Abstract
Interactions between multiple myeloma (MM) cells and the BM microenvironment play a critical role in the pathogenesis of MM and in the development of drug resistance by MM cells. Selectins are involved in extravasation and homing of leukocytes to target organs. In the present study, we focused on adhesion dynamics that involve P-selectin glycoprotein ligand-1 (PSGL-1) on MM cells and its interaction with selectins in the BM microenvironment. We show that PSGL-1 is highly expressed on MM cells and regulates the adhesion and homing of MM cells to cells in the BM microenvironment in vitro and in vivo. This interaction involves both endothelial cells and BM stromal cells. Using loss-of-function studies and the small-molecule pan-selectin inhibitor GMI-1070, we show that PSGL-1 regulates the activation of integrins and downstream signaling. We also document that this interaction regulates MM-cell proliferation in coculture with BM microenvironmental cells and the development of drug resistance. Furthermore, inhibiting this interaction with GMI-1070 enhances the sensitization of MM cells to bortezomib in vitro and in vivo. These data highlight the critical contribution of PSGL-1 to the regulation of growth, dissemination, and drug resistance in MM in the context of the BM microenvironment.
Introduction
The progression and dissemination of multiple myeloma (MM) involves the continuous spread of MM cells in and out of the BM.1,2 Interactions of MM cells with the BM microenvironment play a critical role in the pathogenesis of MM and in the development of drug resistance.3–5 We have shown that blocking the CXCR4/SDF1 axis disrupts the interaction of MM cells with the BM microenvironment, which in turn enhances the efficacy of therapeutic agents against MM cells.6
Selectins are cell-surface adhesion molecules that contain a lectin-like domain with selectivity for binding to specific saccharide chains.7 Each of the 3 types of selectins (E, L, and P) has a unique tissue distribution (in the endothelium, leukocytes, and platelets, respectively),7 and distinct classes of leukocytes use specific combinations of selectins to interact with endothelium.8,9 The binding affinity of selectins to their ligands is relatively low, but is nonetheless strong enough to serve as a biologic brake that rapidly decelerates leukocytes as they roll on endothelial cells (ECs). While rolling, leukocytes are activated by binding to selectin and by chemoattractants such as CXCR4/SDF-1; activation increases the affinity of the integrins on leukocytes for ligands found in the endothelium. The presence of a chemotactic signal outside of a venule induces leukocytes to squeeze between the ECs of the venule and migrate into the target organ (extravasation)10,11; inhibition of rolling by blocking selectins decreases this extravasation.12–14 Small-molecule inhibitors of selectins have clinical activity.12,15–18 Synthetic inhibitors of selectin also have a demonstrated ability to improve the manifestation of psoriasis and allergen-induced asthma in humans19 and in mouse models of skin inflammation.20 These agents are currently being tested in clinical trials for the treatment of inflammatory diseases and in preclinical studies of solid-tumor metastasis.21–24
P-selectin glycoprotein ligand-1 (PSGL-1) is a dimeric, mucin-type glycoprotein ligand that is expressed by all leukocytes and is involved in the homing of leukocytes to target tissues.25 PSGL-1 plays an important role in organ targeting during inflammation, and inhibition of PSGL-1 represents an attractive basis for anti-inflammatory strategies.25,26 Earlier studies have shown that PSGL-1 is highly expressed in MM,22,27 is a novel therapeutic target for mAb-mediated MM immunotherapy, plays a role in humoral immunotherapy of MM, and combined treatment with PSGL-1 mAb and chemotherapy improves tumor cytotoxicity.23,28
In the present study, we targeted PSGL-1 by inhibiting its interaction with selectins in the microenvironment as a therapeutic prospect but with a focus on adhesion dynamics that involve PSGL-1 on MM cells and its interaction with selectins in the BM microenvironment. We show that PSGL-1 regulates the adhesion and homing of MM cells to cells in the BM microenvironment, including ECs and BM stromal cells (BMSCs). We also document that this interaction regulates the proliferation and development of drug resistance by MM cells, both in vitro and in vivo. Furthermore, inhibiting this interaction with GMI-1070, a selective novel therapeutic agent that targets selectins, enhances the sensitization of MM cells to bortezomib in vitro and in vivo. These data highlight the critical contribution of PSGL-1 to the regulation of growth, dissemination, and drug resistance in MM in the context of the BM microenvironment.
Methods
Reagents
The pan-selectin inhibitor GMI-1070 was obtained from GlycoMimetics. Recombinant SDF-1, recombinant selectins, and Abs against E-, L-, and P-selectins were purchased from R&D Systems. mAbs for Western blotting were procured from Cell Signaling Technologies, anti–PSGL-1 Ab was from Millipore, and anti–β1-integrin Ab (clone 12G10) was from Abcam. Calcein-AM cell-labeling dye was obtained from Invitrogen. Scramble-siRNA and SmartPool siRNAs for PSGL-1, E-selectin, L-selectin, and P-selectin were from Dharmacon. Lipofectamin-2000 was from Invitrogen.
Cells
The MM1s cell line was purchased from the ATCC, whereas the OPM1, OPM2, H929, RPMI8226, U266, and U266LR7 lines were the kind gift of Prof Jesús F. San Miguel (Salamanca, Spain). Human umbilical vein ECs (HUVECs) were from Lonza. CD138+ cells were isolated from MM patients by bead selection, and BMSC cultures were established as described previously.6
Informed consent was obtained from all patients in accordance with the Declaration of Helsinki for primary MM patient samples. Approval of the animal study protocol was obtained from the Institutional Review Board of the Dana-Farber Cancer Institute.
Immunohistochemistry
To detect PSGL-1, BM aspirates from 17 MM patients and 3 healthy subjects were rinsed with PBS, fixed with 4% formaldehyde in PBS, dehydrated with ethanol, embedded in paraffin, and sectioned. Tissues were then immunostained with mouse anti–human PSGL-1.
Gene-expression analysis
To determine the gene expression of PSGL-1 in MM, we used datasets from the Gene Expression Omnibus (Mayo Clinic series number GSE 6477). Expression levels of PSGL-1 (probe ID 209879_at) were compared in plasma cells from healthy subjects (n = 15), monoclonal gammopathy of undetermined significance (MGUS) patients (n = 20), smoldering MM patients (n = 23), and newly diagnosed MM patients (n = 68).
Expression of PSGL-1 and selectins by flow cytometry
MM cell lines (MM1s, OPM1, OPM2, H929, RPMI8226, U266, and U266LR7) or HUVECs were treated with mouse anti–human Abs for PSGL-1; with E-, L-, or P-selectin (5 μg/mL); or with an isotype control for 1 hour on ice. Cells were then immersed in FITC-secondary Ab. Expression was determined with flow cytometric analysis and quantified as the ratio of the mean-fluorescence intensity (MFI) of the detected target to the MFI of isotype control.
To detect selectin on primary ECs, mononuclear cells from BM aspirates of MM patients were treated with primary Abs against each selectin, followed by a FITC–anti-mouse secondary Ab, washed, and treated with APC-conjugated anti-CD31 Ab. ECs were gated as APC-CD31+ cells, and the expression of each selectin was quantified as the ratio of the FITC-MFI of each selectin to the FITC-MFI of isotype control.
Knockdown of PSGL-1 in MM1s cells and of E-, L-, and P-selectins in HUVECs
MM1s cells were cultured overnight in 6-well plates in Opti-MEM medium and washed, and cells in each well were immersed overnight in a mixture of Lipofectamin-2000 (7 μL) with scramble-siRNA or SmartPool PSGL-1 siRNA (100 pmol) in a final volume of 2 mL of Opti-MEM. Similarly, HUVECs were transfected with scramble siRNA or with SmartPool siRNA for E-, L-, or P-selectin. Twenty-four hours later, the transfection solution was replaced with complete medium and cells were used after an additional 24-48 hours.
Interaction of recombinant selectins with MM cells
MM1s cells transfected with scramble siRNA or PSGL-1 siRNA or treated with increasing concentrations (0, 250, and 500μM) of GMI-1070, were incubated with free human Fc-chain (isotype control) or with chimeras of human-Fc chain and recombinant human E-, L-, or P-selectin (10 μg/mL), followed by FITC-conjugated mouse anti–human Fc. The interaction of the selectins with MM cells was analyzed by flow cytometry and quantified as the ratio of the MFI of each selectin to the MFI of the isotype control.
Adhesion of MM cells to HUVECs and BMSCs
HUVECs or BMSCs were cultured overnight to confluence in 96-well plates (5 × 103 cells/well) before initiating the adhesion assay. Selectins on HUVECs were inhibited by knockdown with siRNA for E-, L-, or P-selectin; by treatment with blocking Abs against E-, L-, or P-selectin (10 μg/mL for 1 hour); or by increasing concentrations of GMI-1070 (0, 250, and 500mM for 1 hour) before performing the adhesion assay. MM1s, OPM1, and H929 cells were serum-starved for 3 hours, prelabeled with Calcein-AM, added to the HUVECs or BMSCs (1 × 105 cells/well), and allowed to adhere for 2 hours at 37°C. Nonadherent cells were aspirated away, the HUVECS or BMSCs were washed, and fluorescence intensity was measured using a fluorescent-plate reader (Ex/Em = 485/520 nm). In some experiments, MM cells were treated with scramble-siRNA or siRNA for PSGL-1, and in other experiments with isotype control Ab or with a blocking Ab of PSGL1 (clone KPL-1; Millipore).
Adhesion of MM1s cells to HUVECs under shear flow
HUVECs were grown to confluence in 96-well plates (5 × 103 cells/well) before performing the adhesion assay. HUVECs were then activated with vehicle or TNF-α (30 U/mL) for 3 hours or with IL-4 (3 ng/mL) for 24 hours, followed by histamine (2.25mM) for 4 hours. The cells were then treated with mouse anti–human Ab against each of the selectins for 1 hour, followed by a secondary FITC Ab for 1 hour. Nonbound Abs were washed away and the expression of selectins was assessed by measuring the fluorescence intensity (Ex/Em = 485/520 nm).
Adhesion of MM cells to HUVECs under conditions of shear flow was measured with a parallel plate flow chamber. HUVEC monolayers were grown in 35-mm tissue-culture plates, and activated. A MM cell suspension (2 × 106cells/mL) was perfused through the chamber over the HUVEC monolayers at a shear rate corresponding to a wall shear stress of 0.9 dynes/cm2. For each experiment, the cell suspension was allowed to flow through the chamber for 3 minutes, after which time digital images were collected to quantify the results. The IC300 digital image system (Inovision) driven by a Silicon Graphics Indigo2 workstation was used to acquire and analyze the images.
Transendothelial migration and chemotaxis of MM cells
HUVECs (5 × 103 cells/basket) were incubated overnight in the upper chamber of 8-micron pore filters (Costar; Corning) before performing the adhesion assay. Selectins on HUVECs were inhibited by knockdown with siRNA or by 1 hour of treatment with blocking Abs (10 μg/mL) or by 1 hour of treatment with GMI-1070 (0, 250, and 500μM) before carrying out the transendothelial migration assay. MM1s cells were serum-starved for 3 hours and then added to the upper chamber of the basket (2 × 105 cells/well), and left to migrate for 4 hours at 37°C toward the lower chamber, which contained 0 or 30nM of SDF1α. In some cases, PSGL-1 in the MM cells was knocked down by siRNA, and in other experiments, MM cells were treated with isotype control Ab or with a blocking Ab of PSGL1. In other cases, migration was assayed without precoating the filter; in these, MM1s were treated with scramble siRNA, PSGL-1 siRNA, or with increasing concentrations of GMI-1070. Those cells that made it to the lower chambers were counted via flow cytometry.
In vivo extravasation of MM cells
In vivo flow cytometry was used to examine extravasation.6 SCID mice were pretreated for 1 hour with vehicle, anti–P-selectin Ab (250 μg/kg IV), or GMI-1070 (25 mg/kg IP). Calcein-labeled MM1s cells (1 × 106) were injected into anesthetized mice and immediately thereafter, a 488-nm laser was focused on an artery in the mouse's ear. Signals were detected through a 680/25-nm bandpass filter, and analyzed with MATLAB Version 7.2 software. Cell counts were obtained every 5 minutes for 40 minutes after the cell injection.
In vivo homing to the BM
We used in vivo confocal microscopy to test the homing of MM1s cells to the BM in vivo. Calcein-labeled MM1s cells (1 × 106) were injected into anesthetized SCID mice that had been pretreated with vehicle or GMI-1070 (25 mg/kg IP) for 1 hour before the cell injection. For some cases, PSGL-1 in the MM cells was knocked down by siRNA.
In vivo confocal microscopy was performed as described previously.6,29 Briefly, a skin flap was made in the scalps of the mice to expose the underlying skull surface and Evans blue dye was injected intravenously immediately before imaging to visualize blood vessels. High-resolution images with cellular detail were captured 30 minutes after cell injection through the intact mouse skull at depths of up to 250 μm from the surface of the skull using a 30 × 0.9–numerical aperture water-immersion objective lens (Lomo). Multiple imaging depths were acquired and a maximum intensity z-projection was performed in ImageJ software to merge the images. Calcein-labeled MM cells and Evans blue dye were excited with 491-nm and 638-nm single-photon lasers and detected via 528/19-nm and 680/25-nm bandpass filters, respectively.
Adhesion-related signaling
MM1s cells (5 × 106) were serum-starved for 3 hours and stimulated for different amounts of time with recombinant P-selectin (10 μg/mL). MM1s cell were treated with recombinant P-selectin (10 μg/mL for 30 minutes) in the presence or absence of GMI-1070 (500μM). The effect of GMI-1070 on signaling induced in MM cells by adhesion to HUVECs was also tested. HUVECs were treated with 0, 250, and 500μM GMI-1070 for 1 hour, followed by washing with PBS. Nontreated MM cells were cocultured with the HUVECs for 1 hour and then harvested by placing the dishes on ice for 10 minutes and dislodging the MM cells from the HUVECs by gentle pipetting. The MM cells were lysed and immunoblotted for the detection of p-FAK, p-Src, cofilin, p-AKT, p-GSK3, and α-tubulin.
Immunofluorescence for the detection of up-regulation of integrins
MM1s cells were treated for 30 minutes with recombinant P-selectin (10 μg/mL) in the presence or absence of GMI-1070 (500μM); nontreated MM1s cells served as a control. Cells were fixed in formaldehyde for 15 minutes at room temperature, spun onto slides, washed, blocked, stained with 5 μg/mL of anti–β1-integrin Ab at 4°C overnight, followed by FITC-labeled secondary Ab for 1 hour, washed, mounted, and analyzed with a Nikon inverted TE2000 microscope equipped with a 20× Plan-Fluor differential interference contrast 0.5–numerical aperture objective. The percentage of highly labeled cells was calculated for each condition and normalized to the percentage in the nontreated control.
Proliferation assay
HUVECs or BMSCs (5 × 103 cells/well) were plated overnight in 96-well plates. MM1s cells (2 × 105 cells/mL) that were transfected with scrambled siRNA or with siRNA for PSGL-1 were cultured for 24 hours with or without BMSCs or HUVECs. In some cases, HUVECs were pretreated with GMI-1070 (0 or 500μM) before the addition of the MM1s to the culture. In some cases, MM1s cells were treated with vehicle, bortezomib (5nM), or dexamethasone (25nM). Proliferation was quantified with the bromodeoxyuridine assay 24 hours after coculture according to the manufacturer's instructions (BD Biosciences).
Tumor initiation in vivo
MM1s cells transfected with scramble siRNA or PSGL-1 siRNA were injected IV into the tail vein (3 × 106 cells in 100 μL of PBS per mouse) or into the BM of the tibia (2 × 105 cells in 20 μL of PBS per mouse) of SCID/bg mice. Mice were killed after 1 week. BM was extracted from 2 femurs and the tibia of mice injected intravenously. RBCs were lysed and mononuclear cells were incubated for 1 hour with mouse anti–human-CD138 Ab conjugated to PerCP-Cy5.5 (5 μg/mL) and analyzed using a flow cytometer.
Sensitivity of MM cells to chemotherapy in vivo
MM1s cells expressing luciferase were injected intravenously into 40 SCID mice; treatment was started after tumors were first detected by bioluminescence imaging. Mice were divided into 4 groups of 10 each: (1) the control group, which received weekly IP injection of vehicle and were implanted with Alzet-Pump-2002 (flow rate, 0.5 μL/h) loaded with vehicle every 2 weeks for 4 weeks; (2) the GMI-1070–treated group, which received weekly IP injection of vehicle and were implanted with a pump loaded with 200 μL of 150 mg/mL of GMI-1070 that was changed every 2 weeks for 4 weeks; (3) the bortezomib-treated group, which received weekly IP injections of bortezomib 1.5 mg/kg and were implanted with a pump loaded with vehicle that was changed every 2 weeks for 4 weeks; and (4) the combination group, which received weekly IP injection of bortezomib 1.5 mg/kg and were implanted with a pump loaded with 200 μL of 150 mg/mL GMI-1070 that was changed every 2 weeks for 4 weeks. Tumor progression was monitored once a week by bioluminescence imaging while the mice were on treatment (4 weeks) and after the treatment was stopped (4 weeks).
Statistical analysis
Results were reported as the means ± SD for 3 experiments. Samples were compared by the Student t test, 2-factor ANOVA, or the log-rank test. Results were considered significantly different at P < .05.
Results
PSGL-1 is highly expressed on MM cells
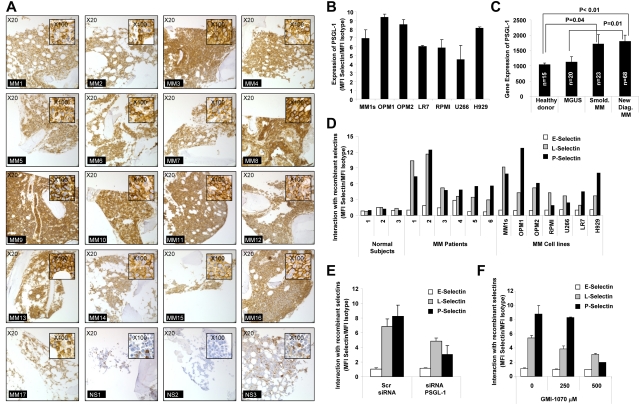
The expression of PSGL-1 on MM cells was examined using immunohistochemistry for BM biopsies from 17 MM patients and using flow cytometry for fresh MM samples. In agreement with previous findings, we confirmed that PSGL-1 was highly expressed on MM cells in all 17 MM patients tested (Figure 1A). The expression of PSGL-1 on MM cells was also confirmed by flow cytometry for 7 MM cells lines and, again, high levels of expression of PSGL-1 were detected on all 7 cell lines tested (Figure 1B). PSGL-1 was also differentially expressed on plasma cells isolated from patients at different stages of MM. PSGL-1 gene expression increases with disease progression from MGUS to smoldering MM and then to active disease, as shown by analysis of the published gene expression datasets by Chng et al30 (Figure 1C). These observations indicate that PSGL-1 is highly expressed on all MM cells.
Figure 1.
PSGL-1 is highly expressed on MM cells and regulates interaction with P-selectins. (A) Expression of PSGL-1 detected in BM biopsies from MM patients (n = 17) and healthy individuals (n = 3) using immunohistochemistry. Images are showing 20× magnification and inserts are showing 100× magnification. All MM patients presented with a higher expression of PSGL-1 compared with healthy individuals. (B) Expression of PSGL-1 evaluated on MM cell lines (MM.1S, OPM1, OPM2, LR7, RPMI.8226, U266, and H929) using flow cytometry and expressed as a ratio between the MFI of PSGL-1 and the MFI of the isotype control. (C) Gene-expression profiling of PSGL-1 from available dataset series number GSE 6477. The expression level of PSGL-1 significantly increases with MM tumor progression from MGUS to smoldering MM to newly diagnosed MM. Significant differences are observed between healthy subjects and MM patients (both smoldering MM and newly diagnosed MM) (P < .01). (D) CD138+ cells isolated from either normal BM (n = 3) or MM BM (n = 6) and MM cell lines (MM.1S, OPM1, OPM2, RPMI.8226, U266, LR7, and H929) incubated with free human Fc-chain (isotype control) or with chimeras of human-Fc chain and recombinant human E-, L-, or P-selectin (10 μg/mL) for 1 hour, followed by FITC-conjugated mouse anti–human Fc. The interaction of the selectins with MM cells was analyzed by flow cytometry and quantified as the ratio of the MFI of each selectin to the MFI of the isotype control. P- and L-selectins (but not E-selectin) interacted with MM primary cells and cell lines, whereas none of the selectins interacted with the normal plasma cells. (E) MM1s cells were transfected with either PSGL-1 siRNA or scramble siRNA. Cells were exposed to recombinant human E-, L-, and P-selectin (10 μg/mL) for 1 hour. The interaction of selectins with MM cells was analyzed by flow cytometry and quantified as ratio of MFI of each selectin to the MFI of the isotype control. Down-regulation of PSGL-1 reduced the interaction of both L- and P-selectins with MM cells. (F) MM.1S cells were treated with increasing concentrations of GMI-1070 (0, 250, and 500μM) for 1 hour and then exposed to recombinant E-, L-, and P-selectin (10 μg/mL, for 1 hour). The interaction of selectins with MM cells was analyzed by flow cytometry and quantified as a ratio of MFI of each selectin to the MFI of the isotype control. Dose-dependent inhibition of the interaction of L- and P-selectins was observed. All data represent means ± SD of triplicate experiments.
We also examined the expression of selectin on MM cells relative to plasma cells obtained from the BM of healthy donors. Unlike the high expression of the selectin ligand, we found no expression of E-, L-, or P- selectins on any of the MM cells tested (primary samples and cell lines; supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Role of PSGL-1 in the interaction of recombinant selectins with MM cells
When we tested the interaction of recombinant selectins with plasma cells derived from healthy subjects, from MM patients, or from MM cell lines, we observed that that E-selectin did not interact with MM cells and normal plasma cells, whereas L- and P-selectins were highly interactive with MM cell lines and MM primary samples but not with normal plasma cells (Figure 1D). This is in agreement with previous reports showing that PSGL-1 interacts with both L- and P-selectins.31 Moreover, the differences in the interaction between MM cells and normal plasma cells were apparently due to lower expression of PSGL-1 in normal plasma cells or potentially because of different glycosylation patterns of the PSGL-1, which is known to alter binding of selectins to the ligand.32,33
To confirm the role of PSGL-1 in the interaction of selectins with MM cells, we knocked down PSGL-1 in MM1s cells using siRNA (as verified by flow cytometry; supplemental Figure 2). Knock-down of PSGL-1 decreased the high level of interaction of L- and P-selectins with MM cells (the effect was stronger for interaction of P-selectin on MM cells). E-selectin did not bind with MM cells and the knockdown of PSGL-1 did not alter this effect (Figure 1E). We further confirmed that GMI-1070, a specific selectin inhibitor that is currently being used in clinical trials, inhibits the interaction of L- and P-selectins with MM cells (Figure 1F). Supplemental Figure 3 shows that GMI-1070 did not induce cytotoxicity in MM cells or HUVECs.
PSGL-1 regulates adhesion of MM cell to ECs
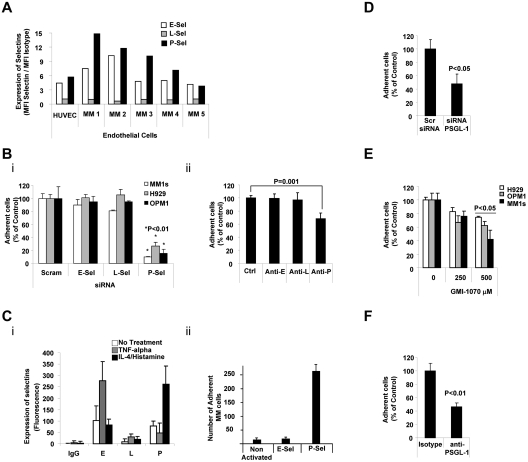
We first determined the expression of selectins on ECs in 5 BM aspirates collected from patients with MM and in HUVECs. E- and P-selectins (but not L-selectin) were highly expressed on ECs isolated from the BM of MM patients and on HUVECs (Figure 2A). To investigate the role of each of selectin in the interaction of ECs with MM cells, we knocked down each of the selectins in HUVECs (as confirmed by flow cytometry; supplemental Figure 4). As shown in Figure 2Bi, knockdown of P-selectin, but not of E- or L-selectins, decreased the adhesion of MM cells to HUVECs. Similarly, blocking Abs for P-selectin (but not for E- or L-selectins) inhibited the adhesion of MM cells to HUVECs (Figure 2Bii). Moreover, increasing the expression of P-selectin (by stimulation with IL-4 and histamine) enhanced the adhesion of MM cells to HUVECs, whereas increasing the expression of E-selectin (by stimulation with TNFα) did not increase this adhesion (Figure 2C). These results indicate that P-selectin regulates the adhesion of MM cells to ECs.
Figure 2.
PSGL-1 regulated adhesion of MM cells to ECs. (A) Expression of E-, L-, and P-selectins evaluated on HUVECs and primary MM BM-derived ECs (n = 5) using flow cytometry and expressed as ratio between MFI of selectin to the MFI of the isotype control. ECs present with higher expression of E- and P-selectins. (B) HUVECs were transfected with siRNAs for E-, L-, or P-selectin. Scramble siRNA was used as a control (panel i) or cells were treated with anti–E-, anti–L-, or anti–P-neutralizing Abs. Isotype control Ab was used as a control (panel ii). Adhesion of MM cells on HUVECs was evaluated: significant inhibition of MM cells to HUVECs was observed in P-selectin knock-down cells (panel i) and in HUVECs treated with neutralizing Ab for P-selectin (panel ii), P < .01. All HUVECs shown in panel C were exposed to TNFα (30 U/mL for 3 hours) or to IL-4 (3 ng/mL for 24 hours) and histamine (2.25mM for 4 hours). Expression of E-, L-, and P-selectins was evaluated by flow cytometry. Induction of E- and P-selectin was observed after activation with TNFα or IL-4/histamine, respectively (panel i). Adhesion of nontreated MM cells to HUVECs was evaluated. MM cells showed increased adhesion to HUVECs with activation of P-selectin (panel ii), P < .01. (D) MM1s cells were transfected with either PSGL-1 siRNA or scramble siRNA. Adhesion of MM cells to HUVECs was evaluated: a significant inhibition of MM cells to HUVECs was observed in PSGL-1 knock-down cells (P < .05). (E) HUVECs were treated with increasing concentrations of GMI-1070 (0, 100, 250, and 500μM) for 1 hour, and adhesion of nontreated MM cells (H929, OPM1, and MM.1S) to HUVECs was evaluated: dose-dependent inhibition of MM cell adhesion to HUVECs was observed (P < .05). All data represent means ± SD of triplicate experiments. (F) MM1s cells were treated with either isotype control or PSGL-1–blocking Ab. Adhesion of MM cells to HUVECs was evaluated: significant inhibition of MM cells to HUVECs was observed in cells treated with PSGL-1–blocking Ab (P < .01).
We next investigated role of PSGL-1 in adhesion of MM cells to ECs. As shown in Figure 2D, knockdown of PSGL-1 in MM cells decreased the adhesion of MM cells to HUVECs. Similarly, inhibition of the interaction between P-selectin in HUVECs and PSGL-1 in MM cells decreased the adhesion of MM cells to HUVECs in a dose-dependent manner (Figure 2E). These results were reconfirmed using a blocking Ab, and we found that blocking PSGL-1 decreased the adhesion of MM cells to HUVECs (Figure 2F). These findings indicate that interactions between selectins and selectin ligands also regulate the adhesion of MM cells to ECs.
PSGL-1 regulates transendothelial migration of MM cells
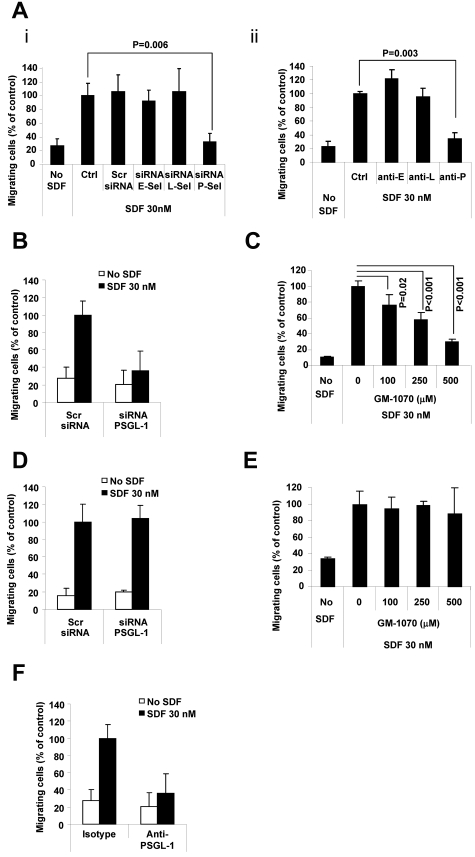
To understand the physiologic process of extravasation of MM cells through ECs, we tested the role of the interaction of PSGL-1 with P-selectin during migration of MM cells through a monolayer of ECs in response to the chemokine SDF1α. Figure 3A shows that blocking P-selectin by siRNA (Figure 3Ai) or blocking Abs (Figure 3Aii) significantly reduced the transendothelial migration of MM cells by 70%, whereas blocking E- or L-selectin had no such effect.
Figure 3.
PSGL-1 regulates transendothelial migration of MM cells. (A) HUVECs were transfected with siRNAs for E-, L-, or P-selectin. Scramble siRNA was used as a control (panel i) or cells were treated with anti–E-, anti–L-, or anti–P-neutralizing Abs. Isotype control Ab was used as a control (panel ii). Transendothelial migration of MM1s cells in response to SDF-1α (30nM) was tested. Significant inhibition of MM cell transendothelial migration was observed in HUVECS with P-selectin knockdown (panel i) and in HUVECs treated with neutralizing Ab for P-selectin (panel ii) (P < .01). (B) MM1s cells were transfected with either PSGL-1 or scramble siRNA, and transendothelial migration of MM cells in response to SDF-1α (30nM) was tested. Significant inhibition of MM cell transendothelial migration was observed in MM cells with PSGL-1 knockdown. (C) HUVECs were treated with increasing concentrations of GMI-1070 (0, 100, 250, and 500μM) for 1 hour, and transendothelial migration of nontreated MM1s cells in response to SDF-1α (30nM) was evaluated: dose-dependent inhibition of MM cell transendothelial migration was observed (P < .02). (D) MM1s cells were transfected with either PSGL-1 or scramble siRNA, and migration of MM cells (with no presence of HUVECs) in response to SDF-1α (30nM) was tested. No difference in cell migration was observed in MM cells transfected with PSGL-1 or scramble siRNA. (E) MM1s cells were treated with increasing concentrations of GMI-1070 (0, 100, 250, and 500μM) for 1 hour, and migration of MM cells (with no presence of HUVECs) in response to SDF-1α (30nM) was tested: no difference in cell migration was observed in MM cells treated with or without GMI-1070. (F) MM1s cells were treated with either isotype control or PSGL-1–blocking Ab. Transendothelial migration of MM cells was evaluated: significant inhibition of MM cells to HUVECs was observed in cells treated with PSGL-1–blocking Ab.
Moreover, down-regulation of PSGL-1 on MM cells by siRNA decreased the transendothelial migration of MM cells through the EC monolayer in vitro (Figure 3B). Similar results were obtained when the interaction between selectin on ECs and PSGL-1 on MM cells was inhibited using the selectin inhibitor GMI-1070 (Figure 3C). To confirm that the effect on transendothelial migration was through the specific interaction between selectins in ECs and PSGL-1 in MM cells, we eliminated the EC component from the experiment and tested the effect of down-regulation of PSGL-1 in MM cells and of GMI-1070 on the trans-well (through the filter) migration of MM cells in response to SDF1 through. Neither down-regulation of PSGL-1 (Figure 3D) nor treatment with GMI-1070 (Figure 3E) had an effect on the migration of MM cells in response to SDF1α. These results were reconfirmed using a blocking Ab, and we showed that blocking PSGL-1 decreased the transendothelial migration of MM cells (Figure 3F). These observations indicate that the interactions of P-selectin in ECs with PSGL-1 in MM cells play a critical role in transendothelial migration, which is the first step of the extravasation process and homing of MM cells into the BM in vivo.
PSGL-1 regulates extravasation and homing of MM cells to BM in vivo
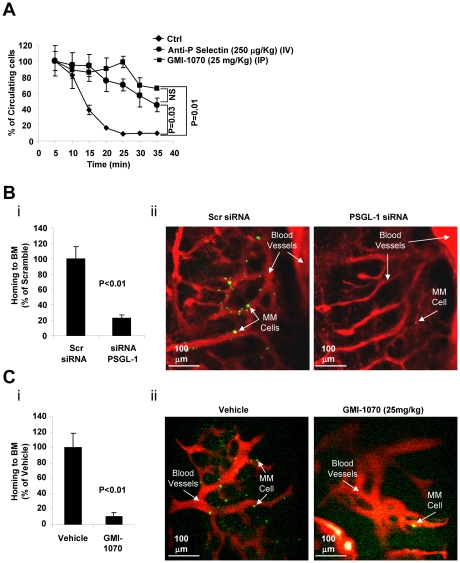
To examine the role of the interaction of PSGL-1/P-selectin on homing of MM cells to the BM in vivo, we used in vivo flow cytometry, which measures the number of circulating cells as the number of cells that pass in an appropriate artery in the mouse ear per minute.29 Figure 4A shows that in control nontreated mice, more than 90% of the MM cells had extravasated within 25 minutes after injection, whereas in mice pretreated with blocking P-selectin Ab or with GMI-1070 (25 mg/kg), MM cells displayed delayed extravasation: more than 50% of the cells were still circulating at 35 minutes after injection.
Figure 4.
The interaction of PSGL-1 with P-selectin regulates extravasation and homing to the BM of MM cells in vivo. (A) Calcein-labeled MM1s cells were injected intravenously into SCID mice treated with either vehicle (Ctrl), anti–P-selectin Ab (250 μg/kg IV), or GMI-1070 (25 mg/kg IP) for 1 hour before injection of MM1s cells (n = 3 per group). The number of circulating cells was followed over time using in vivo flow cytometry. Cells were counted every 5 minutes for 40 minutes. Fluorescence signal was detected on an artery in the ear and digitized for analysis with MATLAB software. Inhibition of P-selectin using GMI-1070 or neutralizing Ab delayed the extravasation of MM cells. (B) MM1s cells were transfected with either PSGL-1 or scramble siRNA, labeled with Calcein, and injected intravenously into SCID mice (n = 3 per group), followed by IV injection of Evans blue. Homing to the BM of mice was imaged by in vivo confocal microscopy 30 minutes after injection. Inhibition of the homing of MM cells to the BM was observed with knockdown of PSGL-1, shown as an average of number of MM cells in 18 images taken from 3 different mice (P < .01; i) and in representative images of the BM (ii; green indicates MM cells; red, blood vessels). (C) Calcein-labeled MM1s cells were injected intravenously into SCID mice, which were treated with either vehicle (Ctrl) or GMI-1070 (25 mg/kg IP) for 1 hour before injection of MM1s cells (n = 3 per group), followed by IV injection of Evans blue. Homing to the BM of mice was imaged by in vivo confocal microscopy 30 minutes after injection. Inhibition of the homing of MM cells to the BM was observed in mice treated with GMI-1070, shown as an average of number of MM cells in 18 images taken from 3 different mice (P < .01; i) and in representative images of the BM (ii; green indicates MM cells; red, blood vessels).
PSGL-1 regulates homing of MM cells to BM in vivo
We also used in vivo live confocal imaging to examine homing of MM cells to the BM, in which we detect the presence of MM cells in the BM niches of the mouse skull. PSGL-1 in MM cells was knocked down or mice were treated with GMI-1070 to interfere with the interaction between ECs and MM cells. Figure 4Bi shows that knockdown of PSGL-1 in MM cells decreased the number of MM cells that had homed to the BM in an average of 18 images taken from 3 different mice. Figure 4Bii shows representative images of the BM in the calvaria of mice, which shows less homing of Calcein-labeled MM cells in cells transfected with PSGL-1 siRNA compared with scramble siRNA. Blood vessels were labeled with Evans blue. Similarly, Figure 4Ci shows that inhibition of selectins in the host environment by GMI-1070 (25 mg/kg) decreased the number of MM cells that homed to the BM. Figure 4Cii shows representative images of homing MM cells in mice treated with GMI-1070 or vehicle: fewer MM cells were present in the BM vascular niches in the GMI-1070–treated mice compared with control mice.
Interaction of P-selectin with PSGL-1 regulates adhesion signaling and activates β1-integrin
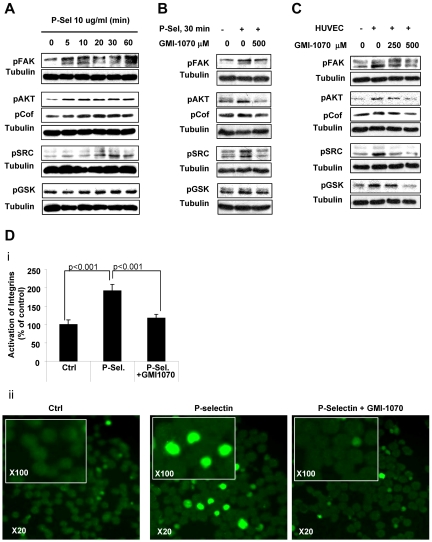
To further understand the nature of the interaction between P-selectin and PSGL-1 in adhesion to ECs, we studied the molecular events and adhesion-related cell signaling in MM cells. Treatment of MM cells with recombinant P-selectin activated cell-adhesion signaling, as evidenced by increased phosphorylation of FAK, Src, cofilin, AKT, and GSK-3α/β. Maximal activation of most proteins was achieved at 30 minutes (Figure 5A). Inhibition of P-selectin by GMI-1070 (500μM for 1 hour) reversed the activation of these kinases induced by recombinant selectin (Figure 5B). Similarly, coculture of HUVECs with MM cells induced activation of the same adhesion-related signaling in MM cells, which was inhibited by GMI-1070 in a dose-dependent manner (Figure 5C). Given that selectin ligands activate adhesion-related kinases, we tested the effect of P-selectin on the activation of integrins in MM cells. Recombinant P-selectin increased the activation and clustering of β1-integrin on the MM-cell surface, which was inhibited by blocking P-selectin with GMI-1070 (Figure 5Di). Representative immunofluorescent images of β1-integrin clustering are shown in Figure 5Dii.
Figure 5.
Interaction of PSGL-1 and P-selectin regulates adhesion-related signaling and β-integrin activation in MM cells. (A) MM1s cells were treated with recombinant P-selectin 10μg/mL for different durations (0, 5, 10, 20, 30, and 60 minutes), lysed, and whole-cell lysates were subjected to Western blotting for pFAK, pAKT, pCoffilin, pSRC, and p-GSK3α/β. Increased adhesion-related signaling was observed after activation with recombinant P-selectin, with maximal activation at 30 minutes. (B) Recombinant P-selectin was incubated with or without GMI-1070 (500mM for 1 hour) and then applied to MM1s cells; nontreated MM cells were used as a control. Cells were then lysed and whole-cell lysates were subjected to Western blotting for pFAK, pAKT, pCoffilin, pSRC, and p-GSK3α/β. GMI-1070 reversed the induction of adhesion-related signaling in MM cells induced by recombinant P-selectin. (C) HUVECs were treated with or without GMI-1070 (500mM for 1 hour), nontreated MM1s cells were cocultured with the HUVECs for 1 hour, and MM1s cells not cocultured with HUVECs served as a control. MM cells were then separated from the HUVECs, lysed, and whole-cell lysates were subjected to Western blotting for pFAK, pAKT, pCoffilin, pSRC, and p-GSK3α/β. Coculture of MM cells with HUVECs induced adhesion-related signaling in MM cells that was reversed by GMI-1070. (D) Recombinant P-selectin was incubated with or without GMI-1070 (500mM for 1 hour) and then applied to MM1s cells; nontreated MM cells were used as a control. Cells were fixed and the expression of activated β1-integrin was detected using FITC-labeled Ab under immunofluorescence microscopy. Increased activation of β1-integrin was observed after activation with recombinant P-selectin, which was reversed by GMI-1070. Results are shown as an average of the percentage of cells with activated integrins, normalized to controls (P < .001; i), and in representative fluorescent images at 20× magnification and at 100× in the inset (ii).
PSGL1/P-selectin interaction regulates proliferation of MM cells in the context of the BM microenvironment in vitro and in vivo
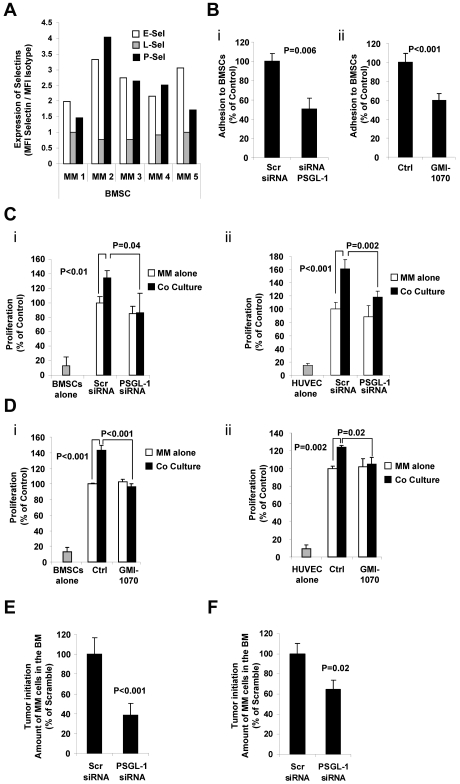
Previous studies have shown that adhesion of MM cells to cells in the BM microenvironment leads to increased proliferation of MM cells.34 We hypothesized that the PSGL-1/P-selectin axis plays a role in the proliferation of MM cells induced by BMSCs and ECs. To examine this possibility, we first determined the expression of selectins on BMSCs derived from the BM of 5 MM patients. Figure 6A shows that, as for ECs derived from BM of MM patients, BMSCs express E- and P-selectins but not L-selectin. Inhibition of the interaction of PSGL-1 in MM and P-selectin with BMSCs and cells by knockdown of PSGL-1 in MM cells (Figure 6Bi) or GMI-1070 (Figure 6Bii) reduced the adhesion of MM cells to BMSCs.
Figure 6.
The interaction of PSGL-1 and P-selectin regulates proliferation of MM cells induced by BMSCs and ECs in vitro and tumor initiation in vivo. (A) Expression of E-, L-, and P-selectins evaluated in primary BMSCs (n = 5) using flow cytometry and expressed as ratio between MFI of selectin to the MFI of the isotype control. BMSCs presented with higher expression of E- and P-selectins. (B) MM1s cells were transfected with either PSGL-1 siRNA or scramble siRNA. Adhesion of MM cells to BMSCs was evaluated: significant inhibition of MM cell adhesion to BMSCs was observed in PSGL-1 knockdown cells (P = .006; i). BMSCs were treated with or without GMI-1070 (500μM for 1 hour) and adhesion of nontreated MM1s cells to BMSCs was evaluated: inhibition of MM cell adhesion to BMSCs was observed in HUVECs treated with GMI-1070 (P < .001; ii). Data represent means ± SD of triplicate experiments. (C) MM1s cells were transfected with either PSGL-1 siRNA or scramble siRNA and cultured with or without BMSCs (i) and HUVECs (ii). Cell proliferation was measured at 24 hours by bromodeoxyuridine incorporation and ELISA. Coculture of MM1s with HUVECs and BMSCs increased the proliferation of MM1s cells transfected with scramble siRNA, an effect that was reversed by PSGL-1 siRNA. Data represent means ± SD of triplicate experiments. (D) HUVECs and BMSCs were treated with or without GMI-1070 and nontreated MM cells were cultured with or without BMSCs (i) and HUVECs (ii). Cell proliferation was measured at 24 hours by bromodeoxyuridine incorporation and ELISA. Coculture of MM1s with nontreated HUVECs and BMSCs increased the proliferation of MM1s cells, an effect that was reversed by GMI-1070. Data represent means ± SD of triplicate experiments. (E) MM1s cells were transfected with either PSGL-1 or scramble siRNA and injected intravenously into SCID mice (n = 4 per group); after 1 week the BM was extracted from the femurs of the mice and tumor initiation was determined as the percentage of CD138+ cells in the BM. Inhibition of tumor initiation in the BM of the mice was observed with knockdown of PSGL-1 (P < .001). (F) MM1s cells were transfected with either PSGL-1 or scramble siRNA and injected into the BM of the tibia of SCID mice; after 1 week the BM was extracted from the tibias and tumor initiation was determined as the percentage of CD138+ cells in the BM. Inhibition of tumor initiation in the BM of the mice was observed with knockdown of PSGL-1, but not to the same extent as that observed after IV injection (P = .02).
We also studied the role of the PSGL-1/P-selectin axis in MM-cell proliferation induced by BMSCs and ECs. Knockdown of PSGL-1 on MM cells reduced the proliferation of MM induced by BMSCs (Figure 6Ci) and ECs (Figure 6Cii). Similarly, inhibition of P-selectin in BMSCs (Figure 6Di) or ECs using GMI-1070 had similar effects (Figure 6Dii). In addition, we found that the mitochondria of MM1s cells incubated with stroma were less primed for apoptosis compared with those cultured in the absence of stroma. This reduction in priming was reversed by selectin inhibition with GMI-1070 (supplemental Figure 5).
To determine the role of PSGL-1/P-selectin in tumor initiation in vivo, we examined the level of MM cells in the BM of mice 1 week after injection of MM cells either IV or directly into the BM of the tibia as models of tumor initiation. Figure 6E shows that, when injected intravenously, knockdown of PSGL-1 in MM cells decreased the tumor initiation in vivo to 40% compared with scramble siRNA–transfected cells. Figure 6F shows that, when injected directly to the tibia, knockdown of PSGL-1 in MM cells decreased tumor initiation in vivo to 65% compared with scramble siRNA–transfected cells.
PSGL-1/P-selectin regulates resistance of MM cells to chemotherapy in the context of BM niches
We showed previously that the interaction of MM cells with the BM microenvironment induces drug resistance6; therefore, in the present study, we examined the role of PSGL-1/P-selectin in this effect in vitro and in vivo.
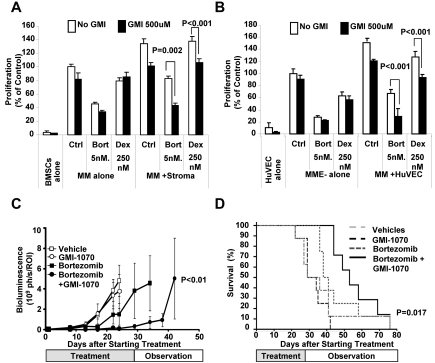
Coculture of MM cells with either BMSCs or ECs induced resistance to bortezomib and dexamethasone, as demonstrated by reduced inhibition of proliferation (compared with MM cells cultured alone). Inhibition of P-selectin by GMI-1070 restored the sensitivity of MM cells to bortezomib and dexamethasone, even when cocultured with BMSCs (Figure 7A) and ECs (Figure 7B).
Figure 7.
The interaction of PSGL-1 and P-selectin regulates drug resistance of MM cells induced by BMSCs and ECs in vitro and tumor progression in vivo. (A) MM1s cells (treated with vehicle, bortezomib 5nM, or dexamethasone 250nM) were cultured with or withoutBMSCs (treated with or without GMI-1070 500μM). Cell proliferation was measured at 24 hours by bromodeoxyuridine incorporation and ELISA. Coculture of MM1s with BMSCs induced resistance to both bortezomib and dexamethasone in MM1s cells which was reversed by GMI-1070. Data represent means ± SD of triplicate experiments. (B) MM1s cells (treated with vehicle, bortezomib 5nM or dexamethasone 250 nM) were cultured with or without the presence of HUVECs (treated with or without GMI-1070 500μM). Cell proliferation was measured at 24 hours by bromodeoxyuridine incorporation and ELISA. Coculture of MM1s with HUVECs induced resistance to both bortezomib and dexamethasone in MM1s cells that was reversed by GMI-1070. Data represent means ± SD of triplicate experiments. (C) Effect of inhibition of the interaction of PSGL-1 with P-selectin by GMI-1070 on the sensitivity of MM tumors to bortezomib in vivo. SCID mice (n = 10 per group) were injected with MM1s cells engineered to express luciferase and tumor growth was determined by bioluminescence imaging. Mice were divided in 4 groups: (1) the control group, which received weekly IP injection of vehicle and were implanted with pumps loaded with vehicle every 2 weeks (for 4 weeks); (2) the GMI-1070–treated group, which received weekly IP injection of vehicle and were implanted with a pump loaded with 200 μL of 150 mg/mL of GMI-1070 that was changed every 2 weeks (for 4 weeks); (3) the bortezomib-treated group, which received weekly IP injection of bortezomib 1.5 mg/kg and were implanted with a pump loaded with vehicle that was changed every 2 weeks (for 4 weeks); and (4) the combination group, which received weekly IP injection of bortezomib 1.5 mg/kg and were implanted with a pump loaded with 200 μL of 150 mg/mL GMI-1070 that was changed every 2 weeks (for 4 weeks). Tumor progression was detected using bioluminescence. Treatment with GMI-1070 alone did not affect the tumor progression, but it increased the sensitivity of MM cells to bortezomib, and decreased tumor progression was observed in the group treated with a combination of GMI-1070 and bortezomib compared with bortezomib alone (P < .01). (D) Kaplan-Meier curves of survival of groups 1 through 4 described in panel C. Increased survival was observed in mice treated with the combination of GMI-1070 and bortezomib compared with bortezomib alone, (P = .012).
We next confirmed the effect of GMI-1070 on the sensitivity of MM cells to bortezomib in vivo. We examined the effect of GMI-1070 and bortezomib used either alone or in combination on MM tumor progression in vivo. MM tumors were established in SCID mice and treatment was initiated after tumor detection by bioluminescence imaging at 4 weeks after the MM-cell injection. Unlike the effect on tumor initiation, inhibition of the PSGL-1/P-selectin axis by treatment of mice with GMI-1070 alone did not affect tumor growth compared with the vehicle-treated group (Figure 7C). As expected, treatment with bortezomib alone delayed MM tumor growth; however, tumor progression was detected even when the animals were still being treated with bortezomib. Interestingly, the combination of bortezomib and GMI-1070 inhibited tumor growth completely while animals were on treatment (first 28 days). Furthermore, tumor growth was delayed after stopping therapy in the mice treated with the combination of GMI-1070 and bortezomib (Figure 7C).
Similarly, GMI-1070 treatment did not affect the survival of mice compared with the vehicle-treated group (50% of the animals died at 28 days in both groups). Bortezomib alone increased the survival of the animals: 50% of the mice died at day 38. The combination of bortezomib and GMI-1070 significantly enhanced survival: up to day 42, 100% of the animals in this group were alive, whereas only 40%, 10%, and 0% of the bortezomib group, vehicle group, and the GMI-1070 group were alive at this time point, respectively. Moreover, the animals in the combination group reached 50% survival at day 52 (Figure 7D).
Discussion
One of the crucial steps in the early phase of tumor dissemination appears to be the interaction of tumor cells with the endothelium for extravasation and homing, which leads to the formation of new metastatic lesions.9,35,36 Selectins are molecules expressed on the cell surface of ECs that have been shown to promote the first interaction between an extravasating cell and the blood-vessel wall.7 Selectins were previously shown to play an important role in seeding of tumor cells in distant organs and in facilitating metastasis.37 Metastasis was dramatically reduced in mice with double deficiency of P- and L-selectin, suggesting a synergistic action of the 2 selectins in this process.8
In the present study, we examined the role of PSGL-1 and its interaction with P-selectin in tumor progression in MM. We found that PSGL-1 was highly expressed in MM cells and that P-selectin was highly expressed on cells in the BM microenvironment, including BMSCs and ECs. Functionally, in vitro studies showed that the interaction of PSGL-1 with P-selectin was the most critical in regulating adhesion to ECs and in transendothelial migration. These results were confirmed by down-regulation of PSGL-1 in MM cells and by down regulation of selectins in ECs. Similar results were obtained using the pan-selectin inhibitor GMI-1070, which is currently in clinical trials for vasoocclusive crisis in sickle cell disease. These findings were confirmed in vivo in mice: down-regulation of PSGL-1 in MM cells or inhibition of selectin using blocking Abs and GMI-1070 decreased the extravasation and homing of MM cells to the BM. The interaction between P-selectin and PSGl-1 mediated adhesion through activation of adhesion-related cell signaling and activation of integrins in MM cells. Future studies to examine mechanisms of interaction of selectins and integrins in MM are warranted, but were beyond the scope of this study.
We have shown previously that the interaction of MM cells with the BM microenvironment, including BMSCs and ECs, induces MM proliferation and drug resistance.6,34 Although selectins are thought to regulate the early steps of homing, in the present study, we show that they are also critical regulators of the interaction of MM with the BM microenvironment, which is critical for MM proliferation and drug resistance. We also showed that E- and P-selectins were expressed in ECs as well as BMSCs isolated from the BM of MM patients. Inhibition of the PSGL-1/P-selectin interaction by down-regulation of PSGL-1 in MM cells or by inhibition of selectin with GMI-1070 reversed the proliferative effects and drug resistance induced by BMSCs and ECs in vitro. These findings were confirmed in vivo in mice, in which inhibition of the PSGL-1/P-selectin interaction decreased tumor initiation, sensitized MM cells to bortezomib, and enhanced survival of the animals. Tumor initiation was decreased in vivo because of knockdown of PSGL-1 after both IV injection and direct injection to the BM in the tibia; however, the decrease after IV injection was more than that after tibial injection. This result indicates that the decrease in tumor initiation is not only due to prevention of homing of MM cells, but also to inhibition of proliferation induced by the interaction with the BM microenvironment. These results confirm our hypothesis that disruption of the interaction of MM cells with the BM microenvironment sensitizes MM cells to therapy,6 and suggests the interaction between PSGL-1 and P-selectin as a therapeutic target.
In summary, these studies delineated the important role of PSGL-1 in the interaction of MM cells with the BM microenvironment as a regulator of adhesion of MM cells to ECs and BMSCs. In addition, it highlights the interaction between PSGL-1 in MM cells and P-selectin in the microenvironment as a therapeutic target for the prevention of tumor progression and drug resistance in MM.
Supplementary Material
Acknowledgments
The authors thank Sonal Jhaveri for editing the manuscript.
This study was supported in part by the Multiple Myeloma Research Foundation and by the National Institutes of Health (grants R01CA125690 and 1R01CA152607).
Footnotes
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.K.A. designed and performed the research, analyzed the data, and wrote the manuscript; P.Q., F.A., C.P., B.T., T.C., J.T.P., V.M., L.M.F., and R.C. performed the research and analyzed the data; P.M., A.S., H.T.N., C.P.L., J.L.M., A.L.K., and A.L. analyzed the data; A.M.R. analyzed the data and wrote the manuscript; and I.M.G. designed the research and wrote the manuscript.
Conflict-of-interest disclosure: I.M.G. is on the advisory board for Millennium/Takeda, Genzyme, Celgene, Onyx, and Novartis. A.M.R. is on the advisory board of Roche. J.T.P. and J.L.M. are employees of GlycoMimetics. The remaining authors declare no competing financial interests.
Correspondence: Irene M. Ghobrial, MD, Medical Oncology, Dana-Farber Cancer Institute, 44 Binney St, Mayer 548A, Boston, MA, 02115; e-mail: irene_ghobrial@dfci.harvard.edu.
References
- 1.Alsayed Y, Ngo H, Runnels J, et al. Mechanisms of regulation of CXCR4/SDF-1 (CXCL12)-dependent migration and homing in multiple myeloma. Blood. 2007;109(7):2708–2717. doi: 10.1182/blood-2006-07-035857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106(6):1901–1910. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- 3.Damiano JS, Cress AE, Hazlehurst LA, Shtil AA, Dalton WS. Cell adhesion mediated drug resistance (CAM-DR): role of integrins and resistance to apoptosis in human myeloma cell lines. Blood. 1999;93(5):1658–1667. [PMC free article] [PubMed] [Google Scholar]
- 4.Hideshima T, Chauhan D, Hayashi T, et al. The biological sequelae of stromal cell-derived factor-1alpha in multiple myeloma. Mol Cancer Ther. 2002;1(7):539–544. [PubMed] [Google Scholar]
- 5.Pagnucco G, Cardinale G, Gervasi F. Targeting multiple myeloma cells and their bone marrow microenvironment. Ann N Y Acad Sci. 2004;1028:390–399. doi: 10.1196/annals.1322.047. [DOI] [PubMed] [Google Scholar]
- 6.Azab AK, Runnels JM, Pitsillides C, et al. CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood. 2009;113(18):4341–4351. doi: 10.1182/blood-2008-10-186668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bevilacqua MP, Nelson RM. Selectins. J Clin Invest. 1993;91(2):379–387. doi: 10.1172/JCI116210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borsig L, Wong R, Hynes RO, Varki NM, Varki A. Synergistic effects of L- and P-selectin in facilitating tumor metastasis can involve nonmucin ligands and implicate leukocytes as enhancers of metastasis. Proc Natl Acad Sci U S A. 2002;99(4):2193–2198. doi: 10.1073/pnas.261704098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Borsig L. Selectins facilitate carcinoma metastasis and heparin can prevent them. News Physiol Sci. 2004;19:16–21. doi: 10.1152/nips.01450.2003. [DOI] [PubMed] [Google Scholar]
- 10.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272(5258):60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 11.Frenette PS, Wagner DD. Adhesion molecules–Part II: Blood vessels and blood cells. N Engl J Med. 1996;335(1):43–45. doi: 10.1056/NEJM199607043350108. [DOI] [PubMed] [Google Scholar]
- 12.Kaila N, Thomas BE. Design and synthesis of sialyl Lewis(x) mimics as E- and P-selectin inhibitors. Med Res Rev. 2002;22(6):566–601. doi: 10.1002/med.10018. [DOI] [PubMed] [Google Scholar]
- 13.Kaila N, Thomas BE, 4th, Thakker P, Alvarez JC, Camphausen RT, Crommie D. Design and synthesis of sialyl Lewis x mimics as E-selectin inhibitors. Bioorg Med Chem Lett. 2001;11(2):151–155. doi: 10.1016/s0960-894x(00)00623-5. [DOI] [PubMed] [Google Scholar]
- 14.Xia L, Sperandio M, Yago T, et al. P-selectin glycoprotein ligand-1-deficient mice have impaired leukocyte tethering to E-selectin under flow. J Clin Invest. 2002;109(7):939–950. doi: 10.1172/JCI14151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaila N, Somers WS, Thomas BE, et al. Quinic acid derivatives as sialyl Lewis(x)-mimicking selectin inhibitors: design, synthesis, and crystal structure in complex with E-selectin. J Med Chem. 2005;48(13):4346–4357. doi: 10.1021/jm050049l. [DOI] [PubMed] [Google Scholar]
- 16.Thoma G, Duthaler RO, Magnani JL, Patton JT. Nanomolar E-selectin inhibitors: 700-fold potentiation of affinity by multivalent ligand presentation. J Am Chem Soc. 2001;123(41):10113–10114. doi: 10.1021/ja0164430. [DOI] [PubMed] [Google Scholar]
- 17.Thoma G, Kinzy W, Bruns C, Patton JT, Magnani JL, Banteli R. Synthesis and biological evaluation of a potent E-selectin antagonist. J Med Chem. 1999;42(23):4909–4913. doi: 10.1021/jm990422n. [DOI] [PubMed] [Google Scholar]
- 18.Thoma G, Magnani JL, Patton JT. Synthesis and biological evaluation of a sialyl Lewis X mimic with significantly improved E-selectin inhibition. Bioorg Med Chem Lett. 2001;11(7):923–925. doi: 10.1016/s0960-894x(01)00092-0. [DOI] [PubMed] [Google Scholar]
- 19.Friedrich M, Bock D, Philipp S, et al. Pan-selectin antagonism improves psoriasis manifestation in mice and man. Arch Dermatol Res. 2006;297(8):345–351. doi: 10.1007/s00403-005-0626-0. [DOI] [PubMed] [Google Scholar]
- 20.Beeh KM, Beier J, Meyer M, Buhl R, Zahlten R, Wolff G. Bimosiamose, an inhaled small-molecule pan-selectin antagonist, attenuates late asthmatic reactions following allergen challenge in mild asthmatics: a randomized, double-blind, placebo-controlled clinical cross-over-trial. Pulm Pharmacol Ther. 2006;19(4):233–241. doi: 10.1016/j.pupt.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 21.Nowakowski GS, Witzig TE, Dingli D, et al. Circulating plasma cells detected by flow cytometry as a predictor of survival in 302 patients with newly diagnosed multiple myeloma. Blood. 2005;106(7):2276–2279. doi: 10.1182/blood-2005-05-1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snapp KR, Ding H, Atkins K, Warnke R, Luscinskas FW, Kansas GS. A novel P-selectin glycoprotein ligand-1 monoclonal antibody recognizes an epitope within the tyrosine sulfate motif of human PSGL-1 and blocks recognition of both P- and L-selectin. Blood. 1998;91(1):154–164. [PubMed] [Google Scholar]
- 23.Tripodo C, Florena AM, Macor P, et al. P-selectin glycoprotein ligand-1 as a potential target for humoral immunotherapy of multiple myeloma. Curr Cancer Drug Targets. 2009;9(5):617–625. doi: 10.2174/156800909789056971. [DOI] [PubMed] [Google Scholar]
- 24.Ulbrich HK, Luxenburger A, Prech P, et al. A novel class of potent nonglycosidic and nonpeptidic pan-selectin inhibitors. J Med Chem. 2006;49(20):5988–5999. doi: 10.1021/jm060468y. [DOI] [PubMed] [Google Scholar]
- 25.Constantin G. PSGL-1 as a novel therapeutic target. Drug News Perspect. 2004;17(9):579–586. doi: 10.1358/dnp.2004.17.9.872571. [DOI] [PubMed] [Google Scholar]
- 26.Rossi B, Constantin G. Anti-selectin therapy for the treatment of inflammatory diseases. Inflamm Allergy Drug Targets. 2008;7(2):85–93. doi: 10.2174/187152808785107633. [DOI] [PubMed] [Google Scholar]
- 27.Florena AM, Tripodo C, Miceli L, Ingrao S, Porcasi R, Franco V. Identification of CD162 in plasma-cell dyscrasia. Lancet Oncol. 2005;6(8):632. doi: 10.1016/S1470-2045(05)70287-4. [DOI] [PubMed] [Google Scholar]
- 28.Di Bernardo A, Macor P, Guarnotta C, et al. Humoral immunotherapy of multiple myeloma: perspectives and perplexities. Expert Opin Biol Ther. 2010;10(6):863–873. doi: 10.1517/14712591003774063. [DOI] [PubMed] [Google Scholar]
- 29.Sipkins DA, Wei X, Wu JW, et al. In vivo imaging of specialized bone marrow endothelial microdomains for tumour engraftment. Nature. 2005;435(7044):969–973. doi: 10.1038/nature03703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chng WJ, Kumar S, Vanwier S, et al. Molecular dissection of hyperdiploid multiple myeloma by gene expression profiling. Cancer Res. 2007;67(7):2982–2989. doi: 10.1158/0008-5472.CAN-06-4046. [DOI] [PubMed] [Google Scholar]
- 31.Sperandio M, Smith ML, Forlow SB, et al. P-selectin glycoprotein ligand-1 mediates L-selectin-dependent leukocyte rolling in venules. J Exp Med. 2003;197(10):1355–1363. doi: 10.1084/jem.20021854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leppänen A, Parviainen V, Ahola-Iivarinen E, Kalkkinen N, Cummings RD. Human L-selectin preferentially binds synthetic glycosulfopeptides modeled after endoglycan and containing tyrosine sulfate residues and sialyl Lewis x in core 2 O-glycans. Glycobiology. 2010;20(9):1170–1185. doi: 10.1093/glycob/cwq083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishimura Y, Wakita T, Shimizu H. Tyrosine sulfation of the amino terminus of PSGL-1 is critical for enterovirus 71 infection. PLoS Pathog. 2010;6(11):e1001174. doi: 10.1371/journal.ppat.1001174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Azab AK, Azab F, Blotta S, et al. RhoA and Rac1 GTPases play major and differential roles in stromal cell-derived factor-1-induced cell adhesion and chemotaxis in multiple myeloma. Blood. 2009;114(3):619–629. doi: 10.1182/blood-2009-01-199281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Konstantopoulos K, Thomas SN. Cancer cells in transit: the vascular interactions of tumor cells. Annu Rev Biomed Eng. 2009;11:177–202. doi: 10.1146/annurev-bioeng-061008-124949. [DOI] [PubMed] [Google Scholar]
- 36.Brooks SA, Lomax-Browne HJ, Carter TM, Kinch CE, Hall DM. Molecular interactions in cancer cell metastasis. Acta Histochem. 2010;112(1):3–25. doi: 10.1016/j.acthis.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 37.Woodward J. Crossing the endothelium: E-selectin regulates tumor cell migration under flow conditions. Cell Adh Migr. 2008;2(3):151–152. doi: 10.4161/cam.2.3.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.