Abstract
Dual oxidases (DUOX1 and DUOX2) are evolutionary conserved reduced nicotinamide adenine dinucleotide phosphate oxidases responsible for regulated hydrogen peroxide (H2O2) release of epithelial cells. Specific maturation factors (DUOXA1 and DUOXA2) are required for targeting of functional DUOX enzymes to the cell surface. Mutations in the single-copy Duox and Duoxa genes of invertebrates cause developmental defects with reduced survival, whereas knockdown in later life impairs intestinal epithelial immune homeostasis. In humans, mutations in both DUOX2 and DUOXA2 can cause congenital hypothyroidism with partial iodide organification defects compatible with a role of DUOX2-generated H2O2 in driving thyroid peroxidase activity. The DUOX1/DUOXA1 system may account for residual iodide organification in patients with loss of DUOX2, but its physiological function is less clear. To provide a murine model recapitulating complete DUOX deficiency, we simultaneously targeted both Duoxa genes by homologous recombination. Knockout of Duoxa genes (Duoxa−/− mice) led to a maturation defect of DUOX proteins lacking Golgi processing of N-glycans and to loss of H2O2 release from thyroid tissue. Postnatally, Duoxa−/− mice developed severe goitreous congenital hypothyroidism with undetectable serum T4 and maximally disinhibited TSH levels. Heterozygous mice had normal thyroid function parameters. 125I uptake and discharge studies and probing of iodinated TG epitopes corroborated the iodide organification defect in Duoxa−/− mice. Duoxa−/− mice on continuous T4 replacement from P6 showed normal growth without an overt phenotype. Our results confirm in vivo the requirement of DUOXA for functional expression of DUOX-based reduced nicotinamide adenine dinucleotide phosphate oxidases and the role of DUOX isoenzymes as sole source of hormonogenic H2O2.
Important steps in thyroid hormone synthesis take place at the apical membrane of follicular thyroid cells. In the first step, iodide is oxidized and covalently bound to tyrosines of thyroglobulin (TG). In the second step, iodinated tyrosyl residues are linked via an ether bond to form iodothyronines (T4 and, to lesser degree, T3, and rT3). Both reactions are catalyzed by thyroid peroxidase (TPO), the activity of which is controlled by the supply of hydrogen peroxide as final electron acceptor.
The catalytic core of this thyroidal H2O2 generator is provided by dual oxidases (DUOX1 and DUOX2; originally called thyroid oxidases or THOX), members of the NOX/DUOX family of reduced nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (1, 2). Their intrinsic activity is acutely stimulated by cytosolic Ca2+ and protein kinase C-mediated phosphorylation in vitro (3) consistent with the regulation of H2O2 production and iodination in thyroid tissue (4, 5).
In heterologous systems, reconstitution of a DUOX-based H2O2 generator requires the formation of a heterodimeric complex of DUOX with another multipass transmembrane protein called dual oxidase maturation factor (DUOXA1 and DUOXA2 paralogs) (6). The evolutionary conserved genetic linkage of DUOX1/DUOXA1 and DUOX2/DUOXA2 into transcriptional units provides the basis for strict coexpression of each DUOX with the corresponding maturation factor (6–8). Only DUOX1/DUOXA1 and DUOX2/DUOXA2 complexes appear to be fully functional in vitro (9, 10).
The DUOX2-based enzyme appears to be most critical for maintaining normal thyroid function, because only mutations in DUOX2 and DUOXA2, but not DUOX1 or DUOXA1 genes, have been identified as cause of dyshormonogenesis in patients with congenital hypothyroidism (CH) (reviewed in Ref. 11), and mice deficient in Duox2 (12), but not Duox1 (13), are hypothyroid. On the other hand, patients with biallelic, complete loss-of-function DUOX2 mutations manifest only partial iodide organification defects and sometimes merely hyperthyrotropinemia with normal thyroid hormone levels (compensated hypothyroidism). These data suggest that other sources of H2O2, presumably DUOX1, can partially compensate for DUOX2 deficiency.
In evolutionary terms, the adaptation of the DUOX system as driver of TPO activity appears to be a rather recent addition to the functional repertoire of DUOX. DUOX/DUOXA are expressed outside the thyroid, particularly in barrier epithelia, and homologs are present in invertebrates lacking a follicular thyroid or an endostyle thyroid equivalent. For instance, high basal and pathogen-inducible DUOX/DUOXA expression in the epithelium of the gastrointestinal tract has been conserved from invertebrates, e.g. nematodes and fruitflies, to mammals including human (14–17). Gene knockdown studies in invertebrate model systems have convincingly demonstrated a crucial function of DUOX-derived H2O2 in host-microbial interaction and barrier defense of the intestinal mucosa. Whether the vertebrate DUOX enzymes play a similar role in epithelial innate immunity remains to be investigated in suitable in vivo model systems. Furthermore, loss-of-function mutations of the single-copy invertebrate Duox and Duoxa genes have been associated with severe, frequently lethal developmental abnormalities (14, 15, 18, 19). Up until now, however, no equivalent complete DUOX deficiency model has been generated in higher vertebrates expressing two DUOX isoenzymes.
Here, we introduce a novel mouse model generated by simultaneous targeting of the contiguous Duoxa1 and Duoxa2 genes (Duoxa−/− mice). Our results indicate that global DUOXA deficiency in Duoxa−/− mice leads to intracellular retention of the DUOX subunits concomitant with a loss of Ca2+-inducible H2O2 release. Loss of DUOX activity in Duoxa−/− mice did not affect intrauterine survival or cause apparent inborn developmental defects. However, due to complete lack of thyroidal iodide organification, they develop the hallmarks of severe primary CH in postnatal life. In contrast, Duoxa−/− mice on continuous postnatal T4 replacement did not display an overt phenotype.
Results
Simultaneous targeting of Duoxa1 and Duoxa2 in embryonic stem cells
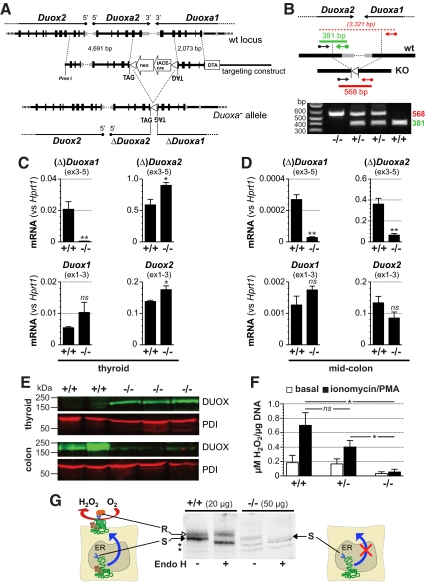
The arrangement of the Duoxa genes and their relative position to the Duox genes is schematically depicted in Fig. 1A. The simultaneous targeting of both Duoxa genes was achieved by deletion of an approximately 2.9-kbp region extending from coding exon 5 of Duoxa2 to coding exon 5 of Duoxa1. Termination of translation at the breakpoints was ensured by the insertion of stop codons (TAG) in the reading frames of the Duoxa genes. The resulting truncation of the Duoxa coding regions has been shown to prevent expression of stable, functional proteins (9) in vitro.
Fig. 1.
Generation of Duoxa−/− mice. A, Structure of the Duoxa1 and Duoxa2 genes, the targeting construct, and the final knockout allele. A unique PmeI endonuclease site was used to linearize the vector before electroporation. Homologous recombination of ES cell DNA with the targeting construct replaced an approximately 2.9-kbp region with the floxed ACN cassette modified by the addition of terminal stop codons in frame of the Duoxa genes. The ACN cassette contained the NeoR-positive selection marker and encodes Cre recombinase expressed specifically during spermatogenesis from a testis-specific promoter (tACE). Passage of the targeted locus through the germ line of male chimeras resulted in the self-excision of the ACN cassette with only a single loxP site flanked by stop codons remaining. B, Strategy of genotyping. The location of the three primers and the amplicons from WT and knockout alleles are indicated. C and D, Expression of Duox and Duoxa mRNA in WT (n = 6) and Duoxa−/− (n = 5) thyroid glands (C) and colon (D). E, DUOX protein expression in thyroid (40 μg total protein) and descending colon (70 μg) of 3-month-old WT and Duoxa−/− mice. Protein disulfide isomerase (PDI) was immunodetected to validate equal protein loading. F, Determination of H2O2 release from thyroid tissue samples of mice (pure 129S6/SvEvTac genetic background) with the indicated Duoxa genotypes. H2O2 concentrations accumulating in the medium were normalized for DNA content of the tissue samples. G, Maturation of DUOX N-glycosylation in WT and Duoxa−/− mice. Cecal protein extracts were analyzed on Western blots with or without prior digestion with Endoglycosidase H (EndoH). Maturation of DUOX N-glycosylation in the Golgi apparatus produces Endo H-resistant glycans (R). DUOX protein from Duoxa−/− mice is only detectable as EndoH-sensitive glycoform (S) indicating complete retention of DUOX in the endoplasmic reticulum (ER). *, P < 0.05; **, P < 0.01; ns, nonsignificant. PMA, phorbol 12-myristate-13-acetate.
Generation of Duoxa−/− mice as a model for complete deficiency of both DUOX isoenzymes
Duoxa−/− mice were generated as detailed in Materials and Methods. Note that the knockout allele is devoid of selection cassettes used in the targeting of embryonic stem (ES) cells, with only a single loxP element (flanked by stop codons) remaining between the truncated Duoxa open reading frames (Fig. 1, A and B). Except where indicated, all mice used in this study were F2 hybrid 129S6×C57BL/6J animals, produced by intercrosses of F1 heterozygotes originating from a single ES cell clone (clone 43). The phenotype of Duoxa−/− mice described below was confirmed in a smaller number of knockout animals derived from two additional ES cell clones.
Duoxa−/− mice express detectable amounts of mRNA from the truncated Duoxa loci as determined by RT-PCR with PCR primers upstream of the introduced stop codons (Fig. 1, C and D). 3′RACE-PCR did not result in a specific product for the truncated Duoxa1 locus (ΔDuoxa1) locus but identified a stable, polyadenylated ΔDuoxa2 transcript in thyroid gland tissue of Duoxa−/− mice. The latter transcript contained, as expected, the in-frame stop codon (Supplemental Fig. 1 published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). Expression of Duox1 and Duox2 was not significantly different in Duoxa−/− and wild type (WT) mice in extrathyroidal tissues, suggesting that the targeting did not alter cis elements crucial for Duox gene expression. In contrast, but compatible with the relatively high level of the polyadenylated ΔDuoxa2 mRNA in the thyroid of Duoxa−/− mice, expression of Duox2 was slightly induced in the thyroid of Duoxa−/− mice.
DUOX protein level was also elevated in the thyroids of Duoxa−/− mice (Fig. 1E). In the colon of Duoxa−/− mice, however, DUOX protein expression was greatly diminished despite intact mRNA expression, a finding consistent with reduced stability of the isolated DUOX subunits expressed without DUOXA heterodimerization partner in heterologous cell lines (21–23). Apart from cell type-specific differences in protein stability, it is conceivable that in the thyroid of Duoxa−/− mice, lower DUOX protein stability may be surmounted by induction of DUOX mRNA expression.
DUOX proteins are normally expressed as high mannose glycosylated forms (180 kDa) in the endoplasmic reticulum (ER) and as fully glycosylated forms (∼190 kDa) found at the plasma membrane (24, 25). By testing the sensitivity of the N-glycosylation to digestion with endoglycosidase H, we were able to show that Duoxa−/− mice express DUOX exclusively in the immature, high-mannose glycosylated form (Fig. 1G). The processing of endogenous DUOX in cells of Duoxa−/− mice is therefore reminiscent of that of recombinant DUOX expressed without DUOXA in heterologous systems in vitro. In the latter systems, the ER-retained DUOX protein is completely inactive.
To more directly confirm the absence of DUOX-based NADPH oxidase activity, we determined H2O2 release from thyroid tissue of WT, Duoxa+/−, and Duoxa−/− mice. We found that basal H2O2 release from thyroid tissue of Duoxa−/− mice, compared with that of WT or Duoxa+/− mice, was more than 5-fold lower (Fig. 1F). H2O2 release from the thyroid tissue samples of WT and Duoxa+/− mice, but not Duoxa−/− mice, was induced by ionomycin/phorbol 12-myristate 13-acetate costimulation. Overall the stimulated H2O2 release from thyroid tissue was more than 12 times higher in WT compared with Duoxa−/− mice (P = 0.02). These results indicate loss of Ca2+-inducible NADPH oxidase activity in the thyroids of Duoxa−/− mice. They also indicate that the amounts of H2O2 arising from cellular metabolism or other NADPH oxidases that permeate into the extracellular milieu are negligible compared with H2O2 generated by DUOX enzymes at the cell surface.
Severe hypothyroidism of Duoxa−/− mice
Duoxa−/− mice appeared normal at birth, and the genotype ratios were consistent with expected single-locus Mendelian inheritance (Duoxa+/+:Duoxa+/−:Duoxa−/− = 73:150:87; P = 0.45, chi-square test). Complete loss of DUOX activity did therefore not cause embryonic lethality or affect prenatal growth. However, Duoxa−/− mice showed severely delayed postnatal development (Fig. 2A). Average age of eye opening, a marker for cerebral maturation, was delayed from 12.2 d in WT to 15.5 d in Duoxa−/− (P < 0.0001) (Fig. 2B). A significant reduction in body weight became apparent at 2 wk of age (Duoxa−/−: 5.8 ± 0.1 g vs. WT 7.7 ± 0.2 g; P < 0.0001) (Fig. 2C). Whole-mount bone stains revealed impaired linear growth of long bones, delayed enchondral ossification, and reduced bone mineralization in Duoxa−/− animals compared with WT littermates (Fig. 2E). Duoxa−/− mice did not survive weaning at P21. However, a substantial proportion of Duoxa−/− mice (>80%) survived into adulthood when weaned at P30. All Duoxa−/−, but neither WT nor Duoxa+/− mice, displayed persistent abnormalities in motor function and coordination reflected by bilateral dystonic hindlimb clasping when lifted up by the tail and failure to invert and climb down when placed on a vertical pole. Between 1 and 3 months of age, approximately one third of Duoxa−/− mice developed signs of respiratory distress accompanied by an audible inspiratory stridor (Supplemental Movie 1). Examination of the laryngotracheal units in these mice revealed subglottic stenoses due to extrinsic compression by massively enlarged thyroid glands.
Fig. 2.
Hypothyroid phenotype of Duoxa−/− mice. A, Exemplar 4-wk-old Duoxa−/− and WT littermates. B, Delayed eye opening of Duoxa−/− pups. The curves depict the fraction of mice with bilateral open eyes at the indicated postnatal age. WT, n = 25; Duoxa−/−, n = 22; Duoxa−/−/l-T4, n = 13. C, Body weight (bw) of male WT (in black), heterozygous (gray), Duoxa−/− mice (red), and Duoxa−/− mice receiving l-T4 replacement starting from P6 (brown). Note that Duoxa−/− without thyroid hormone replacement did not survive weaning at P21 and were, therefore, weaned at P30. D, Serum T4, T3, and TSH concentrations in 10- to 12-wk-old WT, heterozygous, and Duoxa−/− animals. Blue and pink solid symbols denote males and females, respectively. **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. E, Skeletal preparations (rib cage, knee joint, forelimb) from WT and Duoxa−/− mice at P13 stained with Alizarin Red (bone) and Alcian Blue 8GX (cartilage). F, Weights of selected organs in 2-month-old mice normalized for total body weight. Untreated Duoxa−/− (red bars) displayed thyroid hyperplasia and hyposplenia. In contrast, liver weight was reduced proportionally to total body weight. Note that the absolute brain weights (i.e. not normalized for body weight) were not significant different between the genotypes. ****, P < 0.0001 compared with all other groups. Each bar represents the mean ± sem of n = 7–28 animals.
Dwarfism and severe developmental delay are hallmarks of untreated CH. Thyroid function tests using sensitive RIA revealed undetectable level of T4 (<0.25 μg/dl) in Duoxa−/− mice, whereas TSH concentrations were 500-fold (males) to 2500-fold (females) higher than in WT littermates (Fig. 2D). Serum T3 level in Duoxa−/− mice were approximately 50% lower than in WT mice (45.2 ± 3.1 vs. 83.6 ± 3.7 ng/dl; P < 0.0001). Heterozygous animals of every age tested [postnatal d 6 (P6), P14, adult] had thyroid function parameters indistinguishable from age-matched WT animals. Thus, there was no apparent Duoxa haploinsufficiency with respect to thyroid function.
Histological examination of 2-month-old Duoxa−/− mice revealed diffuse, homogeneous hyperplasia of the thyroid gland with residual small follicles lined by columnar epithelium that frequently formed papillary folds protruding into the lumen (Fig. 3, A–C). Immunochemical staining of TSH-β in the anterior pituitary of Duoxa−/− mice showed massive hypertrophy and hyperplasia of the thyrotrophic cells (Fig. 3, D and E). Among TSH-responsive genes expressed in thyroid epithelial cells, thyroid peroxidase (Tpo), sodium iodide symporter (Slc5a5), and TSHR receptor (Tshr) were strongly induced in Duoxa−/− mice (Fig. 3F). In contrast, Tg mRNA expression was unaffected consistent with the concept that, in rodents, Tg expression is dependent on TSH but maximally induced at physiological TSH concentrations (26). Overall, these findings indicate that the primary hypothyroidism of Duoxa−/− mice is due to dyshormonogenesis and consistent with a role of DUOX-derived H2O2 in driving TPO-catalyzed reactions.
Fig. 3.
Pathology of thyroid glands and anterior pituitary. A, Exemplar hematoxylin and eosin (H&E)-stained thyrotracheal units of a 2-month-old Duoxa−/− mouse with manifest inspiratory stridor and a WT littermate. B and C, Immunohistochemical detection of TG on thyroid sections from mice in panel A. D and E, Detection of thyrotrophic cells in the anterior pituitary by immunochemical staining of TSH-β subunit. F, Thyroidal expression of selected TSH-responsive genes determined by real-time RT-PCR. ****, P < 0.0001.
Lack of thyroidal iodide organification in Duoxa−/− mice
To further characterize the hormone synthesis defect in Duoxa−/− mice, we investigated the thyroidal uptake and perchlorate-induced discharge of iodine-125 (125I). To facilitate efficient uptake of the radioisotope in WT and heterozygous animals, all animals were fed a low-iodine diet (LID) for 2 wk. TSH level of WT mice on LID were 40-fold higher compared with those on iodine-sufficient diet (Fig. 4A). LID failed to further increase the markedly elevated TSH level of Duoxa−/− mice, suggesting maximal stimulation of the thyrotrophic cells in the absence of negative feedback by thyroid hormones. To study the kinetics of thyroidal iodide uptake, 125I was injected ip, and the accumulation of radioactivity over the anterior neck was monitored with a γ positioning system (Fig. 4B). The amount of activity detected over the midsternum was used as surrogate for change in background activity due to 125I distribution in extrathyroidal tissues (Fig. 4C). Although both WT and Duoxa−/− animals showed a rapid increase of 125I activity over the neck within the initial 5 min, only WT animals showed a further increase of activity over the ensuing 4 h. Concurrently, nonthyroidal 125I activity measured over the midsternum showed a steep decline from the initial peak (at 6 min) only in WT but not Duoxa−/− mice. These results were consistent with a lack of clearance of 125I from the circulation in Duoxa−/− mice due to limited retention in the thyroid gland. In fact, 4 h after injection of 125I, more than 60% of the initial dose was found in the excised thyroid gland of WT animals, whereas only about 14% of the dose had been retained in the thyroid glands of Duoxa−/− mice (Fig. 4E).
Fig. 4.
Thyroidal 125I organification. A, Effect of 2 wk of LID on serum TSH concentrations of WT and Duoxa−/− mice. B and C, Kinetics of 125I distribution in WT and Duoxa−/− mice. 125I activity over the anterior neck (B) and the midsternum (C) was determined using a γ-positioning system. The plots shown are representative curves from individual animals. D, Perchlorate-induced change in 125I activity recorded over the anterior neck. Perchlorate was given ip 4 h after administration of 125I, and activity over the neck was monitored for 20 min. Representative data from two mice are plotted for each genotype. E, Thyroidal 125I activity before (open bars) and 20 min after (black solid bars) administration of ClO4−. Activity of dissected thyrotracheal units was determined using a γ-scintillation counter. Data are expressed in percent of the administered dose (8 μCi/animal). Significant perchlorate-induced loss of thyroidal 125I activity was only observed in Duoxa−/− mice [53.7% washout (95% CI = 22.7–84.8); P = 0.0027], but not WT (+/+) or heterozygous (+/−) animals. F, Perchlorate-induced appearance of 125I activity in the circulation. Four hours after injection of 125I, blood was collected retroorbitally before and 20 min after administration of KClO4. Bars represent the perchlorate-induced increase of 125I activity in blood, expressed as a percentage of the injected dose. Total blood volume (in milliliters) was estimated to be 7.2% of body weight (in grams) (41). **, P < 0.01; ***, P < 0.001. G, Reducing SDS-PAGE of thyroidal protein extracts (50 μg/lane) from mice on LID. Thyroids were collected at the end of the perchlorate discharge tests. Protein was stained with colloidal Coomassie Brilliant Blue, and 125I incorporation was revealed by autoradiography. S and F denote slow and fast migrating forms of TG, respectively (42, 43). Extracts analyzed are from two different WT and Duoxa−/− mice, respectively. H, Reducing SDS-PAGE of thyroidal protein extracts from mice on an iodine-sufficient diet. Upper panel, Coomassie Blue-stained gel. Lower panel, Western blot probed simultaneously with pan-TG antiserum (green signal) and an antibody specific for iodinated TG (red signal) (38). In the color merge, iodinated and noniodinated forms of TG appear in yellow and green, respectively. Each lane corresponds to protein extract from a different animal.
To test whether the lower 125I accumulation in the thyroid glands of Duoxa−/− mice was due to loss of organification of iodide, the radioactivity over the neck was monitored after the injection of perchlorate (ClO4−). ClO4− is a competitive inhibitor of the sodium-iodide symporter at the basolateral membrane of follicular thyroid cells. Free iodide that has been taken into the gland but not been organified (bound to protein) should be subject to washout after ClO4− administration and leak back into the circulation. Within 1 min of injection of ClO4−, a rapid loss of 125I activity over the neck occurred in Duoxa−/− mice with more than 50% loss of activity after 5 min (Fig. 4D). To corroborate these results, the accumulation of 125I in thyrotracheal units dissected before and 20 min after ClO4− administration was determined by γ counting. Glands of Duoxa−/− animals dissected before ClO4− injection contained 14.4 ± 2.3% of the injected dose compared with only 6.6 ± 0.9% after ClO4− indicating 54% washout (95% CI = 22.7–84.8; P = 0.0027) (Fig. 4E). The concomitant increase of 125I activity in the blood was quantitatively consistent with the loss of activity from the thyroid glands (Fig. 4F). There was no significant 125I washout from the thyroid glands of WT or Duoxa+/− mice.
The high 125I activity in the circulation of Duoxa−/− mice together with the fact that their thyroid glands are massively enlarged and highly vascularized causes a high level of background activity in the perchlorate discharge test and, thus, presumably underestimates the fractional washout. To better assess the severity of the iodide organification defect, we directly tested the iodination status of TG protein. Analysis of thyroidal protein extracts by SDS-PAGE and autoradiography revealed that Duoxa−/− animals, in contrast to WT animals, did not incorporate 125I into TG (Fig. 4G). Note that the amount of thyroidal TG protein in WT animals on LID was not different from that of Duoxa−/− mice kept on either LID or normal diet, but substantially lower compared with WT animals on normal diet (Fig. 4, G and H). These results are consistent with rapid turnover of TG in thyroid glands under excessive TSH stimulation. Consistently, the depletion of TG in the thyroid glands of Duoxa−/− mice was ameliorated by supplementation of l-T4. In these animals fed an iodine-sufficient diet, TG protein of WT, but not that of Duoxa−/− mice, was clearly detectable on Western blots with antibodies specific for iodinated TG epitopes (Fig. 4H). l-T4 replacement increased total but not iodinated TG level in Duoxa−/− mice, confirming that loss of iodide organification is not secondary to altered thyroidal TG metabolism.
Rescue of Duoxa−/− mice by l-T4 replacement
The Duox enzymes are expressed not only in thyroid epithelial cells, but also at high levels in extrathyroidal organs, particularly epithelial cells lining the gastrointestinal tract and the upper airways. To test whether the observed phenotype can be completely attributed to the lack of thyroid hormone production, Duoxa−/− animals were maintained on a hormone replacement regimen starting from P6 with daily sc or ip injections of l-T4. Duoxa−/− mice on chronic l-T4 replacement had normalized circulating levels of the active thyroid hormone T3 [Duoxa−/− 90.0 ± 4.2 (n = 8) vs. WT 87.4 ± 6.1 (n = 7)]. Their bone growth, weight gain (Fig. 2C), and fertility were indistinguishable from that of WT littermates, and they did not display any of the signs described in untreated Duoxa−/− mice. Differences in relative organ weights between Duoxa−/− and WT animals were also obliterated by l-T4 replacement (Fig. 2F). In addition, on a survey including thyroid glands, airways, and the entire gastrointestinal tract no histomorphological differences were observed between WT and l-T4 substituted Duoxa−/− mice.
Discussion
In this study we showed that mice deficient in both Duoxa1 and Duoxa2 lack significant thyroid hormone production due to a total iodine organification defect. In heterologous systems in vitro, expression of recombinant DUOX without coexpression of DUOXA does not reconstitute a functional, surface-expressed NADPH oxidase. Here we showed that in mice with inactivation of both Duoxa genes, endogenous DUOX similarly remains in an immature, ER-retained form and lacks Ca2+/protein kinase C-inducible H2O2 release. Thus Duoxa−/− mice provide a model for complete deficiency of DUOX-based NADPH oxidases. Having undetectable thyroid hormone production and 125I incorporation into TG, these mice provide irrefutable evidence that only DUOX-generated H2O2, but not reactive oxygen species from other sources, is able to drive thyroid hormone synthesis.
Although both DUOX isoenzymes are expressed in follicular thyroid cells, only DUOX2 contribution to thyroid function has been unambiguously established. In humans, loss of function mutations in DUOX2 and DUOXA2 underlie iodide organification defects in a subset of patients with CH (reviewed in Ref. 27). Apart from a large deletion affecting both DUOX systems (28), no mutations in DUOX1/DUOXA1 have been found in CH patients. The in vivo function of the DUOX1 system remains unclear. It is expressed in many of the same tissues as DUOX2, although frequently at lower levels (e.g. in thyroid, gastrointestinal tract). Like DUOX2, it is a Ca2+-stimulated H2O2-generating NADPH oxidase, but there are clearly differences in regulation of gene expression (29) and intrinsic enzyme activity (3).
Two mouse models with deficiency in either the Duox1 or Duox2 system have been described. A model for Duox2 deficiency was provided by the discovery of the thyd strain harboring a spontaneous missense mutation (V674G) at a conserved position of Duox2 (12). Although Duox2thyd mice showed retarded growth similar to Duoxa−/− mice, there is no reported detrimental effect on survival during the weaning or postweaning period. It is likely that residual iodide organification in Duox2thyd mice accounts for this difference. Apart from the possibility that the thyd mutation is hypomorphic, mitigation of the defect could be provided by the intact Duox1 system as has been proposed for patients with CH due to complete loss of DUOX2. Indeed, in male Duox2thyd mice, the average serum T4 level is 0.5 μg/dl (12), whereas T4 level of Duoxa−/− was below the detection limit (<0.25 μg/dl). Similarly, serum TSH levels measured in our RIA (12, 29) were 200-fold and 500-fold over baseline in male Duox2thyd and Duoxa−/− mice, respectively. The TSH of the latter was in the same range as untreated athyreotic mice deficient in the Pax8 gene (30). Because serum TSH was not further augmented in Duoxa−/− mice placed on LID, maximal disinhibition of thyrotrophs from negative feedback had been reached on a normal, iodine-sufficient diet. Collectively these findings indicate that T4 is not only undetectable but essentially excludes the presence of physiologically relevant amounts of thyroid hormones in Duoxa−/− mice to produce TSH feedback suppression.
In contrast to Duox2thyd mice, Duox1-deficient mice did not display an obvious phenotype and have normal thyroid function profiles (13). Clearly, a redundant role of Duox1 in thyroid hormone synthesis would not satisfactorily explain the evolutionary pressure that maintained a duplicated Duox/Duoxa locus since the teleost-amphibian divergence. One plausible explanation for the presence of two functional DUOX systems could have been redundancy for a particularly vital role of DUOX-generated H2O2 in development. For instance, in invertebrates having only one functional copy of each Duox and Duoxa gene, the loss of function mutations in either leads to severe developmental abnormalities with high mortality at an early developmental stage (14, 15, 18, 19). Reduced survival of these mutants has been attributed to an important function of DUOX in driving di-tyrosine cross-linking reactions of extracellular matrix proteins during larval development, peroxidative reactions analogous to the cross-linking of iodotyrosines to iodothyronines (14). However, our results convincingly exclude redundancy of DUOX isoenzymes for an essential role during prenatal murine development because Duoxa−/− mice were born at expected Mendelian ratios and did not show postnatal abnormalities, other than those that could be prevented by early hormone replacement.
It is therefore more likely that the presence of two DUOX paralogs relates to their differential regulation in extrathyroidal organs. A common theme of DUOX function in various model systems is their adaptive response to environmental stressors. In invertebrates, Duox is strongly up-regulated in the intestinal epithelium upon pathogen exposure and RNA interference-mediated knockdown of Duox (15, 18) or Duoxa (18) diminishes reactive oxygen species production and severely impairs the ability to clear pathogens. In acute epithelial injury in zebrafish, H2O2 produced locally by activated DUOX acts as chemoattractant signal to recruit leukocytes (31). There is also ample evidence for strong induction of DUOX in mammalian species as part of innate immune responses, e.g. in bacterial (32) or nematode (33) infections in the intestine, chronic inflammatory bowel diseases (34, 35), or airway inflammation (36).
Duoxa−/− mice on l-T4 replacement should be a useful mammalian model system to screen for extrathyroidal functions of DUOX in response to environmental challenges. The DUOX isoenzymes are often coexpressed in the same tissues raising the possibility of at least partial functional redundancy. Because the proximity of the DUOX genes precludes intergenic meiotic recombinations, double-gene knockouts cannot be readily obtained by crossing lines of mice with single-gene defects (e.g. Duox2thyd and Duox1−/− mice). In this regard, Duoxa−/− mice provide a unique model of complete deficiency of functional DUOX-based NADPH oxidases.
Materials and Methods
Animals
All procedures carried out in mice and described below were approved by the Institutional Animal Care and Use Committees of the University of Chicago, the University of Michigan, and Free University of Brussels, respectively. Animals were maintained in specific pathogen-free conditions on a 12-h light, 12-h dark cycle (lights on at 0600 h) and fed an autoclaved laboratory chow and tap water ad libitum. The mice on l-T4 (l-T4 sodium salt pentahydrate; Sigma, St. Louis, MO) replacement received 40 ng l-T4/g body weight by daily sc injection starting from P6. After weaning, thyroid hormone replacement was achieved by providing albuminized drinking water containing 0.26 mg/liter l-T4.
Generation of targeting construct
The targeting construct for generation of Duoxa−/− mice was designed to delete a 2857-bp region extending from exon 5 of Duoxa2 to coding exon 5 of Duoxa1. Termination of translation at the breakpoints was ensured by the additional insertion of stop codons in the reading frames of the Duoxa genes (after codon 246 in Duoxa1; after codon 232 of Duoxa2). The DTA cassette was excised (via KpnI and NheI) from the pPGKneoDTA vector (kindly provided by Philippe Soriano) and the ACN cassette (via EcoRI and KpnI) from plasmid pACN (kindly provided by Mario Capecchi) and three-fragments ligation performed with the NheI/EcoRI-digested pMCS5 plasmid (MoBiTec). The ACN cassette was flanked with loxP sites and contains the neomycin resistance (NeoR) gene under control of the mouse RNA Pol II promoter, together with Cre recombinase under the control of the testis-specific promoter of angiotensin-converting enzyme (tACE-CRE). The DTA (diphtheria toxin subunit A) cassette was used for negative selection against nonhomologous recombination. The homology arms were then amplified from isogenic genomic DNA from the 126/S6 ES cell line using high-fidelity Pfu polymerase (Stratagene, La Jolla, CA). The 4.7-kb long-arm fragment was cloned via PmeI/BsiWI sites. Subsequently, the 2.1-kb short-arm fragment was prepared with terminal BamHI sites and ligated into the BglII site to obtain the targeting vector. A clone of the final construct was confirmed by restriction digestion and bidirectional sequencing, linearized with PmeI, and purified by gel extraction.
Generation of targeted ES cells and mutant mice
The linearized targeting construct was electroporated into feederless 129S6 ES cells. After selection with G418 (200 μg/ml), nine of 144 (6.25%) neomycin-resistant ES cell clones were found to be correctly targeted as shown by long-range PCR assays and Southern blotting (Supplemental Fig. 2). Positive ES cell clones were injected into C57BL/6J blastocysts at the University of Chicago Transgenic Core Facility. Germline transmitting chimeras were mated with C57BL/6J WT females. In ES cell-derived germ cells of chimeric mice, CRE expression leads to excision of the ACN cassette during spermatogenesis, with only a single loxP element (flanked by the stop codons inserted in the Duoxa open reading frame) remaining. Heterozygous F1 transgenic mice were identified by coat color (agouti) and by PCR of DNA isolated from tail tips. All data reported here were obtained from F2 hybrid (F1 intercross) animals originating from a single ES cell clone (clone 43). The basic characteristics of the phenotype (growth retardation, thyroid function tests) were confirmed in Duoxa−/− mice derived from two additional independently targeted ES cell clones (clones 40 and 60) and in Duoxa−/− mice maintained either on a pure 129S6/SvEvTac background or backcrossed for more than six generations with C57BL/6J mice.
Genotyping
Crude genomic DNA extracts from toe clips (P9–12) and PCR amplification were performed using the REDExtract-N-Amp Tissue PCR Kit (Sigma). PCR was performed with three primers, including the common primer DA-WT/KO-F: 5′-CAGCAGTCCTGGTCAGGGA-3′; a WT allele-specific primer DA-WT-R: 5′-CCTGCTACGGAGACGAACA-3′; and a knockout allele-specific primer DA-KO-R: 5′-ACATCTTCTGCCTAGGTCCTG-3′. PCR products were separated on 1.5% agarose gels. Presence of the WT allele is evident by a 381-bp fragment, whereas the amplicon from the knockout allele has a size of 568 bp.
Thyroid function tests
Serum total T4 and T3 concentrations were measured by coated tube RIA (Siemens Medical Solutions Diagnostics, Los Angeles, CA) modified for measurements in mouse serum. Serum rT3 was measured by RIA using reagents from Adaltis Italia (Rino, Italy). Serum TSH was determined using a sensitive, heterologous, disequilibrium, double-antibody precipitation RIA (28).
Immunohistochemistry
Sections (4-μm) from paraffin-embedded specimens were deparaffinized, rehydrated, and subjected to antigen retrieval in 10 mm citrate buffer, pH 6.0, for 20 min at 95 C. Sections were then washed with PBS and incubated with either mouse monoclonal antibodies against TG (clone 14/14; Cell Sciences, Canton, MA) or rabbit antirat TSHβ-IC-1 (AFP1274789, National Institute of Diabetes and Digestive and Kidney Diseases). Specific antigen-antibody complexes were revealed using horseradish peroxidase-conjugated secondary antibodies (ImmPRESS universal antibody; Vector Laboratories, Burlingame, CA) and the chromogen substrate NovaRED (Vector Laboratories). Sections were counterstained with Mayer's hematoxylin, dehydrated, and permanently mounted in nonaqueous medium (VectaMount; Vector Laboratories).
Whole-mount skeletal preparations
To assess endochondral ossification at early postnatal stages of development, bone and cartilage were simultaneously visualized by whole-mount staining with Alizarin Red and Alcian Blue, respectively. Briefly, after removal of skin and evisceration, carcasses were fixed for 3 d in 95% ethanol. After fat removal by incubation in acetone the specimens were stained for 3 d with Alcian Blue 8GX (15 mg/ml) and Alizarin Red (5 mg/ml) in 5% glacial acetic acid/60% ethanol. Samples were cleared over several weeks in 1% KOH with increasing concentrations (0–80%) of glycerol before storage in 100% glycerol. Photographs were taken using bright-field optics and transillumination with the specimens submerged in glycerol.
SDS-PAGE and Western blotting
For detection of DUOX proteins, mouse tissues were extracted in lysis buffer (50 mm phosphate buffer, 20 mm EDTA, 1% Triton X-100) supplemented with a mixture of protease inhibitors (Complete; Roche Applied Science, Indianapolis, IN) for 1 h at 4 C. The lysate was centrifuged 15 min at 10,000 rpm, and solubilized proteins were denatured in Laemmli buffer (pH 6.8; 1.54% dithiothreitol, 2% sodium dodecyl sulfate, 10% glycerol and 0.75% Tris) before loading and separation by SDS-PAGE. DUOX proteins were detected with DUOX antibody [1:1000; (2)]. Probing with protein disulfide isomerase antibodies (Santa Cruz Biotechnology, Inc., Santa Cruz, CA) was used as loading control. Fluorescent secondary antibodies (1:10,000; IRDye800 antirabbit and IRDye680 antimouse; LI-COR Biosciences, Lincoln, NE) were used for image acquisition and quantification with the Odyssey infrared imaging system (LI-COR Biosciences).
For the deglycosylation experiments, proteins were extracted on ice for 30 min in a 50 mm citrate buffer (pH 5.5), 20 mm EDTA, 1% Triton X-100 supplemented with protease inhibitors. Soluble proteins were treated for 30 min at 30 C with 10 mU of endoglycosidase H (Roche). As negative control, the same soluble fraction was treated without enzyme under the same conditions. Treated proteins were denatured in Laemmli buffer, separated by SDS-PAGE, and detected with the anti-DUOX antibody as described above.
For the detection of TG in mouse thyroids by Western blotting, total protein extracts (3 μg/lane) were separated by 6% SDS-PAGE and transferred to polyvinylidene difluoride membrane (Millipore Corp., Bedford, MA). Total TG proteins were immunodetected with antibovine TG rabbit serum [1:12,000 (37)]; the iodinated TG forms were specifically revealed with a monoclonal antibody [B2, 1:5000 (38)].
To detect thyroidal 125I organification, glands were homogenized in lysis buffer [50 mm Tris-HCl (pH 8.0), 150 mm NaCl, 1 mm dithiothreitol, 1% Nonidet P-40 supplemented with protease inhibitors] and cleared by centrifugation (12,000 × g, 15 min, 4 C). Soluble protein extracts (50 μg/lane) were separated on 4% SDS-PAGE under reducing and nonreducing conditions. Gels were fixed in methanol-acetic acid and stained with colloidal Coomassie Blue (39). Vacuum-dried gels were exposed to autoradiography films.
Assay for H2O2 release from thyroid tissue
The thyroid glands were dissected, cut in small pieces, and rinsed extensively in Krebs-Ringer HEPES medium [KRH (40)]. About two thyroid lobes were used for each experimental condition. The tissues were incubated with or without 2 μm ionomycin (Sigma) + 5 μm phorbol 12-myristate 13-acetate (Sigma) during 3 h at 37 C in the KRH medium supplemented with 0.1 mg/ml horseradish peroxidase type II (Sigma) and 440 mm homovanillic acid (Sigma). The fluorescence intensity from the KRH medium was measured on a microplate reader (TECAN Infinite F200 PRO) with excitation at 315 nm and emission at 415 nm. H2O2 concentrations were estimated based on standard curves obtained with known concentrations of H2O2 run in the same experiment. All measurements were corrected for autofluorescence of the medium. The H2O2 production was normalized to the total DNA content of the incubated tissue and expressed in micromoles of H2O2 per μg of DNA.
Reverse transcription of mRNA and real-time, semiquantitative PCR
Total RNA was prepared using TRIzol reagent (Invitrogen, Carlsbad, CA). After deoxyribonuclease digestion, RNA was further cleaned up with the RNeasy Mini Kit (QIAGEN, Chatsworth, CA). RNA (0.5 μg) was reverse transcribed with Superscript II (Invitrogen). PCR amplifications were performed using a C1000 Thermal Cycler (Bio-Rad Laboratories, Inc., Hercules, CA) with SYBR Green dye (Molecular Probes, Inc., Eugene, OR) and Platinum Taq DNA polymerase (Invitrogen). Each reaction was performed in triplicate with the following conditions: 3 min at 95 C, 40 cycles of 9 sec at 95 C, and 1 min at 60 C, followed by 1 min at 55 C. Melting curve analysis was used to confirm amplification specificity. Gene expression was normalized to Polr2a mRNA. The sequences of primers are listed in Supplemental Table 1.
125I uptake and perchlorate discharge test
125I (8 μCi/mouse; ∼1.7 × 107 cpm) was given ip after feeding mice with LID (<0.05 ppm iodine; Harlan Teklad Co., Madison, WI) for 2 wk. Serum samples for thyroid function tests were obtained 24 h before the uptake experiments. In vivo kinetics of thyroidal 125I accumulation was recorded by counting over the neck using a γ positioning system (Navigator GPS, RMD Instruments Corp., Watertown, MA). To obtain the average uptake rate at 4 h, animals were killed and their thyroid glands dissected immediately. The amount of radioactivity in blood samples and excised thyrotracheal units was measured in a γ-scintillation counter (Cobra II Auto-Gamma, Packard Instruments, Meriden, CT) and expressed as fraction of the injected dose. For the perchlorate-induced iodide-discharge test, 10 μg/g body weight KClO4 was injected ip 4 h after 125I administration, and thyroid glands were obtained 20 min later. To monitor changes of 125I activity in the circulation, blood samples were obtained from the retro-orbital plexus with a heparinated micropipette before and 20 min after perchlorate administration. For calculation of the fraction of 125I dose in the circulation, total blood volume (in milliliters) was estimated as 7.2% of the body weight (in grams) (41).
Statistics
Unless indicated otherwise, statistical analysis was performed using unpaired, two-tailed Student's t test (Prism 5, GraphPad Software, Inc., San Diego, CA) and data presented as means ± sem. Differences were considered significant at P < 0.05.
Acknowledgments
We thank Linda Degenstein and Xiu Chen (Transgenic Core Facility, University of Chicago) for their expert technical assistance.
This work was supported by a Young Investigator Research Grant from the American Thyroid Association (to H.G.), Grants DK15070 and DK20595 from the National Institutes of Health (to S.R.), and the Fonds National de la Recherche Scientifique (to X.D.D.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CH
- Congenital hypothyroidism
- DUOX
- dual oxidase
- ER
- endoplasmic reticulum
- ES
- embryonic stem
- KRH
- Krebs-Ringer HEPES
- LID
- low-iodine diet
- NADPH
- reduced nicotinamide adenine dinucleotide phosphate
- TG
- thyroglobulin
- TPO
- thyroid peroxidase
- WT
- wild type.
References
- 1. Dupuy C, Ohayon R, Valent A, Noël-Hudson MS, Dème D, Virion A. 1999. Purification of a novel flavoprotein involved in the thyroid NADPH oxidase. Cloning of the porcine and human cDNAs. J Biol Chem 274:37265–37269 [DOI] [PubMed] [Google Scholar]
- 2. De Deken X, Wang D, Many MC, Costagliola S, Libert F, Vassart G, Dumont JE, Miot F. 2000. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. J Biol Chem 275:23227–23233 [DOI] [PubMed] [Google Scholar]
- 3. Rigutto S, Hoste C, Grasberger H, Milenkovic M, Communi D, Dumont JE, Corvilain B, Miot F, De Deken X. 2009. Activation of dual oxidases Duox1 and Duox2: differential regulation mediated by cAMP-dependent protein kinase and protein kinase c-dependent phosphorylation. J Biol Chem 284:6725–6734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Corvilain B, van Sande J, Laurent E, Dumont JE. 1991. The H2O2-generating system modulates protein iodination and the activity of the pentose phosphate pathway in dog thyroid. Endocrinology 128:779–785 [DOI] [PubMed] [Google Scholar]
- 5. Björkman U, Ekholm R. 1988. Accelerated exocytosis and H2O2 generation in isolated thyroid follicles enhance protein iodination. Endocrinology 122:488–494 [DOI] [PubMed] [Google Scholar]
- 6. Grasberger H, Refetoff S. 2006. Identification of the maturation factor for dual oxidase. Evolution of an eukaryotic operon equivalent. J Biol Chem 281:18269–18272 [DOI] [PubMed] [Google Scholar]
- 7. Fischer H, Gonzales LK, Kolla V, Schwarzer C, Miot F, Illek B, Ballard PL. 2007. Developmental regulation of Duox1 expression and function in human fetal lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 292:L1506–L1514 [DOI] [PubMed] [Google Scholar]
- 8. Luxen S, Belinsky SA, Knaus UG. 2008. Silencing of Duox NADPH oxidases by promoter hypermethylation in lung cancer. Cancer Res 68:1037–1045 [DOI] [PubMed] [Google Scholar]
- 9. Zamproni I, Grasberger H, Cortinovis F, Vigone MC, Chiumello G, Mora S, Onigata K, Fugazzola L, Refetoff S, Persani L, Weber G. 2008. Biallelic inactivation of the dual oxidase maturation factor 2 (Duoxa2) gene as a novel cause of congenital hypothyroidism. J Clin Endocrinol Metab 93:605–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Morand S, Ueyama T, Tsujibe S, Saito N, Korzeniowska A, Leto TL. 2009. Duox maturation factors form cell surface complexes with Duox affecting the specificity of reactive oxygen species generation. FASEB J 23:1205–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Grasberger H. 2010. Defects of thyroidal hydrogen peroxide generation in congenital hypothyroidism. Mol Cell Endocrinol 322:99–106 [DOI] [PubMed] [Google Scholar]
- 12. Johnson KR, Marden CC, Ward-Bailey P, Gagnon LH, Bronson RT, Donahue LR. 2007. Congenital hypothyroidism, dwarfism, and hearing impairment caused by a missense mutation in the mouse dual oxidase 2 gene, Duox2. Mol Endocrinol 21:1593–1602 [DOI] [PubMed] [Google Scholar]
- 13. Donkó A, Ruisanchez E, Orient A, Enyedi B, Kapui R, Péterfi Z, de Deken X, Benyó Z, Geiszt M. 2010. Urothelial cells produce hydrogen peroxide through the activation of Duox1. Free Radic Biol Med 49:2040–2048 [DOI] [PubMed] [Google Scholar]
- 14. Edens WA, Sharling L, Cheng G, Shapira R, Kinkade JM, Lee T, Edens HA, Tang X, Sullards C, Flaherty DB, Benian GM, Lambeth JD. 2001. Tyrosine cross-linking of extracellular matrix is catalyzed by Duox, a multidomain oxidase/peroxidase with homology to the phagocyte oxidase subunit gp91phox. J Cell Biol 154:879–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ha EM, Oh CT, Bae YS, Lee WJ. 2005. A direct role for dual oxidase in Drosophila gut immunity. Science 310:847–850 [DOI] [PubMed] [Google Scholar]
- 16. El Hassani RA, Benfares N, Caillou B, Talbot M, Sabourin JC, Belotte V, Morand S, Gnidehou S, Agnandji D, Ohayon R, Kaniewski J, Noël-Hudson MS, Bidart JM, Schlumberger M, Virion A, Dupuy C. 2005. Dual oxidase2 is expressed all along the digestive tract. Am J Physiol Gastrointest Liver Physiol 288:G933–G942 [DOI] [PubMed] [Google Scholar]
- 17. Geiszt M, Witta J, Baffi J, Lekstrom K, Leto TL. 2003. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. FASEB J 17:1502–1504 [DOI] [PubMed] [Google Scholar]
- 18. Chávez V, Mohri-Shiomi A, Garsin DA. 2009. Ce-duox1/bli-3 generates reactive oxygen species as a protective innate immune mechanism in Caenorhabditis elegans. Infect Immun 77:4983–4989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xie X, Hu J, Liu X, Qin H, Percival-Smith A, Rao Y, Li SS. 2010. Nip/duoxa is essential for drosophila embryonic development and regulates oxidative stress response. Int J Biol Sci 6:252–267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bunting M, Bernstein KE, Greer JM, Capecchi MR, Thomas KR. 1999. Targeting genes for self-excision in the germ line. Genes Dev 13:1524–1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Grasberger H, De Deken X, Miot F, Pohlenz J, Refetoff S. 2007. Missense mutations of dual oxidase 2 (Duox2) implicated in congenital hypothyroidism have impaired trafficking in cells reconstituted with Duox2 maturation factor. Mol Endocrinol 21:1408–1421 [DOI] [PubMed] [Google Scholar]
- 22. Luxen S, Noack D, Frausto M, Davanture S, Torbett BE, Knaus UG. 2009. Heterodimerization controls localization of Duox-Duoxa NADPH oxidases in airway cells. J Cell Sci 122:1238–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hoste C, Rigutto S, Van Vliet G, Miot F, De Deken X. 2010. Compound heterozygosity for a novel hemizygous missense mutation and a partial deletion affecting the catalytic core of the H2O2-generating enzyme Duox2 associated with transient congenital hypothyroidism. Hum Mutat 31:E1304–E1319 [DOI] [PubMed] [Google Scholar]
- 24. De Deken X, Wang D, Dumont JE, Miot F. 2002. Characterization of Thox proteins as components of the thyroid H2O2-generating system. Exp Cell Res 273:187–196 [DOI] [PubMed] [Google Scholar]
- 25. Morand S, Chaaraoui M, Kaniewski J, Dème D, Ohayon R, Noel-Hudson MS, Virion A, Dupuy C. 2003. Effect of iodide on nicotinamide adenine dinucleotide phosphate oxidase activity and Duox2 protein expression in isolated porcine thyroid follicles. Endocrinology 144:1241–1248 [DOI] [PubMed] [Google Scholar]
- 26. Van Heuverswyn B, Streydio C, Brocas H, Refetoff S, Dumont J, Vassart G. 1984. Thyrotropin controls transcription of the thyroglobulin gene. Proc Natl Acad Sci USA 81:5941–5945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grasberger H, Refetoff S. 2011. Genetic causes of congenital hypothyroidism due to dyshormonogenesis. Curr Opin Pediatr 23:421–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hulur I, Hermanns P, Nestoris C, Heger S, Refetoff S, Pohlenz J, Grasberger H. 2011. A single copy of the recently identified dual oxidase maturation factor (DUOXA) 1 gene produces only mild transient hypothyroidism in a patient with a novel biallelic DUOXA2 mutation and monoallelic DUOXA1 deletion. J Clin Endocrinol Metab 96:E841–E845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Harper RW, Xu C, Eiserich JP, Chen Y, Kao CY, Thai P, Setiadi H, Wu R. 2005. Differential regulation of dual NADPH oxidases/peroxidases, Duox1 and Duox2, by Th1 and Th2 cytokines in respiratory tract epithelium. FEBS Lett 579:4911–4917 [DOI] [PubMed] [Google Scholar]
- 30. Pohlenz J, Maqueem A, Cua K, Weiss RE, Van Sande J, Refetoff S. 1999. Improved radioimmunoassay for measurement of mouse thyrotropin in serum: strain differences in thyrotropin concentration and thyrotroph sensitivity to thyroid hormone. Thyroid 9:1265–1271 [DOI] [PubMed] [Google Scholar]
- 31. Niethammer P, Grabher C, Look AT, Mitchison TJ. 2009. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 459:996–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu X, Lu R, Xia Y, Sun J. 2010. Global analysis of the eukaryotic pathways and networks regulated by salmonella typhimurium in mouse intestinal infection in vivo. BMC Genomics 11:722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Athanasiadou S, Jones LA, Burgess ST, Kyriazakis I, Pemberton AD, Houdijk JG, Huntley JF. 2011. Genome-wide transcriptomic analysis of intestinal tissue to assess the impact of nutrition and a secondary nematode challenge in lactating rats. PLoS One 6:e20771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Csillag C, Nielsen OH, Vainer B, Olsen J, Dieckgraefe BK, Hendel J, Vind I, Dupuy C, Nielsen FC, Borup R. 2007. Expression of the genes dual oxidase 2, lipocalin 2 and regenerating islet-derived 1 α in Crohn's disease. Scand J Gastroenterol 42:454–463 [DOI] [PubMed] [Google Scholar]
- 35. Hamm CM, Reimers MA, McCullough CK, Gorbe EB, Lu J, Gu CC, Li E, Dieckgraefe BK, Gong Q, Stappenbeck TS, Stone CD, Dietz DW, Hunt SR. 2010. Nod2 status and human ileal gene expression. Inflamm Bowel Dis 16:1649–1657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Moskwa P, Lorentzen D, Excoffon KJ, Zabner J, McCray PB, Jr, Nauseef WM, Dupuy C, Bánfi B. 2007. A novel host defense system of airways is defective in cystic fibrosis. Am J Respir Crit Care Med 175:174–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Roger PP, Van Heuverswyn B, Lambert C, Reuse S, Vassart G, Dumont JE. 1985. Antagonistic effects of thyrotropin and epidermal growth factor on thyroglobulin mRNA level in cultured thyroid cells. Eur J Biochem 152:239–245 [DOI] [PubMed] [Google Scholar]
- 38. Den Hartog MT, De Boer M, Veenboer GJ, De Vijlder JJ. 1990. Generation and characterization of monoclonal antibodies directed against noniodinated and iodinated thyroglobulin, among which are antibodies against hormonogenic sites. Endocrinology 127:3160–3165 [DOI] [PubMed] [Google Scholar]
- 39. Encarnación S, Hernández M, Martinez-Batallar G, Contreras S, Vargas Mdel C, Mora J. 2005. Comparative proteomics using 2-d gel electrophoresis and mass spectrometry as tools to dissect stimulons and regulons in bacteria with sequenced or partially sequenced genomes. Biol Proced Online 7:117–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Milenkovic M, De Deken X, Jin L, De Felice M, Di Lauro R, Dumont JE, Corvilain B, Miot F. 2007. Duox expression and related H2O2 measurement in mouse thyroid: onset in embryonic development and regulation by TSH in adult. J Endocrinol 192:615–626 [DOI] [PubMed] [Google Scholar]
- 41. Diehl KH, Hull R, Morton D, Pfister R, Rabemampianina Y, Smith D, Vidal JM, van de Vorstenbosch C. 2001. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J Appl Toxicol 21:15–23 [DOI] [PubMed] [Google Scholar]
- 42. Gentile F, Palumbo G, Salvatore G. 1992. The origin of the electrophoretic doublet of thyroglobulin. Biochem Biophys Res Commun 186:1185–1191 [DOI] [PubMed] [Google Scholar]
- 43. Marinò M, Zheng G, Chiovato L, Pinchera A, Brown D, Andrews D, McCluskey RT. 2000. Role of megalin (gp330) in transcytosis of thyroglobulin by thyroid cells. A novel function in the control of thyroid hormone release. J Biol Chem 275:7125–7137 [DOI] [PubMed] [Google Scholar]