Inflammatory and angiogenic biomarkers were measured in androgen deprivation therapy–treated and control groups of men with prostate cancer. Significantly higher concentrations of some inflammatory biomarkers were found in the treatment group.
Abstract
Introduction.
Angiogenesis and inflammation are both important to the pathogenesis of malignancies. Androgen deprivation therapy (ADT) for prostate cancer causes drastic hormonal changes that alter both disease and host factors. We measured inflammatory and angiogenic biomarkers in ADT-treated and control groups of men with prostate cancer.
Materials and Methods.
Baseline and 12-week plasma samples were collected from 37 ADT-naïve men with locally advanced or recurrent prostate cancer. Of those, 23 initiated ADT with a gonadotropin-releasing hormone (GnRH) agonist and 14 served as nontreatment controls. Samples were tested for a panel of angiogenic and inflammatory biomarkers.
Results.
The treatment group had significantly higher concentrations of the inflammatory biomarkers interleukin (IL)-1β, IL-6, IL-8, tumor necrosis factor (TNF)-α, and stromal cell–derived factor (SDF)-1α. None of the angiogenic biomarkers were significantly different between the groups at baseline. Among patients with a short prostate-specific antigen (PSA) doubling time (<6 months), the proangiogenic factor basic fibroblast growth factor (bFGF) was lower at baseline. In the treatment group, plasma placental growth factor (PlGF) increased and IL-6 decreased after 12 weeks of ADT. Moreover, the treatment group continued to have significantly higher concentrations of the inflammatory biomarkers IL-1β, IL-8, and SDF-1α as well as bFGF than controls.
Discussion.
These men were characterized by elevations in several traditional markers of aggressive disease and also by higher levels of several inflammatory biomarkers. Although ADT decreased IL-6 levels, IL-1β, IL-8, and SDF-1α remained significantly higher than in controls. The role of these biomarkers should be further explored.
Introduction
The natural history of prostate cancer varies greatly. Some prostate cancers are indolent and patients are not likely to benefit from treatment [1]. Others metastasize and progress despite available systemic therapies, making prostate cancer the second leading cause of cancer death among men [2]. Better biomarkers of disease biology and pathogenesis are clearly needed because they would have the potential to improve risk stratification among men with localized disease and to reveal potential therapeutic targets.
Angiogenesis is important to the pathogenesis of a variety of cancers. Vascular endothelial growth factor (VEGF) is a proangiogenic factor that has been validated as a therapeutic target and is known to be present in prostate cancer tissue but not normal prostate tissue or benign prostatic hypertrophy (BPH) [3–5]. Bevacizumab is a monoclonal antibody that inhibits VEGF and has produced benefits for patients with several types of solid tumor [6]. The addition of bevacizumab to conventional chemotherapy for metastatic prostate cancer was initially promising [7] but did not produce a significant benefit in phase III study [8]. Additional insights into the role of antiangiogenesis in prostate cancer therapy are needed.
Inflammation has long been hypothesized to play an important role in the pathogenesis of malignancy [9]. Despite this long-standing knowledge of an association between inflammation and cancer, the mechanisms responsible for this association are not yet well understood. Immunotherapy using sipuleucel-T for advanced prostate cancer has produced clinical benefit [10]. However, a complete mechanistic understanding of the interplay among inflammation, immune function, and prostate cancer is still lacking.
Finally, androgen deprivation therapy (ADT) is the cornerstone systemic therapy for prostate cancer patients. ADT-associated changes in the hormonal environment strongly affect both host and tumor. Previously, we showed that ADT in mice bearing androgen-dependent tumors lowers the level of VEGF in these tumors and “normalizes” their vessels [11]. Little is known about changes in inflammatory cytokines and angiogenic factors during ADT for prostate cancer. Our objective was to better define these changes. Here, we conducted an exploratory analysis of a number of inflammatory and angiogenic biomarkers among men with locally advanced or recurrent prostate cancer.
Materials and Methods
Participants
Study participants were recruited with institutional review board approval at the Massachusetts General Hospital (MGH) in March 2003 to May 2005. Treatment group patients had locally advanced or recurrent prostate cancer. Men with bone metastases on radionuclide bone scan were excluded. Men with a Karnofsky performance status score <90, a history of diabetes mellitus or glucose intolerance, treatment with medications known to alter glucose or insulin levels, or a serum creatinine concentration >2.0 mg/dL were also excluded. Control group participants had prostate cancer and were recruited from the same hospital in the same time frame but were not planned for gonadotropin-releasing hormone (GnRH) agonist therapy. No participant received radiation therapy (RT) during study participation.
Study Design
Treatment group patients were evaluated at the General Clinical Research Center at MGH at baseline and after 12 weeks of treatment. Blood samples were collected on the morning of each visit. Plasma samples were stored at −70°C for subsequent batch measurements. Control group patients were tested in the same way but did not receive prostate cancer treatment during the 12-week interval.
After the baseline visit, treatment group patients received leuprolide 3-month depot (LupronDepot®; TAP Pharmaceuticals Inc., Deerfield, IL; 22.5 mg i.m.). Patients also received bicalutamide (Casodex®; AstraZeneca PLC, London, U.K.; 50 mg by mouth daily) for 4 weeks to prevent the clinical effects of the androgen flare associated with the initiation of a GnRH agonist. The Institutional Review Board of Dana Farber/Harvard Cancer Center reviewed and approved the study and all participants gave written informed consent.
Circulating Biomarker Evaluations
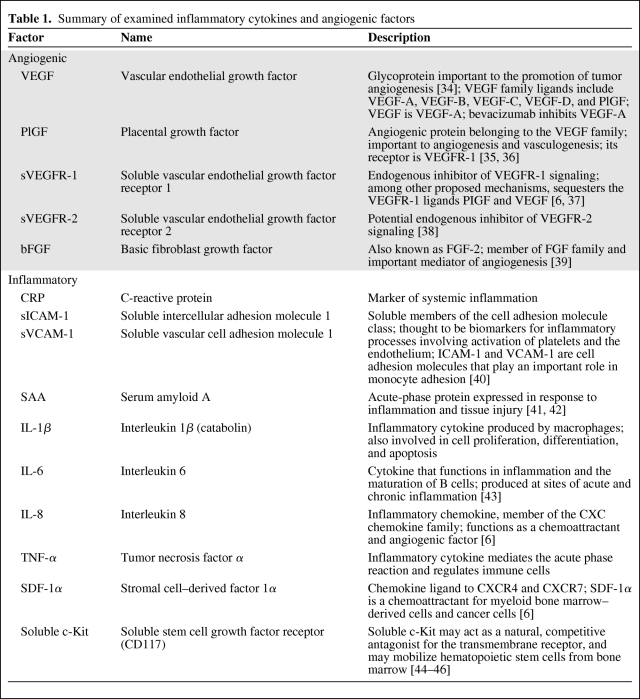
Circulating angiogenic and inflammatory biomarkers were measured in plasma (Table 1). Analysis was carried out for circulating VEGF, placental growth factor (PlGF), soluble VEGF receptor 1 (sVEGFR-1), sVEGFR-2, basic fibroblast growth factor (bFGF), interleukin (IL)-1β, IL-6, IL-8, tumor necrosis factor (TNF)-α, C-reactive protein (CRP), soluble intercellular adhesion molecule 1, soluble vascular cell adhesion molecule 1, and serum amyloid A using multiplex enzyme-linked immunosorbent assay (ELISA) plates from Meso-Scale Discovery (Gaithersburg, MD). Soluble c-Kit and stromal cell–derived factor (SDF)-1α were similarly analyzed using ELISA plates from R&D Systems (Minneapolis, MN). Every sample was run in duplicate.
Table 1.
Summary of examined inflammatory cytokines and angiogenic factors
Statistical Analysis
The objective was to perform an exploratory analysis of the changes in a panel of inflammatory and angiogenic biomarkers in men with prostate cancer managed with or without ADT. Data are reported as medians and interquartile ranges (IQRs) for continuous variables and as percentages for discrete variables. p-values for comparison between groups were determined using the Wilcoxon exact test.
Results
At Baseline, Treatment-Group Patients Had Higher Prostate-Specific Antigen Levels, a Higher Rate of Prostate-Specific Antigen Doubling Time <6 Months, and Higher Levels of IL-1β, IL-6, IL-8, TNF-α, and SDF-1α
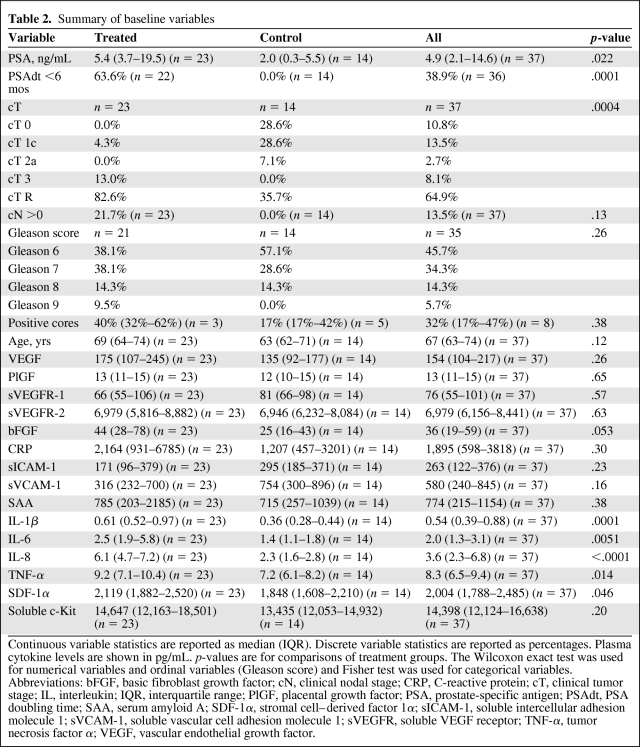
We analyzed plasma samples from all 37 patients enrolled (23 ADT-treated and 14 control participants). The median age for the cohort was 67 years. We measured the plasma concentration of prostate-specific antigen (PSA) and 15 angiogenic and inflammatory biomarkers (Table 2) in patients from the ADT and control groups at baseline. The treatment group featured a higher median PSA level—5.4 ng/mL (IQR, 3.7–19.5 ng/mL) versus 2.0 ng/mL (IQR, 0.3–5.5 ng/mL) (p = .022)—and a higher proportion of patients with a PSA doubling time (PSAdt) <6 months (63.6% versus 0%; p = .0001). In addition, participants in the ADT group had significantly higher levels of the following circulating inflammatory biomarkers in plasma: IL-1β, IL-6, IL-8, TNF-α, and SDF-1α (Table 2). There was no significant difference in the levels of other inflammatory or angiogenic biomarkers between the groups at baseline.
Table 2.
Summary of baseline variables
Continuous variable statistics are reported as median (IQR). Discrete variable statistics are reported as percentages. Plasma cytokine levels are shown in pg/mL. p-values are for comparisons of treatment groups. The Wilcoxon exact test was used for numerical variables and ordinal variables (Gleason score) and Fisher test was used for categorical variables.
Abbreviations: bFGF, basic fibroblast growth factor; cN, clinical nodal stage; CRP, C-reactive protein; cT, clinical tumor stage; IL, interleukin; IQR, interquartile range; PlGF, placental growth factor; PSA, prostate-specific antigen; PSAdt, PSA doubling time; SAA, serum amyloid A; SDF-1α, stromal cell–derived factor 1α; sICAM-1, soluble intercellular adhesion molecule 1; sVCAM-1, soluble vascular cell adhesion molecule 1; sVEGFR, soluble VEGF receptor; TNF-α, tumor necrosis factor α; VEGF, vascular endothelial growth factor.
Treated Patients Had Higher PlGF Levels and Lower Levels of IL-6 in Plasma During ADT
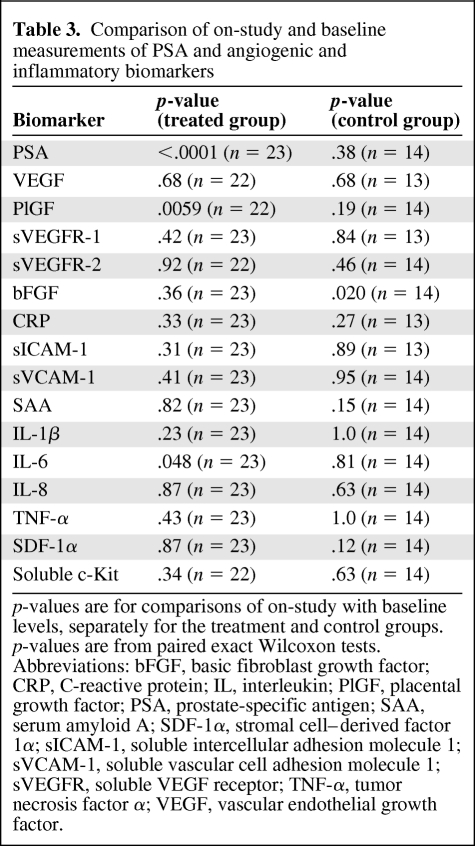
Next, we evaluated biomarker changes at 12 weeks on study. Levels of PSA were significantly lower at 12 weeks than at baseline in treated patients, consistent with the effect of ADT (p < .0001) (Table 3). Plasma PlGF significantly increased during ADT (p < .01), whereas plasma IL-6 decreased (p < .05). In the control group, the levels of bFGF decreased after 12 weeks on study (p < .05). None of the other biomarkers had changed at 12 weeks in the two groups.
Table 3.
Comparison of on-study and baseline measurements of PSA and angiogenic and inflammatory biomarkers
p-values are for comparisons of on-study with baseline levels, separately for the treatment and control groups. p-values are from paired exact Wilcoxon tests.
Abbreviations: bFGF, basic fibroblast growth factor; CRP, C-reactive protein; IL, interleukin; PlGF, placental growth factor; PSA, prostate-specific antigen; SAA, serum amyloid A; SDF-1α, stromal cell–derived factor 1α; sICAM-1, soluble intercellular adhesion molecule 1; sVCAM-1, soluble vascular cell adhesion molecule 1; sVEGFR, soluble VEGF receptor; TNF-α, tumor necrosis factor α; VEGF, vascular endothelial growth factor.
Higher Baseline bFGF Was Correlated with Longer PSAdt (>6 Months)
We next evaluated biomarker changes after stratifying patients from the ADT group based on treatment outcome. PSA at baseline did not significantly correlate with any of the examined markers of disease (i.e., percentage with PSAdt <6 months, node status, Gleason score) or with any of the measured angiogenic or inflammatory biomarkers. When treatment-group patients were dichotomized as baseline PSAdt >6 months versus baseline PSAdt<6 months, the baseline plasma bFGF level was significantly higher in patients with a longer PSAdt—83 pg/mL (IQR, 48–97 pg/mL) versus 39 pg/mL (IQR, 24–55 pg/mL) (p < .05).
After 12 Weeks of ADT, Prostate Cancer Patients Had Lower PSA Levels but Higher Circulating Levels of bFGF, IL-1β, IL-8, and SDF-1α
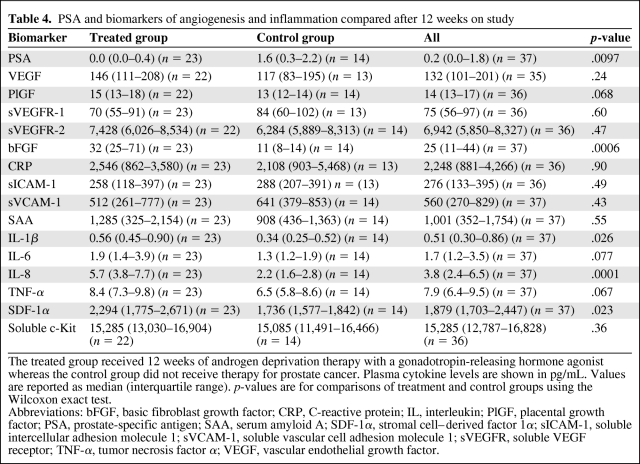
We compared the on-study levels of biomarkers between patients from the treatment group and those from the control group. On-study levels of PSA were lower in treated patients than in the control group, an expected effect of ADT. Plasma bFGF, SDF-1α, IL-1β, and IL-8 levels were all significantly higher in the treatment group (Table 4). There was no difference between the two groups in any of the other biomarkers at week 12, including VEGF.
Table 4.
PSA and biomarkers of angiogenesis and inflammation compared after 12 weeks on study
The treated group received 12 weeks of androgen deprivation therapy with a gonadotropin-releasing hormone agonist whereas the control group did not receive therapy for prostate cancer. Plasma cytokine levels are shown in pg/mL. Values are reported as median (interquartile range). p-values are for comparisons of treatment and control groups using the Wilcoxon exact test.
Abbreviations: bFGF, basic fibroblast growth factor; CRP, C-reactive protein; IL, interleukin; PlGF, placental growth factor; PSA, prostate-specific antigen; SAA, serum amyloid A; SDF-1α, stromal cell–derived factor 1α; sICAM-1, soluble intercellular adhesion molecule 1; sVCAM-1, soluble vascular cell adhesion molecule 1; sVEGFR, soluble VEGF receptor; TNF-α, tumor necrosis factor α; VEGF, vascular endothelial growth factor.
Differential Changes in Circulating VEGF and sVEGFR-1 When Patients Were Dichotomized by Baseline PSAdt
Finally, we dichotomized patients by baseline PSAdt and examined ADT-induced changes in inflammatory and angiogenic biomarkers. We found that VEGF and sVEGFR-1 rose among men with a long PSAdt (median percent of baseline: VEGF, 119%; sVEGFR-1, 111%) and fell among men with a short PSAdt (median percent of baseline: VEGF, 75%; sVEGFR-1, 88%) (p = .013 for VEGF, p = .006 for sVEGFR-1). Changes in other examined biomarkers did not differ between the two groups.
Discussion
Existing data on changes in inflammatory cytokine levels before and during treatment for prostate cancer are limited. Much of the literature is focused on patients undergoing RT. Lopes et al. [12] found that, when inflammatory cytokines (IL-2, IL-4, IL-5, IL-6, TNF-α, microphage inhibitory protein [MIP]-1α, and leukemia inhibitory factor) were measured in patients with localized disease planned for radiotherapy, only IL-2 was significantly elevated at baseline and during treatment. Kovacs et al. [13] found that pretreatment IL-1, macrophage colony-stimulating factor, and transforming growth factor (TGF)-β levels were elevated in patients with prostate cancer and rose during RT. Others found that RT was associated with significant increases in IL-6 [12, 14–16] and TNF-α [15]. Johnke et al. [16] tested IL-1β, IL-6, and TGF-β levels in men undergoing RT with and without ADT and found that IL-1β and IL-6 rose and TGF-β fell after initiation of RT. This pattern was observed with and without ADT, though the ADT group was observed to have greater magnitudes of all three treatment-induced changes.
Both androgens and the prostate cancer disease state have previously been found to influence cytokine levels. Maggio et al. [17] found, in a cross-sectional study, that testosterone levels were inversely associated with soluble IL-6 receptor (sIL-6r) levels but not with levels of the other inflammatory markers they examined (IL-6, TNF-α, IL-1β, and CRP). Malkin et al. [18] found that testosterone supplementation in hypogonadal men caused significant decreases in TNF-α and IL-1β as well as an increase in IL-10. Khosla et al. [19] found that initiation of GnRH agonists in healthy elderly men led to increased levels of TNF-α, IL-1β, and sIL-6r in the short term. In contrast, Maggio et al. [20] found that 12 months of ADT did not affect cytokine levels in men with prostate cancer. They compared men who had received at least 12 months of continuous ADT with age-matched controls with and without a history of prostate cancer and found no difference in serum levels of MIP-1β, TNF-α, IL-7, IL-8, IL-12, IL-13, and IL-10. Similarly, Smith et al. [21] found, in a prospective study, that 12 weeks of ADT did not cause a significant change in the CRP level. Wise et al. [22] compared men with castration-resistant prostate cancer (CRPC) with men with hormone-sensitive prostate cancer and with men with BPH. They found that IL-6, IL-4, and IL-10 were elevated in men with CRPC. George et al. [23] reported that the plasma IL-6 level at baseline was prognostic among men with metastatic CRPC treated in a cooperative group trial, Cancer and Leukemia Group B (CALGB) 9480. The survival time was 19 months (95% confidence interval [CI], 17–22 months) among those with a below-the-median IL-6 level and 11 months (95% CI, 8–14 months) for those with an above-the-median IL-6 level [23].
To gain additional insight into the systemic changes after hormonal therapy for prostate cancer patients, we measured circulating inflammatory and angiogenic biomarkers in plasma. We found that, at baseline, the ADT treatment group was characterized by conventional markers of more aggressive disease as well as elevated levels of several inflammatory cytokines (IL-1β, IL-6, IL-8, TNF-α, and SDF-1α). We also found that PlGF rose and IL-6 fell during ADT. We found that, after 12 weeks of ADT, the treatment group featured higher levels of the proangiogenic factor bFGF as well as the inflammatory cytokines IL-1β, IL-8, and SDF-1α. Finally, we found that patients who progressed faster (i.e., had a shorter PSAdt) had drops in the antiangiogenic molecule sVEGFR-1 and, surprisingly, also in the proangiogenic molecule VEGF. Collectively, these data indicate that elevation in inflammatory biomarkers may reflect characteristics of more aggressive prostate cancers and should be explored as prognostic biomarkers in larger experimental cohorts.
Among biomarkers of angiogenesis, VEGF has been most extensively examined in men with prostate cancer. Prostate tumors stain positively for VEGF, but normal prostate tissue does not [3–5]. The intensity of VEGF staining appears to correlate with Gleason score and to be lower among men who receive ADT prior to surgery [4]. Pallares et al. [24] evaluated prostatectomy specimens for microvessel density, VEGF, bFGF, VEGFR-1, and VEGFR-2 expression by immunohistochemistry. They found statistically significant higher microvessel density and expression levels of VEGF, bFGF, and the receptors Flk-1/KDR and Flt-1 within high-grade prostatic intraepithelial neoplasia and prostate cancer than within normal tissue. Matsumoto et al. [25] found that gene expression levels of PlGF-1 and PlGF-2 were significantly lower in untreated prostate cancer than in BPH or in treated prostate cancer specimens. The accessibility of circulating angiogenic biomarkers makes them more attractive than tissue. Duque et al. [26, 27] found that plasma VEGF levels were significantly higher in men with metastatic disease than in men with localized prostate cancer and in healthy controls. In men with CRPC treated on CALGB protocol 9480, pretreatment urine [28] and plasma [29] VEGF levels were each significantly negatively correlated with survival time.
In our study, in contrast to inflammatory biomarkers, on-treatment angiogenic biomarkers were not different in the treatment and control groups. This is in apparent contrast with preclinical findings, wherein ADT led to a decrease in intratumoral VEGF [11]. Besides the limited sample size, there are several possible explanations for this finding. First, angiogenesis and circulating biomarkers may not differ significantly prior to therapy in indolent versus aggressive prostate cancers. Alternatively, angiogenesis in prostate cancer might be mediated by alternative pathways that were not evaluated in this study. Finally, changes in intratumoral VEGF may not be reflected by changes in circulating VEGF.
The effects of ADT on prostate cancer perfusion were previously examined using an ultrasound-guided transrectal polarographic Eppendorf needle electrode. Measurements before and during treatment with the antiandrogen bicalutamide revealed baseline hypoxia as well as a therapy-induced reduction in tumor hypoxia, indicative of normalization of tumor vasculature [11, 30]. In another study, multiparameter magnetic resonance imaging was used to measure tumor blood flow before and during combined androgen blockade with a GnRH agonist and bicalutamide. The study showed that tumor blood flow fell 79% (p < .0001) during the first month of therapy, suggesting that treatment had potent antivascular effects at that time point [31]. Although neither of those studies measured concurrent levels of intratumoral or circulating VEGF, these data suggest that circulating levels of VEGF might have dropped with ADT-induced reductions in tumor hypoxia and then in blood flow. We observed stable circulating VEGF levels after 12 weeks of ADT. This is in apparent contrast to the clinical observation of ADT-induced declines in tumor blood flow and hypoxia. Potential explanations include a limited sample size, the absence of a true change in VEGF at that time point, and the presence of a change in VEGF that is not reflected in circulating VEGF levels.
In the treatment group, baseline PSA did not correlate with any of the examined markers of disease or with any of the angiogenic or inflammatory markers. It is possible that the sample size was too small to see correlations of modest magnitude. Alternatively, PSA may not significantly correlate with inflammation or angiogenesis. Cohort baseline PSAdt <6 months was only correlated with lower baseline levels of the proangiogenic factor bFGF. Although the reason underlying this correlation is unclear, this finding indicates that bFGF may not be critical for tumor angiogenesis in this setting.
Comparison of baseline and 12-week cytokine levels among those receiving ADT revealed that PlGF rose and IL-6 fell. PlGF is well described to be a proangiogenic cytokine, and its upregulation during ADT may have biologic implications. Several groups have found that circulating IL-6 levels rise in men treated with RT for localized prostate cancer [12, 14–16]. The observation that ADT lowers IL-6 levels stands in intriguing contrast to the reported upregulation of IL-6 with RT. The clinical observation that concurrent ADT improves outcomes in higher risk prostate cancer patients treated with definitive RT suggests that this finding warrants further exploration as potential mechanistic explanation for the clinical efficacy of combined therapy.
Finally, we found that VEGF and sVEGFR-1 rose among men with a long PSAdt and fell among men with a short PSAdt. This finding suggests a differential impact of treatment on angiogenic biomarkers based on more versus less aggressive disease. Retrospective data have suggested that the PSAdt prior to treatment is clinically meaningful. Shulman et al. [32] found that, among men treated with an antiandrogen for CRPC, the median PSAdt at the time of antiandrogen initiation was longer among those who responded (12.7 months for responders versus 7.5 months for nonresponders; p = .037). Keizman et al. [33] found that a short PSAdt before treatment with intermittent ADT was associated with disease progression. Given that a short PSAdt seems to reflect biologically aggressive disease, differential ADT-induced changes in VEGF and sVEGFR-1 among men with a short PSAdt merit further investigation.
The present study features several limitations. First, the conventional disease characteristics of the treatment and control groups differed significantly at baseline, a reflection of the fact that participants had not been randomized to treatment and control groups. This limits the conclusions that can be drawn regarding comparisons between the groups during the on-study interval. Second, the sample sizes are relatively small, thereby limiting the statistical power to detect differences and increasing the risk for chance observations. Despite these limitations, our observations are hypothesis generating and merit validation within larger cohorts. Further study should be done to better define important biomarkers of inflammation and their clinical implications among prostate cancer patients. In particular, the roles of the angiogenic factor PlGF and the proinflammatory cytokine IL-6 in men receiving ADT warrant further clarification.
Conclusion
The objective of our study was to preliminarily characterize profiles of several inflammatory and angiogenic biomarkers among men with locally advanced or recurrent prostate cancer. The ADT treatment group featured conventional markers of more aggressive disease and higher baseline levels of several inflammatory markers. Treatment with ADT was associated with a rise in PlGF and a decline in IL-6. Further work is needed to better define the dynamics of these markers and their clinical implications in this population.
Acknowledgments
We would like to acknowledge Dr. W. Shipley (Radiation Oncology, MGH) for useful discussions and K. Kinzel (Steele Laboratory, MGH) for technical support.
This work was supported by a Bertucci Prostate Cancer Research Grant, and in part by National Cancer Institute grants R01-CA115767, P01-CA080124, R01-CA085140, R01-CA126642, R01-CA159258, and K24-CA121990, and Federal Share Proton Beam Program Income Grants as well as by grant 120733-RSG-11-073-01-TBG from the American Cancer Society. P.J. Saylor is supported by Young Investigator Awards from the Prostate Cancer Foundation and ASCO Cancer Foundation. M.R. Smith is supported by awards from the Prostate Cancer Foundation.
Philip J. Saylor and Kevin R. Kozak contributed equally to this work.
Footnotes
- (C/A)
- Consulting/advisory relationship
- (RF)
- Research funding
- (E)
- Employment
- (H)
- Honoraria received
- (OI)
- Ownership interests
- (IP)
- Intellectual property rights/inventor or patent holder
- (SAB)
- Scientific advisory board
Author Contributions
Conception/Design: Rakesh K. Jain, Dan G. Duda, Kevin R. Kozak, Matthew R. Smith, Marek A. Ancukiewicz, Anthony L. Zietman
Provision of study material or patients: Matthew R. Smith, Anthony L. Zietman
Collection and/or assembly of data: Rakesh K. Jain, Dan G. Duda, Kevin R. Kozak, Matthew R. Smith, Marek A. Ancukiewicz
Data analysis and interpretation: Rakesh K. Jain, Philip J. Saylor, Dan G. Duda, Kevin R. Kozak, Matthew R. Smith, Marek A. Ancukiewicz, Jason A. Efstathiou, Anthony L. Zietman
Manuscript writing: Rakesh K. Jain, Philip J. Saylor, Dan G. Duda, Kevin R. Kozak, Matthew R. Smith, Marek A. Ancukiewicz, Jason A. Efstathiou, Anthony L. Zietman
Final approval of manuscript: Rakesh K. Jain, Philip J. Saylor, Dan G. Duda, Kevin R. Kozak, Matthew R. Smith, Marek A. Ancukiewicz, Jason A. Efstathiou, Anthony L. Zietman
References
- 1.Zietman A. Evidence-based medicine, conscience-based medicine, and the management of low-risk prostate cancer. J Clin Oncol. 2009;27:4935–4936. doi: 10.1200/JCO.2009.24.4533. [DOI] [PubMed] [Google Scholar]
- 2.Surveillance, Epidemiology, and End Results (SEER) SEER Stat Fact Sheets: Prostate Cancer. [accessed January 10, 2012]. Available at http://seer.cancer.gov/statfacts/html/prost.html.
- 3.Ferrer FA, Miller LJ, Andrawis RI, et al. Vascular endothelial growth factor (VEGF) expression in human prostate cancer: In situ and in vitro expression of VEGF by human prostate cancer cells. J Urol. 1997;157:2329–2333. [PubMed] [Google Scholar]
- 4.Mazzucchelli R, Montironi R, Santinelli A, et al. Vascular endothelial growth factor expression and capillary architecture in high-grade PIN and prostate cancer in untreated and androgen-ablated patients. Prostate. 2000;45:72–79. doi: 10.1002/1097-0045(20000915)45:1<72::aid-pros9>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 5.Ferrer FA, Miller LJ, Andrawis RI, et al. Angiogenesis and prostate cancer: In vivo and in vitro expression of angiogenesis factors by prostate cancer cells. Urology. 1998;51:161–167. doi: 10.1016/s0090-4295(97)00491-3. [DOI] [PubMed] [Google Scholar]
- 6.Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Picus J, Halabi S, Kelly WK, et al. A phase 2 study of estramustine, docetaxel, and bevacizumab in men with castrate-resistant prostate cancer: Results from Cancer and Leukemia Group B Study 90006. Cancer. 2011;117:526–533. doi: 10.1002/cncr.25421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly WK, Halabi S, Carducci MA, et al. A randomized, double-blind, placebo-controlled phase III trial comparing docetaxel, prednisone, and placebo with docetaxel, prednisone, and bevacizumab in men with metastatic castration-resistant prostate cancer (mCRPC): Survival results of CALGB 90401. J Clin Oncol. 2010;28(18 suppl) doi: 10.1200/JCO.2011.39.4767. Abstract LBA4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 11.Jain RK, Safabakhsh N, Sckell A, et al. Endothelial cell death, angiogenesis, and microvascular function after castration in an androgen-dependent tumor: Role of vascular endothelial growth factor. Proc Natl Acad Sci U S A. 1998;95:10820–10825. doi: 10.1073/pnas.95.18.10820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopes CO, Callera F. Three-dimensional conformal radiotherapy in prostate cancer patients: Rise in interleukin 6 (IL-6) but not IL-2, IL-4, IL-5, tumor necrosis factor-α, MIP-1-α, and LIF levels. Int J Radiat Oncol Biol Phys. 2011 Jun 1; doi: 10.1016/j.ijrobp.2011.04.040. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13.Kovacs CJ, Daly BM, Evans MJ, et al. Cytokine profiles in patients receiving wide-field + prostate boost radiotherapy (xRT) for adenocarcinoma of the prostate. Cytokine. 2003;23:151–163. doi: 10.1016/s1043-4666(03)00185-6. [DOI] [PubMed] [Google Scholar]
- 14.Christensen E, Pintilie M, Evans KR, et al. Longitudinal cytokine expression during IMRT for prostate cancer and acute treatment toxicity. Clin Cancer Res. 2009;15:5576–5583. doi: 10.1158/1078-0432.CCR-09-0245. [DOI] [PubMed] [Google Scholar]
- 15.Hurwitz MD, Kaur P, Nagaraja GM, et al. Radiation therapy induces circulating serum Hsp72 in patients with prostate cancer. Radiother Oncol. 2010;95:350–358. doi: 10.1016/j.radonc.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnke RM, Edwards JM, Evans MJ, et al. Circulating cytokine levels in prostate cancer patients undergoing radiation therapy: Influence of neoadjuvant total androgen suppression. In Vivo. 2009;23:827–833. [PubMed] [Google Scholar]
- 17.Maggio M, Basaria S, Ble A, et al. Correlation between testosterone and the inflammatory marker soluble interleukin-6 receptor in older men. J Clin Endocrinol Metab. 2006;91:345–347. doi: 10.1210/jc.2005-1097. [DOI] [PubMed] [Google Scholar]
- 18.Malkin CJ, Pugh PJ, Jones RD, et al. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004;89:3313–3318. doi: 10.1210/jc.2003-031069. [DOI] [PubMed] [Google Scholar]
- 19.Khosla S, Atkinson EJ, Dunstan CR, et al. Effect of estrogen versus testosterone on circulating osteoprotegerin and other cytokine levels in normal elderly men. J Clin Endocrinol Metab. 2002;87:1550–1554. doi: 10.1210/jcem.87.4.8397. [DOI] [PubMed] [Google Scholar]
- 20.Maggio M, Blackford A, Taub D, et al. Circulating inflammatory cytokine expression in men with prostate cancer undergoing androgen deprivation therapy. J Androl. 2006;27:725–728. doi: 10.2164/jandrol.106.000141. [DOI] [PubMed] [Google Scholar]
- 21.Smith MR, Lee H, Fallon MA, et al. Adipocytokines, obesity, and insulin resistance during combined androgen blockade for prostate cancer. Urology. 2008;71:318–322. doi: 10.1016/j.urology.2007.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wise GJ, Marella VK, Talluri G, et al. Cytokine variations in patients with hormone treated prostate cancer. J Urol. 2000;164:722–725. doi: 10.1097/00005392-200009010-00024. [DOI] [PubMed] [Google Scholar]
- 23.George DJ, Halabi S, Shepard TF, et al. The prognostic significance of plasma interleukin-6 levels in patients with metastatic hormone-refractory prostate cancer: Results from Cancer and Leukemia Group B 9480. Clin Cancer Res. 2005;11:1815–1820. doi: 10.1158/1078-0432.CCR-04-1560. [DOI] [PubMed] [Google Scholar]
- 24.Pallares J, Rojo F, Iriarte J, et al. Study of microvessel density and the expression of the angiogenic factors VEGF, bFGF and the receptors Flt-1 and FLK-1 in benign, premalignant and malignant prostate tissues. Histol Histopathol. 2006;21:857–865. doi: 10.14670/HH-21.857. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto K, Suzuki K, Koike H, et al. Placental growth factor gene expression in human prostate cancer and benign prostate hyperplasia. Anticancer Res. 2003;23:3767–3773. [PubMed] [Google Scholar]
- 26.Duque JL, Loughlin KR, Adam RM, et al. Measurement of plasma levels of vascular endothelial growth factor in prostate cancer patients: Relationship with clinical stage, Gleason score, prostate volume, and serum prostate-specific antigen. Clinics (Sao Paulo) 2006;61:401–408. doi: 10.1590/s1807-59322006000500006. [DOI] [PubMed] [Google Scholar]
- 27.Duque JL, Loughlin KR, Adam RM, et al. Plasma levels of vascular endothelial growth factor are increased in patients with metastatic prostate cancer. Urology. 1999;54:523–527. doi: 10.1016/s0090-4295(99)00167-3. [DOI] [PubMed] [Google Scholar]
- 28.Bok RA, Halabi S, Fei DT, et al. Vascular endothelial growth factor and basic fibroblast growth factor urine levels as predictors of outcome in hormone-refractory prostate cancer patients: A Cancer and Leukemia Group B study. Cancer Res. 2001;61:2533–2536. [PubMed] [Google Scholar]
- 29.George DJ, Halabi S, Shepard TF, et al. Prognostic significance of plasma vascular endothelial growth factor levels in patients with hormone-refractory prostate cancer treated on Cancer and Leukemia Group B 9480. Clin Cancer Res. 2001;7:1932–1936. [PubMed] [Google Scholar]
- 30.Milosevic M, Chung P, Parker C, et al. Androgen withdrawal in patients reduces prostate cancer hypoxia: Implications for disease progression and radiation response. Cancer Res. 2007;67:6022–6025. doi: 10.1158/0008-5472.CAN-07-0561. [DOI] [PubMed] [Google Scholar]
- 31.Alonzi R, Padhani AR, Taylor NJ, et al. Antivascular effects of neoadjuvant androgen deprivation for prostate cancer: An in vivo human study using susceptibility and relaxivity dynamic MRI. Int J Radiat Oncol Biol Phys. 2011;80:721–727. doi: 10.1016/j.ijrobp.2010.02.060. [DOI] [PubMed] [Google Scholar]
- 32.Shulman MJ, Karam JA, Benaim EA. Prostate-specific antigen doubling time predicts response to deferred antiandrogen therapy in men with androgen-independent prostate cancer. Urology. 2004;63:732–736. doi: 10.1016/j.urology.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 33.Keizman D, Huang P, Antonarakis ES, et al. The change of PSA doubling time and its association with disease progression in patients with biochemically relapsed prostate cancer treated with intermittent androgen deprivation. Prostate. 2011;71:1608–1615. doi: 10.1002/pros.21377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferrara N. Vascular endothelial growth factor. Arterioscler Thromb Vasc Biol. 2009;29:789–791. doi: 10.1161/ATVBAHA.108.179663. [DOI] [PubMed] [Google Scholar]
- 35.De Falco S, Gigante B, Persico MG. Structure and function of placental growth factor. Trends Cardiovasc Medicine. 2002;12:241–246. doi: 10.1016/s1050-1738(02)00168-8. [DOI] [PubMed] [Google Scholar]
- 36.Fischer C, Mazzone M, Jonckx B, et al. FLT1 and its ligands VEGFB and PlGF: Drug targets for anti-angiogenic therapy? Nat Rev Cancer. 2008;8:942–956. doi: 10.1038/nrc2524. [DOI] [PubMed] [Google Scholar]
- 37.Wu FT, Stefanini MO, Mac Gabhann F, et al. A systems biology perspective on sVEGFR1: Its biological function, pathogenic role and therapeutic use. J Cell Mol Med. 2010;14:528–552. doi: 10.1111/j.1582-4934.2009.00941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ebos JM, Bocci G, Man S, et al. A naturally occurring soluble form of vascular endothelial growth factor receptor 2 detected in mouse and human plasma. Mol Cancer Res. 2004;2:315–326. [PubMed] [Google Scholar]
- 39.Presta M, Dell'Era P, Mitola S, et al. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 2005;16:159–178. doi: 10.1016/j.cytogfr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 40.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: The multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 41.Uhlar CM, Whitehead AS. Serum amyloid A, the major vertebrate acute-phase reactant. Eur J Biochem. 1999;265:501–523. doi: 10.1046/j.1432-1327.1999.00657.x. [DOI] [PubMed] [Google Scholar]
- 42.Jensen LE, Whitehead AS. Regulation of serum amyloid A protein expression during the acute-phase response. Biochem J. 1998;334:489–503. doi: 10.1042/bj3340489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heinrich PC, Behrmann I, Haan S, et al. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem J. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turner AM, Bennett LG, Lin NL, et al. Identification and characterization of a soluble c-kit receptor produced by human hematopoietic cell lines. Blood. 1995;85:2052–2058. [PubMed] [Google Scholar]
- 45.Dahlen DD, Lin NL, Liu YC, et al. Soluble Kit receptor blocks stem cell factor bioactivity in vitro. Leuk Res. 2001;25:413–421. doi: 10.1016/s0145-2126(00)00122-3. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura Y, Tajima F, Ishiga K, et al. Soluble c-Kit receptor mobilizes hematopoietic stem cells to peripheral blood in mice. Exp Hematol. 2004;32:390–396. doi: 10.1016/j.exphem.2004.01.004. [DOI] [PubMed] [Google Scholar]