Case presentation
A 16-year-old girl presented to her physician with easy bruising during contact sports. Laboratory evaluation revealed an absolute neutrophil count (ANC) of 554/mm3, platelet count of 11 000/mm3, and hemoglobin of 7.5 g/dL with an absolute reticulocyte count of 21 600/mm3. She was referred to the National Institutes of Health where bone marrow biopsy revealed less than 30% cellularity. The marrow aspirate smear showed normal cellular morphology without dysplastic changes, absence of megakaryocytes, and normal cytogenetics by flow cytometric analysis. These findings were consistent with a diagnosis of severe aplastic anemia (SAA). She received horse antithymocyte globulin 40 mg/kg per day for 4 days and cyclosporine. Transfusion support with leuko-reduced red cells and platelets was given for 6 months, at which time she achieved transfusion independence. Relapse of aplastic anemia occurred 19 months later. She achieved a second remission after treatment with alemtuzumab 10 mg/day for 10 days with prednisolone 1 mg/kg per day; however, relapse occurred again 13 months later, with an ANC of 10/mm3. Cellularity on marrow biopsy was 10%, and a new delq13 karyotypic abnormality was detected in 40% of metaphases, although no dysplastic changes were observed. She subsequently received rabbit ATG 40 mg/kg per day for 4 days, without response. In the following weeks, she was hospitalized on 3 occasions for treatment of neutropenic sepsis.
She was readmitted to the National Institutes of Health 3 months after her last therapy for SAA. She was of both African-American and Eastern European Jewish descent and had neither a related nor an unrelated HLA identical donor. She elected to participate in an allogeneic hematopoietic progenitor cell transplantation (HCT) trial evaluating the cotransplantation of a partially matched umbilical cord blood unit and haploidentical CD34+ cells from a relative after reduced intensity conditioning.
On admission, CT of the lungs revealed nodules in the right upper, right middle, and left lower lobes (Figure 1A). Bronchiolar lavage fluid revealed gram-positive branched filaments suggestive of Nocardia infection, although no growth was seen in culture. She was treated with intravenous sulfamethoxazole-trimethoprim, ceftazidime, and voriconazole but remained febrile during the next 2 weeks with the lung nodules increasing in size. An aspergillus antigen (galactomannan) assay was positive (6.1, reference range < 0.5) leading to a probable diagnosis of invasive pulmonary aspergillosis. Liposomal amphotericin B and caspofungin were added, and voriconazole was discontinued. Despite these interventions, the pneumonia progressed, leading to a significant decline in pulmonary function and performance status and precluding the initiation of transplant conditioning. As the patient had a progressive life-threatening mold infection in the setting of profound neutropenia, a decision was made to initiate a course of granulocyte transfusions. Concurrently, her HLA haploidentical sister underwent a hematopoietic progenitor cell (HPC) collection by apheresis, which was enriched for CD34+ cells by immunomagnetic bead selection and cryopreserved.
Figure 1.
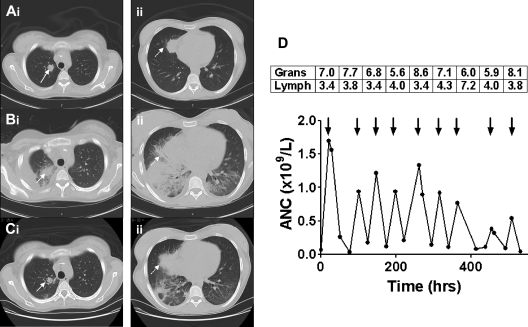
Sequential CT scans of the thorax and response to granulocyte transfusions. CT scan before granulocyte transfusions showed nodules in the right upper lobe (Ai, solid arrow, 2 cm) and right middle lobe (Aii, dotted arrow, 3.5 cm). CT after 4 transfusions of granulocytes revealed a new right pleural effusion, new right lower and middle lobe consolidation, and left lower lobe airspace disease, and enlargement of existing nodules (Bi-ii). CT after 5 additional granulocyte transfusions showed marked improvement in lung consolidations, accompanied by clinical resolution of fever, tachypnea, and hypoxia (Ci-ii). The granulocyte (× 1010) and lymphocyte (× 109) content of each of the 9 transfused granulocyte components (arrows) is shown in the boxes above the arrows, with the corresponding increment in ANC shown below the arrows (D). Mean granulocyte content was 6.9 × 1010 cells per product.
Unrelated community blood donors were stimulated with dexamethasone 8 mg orally and G-CSF 480 μg subcutaneously, and 12 hours later granulocytes were harvested by hetastarch-assisted leukapheresis.1 Granulocyte components were irradiated and transfused in the afternoon of the same day as collection. A total of 9 components were given on an alternate-day schedule over 3 weeks. CT of the chest after 4 transfusions, at a time of increasing dyspnea and hypoxia, revealed increasing size of existing nodules, and new right lower and middle lobe consolidation and left lower lobe airspace disease (Figure 1B). After 5 additional granulocyte doses, the patient became afebrile, her tachypnea resolved, and the lung nodules and consolidations improved substantially (Figure 1C).
The mean posttransfusion ANC increment was 0.92 × 109/L during the first 7 granulocyte transfusions but diminished with the last 2 transfusions (Figure 1D). Although the patient had received approximately 100 leuko-reduced plateletpheresis and 200 leuko-reduced red cell concentrates during the course of her illness, neither class I nor class II HLA antibodies were detectable before granulocyte transfusion. A repeat HLA antibody screen performed 3 weeks after the first granulocyte transfusion revealed antibodies directed against both MHC class I (A25, B8, B45) and class II (DQ2, DQ4, DQ7, DQ8, DQ9, DR7) antigens. Unfortunately, the HLA type of the haploidentical sibling donor whose CD34+ cells had been collected and cryopreserved included DQB1*0302, which is the target of the anti-DQ8 antibody. HLA typing of the 9 granulocyte donors revealed that the first donor had the DQB1*0302 allele, which was probably responsible for the alloimmunization.
A search for an alternative haploidentical donor was initiated. Patient HLA antibodies were directed against maternal, but not paternal, antigens. The haploidentical father met study eligibility criteria and subsequently donated HPC by apheresis. The patient underwent HCT with coinfusion of cord blood and haploidentical CD34-selected paternal HPC. Initial engraftment with the father's cells occurred on day 10, with an ANC of 880/mm3, followed by sustained engraftment of the cord blood unit. Three weeks later, the patient was discharged.
Discussion
Younger patients with SAA and an HLA-matched related or unrelated donor can achieve cure rates of 70% or higher with HCT, which is the treatment of choice. Immunosuppressive therapy can also be successful but is associated with a 35% risk of relapse.2 Cord blood transplantation may be considered in the absence of an HLA-matched donor and has the advantages of rapid availability and tolerance of a limited HLA match but is compromised by relatively low total nucleated cell dose. The latter is associated with delayed neutrophil recovery, increased risk of infection, and graft rejection, which leads to a greater transplant-related mortality, particularly in patients with SAA.3 Our patient was enrolled in a clinical trial to evaluate the safety and efficacy of coinfusion of cord blood and mobilized adult haploidentical CD34+ cells.4 Transient but rapid engraftment of myeloid cells from the adult donor has been reported with this approach, followed by sustained and durable engraftment of cord cells. During the evaluation for HCT, our patient developed a probable fungal pneumonia, which progressed despite antifungal therapy.
Granulocyte transfusion therapy has been used since the 1960s for patients with neutropenic sepsis. Early controlled studies demonstrated impressive survival rates but were limited by study size, heterogeneous patient cohorts, and low granulocyte doses.5 The ability to collect larger cell doses after combined administration of G-CSF and steroids to the donor has renewed interest in this product.6 Collection of 5 to 8 × 1010 granulocytes per component can be expected to increase the recipient's ANC by approximately 1000 cells/μL.1,6 However, provision of granulocytes for transfusion remains a challenge because of high cost, short product shelf-life (24 hours), and the need to recruit donors who are willing to receive oral and parenteral medications the day before the apheresis procedure. Furthermore, donation may not be entirely without risk, as an increased incidence of posterior subcapsular cataracts has been attributed to the repeated administration of steroids in such donors.7 Nevertheless, granulocytes may be of substantial benefit in selected cases.
Fever frequently accompanies the administration of granulocyte concentrates and may be seen in 17.5% of transfusions.6 Pulmonary reactions may also occur, presenting as decreased oxygen saturation or as worsening of preexisting infiltrates, as was observed in our case.8 The decision to continue the transfusions after a pulmonary deterioration can be a difficult one. Worsening infiltrates may reflect the trafficking of transfused cells to sites of infection, with activation and release of microbicidal mediators, and may presage subsequent improvement. This appeared to be the case in our patient, as continued transfusions were followed by clinical and radiologic evidence of improvement.
Fever, chills, and respiratory compromise are more likely to occur in the presence of recipient HLA alloantibodies.9 Granulocyte concentrates contain not only large numbers of granulocytes (expressing class I HLA antigens) but also substantial numbers of lymphocytes (expressing class II), thus providing ample antigenic stimulus (Figure 1D). A retrospective review at our center demonstrated that 17% of SAA patients developed HLA antibodies during the course of granulocyte transfusion therapy and that patients with detectable HLA antibodies had lower posttransfusion white cell increments.1 Ideally, donor-recipient pairs should be HLA matched; however, because the donor pool is limited, this is impossible in practice. Serologic screening for class I and class II HLA antibodies is recommended before initiating a course of granulocyte transfusions, at the end of the course, and whenever adverse pulmonary reactions or lack of the expected count increment occurs.
The development of class I and II HLA antibodies in the patient had a profound impact on her subsequent course. Fortunately, the selected cord blood unit did not express HLA antigens to which she had become sensitized. However, the adult haploidentical donor expressed an incompatible class II antigen. Allosensitization to donor-specific HLA antigens in unrelated HCT has been implicated in early graft failure; donor-specific HLA antibodies were identified in 24% of recipients with graft failure compared with 1% of subjects with sustained engraftment.10
This case illustrates that HLA alloimmunization can be a major complication of granulocyte transfusions, resulting in the development of donor-directed HLA antibodies. A previously compatible HPC component had to be discarded, necessitating collection of a second graft from a different donor. The combination of granulocytes and antifungal therapy was effective in treating this patient's disease, with clinical and radiologic evidence of improvement. Nevertheless, the benefits as well as the risks of granulocyte transfusions should be carefully considered in recipients who are candidates for allogeneic HCT. A multicenter controlled trial of the resolution of infections in neutropenia with granulocyte transfusions (RING Study) is in progress in the United States (Traci Mondoro, Transfusion Medicine/Hemostasis Clinical Trials Network, National Heart, Lung, and Blood Institute/National Institutes of Health, e-mail communication, January 14, 2011) and should lead to a better understanding of the risks and benefits of this therapy.
Authorship
Contribution: D.O. wrote the manuscript; and R.W.C. and S.F.L. edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Susan F. Leitman, Department of Transfusion Medicine, National Institutes of Health, Bldg 10, Clinical Center, Rm 1C-711, Bethesda, MD 20892; e-mail: sleitman@nih.gov.
References
- 1.Quillen K, Wong E, Scheinberg P, et al. Granulocyte transfusions in severe aplastic anemia: an eleven-year experience. Haematologica. 2009;94(12):1661–1668. doi: 10.3324/haematol.2009.010231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosenfeld S, Follmann D, Nunez O, Young NS. Antithymocyte globulin and cyclosporine for severe aplastic anemia: association between hematologic response and long-term outcome. JAMA. 2003;289(9):1130–1135. doi: 10.1001/jama.289.9.1130. [DOI] [PubMed] [Google Scholar]
- 3.Peffault de Latour R, Purtill D, Ruggeri A, et al. Influence of nucleated cell dose on overall survival of unrelated cord blood transplantation for patients with severe acquired aplastic anemia: a study by Eurocord and the Aplastic Anemia Working Party of the European Group for Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2011;17(1):78–85. doi: 10.1016/j.bbmt.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Magro E, Regidor C, Cabrera R, et al. Early hematopoietic recovery after single unit unrelated cord blood transplantation in adults supported by co-infusion of mobilized stem cells from a third party donor. Haematologica. 2006;91(5):640–648. [PubMed] [Google Scholar]
- 5.Higby DJ, Yates JW, Henderson ES, Holland JF. Filtration leukapheresis for granulocyte transfusion therapy. N Engl J Med. 1975;292(15):761–766. doi: 10.1056/NEJM197504102921501. [DOI] [PubMed] [Google Scholar]
- 6.Hübel K, Carter RA, Liles WC, et al. Granulocyte transfusion therapy for infections in candidates and recipients of HPC transplantation: a comparative analysis of feasibility and outcome for community donors versus related donors. Transfusion. 2002;42(11):1414–1421. doi: 10.1046/j.1537-2995.2002.00249.x. [DOI] [PubMed] [Google Scholar]
- 7.Clayton JA, Vitale S, Kim J, et al. Prevalence of posterior subcapsular cataracts in volunteer cytapheresis donors. Transfusion. 2011;51(5):921–928. doi: 10.1111/j.1537-2995.2010.02948.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bow EJ, Schroeder ML, Louie TJ. Pulmonary complications in patients receiving granulocyte transfusions and amphotericin B. Can Med Assoc J. 1984;130(5):593–597. [PMC free article] [PubMed] [Google Scholar]
- 9.Stroncek DF, Leonard K, Eiber G, Malech HL, Gallin JI, Leitman SF. Alloimmunization after granulocyte transfusions. Transfusion. 1996;36(11):1009–1015. doi: 10.1046/j.1537-2995.1996.36111297091747.x. [DOI] [PubMed] [Google Scholar]
- 10.Spellman S, Bray R, Rosen-Bronson S, et al. The detection of donor-directed, HLA-specific alloantibodies in recipients of unrelated hematopoietic cell transplantation is predictive of graft failure. Blood. 2010;115(13):2704–2708. doi: 10.1182/blood-2009-09-244525. [DOI] [PMC free article] [PubMed] [Google Scholar]