts, patients; wk(s), week(s); yr(s), year(s).
The percentage of patients in these five trials with borderline resectable cancers who underwent surgical resection ranged from 33%–64%. Although only three of the five trials specified vessel resection, the rate of resection was high, up to 64% [19]. More interestingly, the rate of R0 resections was high in all studies, 87%–100%. Although small in number, there appears to be good potential for downstaging of borderline resectable tumors to achieve R0 resection.
The benefit of neoadjuvant therapies in borderline resectable pancreatic cancer was retrospectively reviewed by Katz et al. at MDACC [20]. Between 1999 and 2006, 160 (7%) of 2,454 pancreatic cancer patients with borderline resectable disease were scheduled to receive 2–4 months of neoadjuvant chemotherapy followed by radiation in combination with 5-fluorouracil (5-FU), gemcitabine, capecitabine, or paclitaxel. Restaging CT was done every 2 months during the treatment and 4–6 weeks after completion to determine respectability. Patients who progressed or had declined performance status during this period of treatment were excluded from surgery. One-hundred twenty-five (78%) patients completed the restaging, 79 (63% of 125) patients proceeded to surgery, and 66 (53% of 125) patients received pancreaticoduodenectomy. Of all 160 patients with borderline resectable disease, the median survival was 18 months and the 5-year survival was 18%. Importantly, the 66 patients who completed all therapy including surgery had a superior median survival of 40 months and a 5-year survival rate of 36%, compared to a median survival of 13 months in the remaining 94 unresected patients. Patients with greater pathologic response or drop in serum CA19–9 levels during neoadjuvant therapy had better OS. However, 59% of the resected patients eventually had a recurrence, and 45% of patients had recurrence occurring in distant organs such as lung, liver, or bone, 9% had recurrence in the pancreatic bed, and 11% had recurrence in the peritoneum or regional lymph nodes. The median time to progression was 24 months, indicating a need to improve the efficacy of neoadjuvant therapy.
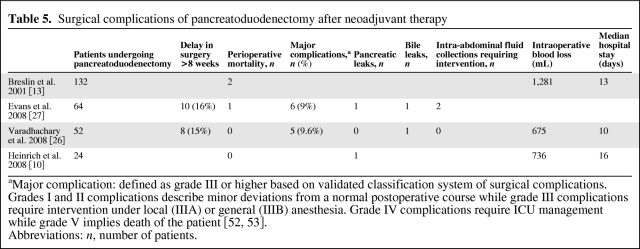
Surgical Complications of Pancreatoduodenectomy after Neoadjuvant Therapy
Pancreatoduodenectomy is associated with a high morbidity (30%–60%). The biggest concern with neoadjuvant therapy for pancreatic cancer is its risk to increase perioperative morbidity. It has been hypothesized that the detrimental effects of neoadjuvant therapy on wound healing place patients at a higher risk for complications like wound infections, anastomotic leaks, intra-abdominal abscesses, and death. However, this association has yet to be seen in clinical studies.
Varadhachary et al. in 2008 conducted a phase II trial of preoperative gemcitabine and cisplatin chemotherapy in addition to chemoradiation (gemcitabine, cisplatin, and radiation) followed by pancreatoduodenectomy for adenocarcinoma of the pancreatic head. Of the 90 patients enrolled, 52 underwent pancreatoduodenectomy. There were no perioperative deaths, and 5 (9.6%) patients developed a major complication, including intraoperative cardiac arrest with successful resuscitation (n = 1), reoperation for a wound hematoma (n = 1), aspiration pneumonia and sepsis from central venous catheter infection (n = 1), and a biliary anastomotic leak (n = 1). There were no clinically significant pancreatic leaks. Evans et al. in 2008 similarly reported a major complication rate of 9% in his review of 64 patients (of 86 enrolled) who underwent pancreatoduodenectomy after preoperative gemcitabine-based chemoradiation. Complications included pancreatic leak (n = 1), intra-abdominal sterile fluid collections requiring percutaneous drainage (n = 2), biliary leak (n = 1), postoperative GI bleed (n = 1), and chylous ascites (n = 1) (Table 5). The morbidity and mortality rates described in these studies are similar to those reported after conventional pancreatoduodenectomy for pancreatic cancer in high-volume centers and suggest that surgery after neoadjuvant chemotherapy is safe.
Table 5.
Surgical complications of pancreatoduodenectomy after neoadjuvant therapy
aMajor complication: defined as grade III or higher based on validated classification system of surgical complications. Grades I and II complications describe minor deviations from a normal postoperative course while grade III complications require intervention under local (IIIA) or general (IIIB) anesthesia. Grade IV complications require ICU management while grade V implies death of the patient [52, 53].
Abbreviations: n, number of patients.
Pathologic Assessment Following Neoadjuvant Therapy
In the literature, there are only a few studies on the pathologic changes of pancreatic ductal adenocarcinoma with neoadjuvant treatment. A recent report from the MDACC showed that post-treatment pathologic American Joint Committee on Cancer (AJCC) stage and lymph node status following neoadjuvant therapy are independent prognostic factors for both disease-free survival and overall survival, whereas tumor grade and margin status are independent factors for disease-free survival for overall survival [21].
Cytologic changes associated with neoadjuvant treatment include cytoplasmic swelling, vacuolization, clearing and eosinophilia, nuclear enlargement, multiple nuclei, bizarre nuclear shape, hyperchromasia, pyknosis, and karyorrhexis. Viable tumor cells are those with fairly preserved morphology including those with nuclear or cytoplasmic swelling, multiple nuclei, or cytoplasmic vacuolization. Nonviable tumor cells are those with bizarre, hyperchromatic, pyknotic, or karyorrhexic nuclei, usually with associated markedly swollen, vacuolated, or deeply eosinophilic cytoplasm. Chemoradiation typically induces stromal fibrosis both within the tumor and the surrounding parenchyma, rendering accurate gross measurement of the tumor size difficult. The mean fibrosis level (defined as percentage of tumor area occupied by fibrosis) in resection specimen was 73% in 61 patients who received neoadjuvant treatment, significantly higher than 38% in 55 patients who did not receive such treatment (p < .0001) (unpublished data).
Limitations of Current Neoadjuvant Therapies
To date, well-designed, randomized studies that directly compare neoadjuvant therapy followed by resection to upfront resection, or comparison of the neoadjuvant approach versus the adjuvant approach, are not available. Recently, a large meta-analysis that included 111 studies and 4,394 patients found no difference in resection rate or median survival between patients with initially resectable disease who were treated with either the neoadjuvant or adjuvant approach [22]. The study showed that about one third of patients with unresectable disease at diagnosis were able to undergo successful resection after neoadjuvant therapy, particularly with combination regimens. Moreover, median survival following resection was comparable to patients with resectable disease [22], which was also consistent with another smaller meta-analysis [23], supporting the ability of neoadjuvant therapy to downstage pancreatic cancer in a subset of patients. Because patients with resectable disease appear to have similar outcomes when treated with the neoadjuvant or adjuvant approach in general, selection of either approach must be individualized and decided by a multidisciplinary team. Upfront surgery should be considered for patients with (a) gastric outlet obstruction, (b) obstructive jaundice that fails to be relieved with a biliary stent, (c) specimens for which a clear diagnosis cannot be made, (d) relatively good preoperative performance status, and (e) expected satisfactory recovery from surgery, which would likely allow adjuvant therapy to be given in time and at the full dose. Patient preference after detailed discussion with the medical oncologist and surgeon should also be a considerable factor in the final decision making.
Currently, a multicenter, prospective randomized phase II study has been launched to compare primary resection versus neoadjuvant chemoradiation with cisplatin and gemcitabine followed by resection for locally resectable or potentially resectable pancreatic cancer [24]. Another phase III study (NEOPAC) attempts to address whether addition of neoadjuvant oxaliplatin plus gemcitabine will improve progression-free survival in patients with resectable pancreatic cancer who also receive adjuvant gemcitabine [25]. Importantly, the lack of effective chemotherapeutic or targeted agents probably explains the relatively modest improvement in survival from current neoadjuvant or adjuvant trials. In the two gemcitabine-based studies published by MDACC, about 60% of resected patients eventually developed recurrence at distal organs at a median time of only 8–13 months [26, 27]. Nonetheless, neoadjuvant therapy clearly provides a way to select for patients who would benefit most from curative resection. On the other hand, patients who have rapidly progressing disease during neoadjuvant treatment can be spared from unnecessary surgery. Recently FOLFIRINOX (oxaliplatin, irinotecan, leucovorin, and 5-FU) was shown to be superior to gemcitabine in patients with metastatic pancreatic cancer, with a median OS of 11.1 months in the FOLFIRINOX group versus 6.8 months in the gemcitabine group (p < .001) and a median progression-free survival of 6.4 months versus 3.3 months (p < .001), although FOLFIRINOX resulted in higher toxicity [28]. Although highly speculative, it is possible that neoadjuvant FOLFIRINOX could improve the surgical outcome in selected patients with good performance status.
What Is on the Horizon?
Cancer treatment has entered a molecular era. A targeted approach to therapy holds promise in many cancers due to its tolerability and high efficacy in specific patient populations. In patients with locally advanced or metastatic pancreatic cancer, gemcitabine plus an epidermal growth factor receptor inhibitor, erlotinib, results in a modest but statistically significant improvement in median OS (6.2 months versus 5.9 months, hazard ratio p = .038) and progression-free survival (3.75 months versus 3.55 months, p = .004) when compared to treatment with gemcitabine alone [29]. However, no further benefit is found with addition of bevacizumab to the gemcitabine/erlotinib combination [30, 31]. For patients with advanced pancreatic cancer, combined use of gemcitabine and other targeted agents such as bevacizumab [32], cetuximab [33], or both [34] and axitinib [31] are not superior to gemcitabine alone. Disappointing results from targeted therapies in pancreatic cancer have not necessarily discouraged researchers in the field of pancreatic cancer but rather inspired them to investigate other molecular targets that may be pertinent to tumorigenesis, such as PI3K, Hedgehog, metalloproteinases, and others [35]. Currently a phase II, multicentered trial by the American College of Surgeons Oncology Group (ACOSOG-Z5041) is ongoing to study the benefit of neoadjuvant or adjuvant use of gemcitabine plus erlotinib in patients with resectable pancreatic cancer. Another phase II trial (NCT00460174) aims to study the effect of neoadjuvant gemcitabine, bevacizumab, and radiation. On the basis of the promising outcome in metastatic pancreatic cancer, discussion on FOLFORINOX as neoadjuvant therapy is being entertained by academic oncologists [28]; however, the treatment-related toxicities have posed a challenge to incorporate this treatment into the neoadjuvant setting.
Furthermore, new areas of targeted therapies are being explored for pancreatic cancer, which include modulating the tumor microenvironment, improving drug delivery, augmenting antitumor immunity, and targeting tumor stem cells. It is now increasingly acknowledged that chemoresistance in pancreatic cancer is greatly ascribed to the presence of a protective desmoplastic, hypovascular stroma around the tumor, and this is primarily driven by paracrine Hedgehog signaling from the tumor cells to the microenvironment. Treatment with IPI-926, a small-molecule inhibitor of the Sonic-Hedgehog pathway, impairs proliferation of stromal myofibroblasts, inproves local vasculature, and greatly improves delivery of gemcitabine to the tumor in a murine model [36]. In another study, combined use of gemcitabine and a CD40 agonist, which augments the tumoricidal activity of T cells, resulted in favorable clinical response in a small number of patients compared to historical data using gemcitabine alone [37].
Improved delivery of radiotherapy such as intensity-modulated radiation therapy (IMRT) has the advantages of minimizing toxicity, allowing higher doses of concurrent chemotherapy, and reducing postoperative complications. A phase II trial has recently been initiated at the University of Michigan using IMRT with active breathing control and target motion management to deliver a high-dose radiation, 50 Gy in 25 fractions, with concurrent full-dose gemcitabine, 1,000 mg/m2. Patients will undergo six cycles of induction FOLFIRINOX, followed by concurrent chemoradiation and two additional cycles of gemcitabine. After completion of neoadjuvant therapy, patients will be assessed for potential surgical resection. The utilization of IMRT limits the significant toxicity associated with full-dose gemcitabine with concurrent radiation and has previously been evaluated in an almost completed phase I/II trial in patients with localized unresectable disease at the University of Michigan.
Though immature at this point, novel diagnostics such as detection of circulating tumor cells and gene expression profiling of tumor samples may allow stratification of patients based on the risks of metastasis and invasion, which could potentially help identify patients who most likely would benefit from neoadjuvant therapies or upfront surgery.
The Future for the Neoadjuvant Approach
Resectable pancreatic cancer represents only about 15%–20% of patients at the time of initial diagnosis, and it is clear that surgical resection alone is inadequate. Development of more effective chemotherapeutic or targeted agents is obviously the key to truly improve the prognosis of this disease. Meanwhile, many neoadjuvant studies incorporating new targeted agents are ongoing, and the results will continue to impact future practice. Therefore, patients with resectable or borderline resectable pancreatic cancer should be encouraged to enroll in clinical trials. In parallel, refinement of radiographic techniques and standardization of imaging criteria on localized disease before and after neoadjuvant therapy will be essential to better define truly operable candidates.
At this point in time, the major benefit of the neoadjuvant approach, once more effective regimens are available, is that it will allow downstaging of borderline resectable disease, and perhaps a small subset of locally advanced pancreatic cancer cases, to improve R0 resection and survival [21, 38].
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor/patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Data analysis and interpretation: Kian-Huat Lim, Eugene Chung, Adeel Khan, Andrea Wang-Gillam
Manuscript writing: Kian-Huat Lim, Eugene Chung, Adeel Khan, Dengfeng Cao, Andrea Wang-Gillam
Final approval of manuscript: Kian-Huat Lim, Eugene Chung, Adeel Khan, Dengfeng Cao, David Linehan, Edgar Ben-Josef, Andrea Wang-Gillam
References
- 1.Howlader NNA, Krapcho M, Neyman N, et al., editors. SEER Cancer Statistics Review, 1975–2008. Bethesda, MD: National Cancer Institute; 2011. [Google Scholar]
- 2.Griffin JF, Smalley SR, Jewell W, et al. Patterns of failure after curative resection of pancreatic carcinoma. Cancer. 1990;66:56–61. doi: 10.1002/1097-0142(19900701)66:1<56::aid-cncr2820660112>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 3.Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–579. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 4.Oettle H, Post S, Neuhaus P, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: A randomized controlled trial. JAMA. 2007;297:267–277. doi: 10.1001/jama.297.3.267. [DOI] [PubMed] [Google Scholar]
- 5.Neuhaus P, Riess H, Post S, et al. Deutsche Krebsgesellschaft (CAO/AIO). CONKO-001: Final results of the randomized, prospective, multicenter phase III trial of adjuvant chemotherapy with gemcitabine versus observation in patients with resected pancreatic cancer (PC) J Clin Oncol. 2008;26 abstr LBA4504. [Google Scholar]
- 6.Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA. 2008;299:1019–1026. doi: 10.1001/jama.299.9.1019. [DOI] [PubMed] [Google Scholar]
- 7.Neoptolemos JP, Stocken DD, Bassi C, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010;304:1073–1081. doi: 10.1001/jama.2010.1275. [DOI] [PubMed] [Google Scholar]
- 8.Ohigashi H, Ishikawa O, Eguchi H, et al. Feasibility and efficacy of combination therapy with preoperative full-dose gemcitabine, concurrent three-dimensional conformal radiation, surgery, and postoperative liver perfusion chemotherapy for T3-pancreatic cancer. Ann Surg. 2009;250:88–95. doi: 10.1097/SLA.0b013e3181ad65cc. [DOI] [PubMed] [Google Scholar]
- 9.Palmer DH, Stocken DD, Hewitt H, et al. A randomized phase 2 trial of neoadjuvant chemotherapy in resectable pancreatic cancer: Gemcitabine alone versus gemcitabine combined with cisplatin. Ann Surg Oncol. 2007;14:2088–2096. doi: 10.1245/s10434-007-9384-x. [DOI] [PubMed] [Google Scholar]
- 10.Heinrich S, Schafer M, Weber A, et al. Neoadjuvant chemotherapy generates a significant tumor response in resectable pancreatic cancer without increasing morbidity: results of a prospective phase II trial. Ann Surg. 2008;248:1014–1022. doi: 10.1097/SLA.0b013e318190a6da. [DOI] [PubMed] [Google Scholar]
- 11.Ishikawa O, Ohigashi H, Imaoka S, et al. Is the long-term survival rate improved by preoperative irradiation prior to Whipple's procedure for adenocarcinoma of the pancreatic head? Arch Surg. 1994;129:1075–1080. doi: 10.1001/archsurg.1994.01420340089017. [DOI] [PubMed] [Google Scholar]
- 12.Stessin AM, Meyer JE, Sherr DL. Neoadjuvant radiation is associated with improved survival in patients with resectable pancreatic cancer: an analysis of data from the surveillance, epidemiology, and end results (SEER) registry. Int J Radiat Oncol Biol Phys. 2008;72:1128–1133. doi: 10.1016/j.ijrobp.2008.02.065. [DOI] [PubMed] [Google Scholar]
- 13.Breslin TM, Hess KR, Harbison DB, et al. Neoadjuvant chemoradiotherapy for adenocarcinoma of the pancreas: treatment variables and survival duration. Ann Surg Oncol. 2001;8:123–132. doi: 10.1007/s10434-001-0123-4. [DOI] [PubMed] [Google Scholar]
- 14.Coia L, Hoffman J, Scher R, et al. Preoperative chemoradiation for adenocarcinoma of the pancreas and duodenum. Int J Radiat Oncol Biol Phys. 1994;30:161–167. doi: 10.1016/0360-3016(94)90531-2. [DOI] [PubMed] [Google Scholar]
- 15.Pilepich MV, Miller HH. Preoperative irradiation in carcinoma of the pancreas. Cancer. 1980;46:1945–1949. doi: 10.1002/1097-0142(19801101)46:9<1945::aid-cncr2820460908>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 16.Vauthey JN, Dixon E. AHPBA/SSO/SSAT Consensus Conference on Resectable and Borderline Resectable Pancreatic Cancer: rationale and overview of the conference. Ann Surg Oncol. 2009;16:1725–1726. doi: 10.1245/s10434-009-0409-5. [DOI] [PubMed] [Google Scholar]
- 17.Varadhachary GR, Tamm EP, Abbruzzese JL, et al. Borderline resectable pancreatic cancer: definitions, management, and role of preoperative therapy. Ann Surg Oncol. 2006;13:1035–1046. doi: 10.1245/ASO.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 18.NCCN. Fort Washington, PA: National Comprehensive Cancer Network; 2008. Pancreatic adenocarcinoma. National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology. [Google Scholar]
- 19.Patel M, Hoffe S, Malafa M, et al. Neoadjuvant GTX Chemotherapy and IMRT-based chemoradiation for borderline resectable pancreatic cancer. J Surg Oncol. 2011;104:155–161. doi: 10.1002/jso.21954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Katz MH, Pisters PW, Evans DB, et al. Borderline resectable pancreatic cancer: the importance of this emerging stage of disease. J Am Coll Surg. 2008;206:833, 846. doi: 10.1016/j.jamcollsurg.2007.12.020. discussion 846–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Estrella JS, Rashid A, Fleming JB, et al. Post-therapy pathologic stage and survival in patients with pancreatic ductal adenocarcinoma treated with neoadjuvant chemoradiation. Cancer. 2011 doi: 10.1002/cncr.26243. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Gillen S, Schuster T, Meyer Zum Buschenfelde C, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 2010;7:e1000267. doi: 10.1371/journal.pmed.1000267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laurence JM, Tran PD, Morarji K, et al. A systematic review and meta-analysis of survival and surgical outcomes following neoadjuvant chemoradiotherapy for pancreatic cancer. J Gastrointest Surg. 2011;15:2059–2069. doi: 10.1007/s11605-011-1659-7. [DOI] [PubMed] [Google Scholar]
- 24.Brunner TB, Grabenbauer GG, Meyer T, et al. Primary resection versus neoadjuvant chemoradiation followed by resection for locally resectable or potentially resectable pancreatic carcinoma without distant metastasis. A multi-centre prospectively randomised phase II-study of the Interdisciplinary Working Group Gastrointestinal Tumours (AIO, ARO, and CAO) BMC Cancer. 2007;7:41. doi: 10.1186/1471-2407-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heinrich S, Pestalozzi B, Lesurtel M, et al. Adjuvant gemcitabine versus NEOadjuvant gemcitabine/oxaliplatin plus adjuvant gemcitabine in resectable pancreatic cancer: A randomized multicenter phase III study (NEOPAC study) BMC Cancer. 2011;11:346. doi: 10.1186/1471-2407-11-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Varadhachary GR, Wolff RA, Crane CH, et al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3487–3495. doi: 10.1200/JCO.2007.15.8642. [DOI] [PubMed] [Google Scholar]
- 27.Evans DB, Varadhachary GR, Crane CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3496–3502. doi: 10.1200/JCO.2007.15.8634. [DOI] [PubMed] [Google Scholar]
- 28.Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 29.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 30.Van Cutsem E, Vervenne WL, Bennouna J, et al. Phase III trial of bevacizumab in combination with gemcitabine and erlotinib in patients with metastatic pancreatic cancer. J Clin Oncol. 2009;27:2231–2237. doi: 10.1200/JCO.2008.20.0238. [DOI] [PubMed] [Google Scholar]
- 31.Kindler HL, Ioka T, Richel DJ, et al. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: A double-blind randomised phase 3 study. Lancet Oncol. 2011;12:256–262. doi: 10.1016/S1470-2045(11)70004-3. [DOI] [PubMed] [Google Scholar]
- 32.Kindler HL, Friberg G, Singh DA, et al. Phase II trial of bevacizumab plus gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol. 2005;23:8033–8040. doi: 10.1200/JCO.2005.01.9661. [DOI] [PubMed] [Google Scholar]
- 33.Philip PA, Benedetti J, Corless CL, et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol. 2010;28:3605–3610. doi: 10.1200/JCO.2009.25.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ko AH, Youssoufian H, Gurtler J, et al. A phase II randomized study of cetuximab and bevacizumab alone or in combination with gemcitabine as first-line therapy for metastatic pancreatic adenocarcinoma. Invest New Drugs. 2011 doi: 10.1007/s10637-011-9691-8. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 35.Stathis A, Moore MJ. Advanced pancreatic carcinoma: current treatment and future challenges. Nat Rev Clin Oncol. 2010;7:163–172. doi: 10.1038/nrclinonc.2009.236. [DOI] [PubMed] [Google Scholar]
- 36.Olive KP, Jacobetz MA, Davidson CJ, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beatty GL, Chiorean EG, Fishman MP, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hartwig W, Hackert T, Hinz U, et al. Pancreatic cancer surgery in the new millennium: better prediction of outcome. Ann Surg. 2011;254:311–319. doi: 10.1097/SLA.0b013e31821fd334. [DOI] [PubMed] [Google Scholar]
- 39.Evans DB, Rich TA, Byrd DR, et al. Preoperative chemoradiation and pancreaticoduodenectomy for adenocarcinoma of the pancreas. Arch Surg. 1992;127:1335–1339. doi: 10.1001/archsurg.1992.01420110083017. [DOI] [PubMed] [Google Scholar]
- 40.Pisters PW, Abbruzzese JL, Janjan NA, et al. Rapid-fractionation preoperative chemoradiation, pancreaticoduodenectomy, and intraoperative radiation therapy for resectable pancreatic adenocarcinoma. J Clin Oncol. 1998;16:3843–3850. doi: 10.1200/JCO.1998.16.12.3843. [DOI] [PubMed] [Google Scholar]
- 41.Pisters PW, Wolff RA, Janjan NA, et al. Preoperative paclitaxel and concurrent rapid-fractionation radiation for resectable pancreatic adenocarcinoma: toxicities, histologic response rates, and event-free outcome. J Clin Oncol. 2002;20:2537–2544. doi: 10.1200/JCO.2002.11.064. [DOI] [PubMed] [Google Scholar]
- 42.Hoffman JP, Weese JL, Solin LJ, et al. A pilot study of preoperative chemoradiation for patients with localized adenocarcinoma of the pancreas. Am J Surg. 1995;169:71–77. doi: 10.1016/s0002-9610(99)80112-3. discussion 77–78. [DOI] [PubMed] [Google Scholar]
- 43.Hoffman JP, Lipsitz S, Pisansky T, et al. Phase II trial of preoperative radiation therapy and chemotherapy for patients with localized, resectable adenocarcinoma of the pancreas: An Eastern Cooperative Oncology Group Study. J Clin Oncol. 1998;16:317–323. doi: 10.1200/JCO.1998.16.1.317. [DOI] [PubMed] [Google Scholar]
- 44.Magnin V, Moutardier V, Giovannini MH, et al. Neoadjuvant preoperative chemoradiation in patients with pancreatic cancer. Int J Radiat Oncol Biol Phys. 2003;55:1300–1304. doi: 10.1016/s0360-3016(02)04157-3. [DOI] [PubMed] [Google Scholar]
- 45.Ohigashi H, Ishikawa O, Eguchi H, et al. Feasibility and efficacy of combination therapy with preoperative and postoperative chemoradiation, extended pancreatectomy, and postoperative liver perfusion chemotherapy for locally advanced cancers of the pancreatic head. Ann Surg Oncol. 2005;12:629–636. doi: 10.1245/ASO.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 46.Mornex F, Girard N, Scoazec JY, et al. Feasibility of preoperative combined radiation therapy and chemotherapy with 5-fluorouracil and cisplatin in potentially resectable pancreatic adenocarci-noma: The French SFRO-FFCD 97-04 Phase II trial. Int J Radiat Oncol Biol Phys. 2006;65:1471–1478. doi: 10.1016/j.ijrobp.2006.02.054. [DOI] [PubMed] [Google Scholar]
- 47.Talamonti MS, Small W, Jr., Mulcahy MF, et al. A multi-institutional phase II trial of preoperative full-dose gemcitabine and concurrent radiation for patients with potentially resectable pancreatic carcinoma. Ann Surg Oncol. 2006;13:150–158. doi: 10.1245/ASO.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 48.Mehta VK, Poen JC, Ford JM, et al. Protracted venous infusion 5-fluorouracil with concomitant radiotherapy compared with bolus 5-fluorouracil for unresectable pancreatic cancer. Am J Clin Oncol. 2001;24:155–159. doi: 10.1097/00000421-200104000-00012. [DOI] [PubMed] [Google Scholar]
- 49.Massucco P, Capussotti L, Magnino A, et al. Pancreatic resections after chemoradiotherapy for locally advanced ductal adenocarcinoma: analysis of perioperative outcome and survival. Ann Surg Oncol. 2006;13:1201–1208. doi: 10.1245/s10434-006-9032-x. [DOI] [PubMed] [Google Scholar]
- 50.Small W, Jr., Berlin J, Freedman GM, et al. Full-dose gemcitabine with concurrent radiation therapy in patients with nonmetastatic pancreatic cancer: A multicenter phase II trial. J Clin Oncol. 2008;26:942–947. doi: 10.1200/JCO.2007.13.9014. [DOI] [PubMed] [Google Scholar]
- 51.Stokes JB, Nolan NJ, Stelow EB, et al. Preoperative capecitabine and concurrent radiation for borderline resectable pancreatic cancer. Ann Surg Oncol. 2011;18:619–627. doi: 10.1245/s10434-010-1456-7. [DOI] [PubMed] [Google Scholar]
- 52.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.DeOliveira ML, Winter JM, Schafer M, et al. Assessment of complications after pancreatic surgery: a novel grading system applied to 633 patients undergoing pancreaticoduodenectomy. Ann Surg. 2006;244:931–937. doi: 10.1097/01.sla.0000246856.03918.9a. discussion 937–939. [DOI] [PMC free article] [PubMed] [Google Scholar]