Abstract
Mucormycosis is a life-threatening infection that occurs in patients who are immunocompromised because of diabetic ketoacidosis, neutropenia, organ transplantation, and/or increased serum levels of available iron. Because of the increasing prevalence of diabetes mellitus, cancer, and organ transplantation, the number of patients at risk for this deadly infection is increasing. Despite aggressive therapy, which includes disfiguring surgical debridement and frequently adjunctive toxic antifungal therapy, the overall mortality rate is high. New strategies to prevent and treat mucormycosis are urgently needed. Understanding the pathogenesis of mucormycosis and the host response to invading hyphae ultimately will provide targets for novel therapeutic interventions. In this supplement, we review the current knowledge about the virulence traits used by the most common etiologic agent of mucormycosis, Rhizopus oryzae. Because patients with elevated serum levels of available iron are uniquely susceptible to mucormycosis and these infections are highly angioinvasive, emphasis is placed on the ability of the organism to acquire iron from the host and on its interactions with endothelial cells lining blood vessels. Several promising therapeutic strategies in preclinical stages are identified.
Mucormycosis is an infection caused by fungi belonging to the order Mucorales [1]. Rhizopus oryzae is the most common organism isolated from patients with mucormycosis and is responsible for ∼70% of all cases of mucormycosis [2–4]. The major risk factors for mucormycosis include uncontrolled diabetes mellitus in ketoacidosis, other forms of metabolic acidosis, treatment with corticosteroids, organ or bone marrow transplantation, neutropenia, trauma and burns, malignant hematologic disorders, and deferoxamine therapy in patients receiving hemodialysis [3, 5, 6]. Because of the increasing prevalence of diabetes mellitus, cancer, and organ transplantation in the aging US population, the number of patients at risk for this deadly infection is dramatically increasing [7]. Unfortunately, despite disfiguring surgical debridement and adjunct antifungal therapy, the overall mortality rate for mucormycosis remains >50%, and it approaches 100% among patients with disseminated disease or those with persistent neutropenia [3, 8]. Clearly new strategies to prevent and treat mucormycosis are urgently needed, and such strategies can be facilitated by clear understanding of the pathogenesis of the disease.
HOST DEFENSE AGAINST MUCORMYCOSIS
Clinical and experimental data clearly demonstrate that individuals who lack phagocytes or have impaired phagocytic function are at higher risk of mucormycosis. For example, severely neutropenic patients are at increased risk for developing mucormycosis. In contrast, patients with AIDS do not seem to be at increased risk for developing mucormycosis [5]. These findings suggest that neutrophils, but not necessarily T lymphocytes, are critical for inhibiting fungal spore proliferation. Furthermore, both mononuclear and polymorphonuclear phagocytes of normal hosts kill Mucorales by the generation of oxidative metabolites and the cationic peptides, defensins [9–11]. A recent study showed that exposure of neutrophils to R. oryzae hyphae results in up-regulation in Toll-like receptor 2 expression and in a robust proinflammatory gene expression with rapid induction of NF-κB pathway–related genes [12]. In the presence of hyperglycemia and low pH, which is found in patients with diabetic ketoacidosis (DKA), phagocytes are dysfunctional and have impaired chemotaxis and defective intracellular killing by both oxidative and nonoxidative mechanisms [13].
Concordant with these clinical observation, inhalation of Mucorales sporangiospores by immunocompetent animals does not result in the development of mucormycosis [9]. In contrast, corticosteroid-immunosuppressed or animals with DKA die of progressive pulmonary and hematogenously disseminated infection [9, 14]. Moreover, the ability of inhaled sporangiospores to germinate and form hyphae in the host is critical for establishing infection. Although pulmonary alveolar macrophages harvested from lungs of immunocompetent mice are able to ingest and inhibit germination of R. oryzae sporangiospores, these bronchoalveolar macrophages have limited capacity to kill the organism in vitro [9]. In contrast, pulmonary alveolar macrophages of immunosuppressed mice are unable even to prevent germination of the sporangiospores in vitro or after intranasal infection [9].
The exact mechanisms by which phagocytes are impaired by ketoacidosis, diabetes mellitus, and corticosteroids are yet to be determined. Furthermore, phagocyte dysfunction alone cannot explain the high incidence of mucormycosis among patients with DKA, because the incidence of mucormycosis among these patients is increased more than the incidence of infections caused by other pathogens [3, 5, 15]. Therefore, Mucorales must possess unique virulence traits that enable the organism to exploit the unique state of immunosuppression and physiologic impairment seen in this subset of patients (see below).
The skin barrier represents a host defense against cutaneous mucormycosis, as evidenced by the increased risk for developing mucormycosis in persons with disruption of this barrier. The agents of mucormycosis are typically incapable of penetrating intact skin. However, burns, traumatic disruption of the skin, and persistent maceration of skin enables the organism to penetrate into deeper tissues. These organisms could originate from traumatic implantation of contaminated soil or water (eg, the outbreaks after natural disasters, as was seen after the tsunami in Indonesia in 2004 and after the destructive tornadoes that occurred in Joplin, Missouri, in June 2011). Contaminated surgical dressings and nonsterile adhesive tape have been shown to be the source of primary cutaneous mucormycosis [16, 17]. Furthermore, mucormycosis can even be introduced through direct access, as was seen with the use of contaminated tongue depressors in neonates [18] or the use of contaminated wooden applicators used to mix drugs given to immunocompromised patients [19]. These cases illustrate an alarming shift in mucormycosis cases from mainly community-acquired infections to nosocomial infections in susceptible hosts.
IRON UPTAKE AND MUCORMYCOSIS PATHOGENESIS
In addition to host factors that predispose patients to mucormycosis, Mucorales possess virulence factors that enable the organism to cause disease. One such trait is the ability to acquire iron from the host. Iron is an essential element for cell growth and development, contributing to many vital processes of the cell [20]. Therefore, successful pathogens use multiple processes for obtaining iron from the host. Recent data demonstrate that the level of available, unbound iron in serum plays a critical factor in uniquely predisposing patients with DKA to mucormycosis [21, 22]. In mammalian hosts, iron is bound to host carrier proteins, such as transferrin, ferritin, and lactoferrin. This sequestration avoids toxic effect of free iron [20, 21]. This strategy of limiting iron availability is also a major universal host defense mechanism against microbes and against Mucorales in particular, because R. oryzae grows poorly in normal serum unless exogenous iron is added [21, 22].
The clinical observation that patients with DKA are uniquely susceptible to mucormycosis lends support to the role of iron uptake in the pathogenesis of the disease. Patients with DKA have elevated levels of free iron in their serum, and such serum supports growth of R. oryzae at acidic pH (7.3–6.88) but not at alkaline pH (7.78–8.38) [21]. Furthermore, adding exogenous iron to serum allowed R. oryzae to grow profusely at acidic conditions but not at pH ≥7.4. Finally, simulated acidic conditions decreased the iron-binding capacity of serum samples collected from healthy volunteers, suggesting that acidosis per se disrupts the capacity of transferrin to bind iron, probably by proton-mediated displacement of ferric iron from transferrin [23]. As proof of principle, animal data showed that mice with DKA were protected from R. oryzae infection by administration of iron chelators, such as deferiprone and deferasirox [24, 25], which are not used by Mucorales as xenosiderophores. However, not all Mucorales have the same susceptibility to effective iron chelators. For example, a study showed that Cunninghamella bertholletiae and Mucor species display higher deferasirox minimal inhibitory and fungicidal concentrations than do Rhizopus species [26].
Another clinical observation highlights the central role of host iron availability in predisposing patients to mucormycosis. Patients receiving dialysis who are treated with the iron chelator deferoxamine are also uniquely susceptible to a deadly form of mucormycosis [27–30]. The bacterial siderophore, deferoxamine, predisposes patients to Rhizopus infection by acting as a xenosiderophore [22]. Deferoxamine strips ferric iron from transferrin and attaches itself on the mold through an inducible receptor, and the iron is transported intracellularly by an active reduction of the ferric form into the more soluble ferrous form [31]. Concordant with these results, administration of deferoxamine worsens survival among guinea pigs infected with Rhizopus but not among those infected with Candida albicans [22, 31, 32]. In addition, in vitro studies of radiolabeled iron uptake from deferoxamine in serum show that Rhizopus is able to incorporate 8-fold and 40-fold more iron than can Aspergillus fumigatus and C. albicans, respectively [22]. Finally, a major risk factor for mucormycosis in transplantation includes underlying myelodysplastic syndrome, which probably predisposes patients to the disease because of iron overload resulting from repeated blood transfusions [33].
Fungi can obtain iron from the host by using high-affinity iron permeases or low-molecular-weight iron chelators (siderophores) [20, 34]. The high-affinity iron permeases are present in fungi as part of a reductive system containing redundant surface reductases that reduce ferric into the more soluble ferrous form. The reduced ferrous iron generated by the surface reductase is, in turn, captured by a protein complex consisting of a multicopper oxidase and a ferrous permease [34–36]. The genome sequencing project identified 3 ferric reductases, 6 copper oxidases, and 1 high-affinity iron permease. Indeed, recent data show that the gene encoding high-affinity iron permease (FTR1) is expressed by R. oryzae during murine infection and inhibition of FTR1 gene expression by RNA-I, or reduction of FTR1 copy number by gene disruption reduces the virulence of the fungus in animal models of mucormycosis [37]. Of importance, passive immunization with anti-Ftr1p immune serum protected mice with DKA from infection with R. oryzae [37]. Thus, FTR1 is a crucial virulence factor for R. oryzae, and anti-Ftr1p passive immunotherapy represents a promising strategy to improve outcomes of deadly mucormycosis.
Rhizopus is known to secrete rhizoferrin, a siderophore that belongs to the polycarboxylate family [38]. This siderophore supplies Rhizopus with iron through a receptor-mediated, energy-dependent process [31, 38]. In this regard, the genome-sequencing project of R. oryzae identified 13 possible siderophore permeases that might act as receptors for siderophores, including rhizoferrin or deferoxamine (Table 1). However, it is not currently known whether rhizoferrin transports iron by release of iron extracellularly or whether the siderophore is internalized before releasing iron in the cytoplasm. What is known is that rhizoferrin is inefficient in obtaining iron from serum [22, 31]; therefore, the contribution of the organism’s endogenous siderophores to its virulence in a mammalian host is likely to be minimal. The lack of rhizoferrin ability to take iron from serum is also highlighted by the adaptation of the organism to use xenosiderophores, such as deferoxamine, which are more efficient in obtaining iron from the host.
Table 1.
Rhizopus oryzae Genes Believed to Be Involved in Iron Uptake.
| Iron Metabolism-Related Genes | Open Reading Frame |
| Reductive system | |
| Ferric reductases | RO3G_05460.1 |
| RO3G_04617.1 | |
| RO3G_10468.1 | |
| Copper oxidases | RO3G_03472.1 |
| RO3G_07290.1 | |
| RO3G_10569.1 | |
| RO3G_06637.1 | |
| RO3G_15489.1 | |
| RO3G_07572.1 | |
| High-affinity permease | RO3G_03471.1 |
| Siderophore permeases | RO3G_16758.1 |
| RO3G_05990.1 | |
| RO3G_00075.1 | |
| RO3G_12627.1 | |
| RO3G_11434.1 | |
| RO3G_09431.1 | |
| RO3G_02798.1 | |
| RO3G_16094.1 | |
| RO3G_02779.1 | |
| RO3G_02337.1 | |
| RO3G_10304.1 | |
| RO3G_03821.1 | |
| RO3G_00019.1 | |
| Uptake from heme (heme oxygenase) | RO3G_07326 |
| RO3G_13316 | |
| SreA | RO3G_07659.3 |
| Ferritin | RO3G_08744.3 |
| RO3G_14254.3 |
These genes were identified from the Fungal Genome Sequencing Initiative at the Broad Institute (http://www.broadinstitute.org/annotation/genome/rhizopus_oryzae/MultiHome.html).
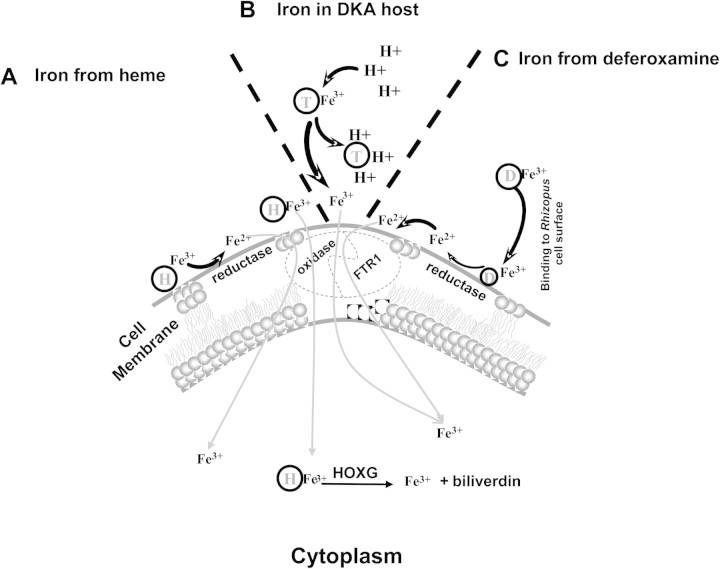
A third mechanism by which fungi can obtain iron from the host is through use of heme [39, 40]. The Rhizopus genome project revealed 2 homologues of the heme oxygenase (Table 1) [41]. These 2 R. oryzae homologues may enable R. oryzae to obtain iron from host hemoglobin and might explain the angioinvasive nature of R. oryzae. Of interest, we found that R. oryzae that had reduced copy numbers of FTR1 also had lagging growth on media supplemented with heme [37]. Therefore, FTR1 in R. oryzae may act as a cytoplasmic membrane permease that facilitates intracellular heme uptake, which is followed by release of ferric iron through degradation with heme oxygenases intracellularly. Other genes likely to be involved in the ability of R. oryzae to take up iron include SreA, a transcriptional regulator that has been described in A. fumigatus to be required for adaptation to the ambient iron availability [42], and 2 orthologes probably encoding ferritin required for intracellular storage of iron. Figure 1 shows the 3 mechanisms of iron uptake that are likely to be operative during mucormycosis.
Figure 1.
Proposed mechanisms of iron uptake by Mucorales during mucormycosis. A, Because of the angioinvasive nature of the disease, heme (H) is likely to represent a source of iron to the invading fungus, which either takes up heme intracellularly or strips ferric iron from heme by the action of the reductase-permease system. If heme is transported intracellulary, ferric iron is obtained by the action of heme oxygenase in the cytoplasm. B, In patients with DKA, proton (H+)–mediated displacement of ferric iron (Fe3+) from transferrin (T) increases the availability of iron, which is transported intracellularly by the reductase-permease system. C, Deferoxamine (D) directly chelates iron from transferrin, resulting in ferrioxamine (iron-deferoxamine complex). The fungus then liberates ferrous iron from ferrioxamine by reduction at the cell surface. In all cases, iron is transported across the cell membrane by the copper oxidase–iron permease (FTR1) complex.
HOST-PATHOGEN INTERACTIONS
Mucormycosis infections are characterized by extensive angioinvasion that results in vessel thrombosis and subsequent tissue necrosis [3, 6, 43]. Ischemic necrosis of infected tissues can prevent delivery of leukocytes and antifungal agents to the foci of infection. This angioinvasion likely contributes to the capacity of the organism to hematogenously disseminate to other target organs. Consequently, damage of and penetration through endothelial cells or the extracellular matrix proteins lining blood vessels is likely to be a critical step in the pathogenetic strategy of R. oryzae. Therefore, understanding the mechanisms by which these processes occur may lead to new approaches to prevent and/or treat mucormycosis.
An earlier study showed that R. oryzae can adhere to the extracellular matrix laminin and type IV collagen [44]. We have found that R. oryzae strains adhere to human umbilical vein endothelial cells in vitro and invade these cells by induced endocytosis [45]. Endocytosed R. oryzae damages endothelial cells, and prevention of endocytosis abrogates the ability of the organisms to cause endothelial cell damage [45]. More recently, glucose-regulated protein (GRP78) was identified to act as a receptor that mediates penetration through and damage of endothelial cells by Mucorales [46]. GRP78 (also known as BiP/HSPA5) was discovered as a cellular protein induced by glucose starvation [47]. It is a member of the HSP70 protein family that is mainly present in the endoplasmic reticulum. It functions as a major chaperone that is involved in many cellular processes, including protein folding and assembly, marking misfolded proteins for proteosome degradation [48], regulating calcium homeostasis, and serving as a sensor for endoplasmic reticulum stress [49]. Despite its main function as a cellular chaperone protein, recent studies reported the translocation of a fraction of GRP78 to the cell surface in a variety of cells [50].
It is of interest that elevated concentrations of glucose and iron consistent with those noted during DKA-enhanced surface GRP78 expression and resulting penetration through and damage of endothelial cells by Mucorales in a receptor-dependent manner. Mice with DKA had enhanced susceptibility to mucormycosis and had increased expression of GRP78 in the sinus, lungs, and brains, compared with normal mice. Note that anti-GRP78 immune serum protected mice with DKA from mucormycosis [46]. Although it is currently unknown whether anti-GRP78 immune serum can protect the neutropenic host from mucormycosis, these observations provide novel insight into the unique susceptibility of patients with DKA to mucormycosis and could provide a foundation for novel therapeutic interventions.
Unlike most fungi (eg, A. fumigatus), which are nonpathogenic in Drosophila melanogaster flies, Mucorales rapidly infect and kill wild-type flies [51]. Whole-genome expression profiling in wild-type flies after infection with R. oryzae, compared with A. fumigatus, identified genes selectively down-regulated by R. oryzae that act in pathogen recognition, immune defense, stress response, detoxification, steroid metabolism, or tissue repair [51].
OTHER PUTATIVE VIRULENCE FACTORS
Some Rhizopus species, especially R. microsporus [52] and R. chinensis [53], are known for their ability to produce the mycotoxin rhizoxin, an antimitotic macrocyclic polyketide metabolite. Recent studies demonstrated that rhizoxin is not biosynthesized by Rhizopus, but rather by an intracellular, symbiotic bacterium of the genus Burkholderia [54]. Rhizoxin has long been known to be crucial to the plant pathogenic strategy of Rhizopus, but it does not seem to have a substantive role in causing mammalian disease. Organisms that have been rendered to be bacteria free by ciprofloxacin treatment or those that cannot produce rhizoxin because of the absence of Burkholderia are still pathogenic in mouse and fruit fly models of infection [55].
Other putative virulence factors include the ability of Rhizopus to secrete lytic enzymes, including aspartic proteinases [56]. In addition, Rhizopus species have an active ketone reductase system, which may be an additional virulence factor by enhancing growth in the acidic and glucose-rich environment seen in ketoacidotic states [57]. To date, none of these potential virulence factors have been definitively proven to be essential for the development of mucormycosis. Finally, a study demonstrated that Mucorales gain increased and transient virulence in 2 infectious models when exposed to voriconazole [14]. Although the study did not explore the mechanism by which voriconazole exposure increases virulence of Mucorales, it may provide an explanation for the emergence of mucormycosis in patients with hematologic malignancies who are receiving voriconazole prophylaxis [58–62].
Differences in virulence across species in the order Mucorales may also provide insight into the complex repertoire of virulence factors causing aggressive invasive disease in some species and infrequent mortality in others, despite environmental ubiquity. Cunninghamella bertholettiae carries the highest mortality rates among reported cases of infection with Mucorales. However, little is known about the properties of this organism that contribute to its pathogenesis. Differences in the aforementioned virulence factors and host evasion may contribute to enhanced infection. Other factors, such as differential thermotolerance and rate of germination at the interface of pulmonary alveolar macrophages, may also contribute to interspecies differences in virulence.
FUTURE DIRECTIONS
The logical extension of the observations of the roles of key virulence factors, such as iron use by R. oryzae, is to develop therapeutic strategies that will translate to interventional clinical trials. Such clinical trials require considerable time and effort in study design, implementation, and analysis. The possible benefits of interventions that would complement existing therapies would be profound for patients with mucormycosis.
Notes
Acknowledgments.
We would like to dedicate this manuscript to Henry Schueller, the heroic child who died of this devastating infection, and his parents who continue to fight for a cure.
Financial support.
This work was supported in part by the US Public Health Service (grants R01 AI063503 and R21 AI082414 to A. S. I.).
Supplement sponsorship.
This article was published as part of a supplement entitled “Advances Against Mucormycosis: A Tribute to the Memory and Courage of Hank Schueler,” sponsored by the Henry Schueler 41&9 Foundation.
Potential conflicts of interest.
A. S. I. has received grant support from Astellas Pharma US, Enzon, Gilead, Merck, Pfizer, NovaDigm Therapeutics, and Novartis and is a shareholder of NovaDigm Therapeutics and Spectral Platforms. B. S. has received grant support from Gilead, Astellas Pharma US, and Novartis; has been a consultant for Merck, Pfizer, Arpida, Theravance, Advanced Life Sciences, Basilea, The Medicines Company, Novo Nordisk, Novartis, and Cerexa; and is a shareholder of NovaDigm Therapeutics. T. W. has provided consultations to Novartis, Trius, iCo Therapeutics, Vestagen, Sigma Tau, and Drais Pharmaceuticals and has received grants from Novartis and Astellas Pharma US, Inc. D. P. K. has received grant support and honoraria from Schering-Plough, Pfizer, Astellas Pharma US Inc., Enzon Pharmaceuticals, and Merck.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Hibbett DS, Binder M, Bischoff JF, et al. A higher-level phylogenetic classification of the Fungi. Mycol Res. 2007;111:509–47. doi: 10.1016/j.mycres.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Ribes JA, Vanover-Sams CL, Baker DJ. Zygomycetes in human disease. Clin Microbiol Rev. 2000;13:236–301. doi: 10.1128/cmr.13.2.236-301.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spellberg B, Edwards J, Jr, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18:556–69. doi: 10.1128/CMR.18.3.556-569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634–53. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- 5.Sugar AM. Agents of mucormycosis and related species. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and practice of infectious diseases. 6th ed. Philadelphia, PA: Elsevier; 2005. p. 2979. [Google Scholar]
- 6.Ibrahim AS, Edwards JE, Filler SG. Zygomycosis. In: Dismukes WE, Pappas PG, Sobel JD, editors. Clinical mycology. New York, NY: Oxford University Press; 2003. pp. 241–51. [Google Scholar]
- 7.Marr KA, Carter RA, Crippa F, Wald A, Corey L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2002;34:909–17. doi: 10.1086/339202. [DOI] [PubMed] [Google Scholar]
- 8.Gleissner B, Schilling A, Anagnostopolous I, Siehl I, Thiel E. Improved outcome of zygomycosis in patients with hematological diseases? Leuk Lymphoma. 2004;45:1351–60. doi: 10.1080/10428190310001653691. [DOI] [PubMed] [Google Scholar]
- 9.Waldorf AR, Ruderman N, Diamond RD. Specific susceptibility to mucormycosis in murine diabetes and bronchoalveolar macrophage defense against Rhizopus. J Clin Invest. 1984;74:150–60. doi: 10.1172/JCI111395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waldorf AR. Pulmonary defense mechanisms against opportunistic fungal pathogens. Immunol Ser. 1989;47:243–71. [PubMed] [Google Scholar]
- 11.Diamond RD, Haudenschild CC, Erickson NF., 3rd Monocyte-mediated damage to Rhizopus oryzae hyphae in vitro. Infect Immun. 1982;38:292–7. doi: 10.1128/iai.38.1.292-297.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chamilos G, Lewis RE, Lamaris G, Walsh TJ, Kontoyiannis DP. Zygomycetes hyphae trigger an early, robust proinflammatory response in human polymorphonuclear neutrophils through toll-like receptor 2 induction but display relative resistance to oxidative damage. Antimicrob Agents Chemother. 2008;52:722–4. doi: 10.1128/AAC.01136-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chinn RY, Diamond RD. Generation of chemotactic factors by Rhizopus oryzae in the presence and absence of serum: relationship to hyphal damage mediated by human neutrophils and effects of hyperglycemia and ketoacidosis. Infect Immun. 1982;38:1123–9. doi: 10.1128/iai.38.3.1123-1129.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamaris GA, Ben-Ami R, Lewis RE, Chamilos G, Samonis G, Kontoyiannis DP. Increased virulence of Zygomycetes organisms following exposure to voriconazole: a study involving fly and murine models of zygomycosis. J Infect Dis. 2009;199:1399–406. doi: 10.1086/597615. [DOI] [PubMed] [Google Scholar]
- 15.Kwon-Chung KJ, Bennett JE. Medical mycology. Philadelphia, PA: Lea & Febiger; 1992. Mucormycosis; pp. 524–59. [Google Scholar]
- 16.Gartenberg G, Bottone EJ, Keusch GT, Weitzman I. Hospital-acquired mucormycosis (Rhizopus rhizopodiformis) of skin and subcutaneous tissue: epidemiology, mycology and treatment. New Engl J Med. 1978;299:1115–8. doi: 10.1056/NEJM197811162992007. [DOI] [PubMed] [Google Scholar]
- 17.Mead JH, Lupton GP, Dillavou CL, Odom RB. Cutaneous Rhizopus infection. Occurrence as a postoperative complication associated with an elasticized adhesive dressing. JAMA. 1979;242:272–4. doi: 10.1001/jama.242.3.272. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell SJ, Gray J, Morgan ME, Hocking MD, Durbin GM. Nosocomial infection with Rhizopus microsporus in preterm infants: association with wooden tongue depressors [see comments] Lancet. 1996;348:441–3. doi: 10.1016/s0140-6736(96)05059-3. [DOI] [PubMed] [Google Scholar]
- 19.Verweij PE, Voss A, Donnelly JP, de Pauw BE, Meis JF. Wooden sticks as the source of a pseudoepidemic of infection with Rhizopus microsporus var. rhizopodiformis among immunocompromised patients. J Clin Microbiol. 1997;35:2422–3. doi: 10.1128/jcm.35.9.2422-2423.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Howard DH. Acquisition, transport, and storage of iron by pathogenic fungi. Clin Microbiol Rev. 1999;12:394–404. doi: 10.1128/cmr.12.3.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Artis WM, Fountain JA, Delcher HK, Jones HE. A mechanism of susceptibility to mucormycosis in diabetic ketoacidosis: transferrin and iron availability. Diabetes. 1982;31:1109–14. doi: 10.2337/diacare.31.12.1109. [DOI] [PubMed] [Google Scholar]
- 22.Boelaert JR, de Locht M, Van Cutsem J, et al. Mucormycosis during deferoxamine therapy is a siderophore-mediated infection: in vitro and in vivo animal studies. J Clin Invest. 1993;91:1979–86. doi: 10.1172/JCI116419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ibrahim AS, Spellberg B, Edwards J., Jr Iron acquisition: a novel perspective on mucormycosis pathogenesis and treatment. Curr Opin Infect Dis. 2008;21:620–5. doi: 10.1097/QCO.0b013e3283165fd1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ibrahim AS, Edwards JE, Jr, Fu Y, Spellberg B. Deferiprone iron chelation as a novel therapy for experimental mucormycosis. J Antimicrob Chemother. 2006;58:1070–3. doi: 10.1093/jac/dkl350. [DOI] [PubMed] [Google Scholar]
- 25.Ibrahim AS, Gebermariam T, Fu Y, et al. The iron chelator deferasirox protects mice from mucormycosis through iron starvation. J Clin Invest. 2007;117:2649–57. doi: 10.1172/JCI32338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis RE, Pongas GN, Albert N, Ben-Ami R, Walsh TJ, Kontoyiannis DP. Activity of deferasirox in Mucorales: influences of species and exogenous iron. Antimicrob Agents Chemother. 2011;55:411–13. doi: 10.1128/AAC.00792-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boelaert JR, Fenves AZ, Coburn JW. Deferoxamine therapy and mucormycosis in dialysis patients: report of an international registry. Am J Kidney Dis. 1991;18:660–7. doi: 10.1016/s0272-6386(12)80606-8. [DOI] [PubMed] [Google Scholar]
- 28.Boelaert JR, Fenves AZ, Coburn JW. Registry on mucormycosis in dialysis patients. J Infect Dis. 1989;160:914. doi: 10.1093/infdis/160.5.914. [DOI] [PubMed] [Google Scholar]
- 29.Boelaert JR, Fenves AZ, Coburn JW. Mucormycosis among patients on dialysis. New Engl J Med. 1989;321:190–1. [PubMed] [Google Scholar]
- 30.Boelaert JR, van Roost GF, Vergauwe PL, Verbanck JJ, de Vroey C, Segaert MF. The role of desferrioxamine in dialysis-associated mucormycosis: report of three cases and review of the literature. Clin Nephrol. 1988;29:261–6. [PubMed] [Google Scholar]
- 31.de Locht M, Boelaert JR, Schneider YJ. Iron uptake from ferrioxamine and from ferrirhizoferrin by germinating spores of Rhizopus microsporus. Biochem Pharmacol. 1994;47:1843–50. doi: 10.1016/0006-2952(94)90314-x. [DOI] [PubMed] [Google Scholar]
- 32.Boelaert JR, Van Cutsem J, de Locht M, Schneider YJ, Crichton RR. Deferoxamine augments growth and pathogenicity of Rhizopus, while hydroxypyridinone chelators have no effect. Kidney Int. 1994;45:667–71. doi: 10.1038/ki.1994.89. [DOI] [PubMed] [Google Scholar]
- 33.Maertens J, Demuynck H, Verbeken EK, et al. Mucormycosis in allogeneic bone marrow transplant recipients: report of five cases and review of the role of iron overload in the pathogenesis. Bone Marrow Transplant. 1999;24:307–12. doi: 10.1038/sj.bmt.1701885. [DOI] [PubMed] [Google Scholar]
- 34.Stearman R, Yuan DS, Yamaguchi-Iwai Y, Klausner RD, Dancis A. A permease-oxidase complex involved in high-affinity iron uptake in yeast. Science. 1996;271:1552–7. doi: 10.1126/science.271.5255.1552. [DOI] [PubMed] [Google Scholar]
- 35.Knight SA, Vilaire G, Lesuisse E, Dancis A. Iron acquisition from transferrin by Candida albicans depends on the reductive pathway. Infect Immun. 2005;73:5482–92. doi: 10.1128/IAI.73.9.5482-5492.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jung WH, Sham A, Lian T, Singh A, Kosman DJ, Kronstad JW. Iron source preference and regulation of iron uptake in Cryptococcus neoformans. PLoS Pathog. 2008;4:e45. doi: 10.1371/journal.ppat.0040045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ibrahim AS, Gebremariam T, Lin L, et al. The high affinity iron permease is a key virulence factor required for Rhizopus oryzae pathogenesis. Mol Microbiol. 2010;77:587–604. doi: 10.1111/j.1365-2958.2010.07234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thieken A, Winkelmann G. Rhizoferrin: a complexone type siderophore of the Mucorales and entomophthorales (Zygomycetes) FEMS Microbiol Lett. 1992;73:37–41. doi: 10.1016/0378-1097(92)90579-d. [DOI] [PubMed] [Google Scholar]
- 39.Santos R, Buisson N, Knight S, Dancis A, Camadro JM, Lesuisse E. Haemin uptake and use as an iron source by Candida albicans: role of CaHMX1-encoded haem oxygenase. Microbiology. 2003;149:579–88. doi: 10.1099/mic.0.26108-0. [DOI] [PubMed] [Google Scholar]
- 40.Worsham PL, Goldman WE. Quantitative plating of Histoplasma capsulatum without addition of conditioned medium or siderophores. J Med Vet Mycol. 1988;26:137–43. [PubMed] [Google Scholar]
- 41.Ma LJ, Ibrahim AS, Skory C, et al. Genomic analysis of the basal lineage fungus Rhizopus oryzae reveals a whole-genome duplication. PLoS Genet. 2009;5:e1000549. doi: 10.1371/journal.pgen.1000549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schrettl M, Kim HS, Eisendle M, et al. SreA-mediated iron regulation in Aspergillus fumigatus. Mol Microbiol. 2008;70:27–43. doi: 10.1111/j.1365-2958.2008.06376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ben-Ami R, Luna M, Lewis RE, Walsh TJ, Kontoyiannis DP. A clinicopathological study of pulmonary mucormycosis in cancer patients: extensive angioinvasion but limited inflammatory response. J Infect. 2009;59:134–8. doi: 10.1016/j.jinf.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bouchara JP, Oumeziane NA, Lissitzky JC, Larcher G, Tronchin G, Chabasse D. Attachment of spores of the human pathogenic fungus Rhizopus oryzae to extracellular matrix components. Eur J Cell Biol. 1996;70:76–83. [PubMed] [Google Scholar]
- 45.Ibrahim AS, Spellberg B, Avanessian V, Fu Y, Edwards JE., Jr Rhizopus oryzae adheres to, is phagocytosed by, and damages endothelial cells in vitro. Infect Immun. 2005;73:778–83. doi: 10.1128/IAI.73.2.778-783.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu M, Spellberg B, Phan QT, et al. The endothelial cell receptor GRP78 is required for mucormycosis pathogenesis in diabetic mice. J Clin Invest. 2010;120:1914–24. doi: 10.1172/JCI42164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee AS. GRP78 induction in cancer: therapeutic and prognostic implications. Cancer Res. 2007;67:3496–9. doi: 10.1158/0008-5472.CAN-07-0325. [DOI] [PubMed] [Google Scholar]
- 48.Ni M, Lee AS. ER chaperones in mammalian development and human diseases. FEBS Lett. 2007;581:3641–51. doi: 10.1016/j.febslet.2007.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li J, Lee AS. Stress induction of GRP78/BiP and its role in cancer. Curr Mol Med. 2006;6:45–54. doi: 10.2174/156652406775574523. [DOI] [PubMed] [Google Scholar]
- 50.Wang M, Wey S, Zhang Y, Ye R, Lee AS. Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxid Redox Signal. 2009;11:2307–16. doi: 10.1089/ars.2009.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chamilos G, Lewis RE, Hu J, et al. Drosophila melanogaster as a model host to dissect the immunopathogenesis of zygomycosis. Proc Natl Acad Sci U S A. 2008;105:9367–72. doi: 10.1073/pnas.0709578105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jennessen J, Nielsen KF, Houbraken J, et al. Secondary metabolite and mycotoxin production by the Rhizopus microsporus group. J Agric Food Chem. 2005;53:1833–40. doi: 10.1021/jf048147n. [DOI] [PubMed] [Google Scholar]
- 53.White JD, Blakemore PR, Green NJ, et al. Total synthesis of rhizoxin D, a potent antimitotic agent from the fungus Rhizopus chinensis. J Org Chem. 2002;67:7750–60. doi: 10.1021/jo020537q. [DOI] [PubMed] [Google Scholar]
- 54.Partida-Martinez LP, Hertweck C. Pathogenic fungus harbours endosymbiotic bacteria for toxin production. Nature. 2005;437:884–8. doi: 10.1038/nature03997. [DOI] [PubMed] [Google Scholar]
- 55.Ibrahim AS, Gebremariam T, Liu M, et al. Bacterial endosymbiosis is widely present among zygomycetes but does not contribute to the pathogenesis of mucormycosis. J Infect Dis. 2008;198:1083–90. doi: 10.1086/591461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Farley PC, Sullivan PA. The Rhizopus oryzae secreted aspartic proteinase gene family: an analysis of gene expression. Microbiology. 1998;144:2355–66. doi: 10.1099/00221287-144-8-2355. [DOI] [PubMed] [Google Scholar]
- 57.Anand VK, Alemar G, Griswold JA., Jr Intracranial complications of mucormycosis: an experimental model and clinical review. Laryngoscope. 1992;102:656–62. doi: 10.1288/00005537-199206000-00011. [DOI] [PubMed] [Google Scholar]
- 58.Imhof A, Balajee SA, Fredricks DN, Englund JA, Marr KA. Breakthrough fungal infections in stem cell transplant recipients receiving voriconazole. Clin Infect Dis. 2004;39:743–6. doi: 10.1086/423274. [DOI] [PubMed] [Google Scholar]
- 59.Kontoyiannis DP, Lionakis MS, Lewis RE, et al. Zygomycosis in a tertiary-care cancer center in the era of Aspergillus-active antifungal therapy: a case-control observational study of 27 recent cases. J Infect Dis. 2005;191:1350–60. doi: 10.1086/428780. [DOI] [PubMed] [Google Scholar]
- 60.Marty FM, Cosimi LA, Baden LR. Breakthrough zygomycosis after voriconazole treatment in recipients of hematopoietic stem-cell transplants. N Engl J Med. 2004;350:950–2. doi: 10.1056/NEJM200402263500923. [DOI] [PubMed] [Google Scholar]
- 61.Oren I. Breakthrough zygomycosis during empirical voriconazole therapy in febrile patients with neutropenia. Clin Infect Dis. 2005;40:770–1. doi: 10.1086/427759. [DOI] [PubMed] [Google Scholar]
- 62.Siwek GT, Dodgson KJ, de Magalhaes-Silverman M, et al. Invasive zygomycosis in hematopoietic stem cell transplant recipients receiving voriconazole prophylaxis. Clin Infect Dis. 2004;39:584–7. doi: 10.1086/422723. [DOI] [PubMed] [Google Scholar]