Abstract
Recent evidence suggests chronic myeloid leukemia (CML) stem cells are insensitive to kinase inhibitors and responsible for minimal residual disease in treated patients. We investigated whether CML stem cells, in a transgenic mouse model of CML-like disease or derived from patients, are dependent on Bcr-Abl. In the transgenic model, after retransplantation, donor-derived CML stem cells in which Bcr-Abl expression had been induced and subsequently shut off were able to persist in vivo and reinitiate leukemia in secondary recipients on Bcr-Abl reexpression. Bcr-Abl knockdown in human CD34+ CML cells cultured for 12 days in physiologic growth factors achieved partial inhibition of Bcr-Abl and downstream targets p-CrkL and p-STAT5, inhibition of proliferation and colony forming cells, but no reduction of input cells. The addition of dasatinib further inhibited p-CrkL and p-STAT5, yet only reduced input cells by 50%. Complete growth factor withdrawal plus dasatinib further reduced input cells to 10%; however, the surviving fraction was enriched for primitive leukemic cells capable of growth in a long-term culture-initiating cell assay and expansion on removal of dasatinib and addition of growth factors. Together, these data suggest that CML stem cell survival is Bcr-Abl kinase independent and suggest curative approaches in CML must focus on kinase-independent mechanisms of resistance.
Introduction
In chronic myeloid leukemia (CML), the malignant clone is driven by the oncogene Bcr-Abl. Although there is strong evidence that Bcr-Abl is sufficient to induce CML-like disease in transduction/transplantation and transgenic murine models,1,2 it is less clear that Bcr-Abl is always the first hit in CML in humans or that the disease in chronic phase is maintained by Bcr-Abl as the only driver mutation, rather than by additional genetic and/or epigenetic changes, as has been shown recently for Philadelphia-positive and -negative acute lymphoblastic leukemia.3,4 Therefore, comparisons between mouse models and primary CML should be interpreted with caution. In primary CML studies, kinase inhibitors, imatinib,5 dasatinib,6 or nilotinib7 induced high rates of apoptosis. Similarly, in transduction/transplantation1 and transgenic1,2,8 murine models of Ph+ B-cell leukemia or CML, inhibition of Bcr-Abl kinase, by kinase inhibitors or by switching off transgene expression, resulted in proliferation arrest, apoptosis, and complete remissions.
Kinase inhibitors have transformed the natural history of CML by inducing cytogenetic and molecular responses in the majority of patients in chronic phase9 and resulting in transient deep responses in many cases of advanced disease.10 A proportion of CML cases develops drug resistance, with the incidence increasing in more advanced disease.11 In most cases, the leukemic cells continue to express Bcr-Abl and often retain their dependence on the oncogene. For example, where the underlying mechanism for drug resistance is expansion of a clone expressing a Bcr-Abl kinase domain mutation resulting in imatinib resistance, the leukemic cells retain sensitivity to second-generation kinase inhibitors.7,12
Those working on CML now believe we are on a “pathway to cure”; however, this belief is tempered by the insensitivity of CML stem cells to kinase inhibitors. We have consistently demonstrated that kinase inhibitors, imatinib,13,14 nilotinib,15,16 dasatinib17,18 and bosutinib,19 although exhibiting potent antiproliferative effects, are only weak inducers of apoptosis in CML stem and progenitor cells. In primitive stem/progenitor cells (CD34+38−) harvested from CML patients in durable complete cytogenetic response over 5 years, Bcr-Abl transcripts show no suggestion of a downward trend over time.20 Furthermore, genomic PCR and PCR on individual long-term culture-initiating cell (LTC-IC) colonies reveals that CML patients on imatinib who achieve complete molecular response remain Bcr-Abl positive.21,22 These in vitro and in vivo results, derived from primary human CML stem and progenitor cells, at least hint that CML stem cells may not be “oncogene addicted.” However, in these studies, no conclusive evidence was provided that Bcr-Abl activity had been fully suppressed in the surviving cells and in particular in leukemic stem cells with repopulating potential, with many groups focused on issues of drug transport23,24 and more potent kinase inhibitors.15–19 These data have recently been strengthened by a carefully conducted study which concluded that primary CML stem cells are insensitive to imatinib despite inhibition of Bcr-Abl.25 Similarly, in transgenic mice, multiple rounds of induction and reversion of Bcr-Abl are possible,2,8 suggesting long-term persistence of leukemic stem cells. However, as these studies were performed in primary recipients only, there was no proof that transplantable stem cells were responsible for disease reinitiation. To address these critical points, we used complementary in vivo and in vitro approaches to determine whether Bcr-Abl activity is required for the survival of transplantable murine CML-like and primary human CML stem cells and conclude that this critical population is independent of Bcr-Abl kinase activity for survival.
Methods
Reagents
Dasatinib was obtained from Bristol-Myers Squibb (BMS). Stock solution (10 mg/mL) in DMSO (Sigma-Aldrich) was prepared and stored in aliquots at −20°C.
Primary cell samples
After informed consent, CD34+ cells were enriched from CML chronic-phase samples at diagnosis and non-CML controls and cryopreserved.17
In vitro cell culture
HT1080 and 293FT cells were cultured in DMEM, K562, KCL22, and BaF3 cells in RPMI 1640 medium, supplemented with 1% (vol/vol) penicillin/streptomycin (10 000 μg/mL/10 000 U/mL), 1% L-glutamine, and 10% (vol/vol) FCS (all Invitrogen). IL-3 (10 ng/mL; StemCell Technologies) was added to parental BaF3 cells. Primary CML cells were cultured in serum-free medium,17 supplemented with a high growth factor cocktail, 100 ng/mL Flt-3 ligand and SCF, and 20 ng/mL each of IL-3, IL-6 (StemCell Technologies) and G-CSF (Chugai Pharma Europe Ltd), growth factor cocktail for transduction, 50 ng/mL SCF, 100 ng/mL thrombopoietin (TPO) and Flt-3 ligand, physiologic growth factor cocktail, 0.2 ng/mL SCF, GM-CSF, and MIP-α, 1.0 ng/mL G-CSF and IL-6, 0.05 ng/mL Leukemia inhibitory factor (LIF; StemCell Technologies) or with no added growth factors. Primary mouse cells were cultured in serum-free expansion medium (SFEM) supplemented with 10 ng/mL IL-3, IL-6, and mSCF (StemCell Technologies).
FISH
FISH was performed as previously described.17
Lentivirus production
The pHIV7-GFP transfer vector containing the shRNA expression cassette specific for 2 different splice forms of Bcr-Abl, pCMV-VSV-G and pCMV-Hiv1, were provided by Dr John Rossi (Dept of Molecular and Cellular Biology, Beckman Institute of City Hope, Duarte, CA).26 Trans-duction of K562 or KCL22 was performed at a MOI 1-10 with 70%-95% of the cells expressing GFP after 48 hours. Primary human CD34+ cells were preincubated on retronectin (8 μg/cm2) in medium containing growth factor cocktail for transduction for 2 days. Concentrated lentivirus was added twice for 24 hours at MOI 40. Forty-eight hours later, cells were FACS-sorted on GFP and cultured in medium containing physiologic growth factors.
Western blotting and flow cytometry
Western blotting was performed17 using Abs against Bcr-Abl/Abl, p-CrkL, p-STAT5, Tubulin (Cell Signaling), and actin (New England Biolabs Ltd and Sigma-Aldrich). Detection was by ECL (GE Healthcare/Amersham) using a HRP-linked secondary Ab. Bcr-Abl activity was determined as described previously.27
Assessment of apoptosis and CFSE tracking of cell division
Apoptosis and CFSE tracking were performed as previously described.16,17,28
High-resolution cell-cycle analysis
High-resolution cell-cycle analysis was performed using Ki-67 and 7AAD (BD Biosciences).29
CFC and LTC-IC assays
Colony forming cell (CFC) and LTC-IC were performed as previously.28
Q-PCR for Bcr-Abl and Abl-kinase domain mutation analyses
Quantitative PCR (Q-PCR) was performed by standardized Europe Against Cancer (EAC) protocols30 and final results expressed according to the international scale.31 Kinase domain mutation was investigated by direct sequencing.32,33
Q-PCR
Isolation of DNase-treated RNA was performed using RNeasy Mini or Micro Kits (QIAGEN). For colony PCR, cDNA was made using the TaqMan Gene Express Cells-to-CT Kit (Ambion). Bcr-Abl was detected using a real-time TaqMan assay (Eurogentec) or Evergreen (Web Scientific) using the ABI 7500 Fast Real-Time PCR system (Applied Biosystems). GAPDH was used as control, setting the cutoff at 35 cycles.
Mice and genotyping
Genotyping of SCLtTA/Bcr-Abl dtg mice was described previously.2 Approval for the animal research was obtained from the local authorities of North-Rhine (Westphalia, Germany). For first-round transplantation, 2 × 106 BM cells were tail vein injected into 12-week-old FVB/N CD45.2 mice after 8 Gy irradiation. For secondary transplantation, 1.2 × 106 FACS-sorted CD45.1+ cells were injected into 9 Gy irradiated FVB/N CD45.2 recipients. Mice were treated with cotrimoxazole (Ratiopharm) until 2 weeks after transplantation. Peripheral blood (PB) was collected from the retro-orbital plexus. Tissues were fixed with 5% paraformaldehyde/PBS and paraffin-embedded. Sections from spleen were stained using N-acetyl-chloroacetate esterase (NACE).
Flow cytometry analysis of mice
After red cell lysis, cells were stained with CD45.1, CD45.2, CD3, B220, Gr1, CD41, Ter119, IgG2a, and IgG2b (BD Biosciences) and CD11b (Invitrogen). FACS analysis for Lineage−Sca-1+c-kit+ (LSK) cells was performed using red cell–lysed BM stained with rat Abs specific for CD3, CD4, CD8a, B220, and Gr1 (Invitrogen), CD11b, Ter119. LSK cells were stained using CD117 (c-kit) and Sca-1 (BD Biosciences). Isolation of lineage-negative cells was performed using MACS mouse lineage depletion kit (Miltenyi Biotec Inc). For purification of LSK cells, lineage-negative cells were stained with rat Abs specific for CD3, CD4, CD8a, B220, and Gr1 (Invitrogen), CD11b, Ter119, CD117 (c-kit), and Sca-1 (BD Biosciences) and FACS-sorted.
Statistical analysis
Error bars represent SEM. Statistical analyses were performed using the 2-tailed Student t test. A level of P ≤ .05 was taken to be statistically significant.
Results
Leukemic stem cells retain their transplantation and leukemogenic potential after complete reversion of Bcr-Abl
We previously described an inducible transgenic mouse model of CML in which p210-Bcr-Abl expression is targeted to stem and progenitor cells of murine BM using the tet-off system.2,8 On tetracycline withdrawal, Bcr-Abl expression is induced and mice demonstrate leukocytosis, splenomegaly, and myeloid hyperplasia. The disease is transplantable using Bcr-Abl+ unfractionated (uf) BM or LSK cells and can be reverted after tetracycline treatment or to a much lesser extent using imatinib.8 In both transgenic and primary transplant recipients, the CML-like disease is fatal after 29-122 days.2,8
Here we used the CD45.1/45.2 system to discriminate donor and host cells in a transplantation setting and were thus able to serially transplant the initially leukemic cells after abrogation of Bcr-Abl expression and reversion of the CML phenotype (Figure 1Ai). SCLtTA/Bcr-Abl double-transgenic (dtg) BM cells from CD45.1 donors were transplanted into 8Gy sublethally irradiated CD45.2 recipients (n = 12). Wild-type (wt) CD45.1 donors were used as controls (n = 12). An alternative approach would have been to use dtg mice as donors and to maintain one cohort on and the other one off tetracycline throughout the experiment. This was considered but decided against in view of concern that tetracycline might lead to undesired effects on either normal or leukemic hemopoiesis. Recipient mice were maintained off tetracycline to induce Bcr-Abl expression as shown previously.2,8 PB analysis on day 21 confirmed that dtg recipient mice had developed disease and this was confirmed in BM and spleen on day 25 when mice were killed. At this time point, donor BM LSK showed a slight but significant 1.2-fold expansion compared with controls (supplemental Figure 1A-G, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
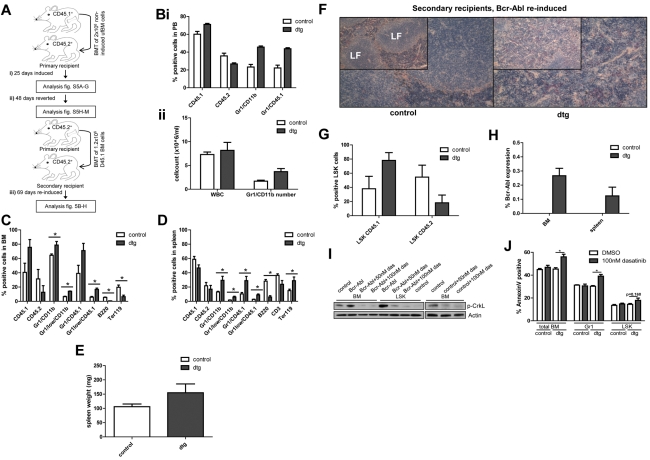
Figure 1.
CML stem cells retain transplantation and oncogenic capacity following Bcr-Abl reversion. (A) Irradiated CD45.2+ mice (8 Gy) transplanted using unfractionated (uf) CD45.1+ BM cells isolated from SCLtTA/Bcr-Abl dtg or wt controls (n = 12/12). (i) Bcr-Abl expression in recipient mice was controlled by absence (expression) or presence (no expression) of tetracycline in drinking water. Bcr-Abl expression was induced after transplantation for 25 days and the phenotype analyzed in dtg and controls (n = 3/3) by FACS of BM and spleen cells, spleen weight and histology, ratio of donor:host LSK cells, and Bcr-Abl expression. (ii) Bcr-Abl expression was reverted for 48 days and a cohort of remaining mice (n = 4/4) analyzed. (iii) BM cells from reverted dtg and controls were retransplanted using CD45.1+ FACS-sorted cells (n = 5/5). Bcr-Abl expression was reinduced and the phenotype was assessed 69 days after retransplantation. (Bi-ii) PB was analyzed in CD45.1+ retransplanted and reinduced mice 34 days after secondary transplantation. FACS of (C) BM and (D) spleen. Mean percentages of donor cells (CD45.1+), recipient cells (CD45.2+), granulocytes (Gr1+/CD11b+), immature granulocytes (Gr1low/CD11b+), donor granulocytes (Gr1+/CD45.1+), immature donor granulocytes (Gr1low/CD45.1+), B cells (B220+), T cells (CD3+), or erythroid cells (Ter119+). Spleen weights on (E) autopsy, (F) spleen histology, (G) donor:host ratio within BM LSK, and (H) Bcr-Abl expression in secondary recipients. (I) BM and LSK cells from 4-week induced dtg and control mice were cultured with either 50nM dasatinib, 100nM dasatinib, or DMSO for 48 hours. The level of p-CrkL was measured by Western blotting. (J) Apoptosis in total BM, Gr1, and LSK cells was assessed by annexin V staining in cells from 4-week induced dtg (n = 7) and control (n = 5) mice that were treated for 48 hours using 100nM dasatnib or DMSO. Histologic analyses of spleen were performed using NACE stain and are shown at magnifications of (insets) 10× and 20×. Shown is 1 representative spleen histology from each group. LF indicates lymphoid follicle. Data represented as mean ± SEM; *P < .05.
Tetracycline was then administered to the remaining mice to abrogate Bcr-Abl expression and revert the phenotype (Figure 1Aii). By day 41 on PB sampling the disease had been completely reverted with no difference between dtg and controls (supplemental Figure 1Hi-ii) and this was confirmed at the time of sacrifice on day 48, with no evidence of leukemia in BM or spleen (supplemental Figure 1I-M). Strikingly, the percentages of mature and immature granulocytic donor cells decreased to control levels in dtg BM and spleen on Bcr-Abl abrogation (compare supplemental Figure 1B-C, I-J), showing that proliferation and survival of mature cells are affected by Bcr-Abl abrogation. Conversely, BM LSK donor cells had continued to increase by equivalent amounts in control and dtg mice (supplemental Figure 1M) suggesting that dtg donor LSK cells showed similar chimerism dynamics as controls. Bcr-Abl was neither detectable in total BM nor spleen cells, nor in FACS-sorted CD45.1+ BM cells from either cohort. Histology of spleen showed no evidence of leukemic infiltration and there was no evidence of splenomegaly. To assess potential residual Bcr-Abl expression in reverted LSK cells, we FACS-sorted these cells from a cohort of primary, transgenic mice that had either been induced for 3 weeks or reverted for an additional 6 weeks. Analysis of BM, spleen, and PB confirmed neutrophilia and splenomegaly restricted to induced, but not reverted dtg or control mice (supplemental Figure 2A-B). RT-PCR using LSK cells showed a > 96% reduction of Bcr-Abl expression in reverted mice back to control levels (supplemental Figure 2C). To assess Bcr-Abl activity, we performed Western blot using lineage-negative BM cells from mice that had either been induced for 4 weeks (supplemental Figure 2E) or mice that had been reverted for 68 days after a 3-week induction period (supplemental Figure 2F). Level of CrkL phosphorylation was increased on induction of Bcr-Abl (supplemental Figure 2E) but was decreased to control level on reversion (supplemental Figure 2F). These results confirmed that by administration of tetracycline the leukemic phenotype had been completely reverted.
As a final step, FACS-sorted, BM-derived, CD45.1+ cells from each cohort were retransplanted, at 1.2 × 106 cells/mouse, into 9 Gy sublethally irradiated, secondary recipients (CD45.2+, n = 5/5, Figure 1Aiii). These mice were maintained off tetracycline to reinduce Bcr-Abl expression to determine whether cells with oncogenic potential had survived the reversion period. PB analyses 34 days after transplantation again showed an increased donor-to-host cell ratio, increasing percentages of donor granulocytes (Figure 1Bi) and increased numbers of granulocytes (Figure 1Bii). After a further 35 days, the secondary recipients were killed at 69 days after retransplantation and reinduction of Bcr-Abl expression. CD45.1+ dtg donor cells had engrafted in BM and spleen (Figure 1C-D). Although the phenotype was weaker than in the primary recipients, there was still significant expansion of immature myeloid donor cells in BM and spleen (Figure 1C-D). Development of splenomegaly was observed, but did not reach statistical significance (Figure 1E), unless the dtg mice were corrected for loss of body weight on disease development. In addition, disruption of spleen morphology and myeloid cell infiltration of the spleen were clearly observed in dtg mice (Figure 1F). There was a nonsignificant trend toward an increase of dtg donor BM LSK compared with control LSK cells (2.1-fold increase, Figure 1G). Bcr-Abl was expressed in BM and spleen of all secondary dtg recipients (Figure 1H). As an alternative to switching off Bcr-Abl transgene expression in transplanted CD45.1 dtg LSK, we treated total BM and LSK cells with dasatinib. Western blot showed that p-CrkL level was increased in total BM and LSK of Bcr-Abl–expressing cells but was completely reverted on 100nM dasatinib treatment (Figure 1I). In addition we demonstrated that dasatinib treatment significantly induced apoptosis in leukemic total BM and mature Gr1-positive cells, whereas Bcr-Abl–expressing dtg LSK were comparatively resistant (Figure 1J).
Partial Bcr-Abl knockdown inhibits proliferation of CML CD34+ cells
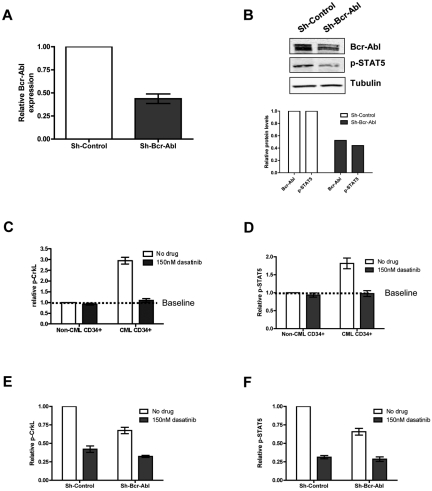
Recently Shah et al argued for evaluation of more than one end point for inhibition of Bcr-Abl to ensure coverage of an adequate dynamic range.34 Measurement of inhibition of phosphorylation of STAT5 and CrkL by flow cytometry and Western blotting was therefore optimized in BaF3 cells expressing Bcr-Abl (supplemental Figure 3A-C). A total of 150nM dasatinib reduced phosphorylation of both CrkL and STAT5 to the baseline seen in parental BaF3 cells, with no effect on Bcr-Abl T315I mutant and no additional effect with increasing concentrations of dasatinib (supplemental Figure 3A-C). Previous data using lentiviral-mediated shRNA delivery confirmed efficient and specific knockdown of Bcr-Abl in Ph+ cell lines expressing B3A226,35 (supplemental Figure 3D-E). Here Bcr-Abl was knocked down in CML CD34+ cells expressing B3A2, cultured in SFM supplemented with physiologic growth factors, and viral transduction of the most primitive CD34+38− population verified (supplemental Figure 4A-B). To measure the extent of Bcr-Abl knockdown, transduced CD34+ CML cells were FACS sorted. Q-PCR confirmed 56% specific down-regulation of Bcr-Abl mRNA (Figure 2A) and Western blot showed clear reduction in Bcr-Abl protein level (48% by densitometry; Figure 2B). We then sought to demonstrate inhibition of Bcr-Abl downstream targets. In primary CML CD34+ cells, 150nM dasatinib was sufficient to reduce p-CrkL27 and p-STAT5 to the same level (described as baseline) as non-CML CD34+ cells (Bcr-Abl-negative control; Figure 2C-D). In Bcr-Abl knockdown, CML cell inhibition of phosphorylation of both CrkL and STAT5 was observed in CD34+ and CD34+38− cells by flow cytometry (gated on GFP+ cells; supplemental Figure 4C), and in CD34+ cells for STAT5 by Western blotting (Figure 2B), although the level of inhibition was not as profound as seen with dasatinib.
Figure 2.
Bcr-Abl knockdown and dasatinib treatment of CML cells in physiologic growth factors. CD34+ CML cells were infected with sh-Bcr-Abl or sh-Control. (A) Forty-eight hours following second infection, cells were sorted for GFP and RNA, protein extracted, and relative expression of Bcr-Abl measured by Q-PCR (n = 5, P = .0004) and (B) Bcr-Abl and p-STAT5 by Western (n = 1). CD34+ non-CML (n = 3) and CML cells (n = 3) were cultured in SFM ± growth factors ± 150nM dasatinib. Twenty-four hours after treatment, (C) p-CrkL and (D) p-STAT5 were measured by flow cytometry. Lentivirally infected CD34+ cells (n = 3) were cultured in physiologic growth factors and treated ± 150nM dasatinib. Twenty-four hours after treatment, (E) p-CrkL and (F) p-STAT5 were measured in infected cells (gated on GFP) cells by flow cytometry.
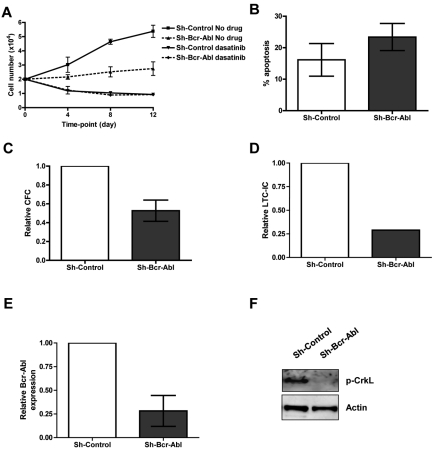
While sh-Control expressing cells were able to expand ∼ 3-fold over 12 days in the presence of physiologic growth factors, sh-Bcr-Abl cells showed little expansion (Figure 3A), with some induction of apoptosis (Figure 3B). To examine the effect of Bcr-Abl knockdown on survival/proliferation of functional CML stem and progenitor cells, GFP+ cells were plated into CFC and LTC-IC assays (Figure 3C-D). Bcr-Abl knockdown resulted in 47% and 71% reduction in CFC and LTC-IC colonies, respectively. Q-PCR on single colonies for Bcr-Abl and Western blotting for p-CrkL on pooled colonies confirmed Bcr-Abl inhibition in cells expressing sh-Bcr-Abl (Figure 3E-F).
Figure 3.
Functional analysis of CML CD34+ cells following Bcr-Abl knockdown ± dasatinib. (A) Proliferation of GFP+ sorted cells treated ± 150nM dasatinib in physiologic growth factors over 12 days (n = 3). (B) Apoptosis measured in infected cells (gated on GFP) by annexin V and viaprobe (n = 2). (C) CFC (n = 4, P = .025) and (D) LTC-IC assays (n = 1). (E) Bcr-Abl levels were measured by Q-PCR on colonies from CFC assay (n = 7 colonies for sh-scrambled and 9 colonies for sh-Bcr-Abl). (F) The level of total p-CrkL was measured by Western blotting on pooled colonies from CFC assay (n = 1).
A proportion of CML CD34+ cells is sensitive to enhanced levels of Bcr-Abl inhibition
To determine whether CML CD34+ cells would be susceptible to further inhibition of Bcr-Abl activity, GFP+ knockdown and control cells were cultured in physiologic growth factors in the presence and absence of 150nM dasatinib for 12 days, with fresh medium and dasatinib replaced on days 4 and 8. When GFP+ CML CD34+ cells were exposed to 150nM dasatinib (Figure 2E-F), further reductions in phosphorylation of CrkL and STAT5—over those seen with sh-Bcr-Abl, and to previously described baseline levels—were seen, confirming complete inhibition of Bcr-Abl kinase activity. For dasatinib, cell counts on days 4, 8, and 12 illustrated a sharp reduction in cell numbers evident by day 4, irrespective of Bcr-Abl knockdown (Figure 3A). Most interestingly, ∼ 50% of input cells survived combined partial Bcr-Abl knockdown and dasatinib treatment for 12 days, indicating that, at least in medium supplemented with physiologic growth factors, a proportion of CD34+ cells can survive without Bcr-Abl kinase activity. However, with the limited number of cells available it was not feasible to confirm complete Bcr-Abl inhibition at all time points, neither was it possible to examine whether, as expected, surviving cells were enriched for primitive stem cells.
Complete Bcr-Abl kinase inhibition is achievable at the stem/progenitor cell level
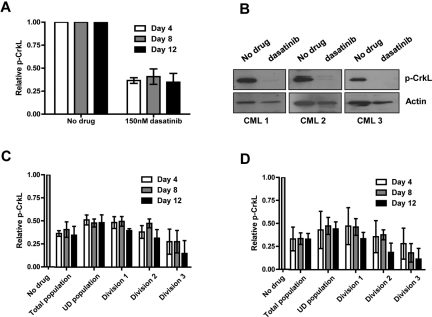
To investigate in more detail whether CML stem/progenitor cells would be susceptible to complete inhibition of Bcr-Abl activity, CML CD34+ cells were cultured in SFM, in the presence or absence of dasatinib, which was replaced with fresh medium on days 4 and 8. After preliminary experiments (supplemental Figure 5A-D, supplemental Table 1), these cells were cultured without growth factors to exclude any additional survival signals. In the remaining viable cells on days 4, 8, and 12 Bcr-Abl activity was fully inhibited as demonstrated by complete inhibition of p-CrkL by flow cytometry and by Western blotting (Figure 4A-B). Because genuine CML stem cells likely represent a small fraction of total CD34+ cells, previous work has focused on those CD34+ cells that remain quiescent in culture by tracking cell division with CFSE.16,17,28 Here the level of inhibition of p-CrkL by continuous exposure to dasatinib 150nM was determined on days 4, 8, and 12 for viable CML CD34+ cells that remained undivided in culture or entered cell divisions 1, 2, or 3. The level of inhibition of p-CrkL achieved across the entire time course showed no significant difference for primitive undivided CD34+ CML cells compared with more mature cells able to enter division (Figure 4C, supplemental Table 2). Inhibition of p-STAT5 by dasatinib gave a very similar profile as seen for p-CrkL (supplemental Figure 6A). Although no significant differences were detected in levels of inhibition between undivided and cells in divisions 1-3, we repeated the experiment including a higher concentration of dasatinib to ensure that inhibition of p-CrkL was complete (Figure 4D). Critically, residual levels of p-CrkL were equivalent for 150 and 1000nM dasatinib.
Figure 4.
Complete Bcr-Abl inhibition is achieved by dasatinib within primitive subpopulations of CML cells. CFSE-stained CD34+ CML cells (n = 3) were cultured in SFM ± 150 or 1000nM dasatinib for 12 days. (A) The levels of total p-CrkL were measured by flow cytometry and (B) by Western blotting at day 12. p-CrkL was also measured within each cell division after treatment with either (C) 150 or (D) 1000nM dasatinib.
The most primitive, quiescent CML stem and progenitor cells are independent of Bcr-Abl kinase for survival
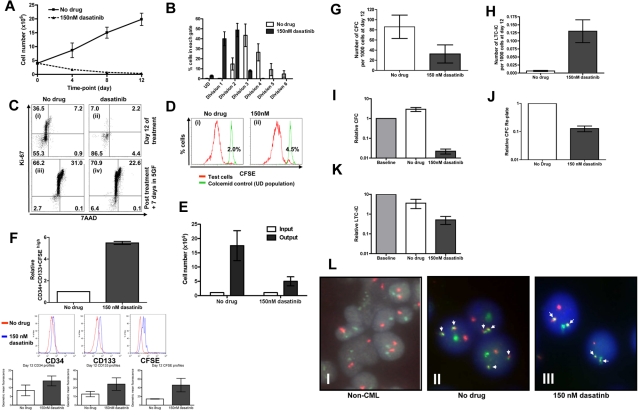
In the mouse, hemopoietic stem cells can be selected to near purity (1 in 2) based on surface markers. However, this is not yet possible for normal or leukemic human stem cells. The human stem and progenitor compartment should therefore be considered as a continuum and not made up of discrete populations. Here we have combined accepted surrogate markers for primitive cells based on phenotype, CFSE retention, absence of Ki-67 staining, expansion kinetics, CFC, CFC replating, and LTC-IC to demonstrate which CML cells are growth factor and Bcr-Abl kinase independent. Over the same 12 days, in the absence of growth factors, untreated CML CD34+ cells expanded by ∼ 4-fold, in keeping with their autocrine production of cytokines,36,37 whereas with 150nM dasatinib viable cells decreased to 10% of input (Figure 5A). CFSE profiling demonstrated that even in the absence of growth factors and with addition of dasatinib, CML CD34+ cells were able to enter divisions 1 and 2, with a few cells progressing as far as division 3 (Figure 5B). The untreated control CML CD34+ cells proliferated more actively, with the majority of cells found in divisions 3 and 4. Although CFSE profiling confirmed that most CML CD34+ cells had gone through one or more divisions in the presence of dasatinib, by day 12, > 85% of residual cells had become quiescent as demonstrated by low Ki-67 staining (Figure 5Ci-ii). To confirm that the viable residual cells were functional and not irreversibly arrested or senescent, on day 12, both experimental arms were washed and reestablished in culture in SFM with added growth factors. Ki-67 and 7AAD analysis confirmed that the majority of CML CD34+ cells that had been cultured in the absence of growth factors and in the presence of dasatinib at 150nM for 12 days rapidly entered cell division with only 6% remaining Ki-67 low after a further 7 days of culture (Figure 5Ciii-iv). The pattern of Ki-67 and 7AAD staining was very similar for the untreated control (Figure 5Ciii-iv). Further confirmation that almost all the dasatinib-treated cells entered cell division again by day 7 was provided through CFSE staining (Figure 5Di-ii). Although the residual quiescent fraction was < 10% in both arms, the dasatinib-treated cells expanded more slowly over 7 days with a total 5-fold expansion compared with untreated control, 17-fold expansion (Figure 5E). Both populations showed complete reactivation of Bcr-Abl kinase activity, as measured by p-CrkL staining (data not shown). Enrichment for primitive cells was confirmed by CFSE retention, phenotyping for CD34 and CD133 (Figure 5F) and by residual CFC, CFC replating potential, and LTC-IC activity in the dasatinib-treated cells (Figure 5G-K). Considering that the untreated cells expanded by 4-fold and the dasatinib-treated cells decreased to 10% of input, the final frequency of LTC-IC was 10-fold higher in the dasatinib-treated versus untreated arms. The residual viable cells present at day 12 were also confirmed to express single copy Bcr-Abl by D-FISH (Figure 5L), to express Bcr-Abl by Q-PCR (supplemental Figure 6B) and showed no evidence of mutation by direct sequencing of the kinase domain, thus confirming that these cells were leukemic and that their dasatinib resistance could not be explained by mutation.
Figure 5.
A primitive subset of CD34+ CML cells survives complete Bcr-Abl inhibition in growth factor–free medium for 12 days. (A) CD34+ CML cells (n = 3) were cultured in SFM ± 150nM and viable cell counts performed on days 4, 8, and 12, and (B) percentages of cells residing within each cell division determined by CFSE staining. After 12 days, cells were washed with PBS, added to CFC and LTC-IC assays, cultured in SFM plus high growth factor cocktail for 7 days. At day 7, (C) Ki-67/7AAD and (D) CFSE were measured by flow cytometry and (E) viable cell counts performed. (Ci) Cells at day 12 without drug treatment; (ii) cells at day 12 following dasatinb treatment; (iii) cells from (i) cultured for additional 7 days in SFM plus high growth cocktail; and (iv) cells from (ii) cultured for additional 7 days in SFM plus high growth factor cocktail. (Di) Cells from panel Ci were cultured for additional 7 days in SFM plus high growth factor cocktail, and (ii) cells from panel Cii were cultured for additional 7 days in SFM plus high growth factor cocktail. (F) The relative increase in CD34+CD133+CFSEhigh was calculated (n = 3, P = .001) and the numbers of (G) CFC (n = 7, P = .048) and (H) LTC-IC (n = 3, P = .025) per 1000 cells at day 12 were calculated. The changes in (I) CFC, (J) CFC replated (n = 2), and (K) LTC-IC numbers compared with baseline cells (1.0) were also calculated. (L) The presence of Bcr-Abl (denoted by the white arrows) was measured by D-FISH in the remaining CML cells following the 12 day time course. A representative profile from (i) non-CML CD34+ cells at baseline, (ii) untreated CD34+ CML cells and (iii) 150nM dasatinib-treated CD34+ cells is demonstrated.
Discussion
The discovery that Bcr-Abl exhibited oncogenic properties38 and was sufficient to induce CML-like disease in mice1 provided clear evidence that Bcr-Abl was the key initiating event in CML. However, for many years investigators were skeptical that a tumor would remain dependent on the initiating oncogene in view of accumulation of additional genetic and epigenetic events occurring over time. The early studies with imatinib, a relatively selective Bcr-Abl inhibitor, resulted in a genuine paradigm shift with many drug discovery efforts now focused on single oncogenic lesions and the belief that a variety of tumors may depend on the driver oncogene. This concept of oncogene addiction was further strengthened when CML patients with blast crisis, a cancer more akin to solid tumors, also showed dramatic responses to imatinib, providing compelling proof that even these genetically complex cancers can remain dependent on the initiating driver mutation. The phenomenon of oncogene addiction, while well recognized, is poorly understood. Despite genetic complexity cancer cells become dependent, by an unknown mechanism, on a single oncoprotein-driven signaling cascade, which, if disrupted acutely, results in cell-cycle arrest and apoptosis. There is ongoing debate regarding whether this is a passive process resulting from withdrawal of prosurvival signaling, or an active process driven by accumulation of proapoptotic signals.39–41 Furthermore, responses of tumors to oncogene inhibition are highly variable and dependent on the cellular and genetic context. These responses in human cancer are paralleled in transgenic murine models in which oncogene inactivation gives the impression of tumor regression, yet on oncogene reactivation the disease can recur.2,8,42,43
For CML, there is now convincing evidence that the malignant stem cell population is intrinsically resistant to kinase inhibitors13,14,16–19,25 and behaves quite differently to the bulk population of more mature cells that are oncogene addicted. This also appears to be the case in the transgenic mouse model in which Bcr-Abl+ mature myeloid cells undergo apoptosis in response to in vitro dasatinib treatment, with the LSK population being relatively resistant. In vivo Bcr-Abl is likely induced in ∼ 50% of donor CD45.1+ long-term repopulating HSCs, 20% of LSK and 17% of lin+c-kit+ cells based on a comparable model using the SCL 3′ enhancer44 and unpublished data (R.B., 2011). This fits well with our data showing that on reversion of the leukemic phenotype the proportion of donor CD45.1+ cells returns to those of control mice rather than being eliminated altogether. We considered that reversion of disease in this model may either occur because transgenic stem cells undergo apoptosis in response to oncogene inactivation39–41 or because they revert back to the behavior of normal stem cells and no longer produce excess numbers of more mature myeloid cells. Our data show that despite complete reversion of disease the leukemic LSK persist, but proliferate less, and do not undergo apoptosis on oncogene withdrawal. These surviving LSK, in which Bcr-Abl expression has been suppressed by > 96%, are capable of reinitiating disease after secondary transplantation and reinduction of Bcr-Abl expression.
These results with the transgenic CML model were closely replicated by our work on primary CML cells. Functional CML stem and progenitor cells were able to survive in vitro for prolonged periods of time despite complete oncogene inactivation and withdrawal of cytokines. Using similar experimental approaches to Corbin et al,25 we made every effort to demonstrate that in the surviving CML stem cells Bcr-Abl activity was completely shut off. We further confirmed that the drug-resistant population belonged to the leukemic clone, was enriched for primitive cells, and showed neither Bcr-Abl amplification nor kinase domain mutation, further mechanisms that might explain their resistance.
Taken together, these results have firmly shifted our focus toward Bcr-Abl kinase–independent resistance mechanisms that might involve stem cell phenotype, stem cell signaling, or other survival pathways that are either not suppressed by or are induced by kinase inhibitors and are the subject of current investigation by ourselves and others. These involve approaches to (1) reverse CML stem cell quiescence by interfering with Foxo transcription factor activity using inhibitors of TGFβ, BCL6 activity using a retro-inverso peptide inhibitor,45,46 or promyeloctic leukemia protein activity using arsenic trioxide; (2) inhibit self-renewal in favor of differentiation through inhibition of WNT or hedgehog signaling; (3) mobilize CML stem cells from the niche using CXCR4 antagonists; (4) inhibit JAK signaling; (5) inhibit kinase inhibitor–induced autophagy; (6) activate PP2A; or (7) exploit differences in epigenetic regulation between normal and CML stem cells. These approaches have been comprehensively reviewed.47
When considering mechanisms of resistance, it is important to consider both the kinase activity of Bcr-Abl and other nonkinase domains of Bcr-Abl that might confer resistance. Using a mouse genetics approach, Chen et al were able to reveal Alox5 as a target whose expression was dependent on Bcr-Abl expression, but not modulated by kinase inhibition.48 To take account of this possibility, we used the combination of Bcr-Abl knockdown with kinase inhibition. However, because we were unable to achieve > 50% inhibition of Bcr-Abl using an shRNA specific for the breakpoint and believe that this is not technically possible in primary CML stem cells at this time, we have not formally been able to exclude ongoing signaling via nonkinase Bcr-Abl motifs or proteins within the Bcr-Abl interactome such as Jak2, within the primary cells.49,50 Although complete abrogation of Bcr-Abl expression was achieved in the transgenic mouse model, we accept that relatively short-term transgenic expression may not allow for further genetic or epigenetic changes that likely occur in the primary setting that might affect the degree of oncogene addiction and would again suggest caution comparing mouse models with human disease. Future efforts toward cure in CML patients who are responding well to kinase inhibitors, but continue to show evidence of minimal residual disease, should focus on understanding the mechanisms of proliferation arrest and dormancy on oncogene inactivation in the CML stem cell population and aim to target Bcr-Abl kinase–independent survival pathways that remain active in these cells or are activated on kinase inhibition. In the latter scenario, it is possible that kinase inhibition will be required alongside a second agent to achieve synthetic lethality. Similarly, in solid tumors driven by a single oncogene and sustained by cancer stem cells an enhanced understanding of tumor dormancy will be a critical step to uncovering novel targets in this drug-resistant population.
Supplementary Material
Acknowledgments
The authors thank all CML patients and UK hematology departments who contributed samples, Dr Alan Hair for sample processing, Dr Sandrine Hayette for performing Q-PCR for Bcr-Abl and Abl-kinase domain mutation analyses, Linda Kamp for excellent technical assistance, John Dick and Austin Smith for helpful discussions, and Richard and David Rockefeller for supporting this work.
This work was supported by the Medical Research Council United Kingdom (M.S., A.H., and G.V.H.), Cancer Research UK (T.L.H.), Leukaemia & Lymphoma Research United Kingdom (A.H.), the Kay Kendall Leukaemia Fund (G.V.H.), the German Research Foundation (DFG; KO2155/2-2, S.K.), National Institutes of Health grant R01 CA95684 (R.B.), and Leukemia & Lymphoma Society Translational Research Grant (R.B.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.H., G.V.H., and M.S. performed research, analyzed data, and wrote the manuscript; B.Z. and F.E.N. performed research and analyzed the data; S.M. performed research; E.K.A. and C.M.-T. performed research and wrote the manuscript; R.B. contributed vital new reagents, designed research, analyzed data, and wrote the manuscript; V.G.B. designed and performed research, and wrote the manuscript; and S.K. and T.L.H. designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: T.L.H. has previously received research support from Bristol-Myers Squibb and Novartis. S.K. is an Advisory Board honorarium and has received research support from Bristol-Myers Squibb and Novartis. The remaining authors declare no competing financial interests.
The current affiliation for S.K. is Department of Medicine, Oncology, Hematology, and Stem Cell Transplantation, University of Aachen, Aachen, Germany.
Correspondence: Prof Tessa Holyoake, Paul O'Gorman Leukaemia Research Centre, Institute of Cancer Sciences, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow, G12 OZD, United Kingdom; e-mail: tessa.holyoake@glasgow.ac.uk.
References
- 1.Daley GQ, Van Etten RA, Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990;247(4944):824–830. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- 2.Koschmieder S, Gottgens B, Zhang P, et al. Inducible chronic phase of myeloid leukemia with expansion of hematopoietic stem cells in a transgenic model of BCR-ABL leukemogenesis. Blood. 2005;105(1):324–334. doi: 10.1182/blood-2003-12-4369. [DOI] [PubMed] [Google Scholar]
- 3.Anderson K, Lutz C, van Delft FW, et al. Genetic variegation of clonal architecture and propagating cells in leukaemia. Nature. 2011;469(7330):356–361. doi: 10.1038/nature09650. [DOI] [PubMed] [Google Scholar]
- 4.Notta F, Mullighan CG, Wang JC, et al. Evolution of human BCR-ABL1 lymphoblastic leukaemia-initiating cells. Nature. 2011;469(7330):362–367. doi: 10.1038/nature09733. [DOI] [PubMed] [Google Scholar]
- 5.Druker BJ, Tamura S, Buchdunger E, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2(5):561–566. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- 6.Lombardo LJ, Lee FY, Chen P, et al. Discovery of N-(2-chloro-6-methyl- phenyl)-2-(6-(4-(2-hydroxyethyl)- piperazin-1-yl)-2-methylpyrimidin-4- ylamino)thiazole-5-carboxamide (BMS-354825), a dual Src/Abl kinase inhibitor with potent antitumor activity in preclinical assays. J Med Chem. 2004;47(27):6658–6661. doi: 10.1021/jm049486a. [DOI] [PubMed] [Google Scholar]
- 7.Weisberg E, Manley PW, Breitenstein W, et al. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell. 2005;7(2):129–141. doi: 10.1016/j.ccr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 8.Schemionek M, Elling C, Steidl U, et al. BCR-ABL enhances differentiation of long-term repopulating hematopoietic stem cells. Blood. 2010;115(16):3185–3195. doi: 10.1182/blood-2009-04-215376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Druker BJ, Guilhot F, O'Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 10.Druker BJ, Sawyers CL, Kantarjian H, et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome. N Engl J Med. 2001;344(14):1038–1042. doi: 10.1056/NEJM200104053441402. [DOI] [PubMed] [Google Scholar]
- 11.Gorre ME, Mohammed M, Ellwood K, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293(5531):876–880. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- 12.Talpaz M, Shah NP, Kantarjian H, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354(24):2531–2541. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 13.Bhatia R, Holtz M, Niu N, et al. Persistence of malignant hematopoietic progenitors in chronic myelogenous leukemia patients in complete cytogenetic remission following imatinib mesylate treatment. Blood. 2003;101(12):4701–4707. doi: 10.1182/blood-2002-09-2780. [DOI] [PubMed] [Google Scholar]
- 14.Graham SM, Jorgensen HG, Allan E, et al. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood. 2002;99(1):319–325. doi: 10.1182/blood.v99.1.319. [DOI] [PubMed] [Google Scholar]
- 15.Konig H, Holtz M, Modi H, et al. Enhanced BCR-ABL kinase inhibition does not result in increased inhibition of downstream signaling pathways or increased growth suppression in CML progenitors. Leukemia. 2008;22(4):748–755. doi: 10.1038/sj.leu.2405086. [DOI] [PubMed] [Google Scholar]
- 16.Jorgensen HG, Allan EK, Jordanides NE, Mountford JC, Holyoake TL. Nilotinib exerts equipotent antiproliferative effects to imatinib and does not induce apoptosis in CD34+ CML cells. Blood. 2007;109(9):4016–4019. doi: 10.1182/blood-2006-11-057521. [DOI] [PubMed] [Google Scholar]
- 17.Copland M, Hamilton A, Elrick LJ, et al. Dasatinib (BMS-354825) targets an earlier progenitor population than imatinib in primary CML but does not eliminate the quiescent fraction. Blood. 2006;107(11):4532–4539. doi: 10.1182/blood-2005-07-2947. [DOI] [PubMed] [Google Scholar]
- 18.Konig H, Copland M, Chu S, Jove R, Holyoake TL, Bhatia R. Effects of dasatinib on SRC kinase activity and downstream intracellular signaling in primitive chronic myelogenous leukemia hematopoietic cells. Cancer Res. 2008;68(23):9624–9633. doi: 10.1158/0008-5472.CAN-08-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Konig H, Holyoake TL, Bhatia R. Effective and selective inhibition of chronic myeloid leukemia primitive hematopoietic progenitors by the dual Src/Abl kinase inhibitor SKI-606. Blood. 2008;111(4):2329–2338. doi: 10.1182/blood-2007-05-092056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chu S, McDonald T, Lin A, et al. Persistence of leukemia stem cells in chronic myelogenous leukemia patients in prolonged remission with imatinib treatment. Blood. 2011;118(20):5565–5572. doi: 10.1182/blood-2010-12-327437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sobrinho-Simoes M, Wilczek V, Score J, Cross NC, Apperley JF, Melo JV. In search of the original leukemic clone in chronic myeloid leukemia patients in complete molecular remission after stem cell transplantation or imatinib. Blood. 2010;116(8):1329–1335. doi: 10.1182/blood-2009-11-255109. [DOI] [PubMed] [Google Scholar]
- 22.Chomel JC, Bonnet ML, Sorel N, et al. Leukemic stem cell persistency in chronic myeloid leukemia patients with sustained undetectable molecular residual disease. Blood. 2011;118(13):3657–3660. doi: 10.1182/blood-2011-02-335497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White DL, Saunders VA, Dang P, et al. OCT-1-mediated influx is a key determinant of the intracellular uptake of imatinib but not nilotinib (AMN107): reduced OCT-1 activity is the cause of low in vitro sensitivity to imatinib. Blood. 2006;108(2):697–704. doi: 10.1182/blood-2005-11-4687. [DOI] [PubMed] [Google Scholar]
- 24.Jordanides NE, Jorgensen HG, Holyoake TL, Mountford JC. Functional ABCG2 is overexpressed on primary CML CD34+ cells and is inhibited by imatinib mesylate. Blood. 2006;108(4):1370–1373. doi: 10.1182/blood-2006-02-003145. [DOI] [PubMed] [Google Scholar]
- 25.Corbin AS, Agarwal A, Loriaux M, Cortes J, Deininger MW, Druker BJ. Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. J Clin Invest. 2011;121(1):396–409. doi: 10.1172/JCI35721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Myssina S, Helgason GV, Serrels A, et al. Combined BCR-ABL inhibition with lentiviral-delivered shRNA and dasatinib augments induction of apoptosis in Philadelphia-positive cells. Exp Hematol. 2009;37(2):206–214. doi: 10.1016/j.exphem.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 27.Hamilton A, Elrick L, Myssina S, et al. BCR-ABL activity and its response to drugs can be determined in CD34+ CML stem cells by CrkL phosphorylation status using flow cytometry. Leukemia. 2006;20(6):1035–1039. doi: 10.1038/sj.leu.2404189. [DOI] [PubMed] [Google Scholar]
- 28.Holyoake T, Jiang X, Eaves C, Eaves A. Isolation of a highly quiescent subpopulation of primitive leukemic cells in chronic myeloid leukemia. Blood. 1999;94(6):2056–2064. [PubMed] [Google Scholar]
- 29.Jordan CT, Yamasaki G, Minamoto D. High-resolution cell cycle analysis of defined phenotypic subsets within primitive human hematopoietic cell populations. Exp Hematol. 1996;24(11):1347–1355. [PubMed] [Google Scholar]
- 30.Gabert J, Beillard E, van der Velden VH, et al. Standardization and quality control studies of ‘real-time’ quantitative reverse transcriptase polymerase chain reaction of fusion gene transcripts for residual disease detection in leukemia–a Europe Against Cancer program. Leukemia. 2003;17(12):2318–2357. doi: 10.1038/sj.leu.2403135. [DOI] [PubMed] [Google Scholar]
- 31.Hughes T, Deininger M, Hochhaus A, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108(1):28–37. doi: 10.1182/blood-2006-01-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Branford S, Rudzki Z, Walsh S, et al. High frequency of point mutations clustered within the adenosine triphosphate-binding region of BCR/ABL in patients with chronic myeloid leukemia or Ph-positive acute lymphoblastic leukemia who develop imatinib (STI571) resistance. Blood. 2002;99(9):3472–3475. doi: 10.1182/blood.v99.9.3472. [DOI] [PubMed] [Google Scholar]
- 33.Hayette S, Michallet M, Baille ML, Magaud JP, Nicolini FE. Assessment and follow-up of the proportion of T315I mutant BCR-ABL transcripts can guide appropriate therapeutic decision making in CML patients. Leuk Res. 2005;29(9):1073–1077. doi: 10.1016/j.leukres.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 34.Shah NP, Kasap C, Weier C, et al. Transient potent BCR-ABL inhibition is sufficient to commit chronic myeloid leukemia cells irreversibly to apoptosis. Cancer Cell. 2008;14(6):485–493. doi: 10.1016/j.ccr.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 35.Li MJ, McMahon R, Snyder DS, Yee JK, Rossi JJ. Specific killing of Ph+ chronic myeloid leukemia cells by a lentiviral vector-delivered anti-bcr/abl small hairpin RNA. Oligonucleotides. 2003;13(5):401–409. doi: 10.1089/154545703322617087. [DOI] [PubMed] [Google Scholar]
- 36.Wang Y, Cai D, Brendel C, et al. Adaptive secretion of granulocyte-macrophage colony-stimulating factor (GM-CSF) mediates imatinib and nilotinib resistance in BCR/ABL+ progenitors via JAK-2/STAT-5 pathway activation. Blood. 2007;109(5):2147–2155. doi: 10.1182/blood-2006-08-040022. [DOI] [PubMed] [Google Scholar]
- 37.Jiang X, Lopez A, Holyoake T, Eaves A, Eaves C. Autocrine production and action of IL-3 and granulocyte colony-stimulating factor in chronic myeloid leukemia. Proc Natl Acad Sci U S A. 1999;96(22):12804–12809. doi: 10.1073/pnas.96.22.12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lugo TG, Pendergast AM, Muller AJ, Witte ON. Tyrosine kinase activity and transformation potency of bcr-abl oncogene products. Science. 1990;247(4946):1079–1082. doi: 10.1126/science.2408149. [DOI] [PubMed] [Google Scholar]
- 39.Sharma SV, Gajowniczek P, Way IP, et al. A common signaling cascade may underlie “addiction” to the Src, BCR-ABL, and EGF receptor oncogenes. Cancer Cell. 2006;10(5):425–435. doi: 10.1016/j.ccr.2006.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weinstein IB. Cancer. Addiction to oncogenes–the Achilles heal of cancer. Science. 2002;297(5578):63–64. doi: 10.1126/science.1073096. [DOI] [PubMed] [Google Scholar]
- 41.Sharma SV, Settleman J. Exploiting the balance between life and death: targeted cancer therapy and “oncogenic shock.”. Biochem Pharmacol. 2010;80(5):666–673. doi: 10.1016/j.bcp.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 42.Felsher DW, Bishop JM. Reversible tumorigenesis by MYC in hematopoietic lineages. Mol Cell. 1999;4(2):199–207. doi: 10.1016/s1097-2765(00)80367-6. [DOI] [PubMed] [Google Scholar]
- 43.Shachaf CM, Kopelman AM, Arvanitis C, et al. MYC inactivation uncovers pluripotent differentiation and tumour dormancy in hepatocellular cancer. Nature. 2004;431(7012):1112–1117. doi: 10.1038/nature03043. [DOI] [PubMed] [Google Scholar]
- 44.Gothert JR, Gustin SE, Hall MA, et al. In vivo fate-tracing studies using the Scl stem cell enhancer: embryonic hematopoietic stem cells significantly contribute to adult hematopoiesis. Blood. 2005;105(7):2724–2732. doi: 10.1182/blood-2004-08-3037. [DOI] [PubMed] [Google Scholar]
- 45.Hurtz C, Hatzi K, Cerchietti L, et al. BCL6-mediated repression of p53 is critical for leukemia stem cell survival in chronic myeloid leukemia. J Exp Med. 2011;208(11):2163–2174. doi: 10.1084/jem.20110304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pellicano F, Holyoake T. Assembling defenses against therapy-resistant leukemic stem cells: Bcl6 joins the ranks. J Exp Med. 2011;208(11):2155–2158. doi: 10.1084/jem.20112087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scott MT, McCaig AM, Holyoake TL. Hematology education–the education program for the annual congress of the European Hematology Association.. 16th Congress of the European Hematology Association; June 9-12, 2011; London, United Kingdom. 2011. pp. 112–119. [Google Scholar]
- 48.Chen Y, Hu Y, Zhang H, Peng C, Li S. Loss of the Alox5 gene impairs leukemia stem cells and prevents chronic myeloid leukemia. Nat Genet. 2009;41(7):783–792. doi: 10.1038/ng.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samanta AK, Chakraborty SN, Wang Y, et al. Jak2 inhibition deactivates Lyn kinase through the SET-PP2A-SHP1 pathway, causing apoptosis in drug-resistant cells from chronic myelogenous leukemia patients. Oncogene. 2009;28(14):1669–1681. doi: 10.1038/onc.2009.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Samanta AK, Lin H, Sun T, Kantarjian H, Arlinghaus RB. Janus kinase 2: a critical target in chronic myelogenous leukemia. Cancer Res. 2006;66(13):6468–6472. doi: 10.1158/0008-5472.CAN-06-0025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.