Abstract
Aims
Angiotensin II (Ang II) has been shown to have both central and peripheral effects in mediating hypertension, for which the hypothalamic paraventricular nucleus (PVN) is an important brain cardio-regulatory centre. Angiotensin-converting enzyme 2 (ACE2) has been identified as a negative regulator of the pro-hypertensive actions of Ang II. Recent findings from our laboratory suggest that Ang II infusion decreases ACE2 expression in the PVN. In the present study, we hypothesized that ACE2 overexpression in the PVN will have beneficial effects in counteracting Ang II-induced hypertension.
Methods and results
Male Sprague-Dawley rats were used in this study. Bilateral microinjection of an adenovirus encoding hACE2 (Ad-ACE2) into the PVN was used to overexpress ACE2 within this region. Mean arterial pressure measured by radiotelemetry was significantly increased after 14 days in Ang II-infused (200 ng/kg/min) rats vs. saline-infused controls (162.9 ± 3.6 vs. 102.3 ± 1.5 mmHg). Bilateral PVN microinjection of Ad-ACE2 attenuated this Ang II-induced hypertension (130.2 ± 5.7 vs. 162.9 ± 3.6 mmHg). ACE2 overexpression also significantly decreased AT1R and ACE expression and increased AT2R and Mas expression in the PVN. Additionally, ACE2 overexpression in the PVN attenuated the Ang II-induced increase in the expression of the pro-inflammatory cytokines tumour necrosis factor-α, interleukin (IL)-1β and IL-6 in the PVN.
Conclusion
Our findings suggest that attenuation of pro-inflammatory cytokines in the PVN in combination with the shift of the renin–angiotensin system towards the anti-hypertensive axis (ACE2/Ang-(1–7)/Mas) may be responsible for the overall beneficial effects of ACE2 overexpression in the PVN on the Ang II-induced hypertensive response.
Keywords: Angiotensin II, ACE2, Cytokines, Hypertension, Paraventricular nucleus
1. Introduction
The renin–angiotensin system (RAS) plays an important role in the regulation of blood pressure and volume homeostasis through its effects on vasoconstriction, cardiac remodelling, sympathetic outflow, and vasopressin synthesis and release.1 Angiotensin II (Ang II), the main physiologically active effector peptide of the RAS, exerts its actions mainly via interaction with the angiotensin II type-1 receptor (AT1R), thereby contributing to blood pressure regulation.2 In addition to the classic endocrine system, the discovery of RAS components in various tissues throughout the body confirmed the existence of a local or tissue RAS, which participates in the local synthesis, release, and action of all angiotensin peptides.3,4 Components of the RAS have been identified in multiple brain areas that are involved in the central regulation of blood pressure, including the paraventricular nucleus (PVN), subfornical organ (SFO), rostral ventrolateral medulla (RVLM), area postrema and nucleus tractus solitarius, among others.5 Moreover, increased circulating Ang II by peripheral infusion can increase neuronal activity in these regions and is implicated in autonomic and cardiovascular regulation leading to sympathetic hyperactivity and neurogenic hypertension.6–8
The hypothalamic PVN is recognized as a critical central nervous system centre for the coordination of autonomic and neuroendocrine homeostatic responses.9,10 The PVN contains a complex profile of excitatory and inhibitory neurotransmitters and neuromodulators, and receives inputs from a wide variety of sources, including peripheral receptors, higher brain centres (e.g. cortex and amygdala), and the circumventricular organs.9,11
Several studies support the role of the PVN in blood pressure control. Microinjection of Ang II into the PVN of rats increases mean arterial pressure (MAP), which is attenuated following systemic administration of losartan, an AT1R antagonist.12 It has also been shown that electrolytic or chemical ablation of the PVN attenuated the development of high blood pressure in spontaneously hypertensive (SH) rats, DOCA-salt hypertensive rats, and Dahl salt-sensitive rats.13–15 Others have shown that activation of the PVN results in sympathoexcitation16 and increased blood pressure.17 Recent evidence also suggests that hypertension is an inflammatory condition where various pro-inflammatory cytokines (PICs) such as tumour necrosis factor (TNF)-α, interleukin (IL)-6, and IL-1β, both centrally and in the periphery, have been shown to play an important role in the pathogenesis of hypertension.18–21 We have recently reported that chronic peripheral Ang II infusion results in an increased production of PICs in the PVN.22
Previous studies on the RAS have identified new components and have proposed alternate signalling pathways, such as the ACE2/Ang-(1–7)/Mas receptor pathway, in addition to the classical ACE/Ang II/AT1R pathway.23 ACE2 is considered a critical enzyme of the RAS cascade that is potentially important in counterbalancing the vasoconstrictor and proliferative effects of Ang II with the vasodilatory and anti-proliferative effects of Ang-(1–7) and other-related peptides.24,25 Several recent studies showed that alterations in ACE2 expression are implicated in cardiovascular diseases. Targeted disruption of ACE2 in mice results in severe cardiac contractility defects with elevated cardiac Ang II levels.26 ACE2 overexpression by systemic lentiviral delivery prevents cardiac hypertrophy in an Ang II infusion rat model of hypertension,25 and exerts protective effects on high blood pressure and cardiac pathophysiology induced by hypertension in SH rats.27 Furthermore, lentiviral-mediated overexpression of ACE2 in the RVLM decreases high blood pressure in SH rats.28 Recently, it has been shown that brain-targeted overexpression of an adenovirus coding for human ACE2 (Ad-ACE2) in the SFO reduces the acute Ang II-mediated pressor and drinking responses.29
Based on these observations, we hypothesized that ACE2 overexpression within the PVN will have beneficial effects in counteracting the Ang II-induced hypertensive response. To test this hypothesis, rats were injected with an Ad-ACE2 or its control (Ad-eGFP) bilaterally into the PVN to test against the Ang II-mediated hypertensive response. Our observations demonstrate that overexpression of ACE2 in the PVN attenuated the Ang II-induced inflammatory and hypertensive response.
2. Methods
Detailed description of methodologies can be found in the accompanying Supplementary material online.
2.1. Animals
Male Sprague-Dawley rats (12 weeks old) were used in this study. Animals were housed in a temperature-controlled room (25 ± 1°C) and maintained on a 12:12 h light:dark cycle with free access to water and normal laboratory rat chow (0.4% salt). This study conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication no. 85–23, revised 1996). All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee at Louisiana State University.
2.2. Experimental design
Rats were anaesthetized with a ketamine (90 mg/kg) and xylazine (10 mg/kg) mixture (ip) and implanted with radio-telemetry transmitters for continuous blood pressure recording. Following a 7-day surgical recovery, Ad-ACE2 or Ad-eGFP was injected bilaterally intra-PVN using a pressure injector. Osmotic minipumps (Alzet, model 2002) with an infusion rate of 0.5 µL/h for 14 days were filled with Ang II (Bachem, 200 ng/kg/min) dissolved in 0.9% saline or saline alone. The minipumps were implanted subcutaneously (sc) in the retroscapular area at the time of the adenoviral injections. The rats were divided into four groups: (i) Ad-eGFP group: saline minipump (sc) + intra-PVN Ad-eGFP, (ii) Ad-ACE2 group: saline minipump (sc) + intra-PVN Ad-ACE2, (iii) Ang II + Ad-eGFP group: Ang II minipump (sc) + intra-PVN Ad-eGFP, (iv) Ang II + Ad-ACE2 group: Ang II minipump (sc) + intra-PVN Ad-ACE2. Blood pressure was measured using radio telemetry for 2 weeks. Rats were euthanized after 14 days with CO2 inhalation and brains collected for mRNA and protein measurements.
2.3. Blood pressure measurement
Blood pressure was measured continuously in conscious rats implanted with radio-telemetry transmitters (Model TA11PA-C40, Data Sciences International, St Paul, MN, USA). Rats were anaesthetized with a ketamine (90 mg/kg) and xylazine (10 mg/kg) mixture (ip) and placed dorsally on a heated surgical table. The adequacy of anaesthesia was monitored by limb withdrawal response to toe pinching. An incision was made on the ventral surface of the left leg, and the femoral artery and vein were exposed and bluntly dissected apart. The femoral artery was ligated distally, and a small clamp was used to temporarily interrupt the blood flow. The catheter tip was introduced through a small incision in the femoral artery, advanced ∼6 cm into the abdominal aorta such that the tip was distal to the origin of the renal arteries, and secured into place. The body of the transmitter was placed into the abdominal cavity and sutured to the abdominal wall. The abdominal musculature was sutured, and the skin layer was closed. Rats received benzathine penicillin (30 000 U, im) and buprenorphine (0.1 mg/kg, sc) immediately following surgery and 12 h postoperatively. The rats were allowed to recover for 7 days after the surgery.
2.4. Bilateral intra-PVN injections
Rats were anaesthetized with a ketamine (90 mg/kg) and xylazine (10 mg/kg) mixture (ip). The adequacy of anaesthesia was monitored by limb withdrawal response to toe pinching. The rats were placed in a stereotaxic instrument (Kopf instruments; Tujunga, CA, USA) and the skull was exposed through an incision on the midline of the scalp. After bregma was identified, the coordinates for the PVN were determined from the Paxinos and Watson (2007) rat atlas, at 1.8 mm posterior, 0.4 mm lateral to the bregma, and 7.9 mm ventral to the zero level. Ad-ACE2 or Ad-eGFP virus was injected bilaterally intra-PVN [2 × 106 plaque-forming units (p.f.u.), 200 nL] using a pressure injector (Tritech Research, Los Angeles, CA, USA), as described previously.29 The Ad-ACE2 virus was generated by cloning 2418-bp hACE2 fragment (GenBank accession no. AF291820) into a pacAd5 CMV IRES eGFP pA shuttle vector.29
2.5. Real-time RT–PCR
The rats were euthanized using CO2 inhalation, brains were quickly removed, flash frozen in liquid nitrogen and stored at −80°C. Coronal sections of brains were made using a cryostat microtome and the PVN punches were made from frozen brain sections using a Stoelting brain punch with a diameter of 1.0 mm (Stoelting). Total RNA was isolated from PVN tissue using RNeasy plus micro kit (Qiagen), and cDNA was synthesized using the iScript cDNA synthesis kit (Bio-Rad). Real-time PCR amplification reactions were performed with iQ SYBR Green Super mix with ROX (Bio-Rad) using the ABI Prism 7900 Real-time PCR machine (Applied Biosystems). The primer sequences used for real-time PCR were given in Supplementary material online. Data were normalized to GAPDH expression by the ▵▵CT comparative method.
2.6. Western blot analysis
Western blot analysis was performed according to standard protocols. The PVN tissue was homogenized with RIPA lysis buffer. Equal amounts of protein (5 µg) were separated by SDS–PAGE on 10% (wt/vol) gels and transferred on to PVDF membrane (Immobilon-P, Millipore), and blocked with 1% BSA in TBS-T at room temperature for 60 min. The membranes were subjected to immunoblot analyses with anti-AT1 (Santa Cruz), anti-AT2 (Santa Cruz), anti-ACE2 (Santa Cruz), anti-hACE2 (R&D systems), anti-Mas (Almone Labs), and anti-GAPDH (Santa Cruz) antibodies (1:200 dilution). Immunodetection was accomplished with a horse radish anti-rabbit or anti-goat secondary antibody (1:2000 dilution) using an enhanced chemiluminescence kit (Amersham). The data were quantified by the densitometry using Chemidoc XRS system and Quantity-One software (Bio-Rad), and were normalized to GAPDH expression.
2.7. Immunohistochemical analysis
Rats (n= 6 in each group) were euthanized using CO2 inhalation and transcardially perfused with 200 mL of ice-cold PBS (7.4 pH; 0.1 M) followed immediately by 200 mL of 4% paraformaldehyde in PBS. The brains were removed, post-fixed in 4% paraformaldehyde solution for 2 h, and transferred to a 0.1% phosphate buffer containing 20% sucrose (pH 7.4) and stored overnight. Frozen brain tissues were sectioned in the coronal plane (10 µm) and sections without immunostaining were used to examine GFP expression. For immunostaining, 10-µm coronal sections from paraffin embedded brains were collected on slides. First the sections were incubated with 0.3% H2O2 in methanol for 10 min. For antigen retrieval, citrate buffer with microwave heating technique is used. Then the sections were incubated with 1.5% goat or rabbit serum in PBS containing 0.3% Triton X100 for 30 min. The sections were incubated with primary antibodies anti-hACE2 (R&D systems), anti AT1R (Santa Cruz) 1:50 dilution overnight at 4°C followed by incubation with biotinylated goat-anti rabbit or rabbit-anti goat secondary antibodies 1:100 dilution for 60 min, and stained with Vectastain ABC kit (Vector Laboratories) according to the manufacturer's instructions. Each step was followed by washing the sections with PBS containing 0.3% Triton X-100. Sections incubated without primary antibody were used as negative controls.
2.8. ACE2 activity assay
The rats were euthanized using CO2 inhalation; the brains were quickly removed, frozen on dry ice and stored at −80°C until use. The tissue from brain hypothalamus containing the PVN was collected and ACE2 activity was measured as described previously.29–31
2.9. Statistical analysis
Data are presented as mean ± SEM. Data were analysed, when appropriate, by Student's t-test, repeated measures ANOVA, or one-way ANOVA followed by Newman–Keuls correction for multiple comparisons between means. Statistical comparisons were performed using Prism5 (GraphPad Software). Differences were considered statistically significant at P< 0.05.
3. Results
3.1. Ad-ACE2 gene transfer increases the expression of ACE2 in the PVN
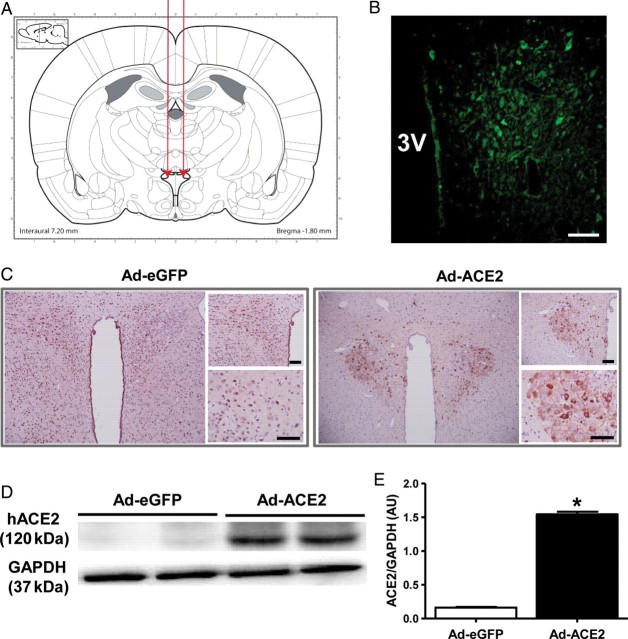
To determine the role of ACE2 in the central regulation of blood pressure, we used an adenovirus encoding human ACE2 gene to overexpress ACE2 in the PVN (Figure 1A). Figure 1B shows the localization of ACE2 gene expression in the PVN as indicated by enhanced green fluorescence protein (eGFP) expression. Figure 1C shows increased ACE2 immunoreactivity, using an antibody staining against hACE2, in the PVN region that received Ad-ACE2 injection when compared with the PVN region that received Ad-eGFP. Similarly, hACE2 protein expression was detected only in the PVN of Ad-ACE2 injected rats (Figure 1D and E). To determine the functionality of the protein expressed, following the initial intra-PVN injection of Ad-ACE2, ACE2 activity was measured in rat PVNs at different time points (Supplemental material online, Figure S1). ACE2 activity in the PVN was significantly increased 7 days after injection and achieved a maximum at 14 days post-injection. ACE2 activity had returned to baseline 21 days following Ad-ACE2 injection. These results demonstrate that ACE2 expression and activity are increased in the PVN following bilateral Ad-ACE2 microinjection.
Figure 1.
Overexpression of ACE2 in the PVN. (A) Schematic showing delivery of Adenovirus to the PVN. (B) Increased ACE2 immunofluorescence within the PVN after Ad-ACE2 injection (C) ACE2 immunoreactivity was increased in the PVN following Ad-ACE2 injection when compared with Ad-eGFP injection. Scale bar, 100 µm. (D) Western blot using specific antibody against human ACE2 (hACE2) and (E) densitometric analysis showing a significant increase in hACE2 protein expression in the PVN compared with control Ad-eGFP. *P< 0.05 compared with Ad-eGFP.
3.2. ACE2 overexpression and Ang II-induced hypertension
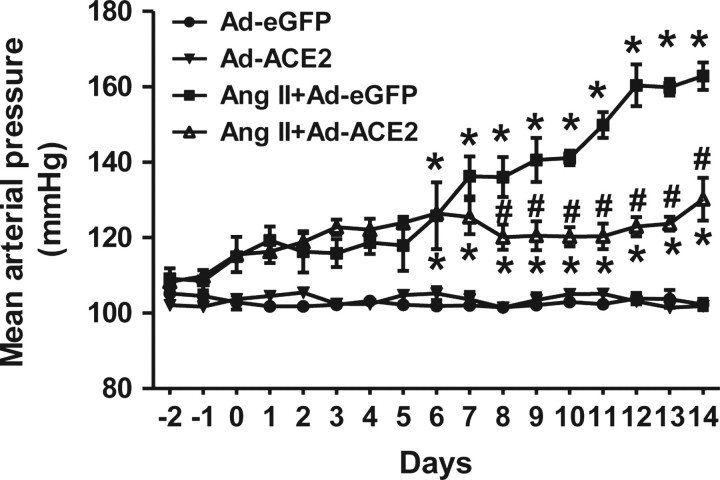
To assess the effect of overexpression of ACE2 in the PVN on Ang II-induced hypertensive response, BP was measured continuously. Figure 2 shows the effects of bilateral ACE2 overexpression on MAP. Ad-ACE2 alone did not produce any significant change in MAP when compared with control Ad-eGFP rats. Chronic 14-day Ang II infusion significantly increased MAP in rats that had received bilateral PVN microinjection of Ad-eGFP virus (102.3 ± 1.5 mmHg in Ad-eGFP vs.162.9 ± 3.6 mmHg in Ang II + Ad-eGFP). In contrast, Ang II-treated rats that had received bilateral PVN microinjection of Ad-ACE2 virus had a significantly decreased MAP (130.2 ± 5.7 mmHg in Ang II + Ad-ACE2 vs.162.9 ± 3.6 mmHg in Ang II + Ad-eGFP), though the MAP was not decreased to control levels. These data indicate that ACE2 expression within the PVN plays an important regulatory role in Ang II-induced hypertension.
Figure 2.
Overexpression of ACE2 in the PVN reduces Ang II hypertension. Ang II infusion significantly increased MAP in Ad-eGFP injected control rats. Overexpression of ACE2 by bilateral PVN microinjection of Ad-ACE2 virus alone did not produce any significant change in MAP when compared with control Ad-eGFP rats. In contrast, bilateral ACE2 overexpression in the PVN significantly reduced Ang II-mediated hypertension, though the MAP was not decreased to control levels. n= 5–6/group; *P< 0.05 compared with Ad-eGFP; #P< 0.05 compared with Ang II + Ad-eGFP.
3.3. ACE2 overexpression and RAS components
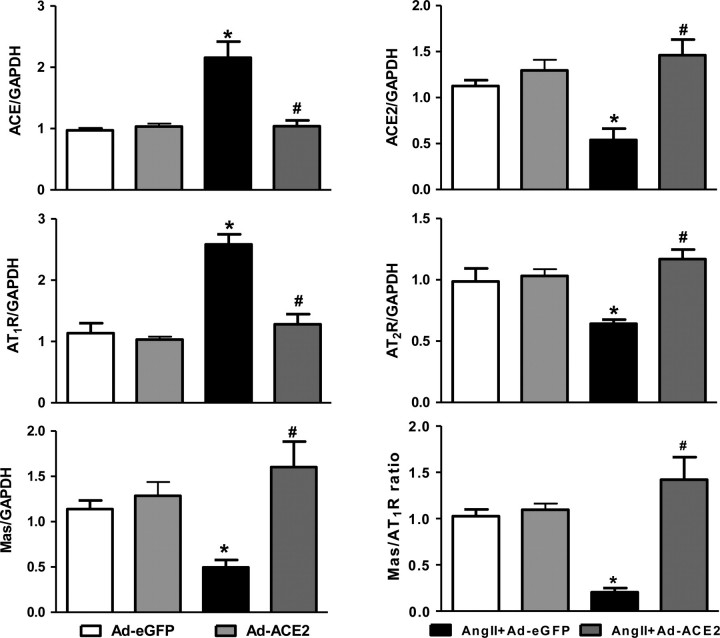
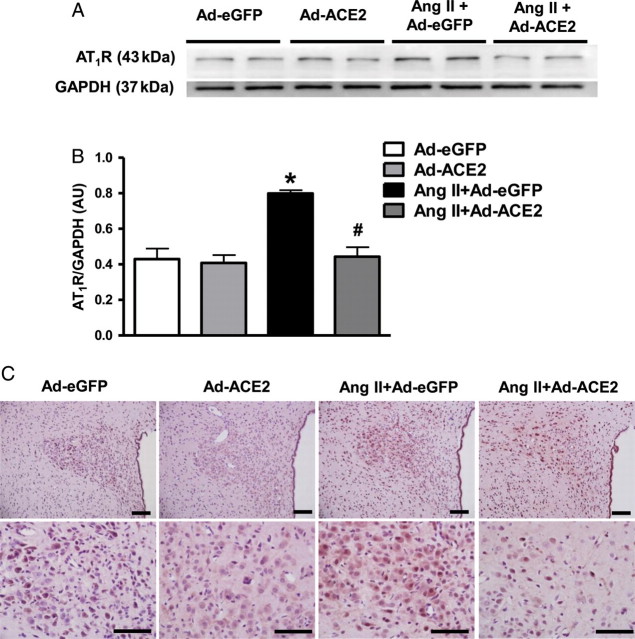
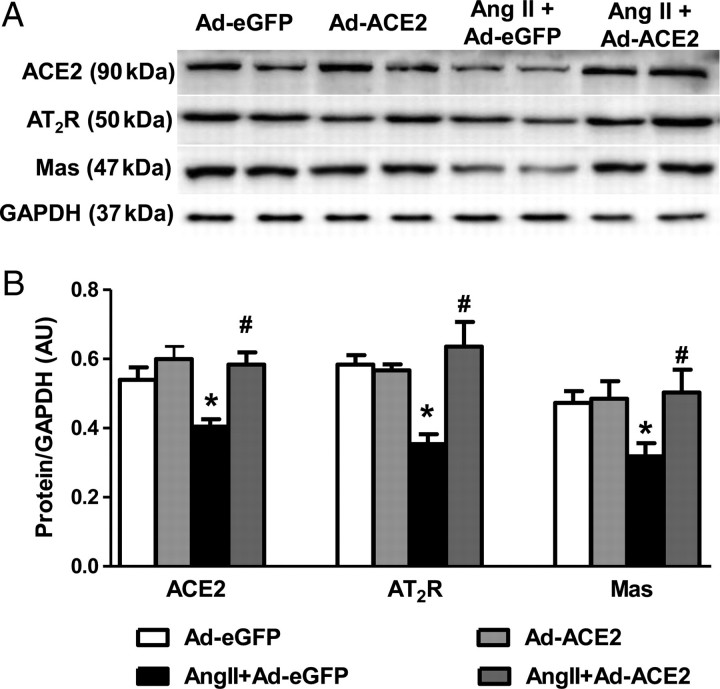
To study the effect of ACE2 overexpression on other RAS components, we measured the expression levels of ACE, ACE2, AT1R, AT2R and the Mas receptor by real-time PCR, western blot, and immunohistochemistry. Ang II infusion significantly increased mRNA expression of ACE and AT1R, and decreased ACE2, AT2R, and Mas mRNA levels in the PVN when compared with Ad-eGFP rats. Bilateral overexpression of ACE2 in the PVN reversed these gene expression changes (Figure 3). These results were further confirmed by western blot and immunohistochemical analysis for AT1R protein levels in the PVN (Figure 4). Similarly, the changes in protein expression were examined by western blot using specific antibodies against ACE2, AT2R, and Mas receptor. Ang II infusion significantly decreased ACE2, AT2R, and Mas receptor protein expression in the PVN when compared with saline infused control rats; this was prevented by bilateral PVN ACE2 overexpression (Figure 5).
Figure 3.
Effect of ACE2 overexpression on RAS components expression in the PVN. Ang II infusion significantly increased mRNA expression of ACE and AT1R, and decreased ACE2, AT2R, Mas mRNA expression, and Mas/AT1 ratio in the PVN when compared with control rats. Bilateral overexpression of ACE2 in the PVN reversed these gene expression changes. n= 9/group; *P< 0.05 compared with Ad-eGFP; #P< 0.05 compared with Ang II + Ad-eGFP.
Figure 4.
Overexpression of ACE2 down-regulates AT1R expression in the PVN of Ang II-infused rats. Western blot (A), and quantitative data (B) showing a reduction in AT1R expression in the PVN of Ang II-infused rats following ACE2 overexpression. (C) This was further confirmed by immunohistochemistry against AT1R. Scale bar, 100 µm; n= 6/group; *P< 0.05 compared with Ad-eGFP; #P< 0.05 compared with Ang II + Ad-eGFP.
Figure 5.
Effect of ACE2 overexpression on ACE2, AT2R and Mas protein expression in the PVN. Protein expression was measured by western blot using specific antibodies against ACE2, AT2R and the Mas receptor and data were normalized using GAPDH expression. Densitometric analysis of western blot shows that 14 days Ang II infusion reduced protein expression of ACE2, AT2R and Mas receptor in the PVN. ACE2 overexpression reversed these changes in the PVN. n= 6/group; *P< 0.05 compared with Ad-eGFP; #P< 0.05 compared with Ang II + Ad-eGFP.
3.4. ACE2 overexpression and pro-inflammatory cytokines
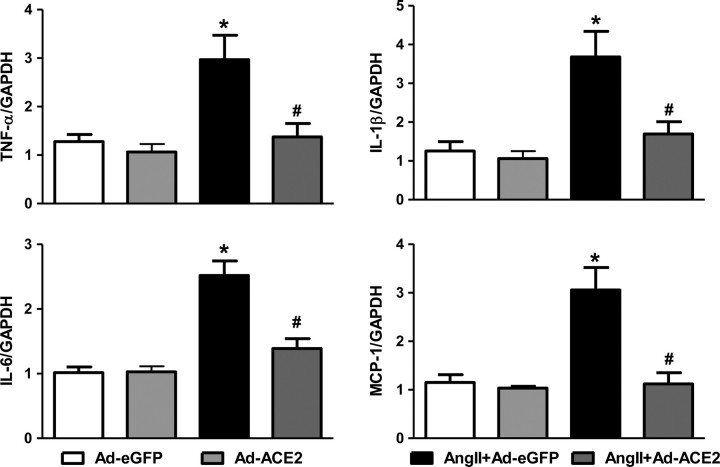
To study the effect of ACE2 overexpression on PIC production in the PVN, we measured the expression levels of PICs by real-time PCR. Ang II infusion significantly increased the PIC mRNA expression of TNF-α, IL-1β, IL-6, and the chemokine MCP-1 in the PVN vs. control Ad-eGFP rats. ACE2 overexpression attenuated the Ang II-induced increase in TNF-α, IL-1β, IL-6, and MCP-1 gene expression in the PVN (Figure 6). These results suggest that the beneficial effects of ACE2 overexpression on Ang II-induced hypertensive response might be, in part, mediated by reduction in the expression of PICs.
Figure 6.
Overexpression of ACE2 reduces pro-inflammatory cytokine and chemokine expression in the PVN. mRNA expression in the PVN was measured using real-time-RT–PCR and normalized to GAPDH expression. ACE2 overexpression significantly reduced the Ang II-induced increases in TNF-α, IL-1β, IL-6 and MCP-1 mRNA expression in the PVN. n= 6–9/group; * P< 0.05 compared with Ad-eGFP; #P< 0.05 compared with Ang II + Ad-eGFP.
4. Discussion
In this study, we investigated the effects of bilateral overexpression of ACE2 in the hypothalamic PVN of the brain on Ang II-induced hypertension. The salient findings of this study are: (i) Chronic Ang II infusion significantly increased MAP and reduced the mRNA and protein expression of ACE2 in the PVN; (ii) ACE2 overexpression resulted in increased ACE2 activity and the down-regulation of AT1R expression in the PVN, thus reducing the Ang II-mediated hypertension; (iii) Ang II infusion significantly increased the expression of PICs in the PVN, which was attenuated by ACE2 overexpression; (iv) ACE2 overexpression resulted in increased expression of the anti-hypertensive components of RAS (ACE2, Mas, and AT2R). These findings indicate that the Ang II-induced hypertensive effects are attenuated via bilateral PVN ACE2 overexpression. This demonstrates the importance of ACE2 within the PVN, an important central cardiovascular regulatory region, as well as the implications an imbalanced RAS can have on hypertensive drive.
In the present study, we found that overexpression of ACE2 in the PVN alone without Ang II infusion did not have any effect on BP response, but that overexpression of ACE2 within the PVN attenuated the Ang II-mediated hypertension. It has been shown that lentiviral-mediated overexpression of ACE2 in the heart27 or RVLM28 of Wistar-Kyoto rats had no effect on BP. Also, transgenic mice with brain-targeted overexpression of hACE2 remained normotensive;32 supporting the idea that overexpression of ACE2 does not have any effect on basal BP regulation, but plays a major role in the pathophysiology of hypertension.
In our study, ACE2 overexpression resulted in decreased AT1R expression and increased the expression of Mas and AT2R, shifting the RAS balance towards the protective axis. Studies examining the anti-hypertensive effects of ACE2 overexpression in SH rats27,28 and Ang II-infused mice29 are consistent with the current results of our study. Previous studies also showed down-regulation of ACE2 in the heart, kidney, and brain of several hypertensive animal models and in patient populations,33 with this ACE2 down-regulation commonly being attributed to AT1R activation. Furthermore, it has been shown that when ACE2 is overexpressed in the SFO of mice, AT1R expression was also ultimately decreased, suggesting that ACE2 can, directly or indirectly, affect AT1R transcription and/or internalization.29 Another recent study in mice with human ACE2 overexpression targeted selectively to neurons by using a synopsin promoter (Syn-hACE2 transgenic mice) showed that ACE2 overexpression in the brain resulted in AT1R down-regulation and attenuation of neurogenic hypertension.32 These findings suggest an important interplay between ACE2 and AT1R activity in hypertension. Furthermore, this corroborates with the present study, in that chronic Ang II infusion resulted in both decreased mRNA and protein expression of ACE2, and an increased expression of AT1R in the PVN, and that ACE2 overexpression reversed these changes. These results further confirm that the AT1R in the PVN mediates the central inhibitory effects on ACE2 in Ang II-mediated hypertension, as previously suggested.31 Furthermore, the prevention of hypertension by ACE2 overexpression in Syn-hACE2 mice was reversed by blockade of Ang-(1–7) receptor by d-Ala7-Ang-(1–7). Following Ang II infusion, both Mas/AT1R and AT2R/AT1R ratios were increased in the presence of overexpressed ACE2 in these mice. In our study, ACE2 overexpression in the PVN caused an up-regulated expression of ACE2, Mas, and AT2R, resulting in an increased Mas/AT1R ratio, thus favouring the anti-hypertensive axis of the RAS.
Accumulating evidence shows that hypertension is a chronic low grade inflammatory condition where various PICs, such as TNF-α, IL-6 and IL-1β, have been shown to play an important role.18–21 A recent study showed that microinjection of IL-1β into the PVN increases BP via the AT1R, and also that microinjection of a subpressor dose of IL-1β into the PVN enhances its sensitivity to the central Ang II pressor response.20 We have previously demonstrated that chronic Ang II infusion increases PIC expression in the PVN.22 Moreover, Ang II-induced increases in the cellular adhesion molecules VCAM-1 and MCP-1 were attenuated in endothelial cells that had adenovirus-mediated ACE2 over-expression.34 A recent study showed that Ang II-mediated hypertension is caused by central mechanisms and described a feed-forward process in which the central pressor effects of Ang II lead to activation of T cells, which, in turn, promotes vascular inflammation and further raises BP, leading to severe hypertension.35 The current study shows that Ang II infusion significantly increased the mRNA expression of PICs such as TNF-α, IL-1β, IL-6, and the chemokine MCP-1 in the PVN. This increase was attenuated by bilateral ACE2 overexpression in the PVN. These findings suggest that the beneficial effects of ACE2 overexpression are, at least in part, mediated by decreased PIC expression in the PVN. A study by Sinnayah et al. reported that injection of an adenoviral vector into the mouse brain results in gene transduction of both neuronal and glial cells (a primary source of central PICs) with approximately equal affinity.36 It has also been shown that overexpression of human ACE2 in the SFO using an adenoviral vector results in expression of ACE2 in both neurons and glial cells. Once transfected, overexpression of human ACE2 in these cell types can be targeted by endogenous sheddases, thus resulting in secreted ACE2 being released in the surrounding milieu and potentially acting on every cell type in the proximity, including glial cells.29 Since glial cells are the primary source of PICs in the brain, the reduction in PICs within the brain can potentially be attributed to beneficial effects exerted by ACE2 overexpression.
As evidenced extensively over the years, overactivity of the RAS has been implicated in the development and maintenance of several cardiovascular diseases, including hypertension, and participation of the brain RAS in the pathophysiology of hypertension is now well established.5,37 Levels of Ang II and AT1R in the PVN are increased in many animal models of hypertension.37 Furthermore, Ang II up-regulates ACE and down-regulates ACE2 in patients with hypertension.38 Previous studies have shown that the Mas and AT2 receptors oppose the actions of the AT1R in the brain and periphery.39,40 Thus, it is the imbalance between the hypertensive (ACE, Ang II, and AT1R) and anti-hypertensive components (ACE2, Ang1–7, Mas, and AT2R) of the RAS that results in hypertension. In our study, the use of ACE2 overexpression determined the relevance of this imbalance in perpetuating the hypertensive state. ACE2 overexpression caused a decreased expression of ACE and AT1R, and an increased expression of ACE2, Mas, and AT2R, resulting in an increased Mas/AT1 receptor ratio and therefore counter balancing the Ang II-induced hypertensive response.
In summary, Ang II infusion resulted in a hypertensive state associated with increased AT1R and ACE gene expression, and decreased ACE2 and Mas gene expression in the PVN. Ang II infusion also increased gene expression of the PICs TNF-α, IL-1β and IL-6 in the PVN. Bilateral microinjection of Ad-ACE2 into the PVN attenuated the Ang II-induced hypertension and reversed these gene expression changes. Our findings, together with previous reports, suggest that attenuation of PICs in combination with the shift of the RAS towards the anti-hypertensive axis may be responsible for the overall beneficial effects of ACE2 overexpression on Ang II-induced hypertension.
Hypertension, while easily detectable, remains poorly controlled with current therapeutic regimes, with more than half of treated patients showing a poor response to standard drug therapy, and highlighting the need for additional avenues for hypertensive treatments. Recent studies have begun to clarify the complexity of the RAS both systemically and locally, including the less understood anti-hypertensive axis.23 The present study indicates that bilateral overexpression of ACE2 in the hypothalamic PVN of the brain attenuated the Ang II-mediated pressor response. The anti-hypertensive effects of ACE2 overexpression are likely a result from the net effect of shifting the RAS balance towards decreased expression of ACE and AT1R, and increased expression of ACE2, Mas, and AT2R, resulting in attenuation of PICs and the hypertensive state. This study demonstrates the importance of controlling ACE2 in neurogenic hypertension and a possible new focus for the development of future therapeutics towards up-regulating the actions of the central anti-hypertensive axis of the RAS, thereby potentially negating the effects of brain Ang II in perpetuating the hypertensive state.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
This work was supported by National Heart Lung and Blood Institute Grant (HL80544-01) to J.F.
Supplementary Material
References
- 1.Brunner HR. Experimental and clinical evidence that angiotensin II is an independent risk factor for cardiovascular disease. Am J Cardiol. 2001;87:3C–9C. doi: 10.1016/s0002-9149(01)01538-7. [DOI] [PubMed] [Google Scholar]
- 2.Allen AM, Zhuo J, Mendelsohn FA. Localization and function of angiotensin AT1 receptors. Am J Hypertens. 2000;13:31S–38S. doi: 10.1016/s0895-7061(99)00249-6. [DOI] [PubMed] [Google Scholar]
- 3.Lavoie JL, Sigmund CD. Minireview: overview of the renin-angiotensin system—an endocrine and paracrine system. Endocrinology. 2003;144:2179–2183. doi: 10.1210/en.2003-0150. [DOI] [PubMed] [Google Scholar]
- 4.Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- 5.Davisson RL. Physiological genomic analysis of the brain renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol. 2003;285:R498–R511. doi: 10.1152/ajpregu.00190.2003. [DOI] [PubMed] [Google Scholar]
- 6.Ferguson AV, Bains JS. Actions of angiotensin in the subfornical organ and area postrema: implications for long term control of autonomic output. Clin Exp Pharmacol Physiol. 1997;24:96–101. doi: 10.1111/j.1440-1681.1997.tb01790.x. [DOI] [PubMed] [Google Scholar]
- 7.Davern PJ, Head GA. Fos-related antigen immunoreactivity after acute and chronic angiotensin II-induced hypertension in the rabbit brain. Hypertension. 2007;49:1170–1177. doi: 10.1161/HYPERTENSIONAHA.106.086322. [DOI] [PubMed] [Google Scholar]
- 8.McKinley MJ, Allen AM, Burns P, Colvill LM, Oldfield BJ. Interaction of circulating hormones with the brain: the roles of the subfornical organ and the organum vasculosum of the lamina terminalis. Clin Exp Pharmacol Physiol Suppl. 1998;25:S61–S67. doi: 10.1111/j.1440-1681.1998.tb02303.x. [DOI] [PubMed] [Google Scholar]
- 9.Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- 10.Dampney RA. Functional organization of central pathways regulating the cardiovascular system. Physiol Rev. 1994;74:323–364. doi: 10.1152/physrev.1994.74.2.323. [DOI] [PubMed] [Google Scholar]
- 11.Tribollet E, Dreifuss JJ. Localization of neurones projecting to the hypothalamic paraventricular nucleus area of the rat: a horseradish peroxidase study. Neuroscience. 1981;6:1315–1328. doi: 10.1016/0306-4522(81)90190-1. [DOI] [PubMed] [Google Scholar]
- 12.Bains JS, Potyok A, Ferguson AV. Angiotensin II actions in paraventricular nucleus: functional evidence for neurotransmitter role in efferents originating in subfornical organ. Brain Res. 1992;599:223–229. doi: 10.1016/0006-8993(92)90395-p. [DOI] [PubMed] [Google Scholar]
- 13.Allen AM. Inhibition of the hypothalamic paraventricular nucleus in spontaneously hypertensive rats dramatically reduces sympathetic vasomotor tone. Hypertension. 2002;39:275–280. doi: 10.1161/hy0202.104272. [DOI] [PubMed] [Google Scholar]
- 14.Ito S, Hiratsuka M, Komatsu K, Tsukamoto K, Kanmatsuse K, Sved AF. Ventrolateral medulla AT1 receptors support arterial pressure in Dahl salt-sensitive rats. Hypertension. 2003;41:744–750. doi: 10.1161/01.HYP.0000052944.54349.7B. [DOI] [PubMed] [Google Scholar]
- 15.Nakata T, Takeda K, Itho H, Hirata M, Kawasaki S, Hayashi J, et al. Paraventricular nucleus lesions attenuate the development of hypertension in DOCA/salt-treated rats. Am J Hypertens. 1989;2:625–630. doi: 10.1093/ajh/2.8.625. [DOI] [PubMed] [Google Scholar]
- 16.Kannan H, Hayashida Y, Yamashita H. Increase in sympathetic outflow by paraventricular nucleus stimulation in awake rats. Am J Physiol. 1989;256:R1325–R1330. doi: 10.1152/ajpregu.1989.256.6.R1325. [DOI] [PubMed] [Google Scholar]
- 17.Tagawa T, Dampney RA. AT(1) receptors mediate excitatory inputs to rostral ventrolateral medulla pressor neurons from hypothalamus. Hypertension. 1999;34:1301–1307. doi: 10.1161/01.hyp.34.6.1301. [DOI] [PubMed] [Google Scholar]
- 18.Chae CU, Lee RT, Rifai N, Ridker PM. Blood pressure and inflammation in apparently healthy men. Hypertension. 2001;38:399–403. doi: 10.1161/01.hyp.38.3.399. [DOI] [PubMed] [Google Scholar]
- 19.Sriramula S, Haque M, Majid DS, Francis J. Involvement of tumor necrosis factor-alpha in angiotensin II-mediated effects on salt appetite, hypertension, and cardiac hypertrophy. Hypertension. 2008;51:1345–1351. doi: 10.1161/HYPERTENSIONAHA.107.102152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu Y, Chen J, Yin X, Zhao H. Angiotensin II receptor 1 involved in the central pressor response induced by interleukin-1 beta in the paraventricular nucleus. Neurol Res. 2009;31:420–424. doi: 10.1179/174313208X353677. [DOI] [PubMed] [Google Scholar]
- 21.Sun C, Li H, Leng L, Raizada MK, Bucala R, Sumners C. Macrophage migration inhibitory factor: an intracellular inhibitor of angiotensin II-induced increases in neuronal activity. J Neurosci. 2004;24:9944–9952. doi: 10.1523/JNEUROSCI.2856-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang YM, Ma Y, Zheng JP, Elks C, Sriramula S, Yang ZM, et al. Brain nuclear factor-kappa B activation contributes to neurohumoral excitation in angiotensin II-induced hypertension. Cardiovasc Res. 2009;82:503–512. doi: 10.1093/cvr/cvp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu P, Sriramula S, Lazartigues E. ACE2/ANG-(1-7)/Mas pathway in the brain: the axis of good. Am J Physiol Reg, Integr Compar Physiol. 2011;300:R804–R817. doi: 10.1152/ajpregu.00222.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferrario CM. Angiotensin-(1-7) and antihypertensive mechanisms. J Nephrol. 1998;11:278–283. [PubMed] [Google Scholar]
- 25.Huentelman MJ, Grobe JL, Vazquez J, Stewart JM, Mecca AP, Katovich MJ, et al. Protection from angiotensin II-induced cardiac hypertrophy and fibrosis by systemic lentiviral delivery of ACE2 in rats. Exp Physiol. 2005;90:783–790. doi: 10.1113/expphysiol.2005.031096. [DOI] [PubMed] [Google Scholar]
- 26.Crackower MA, Sarao R, Oudit GY, Yagil C, Kozieradzki I, Scanga SE, et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 27.Diez-Freire C, Vazquez J, Correa de Adjounian MF, Ferrari MF, Yuan L, Silver X, et al. ACE2 gene transfer attenuates hypertension-linked pathophysiological changes in the SHR. Physiol Genomics. 2006;27:12–19. doi: 10.1152/physiolgenomics.00312.2005. [DOI] [PubMed] [Google Scholar]
- 28.Yamazato M, Yamazato Y, Sun C, Diez-Freire C, Raizada MK. Overexpression of angiotensin-converting enzyme 2 in the rostral ventrolateral medulla causes long-term decrease in blood pressure in the spontaneously hypertensive rats. Hypertension. 2007;49:926–931. doi: 10.1161/01.HYP.0000259942.38108.20. [DOI] [PubMed] [Google Scholar]
- 29.Feng Y, Yue X, Xia H, Bindom SM, Hickman PJ, Filipeanu CM, et al. Angiotensin-converting enzyme 2 overexpression in the subfornical organ prevents the angiotensin II-mediated pressor and drinking responses and is associated with angiotensin II type 1 receptor downregulation. Circ Res. 2008;102:729–736. doi: 10.1161/CIRCRESAHA.107.169110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huentelman MJ, Zubcevic J, Katovich MJ, Raizada MK. Cloning and characterization of a secreted form of angiotensin-converting enzyme 2. Regul Pept. 2004;122:61–67. doi: 10.1016/j.regpep.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 31.Xia H, Feng Y, Obr TD, Hickman PJ, Lazartigues E. Angiotensin II type 1 receptor-mediated reduction of angiotensin-converting enzyme 2 activity in the brain impairs baroreflex function in hypertensive mice. Hypertension. 2009;53:210–216. doi: 10.1161/HYPERTENSIONAHA.108.123844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feng Y, Xia H, Cai Y, Halabi CM, Becker LK, Santos RA, et al. Brain-selective overexpression of human angiotensin-converting enzyme type 2 attenuates neurogenic hypertension. Circ Res. 2010;106:373–382. doi: 10.1161/CIRCRESAHA.109.208645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xia H, Lazartigues E. Angiotensin-converting enzyme 2 in the brain: properties and future directions. J Neurochem. 2008;107:1482–1494. doi: 10.1111/j.1471-4159.2008.05723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lovren F, Pan Y, Quan A, Teoh H, Wang G, Shukla PC, et al. Angiotensin converting enzyme-2 confers endothelial protection and attenuates atherosclerosis. Am J Physiol Heart Circ Physiol. 2008;295:H1377–H1384. doi: 10.1152/ajpheart.00331.2008. [DOI] [PubMed] [Google Scholar]
- 35.Marvar PJ, Thabet SR, Guzik TJ, Lob HE, McCann LA, Weyand C, et al. Central and peripheral mechanisms of T-lymphocyte activation and vascular inflammation produced by angiotensin II-induced hypertension. Circ Res. 2010;107:263–270. doi: 10.1161/CIRCRESAHA.110.217299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sinnayah P, Lindley TE, Staber PD, Cassell MD, Davidson BL, Davisson RL. Selective gene transfer to key cardiovascular regions of the brain: comparison of two viral vector systems. Hypertension. 2002;39:603–608. doi: 10.1161/hy0202.103295. [DOI] [PubMed] [Google Scholar]
- 37.Veerasingham SJ, Raizada MK. Brain renin-angiotensin system dysfunction in hypertension: recent advances and perspectives. Br J Pharmacol. 2003;139:191–202. doi: 10.1038/sj.bjp.0705262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koka V, Huang XR, Chung AC, Wang W, Truong LD, Lan HY. Angiotensin II up-regulates angiotensin I-converting enzyme (ACE), but down-regulates ACE2 via the AT1-ERK/p38 MAP kinase pathway. Am J Pathol. 2008;172:1174–1183. doi: 10.2353/ajpath.2008.070762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferrario CM. Angiotensin-converting enzyme 2 and angiotensin-(1-7): an evolving story in cardiovascular regulation. Hypertension. 2006;47:515–521. doi: 10.1161/01.HYP.0000196268.08909.fb. [DOI] [PubMed] [Google Scholar]
- 40.Widdop RE, Matrougui K, Levy BI, Henrion D. AT2 receptor-mediated relaxation is preserved after long-term AT1 receptor blockade. Hypertension. 2002;40:516–520. doi: 10.1161/01.hyp.0000033224.99806.8a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.