Abstract
Sik-similar protein (Sik-SP), a small nucleolar ribonucleoprotein, has been shown to be primarily involved in ribosome biogenesis. However, its role in the hormone-directed nuclear receptor signaling is largely unknown. Here, we provide novel evidence that Sik-SP is required for appropriate regulation of estrogen receptor (ER)α-mediated estradiol-17β (E2)-dependent uterine physiologic responses in mice. Studies by Western blotting using the newly developed antibodies for Sik-SP showed that this protein is up-regulated in both the ovariectomized wild-type and ERα null uteri by E2. Immunohistochemical analyses in uterine sections showed that this protein is induced in the epithelial and stromal cells. Coimmunoprecipitation studies revealed that E2 directs molecular interaction between Sik-SP and ERα. Furthermore, gel-mobility shift and chromatin immunoprecipitation analyses provided evidence that Sik-SP is recruited with ERα to estrogen-responsive uterine gene promoters. Overexpression of Sik-SP in vitro demonstrated a role for Sik-SP in cellular growth and viability. In a primary uterine epithelial-stromal coculture system, E2 exhibited early induction of Sik-SP in both the epithelial and stromal cells. Interestingly, suppression of Sik-SP in this coculture model, for the stromal but not epithelial cells, caused perturbation of E2-dependent proliferation in the epithelial cell layer. Similarly, in vivo uterine suppression of Sik-SP also caused inhibition of epithelial cell proliferation and aberrant prolongation of water imbibition in the late phase by E2. Finally, studies showed that Sik-SP is physiologically important during the onset of implantation by E2. In conclusion, Sik-SP, an early E2-responsive nucleolar protein, is necessary to induce E2-dependent ERα-mediated appropriate physiologic responses in the uterus.
Estrogens exert diverse biological actions in target organs, and many of them are mediated via their interactions with nuclear estrogen receptors (ER) (ERα and/or ERβ) (1). Classically, estrogenic action is thought to be mediated by interaction with ER; the ligand-bound ER undergoes dimerization before its binding to cis-acting estrogen response elements (ERE) in the promoter regions of responsive genes, such as lactoferrin (Ltf) and progesterone (P4) receptor (Pgr) in the mouse uterus (2, 3). The alternative, nonclassical pathway involves indirect modulation of transcription by interaction of the ER with components of other transcription complexes via protein-protein interactions. For example, ER can interact with c-fos and c-jun of the activator protein-1 (AP-1) transcription complex, up-regulating AP-1-responsive genes, such as uterine cyclin D1 (2). In addition, estrogen can signal through membrane receptors via use of ERK1/2 signaling. In fact, such a signaling downstream of G protein-coupled receptor 30 (GPR30) appears to be involved in the negative regulation of estradiol-17β (E2)-dependent uterine growth responses in mice (4).
Estrogen effects in the uterus are biphasic: the early phase (phase-I) responses that occur within 6 h are characterized by water imbibition and macromolecular uptake, whereas the late phase (phase-II) responses that occur by 18–30 h are reflected in DNA synthesis and uterine epithelial cell proliferation (5, 6). Although molecular cross talk between the early and late phases remains poorly understood, recent studies suggest that they are coordinately controlled via ER-dependent and ER-independent mechanisms (3, 7–11). In mice, it is well recognized that uterine estrogen signaling for late growth response is critically dependent on ERα (12). The manifestation of this signaling is believed to be the result of ERα-dependent gene transactivation functioning via interaction with nuclear transcription protein factors on the promoter of target genes. Furthermore, accumulating evidence based on physiologic, pharmacologic, and genetic studies suggests that E2 can elicit a variety of early signaling events that do not require classical signaling via ER in the uterus (3, 7–11, 13, 14). Our long-standing hypothesis is that estrogen-dependent early nonclassical responders participate in a concerted manner to ultimately control the ERα-mediated functions conducive for the induction of late uterine growth responses (2, 3, 10). In this regard, our previous studies have shown that the expression of Sik-similar protein (Sik-SP) is stimulated by E2 at the level of mRNA during the early phase without involving nuclear ER in the mouse uterus (8). However, the role of Sik-SP in uterine biology remains unknown.
Sik-SP (also known as nucleolar protein (Nop) 58/Nop5/nucleolar protein (Nol) 5 is a member of the conserved nop5 (Drosophila)/sik1/Nop56p (yeast) gene family that encodes components of small nucleolar ribonucleoproteins (snoRNP), which consist of a snoRNA and a complex of proteins. Sik-SP belongs to box C/D snoRNP, which is named for the short, conserved box C (UGAUGA) and box D (CUGA) sequences present in their RNA moiety (15). snoRNP mainly catalyze the cleavage and modification of rRNA during the ribosome biogenesis (16), nucleolar sizing (17), and snoRNA trafficking (18). In yeast, functional mutation studies of Nop56p/sik1 have been shown to cause reduced cell growth and proliferation (19, 20). Although snoRNP have been recognized mostly for the nucleolar functions, to our knowledge, there is no report that indicates that snoRNP molecules can also be recognized for nuclear receptor functioning in the control of gene transcriptional mechanisms and the physiologic responses under the direction of a steroid hormone.
The present study highlights a function of nucleolar protein Sik-SP that is tightly regulated by E2 in an ERα-independent manner but is still required for the control of ERα-mediated gene regulation. Using both the in vitro coculture and in vivo approaches, we show here in a comprehensive manner that E2-induced Sik-SP directly controls ERα-dependent uterine physiologic responses, whereas the suppression of Sik-SP produces attenuation of epithelial cells proliferation and aberrant prolongation of water imbibition during the late phase. These results suggest that Sik-SP is required for the maintenance of appropriate regulation of E2-dependent biphasic responses in the mouse uterus.
Results
Detection of Sik-SP in various adult mouse tissues, including the uterus
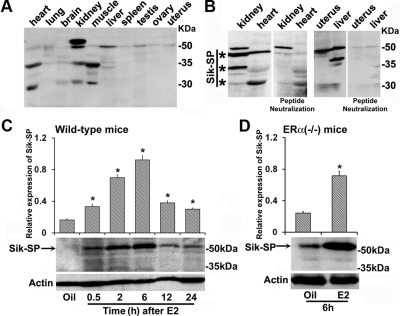
To analyze the expression of Sik-SP, we generated rabbit polyclonal antibodies against the mouse Sik-SP sequence available in the GenBank. Two separate peptides, either for the N- or C-terminal regions of Sik-SP, were designed as described in Materials and Methods. The affinity purified antibodies for the N terminus (Fig. 1A), as well as the C terminus (data not shown), were able to detect a main protein band primarily at approximately 54–58 kDa in all adult mice tissues, such as heart, lung, brain, kidney, muscle, liver, spleen, ovary, and uterus; three additional bands at approximately 65–68, 35–38, and 30–32 kDa were also detected in some tissues. However, the antibody neutralization experiments with excess antigenic peptides revealed that the bands at approximately 54–58, 35–38, or 30–32 kDa are primarily specific and exhibit a differential pattern of expression in the uterus, liver, kidney, and heart (Fig. 1B). The identity of other immunoreactive band (∼65–68 kDa) remains unclear.
Fig. 1.
Analysis of uterine Sik-SP and its regulation by E2. A, Analysis of Sik-SP in different adult mouse tissues (heart, lung, brain, kidney, muscle, liver, spleen, testis, ovary, and uterus) by Western blotting. B, Analysis of Sik-SP in tissues (kidney, heart, liver, and uterus) by the antibody neutralization experiment using the immunizing peptide. *, Bands represent actual detected by the antibody for Sik-SP. C, Temporal analysis of E2-dependent regulation of uterine Sik-SP in the wild type. Adult ovariectomized wild-type mice given a single injection (0.1 ml/mouse, sc) of E2 (100 ng/mouse) were killed at indicated times. Mice injected with sesame seed oil (0.1 ml/mouse, sc) were served as vehicle control and killed after 6 h for comparison. D, E2-dependent regulation of Sik-SP in the ERα null mice. Adult ovariectomized ERα null mice were given a single injection of E2 (100 ng/mouse) or oil (as vehicle control) (0.1 ml/mouse, sc) and killed after 6 h. Uterine tissue extracts were analyzed by Western blotting (C and D) using the antibodies for Sik-SP and actin. These experiments were repeated at least four times with independent samples, and a representative blot is presented. The quantitation of protein levels from the Western blottings was determined by densitometric scanning. Relative expression levels were calculated against actin levels. The error bars represent se. *, Values are statistically different compared with the vehicle control (P < 0.01, ANOVA followed by Newman-Keul's multiple range test).
Up-regulation of uterine Sik-SP by E2 without involving ERα
Previously, it was shown that E2 causes an early uterine up-regulation of Sik-SP mRNA in an ER-independent manner in the adult ovariectomized mice (8). We wanted to examine whether Sik-SP protein can also be detected in a manner similar to mRNA. Western blot analyses of Sik-SP in the uteri of ovariectomized wild-type mice, after an injection of E2 (100 ng/mouse) or oil (a vehicle control) at indicated times, are shown in Fig. 1C. Consistent with the previous report (8), our results showed that the expression of Sik-SP was sharply induced by E2 after 0.5 h, peaked at 6 h, and thereafter declined, although expression remained induced at 12 and 24 h. To further examine whether this E2-dependent expression of Sik-SP requires the presence of ERα, we also analyzed the expression in ovariectomized ERα null mice after an injection of E2 or oil by 6 h (Fig. 1D). Our results showed that E2 was indeed capable of inducing Sik-SP in the null uteri as compared with oil, suggesting that E2-dependent induction of Sik-SP is independent of ERα.
Molecular interaction of Sik-SP with ERα under the direction of E2
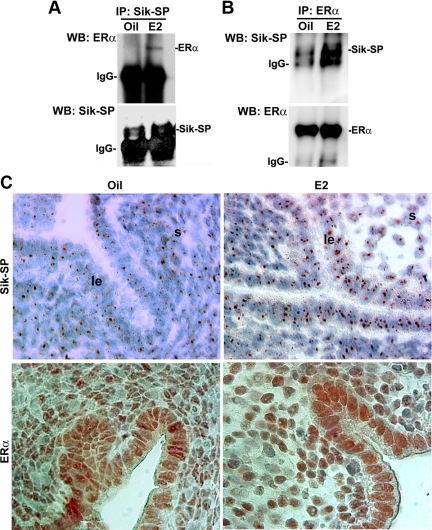
Our long-standing hypothesis is that the early estrogen-responsive ER-independent signaling molecules control ERα function to successfully mediate its late estrogen-responsive signaling in the uterus (2, 3, 10). In a similar notion, we wanted to examine whether E2-driven expression of uterine Sik-SP exhibits any molecular interaction with ERα during the early phase in ovariectomized wild-type mice after the treatments of oil (a control) or E2 after 6 h. Our immunoprecipitation analyses of uterine extracts, using the Sik-SP (Fig. 2A)- or ERα (Fig. 2B)-specific antibodies, were followed by Western blotting for Sik-SP and ERα and provided evidence that E2 was indeed capable of inducing molecular association between the Sik-SP and ERα, as compared with that of oil-treated samples. Furthermore, estrogen-responsive Pgr was not pulled down by Sik-SP antibody (data not shown), suggesting that the interaction between Sik-SP and ERα is specific. Immunoprecipitation using the preimmune serum did not detect any bands for Sik-SP or ERα by Western blotting (data not shown).
Fig. 2.
Analysis of E2-dependent molecular association between Sik-SP and ERα in the uterus. Adult ovariectomized wild-type mice given a single injection of E2 (100 ng/mouse) or oil (as vehicle control) (0.1 ml/mouse, sc) were killed after 6 h. Whole uterine tissue extracts were immunoprecipitated (IP) with Sik-SP (A)- or ERα (B)-specific antibodies followed by Western blotting (WB) for Sik-SP and ERα. In control experiments, immunoprecipitation using normal serum did not detect any specific bands by Western blotting (data not shown). These experiments were repeated at least three times with similar results. C, Immunohistochemical analyses of Sik-SP and ERα in the uterus by oil or E2. Ovariectomized wild-type mice were treated with oil or E2 (100 ng/mouse) and killed after 6 h. Representative pictures are shown at ×1000. le, Luminal epithelium; s, stroma. These experiments were repeated twice with three to four mice in each group, and similar results were obtained.
We further analyzed cell-specific expression of Sik-SP and ERα in the uterus after an administration of oil or E2 by 6 h. Consistent with previous literatures (21, 22), our immunohistochemical analyses in wild-type mice showed that E2 caused an increase in uterine accumulation of Sik-SP with punctate nucleolar staining in both the epithelial and subluminal stromal cells, as compared with that of oil-treated uterus (Fig. 2C, upper panels). Similar results were also seen in the ERα null mice (data not shown), again suggesting that E2-dependent regulation of Sik-SP does not require ERα. Furthermore, it should be noted that uterine cell-specific expression of Sik-SP is consistent with the in situ hybridization results (8). In respect to ERα expression, our results show that the nuclear accumulation of this protein is primarily distributed in both the above cell types after injections of oil or E2 (Fig. 2C, lower panels). Overall results suggest that Sik-SP molecularly interacts with ERα under the direction of E2 in the mouse uterus.
Coordinated recruitment of Sik-SP, in conjunction with ERα, to estrogen-responsive gene promoters by E2
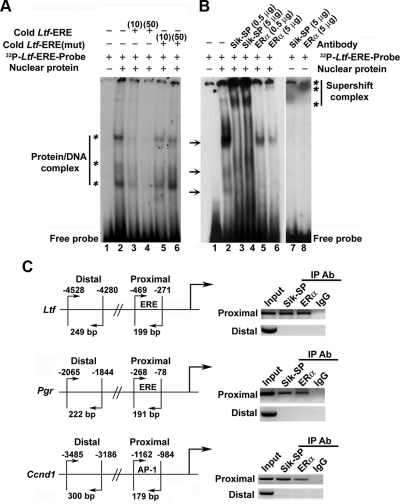
We next wanted to examined whether the above molecular complex can also be detected at the level of DNA interacting site using a defined ERE for the estrogen-responsive Ltf gene promoter, as described in Materials and Methods. As shown in Fig. 3A, E2-treated uterine nuclear extracts were incubated with 32P-labeled ERE in the absence (Fig. 3A, lane 2) or presence (lanes 3 and 4) of different concentrations of cold Ltf-ERE (10- or 50-fold molar excess). The specificity of the reaction was tested after incubation with the mutant sequence Ltf-ERE(mut) (10- or 50-fold molar excess) (Fig. 3A, lanes 5 and 6). Our analyses revealed that the bands (Fig. 3A, marked by asterisks on the left) were specifically detected with retarded mobility on the SDS-PAGE gel (Fig. 3A), suggesting that the above nuclear proteins indeed form complex with the ERE. We also analyzed the complex by gel supershift assay using antibodies specific to Sik-SP or ERα proteins. As shown in Fig. 3B, the addition of Sik-SP or ERα antibodies clearly exhibited the formation of supershift complexes, as judged by the detection of additional bands with more retarded mobility (Fig. 3B, lanes 3–6, marked by asterisks on the right) in conjunction with the complete or partial loss of three original bands (Fig. 3B, marked by arrows on the left). In case of ERα, the appearance of a comigrated major protein-DNA complex band and its gradual loss by increasing concentrations of the antibody strongly suggest that this band is one of the original three bands before its complete supershift. However, each antibody that was incubated separately with the labeled ERE, but without the addition of nuclear proteins, did not reveal such shifts (Fig. 3B, lanes 7 and 8), suggesting that antibodies do not possess any direct interactions with the probes. Overall, these results provide strong evidence to suggest that both ERα and Sik-SP can be recruited as a complex to ERα-responsive DNA consensus site by E2.
Fig. 3.
Analysis of E2-dependent recruitment of Sik-SP in conjunction with ERα to gene-specific promoter. A, EMSA for Sik-SP recruitment to ERE via ERα binding. Primer sequence designed from half consensus ERE of mouse Ltf gene was used: 5′-TGTCACAGGTCAAGGTAACCCACAAAT-3′. The mutant (mut) primer was designed after randomization of the above sequence. 32P-end-labeled probes were generated by kinase reaction using [γ32P]ATP and T4 polynucleotide kinase. The double stranded primers were prepared after heating to 90 C for 2–3 min, followed by slow cooling. Binding of E2-treated uterine nuclear proteins to ERE probes was achieved as described in Materials and Methods. For competition assays, cold or mut primers were added at 10- or 50-fold molar excess. Samples were loaded onto 5% nondenaturing polyacrylamide gel and run at 150 V for 2.5 h. The gel was dried and exposed to x-ray film (Kodak) at −80 C. Bands as shown by asterisks on the left indicate specific shifts due to formation of protein-DNA complexes. B, Supershift assays were performed by adding Sik-SP or ERα antibody at indicated concentrations to the above reaction. Supershift bands are indicated by asterisks on the right. Arrows denote the position of bands that are abolished after the addition of either type of antibodies, suggesting both Sik-SP and ERα recruit as a complex to the ERE. C, ChIP analysis of Sik-SP and ERα recruitment to E2-responsive Ltf, Pgr, and Ccnd1 genes. ChIP analysis was performed using Sik-SP or ERα antibody (Ab) or normal rabbit serum (as IgG control) as described by us (47). Primers designed for the proximal and distal regions were previously described by us (3). The presence of the promoter DNA before immunoprecipitation (IP) was confirmed by PCR (input).
We further examined whether this complex interaction can also be detected by chromatin immunoprecipitation (ChIP) studies at the promoter regions for estrogen-responsive Ltf, Pgr, and Ccnd1 genes in the mouse uterus. Previously, we have shown that these genes are regulated by ERα through specific interactions with ERE (Ltf and Pgr) or AP-1 (Ccnd1) sites at the level of promoters (2, 3). For PCR analyses, as illustrated in Fig. 3C, primers were designed for the ERα binding site (proximal) or a nonspecific region (distal), as described in Materials and Methods. Using both the ERα- and Sik-SP-specific antibodies, we were able to amplify the desired products only in the proximal region but not in the distal region (Fig. 3C). The presence of promoter DNA, before ChIP, was confirmed by PCR (input); additionally, the assays using preimmune serum (IgG) did not detect any amplified PCR products, suggesting that Sik-SP- or ERα-directed DNA recovery was promoter specific (Fig. 3C). Collectively, our results suggest that Sik-SP can be recruited with ERα to E2-responsive gene promoters.
Up-regulation of Sik-SP in the primary uterine coculture by E2
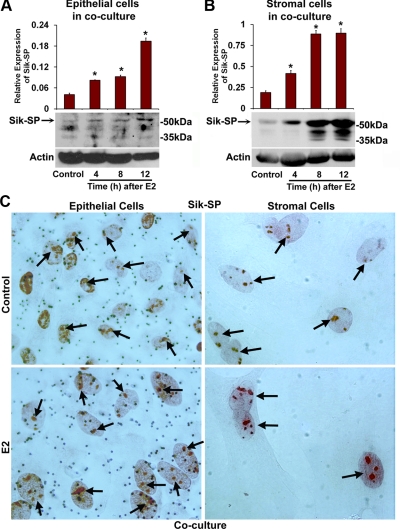
Recently, we have established a mouse primary uterine epithelial-stromal coculture system, in which the aspects of cell-specific proliferation mimic the in vivo situation in ovariectomized mice under the direction of ovarian hormones (23). Because E2 caused up-regulation of Sik-SP in the ovariectomized mouse uterus, we next wanted to examine whether the above coculture system also exhibited E2-dependent regulation of Sik-SP. Cells in the coculture were treated with E2 (10 nm) or vehicle control (0.01% ethanol) as described in Materials and Methods, and cellular extracts were analyzed at indicated times by Western blotting using antibodies specific to Sik-SP and actin (loading control). Consistent with the above in vivo results, E2 indeed caused up-regulation of Sik-SP by 4–12 h in both the epithelial (Fig. 4A) and stromal (Fig. 4B) cell layers, as compared with control. We also analyzed the localization of Sik-SP in both cell types by immunostaining (Fig. 4C). Our results show that the E2-dependent induction of Sik-SP was primarily detected as punctate nucleolar staining in both the cell layers (Fig. 4C). Overall, these results suggest that E2-dependent regulation of Sik-SP in the primary uterine coculture system mimics the in vivo situation.
Fig. 4.
Analysis of Sik-SP regulation by E2 in the primary uterine epithelial-stromal coculture system. Expression of Sik-SP in epithelial (A) and stromal (B) cell layers was analyzed after the culture treated with E2 (10 nm) or vehicle control (0.01% ethanol), as described in Materials and Methods. Cells treated with E2 were analyzed at indicated times, whereas the cells treated with the vehicle for 12 h were served as control for comparison. Cellular extracts were analyzed separately by Western blotting for the expression of Sik-SP and actin. These experiments were repeated at least three times with independent samples, and the representative blots are presented. The quantitation of protein levels from the Western blottings was determined by densitometric scanning. Relative protein levels were calculated against actin levels. The error bars represent se. *, Values are statistically different compared with the vehicle control (P < 0.01, ANOVA followed by Newman-Keul's multiple range test). C, Immunocytochemical analyses of Sik-SP. Primary epithelial-stromal cocultured cells were treated with vehicle (0.01% ethanol) or E2 (10 nm) for 12 h. Red nucleolar staining indicates the localization of Sik-SP (indicated by arrows). Representative pictures are shown at ×1000.
Expressional manipulation of Sik-SP affects cell viability and E2-dependent control of uterine epithelial cell proliferation in vitro
Analysis of cell viability in Cos-7 cells
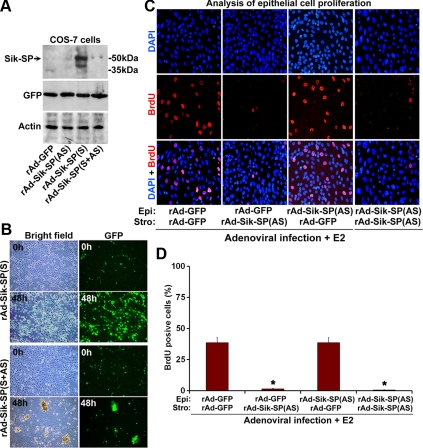
To examine the role of Sik-SP in cell biology, we applied adenovirus-driven gene targeting strategy to manipulate Sik-SP expression in vitro. We made recombinant adenoviruses carrying either the sense [rAd-Sik-SP(S)] or antisense [rAd-Sik-SP(AS)] cDNA for Sik-SP, in conjunction with a green fluorescence protein (GFP) reporter, under the control of two independent cytomegalovirus (CMV) promoters. The control virus (rAd-GFP) expresses only GFP under the direction of CMV. In our initial studies, we induced infection with these viruses in Cos-7 cells for 48 h. Cellular extracts were then prepared and analyzed by Western blotting using Sik-SP, GFP, and actin antibodies (Fig. 5A). Our results show that infection with rAd-Sik-SP(S) caused overexpression of Sik-SP, whereas the infections with either rAd-Sik-SP(AS) or rAd-GFP (control) did not reveal any such expression. Moreover, infection with mixed viruses rAd-Sik-SP(S+AS) completely eliminated rAd-Sik-SP(S)-driven expression (Fig. 5A), suggesting that rAd-Sik-SP(AS) was effective in suppression of Sik-SP overexpression directed by rAd-Sik-SP(S). Overall, these results suggest that adenoviral vector-mediated Sik-SP gene delivery is effective in manipulation of gene expression.
Fig. 5.
Functional analyses of Sik-SP on cellular viability and on E2-driven epithelial cell proliferation in a primary uterine coculture. A, Analysis of the effects mediated by Sik-SP adenoviruses in Cos-7 cells. Cos-7 cells were infected with adenoviruses (10 pfu) for GFP (as a control), Sik-SP(AS), Sik-SP(S), or mixture (1:1 ratio) of Sik-SP(S+AS) for a period of 48 h. Cell extracts were then collected for analyses of Sik-SP, GFP, and actin expression by Western blotting. B, Cell viability in Cos-7 cells after serum deprivation. Cos-7 cells were subjected to infection with GFP (data not shown), Sik-SP(AS) (data not shown), Sik-SP(S), or Sik-SP(S+AS) adenoviruses at 10 pfu level as indicated above. After 12 h after infection, cells were subjected to serum deprivation, as described in Materials and Methods, and continued the culture for an additional 48-h period. Cells at indicated times were visualized by fluorescence microscopy using bright field or GFP. Note, Sik-SP(S) virus, as opposed to Sik-SP(S+AS), control GFP (data not shown), or Sik-SP(AS) (data not shown), provides cellular protection, as judged by the maintenance of growth/viability even after serum deprivation. C, The role of Sik-SP in the E2-treated primary uterine coculture system. Epithelial (Epi) and stromal (Stro) cells were separately infected with rAd-GFP (control) and/or rAd-Sik-SP(AS) before the initiation of coculture and the treatment of E2 (10 nm), as described in Materials and Methods. Confocal analysis of epithelial cell proliferation was determined by the incorporation of BrdU (red). 4′,6-diamidino-2-phenylindole (DAPI) (blue) was used to stain all nuclei. Stromal cells did not reveal any proliferation under this condition. D, Quantitative analysis of cellular proliferation as shown in C. The data presented here are after the analysis of at least 1000 cells from each group. The error bars represent se. *, Values are statistically different against the control group (P < 0.01, ANOVA followed by Newman-Keul's multiple range test). These experiments were repeated at least three times with similar results.
Once the effectiveness of these constructs was established, we analyzed their influences on cellular viability under the condition of serum deprivation for 48 h (Fig. 5B). Our results show that infection with rAd-Sik-SP(S) was able to support cell viability or growth, as judged by the number of viable cells retained after 48 h, i.e. approximately 80% of the original count (0 h). In contrast, the infection with rAd-Sik-SP(S+AS) showed approximately 2% level of viability (Fig. 5B). In addition, infection with rAd-GFP or rAd-Sik-SP(AS) viruses alone also did not exhibit any protection for these cells (data not shown). Overall, these results suggest that the ectopic expression of Sik-SP provides a protective role to support cell survival/growth.
Analysis of E2-dependent regulation of epithelial cell proliferation in the uterine primary coculture system
Given the above finding of E2-dependent up-regulation of Sik-SP in both the epithelial and stromal compartments in the coculture system, we next wanted to determine the cell-specific contribution of Sik-SP for the progression of epithelial cell proliferation in the E2-stimulated coculture condition, as recently reported by us (23). As shown in Fig. 5, C and D, rAd-Sik-SP(AS) and/or rAd-GFP (as control) viruses were applied separately in different combinations to both the epithelial and stromal cells. Similar to the above, when the degree of virus infection was about 70–80%, as judged by GFP expression, our analysis of expression by Western blotting showed that rAd-Sik-SP(AS), as opposed to rAd-GFP, was indeed able to suppress E2-stimulated Sik-SP in both the epithelial and stromal cells (data not shown). Given this effective manipulation of Sik-SP, E2-dependent epithelial cell proliferation status was evaluated by bromodeoxyuridine (BrdU) incorporation. Our results showed that E2 was able to induce epithelial cell proliferation in the presence of GFP viruses only (Fig. 5, C and D). However, the suppression of Sik-SP in either the stromal cell layer alone or in both cell layers was inhibitory to E2-dependent epithelial cell proliferation (Fig. 5, C and D). In contrast, suppression only in the epithelial cell layer did not reveal any inhibition of proliferation under these conditions (Fig. 5, C and D). Overall, the results strongly suggest that stromal-derived Sik-SP essentially controls E2-dependent epithelial cell proliferation.
Aberration of E2-dependent uterine biphasic responses in the ovariectomized mice after suppression of Sik-SP
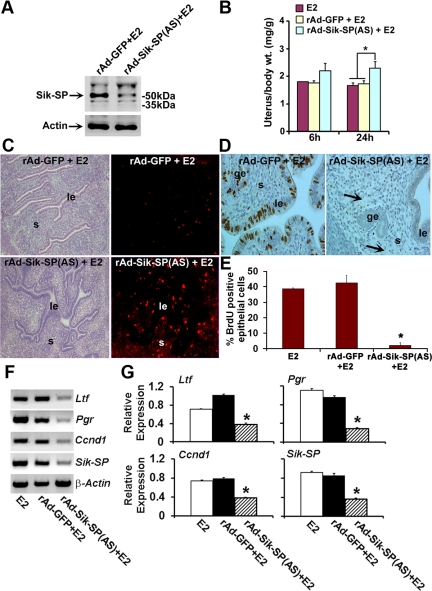
Because uterine Sik-SP was induced early and recruited with ERα for gene regulation, and because suppression of Sik-SP caused elimination of E2-dependent epithelial cell proliferation in vitro, we next wanted to examine whether in vivo suppression of Sik-SP elicits aberration of E2-regulated uterine biphasic responses in mice. rAd-Sik-SP(AS) or rAd carrying the empty vector (rAd-GFP) (as control) were applied to the ovariectomized uterus, as previously described by us (2–4, 10). Our analysis revealed that rAd-Sik-SP(AS), as opposed to the control (rAd-GFP), was indeed capable of suppression of E2-induced uterine Sik-SP (Fig. 6A). However, the clear demonstration for cell-specific suppression of Sik-SP was remained challenging. To circumvent this problem, we analyzed the expression of virus-driven GFP, because this was used as a secondary reporter in the rAd-Sip-SP(AS) virus. Our analysis of uterine GFP by direct visualization under the fluorescence microscopy revealed that the expression is primarily localized in the subluminal stroma and in the apical location of epithelial cell but not in the open lumen space (as judged by the analysis of uterine sections without injection of viruses), indicating that both uterine epithelial and stromal cells were indeed targeted to suppress the Sik-SP, after administration of rAd-Sik-SP(AS) viruses (see Supplemental Fig.1, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). Our analysis of E2-induced uterine wet weight response occurring at 6 h in mice administered with rAd-Sik-SP(AS) was comparable with those receiving the control virus (Fig. 6B), thus suggesting that the early response is not dependent on Sik-SP. However, during the late phase of E2 action (at 24 h), mice injected with the rAd-Sik-SP(AS) virus demonstrated a significant increase in uterine wet weight response as compared with those treated with control virus (Fig. 6B). Further analysis of these results revealed that administration of rAd-Sik-SP(AS) virus in conjunction with E2 caused an aberrant increase in uterine water imbibition at 24 h, whereas the control virus did not show such response (data not shown). These results are further consistent with the finding that E2-dependent uterine uptake of BSA was aberrantly induced in the endometrial stoma during the late phase after injection of rAd-Sik-SP(AS) (Fig. 6C); the control virus was unable to show such response (Fig. 6C). Taken together, these results suggest that E2-induced early responses, at least in respect to water imbibition and macromolecular uptake, are aberrantly prolonged by uterine suppression of Sik-SP. Moreover, the suppression of Sik-SP also caused elimination of E2-stimulated uterine epithelial cell proliferation, as judged by the analysis of BrdU incorporation (Fig. 6, D and E). In addition, to determine the impact of in vivo inhibition of Sik-SP on endogenous ERα activity, we examined the expression of E2-induced ERα-dependent uterine genes Ltf, Pgr, and Ccnd1. Our results show that rAd-Sik-SP(AS)-mediated perturbation of uterine Sik-SP indeed caused dramatic attenuation of expression of the above genes (Fig. 6, F and G). Overall, these findings provide strong evidence that Sik-SP is critical to maintaining appropriate regulation of uterine biphasic responses induced by E2.
Fig. 6.
Functional analyses of Sik-SP on uterine biphasic responses in ovariectomized wild-type mice after administration of E2. Ovariectomized mice were administrated with intraluminal injections of adenoviruses rAd-GFP (control) or rAd-Sik-SP(AS), and uterine tissues were collected after injections of E2 (100 ng/mouse) at indicated time points. A, Western blot analysis of uterus after adenovirus infection followed by E2 for 6 h. B, Analysis of uterine wet weights as examined at 6 and 24 h after E2 injection. *, This was statistically different (P < 0.05) as compared with corresponding groups. C, Analysis of uterine macromolecular uptake after E2 by 24 h. The fluorescence for the uptake of rhodamine-BSA in uterine tissue sections was visualized by direct fluorescence (right panels) and phase-contrast (left panels) microscope. le, Luminal epithelium; s, stroma. D, Analysis of uterine epithelial cell proliferation (at 24-h time point after E2) by immunostaining with BrdU. Reddish-brown nuclear deposits indicate the sites of positive immunostaining. ge, Glandular epithelium. Note, induction of uterine edema within the stromal compartment (shown by arrow) is visible in the rAd-Sik-SP(AS)+E2 group. E, Quantitation of BrdU-positive cells. Data presented here after the analysis of at least five different mice from each group. *, This was statistically different (P < 0.01) as compared with corresponding control groups. F, RT-PCR analyses of Ltf, Pgr, Ccnd1, Sik-SP, and β-actin uterine genes after the virus-mediated manipulation in mice. Uterine tissues were collected after administration of adenoviruses rAd-GFP (control) and rAd-Sik-SP(AS) in mice as described in Materials and Methods, followed by injection of E2 (100 ng/mouse) for 24 h. E2-treated uterine sample (24 h) without any virus injection was used as a reference. β-Actin was used as a constitutive gene. G, Quantitation of relative expression as shown in F. *, This was statistically different (P < 0.01) as compared with corresponding control groups.
Expression of Sik-SP during the development of the periimplantation uteri and under the control of E2 in the delayed implantation model
The deletion of Sik-SP in mice has not yet been reported. Thus, the role of Sik-SP in respect of fertility in mice remains unknown. However, in a previous report (24), we have shown that the expression of Sik-SP is up-regulated at the site of implantation in mice, based on a comparison of the implantation and interimplantation sites by microarray studies. Further, in situ hybridization analysis showed that Sik-SP is specifically up-regulated in the stroma surrounding the implanting blastocyst on d 4 (2400 h) of pregnancy (24). However, the periimplantation uterine expression of Sik-SP remains unknown, thus the following study shows the analysis along this line by in situ hybridization (Fig. 7). Sik-SP was primarily expressed in uterine epithelial cells on d 1, and this pattern of expression was apparently declined on d 2 (Fig. 7A). However, on d 3, the expression was distinctly localized in the stroma but still retained a low level of expression in the epithelial cells. In contrast, in the receptive uterus on d 4 morning, the expression was intensely localized in the subluminal stroma, as well as low level in the epithelial cells (Fig. 7A). As reported before (24), after the embryonic attachment, the expression was primarily distributed in the subluminal stroma at the site of implantation on d 5, and this stromal expression specifically induced during decidualization throughout the decidual bed but without exhibiting expression in the primary decidual zone, on d 6, and thereafter, the expression was similarly maintained with reduced intensity on d 7 and 8 (Fig. 7A).
Fig. 7.
Spatiotemporal expression of uterine Sik-SP during the periimplantation period and in the delayed and activated model. A, In situ analysis of Sik-SP. Representative pictures are shown at ×40 (d 1–6), and ×20 (d 7 and 8). B, Expression of Sik-SP in the delayed and activated uteri. le, Luminal epithelium; s, stroma; e, implanting embryo; pdz, primary decidual zone; sdz, secondary decidual zone; M, mesometrial pole; AM, antimesometrial pole.
Because our study showed that E2 critically controls uterine expression of Sik-SP, we further wanted to examine whether the termination of the delayed implantation by administration of E2 can enhance the uterine expression of Sik-SP with the onset of implantation. Indeed, our results showed that Sik-SP was specifically up-regulated in the subluminal stromal cells at the site of implanting embryo after the activation with E2, whereas the delayed uterus did not reveal any specific uterine expression at the site of embryo (Fig. 7B). Overall, these results suggest that Sik-SP plays a vital role in normal uterine implantation under the direction of E2.
Discussion
It has been well established that estrogen regulates diverse but interdependent signaling mechanisms with or without involving ER. We have previously demonstrated by mRNA differential display studies that Sik-SP is up-regulated early as one of the uterine genes that occur in an ER-independent manner by E2 in mice. Here, using both the in vivo and in vitro primary uterine coculture model systems, we provide evidence that Sik-SP plays a critical role via ERα in maintaining the appropriate regulation of E2-dependent uterine biphasic responses in mice. More specifically, E2-responsive early nucleolar Sik-SP exhibits molecular association with ERα in the uterus. This interactive complex also results in recruitment at the level of E2-regulated gene promoters via ERα- interacting DNA consensus sites. Further studies have revealed that down-regulation of Sik-SP is strongly correlated with both the loss of estrogen-dependent late growth responses and the abnormally prolonged early estrogenic responses into the late phase. This suggests that Sik-SP is critical to coordinately control phase-I and phase-II uterine responses as induced by E2 in mice.
Although tagged-epitope-based localization experiments have strongly suggested that Sik-SP is a nucleolar protein (21, 22), and that this and other members of the protein family play a major role in ribosome biogenesis and the control of nucleolar size (17), identifying the role of Sik-SP in uterine estrogen signaling and its interaction with ERα is clearly novel. Our analysis of Sik-SP localization in mouse uterine tissue (Fig. 2C) or the cocultured cells (Fig. 4C), using the newly developed antibodies, provides consistent nucleolar staining results as previously observed (21, 22). Moreover, this staining pattern is consistent with findings from another analysis using nucleolar protein marker Nol1 (Supplemental Fig. 2).
It has been suggested that estrogenic regulation of various early uterine genes, including Sik-SP, is ER independent (8). Consistently, our present study also provides evidence to suggest that Sik-SP is regulated by E2 without involvement from ERα. The mechanism of this regulation by E2 remains unknown. However, it is interesting to note that our recent analysis of various ER-independent uterine genes, after the application of GPR30-selective agonists in wild-type ovariectomized mice, appeared to indicate that GPR30 is probably not involved in this regulation. Whether other orphan or not yet identified receptors play roles in these responses remains an open question. Nonetheless, our accumulating evidence for the ER-independent genes and their cooperative roles with ERα are intriguing and likely to stimulate further research in identifying the signaling mechanism for these responses.
Consistent to our previous observations for gene specific mRNA (8), uterine Sik-SP protein is indeed induced early by E2 without involving ERα (Fig. 1, C and D). However, it is quite interesting to note that Sik-SP is also involved in the regulation of ERα-mediated uterine functions that result from direct interaction with ERα (Figs. 2, A and B, and 3). Furthermore, abrogation of Sik-SP functions both in vivo and in vitro in the primary coculture system, in conjunction with elimination of uterine epithelial cell proliferation under the direction of E2, is indeed supportive of a link between Sik-SP and ERα-mediated actions in the uterus (Figs. 5, C and D, and 6). In this regard, it is worth to mention that our previous study (23) has already examined the status of ERα in the coculture system by the Western blotting and immunofluorescence studies. The results revealed that ERα was present with or without E2, although the level of ERα was partially declined in both the epithelial and stromal cell compartments by E2. Overall, these observations are, in general, very much analogous with our previous findings in respect to immunoglobulin heavy chain-binding protein (Bip) (3). Bip, a member of the heat shock protein 70 family of chaperones involved in the proper folding of numerous proteins in the endoplasmic reticulum, also interacts with ERα and mediates E2-dependent regulation of uterine proliferative aspects in mice (3, 23). Similar to Bip (3, 25), Sik-SP is also likely to be involved in the regulation of cellular protection against apoptosis, as revealed by the adenovirus-driven overexpression of Sik-SP studies in Cos-7 cells (Fig. 5, A and B). However, the relationship between Bip and Sik-SP in controlling ERα-dependent estrogen signaling remains unknown. It is worth mentioning that cell fractionation and subsequent protein detection by Western blotting appeared to show that Sik-SP, Bip, and ERα are localized on the endoplasmic reticulum in the presence of E2 (Ray, S., and S. K. Das, unpublished observations), suggesting a possible common link between Sik-SP, Bip, and ERα in uterine estrogen signaling. In this regard, it is also important to mention that the translocation of ERα to the endoplasmic reticulum has been previously reported (26, 27). Consistent with the above growth regulation, Sik-SP, under the direction of E2, is directly recruited together with ERα to the growth regulatory genes cyclin D1 and Pgr via ERα interactive sites on the promoter (Fig. 3C). These results are strongly supported by in vitro DNA binding analyses of Sik-SP and ERα in the EMSA studies (Fig. 3, A and B). Although our studies clearly document that E2-regulated early elevated Sik-SP in the uterus is required for the regulation of ERα-mediated late signaling, whether the participation of any other E2-driven early emergent signaling involves in the regulation of Sik-SP's functionality before the interaction with ERα remains unknown.
Previously, tissue recombination studies using the ERα knockout and wild-type uteri from mice showed that E2-induced epithelial cell DNA synthesis is critically mediated by stromal ERα (28, 29). Furthermore, it has been well documented that the complex coregulatory proteins also play major roles in ERα-regulated control of uterine cell proliferation and gene regulation (2, 3, 23). Consistent to this notion, the epithelial-stromal coculture studies found that suppression of Sik-SP in the stromal cells either individually or jointly in both compartments caused a dramatic abrogation of E2-induced epithelial cell proliferation (Fig. 5, C and D). A similar situation appeared to be induced in utero via stromal Sik-SP, because adenoviral vector-driven expression of GFP was indeed exhibited in the epithelial and stromal compartment (Supplemental Fig. 1), as previously reported in other studies (3, 4). Given the fact that Sik-SP is regulatory for ERα, it is thus possible that the above stromal Sik-SP plays an essential role in E2-induced cell proliferation, presumably through ERα. In this regard, the expression of ERα in both the stromal and epithelial compartments has been detected in the ovariectomized uteri or the coculture model by E2 (23, 30). Furthermore, it has recently been observed that the stromal activation of ERK1/2 phosphorylation acts as an upstream regulator for E2-induced epithelial cell proliferation, predominantly through the activation of stromal ERα phosphorylation signal (4). Thus, it is possible that the ERK1/2 activation may be an upstream signal for the association of Sik-SP and ERα in the stromal compartment. Clearly, the identification of paracrine signaling mediators originated from the stroma downstream of Sik-SP will be critically important to determine the E2-dependent regulation of epithelial cell proliferation, and this will be the subject of our future study. We have previously showed that E2 regulates wingless (Wnt) 4 and 5a, and their downstream effectors lymphoid enhancer binding factor 1 (Lef1) and transcription factor 3 (Tcf3) as early signaling mediators again without involving ER in the uterine epithelium and subluminal stroma (2, 10). However, whether Sik-SP plays a role in the control of Wnt signaling ligands or their effectors in uterine endometrial cells remains an interesting possibility for future study.
E2-regulated uterine biphasic responses have been well recognized, although the link between the early and late responses remains unknown. It has also been well documented that E2 elicits early biologic responses in the mouse uterus with or without involving ERα or ERβ, whereas the late growth responses are dependent on the participation of ERα (7–11). Because ERβ in the uterus is extremely low and is not involved in influencing uterine biology (30, 31), our present study primarily focused on ERα-mediated function in the uterus. Our in vivo observation of unopposed increase in vascular permeability and water imbibition in the absence of true uterine growth and gene expression during the late phase after adenovirus-driven suppression of Sik-SP (Fig. 6) suggests that Sik-SP-mediated ERα activity is critical to coordinating the phase-I and phase-II responses in the uterus under the direction of E2.
Because uterine Sik-SP is primarily detected in the nucleolus, the molecular association between Sik-SP and ERα under the direction of E2 is likely to be mediated at the nucleolar region. The nucleolus is a large intranuclear complex area that typically has the most concentrated mass in the cell. In addition to ribosome biogenesis, studies have implicated that the nucleolar sites can also be involved in other aspects of eukaryotic cell biology, such as gene transcriptional regulation, cell cycle progression, and senescence (32–35). Therefore, it is possible that the nucleolar Sik-SP may be involved not only in the regulation of ERα-mediated gene expression but could also be involved in targeting the ERα to the nucleolar location to perform gene regulatory function. In this regard, it is worth mentioning that several recent reports have shown that estrogen enhances nuclear redistribution of ERα, fused with yellow fluorescent protein or GFP in living cells (36–39). However, the molecular mechanism for this regulation remains unknown. Overall, our study provides evidence to suggest that Sik-SP, probably at the site of nucleolar location, acts as a novel coactivator for ERα, thus expanding the repertoire of ERα coactivators in uterine function (2, 3) and suggesting the complexity of ERα-mediated transcription, similar to that described for Pgr coregulators and Pgr-regulated transactivation in reproductive tissues (40).
It is well documented that the early embryo implantation can be manipulated by the removal of ovary in the early morning on d 4, the day of uterine receptivity. The daily supplementation of P4 can delay the implantation, but a single injection of E2 can terminate the delay process with the initiation of embryo implantation (6, 41). In our analysis of Sik-SP expression using this model system, as well as the periimplantation uterus, we provide some in vivo evidence to support for a physiological role for Sik-SP in the uterus during the onset of implantation, particularly during the modulation of implantation with E2 (Fig. 7).
In conclusion, this study provides evidence that the nucleolar protein Sik-SP-ERα-mediated signaling axis plays a critical role to coordinate the biphasic responses in the uterus for its appropriate growth under the direction of E2. This remarkable finding of ERα-independent early Sik-SP contributing to ERα-regulated events in the mouse uterus adds new insights to our understanding of uterine biology.
Materials and Methods
Animals
Wild-type and ERα(−/−) mice of the same genetic background (C57BL/6J/129/J) were produced by crossing heterozygous females and males (12). All mice were housed in the animal care facility at Cincinnati Children's Hospital Medical Center according to the National Institutes of Health and institutional guidelines for the use of laboratory animals. All protocols for the present study were reviewed and approved by the Institutional Animal Care and Use Committee (approval no. 1D05043). In general, 8- to 10-wk-old adult mice were ovariectomized and rested for 10 d before they received any injections. Mice were given a single injection (0.1 ml/mouse, sc) of E2 (100 ng/mouse) dissolved in sesame seed oil and then killed at indicated times as referred in the text. The mice injected with oil (as vehicle control, 0.1 ml/mouse, sc) were killed at 6 h for comparison.
Induction of pregnancy and delayed implantation model
Adult CD-1 females (Charles River Laboratories, Raleigh, NC) were mated with fertile males of the same strain to induce pregnancy (d 1, vaginal plug). Mice on d 1–8 were killed between 0830 and 0930 h. Implantation sites on d 5 through 6 were visualized by iv injections (0.1 ml/mouse) of a Chicago Blue B dye solution (1% in saline) and killing mice 5 min later. To induce and maintain delayed implantation, mice were ovariectomized in the morning (0800–0900 h) of d 4 and received daily injections of P4 (2 mg/mouse) from d 5 to 7, as described by us (42). To terminate delayed implantation and to induce blastocyst activation, the P4-primed delayed pregnant mice were given an injection of E2 (25 ng/mouse) on the third day of the delay (d 7). Mice were killed at 24 h after E2 injection.
Generation of rabbit antimouse Sik-SP polyclonal antibodies
Mouse-specific peptide sequences corresponding to the N-terminal [MEGKINKQLKKVLK (amino acids 64–77)] or C-terminal [GIENRAKLEARLRIL (amino acids 384–398)] regions of Sik-SP were synthesized and immunized in New Zealand white rabbits through the help of commercial resources for generation of anti-Sik-SP polyclonal antibodies (Invitrogen, Carlsbad, CA). Peptide-affinity purified antibody was obtained through affi-Gel purification columns (1.5 ml) (Bio-Rad, Hercules, CA) according to the commercial protocol.
Western blotting and immunoprecipitation analyses
These procedures were previously described by us (43). Antibody neutralization was carried out by previous incubation of the primary antibody using neutralizing peptides (200-fold molar excess).
Immunohistochemical staining
This was followed as previously described by us (43).
Gel mobility shift assay
Uterine nuclear extracts were prepared as previously described (44). The gel mobility shift assays were followed with a minor modification as previously described (45). In brief, double stranded oligos derived from the promoter of mouse Ltf ERE (5′-TGTCACAGGTCAAGGTAACCCACAAAT-3′) (46) were 5′-end-labeled with [32P]-ATP using T4 polynucleotide kinase. The cold double stranded mouse Ltf ERE or the mutant Ltf ERE (5′-AACAACGAGTCATGACCATGGTTAACC-3′) was used as competitors (10- or 50-fold molar excess). The binding reaction was achieved in a 20-μl mixture containing: 5× buffer [20% (vol/vol) glycerol, 5 mm MgCl2, 2.5 mm EDTA, 2.5 mm dithiothreitol, 50 mm NaCl, and 50 mm Tris-HCl (pH 7.5)], 5–10 μg of nuclear protein, 4 μg of poly dI-dC, and 60,000 cpm of the labeled probe. Reactions were incubated for 1 h at 4 C. For competition assays, cold Ltf ERE or mutant ERE were added at 10- to 50-fold molar excess. Supershift assays were performed by addition of anti-Sik-SP (0.5 or 5.0 μg) or anti-ERα (0.5 or 5.0 μg) antibodies to the mixture. Samples were separated by 5% nondenaturing polyacrylamide gel using a running buffer (0.5× Tris-borate-EDTA) at 150 V for 2.5 h. The gel was dried and exposed to x-ray film (Kodak, New York, NY) at −80 C.
ChIP assay
The primers used for PCR and this protocol were essentially the same as previously described (3, 47).
Primary coculture of uterine epithelial and stromal cells
This was essentially the same as previously described by us (23). In brief, uterine epithelial and stromal cells were isolated by enzymatic digestion of uterine tissues on d 4 of pseudopregnancy (48). Epithelial sheets were grown on Matrigel-coated cell culture inserts and the stromal cells were grown on cover slips in six-well plates. The cells were depleted in phenol red-free DMEM/F12 media supplemented with charcoal-stripped 1% FBS for overnight before the initiation of treatments with E2 (10 nm) or vehicle (0.01% ethanol). Both cell layers were analyzed separately at indicated times.
Analysis of estrogenic phase-I and phase-II responses in the uterus
To examine the effects of E2 on uterine biphasic responses in mice, uterine wet weights were initially recorded. In addition, the early effect was determined by macromolecular uptake after iv injection of rhodamine-tagged BSA, 15 min before killing. Frozen sections were directly analyzed by fluorescence microscopy (3). The late effect was analyzed by epithelial cell proliferation using the BrdU incorporation into DNA (3).
Recombinant adenoviral plasmids and generation of viral particles
The full-length coding region of mouse Sik-SP cDNA was generated by RT-PCR using mouse uterine total RNA on d 5 of pregnancy. Primers carrying the linkers for XhoI at 5′-ends were used for RT-PCR as follows: 5′-GGCTCGAGATGTTGGTCCTGTTTGAAAC-3′ (sense) and 5′-GGCTCGAG GTCTTCAGCATCTTTCTTCT-3′ (antisense). Cloning of the amplified DNA in a shuttle vector pAd-track CMV, and followed by recombination with pAdEasy-1 in Escherichia coli BJ5183, was as described (3). Viral packaging was carried out in 293 cells, and the viral particles were purified through CsCl density gradient centrifugation and stored at −70 C (3).
Adenoviral infection in Cos-7 cells
Cos-7 cells (at 70–80% confluence) grown in DMEM plus 10% FBS were subjected to infection with adenoviruses at 10 plaque-forming unit (pfu) for a period of 48 h. For testing cellular viability, 12-h postinfected cells were subjected to serum deprivation by transferring cells in DMEM that did not contain FBS and continued the culture for an additional 48-h period. Viable cells were counted by trypan blue exclusion assay.
Adenoviral infection in primary uterine epithelial-stromal coculture
This procedure was the same as described (23). Cells at 70–80% confluence were subjected to adenoviral infection at 10 multiplication of infection separately before initiation of coculture.
In vivo delivery of adenoviruses
Virus particles (20-μl solution in saline containing 1 × 1011 virus particles per horn) were inoculated directly into the uterine lumen of both horns from the oviductal end just before ovariectomy, as described by us (2, 3). They were given rest for 10 d before injections of E2 or oil for indicated times. Uterine tissues were appropriately collected for subsequent analysis.
Reverse transcription-polymerase chain reaction
Probes and in situ hybridization
Frozen sections (10 μm) were hybridized with 35S-labeled cRNA probes as described previously (42). Sections hybridized with sense probes served as negative controls and showed no positive signals.
Acknowledgments
We thank Serenity Curtis for editing the manuscript and Bert Vogelstein (Johns Hopkins University, Baltimore, MD) for providing reagents to generate recombinant adenoviral clones.
This work was supported in part by National Institutes of Health Grants R01 ES07814 and R01 HD56044.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AP-1
- Activator protein-1
- Bip
- immunoglobulin heavy chain-binding protein
- BrdU
- bromodeoxyuridine
- ChIP
- chromatin immunoprecipitation
- CMV
- cytomegalovirus
- E2
- estradiol-17β
- ER
- estrogen receptor
- ERE
- estrogen response element
- GFP
- green fluorescence protein
- GPR30
- G protein-coupled receptor 30
- Ltf
- lactoferrin
- Nop
- nucleolar protein
- P4
- progesterone
- pfu
- plaque-forming unit
- Pgr
- P4 receptor
- rAd-Sik-SP(AS)
- recombinant adenovirus carrying the antisense cDNA for Sik-SP
- rAd-Sik-SP(S)
- recombinant adenovirus carrying the sense cDNA for Sik-SP
- Sik-SP
- Sik-similar protein
- snoRNP
- small nucleolar ribonucleoprotein
- Wnt
- wingless.
References
- 1. Couse JF, Korach KS. 1999. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev 20:358–417 [DOI] [PubMed] [Google Scholar]
- 2. Ray S, Xu F, Wang H, Das SK. 2008. Cooperative control via lymphoid enhancer factor 1/T cell factor 3 and estrogen receptor-α for uterine gene regulation by estrogen. Mol Endocrinol 22:1125–1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ray S, Hou X, Zhou HE, Wang H, Das SK. 2006. Bip is a molecular link between the phase I and phase II estrogenic responses in uterus. Mol Endocrinol 20:1825–1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gao F, Ma X, Ostmann AB, Das SK. 2011. GPR30 activation opposes estrogen-dependent uterine growth via inhibition of stromal ERK1/2 and estrogen receptor α (ERα) phosphorylation signals. Endocrinology 152:1434–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Huet-Hudson YM, Dey SK. 1990. Differential effects of ovarian steroids and triphenylethylene compounds on macromolecular uptake and thymidine incorporation in the mouse uterus. J Steroid Biochem 35:23–27 [DOI] [PubMed] [Google Scholar]
- 6. Huet YM, Dey SK. 1987. Role of early and late oestrogenic effects on implantation in the mouse. J Reprod Fertil 81:453–458 [DOI] [PubMed] [Google Scholar]
- 7. Das SK, Taylor JA, Korach KS, Paria BC, Dey SK, Lubahn DB. 1997. Estrogenic responses in estrogen receptor-α deficient mice reveal a distinct estrogen signaling pathway. Proc Natl Acad Sci USA 94:12786–12791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Das SK, Tan J, Raja S, Halder J, Paria BC, Dey SK. 2000. Estrogen targets genes involved in protein processing, calcium homeostasis, and Wnt signaling in the mouse uterus independent of estrogen receptor-α and -β. J Biol Chem 275:28834–28842 [DOI] [PubMed] [Google Scholar]
- 9. Hewitt SC, Deroo BJ, Hansen K, Collins J, Grissom S, Afshari CA, Korach KS. 2003. Estrogen receptor-dependent genomic responses in the uterus mirror the biphasic physiological response to estrogen. Mol Endocrinol 17:2070–2083 [DOI] [PubMed] [Google Scholar]
- 10. Hou X, Tan Y, Li M, Dey SK, Das SK. 2004. Canonical Wnt signaling is critical to estrogen-mediated uterine growth. Mol Endocrinol 18:3035–3049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Das SK, Tan J, Johnson DC, Dey SK. 1998. Differential spatiotemporal regulation of lactoferrin and progesterone receptor genes in the mouse uterus by primary estrogen, catechol estrogen, and xenoestrogen. Endocrinology 139:2905–2915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. 1993. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA 90:11162–11166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. O'Brien JE, Peterson TJ, Tong MH, Lee EJ, Pfaff LE, Hewitt SC, Korach KS, Weiss J, Jameson JL. 2006. Estrogen-induced proliferation of uterine epithelial cells is independent of estrogen receptor α binding to classical estrogen response elements. J Biol Chem 281:26683–26692 [DOI] [PubMed] [Google Scholar]
- 14. Watanabe H, Suzuki A, Kobayashi M, Takahashi E, Itamoto M, Lubahn DB, Handa H, Iguchi T. 2003. Analysis of temporal changes in the expression of estrogen-regulated genes in the uterus. J Mol Endocrinol 30:347–358 [DOI] [PubMed] [Google Scholar]
- 15. Lyman SK, Gerace L, Baserga SJ. 1999. Human Nop5/Nop58 is a component common to the box C/D small nucleolar ribonucleoproteins. RNA 5:1597–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang Y, Isaac C, Wang C, Dragon F, Pogacic V, Meier UT. 2000. Conserved composition of mammalian box H/ACA and box C/D small nucleolar ribonucleoprotein particles and their interaction with the common factor Nopp140. Mol Biol Cell 11:567–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nakamoto K, Ito A, Watabe K, Koma Y, Asada H, Yoshikawa K, Shinomura Y, Matsuzawa Y, Nojima H, Kitamura Y. 2001. Increased expression of a nucleolar Nop5/Sik family member in metastatic melanoma cells: evidence for its role in nucleolar sizing and function. Am J Pathol 159:1363–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maxwell ES, Fournier MJ. 1995. The small nucleolar RNAs. Annu Rev Biochem 64:897–934 [DOI] [PubMed] [Google Scholar]
- 19. Gautier T, Bergès T, Tollervey D, Hurt E. 1997. Nucleolar KKE/D repeat proteins Nop56p and Nop58p interact with Nop1p and are required for ribosome biogenesis. Mol Cell Biol 17:7088–7098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Venema J, Tollervey D. 1999. Ribosome synthesis in Saccharomyces cerevisiae. Annu Rev Genet 33:261–311 [DOI] [PubMed] [Google Scholar]
- 21. Irigoyen M, Mizuno T, Zhong X, Negm RS, Schneider TJ, Rothstein TL. 2002. B cell activation leads to upregulated expression of the murine Sik-similar protein gene. Mol Immunol 38:861–866 [DOI] [PubMed] [Google Scholar]
- 22. Nelson SA, Aris JP, Patel BK, LaRochelle WJ. 2000. Multiple growth factor induction of a murine early response gene that complements a lethal defect in yeast ribosome biogenesis. J Biol Chem 275:13835–13841 [DOI] [PubMed] [Google Scholar]
- 23. Chung D, Das SK. 2011. Mouse primary uterine cell coculture system revisited: ovarian hormones mimic the aspects of in vivo uterine cell proliferation. Endocrinology 152:3246–3258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reese J, Das SK, Paria BC, Lim H, Song H, Matsumoto H, Knudtson KL, DuBois RN, Dey SK. 2001. Global gene expression analysis to identify molecular markers of uterine receptivity and embryo implantation. J Biol Chem 276:44137–44145 [DOI] [PubMed] [Google Scholar]
- 25. Luo S, Mao C, Lee B, Lee AS. 2006. GRP78/BiP is required for cell proliferation and protecting the inner cell mass from apoptosis during early mouse embryonic development. Mol Cell Biol 26:5688–5697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Steinsapir J. 1992. Microsomal steroid receptors in target tissues. Receptor 2:45–76 [PubMed] [Google Scholar]
- 27. Qualmann B, Kessels MM, Thole HH, Sierralta WD. 2000. A hormone pulse induces transient changes in the subcellular distribution and leads to a lysosomal accumulation of the estradiol receptor α in target tissues. Eur J Cell Biol 79:383–393 [DOI] [PubMed] [Google Scholar]
- 28. Cooke PS, Buchanan DL, Young P, Setiawan T, Brody J, Korach KS, Taylor J, Lubahn DB, Cunha GR. 1997. Stromal estrogen receptors mediate mitogenic effects of estradiol on uterine epithelium. Proc Natl Acad Sci USA 94:6535–6540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cooke PS, Buchanan DL, Lubahn DB, Cunha GR. 1998. Mechanism of estrogen action: lessons from the estrogen receptor-α knockout mouse. Biol Reprod 59:470–475 [DOI] [PubMed] [Google Scholar]
- 30. Tan J, Paria BC, Dey SK, Das SK. 1999. Differential uterine expression of estrogen and progesterone receptors correlates with uterine preparation for implantation and decidualization in the mouse. Endocrinology 140:5310–5321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. 1998. Generation and reproductive phenotypes of mice lacking estrogen receptor β. Proc Natl Acad Sci USA 95:15677–15682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pederson T. 1998. Growth factors in the nucleolus? J Cell Biol 143:279–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cockell MM, Gasser SM. 1999. The nucleolus: nucleolar space for RENT. Curr Biol 9:R575–R576 [DOI] [PubMed] [Google Scholar]
- 34. Garcia SN, Pillus L. 1999. Net results of nucleolar dynamics. Cell 97:825–828 [DOI] [PubMed] [Google Scholar]
- 35. Politz JC, Polena I, Trask I, Bazett-Jones DP, Pederson T. 2005. A nonribosomal landscape in the nucleolus revealed by the stem cell protein nucleostemin. Mol Biol Cell 16:3401–3410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Htun H, Holth LT, Walker D, Davie JR, Hager GL. 1999. Direct visualization of the human estrogen receptor α reveals a role for ligand in the nuclear distribution of the receptor. Mol Biol Cell 10:471–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hager GL, Lim CS, Elbi C, Baumann CT. 2000. Trafficking of nuclear receptors in living cells. J Steroid Biochem Mol Biol 74:249–254 [DOI] [PubMed] [Google Scholar]
- 38. Stenoien DL, Mancini MG, Patel K, Allegretto EA, Smith CL, Mancini MA. 2000. Subnuclear trafficking of estrogen receptor-α and steroid receptor coactivator-1. Mol Endocrinol 14:518–534 [DOI] [PubMed] [Google Scholar]
- 39. Ochiai I, Matsuda K, Nishi M, Ozawa H, Kawata M. 2004. Imaging analysis of subcellular correlation of androgen receptor and estrogen receptor α in single living cells using green fluorescent protein color variants. Mol Endocrinol 18:26–42 [DOI] [PubMed] [Google Scholar]
- 40. Han SJ, DeMayo FJ, O'Malley BW. 2007. Dynamic regulation of progesterone receptor activity in female reproductive tissues. Ernst Schering Found Symp Proc 25–43 [DOI] [PubMed] [Google Scholar]
- 41. Yoshinaga K, Adams CE. 1966. Delayed implantation in the spayed, progesterone treated adult mouse. J Reprod Fertil 12:593–595 [DOI] [PubMed] [Google Scholar]
- 42. Das SK, Wang XN, Paria BC, Damm D, Abraham JA, Klagsbrun M, Andrews GK, Dey SK. 1994. Heparin-binding EGF-like growth factor gene is induced in the mouse uterus temporally by the blastocyst solely at the site of its apposition: a possible ligand for interaction with blastocyst EGF-receptor in implantation. Development 120:1071–1083 [DOI] [PubMed] [Google Scholar]
- 43. Tan J, Raja S, Davis MK, Tawfik O, Dey SK, Das SK. 2002. Evidence for coordinated interaction of cyclin D3 with p21 and cdk6 in directing the development of uterine stromal cell decidualization and polyploidy during implantation. Mech Dev 111:99–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lim H, Gupta RA, Ma WG, Paria BC, Moller DE, Morrow JD, DuBois RN, Trzaskos JM, Dey SK. 1999. Cyclo-oxygenase-2-derived prostacyclin mediates embryo implantation in the mouse via PPARδ. Genes Dev 13:1561–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang F, Porter W, Xing W, Archer TK, Safe S. 1997. Identification of a functional imperfect estrogen-responsive element in the 5′-promoter region of the human cathepsin D gene. Biochemistry 36:7793–7801 [DOI] [PubMed] [Google Scholar]
- 46. Liu YH, Teng CT. 1991. Characterization of estrogen-responsive mouse lactoferrin promoter. J Biol Chem 266:21880–21885 [PubMed] [Google Scholar]
- 47. Ray S, Das SK. 2006. Chromatin immunoprecipitation assay detects ERα recruitment to gene specific promoters in uterus. Biol Proced Online 8:69–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tan Y, Li M, Cox S, Davis MK, Tawfik O, Paria BC, Das SK. 2004. HB-EGF directs stromal cell polyploidy and decidualization via cyclin D3 during implantation. Dev Biol 265:181–195 [DOI] [PMC free article] [PubMed] [Google Scholar]