Abstract
Hepatic insulin resistance (IR) is associated with liver inflammatory diseases, but molecular mechanisms for the association remained elusive. IR is known to increase activity of forkhead box-containing protein O subfamily-1 (FOXO1), a transcription factor that was recently shown to enhance proinflammatory cytokine production in macrophages and adipocytes. Here we report that overexpression of constitutively active FOXO1 markedly increased chemokine ligand 20 (CCL20) expression and secretion in HepG2 hepatoma cells treated with TNF-α. The opposite was seen when endogenous FOXO1 was silenced. FOXO1 did not bind CCL20 promoter directly; instead, it potentiated CCL20 transcription through increasing the binding of p65/p50 heterodimer to a functional nuclear factor-κB site in the human CCL20 promoter. The conditional medium from TNF-α-treated HepG2 cells stimulated migration of human peripheral blood mononuclear cells. This stimulation was significantly enhanced when FOXO1 was overexpressed, and attenuated when FOXO1 was silenced. CCL20 antibody partly blocked the synergistic effect of FOXO1 and TNF-α on peripheral blood mononuclear cells migration. Additionally, TNF-α antagonizes the insulin/Akt signal transduction, thus leading to activation of FOXO1, which is capable of mediating a transcriptional activation role in response to TNF-α on CCL20 gene expression in HepG2 cells and promotes lymphocyte chemotaxis. Furthermore, we found that FOXO1 and CCL20 were coordinately up-regulated in the insulin resistant and inflammatory cell-infiltrated liver of db/db mice, an animal model that displayed hepatic and systemic low-grade inflammation. In conclusion, our data suggest that FOXO1 links IR to lymphocyte chemotaxis in the insulin-resistant hepatocytes and livers by amplifying nuclear factor-κB-dependent hepatic CCL20 production.
Liver insulin resistance (IR) and inflammatory cell recruitment play critical roles in the development of hepatic steatosis and its progression to steatohepatitis, a major health problem in developed countries (1). It has been well established that IR increases the activity of forkhead box-containing protein O subfamily-1 (FOXO1) by reducing FOXO1 phosphorylation and its retention in the cytoplasm and therefore increasing FOXO1 translocation into the nucleus to regulate transcription of its target genes (2–4). In liver, FOXO1 plays important roles in controlling the expression of genes involved in gluconeogenesis (5–7), very low-density lipoprotein production (8, 9), oxidative stress (10, 11), and apoptosis (12). Recent evidence suggests that FOXO1 may link IR to inflammation. It has been reported that FOXO1 activation increases the expression of proinflammatory cytokines, such as IL-1β in macrophages (13) and monocyte chemoattractant protein-1 in adipocytes (14).
Knockdown of FOXO1 expression was shown to improve hepatic and peripheral insulin action in diet-induced obese mice (15). Augmented FOXO1 expression and activity were reported in the liver of human patients with nonalcoholic steatohepatitis (NASH) and were independently associated with the hepatic necroinflammatory activity (16). It is largely unknown how FOXO1 promotes hepatic inflammation. Chemokines comprise a large group of closely related proteins that play important roles in inflammation and immune response regulation (17–20). So far, approximately 50 chemokines have been identified and subdivided into four families defined by the number of amino acids between the conserved N-terminal cysteine residues (CC, CXC, CCX, and CX3C) (17). The largest families are the CC and the CXC chemokines, the members of which have been repeatedly detected in the liver (21, 22). Chemokine (C-C motif) ligand 20 (CCL20) was simultaneously identified by three groups using a bioinformatics approach. Hieshima et al. (23) identified the gene from HepG2 hepatocarcinoma cells and human liver cDNA library and thus named the gene “liver and activation-related chemokine.” Rossi et al. (24) obtained the CCL20 gene from differentiated monocytes and therefore called the gene “macrophage inflammatory protein (MIP)-3α.” Hromas et al. (25) cloned the CCL20 gene from pancreatic islet cells, and thus designated the gene as “Exodus.” By binding specifically to its CC chemokine receptor 6 (CCR6), CCL20 attracts memory T lymphocytes, immature dendritic cells (26), and perhaps other inflammatory cells that may express CCR6 under circumstances, such as phytohemagglutinin-, or TNF-α-stimulated human peripheral blood mononuclear cells (PBMC) (27). It was shown that TNF-α induces CCL20 expression via increasing nuclear factor-κB (NF-κB) binding to a NF-κB-binding site in the proximal CCL20 promoter (28).
Interestingly, CCL20 expression is increased in the adipocytes from obese human subjects relative to normal humans, and the adipocyte-released CCL20 can promote lymphocyte recruitment (29). CCL20 secretion by endometriotic stromal cells is also induced by inflammatory factors such as IL-1β, TNF-α, and IL-17A (30). CCL20 mRNA is most abundantly expressed in human liver relative to other human tissues (23). Immunohistochemistry of livers from patients with hepatitis showed that CCL20 or MIP-3α is enriched in hepatic piecemeal necrotic regions in which dendritic cells/macrophages mostly exist, but it also reveal hepatocyte expression of MIP-3α, although not as abundant as in the necrotic region (31). It was shown that CCL20 expression in mucosal epithelial cells and epidermal keratinocytes is up-regulated by proinflammatory cytokines (32, 33). In contrast, hepatitis C virus core protein was shown to suppress CCL20 expression in HepG2 cells (34). It is currently unknown whether CCL20 expression in hepatocytes can be regulated by inflammatory cytokines. It is also unknown whether IR increases hepatocyte CCL20 expression and secretion. The role of hepatocyte CCL20 in inflammatory cell recruitment has yet to be examined.
In this study, we tested a hypothesis that, in insulin-resistant hepatocytes, activation of FOXO1 may augment inflammatory cell recruitment by inducing CCL20 expression when a baseline inflammation exists. Consistently with our hypothesis, we found that FOXO1 dramatically enhances CCL20 expression in a NF-κB-dependent manner in cultured HepG2 cells and induces lymphocytes chemotaxis. To the best of our knowledge, this is the first observation showing that FOXO1 links hepatic CCL20 production to inflammatory cell recruitment. Our observation implies that insulin-resistant hepatocytes relative to normal hepatocytes may recruit more inflammatory cells to the liver via secreting more CCL20 in a FOXO1-dependent manner when a baseline inflammation exists.
Results
FOXO1 overexpression increases CCL20 expression in TNF-α-treated HepG2 cells
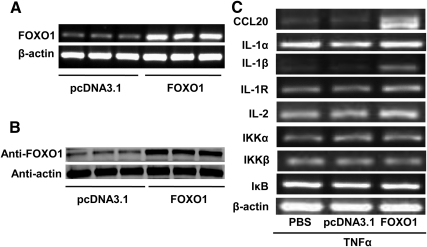
We aimed to identify the molecules linking FOXO1 to production of proinflammatory cytokines in hepatocytes in a state of IR. A constitutively active FOXO1 (FOXO1-AAA) was first overexpressed in HepG2 cells via transient transfection of FOXO1-AAA-expressing plasmid. We chose the constitutively active form of FOXO1 because it cannot be phosphorylated, thus mimicking the predominant form of FOXO1 under insulin-resistant condition (13, 14). Results showed that ectopic gene transfer increased mRNA and protein expression of FOXO1 in HepG2 cells (Fig. 1, A and B). To mimic a low-grade inflammation often associated with obesity and IR, HepG2 cells were primed with a low dose of TNF-α (10 ng/ml), and the effect of increased FOXO1 expression on inflammation-related genes was determined by RT-PCR using primers listed in Table 1. Results showed that overexpression of FOXO1 dramatically potentiated TNF-α induction of CCL20 and IL-1β with moderate or no effects on mRNA levels of IL-1α, IL-1 receptor, IL-2, IκB kinase (IKK)α, IKKβ, and IκB (Fig. 1C). FOXO1 therefore differentially modulated expression of cytokines in TNF-α-treated HepG2 cells. It has been shown that FOXO1 enhances lipopolysaccharide induction of IL-1β in macrophages (13). However, we found no reports on FOXO1 augmentation of TNF-α induction of CCL20.
Fig. 1.
Ectopic overexpression of FOXO1 increases proinflammatory gene expression in TNF-α-treated HepG2 cells. HepG2 cells in six-well plates were transfected with a plasmid expressing constitutively active FOXO1-AAA or a control vector pcDNA3.1 at a fixed dose (0.4 μg/1ml Optimem). Cells were harvested for mRNA and protein analyses 24 h after transfection. A, FOXO1 mRNA levels determined by RT-PCR using β-actin as an internal control. B, FOXO1 protein levels determined by Western blotting. C, Effects of FOXO1 on proinflammatory cytokines gene expression. HepG2 cells were transfected as described above. Cells were treated 6 h after transfection with 10 ng/ml recombinant human TNF-α for 24 h, and then harvested for RT-PCR analysis of a panel of proinflammatory genes. Each of the experiments was repeated at least three times, and similar results were obtained.
Table 1.
RT-PCR primers for FOXO1 and inflammation-related genes
| Gene | Cycle | Tm (°C) | Product (bp) | Primers (5′ to 3′) |
|---|---|---|---|---|
| β-Actin | 28 | 60 | 308 | Forward: CATCTCTTGCTCGAAGTCCA |
| Reverse: ATCATGTTTGAGACCTTCAACA | ||||
| FOXO1 | 35 | 62 | 188 | Forward: GCTGCATCCATGGACAACAACA |
| Reverse: CGAGGGCGAAATGTACTCCAGTT | ||||
| CCL20 | 38 | 58 | 342 | Forward: GCAAGCAACTTTGACTGCTG |
| Reverse: CAAGTCCAGTGAGGCACAAA | ||||
| IL-1α | 38 | 65 | 234 | Forward: GTAAGCTATGGCCCACTCCA |
| Reverse: AGGTGCTGACCTAGGCTTGA | ||||
| IL-1β | 38 | 57 | 184 | Forward: CTGAAAGCTCTCCACCTC |
| Reverse: GATCTACACTCTCCAGCTG | ||||
| IL-1R | 38 | 58 | 271 | Forward: TGTGGAAAATCCTGCAAACA |
| Reverse: GGAATCCCTGTACCAAAGCA | ||||
| IL-2 | 38 | 58 | 207 | Forward: GCAACTCCTGTCTTGCATTG |
| Reverse: GCCTTCTTGGGCATGTAAAA | ||||
| IKKα | 38 | 58 | 181 | Forward: GAAGGTGCAGTAACCCCTCA |
| Reverse: ATTGCCCTGTTCCTCATTTG | ||||
| IKKβ | 38 | 60 | 232 | Forward: AACCAGCATCCAGATTGACC |
| Reverse: CTCTAGGTCGTCCAGCGTTC | ||||
| GAPDH | 60 | 77 | Forward: TGTGTCCGTCGTGGATCTGA | |
| Reverse: CCTGCTTCACCACCTTCTTGAT | ||||
| F4/80 | 60 | 223 | Forward: TTTCCTCGCCTGCTTCTTC | |
| Reverse: CCCCGTCTCTGTATTCAACC | ||||
| CD3 | 60 | 111 | Forward: AAGGGAGCCCCTTCAAGGTA | |
| Reverse: TCTTTGCAAACCATCCTTCC |
Tm, Temperature.
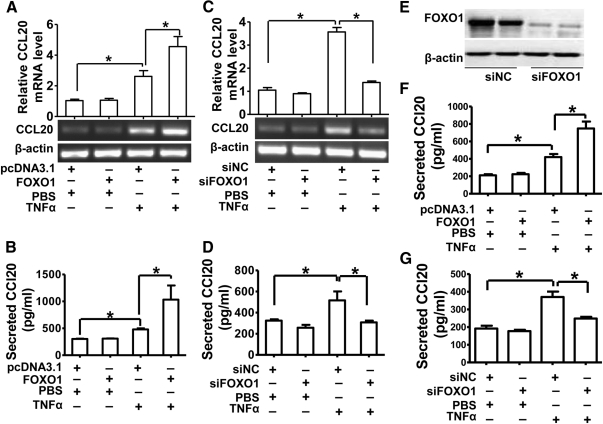
CCL20 is known to recruit lymphocytes via binding to its receptor CCR6 on the cell surface of lymphocytes (30, 37). Lymphocyte infiltration is often observed in inflammatory livers, playing important roles in modulating inflammatory and immune responses in liver (18, 31). Thus, we focused on the molecular mechanism underlying FOXO1 induction of CCL20 production. As an initial step to confirm the stimulatory effect of FOXO1 on CCL20 gene expression, we measured both CCL20 mRNA levels with RT-PCR and protein levels with ELISA by collecting cultured medium in HepG2 cells with or without overexpression of FOXO1-AAA and treated with or without TNF-α. CCL20 mRNA levels (Fig. 2A) and protein secretion (Fig. 2B) were not affected by FOXO1 overexpression in nonstimulated cells but were significantly enhanced in TNF-α-treated HepG2 cells.
Fig. 2.
FOXO1 increases CCL20 expression and secretion in cultured hepatic cells. HepG2 cells or L02 cells were transfected and treated with TNF-α as described in Fig. 1 and then harvested for the determination of CCL20 mRNA and (or) protein levels. A, Relative CCL20 mRNA levels in HepG2 cells determined by RT-PCR. B, Secreted CCL20 protein in HepG2 cells determined by ELISA. C, Effects of FOXO1 silencing on CCL20 mRNA levels in HepG2 cells. HepG2 cells were transfected with siFOXO1 or scrambled siRNA (siNC) as a control at a final concentration of 20 nmol/ml. Cells were treated with 10 ng/ml TNF-α or vehicle PBS 24 h after transfection and then harvested for analysis of CCL20 mRNA levels by RT-PCR. D, Effects of FOXO1 silencing on CCL20 protein secretion in HepG2 cells. HepG2 cells were transfected and treated with TNF-α (10 ng/ml) as described above, and ELISA was performed to determine the CCL20 protein secreted into the cultured cell supernatant. E, Effects of FOXO1-specific siRNA (siFOXO1) on endogenous FOXO1 expression in HepG2 cells. HepG2 cells were transfected with siFOXO1 or scrambled siRNA (siNC) as a control at a final concentration of 20 nmol/ml. Cells were harvested 48 h after siRNA transfection, and the endogenous FOXO1 expression was measured by Western blotting. F, Secreted CCL20 protein in L02 cells determined by ELISA. L02 cells were transfected and treated as described in panel B, and the secreted CCL20 protein in the supernatant was measured by ELISA. G, Effects of FOXO1 silencing on CCL20 protein secretion in L02 cells. L02 cells were transfected and treated as in panel D, and ELISA was performed to determine the CCL20 protein in the supernatant. Each of the experiment was repeated at least three times, and similar results were obtained. *, P < 0.05 vs. controls.
To confirm the role of FOXO1 in TNF-α-induced CCL20 gene expression, small interfering RNA (siRNA) specifically targeting human FOXO1 (siFOXO1) was used to silence endogenous FOXO1 expression in HepG2 cells (Fig. 2E). Intriguingly, although CCL20 mRNA expression (Fig. 2C) and protein secretion (Fig. 2D) were unaffected by silencing FOXO1 in nonstimulated cells (PBS group), their induction by TNF-α was virtually abolished in FOXO1 siRNA-treated HepG2 cells. These data suggested that TNF-α requires FOXO1 to efficiently induce CCL20 expression. To determine whether a similar finding could be recapitulated in hepatic cell lines of different origin, we transfected the constitutively active FOXO1 or siFOXO1 into the human hepatic cell line L02, treated these cells with TNF-α or PBS, and then measured CCL20 production in the cells. Consistent with the data from HepG2 cells, CCL20 secretion in response to TNF-α was also FOXO1 dependent in these L02 cells (Fig. 2, F and G).
A NF-κB-binding element is required for FOXO1 stimulation of TNF-α-induced increase in CCL20 promoter activity
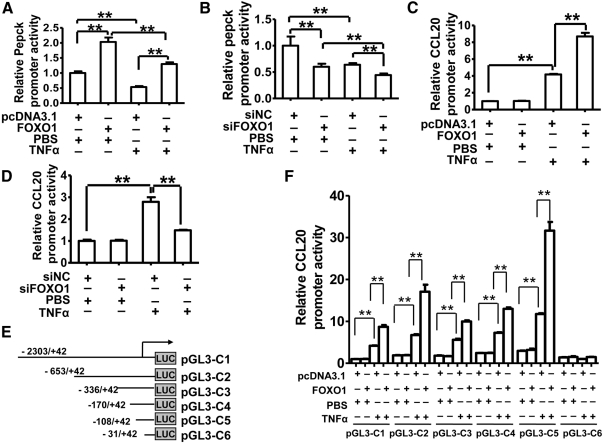
To molecularly define how FOXO1 augments TNF-α induction of CCL20 expression, the human CCL20 promoter activity was examined in HepG2 cells overexpressing constitutively active FOXO1 or treated with FOXO1 siRNA. The promoter of human phosphoenolpyruvate carboxykinase (Pepck) gene, a known target of FOXO1 (38), was used as a positive control for the transcription activity of FOXO1 in this experiment. As expected, FOXO1 overexpression increased (Fig. 3A), and silencing of endogenous FOXO1 decreased (Fig. 3B), Pepck promoter activity significantly in HepG2 cells regardless of TNF-α treatment, confirming the FOXO1 transcriptional activity on target gene expression. Similar effects of FOXO1 on human CCL20 promoter activity were observed when HepG2 cells were treated with TNF-α (Fig. 3, C and D) using a 2345-bp (−2303/+42) DNA fragment harboring the human CCL20 promoter (pGL3-C1) (Fig. 3E). This finding indicates that the human CCL20 promoter may not have a direct and functional binding site for activated FOXO1. Indeed, we mutated a potential FOXO1 site (−161/−152) in the promoter and found that this mutation had no effects on FOXO1 stimulation of TNF-α-induced increases in CCL20 promoter activity (data not shown).
Fig. 3.
FOXO1 stimulates promoter activities of PEPCK and CCL20. A, FOXO1 stimulates promoter activity of its known target gene PEPCK in HepG2 cells. A 593-bp (−523/+70) DNA fragment harboring the human PEPCK promoter was cloned into pGL3-Basic vector and used for luciferase assays 24 h after cotransfection with either a plasmid expressing constitutively active FOXO1-AAA or the control plasmid pcDNA3.1 with TNF-α (10 ng/ml) or PBS treatment for 24 h. B, Silencing of FOXO1 suppresses human PEPCK promoter activity in HepG2 cells. The PEPCK promoter construct was cotransfected with FOXO1 siRNA (siFOXO1) or a scrambled siRNA (siNC) into HepG2 cells, and luciferase activities were assayed 48 h after transfection with or without TNF-α treatment for 24 h. C, FOXO1 stimulates CCL20 promoter activity in TNF-α-treated HepG2 cells. A 2345-bp (−2303/+42) DNA fragment harboring the human CCL20 promoter was cloned into the pGL3-basic (pGL3–C1) vector. The construct was transfected alone (Control) or cotransfected with either FOXO1-AAA plasmid or pcDNA3.1. Cells were treated 6 h after transfection with TNF-α (10 ng/ml) for an additional 24 h and then harvested for luciferase assays. D, FOXO1 silencing decreases CCL20 promoter activity in TNF-α-treated HepG2 cells. Cells were transfected with FOXO1 siRNA (siFOXO1) or control siRNA (siNC) for 24 h, and then treated with TNF-α (10 ng/ml) for 24 h before harvesting for assays. E, A schematic depiction of different human CCL20 promoter regions cloned into the pGL3-basic plasmid. The constructs were designated as pGL3–C1-C6. F, Effects of FOXO1 overexpression on promoter activities of different CCL20 promoter constructs. HepG2 cells were cotransfected with FOXO1-AAA plasmid or pcDNA3.1 and each of the truncated promoter constructs. Cells were treated 6 h later with TNF-α (10 ng/ml) for an additional 24 h and then harvested for assays. Results are expressed as the relative luciferase activity normalized by the activity from the pcDNA3.1 and pGL3–C1 cotransfected group without TNF-α treatment (the first bar), which are arbitrarily defined as 1. Each of the experiments was repeated at least three times, and similar results were obtained. **, P < 0.001 vs. control.
To locate the promoter region required for FOXO1 regulation of CCL20 expression, several pGL3 reporter plasmids harboring different fragments of the 5′-flanking region of the human CCL20 promoter were constructed and designated as pGL3-C2 to -C6 (Fig. 3E). HepG2 cells were cotransfected with FOXO1-AAA expression vector and each of the reporter plasmids. As shown in Fig. 3F, in the absence of TNF-α, FOXO1 had no effects on CCL20 promoter activity of all constructs, whereas in the presence of TNF-α, FOXO1 significantly enhanced TNF-α-induced CCL20 promoter activity in constructs pGL3-C1 to -C5, but not pGL3-C6. Our findings indicate that the 5′-flanking region located at −108/−31 upstream of the human CCL20 gene transcriptional start site is required for FOXO1 to augment TNF-α induction of CCL20 promoter activity.
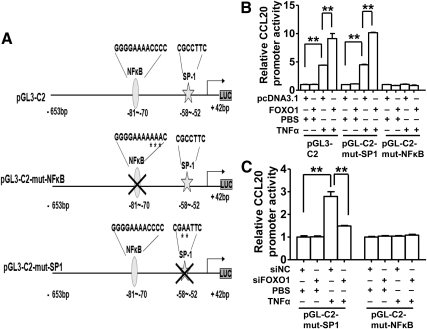
Previous studies have demonstrated that a NF-κB element (GGGGAAAACCCC) corresponding to nucleotides −70/−81 in the human CCL20 promoter binds p50/p65 NF-κB heterodimers and plays a major role for TNF-α to activate CCL20 expression in G-361 human melanoma cells (28). An adjacent stimulatory protein 1 (SP1) element (CGCCTTC) (−52/−58) binds SP1 and regulates basal CCL20 gene transcription in Caco-2 human colonic epithelial cells (39). To determine if these cis-elements are involved in FOXO1 stimulation of TNF-α activation of CCL20 expression, we performed site-directed mutagenesis on these elements on the construct pGL3-C2, and the resultant mutants were designated as pGL3-C2-mut-NF-κB and pGL3-C2-mut-SP1 (Fig. 4A). HepG2 cells were cotransfected with FOXO1-AAA expression vector or FOXO1 siRNA and the wild-type or mutant CCL20 promoter gene reporter plasmid. Although the luciferase activity did not differ between FOXO1 manipulated groups in nonstimulated HepG2 cells transfected with a wild-type construct or a mutant, it was substantially decreased in TNF-α-treated and FOXO1-overexpressing HepG2 cells transfected with the NF-κB mutant, not the SP1 mutant (Fig. 4B). The opposite result was obtained for the NF-κB mutant in FOXO1-silenced and TNF-α-treated HepG2 cells (Fig. 4C). These data demonstrated that the NF-κB element located at −70/−81 of the human CCL20 promoter is required for FOXO1 to enhance TNF-α-induced increase in CCL20 promoter activity in HepG2 cells.
Fig. 4.
FOXO1 stimulation of CCL20 promoter activity requires a functional NF-κB cis-element located −80/−70 bp upstream of its transcription start site. A, Schematic illustration of the consensus NF-κB and SP1-binding elements on the human CCL20 promoter and their mutant forms. Site-directed mutagenesis of NF-κB site or SP1 site was performed using the CCL20 promoter construct (pGL3–C2) as a template, and the mutant constructs were named “pGL3–C2-mut-NF-κB” or “pGL3–C2-mut-SP1.” B, Effects of FOXO1 on the activity of mutant CCL20 promoters. HepG2 cells were cotransfected with one of CCL20 promoter constructs and FOXO1-AAA plasmid or pcDNA3.1. Cells were treated 24 h after cotransfection with 10 ng/ml TNF-α or vehicle (PBS) for an additional 24 h and then harvested for the determination of relative luciferase activities. C, Effects of FOXO1 silencing on the activity of mutant CCL20 promoters. HepG2 cells were cotransfected with each of mutant constructs and FOXO1 siRNA (siFOXO1) or a scrambled siRNA (siNC). After a 24-h cotransfection, cells were treated with 10 ng/ml TNF-α or PBS for an additional 24 h and then harvested for the determination of relative luciferase activities. Each of the experiments was repeated at least three times, and similar results were obtained. *, P < 0.05 and **, P < 0.001 vs. control.
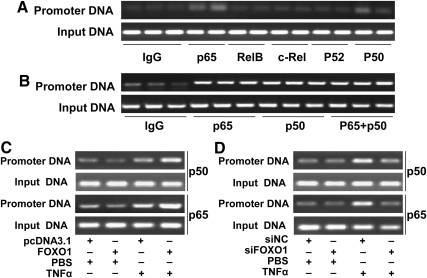
FOXO1 enhances binding of p65/p50 protein heterodimer to CCL20 promoter in TNF-α-stimulated HepG2 cells
Potential mechanisms responsible for the stimulatory effect of FOXO1 may include 1) up-regulation of NF-κB expression, 2) activation of NF-κB signal pathway, and 3) increase of NF-κB protein binding to CCL20 promoter. Using chromatin immunoprecipitation (ChIP) assays, here we showed that p65 and p50, but not RelB, C-rel, or p52 subunit of NF-κB, interacted with CCL20 promoter DNA in regularly cultured HepG2 cells (Fig. 5A), consistent with previous studies in human melanoma cells (28). Because NF-κB proteins may bind to DNA through forming either homodimers or heterodimers, we next determined the possible binding form of NF-κB to its cognate site on the CCL20 promoter. We performed ChIP assays using anti-p65 and/or anti-p50 antibodies and found that the amount of CCL20 promoter DNA immunoprecipitated with anti-p65 alone, anti-p50 alone, or both antibodies simultaneously were quantitatively the same (Fig. 5B). This finding indicates that NF-κB most likely binds CCL20 promoter as a heterodimer (p65/p50) to stimulate its expression in response to TNF-α in HepG2 cells. Importantly, ChIP assay showed that FOXO1 significantly enhanced binding of p65/p50 to CCL20 promoter DNA in the presence of TNF-α (Fig. 5C). It is unlikely that FOXO1 enhanced p65/p50 binding to CCL20 promoter via increasing p65/p50 expression because we found that FOXO1 had no effects on p65 or p50 mRNA expression in HepG2 cells (data not shown). We also showed that FOXO1 had no effect on the mRNA level of IKKα, IKKβ, or IκB (a downstream target of IKK) (Fig. 1C). Although our observations could not exclude the possibility that FOXO1 directly enhances the activity of IKK or IκB protein to promote NF-κB translocation into the nucleus and hence increasing NF-κB DNA binding activity, the absence of increased p65 or p50 binding to CCL20 promoter in nonstimulated HepG2 cells (PBS group) also makes this possibility most unlikely.
Fig. 5.
FOXO1 increases NF-κB and CCL20 promoter interaction. A, NF-κB and CCL20 promoter interaction determined by ChIP assays. HepG2 cells incubated in normal culture medium were harvested for ChIP assays as described in Materials and Methods. The interaction between NF-κB subunits (p65, RelB, C-rel, p52, and p50) and CCL20 promoter was determined. B, Interaction between NF-κB proteins (p65, p50 or p65/p50) and CCL20 promoter. HepG2 cells treated with TNF-α (10 ng/ml) for 24 h were harvested as described in Materials and Methods and immunoprecipitated with anti-p65, anti-p50, or both antibodies. C, FOXO1 stimulation of NF-κB (p65 and p50) and CCL20 promoter interaction determined by ChIP assays using HepG2 cells transfected with FOXO1-AAA or pcDNA3.1 plasmid for 24 h, followed by 24-h treatment of TNF-α (10 ng/ml). D, FOXO1 silencing inhibits NF-κB (p65 and p50) and CCL20 promoter interaction as determined by ChIP assays using HepG2 cells transfected with FOXO1 siRNA (siFOXO1) or a scrambled siRNA (siNC) for 24 h, followed by 24 h of TNF-α treatment (10 ng/ml). Each of the experiments was repeated two to four times, and representative results were displayed.
To provide additional evidence in support of FOXO1 in the regulation of NF-κB binding to CCL20 promoter, FOXO1 siRNA was transfected into HepG2 cells in the absence or presence of TNF-α. ChIP assays were performed with these cells. Consistently, FOXO1 silencing did not affect p65 or p50 binding activity in nonstimulated HepG2 cells but significantly decreased the binding of NF-κB to CCL20 promoter in TNF-α-stimulated cells (Fig. 5D). Taken together, these results indicated that FOXO1 stimulated TNF-α-induced CCL20 transcription via increasing the binding activity of NF-κB p65/p50 to its cognate binding site.
FOXO1 promotes PBMC migration in a CCL20-dependent manner
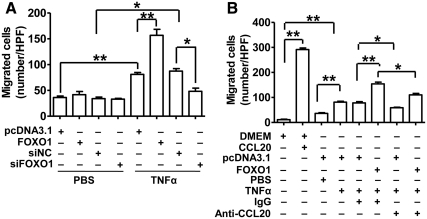
To explore the functional role of our molecular and biochemical findings, monocyte-deprived human PBMC (mainly lymphocytes) were subjected to migration tests in Boyden chambers. The migrated cell numbers significantly increased with TNF-α treatment and were further enhanced with the overexpression of FOXO1. In contrast, the migrated PBMC significantly decreased with the silencing of FOXO1 in the presence of TNF-α treatment (Fig. 6A). FOXO1 manipulations had no effects on PBMC migration in the absence of TNF-α. Our data indicated that FOXO1 may increase release of bioactive chemoattractant substance(s) in TNF-α-stimulated HepG2 cells.
Fig. 6.
CCL20 antibody partly inhibits FOXO1 stimulation of PBMC migration in the presence of TNF-α. A, Effects of FOXO1 on PBMC migration. HepG2 were incubated in six-well plates and transfected with pcDNA3.1 (0.4 μg), FOXO1-AAA plasmid (0.4 μg), siFOXO1 (20 nmol/ml), or siNC (20 nmol/ml). Cells were treated 24 h after transfection with 10 ng/ml TNF-α or vehicle PBS for an additional 24 h, and the cell culture supernatant were used for migration test of monocyte-deprived PBMC (mainly lymphocytes) in the six-well Boyden chamber system. B, Effects of CCL20 antibody on PBMC migration. HepG2 cells were transfected with pcDNA3.1 or FOXO1-AAA plasmid and treated with TNF-α as described above in the presence of anti-human CCL20 antibody (10 ng/ml) or IgG. The supernatants were harvested for PBMC migration test. DMEM and DMEM plus purified human CCL20 protein (1 ng/ml) were used as negative and positive controls, respectively. Results were from five high-power fields (HPF) of each of triplicate wells. *, P < 0.05; and **, P < 0.001 vs. control.
To determine whether the chemokine CCL20 was involved in attracting PBMC in TNF-α-stimulated HepG2 cells, the purified CCL20 protein, as a positive control, or an antihuman CCL20 antibody, as an inhibitor of CCL20, was included in the PBMC migration assays. As expected, migration of PBMC was potently stimulated by recombinant human CCL20 protein dissolved in DMEM, confirming its chemoattractant activity (Fig. 6B). The IgG-added supernatant from TNF-α-treated HepG2 cells significantly stimulated PBMC migration compared with the DMEM control, which was potentiated by FOXO1 overexpression. Importantly, both of these effects were substantially blocked by CCL20 antibody. These data indicated that CCL20 likely functions as one of the major chemoattractants contributing to the enhanced PBMC migration by the supernatant from FOXO1-transfected and TNF-α-stimulated HepG2 cells.
FOXO1-CCL20 pathway mediates antagonistic effects of TNF-α on insulin function in HepG2 cells
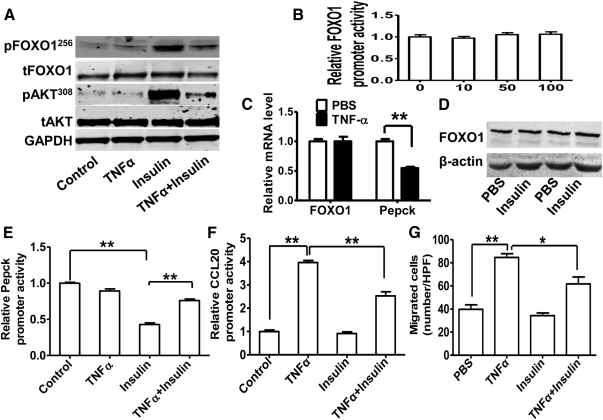
TNF-α is known to impair insulin signaling (40). The impairment of insulin signaling leads to a pathophysiological state known as IR. It is well established that IR activates FOXO1 function. Given that FOXO1 increased CCL20 expression and lymphocyte chemotaxis in TNF-α-stimulated HepG2 cells, we tested whether attenuated insulin signaling by TNF-α stimulates FOXO1 activities, thus leading to increased expression of CCL20 and lymphocyte migration. HepG2 cells were treated with insulin, TNF-α, or both. As expected, acute insulin treatment for 10 min substantially increased the phosphorylation of AKT, a downstream marker of insulin-signaling activation. Phosphorylated FOXO1 was also markedly increased under this condition. This insulin-stimulated phosphorylation of AKT and FOXO1 was substantially inhibited by TNF-α pretreatment for 24 h (Fig. 7A). These observations prove that FOXO1 acts as a downstream effector of insulin and TNF-α antagonizes the insulin/Akt/FOXO1 signal transduction. Additionally, we found TNF-α had no effect on human FOXO1 promoter activity (Fig. 7B) or mRNA expression (Fig. 7C). Although it has been reported that acute insulin treatment induces FOXO1 protein degradation in mouse embryo fibroblasts (41) and HepG2 cells (42), we found that prolonged insulin treatment (24 h) had no significant effects on FOXO1 protein levels in HepG2 cells (Fig. 7D), which is consistent with a report showing that the prolonged insulin treatment restores FOXO1 protein expression in mouse embryo fibroblasts (41). We next tested whether attenuated insulin signaling by TNF-α affects FOXO1 target gene expression. Indeed, the human PEPCK, a well known FOXO1 target gene in liver, was found inhibited by TNF-α at the mRNA level (Fig. 7C) and the promoter activity level (Fig. 7E). Furthermore, the insulin suppression effect on Pepck promoter activity was markedly disrupted by TNF-α (Fig. 7E). Interestingly, human CCL20 promoter activity stimulated by TNF-α was also antagonized by insulin (Fig. 7F). Moreover, we showed that insulin treatment inhibited TNF-α-stimulated PBMC migration (Fig. 7G). Together, these results suggest that FOXO1 functions downstream of insulin signaling and is capable of mediating a transcriptional activation role in response to TNF-α on CCL20 gene expression and lymphocyte chemotaxis in HepG2 cells.
Fig. 7.
FOXO1-CCL20 pathway mediating antagonistic effects of TNF-α on insulin function in HepG2 cells. A, TNF-α inhibits insulin-induced phosphorylation of AKT and FOXO1 in HepG2 cells. HepG2 cells treated with TNF-α (10 ng/ml) for 24 h were stimulated with insulin (50 nm) for 10 min and harvested for determination of p-FOXO1 (S256), FOXO1, p-AKT (T308), AKT, and GAPDH as control. B, Effect of TNF-α on the promoter of human FOXO1. A 2963-bp (−2936/+27) DNA fragment harboring the human FOXO1 promoter was cloned into pGL3-Basic vector and used for luciferase assays 24 h after transfection into HepG2 cells with TNF-α (0, 10, 50, 100 ng/ml) treatment. C, Effect of TNF-α on FOXO1 and Pepck mRNA levels. HepG2 cells were treated with TNF-α (10 ng/ml) for 24 h, and harvested for RNA analysis by quantitative RT-PCR. D, Effect of insulin on FOXO1 protein level. HepG2 cells were treated with insulin (50 nm) for 24 h and harvested for protein extraction and Western blotting. E, TNF-α antagonizes insulin-decreased PEPCK promoter activity. HepG2 cells were transfected with the human PEPCK promoter construct. TNF-α (10 ng/ml) or 50 nm insulin was added to the cells 6 h later before harvesting for luciferase assays 24 h after transfection. F, Insulin decreases TNF-α-induced CCL20 promoter activity. HepG2 cells were transfected with pGL3–C1 construct and treated with TNF-α or insulin as described above, followed by luciferase assays. G, Insulin attenuates TNF-α-induced migration of PBMC by conditional medium from cultured HepG2 cells treated with TNF-α or insulin as described above. *, P < 0.05; and **, P < 0.001 vs. control. HPF, High-power field.
FOXO1 and CCL20 levels are coordinately increased in the livers of db/db mice
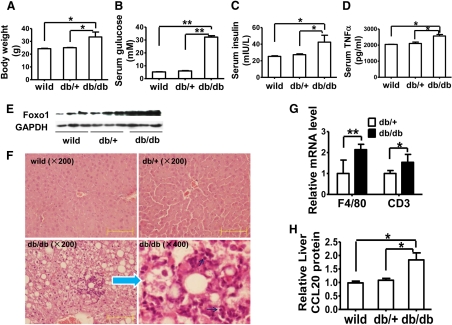
To determine whether FOXO1 and CCL20 are coordinately regulated in vivo, we measured hepatic expression levels of FOXO1 and CCL20 in db/db mice, an obese and diabetic mouse model. As expected, these mice showed increased body weight (Fig. 8A) and fasting serum concentrations of glucose (Fig. 8B), insulin (Fig. 8C), and TNF-α (Fig. 8D). Consistent with previous reports (43, 44), these mice expressed increased amounts of FOXO1 protein in the liver (Fig. 8E) and showed a severe fatty liver (Fig. 8F). Focal inflammatory infiltration was also observed on the fatty liver background, and inflammatory cells were obvious, including lymphocytes. Quantitative real-time PCR also showed increased mRNA levels of macrophage marker F4/80 and lymphocyte marker CD3 (Fig. 8G). Intriguingly, hepatic CCL20 protein content was also coordinately increased (Fig. 8H). Together with our in vitro findings, this observation implies that CCL20 may function as a new chemoattractant molecule downstream of FOXO1 to promote liver inflammation, especially in insulin-resistant livers.
Fig. 8.
FOXO1 and CCL20 are increased in the liver of db/db mice. C57BL/6 obese and diabetic db/db mice (n = 5; 30 wk of age) and two control groups [db/+ littermates (n = 3) and wild-type C57BL/6 mice (n = 3)], were fed a standard chow diet. The mice were fasted overnight and then killed for analyses of the following parameters: body weight (A), serum glucose (B), serum insulin (C), and serum TNF-α (D). E, Western blots of hepatic total FOXO1 protein. F, Hematoxylin and eosin staining of liver sections showing lipid vacuoles and a focal inflammation. The black arrows point to lymphocytes. Scale bar, 100 μm. G, Relative hepatic mRNA levels of macrophage marker F4/80 and lymphocyte marker CD3 measured by quantitative real-time PCR (n = 5). H, Hepatic content of CCL20 protein determined by ELISA. *, P < 0.05; and **, P < 0.001 vs. one of controls.
Discussion
IR and hepatic inflammatory cell recruitment play critical roles in the development of hepatic steatosis and its progression to steatohepatitis (45). In this study, we demonstrated that the chemokine CCL20 released by hepatocytes links IR-induced activation of FOXO1, at least in part, to recruitment of inflammatory cells. We showed that forced overexpression of constitutively active FOXO1 (FOXO1-AAA) dramatically increases CCL20 expression only in TNF-α-treated, but not in nonstimulated HepG2 cells, and the increased CCL20 expression is associated with an increased CCL20 protein secretion into the medium of these cells. We chose the constitutively active form of FOXO1 because it cannot be phosphorylated and is mainly located in the nucleus, thus mimicking its form in the insulin-resistant state. We speculate that the wild-type form would produce similar effects because the overexpressed wild-type FOXO1 accumulated mainly in the nucleus (thus active) in cells treated with TNF-α (14) or lipopolysaccharide (13). The opposite results were observed when the endogenous FOXO1 expression was silenced by FOXO1 siRNA in hepatic cell lines.
The detailed molecular mechanism for FOXO1 to potentiate TNF-α-induced CCL20 expression has yet to be elucidated. Previous studies demonstrated a NF-κB binding site corresponding to the sequence −81/−70 in our pGL3-promoter constructs as a key element in the regulation of human and mouse CCL20 promoter activity (39, 46). In this study, we not only confirmed the previous finding by demonstrating NF-κB binding to this site on human CCL20 promoter but also showed that the specific NF-κB protein is the heterodimer p65/p50, but not RelB, C-rel, or p52 in response to TNF-α treatment. Importantly, by using a ChIP assay, we showed that FOXO1 overexpression increased, and FOXO1 silencing decreased, p65/p50 binding to the NF-κB-element on the human CCL20 promoter under inflammatory conditions (Fig. 5, C and D). To our knowledge, this study is the first to show that the interaction of FOXO1 pathway and NF-κB signaling induces a proinflammatory gene expression in hepatocytes. It has been shown that FOXO1 can function as a corepressor of androgen receptor (47) and a coactivator of constitutive androstane receptor and pregnane X receptor (7). Given that FOXO1 has no effects on CCL20 expression in nonstimulated HepG2 cells (Figs. 2–4), it is tempting to propose that FOXO1 may serve as a coactivator of NF-κB in the nucleus to amplify NF-κB signaling. Because we found no interactions between FOXO1 protein and several components of NF-κB, including p65, RelB, C-rel, p52, and P50 (data not shown), we concluded that FOXO1 might enhance NF-κB activity indirectly through other pathways.
We have also demonstrated that increased expression of CCL20 stimulates migration of human PBMC. This stimulation is significantly enhanced when FOXO1 is overexpressed and attenuated when the endogenous FOXO1 is silenced. In addition, we found that FOXO1 and CCL20 are coordinately up-regulated in the fatty and insulin-resistant liver of obese and diabetic db/db mice, animals that are accompanied by liver and systemic low-grade inflammation. Increased hepatic FOXO1 and CCL20 are associated with increased expression of a lymphocyte marker CD3 and a macrophage marker F4/80 in the liver of these animals. Collectively, our findings suggest that hepatic IR, via activation of FOXO1, may exacerbate inflammation-induced production of some cytokines, such as CCL20, by hepatocytes, to enhance the recruitment of inflammatory cells, thereby promoting the progression of simple hepatic steatosis to steatohepatitis, particularly in animals and humans with obesity, a condition that is often associated with a systemic low-grade inflammation (48).
It has been reported that FOXO1 up-regulates proinflammatory gene production, including IL-1β (13) and Toll-like receptor 4 (49) in macrophages, and IL-6, monocyte chemoattractant protein-1, and CCAAT/enhancer binding protein (C/EBPβ) in adipocytes (14), and that FOXO1 directly binds to signal transducer and mediator of transcription 3 protein to promote inflammation in HepG2 cells (50). In the present study, the mRNA for IL-1β was also found substantially up-regulated by FOXO1 in TNF-α-treated HepG2 cells (Fig. 1C). The proinflammatory cytokines induced by FOXO1 may enhance NF-κB activity in an autocrine manner, thus increasing CCL20 expression.
Because NF-κB is a master switch governing the production of a host of inflammatory markers and mediators, such as C-reative protein, type 1 plasminogen activator inhibitor, IL-6, TNF-α, IL-1β, and leukocyte adhesion molecules (51, 52), our studies underscore the importance of a FOXO1-NF-κB transcriptional mechanism in linking IR to the initiation and amplification of inflammation in liver diseases. In fatty liver, NF-κB is activated in the hepatocyte, and cytokines including IL-6, TNF-α, and IL-1β are overproduced (53). These proinflammatory cytokines act in a paracrine or autocrine fashion to activate Kupffer cells, the resident hepatic macrophages, leading to a vicious circle that causes worsening of liver damage, inflammation development, and disease progression (54–57). Here in this study, we showed that FOXO1 and CCL20 proteins are coordinately elevated in the liver of db/db mice (Fig. 8D), an obese and IR model that is associated with a low-grade inflammation, which was associated with increased infiltration of inflammatory cells (Fig. 8, F and G). Our findings thus uncover a novel molecular pathway linking impaired insulin signaling to the development of hepatic inflammation (Fig. 9). Given that pharmacological or genetic inhibition of inflammatory mediators was shown to markedly suppress the initiation or severity of NASH in rodent models (58, 59), and that insulin-sensitizing agents were also demonstrated to have beneficial effects on liver steatosis and NASH (60) in clinical trials, our data suggest that targeting FOXO1 may provide a potential therapeutic means to counteract IR and its associated inflammatory liver diseases.
Fig. 9.
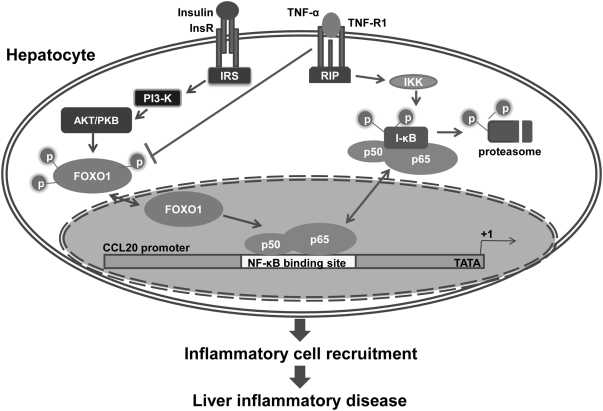
Proposed model for FOXO1-CCL20 pathway to enhance inflammatory infiltration in the insulin-resistant liver. Normally, insulin suppresses FOXO1 activation by sustaining FOXO1 phosphorylation through phosphatidylinositol 3 kinase/AKT pathway, preventing FOXO1 translocation to the nucleus. In the IR state, phosphorylated FOXO1 is reduced, and dephosphorylated FOXO1 is translocated into the nucleus to regulate transcription of its target genes. CCL20 may not be the direct target of FOXO1 because no FOXO1-binding sites are found in the proximal promoter of CCL20, and FOXO1 increases CCL20 promoter activity and expression only when the NF-κB pathway is activated by TNF-α. FOXO1 may somehow function as a coactivator of NF-κB to stimulate CCL20 production in hepatocytes, thereby exacerbating inflammatory infiltration in insulin-resistant livers. IRS, Insulin receptor substrate; insR, insulin receptor; RIP, receptor-interacting protein.
Materials and Methods
Materials
Recombinant human TNF-α was purchased from eBioscience. DMEM, fetal calf serum (FCS), and other culture media were purchased from Hyclone Laboratories, Inc. (Logan, UT). Optimem was provided by Life Technologies, Inc. (Gaithersburg, MD). Lipofectamine-2000 was purchased from Invitrogen (Carlsbad, CA). ELISA kits for human CCL20, mouse CCL20, and TNF-α were obtained from (R&D Systems, Inc.) Insulin was obtained from Sigma Chemical Co. (St. Louis, MO). Boyden chambers and ChIP assay kit were from Millipore Corp. (Bedford, MA). Human FOXO1-AAA plasmid, a constitutive active form construct with the three conserved Akt/PKB sites (Thr24, Ser 256, and Ser319) within FOXO1 specifically mutated into alanine, was kindly provided by Dr. Terry Unterman (University of Illinois at Chicago). Anti-pFOXO1 (S256) (catalog no. 9461), anti-tAKT (catalog no. 4691), anti-pAKT (Thr 308) (catalog no. 2965), anti-RelB (catalog no. 4922), anti-c-Rel (catalog no. 4727), anti-p50 (catalog no. 3035) and anti-p52 (catalog no. 4882) antibodies were purchased from Cell Signaling Technology (Danvers, MA). Anti-p65 (sc-109), anti-FOXO1 (sc-67140), anti-β-actin (sc-130656), and anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (sc-166574) antibodies were all from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
Cell culture and treatment
HepG2 cells were grown in DMEM supplemented with 10% FCS at 37 C in a humidified 5% CO2 atmosphere. Cells at approximately 70–80% confluence were washed with PBS and preincubated in serum-free medium for 6 h before treatment with TNF-α (10 ng/ml) or insulin (50 nm).
RNA isolation and RT-PCR
Total RNA were extracted from HepG2 cells using the Trizol reagent (Invitrogen) according to the manufacturer's protocol. The RT-PCR was performed using Revertra Ace-α-TM and KOD PLUS Kit (Toyobo, Osaka, Japan). Briefly, 12 μl volume of system containing 1 μg total RNA and 1 μl Oligo(dT)20 (10 pmol/μl) was preheated at 65 C for 5 min followed by addition of 4 μl 5 × reverse transcriptase buffer (including 25 mm Mg2+), 2 μl deoxynucleotide diphosphate mixture (10 mm each), 1 μl ribonuclease inhibitor (10 U/μl), and 1 μl ReverTra Ace. Reverse transcription was performed for 20 min at 42 C, 5 min at 85 C, and 5 min at 4 C. The 25-μl PCR system contains: template cDNA, 100 ng, 2.5 μl 10×PCR buffer, 0.2 mm deoxynucleotide diphosphate, 2.5 mm Mg2+, 0.4 μm primers, 0.02 U/μm DNA polymerase. The primers used in these RT-PCR are listed in Table 1. The DNA products were subjected to 1.2% agarose, and the relative DNA band intensity (agarose gel band intensity normalized with the intensity of β-actin for each sample) was quantified by Quantity One image software (Bio-Rad Laboratories, Inc., Hercules, CA). To evaluate the mRNA level of a macrophage marker F4/80 and a lymphocyte marker CD3 in mouse livers (GAPDH as internal control, primers listed in Table 1), quantitative real-time RT-PCR was employed as described previously (35).
Western blotting
Proteins were extracted using radioimmune precipitation assay lysis buffer (Beyotime) and quantified by the BCA kit (Beyotime) according to the provided protocol and then separated by 8 or 10% SDS-PAGE and transfered to a polyvinylidene difluoride membrane. The membrane was blocked with 5% nonfat milk and incubated with primary antibodies for 10 h at 4 C. The membranes were rinsed 5 times with PBS containing 0.1% Tween 20 and incubated for 1 h with the appropriate horseradish peroxidase-conjugated secondary antibody at 37 C. Membranes were extensively washed with PBS containing 0.1% Tween 20 and incubated with enhanced chemiluminescence substrate (Pierce Chemical Co., Rockford, IL) for 1 min and placed on film.
ELISA
The concentrations of TNF-α and CCL20 from the cultured HepG2 supernatant or mouse livers or mouse sera were measured using an ELISA kit (R&D Systems, Inc.) according to the manufacturer's protocol. Briefly, 100 μl CCL20 protein standards or samples in triplicate were added into monoclonal antibody-coated 96-well plates for 2 h at 37 C. The plates were washed five times after which 100 μl biotinylated antibody were added for 1 h at 37 C. After plates were washed, 100 μl/well enzyme conjugate were added and the plates incubated for 30 min at 37 C. The plates were washed again, and 100 μl 3,3′,5,5′-tetramethylbenzidine substrate solution was added for 15 min in the dark at 37 C. The reaction was terminated by the addition of 100 μl of stop solution. The optical density at 450 nm was measured using an ELISA plate reader (Thermo Electron Corp., Albany, NY) within 30 min. The standard curve was made with recombinant human (or mouse) CCL20 or TNF-α at concentrations ranging from 10 pg/ml to 10 ng/ml.
DNA constructs, cell transfection, and reporter gene assays
The DNA fragments for the six human CCL20 promoter fusion reporter constructs shown in Fig. 3E were generated from HepG2 cell genomic DNA by PCR amplification using KOD-Plus (Toyobo). The primers used for the DNA fragments are listed in Table 2. Amplified DNA fragments were subcloned into pGL3-Basic vector. Transient transfections were carried out using Lipofectamine-2000 according to the protocol from the manufacturer. Briefly, cells were plated 24 h before transfection at a density of 1 × 105/well on a 24-well tissue culture dish (Corning Costar, Wilkes-Barre, PA). HepG2 cells were incubated 3 h before transfection with fetal calf serum-free, antibiotic-free media and then transfected with 0.8 μg of CCL20 reporter gene plasmid or equal molar amounts of its truncated promoter reporter constructs. After transfection for 6 h, the media were removed and replaced with complete growth medium containing sterile PBS as control or 10 ng/ml TNF-α. After a further 24 h, HepG2 cells were washed twice with PBS and lysed with specific reporter lysis buffer. Then, the luciferase activities of the cell lysate were evaluated according to the manufacturer's instructions (Promega Corp., Madison, WI), and the total protein concentration in each well was measured as an internal control.
Table 2.
Primers used for CCL20 promoter fusion reporter constructions
| Promoter regions | Primers (5′ to 3′) |
|---|---|
| −2303/+42 | Forward: CGACGCGTCTCTAATCTTCATTTCCCTC |
| Reverse: GGCAGATCTCAGTGTTGAGTACCCAGTT | |
| −653/+42 | Forward: CGACGCGTGCCATGTGAATGTATAAGA |
| Reverse: GGCAGATCTCAGTGTTGAGTACCCAGTT | |
| −336/+42 | Forward: CGACGCGTTGACCTTTGTATCGCTGTT |
| Reverse: GGC AGATCTCAGTGTTGAGTACCCAGTT | |
| −170/+42 | Forward: CGACGCGT ACCTTCGCACCTTCCCAAT |
| Reverse: GGC AGATCTCAGTGTTGAGTACCCAGTT | |
| −108/+42 | Forward: CGACGCGTATGACATGATGGGGCCAGT |
| Reverse: GGCAGATCTCAGTGTTGAGTACCCAGTT | |
| −31/+42 | Forward: CGACGCGTGCTATAAATAGGGCCATCC |
| Reverse: GGCAGATCTCAGTGTTGAGTACCCAGTT |
Site-directed reporter gene mutation
NF-κB and SP1 specific binding sites in the human CCL20 promoter region were mutated using MutanBEST Kit (Takara Bio, Inc., Shiga, Japan) according to the manufacturer's protocol. Briefly, the required primers were designed as follows: for mut-NF-κB, the forward: 5′-aaacatgtggcaacacgccttct-3′, reverse: 5′-ttttccccattgatcaactgGC-3′; for mut-SP1, forward: 5′-AattctGtgtacattcccaa-3′, reverse: 5′-CGTGTTGCCACATGGGGTT-3′. PCR was performed using Pyrobest DNA Polymerase, followed by blunting, kination, and ligation reaction. Finally, the mutant plasmid was transformed into Escherichia coli DH5α, and the positive clone was selected and confirmed by DNA sequencing.
FOXO1 knockdown by RNA interference
Human FOXO1-specific siRNA (si-FOXO1): 5-AAGCCCTGGCTCTCACAGCAA-3 was designed according to a previous report (36) to knock down the expression of endogenous FOXO1 genes and the scrambled control RNA (siNC): 5′-UUCUCCGAACGUGUCACGUTT-3′ was provided by GenePharma (Shanghai, China). HepG2 cells were transfected using Lipofectamine-2000 following the manufacturer's instructions as previously described. TNF-α was added at 10 ng/ml for an additional 24 h after 24 h of transfection with 20 nmol/ml of siRNA. The samples were prepared for assays of RT-PCR, ELISA, Western blotting, Reporter assay, ChIP, or migration test as indicated.
ChIP assays
ChIP was used to study TNF-α- and FOXO1-induced interaction between NF-κB and the CCL20 promoter DNA in HepG2 cells. In brief, 1 × 106 HepG2 cells incubated in six-well dishes were cross-linked with 1% formaldehyde, followed by sonication in a Microson 500-W Ultrasonicator (Sonics & Materials, Inc., Newtown, CT) at 38% of maximum power for approximately eight to 10 consecutive cycles of 10-sec pulses. After centrifugation at 18,000 × g for 10 min, the supernatant with equal amounts of protein were immunoprecipitated with 1 μg of special antibody (anti-p65, anti-RelB, anti-C-rel, anti-p52, or anti-p50) or rabbit IgG as control using the ChIP kit (Millipore Corp.) according to the manufacturer's protocol. The immunoprecipitates were analyzed by PCR for detecting the coimmunoprecipitated DNA containing the functional κB-element in the CCL20 promoter; the primers were designed as: forward: 5′-tgaggaaaaagcaggaagtttt-3′, reverse: 5′-gtacacagaaggcgtgttgc-3′. Amplification conditions were denaturation at 94 C for 30 sec, annealing at 60 C for 30 sec, and extension at 72 C for 30 sec for 38 cycles, and the expected product was 106 bp.
PBMC migration test
Human PBMC were isolated from healthy donors by Ficoll-Hypaque gradient centrifugation (Pharmacia Biotech, Piscataway, NJ), washed three times in PBS, and resuspended in complete RPMI 1640 medium containing 10% FCS, 100 U/ml penicillin, 100 mg/ml streptomycin sulfate, and 2.5 mg/ml amphotericin B. The cells were allowed to adhere in a volume of 25 ml (2–3 × 106 cells/ml) to 150-cm2 plastic tissue culture flasks for 10 h at 37 C in humidified 5% CO2 to remove monocytes and macrophages. The nonadherent cells (mainly lymphocytes) were harvested for the migration test. The Migration studies were performed using series of six-well Boyden chambers (Millipore). The top and bottom chambers were separated by a polycarbonate filter with 8-μm pores. The bottom wells were loaded with 4.2 ml of cultured HepG2 cell supernatant from the designated treatments. Purified CCL20 (from Biotechnology Systems) at a concentration of 10 ng/ml DMEM was used as a positive control and DMEM medium alone was used as a negative control. The top wells were loaded with 2 ml of PBMC at a concentration of 5 × 105 cells/ml, followed by incubation at 37 C for 2 h. The migrated cells in the bottom chamber of each well were counted in five random fields using a microscope with a ×20 objective lens. The experiment was performed in triplicate wells of cells.
Animal studies
All animal experiments were conducted in accordance with the Third Military Medical University guidelines for the care and use of laboratory animals and were approved by the University Animal Care and Use Committee. Male homozygous db/db and heterozygous littermates db/+ were obtained at approximately 3–4 wk of age from The Jackson Laboratory. Age- and sex-matched C57BL/6 mice were purchased from the experimental animal center of the Third Military Medical University. Each group of mice was housed five per cage supplied with a normal rodent diet ad libitum in a pathogen-free facility with a 12-h light, 12-h dark cycle. Mice, 30 wk of age, fasted overnight, were anesthetized using pentobarbital sodium, blood samples (∼150 μl) were collected from the retroorbital vein for serum glucose, insulin, and TNF-α evaluation, and livers were isolated for RNA, protein assay, and hematoxylin-eosin staining.
Statistics
Data are generated as mean ± sd. Comparisons were performed using ANOVA for multiple groups or Student's t test for two groups. P < 0.05 is considered statistically significant.
Acknowledgments
We thank Dr. Hang Shi and Bingzhong Xue (Wake Forest University School of Medicine, Winston Salem, NC) for providing hepatic RNA samples from db/db mice. The manuscript was kindly read by Professor Arthur Cederbaum from the Department of Pharmacology and Systems Therapeutics, Mount Sinai School of Medicine (New York, NY).
This work was mainly supported by grants (30670998, 30971082) from the National Natural Science Foundation of China (to L.G.) and in part by Award No. R01DK085176 (to L.Y.) from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Health.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CCR6
- CC chemokine receptor 6
- ChIP
- chromatin immunoprecipitation
- CCL20
- chemokine (C-C motif) ligand 20
- FCS
- fetal calf serum
- FOXO1
- Forkhead box-containing protein O subfamily-1
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- IKK
- IκB kinase
- IR
- insulin resistance
- NASH
- nonalcoholic steatohepatitis
- NF-κB
- nuclear factor-κB
- PEPCK
- phosphoenolpyruvate carboxykinase
- PBMC
- peripheral blood mononuclear cell
- siRNA
- small interfering RNA
- SP1
- stimulatory protein 1.
References
- 1. Browning JD, Horton JD. 2004. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest 114:147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guo S, Rena G, Cichy S, He X, Cohen P, Unterman T. 1999. Phosphorylation of serine 256 by protein kinase B disrupts transactivation by FKHR and mediates effects of insulin on insulin-like growth factor-binding protein-1 promoter activity through a conserved insulin response sequence. J Biol Chem 274:17184–17192 [DOI] [PubMed] [Google Scholar]
- 3. Rena G, Guo S, Cichy SC, Unterman TG, Cohen P. 1999. Phosphorylation of the transcription factor forkhead family member FKHR by protein kinase B. J Biol Chem 274:17179–17183 [DOI] [PubMed] [Google Scholar]
- 4. del Peso L, González VM, Hernández R, Barr FG, Núñez G. 1999. Regulation of the forkhead transcription factor FKHR, but not the PAX3-FKHR fusion protein, by the serine/threonine kinase Akt. Oncogene 18:7328–7333 [DOI] [PubMed] [Google Scholar]
- 5. Nakae J, Kitamura T, Silver DL, Accili D. 2001. The forkhead transcription factor Foxo1 (Fkhr) confers insulin sensitivity onto glucose-6-phosphatase expression. J Clin Invest 108:1359–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barthel A, Schmoll D, Krüger KD, Bahrenberg G, Walther R, Roth RA, Joost HG. 2001. Differential regulation of endogenous glucose-6-phosphatase and phosphoenolpyruvate carboxykinase gene expression by the forkhead transcription factor FKHR in H4IIE-hepatoma cells. Biochem Biophys Res Commun 285:897–902 [DOI] [PubMed] [Google Scholar]
- 7. Kodama S, Koike C, Negishi M, Yamamoto Y. 2004. Nuclear receptors CAR and PXR cross talk with FOXO1 to regulate genes that encode drug-metabolizing and gluconeogenic enzymes. Mol Cell Biol 24:7931–7940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kamagate A, Qu S, Perdomo G, Su D, Kim DH, Slusher S, Meseck M, Dong HH. 2008. FoxO1 mediates insulin-dependent regulation of hepatic VLDL production in mice. J Clin Invest 118:2347–2364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sparks JD, Dong HH. 2009. FoxO1 and hepatic lipid metabolism. Curr Opin Lipidol 20:217–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Candia P, Blekhman R, Chabot AE, Oshlack A, Gilad Y. 2008. A combination of genomic approaches reveals the role of FOXO1a in regulating an oxidative stress response pathway. PLoS One 3:e1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kumashiro N, Tamura Y, Uchida T, Ogihara T, Fujitani Y, Hirose T, Mochizuki H, Kawamori R, Watada H. 2008. Impact of oxidative stress and peroxisome proliferator-activated receptor γ coactivator-1α in hepatic insulin resistance. Diabetes 57:2083–2091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Park SJ, Sohn HY, Yoon J, Park SI. 2009. Down-regulation of FoxO-dependent c-FLIP expression mediates TRAIL-induced apoptosis in activated hepatic stellate cells. Cell Signal 21:1495–1503 [DOI] [PubMed] [Google Scholar]
- 13. Su D, Coudriet GM, Hyun Kim D, Lu Y, Perdomo G, Qu S, Slusher S, Tse HM, Piganelli J, Giannoukakis N, Zhang J, Dong HH. 2009. FoxO1 links insulin resistance to proinflammatory cytokine IL-1β production in macrophages. Diabetes 58:2624–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ito Y, Daitoku H, Fukamizu A. 2009. Foxo1 increases pro-inflammatory gene expression by inducing C/EBPβ in TNF-α-treated adipocytes. Biochem Biophys Res Commun 378:290–295 [DOI] [PubMed] [Google Scholar]
- 15. Samuel VT, Choi CS, Phillips TG, Romanelli AJ, Geisler JG, Bhanot S, McKay R, Monia B, Shutter JR, Lindberg RA, Shulman GI, Veniant MM. 2006. Targeting foxo1 in mice using antisense oligonucleotide improves hepatic and peripheral insulin action. Diabetes 55:2042–2050 [DOI] [PubMed] [Google Scholar]
- 16. Valenti L, Rametta R, Dongiovanni P, Maggioni M, Fracanzani AL, Zappa M, Lattuada E, Roviaro G, Fargion S. 2008. Increased expression and activity of the transcription factor FOXO1 in nonalcoholic steatohepatitis. Diabetes 57:1355–1362 [DOI] [PubMed] [Google Scholar]
- 17. Charo IF, Ransohoff RM. 2006. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med 354:610–621 [DOI] [PubMed] [Google Scholar]
- 18. Oo YH, Shetty S, Adams DH. 2010. The role of chemokines in the recruitment of lymphocytes to the liver. Dig Dis 28:31–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rot A, von Andrian UH. 2004. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu Rev Immunol 22:891–928 [DOI] [PubMed] [Google Scholar]
- 20. Schaerli P, Moser B. 2005. Chemokines: control of primary and memory T-cell traffic. Immunol Res 31:57–74 [DOI] [PubMed] [Google Scholar]
- 21. Berres ML, Nellen A, Wasmuth HE. 2010. Chemokines as immune mediators of liver diseases related to the metabolic syndrome. Dig Dis 28:192–196 [DOI] [PubMed] [Google Scholar]
- 22. Bertola A, Bonnafous S, Anty R, Patouraux S, Saint-Paul MC, Iannelli A, Gugenheim J, Barr J, Mato JM, Le Marchand-Brustel Y, Tran A, Gual P. 2010. Hepatic expression patterns of inflammatory and immune response genes associated with obesity and NASH in morbidly obese patients. PLoS One 5:e13577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hieshima K, Imai T, Opdenakker G, Van Damme J, Kusuda J, Tei H, Sakaki Y, Takatsuki K, Miura R, Yoshie O, Nomiyama H. 1997. Molecular cloning of a novel human CC chemokine liver and activation-regulated chemokine (LARC) expressed in liver. Chemotactic activity for lymphocytes and gene localization on chromosome 2. J Biol Chem 272:5846–5853 [DOI] [PubMed] [Google Scholar]
- 24. Rossi DL, Vicari AP, Franz-Bacon K, McClanahan TK, Zlotnik A. 1997. Identification through bioinformatics of two new macrophage proinflammatory human chemokines: MIP-3α and MIP-3β. J Immunol 158:1033–1036 [PubMed] [Google Scholar]
- 25. Hromas R, Gray PW, Chantry D, Godiska R, Krathwohl M, Fife K, Bell GI, Takeda J, Aronica S, Gordon M, Cooper S, Broxmeyer HE, Klemsz MJ. 1997. Cloning and characterization of exodus, a novel β-chemokine. Blood 89:3315–3322 [PubMed] [Google Scholar]
- 26. Schutyser E, Struyf S, Van Damme J. 2003. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev 14:409–426 [DOI] [PubMed] [Google Scholar]
- 27. Yamashiro S, Wang JM, Yang D, Gong WH, Kamohara H, Yoshimura T. 2000. Expression of CCR6 and CD83 by cytokine-activated human neutrophils. Blood 96:3958–3963 [PubMed] [Google Scholar]
- 28. Harant H, Eldershaw SA, Lindley IJ. 2001. Human macrophage inflammatory protein-3α/CCL20/LARC/Exodus/SCYA20 is transcriptionally upregulated by tumor necrosis factor-α via a non-standard NF-κB site. FEBS Lett 509:439–445 [DOI] [PubMed] [Google Scholar]
- 29. Duffaut C, Zakaroff-Girard A, Bourlier V, Decaunes P, Maumus M, Chiotasso P, Sengenès C, Lafontan M, Galitzky J, Bouloumié A. 2009. Interplay between human adipocytes and T lymphocytes in obesity: CCL20 as an adipochemokine and T lymphocytes as lipogenic modulators. Arterioscler Thromb Vasc Biol 29:1608–1614 [DOI] [PubMed] [Google Scholar]
- 30. Hirata T, Osuga Y, Takamura M, Kodama A, Hirota Y, Koga K, Yoshino O, Harada M, Takemura Y, Yano T, Taketani Y. 2010. Recruitment of CCR6-expressing Th17 cells by CCL 20 secreted from IL-1 β-, TNF-α-, and IL-17A-stimulated endometriotic stromal cells. Endocrinology 151:5468–5476 [DOI] [PubMed] [Google Scholar]
- 31. Shimizu Y, Murata H, Kashii Y, Hirano K, Kunitani H, Higuchi K, Watanabe A. 2001. CC-chemokine receptor 6 and its ligand macrophage inflammatory protein 3α might be involved in the amplification of local necroinflammatory response in the liver. Hepatology 34:311–319 [DOI] [PubMed] [Google Scholar]
- 32. Fujiie S, Hieshima K, Izawa D, Nakayama T, Fujisawa R, Ohyanagi H, Yoshie O. 2001. Proinflammatory cytokines induce liver and activation-regulated chemokine/macrophage inflammatory protein-3α/CCL20 in mucosal epithelial cells through NF-κB [correction of NK-kappaB]. Int Immunol 13:1255–1263 [DOI] [PubMed] [Google Scholar]
- 33. Nakayama T, Fujisawa R, Yamada H, Horikawa T, Kawasaki H, Hieshima K, Izawa D, Fujiie S, Tezuka T, Yoshie O. 2001. Inducible expression of a CC chemokine liver- and activation-regulated chemokine (LARC)/macrophage inflammatory protein (MIP)-3 α/CCL20 by epidermal keratinocytes and its role in atopic dermatitis. Int Immunol 13:95–103 [DOI] [PubMed] [Google Scholar]
- 34. Nguyen H, Sankaran S, Dandekar S. 2006. Hepatitis C virus core protein induces expression of genes regulating immune evasion and anti-apoptosis in hepatocytes. Virology 354:58–68 [DOI] [PubMed] [Google Scholar]
- 35. Yang J, Goldstein JL, Hammer RE, Moon YA, Brown MS, Horton JD. 2001. Decreased lipid synthesis in livers of mice with disrupted Site-1 protease gene. Proc Natl Acad Sci USA 98:13607–13612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Alikhani M, Alikhani Z, Graves DT. 2005. FOXO1 functions as a master switch that regulates gene expression necessary for tumor necrosis factor-induced fibroblast apoptosis. J Biol Chem 280:12096–12102 [DOI] [PubMed] [Google Scholar]
- 37. Baba M, Imai T, Nishimura M, Kakizaki M, Takagi S, Hieshima K, Nomiyama H, Yoshie O. 1997. Identification of CCR6, the specific receptor for a novel lymphocyte-directed CC chemokine LARC. J Biol Chem 272:14893–14898 [DOI] [PubMed] [Google Scholar]
- 38. Durham SK, Suwanichkul A, Scheimann AO, Yee D, Jackson JG, Barr FG, Powell DR. 1999. FKHR binds the insulin response element in the insulin-like growth factor binding protein-1 promoter. Endocrinology 140:3140–3146 [DOI] [PubMed] [Google Scholar]
- 39. Kwon JH, Keates S, Simeonidis S, Grall F, Libermann TA, Keates AC. 2003. ESE-1, an enterocyte-specific Ets transcription factor, regulates MIP-3α gene expression in Caco-2 human colonic epithelial cells. J Biol Chem 278:875–884 [DOI] [PubMed] [Google Scholar]
- 40. Lorenzo M, Fernández-Veledo S, Vila-Bedmar R, Garcia-Guerra L, De Alvaro C, Nieto-Vazquez I. 2008. Insulin resistance induced by tumor necrosis factor-α in myocytes and brown adipocytes. J Anim Sci 86:E94–E104 [DOI] [PubMed] [Google Scholar]
- 41. Guo S, Dunn SL, White MF. 2006. The reciprocal stability of FOXO1 and IRS2 creates a regulatory circuit that controls insulin signaling. Mol Endocrinol 20:3389–3399 [DOI] [PubMed] [Google Scholar]
- 42. Matsuzaki H, Daitoku H, Hatta M, Tanaka K, Fukamizu A. 2003. Insulin-induced phosphorylation of FKHR (Foxo1) targets to proteasomal degradation. Proc Natl Acad Sci USA 100:11285–11290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Altomonte J, Cong L, Harbaran S, Richter A, Xu J, Meseck M, Dong HH. 2004. Foxo1 mediates insulin action on apoC-III and triglyceride metabolism. J Clin Invest 114:1493–1503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cheng Z, Guo S, Copps K, Dong X, Kollipara R, Rodgers JT, Depinho RA, Puigserver P, White MF. 2009. Foxo1 integrates insulin signaling with mitochondrial function in the liver. Nat Med 15:1307–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tilg H, Moschen AR. 2008. Insulin resistance, inflammation, and non-alcoholic fatty liver disease. Trends Endocrinol Metab 19:371–379 [DOI] [PubMed] [Google Scholar]
- 46. Sugita S, Kohno T, Yamamoto K, Imaizumi Y, Nakajima H, Ishimaru T, Matsuyama T. 2002. Induction of macrophage-inflammatory protein-3α gene expression by TNF-dependent NF-κB activation. J Immunol 168:5621–5628 [DOI] [PubMed] [Google Scholar]
- 47. Fan W, Yanase T, Morinaga H, Okabe T, Nomura M, Daitoku H, Fukamizu A, Kato S, Takayanagi R, Nawata H. 2007. Insulin-like growth factor 1/insulin signaling activates androgen signaling through direct interactions of Foxo1 with androgen receptor. J Biol Chem 282:7329–7338 [DOI] [PubMed] [Google Scholar]
- 48. Gregor MF, Hotamisligil GS. 2011. Inflammatory mechanisms in obesity. Annu Rev Immunol 29:415–445 [DOI] [PubMed] [Google Scholar]
- 49. Fan W, Morinaga H, Kim JJ, Bae E, Spann NJ, Heinz S, Glass CK, Olefsky JM. 2010. FoxO1 regulates Tlr4 inflammatory pathway signalling in macrophages. EMBO J 29:4223–4236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kortylewski M, Feld F, Krüger KD, Bahrenberg G, Roth RA, Joost HG, Heinrich PC, Behrmann I, Barthel A. 2003. Akt modulates STAT3-mediated gene expression through a FKHR (FOXO1a)-dependent mechanism. J Biol Chem 278:5242–5249 [DOI] [PubMed] [Google Scholar]
- 51. Lawrence T. 2009. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb Perspect Biol 1:a001651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shoelson SE, Lee J, Goldfine AB. 2006. Inflammation and insulin resistance. J Clin Invest 116:1793–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. 2005. Local and systemic insulin resistance resulting from hepatic activation of IKK-β and NF-κB. Nat Med 11:183–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Marra F, Gastaldelli A, Svegliati Baroni G, Tell G, Tiribelli C. 2008. Molecular basis and mechanisms of progression of non-alcoholic steatohepatitis. Trends Mol Med 14:72–81 [DOI] [PubMed] [Google Scholar]
- 55. Rivera CA. 2008. Risk factors and mechanisms of non-alcoholic steatohepatitis. Pathophysiology 15:109–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Syn WK, Choi SS, Diehl AM. 2009. Apoptosis and cytokines in non-alcoholic steatohepatitis. Clin Liver Dis 13:565–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tilg H, Moschen AR. 2010. Evolution of inflammation in nonalcoholic fatty liver disease: the multiple parallel hits hypothesis. Hepatology 52:1836–1846 [DOI] [PubMed] [Google Scholar]
- 58. Beraza N, Malato Y, Vander Borght S, Liedtke C, Wasmuth HE, Dreano M, de Vos R, Roskams T, Trautwein C. 2008. Pharmacological IKK2 inhibition blocks liver steatosis and initiation of non-alcoholic steatohepatitis. Gut 57:655–663 [DOI] [PubMed] [Google Scholar]
- 59. Mas E, Danjoux M, Garcia V, Carpentier S, Ségui B, Levade T. 2009. IL-6 deficiency attenuates murine diet-induced non-alcoholic steatohepatitis. PLoS One 4:e7929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lewis JR, Mohanty SR. 2010. Nonalcoholic fatty liver disease: a review and update. Dig Dis Sci 55:560–578 [DOI] [PubMed] [Google Scholar]