Abstract
Imaging studies using ROI morphometry and PET have contributed to our understanding of structural and functional abnormalities in BPD; however, both methods have practical limitations to their usefulness for exploratory studies of brain-behavior relationships. We used voxel based morphometry (VBM) in 34 subjects with BPD and 30 healthy controls (HC) to study effects of diagnosis, gender, childhood sexual abuse, depressed mood, impulsivity and aggression on group differences. VBM is a computer-based method for whole brain analysis that combines the advantages of a functional study with a structural method. The BPD subjects, diagnosed with the Diagnostic Interview for Borderline Patients and the International Personality Disorders Examination, were compared with 30 HC, with age and gender covaried. Analyses were repeated separately by gender and, in women, by histories of childhood sexual abuse. Depressed mood, impulsivity, and aggression were covaried in separate analyses. Compared with HC, BPD subjects had significant bilateral reductions in gray matter concentrations in ventral cingulate gyrus and several regions of the medial temporal lobe, including the hippocampus, amygdala, parahippocampal gyrus, and uncus. BPD women (and abused BPD women), but not BPD men, had significant reductions in medial temporal lobe, including the amygdala. BPD men, but not BPD women, showed diminished gray matter concentrations in the anterior cingulate gyrus compared with findings HC. Covarying for depressed mood rendered group differences non-significant in the ventral cingulate but had little effect on differences in medial temporal cortex. Covarying for aggression (LHA) had relatively little effect on group differences, while covarying for impulsivity (BIS) rendered all previously noted voxel-level group differences non-significant. Diminished gray matter in the prefrontal cortex and the medial temporal cortex may mediate the dysregulation of impulse and affect in BPD. Group differences varied greatly by gender, levels of depression, and impulsivity. VBM is an efficient method for exploratory study of brain-behavior relationships.
Keywords: Magnetic resonance imaging, Personality Disorders, Suicide, Aggression
1. Introduction
Impulsivity and impulsive-aggression are core characteristics of patients with borderline personality disorder (BPD). They are heritable traits of temperament associated with suicidal and aggressive behaviors in patients with BPD, other impulsive personality disorders (PDs), and across diagnostic categories (Mann et al., 1999; Oquendo and Mann, 2000; Soloff et al., 2000a). Functional and structural brain imaging studies have contributed greatly to our understanding of the neurobiology of impulsivity and impulsive-aggression in BPD. For example, PET studies have measured glucose utilization and metabolic responses to serotonergic challenge in patients with BPD (and other impulsive PDs), ascertained for high degrees of impulsive-aggression (Siever et al., 1999; Soloff et al., 2000b, New et al., 2002; Soloff et al., 2003). Aggression in impulsive PDs (including BPD) is inversely related to glucose utilization in discrete areas of frontal cortex (e.g. anterior medial frontal, orbital frontal) and in Rt. temporal cortex (Goyer et al., 1994). Impulsive subjects with BPD have diminished glucose uptake relative to controls in medial orbital cortex bilaterally (BA 9, 10, 11) (Soloff et al., 2003). Following challenge with fenfluramine, subjects with BPD (and other impulsive PDs) demonstrate blunted cortical metabolic responses relative to healthy controls in orbital frontal, adjacent ventral medial, and cingulate cortex (Siever et al., 1999; Soloff et al., 2000). Similarly, decreased metabolic response is found in anteromedial orbital cortex and anterior cingulate in impulsive PD subjects following challenge with meta-chlorophenylpiperazine (m-CPP) (New et al., 2002). An inverse relationship was noted between aggression and metabolic response to m-CPP in the Rt. inferior frontal cortex (BA 45).
Structural imaging studies using ROI morphometry have compared BPD to healthy control subjects and reported volume loss in hippocampus (Driessen et al., 2000; Tebartz van Elst et al.,2003; Schmahl et al., 2003; Brambilla et al., 2004; Irle et al., 2005; Zetzsche et al., 2007) and amygdala (Driessen et al., 2000; Tebartz van Elst et al., 2003; Schmahl et al., 2003). Diminished volumes in hippocampus have been related to childhood histories of trauma or abuse in some studies (Driessen et al., 2000; Schmahl et al., 2003; Brambilla et al., 2004; Irle et al., 2005), though not all (Zetzsche et al., 2007). Zetzsche et al. (2007) found decreased hippocampal volume in BPD patients more pronounced among patients with histories of multiple hospitalizations. An inverse relationship was found between Lt. hippocampal volumes and measures of aggression and hostility (though not impulsivity). Compared to healthy controls, volume loss in BPD subjects has also been reported in frontal lobes (Lyoo et al., 1998), Lt. orbitofrontal and Rt. anterior cingulate cortex (Tebartz van Elst et al., 2003, Hazlett et al., 2005), and in Rt. parietal cortex, where reduced leftward asymmetry has been related to increased psychotic symptoms and schizotypal personality traits (Irle et al.,2005).
fMRI studies demonstrate increased activation of amygdala bilaterally in BPD subjects compared to controls in response to aversive slides (Herpertz et al., 2001). BPD subjects also have higher Lt. amygdala activation in response to expressions of emotion depicted in the Ekman faces (Donegan et al., 2003). BPD subjects tend to project negative attributes onto the Ekman faces, especially the ambiguous “neutral” expression, suggesting a role for abnormal amygdala function in the borderline patient’s emotion dysregulation.
Both PET and ROI morphometry have practical limitations to their usefulness for exploratory studies of brain-behavior relationships. Exploratory studies are not strictly limited to hypothesis-driven designs, or a few pre-selected ROIs, but may assess the effects of multiple risk factors on structural (or functional) differences between BPD and control subjects across the entire brain. Important factors to be considered in BPD studies include gender, Axis I co-morbidity, lifetime number of suicide attempts, depressed mood, impulsivity, aggression, and a history of childhood sexual abuse (Soloff 2005, for review). For statistical power, large samples are preferred. PET studies have the great advantage of assessing the whole brain using Statistical Parametric Mapping (SPM); however, anatomical localization of findings is often imprecise. Cost may be prohibitive for large samples, and subjects may object to studies involving ionizing radiation and arterial cannulation. Hand-drawn ROI morphometry is the “gold standard” for anatomical localization, allowing for detection of small volume differences without deformation of original anatomical structures. However, ROI morphometry studies are very labor intensive, and subject to error from loss of inter-rater reliability or drift from template standards. As a result, ROI morphometry studies are generally hypothesis-driven, small sample confirmatory studies which typically focus on a few pre-defined ROIs, and do not address multiple risk factors in the same patients.
This paper describes the use of Voxel-Based Morphometry (VBM) as an exploratory technique to study brain-behavior relationships. VBM is a computer based morphometric technique for voxel-wise comparisons between groups of subjects (Ashburner and Friston, 2000). It assesses total brain using Statistical Parametric Mapping (SPM), does not require a priori definitions of anatomical areas, and is independent of hypotheses. It is fully automated and highly efficient for large samples. VBM requires normalizing images to a standard template, which results in some deformation of anatomical structures and possible loss of small volume differences. While less sensitive than hand-drawn ROI morphometry, VBM is free of rater bias, inter-rater variability and drift from template standards. VBM combines some of the advantages of a whole brain functional study in a structural method, enhancing its value for exploratory studies. Convergent findings have been reported when VBM and hand-drawn ROI morphometry are used concurrently in the same subjects (Diwadkar et al., 2003).
Based upon published ROI morphometry studies, we hypothesized that VBM analyses would demonstrate diminished gray matter concentrations in BPD subjects compared to healthy controls in areas of prefrontal cortex, hippocampus and amygdala. Differences between BPD and control subjects would be related, in part, to measures of impulsivity and aggression.
2. Methods
This study was approved by the Institutional Review Board of the University of Pittsburgh. All subjects gave informed consent and were enrolled in a longitudinal study of risk factors for suicidal behavior in BPD. Subjects were recruited from treatment clinics, and, by advertisement, from the surrounding community. Diagnoses were determined by trained raters using structured interviews. Axis I disorders were diagnosed using the Structured Clinical Interview for DSM III-R (to preserve continuity with ongoing longitudinal studies) (Spitzer et al., 1988), Axis II (including BPD) with the International Personality Disorders Examination (IPDE), which has a lifetime framework (Loranger et al., 1997). The Diagnostic Interview for Borderline Patients (DIB; Gunderson et al., 1981) was administered as an independent measure of diagnosis and recent symptom severity, with a timeframe of 3 months for the Affect and Psychosis subscales, and 2 years for the Social Adaptation, Impulse Action Pattern, and Interpersonal Relations subscales. (The DIB is used, rather than the DIB-R, for continuity with the longitudinal database). For inclusion, participants had to meet diagnostic criteria for BPD on the IPDE (probable or definite), and have a score of 7 or more (definite) on the DIB. Exclusion criteria included any past or current Axis I diagnosis of schizophrenia, delusional (paranoid) disorder, schizoaffective disorder, bipolar disorder, or psychotic depression. Subjects were also excluded for physical disorders of known psychiatric consequence (e.g., hypothyroidism, seizure disorder, or CNS pathology of any etiology), and borderline mental retardation. Medical records were reviewed where available to confirm inclusion and exclusion criteria. Final diagnoses were determined by consensus of raters using all available data. Control subjects were free of all Axis I and II disorders. Attempter status and lifetime number of suicide attempts were obtained by interview using the Columbia Suicide History Form (Oquendo et al., 2003). A history of childhood abuse was obtained using a semi-structured 19 item Abuse History, derived from the Dissociative Disorders Interview Schedule (Ross et al., 1989). The Abuse History surveys occurrence, severity, and duration of physical and sexual abuse separately (Soloff et al., 2002), and has an interclass correlation coefficient of 0.88 for the presence of sexual abuse, 0.71 for physical abuse, and 0.89 for any abuse compared to historical data obtained 2 wks.-1 month later by a separate interviewer. Clinical measures obtained prior to the MRI scan included: the Hamilton Rating Scale for Depression (HamD-24) (Guy, 1976), Barratt Impulsiveness Scale (BIS) (Barratt and Slaughter, 1998), and Brown-Goodwin Lifetime History of Aggression (LHA) (Brown et al., 1982).
All subjects were physically healthy, free of psychoactive medication (for two to six weeks, depending on half life) and oral contraceptives, and free of drugs of abuse and alcohol for at least one week. Subjects were required to have a negative urine toxicology screen for drugs of abuse prior to the scan. Female subjects had a negative screen for pregnancy.
2.1. Imaging method
MRI scans were acquired with a 1.5T GE Signa Imaging System running version Signa 5.4.3 software (General Electric Medical Systems, Milwaukee, WI). A T1-weighted sagittal scout image was obtained for graphic prescription of the coronal and axial images. 3D gradient echo imaging (Spoiled Gradient Recalled Acquisition, SPGR) was performed in the coronal plane (TR=25 ms, TE=5 ms, nutation angle=40°, FOV=24 cm, slice thickness=1.5 mm, NEX=1, matrix size=256×192) to obtain 124 images covering the entire brain. Additionally, a double echo-spin echo sequence was used to obtain T2 and proton density images in the axial plane to screen for neuroradiological abnormalities.
Voxel-based image analysis was achieved using optimized voxel-based morphometry processing (Good et al., 2001). All preprocessing was conducted in SPM2 and statistical modeling was conducted in SPM5b (http://www.fil.ion.ucl.ac.uk/spm/). The standard optimized preprocessing stream was employed, including the creation of a study-specific template, and optimized re-segmentation and normalization of gray matter segments. Unmodulated gray matter segments were smoothed using a standard Gaussian smoothing kernel (12 FWHM) (Ashburner and Friston, 2000).
The following contrasts were conducted: a.) Combined gender sample: BPD vs. Healthy Controls (HC) (e.g., HC>BPD, BPD>HC), with age and gender covaried, b.) Female subjects only: BPD vs. HC, with age covaried, c.) Male subjects only: BPD vs. HC, with age covaried, d.) Female BPD with childhood sexual abuse vs. non-abused female BPD, e). In exploratory analyses, depressed mood (HamD), impulsivity (BIS) and aggression (LHA) were each covaried in separate analyses of the combined gender sample. Age and gender were covaried in each analysis. Resulting SPM (t) maps were thresholded using a hierarchical inferential scheme. Following the identification of supra-threshold clusters (using an uncorrected threshold of p.<.001), within-cluster analysis was conducted using small volume correction. We used the Wake Forest University (WFU) PickAtlas and its compendium of regions defined in stereotactic space to conduct post-hoc multiple comparison corrections in amygdala and hippocampus (Maldjian et al., 2003), which were regions of interest based on prior anatomical hypotheses. Post-hoc analyses within each region were thresholded at pFWE<.05 to investigate medial temporal lobe reductions in gray matter density. Supra-threshold clusters which implicate lower gray matter density in the patients in those regions were subsequently rendered onto a template brain to visualize regions of significant difference. The MNI (Montreal Neurological Institute) coordinates generated by SPM were referenced to the AAL (Anatomical Automatic Labeling) atlas for structure specification. We also converted MNI to Talairach coordinates for identification of Brodmann areas (BA), using the Talairach atlas (Talairach and Tournoux, 1988). Inconsistencies between atlases were noted in several boundary regions: insula vs. putamen, parahippocampal vs. lingual gyrus, parahippocampus vs. uncus, cerebellum vs. fusiform gyrus. Differences between atlases in labeling adjoining brain regions were resolved by direct inspection of images. Results are reported in Tables 2–4 if significant at the voxel-level (P FWE-corr ≤0.05) or cluster-level (P corr.≤0.05).
Table 2.
Combined-gender sample: Anatomical locations of significant voxel and cluster peaks
| CONTRAST | Cluster size K, (P. corr). | T, P (FWE-corr) | Z | Peak Voxel MNI :x,y,z (mm) | ** Anatomical locations of peak voxels |
|---|---|---|---|---|---|
| I. 34 BPD vs. 30 HC* | |||||
| A. HC>BPD | |||||
| 1565 (< 0.001) | 5.06 (<0.001) | 4.59 | −23,−2,−19 | Lt. amygdala(!) | |
| 19527 (<0.001) | 5.80 (0.009) | 5.14 | −21,−15,−25 | Lt. parahippocampal g. (BA28) | |
| 1033 (0.002) | 5.73 (<0.001) | 5.09 | −21,−14,−24 | Lt. hippocampus(!) | |
| 643 (0.003) | 4.79 (0.001) | 4.38 | 23,−1,−22 | Rt. amygdala(!) | |
| 3967 (<0.001) | 5.25 (0.05) | 4.73 | 14,5,−26 | Rt. parahippocampal g. (BA 34) | |
| 157 (0.07) | 4.85( 0.003) | 4.43 | 22,−3,−23 | Rt. hippocampus(!) | |
| 12859 (<0.001) | 6.45 (0.001) | 5.59 | 62,−49,9 | Rt. middle temporal g. (BA 21) | |
| 5.93 (0.006) | 5.23 | 42,−8,−6 | Rt. insula (BA 13) | ||
| 5.76 (0.01) | 5.11 | 41,14,4 | Rt. insula (BA 13) | ||
| 4631 (<0.001) | 6.01 (0.004) | 5.29 | −62,−49,12 | Lt. superior temporal g. (BA 22) | |
| 5.59(0.018) | 4.99 | −45,−11,−8 | Lt.superior temporal g. (BA 22) | ||
| 5.46 (0.027) | 4.89 | −64,−26,−4 | Lt. middle temporal g. (BA 21) | ||
| 1071 (0.10) | 6.32 (0.002) | 5.50 | 17,−51,−3 | Rt. lingual g. (BA19) | |
| Cluster-Level: | 6858 (<0.001) | n.s. | 4.40 | 8, 35, 17 | Rt. anterior cingulate g. (BA32) |
| n.s | 4.35 | −8,40,0 | Lt. anterior cingulate g. (BA32) | ||
| 5762 (<0.001) | n.s. | 4.13 | −40,−75,−18 | Lt. fusiform g. (BA 19) | |
| 1927 (0.01) | n.s. | 4.01 | 27,−80,−19 | Rt. fusiform g. (BA19) | |
| B. BPD>HC | |||||
| 84496 (<0.001) | 6.67(<0.001) | 5.75 | 7,−9,62 | Rt. medial frontal g. (BA 6) | |
| 6.46 (<0.001) | 5.59 | 6,−62,35 | Rt. parietal, precuneus, (BA 7) | ||
| 6.20 (0.002) | 5.42 | −17,47,42 | Lt. superior frontal g. (BA 8) | ||
| Cluster-Level: | 3802 (<0.001) | n.s. | 4.07 | −46,−39,48 | Lt. inferior parietal g. (BA 40) |
| 2791, (0.002) | n.s. | 4.30 | −40,7,6 | Lt. insula (BA 44) | |
| 2078, (0.008) | n.s. | 4.47 | 27,7,10 | Rt. putamen |
ANCOVA with age, gender. P (2 tailed), n.s.= not significant at voxel level
AAL atlas in MNI space (approximate Brodmann areas from MNI to TAL transformation)
post hoc analysis: small volume correction using region-of-interest masks.
Table 4.
Combined-gender sample, covarying for depressed mood (HamD), impulsivity (BIS), aggression (LHA).
| CONTRAST | Cluster size K, ( p. corr). | T, p (FWE-corr) | Z | Peak Voxel MNI :x,y,z (mm) | ** Anatomical locations of peak voxels |
|---|---|---|---|---|---|
| I. 34 BPD vs. 30 HC | |||||
| A. HAMD: HC>BPD | |||||
| 4.14 (0.008) | 4.14 | 19,1,−23 | Rt. parahippocampal g. (BA 34) | ||
| 4.06(0.01) | 3.76 | −23,−15,−22 | Lt. parahippocampal g, (BA28) | ||
| BPD>HC | |||||
| 6.20(0.004) | 5.31 | −50,−21,46 | Lt. parietal l, post-central g, (BA3) | ||
| Cluster-level: | 2142 (0.006) | n.s | 4.02 | 46,−16,53 | Rt. parietal l, post-central g (BA4) |
| 7089 (<0.001) | n.s | 4.61 | 10,−16,67 | Rt. medial frontal g.(BA6) | |
| 3216 (<0.001) | n.s | 4.71 | −12,62,24 | Lt. sup. frontal g. (BA10) | |
| B. LHA: HC>BPD | |||||
| 5.88 (0.01) | 5.12 | 16,3,−24 | Rt. uncus (BA 34) | ||
| 5.64, (0.02) | 4.95 | 43,−8,−6 | Rt. insula (BA13) | ||
| 1486 (0.03) | 5.32 (0.06) | 4.72 | 17,−51,−3 | Rt. parahippocampal g (BA19) | |
| Cluster-level: | 25,152 (0.001) | n.s | 4.70 | 25,−75,−22 | Rt. cerebellum |
| 1347 (0.04) | n.s | 4.41 | 57,4,5 | Rt. sup. temp. g.(BA22) | |
| 1474 (0.03) | n.s | 4.27 | −30,−95,13 | Lt. middle occip. g. (BA19) | |
| BPD>HC | |||||
| 10,750 (<0.001) | 5.78 (0.015) | 5.05 | −11,39,53 | Lt. sup. frontal g (BA8) | |
| 5.71(0.018) | 5.00 | −7,62,25 | Lt. sup. frontal g (BA10) | ||
| 5.51(0.03) | 4.86 | −42, 6,42 | Lt. middle frontal g (BA9) | ||
| Cluster-level: | 2656 (0.002) | n.s | 4.21 | 3,−19,38 | Rt. cingulate g (BA 24) |
| 1594 (0.02) | n.s | 3.89 | −6,24,40 | Lt. cingulate g (BA32) | |
| 2335 (0.003) | n.s | 4.36 | 27,9,9 | Rt. putamen | |
| 3319 (<0.001) | n.s | 4.19 | −27,8,6 | Lt. putamen | |
| C. BIS: HC>BPD | |||||
| Cluster-level: | 2746 (0.001) | 5.35 (0.06) | 4.71 | −48,−48,49 | Lt. inf.parietal (BA40) |
| 1590, (0.02) | n.s | 4.45 | 63,−48,13 | Rt. sup. temp. g (BA 22) | |
| 2922 (0.001) | n.s | 4.54 | −42,−67,−19 | Lt. fusiform g (BA19) | |
| 1769 (0.01) | n.s | 4.34 | 23,−81,−20 | Rt. fusiform g (BA19) | |
| BPD>HC | |||||
| Cluster-level: | 1419 (0.04) | 5.37 (0.06) | 4.72 | 8,−15,63 | Rt. medial frontal g (BA6) |
| 2222 (0.004) | n.s | 4.11 | 6,−60,32 | Rt. parietal precuneus (BA 7) |
ANCOVA with age, gender. P (2-tailed), n.s.= not significant at voxel level
AAL atlas in MNI space (approximate Brodmann areas from MNI to TAL transformation)
There were 34 BPD subjects (22 females, 12 males) and 30 healthy controls (HC: 19 females, 11 males). Mean (S.D.) age of BPD subjects was 27.5 (8.0) years, not significantly different from mean age of the HC group (25.6 (7.7) years, t=0.95, df=62, P=0.35). Current co-morbid Axis I diagnoses among BPD subjects included seven subjects with MDD (20.6%), 11 with dysthymic D/O (32.3%), two with PTSD (5.9%), and three (8.8%) with an alcohol use disorder (AUD, abuse or dependence). A pre-scan HamD was obtained on 32 BPD subjects, LHA on 33, and BIS on 29 subjects. Compared with healthy controls, BPD subjects had significantly higher scores on the HamD, BIS, and LHA (Table 1). There were no significant correlations between age and HamD, BIS, or LHA in the total sample, or separately by gender, for all males, all females, male BPD, or female BPD subjects.
Table 1.
Characteristics of Sample
| Subjects (N) | Control-all (30) | Control:F (19) | Control:M (11) | BPD-all (34) | BPD-F (22) | BPD-M (12) | T (p)/X2 (p) ** |
|---|---|---|---|---|---|---|---|
| Age: mean(sd) | 25.6 (7.7) | 26.8 (8.1) | 23.6 (6.9) | 27.5 (8.0) | 26.1 (8.0) | 30 (7.8) | p. ns |
| ICV-total, mean (s.d) | 1629.2 (146.2) | 1564.3 (96.7) (!) | 1741.2 (152.9) | 1595.5 (120.9) | 1540.2 (100.2) | 1696.9 (86.0) (!!) | 1.01, (ns) |
| Ham-D | 0.62 (1.1) | 0.60(0.83) | 0.64 (1.5) | 14.8(8.7) | 15.1 (9.7) | 14.2 (6.3) | 9.18, p<.001 |
| BIS | 57.5 (7.2) | 57.2 (7.6) | 57.9 (7.0) | 79.9 (11.4) | 81.1(11.3) | 77.6 (12.0) | 8.74, p.<.001 |
| LHA | 14.8 (3.4) | 12.9 (1.9) | 18.3 (2.6)(!!) | 21.2 (7.5) | 19.1 (7.5) | 24.9 (6.2)(!!) | 4.30, p.<.001 |
| MDD* | 7 | 5 | 2 | ||||
| Dysthymic | 11 | 6 | 5 | ||||
| Depress NOS | 1 | 1 | 0 | ||||
| PTSD | 2 | 1 | 1 | ||||
| Alc.Use D/O | 3 | 2 | 1 | ||||
| Any Anxiety Dx | 11 | 4 | 7 |
Current Axis I diagnoses by SCID I;
All BPD vs. All Control subjects
ICV-Total, Control: M>F t 3.90, 28 df, p.001; BPD: M>F t 4.57, 32 df, p.<.001;
LHA, Control: M vs. F. t 6.06, 23 df, p.<.001; BPD: M vs. F: t2.27 31 df, p.03
3. Results
3.1. Combined gender sample: BPD vs. HC (Fig. 1, Fig. 2a, Figs. 3a,b)
Figure 1.
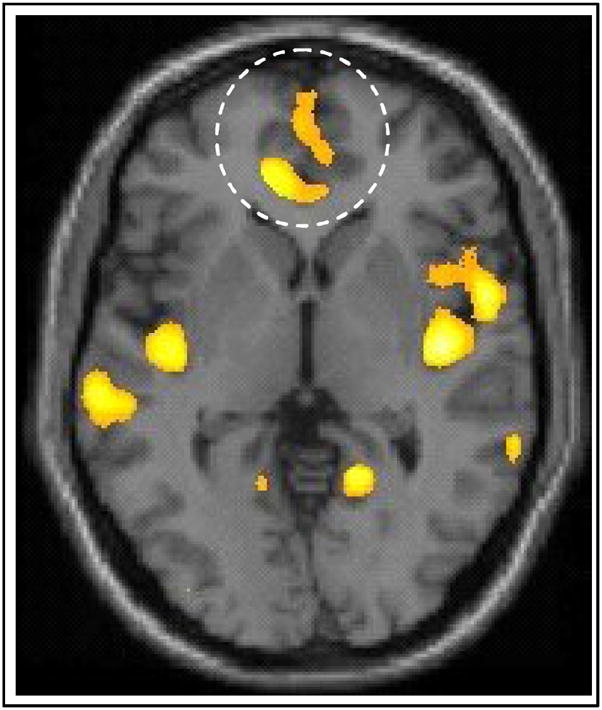
The inset on the axial slice depicts reductions in BPD subjects in ventral cingulate gyrus (Cluster peak: t59 =3.48, x=−1, y=61, z=1).
Figure 2.
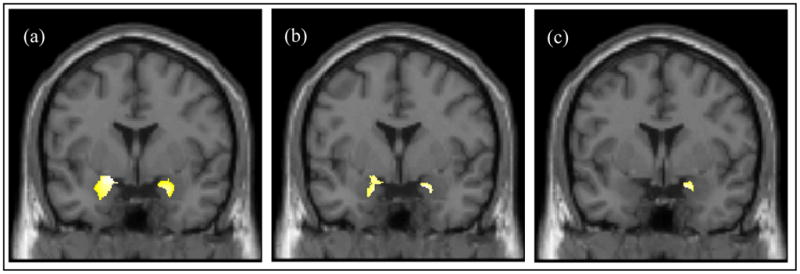
(a) Significant reductions in gray matter concentration in BPD compared to HC; insets depict bilateral reductions in the medial temporal lobe. (Cluster peaks: Left Amygdala, t59 =5.06, x= −23, y= −2, z= −19; Right Amygdala, t59 =4.79, x= 23, y= −1, z= −22). (b) Significant reductions in gray matter concentration in female BPD compared to female HC. (Cluster peaks: Left Amygdala, t37 =3.94, x= −17, y= −0, z= −21; Right Amygdala, t37 =4.31, x= 20, y= 5, z= −19). (c) Significant reductions in gray matter concentration in sexually abused compared to non-abused female BPD subjects in Rt. medial temporal lobe. (Cluster peak: Right Amygdala, t18 =4.94, x= 18 y= 4, z= −19).
Figure 3.
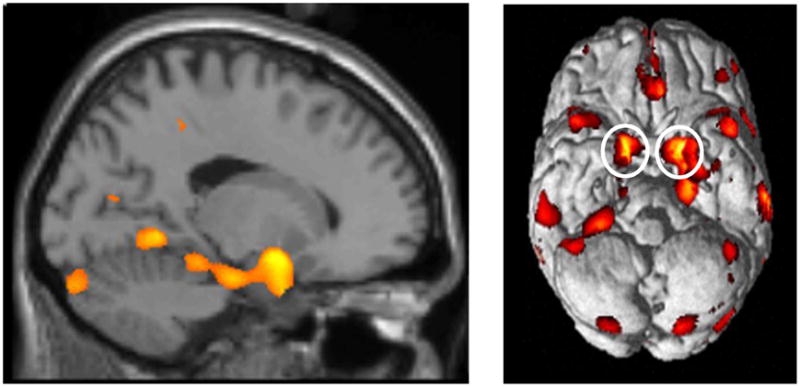
Figure 3a. Sagittal view emphasizing significant gray matter reductions observed through the medial temporal lobe (HC>BPD).
Figure 3b. Ventral view of surface rendering showing significant clusters with reductions in gray matter concentration (HC>BPD). Note the bilateral reductions in the amygdala (insets) in particular, and the medial temporal lobe in general.
The 34 BPD subjects were compared with the 30 HC, with age and gender covaried. Significant bilateral reductions in gray matter concentrations were noted in the BPD sample compared with the HC sample in the ventral aspect of the cingulate gyrus and several regions of the medial temporal lobe, including areas of the hippocampus, amygdala, parahippocampal gyrus and uncus. In the frontal lobe, significant cluster-level peaks were located bilaterally in the anterior cingulate gyrus (BA32) (Table 2). In the medial temporal lobe, significant voxel level peaks were centered in the hippocampus, amygdala, and parahippocampal gyrus bilaterally. Elsewhere in the temporal lobe, significant voxel-level peaks were noted bilaterally in the middle temporal gyrus (BA 22), the Lt. superior temporal gyrus (BA 22), Rt. insula (BA13) and Rt. occipitotemporal (lingual) gyrus (BA19). BPD subjects also had significant cluster-level peaks in the occipitotemporal (fusiform) gyrus, bilaterally (BA 19). (Table 2).
In the reverse contrast (BPD>HC), significant increases in gray matter concentrations were found in BPD subjects compared to HC in a very large contiguous area (K = 84,496) including the dorsal medial frontal cortex bilaterally, extending posteriorly to Rt. premotor and parietal cortex. Voxel-level peaks were found in the Rt. medial frontal gyrus (BA 6), Lt. superior frontal gyrus (BA 8), and posteriorly, in the precuneus of the Rt. parietal lobe (BA 7). At the cluster level, significant peaks were also noted in the area of the Lt. insula (BA 44), Lt. Inferior parietal gyrus (BA 40), and Rt. putamen (Table 2).
3.2. Female-only: BPD vs. HC (Fig. 2b.)
PET studies have demonstrated marked gender differences in glucose utilization in impulsive subjects with BPD, at rest, and following serotonergic activation (Soloff et al., 2005). To explore possible gender differences, we analyzed female and male subjects separately. Twenty-two female BPD subjects were compared with 19 HC females, with age as covariate. As in the combined gender sample (above), there were large areas of reduction in gray matter concentrations in BPD relative to HC subjects in the medial temporal lobe, bilaterally, but not in the ventral cingulate gyrus (Fig. b). Significant voxel-level reductions in gray matter concentrations were noted in BPD subjects compared to HC in hippocampus and amygdala, bilaterally. Significant reductions were also noted at the cluster level in the Rt. uncus (BA 34), and parahippocampal gyrus, bilaterally. As in the combined gender sample (above), significant cluster-level reductions in gray matter concentrations were also noted in BPD subjects compared with the HC group in the middle temporal lobe (BA 21) bilaterally, and in the Lt. occipitotemporal fusiform gyrus (BA 19) (Table 3).
Table 3.
Male and Female samples: Anatomical locations of significant voxel and cluster peaks
| Contrasting Groups | Cluster size K, ( P. corr). | T,P (FWE-corr) | Z | Peak Voxel x,y,z (mm) | ** Anatomical locations of peak voxels |
|---|---|---|---|---|---|
| I. Female subjects 22 BPD vs. 19 HC* | |||||
| A. HC>BPD | 83 (n.s.) | 4.13 (0.045) | 3.72 | −22,−16,−23 | Lt. hippocampus (!) |
| 318 (0.008) | 3.94(0.02) | 3.57 | −17, 0,−21 | Lt. amygdala (!) | |
| 45 (n.s.) | 4.43 (0.02) | 3.94 | 21,−2,−22 | Rt. hippocampus (!) | |
| 263 (0.01) | 4.13 (0.008) | 3.86 | 20, 5,−19 | Rt. amygdala (!) | |
| 710 (n.s.) | 5.49 (<0.1) | 4.66 | −61,−29,−3 | Lt. middle temporal g.(BA21) | |
| Cluster-level: | 1265 (0.04) | n.s. | 4.54 | 42,−8,−8 | Rt. middle temporal l (BA21) |
| 3259 (<0.001) | 5.53, (<0.1) | 4.69 | 10,4,−24 | Rt. limbic lobe, uncus (BA34) | |
| n.s. | 4.42 | 18,−2,−24 | Rt. parahippocampal g. (BA 28) | ||
| 1449 (0.023) | n.s. | 3.72 | −13, 0,−22 | Lt. parahippocampal g. (BA 34) | |
| −21, 3,−31 | Lt. parahippocampal g. (BA 34) | ||||
| 1311 (0.035) | n.s. | 3.71 | −44,−73,−17 | Lt. fusiform g (BA19) | |
| B. BPD>HC | |||||
| 57701(<0.001) | 6.65 (0.004) | 5.36 | 11,−2,71 | Rt. superior frontal g.(BA6) | |
| 6.41 (0.008) | 5.22 | 5,−62,35 | Rt. parietal l, precuneus, (BA 7) | ||
| 6.35 (0.01) | 5.19 | 21,57,26 | Rt. superior frontal g, (BA 10) | ||
| 2521 (0.001) | 5.75 (0.046) | 4.83 | −55,0,4 | Lt. superior temporal g, (BA 22) | |
| Cluster level: | 1869 (0.007) | n.s | 4.28 | 11,−46,−25 | Rt.cerebellum, anterior l. |
| 1752 (0.01) | n.s. | 4.06 | −47,23,27 | Lt. middle frontal g.(BA46) | |
| II. Male subjects 12 BPD vs. 11 HC* | |||||
| C. HC>BPD | |||||
| Cluster level: | 2911 (<0.001) | n.s. | 4.61 | 9,36,0 | Rt. anterior cingulate (BA 24) |
| n.s. | 3.96 | −6,37,−2 | Lt. anterior cingulate (BA32) | ||
| 1092 (0.04) | n.s. | 4.20 | 28,−41,−30 | Rt. cerebellum, anterior l | |
| 1108 (0.04) | n.s. | 4.00 | −60,−10,−9 | Lt. middle temporal g. (BA21) | |
| D. BPD>HC | |||||
| 1250 (0.024) | n.s. | 3.95 | 28,10,6 | Rt. putamen | |
| III. BPD Females: 14 Non-Abused vs. 8 Abused* | |||||
| E. Non-Abused .> Abused | |||||
| 201 (n.s.) | 4.94 (0.01) | 3.88 | 18, 4,−19 | Rt. amygdala (!) | |
| 3455 (<0.001) | 8.93 (0.007) | 5.45 | −39,22,−16 | Lt. inferior frontal g, (BA 47) | |
| Cluster level: | 3215 (<0.001) | n.s. | 4.25 | 23,−20,−23 | Rt. parahippocampal g. (BA28) |
| 985 (0.04) | n.s. | 4.74 | −56,−32,−4 | Lt. middle temporal g. (BA21) | |
| 2320 (<0.001) | n.s | 4.70 | 46,24,−11 | Rt.inferior frontal g (BA47) | |
| 1667 (0.003) | n.s. | 4.63 | −7,−3, 46 | Lt. cingulate gyrus (BA24) | |
| F. Abused > Non-abused | |||||
| 772 (<0.1) | 8.32 (0.015) | 5.27 | −24,29,57 | Lt. superior frontal g,(BA 8) |
ANCOVA with age, P (2-tailed), n.s.= not significant at voxel level
AAL atlas in MNI space (approximate Brodmann areas from MNI to TAL transformation)
post hoc analysis: small volume correction using region-of-interest masks.
In the reverse contrast (BPD>HC), results resembled the analysis of the combined gender sample (above). BPD subjects had increased gray matter concentration compared to HC in a large contiguous area of the Rt. cerebrum (k = 57,701), extending from the Rt. superior frontal gyrus (BA 6) posteriorly to the precuneus of the Rt. parietal lobe (BA 7). Significant voxel-level peaks were noted in Rt. superior frontal gyrus (BA 6, 10), Rt. parietal precuneus (BA 7), and Lt. superior temporal gyrus (BA 22). At the cluster-level, significant increases in BPD relative to HC subjects were noted in the Lt. middle frontal gyrus (BA 46) and Rt. anterior cerebellum (Table 3).
3.3. Males-only: BPD vs. HC
Twelve (12) male BPD subjects were compared to 11 HC males, with age as covariate. As in the combined gender analysis (above), BPD males demonstrated diminished gray matter concentration in anterior cingulate bilaterally, a result not found in the female-only study. BPD males also had diminished concentrations in the Lt. middle temporal gyrus (BA 21), and an area of Rt. anterior cerebellum. In the reverse contrast, BPD males had increased gray matter concentration relative to HC in the Rt. putamen, similar to the combined-gender sample, but not found in the female-only study (Table 3).
3.4. Non-abused vs. abused female BPD (Fig. 2c)
ROI morphometry studies of BPD females demonstrate volume loss in hippocampus and amygdala (Tebartz van Elst et al., 2003), especially associated with histories of childhood abuse (Driessen et al., 2000; Schmahl et al., 2003; Brambilla et al., 2004; Irle et al., 2005). All of these studies compared female BPD subjects to healthy controls. To control for the effects of diagnosis, we contrasted abused and non-abused female BPD subjects. Eight (8) female BPD subjects with a history of childhood sexual abuse were compared to 14 female BPD subjects with no history of abuse, with age as covariate. Significant reductions in gray matter concentrations at the voxel level were noted in abused BPD females compared to non-abused in the Rt. medial temporal lobe, with a significant voxel-level peak in the Rt. amygdala. Abused BPD females also had diminished gray matter concentrations bilaterally in the inferior frontal gyrus (BA47), with a significant voxel-level peak on the left (Table 3). (The voxel peak in the Rt. inferior frontal gyrus is significant only at the cluster level, with K = 2320, p.<.001.) Significant cluster-level peaks were noted in abused BPD females compared to non-abused in the Rt. parahippocampal gyrus (BA 28), Rt. inferior frontal gyrus (BA 47), Lt. cingulate gyrus (BA24) and Lt. middle temporal gyrus (BA 21). In the reverse contrast, abused subjects had increased gray matter concentrations at the voxel level in the Lt. superior frontal gyrus (BA 8) (Table 3). These analyses should be viewed as preliminary given the small sample sizes.
3.5. Effect of depressed mood (HamD) on the combined gender sample
Dysphoric mood in patients with MDD, and provoked sadness in healthy volunteers, are each associated with specific changes in regional cerebral blood flow and metabolism in limbic, paralimbic, and neocortical sites (Mayberg et al., 1999). To explore potential effects of depressed mood on gray matter concentrations in BPD, we covaried for HamD (with age and gender) in a re-analysis of the combined-gender sample. Covarying for HamD had little effect on previously noted decreases in gray matter concentrations in BPD subjects compared to HC in the medial temporal lobes, even after small volume correction (10.0 mm) (Table 4). However, previously noted decreases in gray matter concentrations in the ventral cingulate gyrus among BPD subjects were no longer apparent. (Peak voxels and significant clusters for both contrasts are given in Table 4.)
3.6. Effect of impulsivity (BIS) and aggression (LHA) on the combined gender sample
Regulation of impulsive and aggressive behavior involves the inhibitory function of the prefrontal cortex (PFC), especially the orbital frontal and ventromedial cortex, and functional connectivity with other cortical and limbic circuits (Weinberger, 1993). PET studies in BPD demonstrate hypometabolism and diminished response to serotonergic agonists in these same areas (Siever et al., 1999; New et al., 2000; Soloff et al., 2003; Soloff et al., 2005). To examine the effects of impulsivity (BIS) and aggression (LHA) on gray matter concentrations in BPD, we covaried separately for LHA and BIS in exploratory re-analyses of the combined gender sample.
Covarying for LHA (with age and gender), produced differences in gray matter concentrations in BPD subjects relative to HC in areas similar to those previously noted in the combined gender analysis (above) (Table 4). However, after covarying for BIS (with age and gender), no significant differences remained in gray matter concentrations at the voxel level among BPD subjects relative to HC, reflecting a marked statistical effect (Table 4).
4. Discussion
Using VBM, we demonstrated decreased gray matter concentrations in BPD compared to control subjects in ventral cingulate gyrus and several regions of the medial temporal cortex, including hippocampus, amygdala, parahippocampal gyrus and uncus. Decreased gray matter concentrations in medial temporal cortex were attributable primarily to female BPD subjects, especially those with histories of childhood abuse, while male, but not female, BPD subjects showed diminished gray matter concentrations in anterior cingulate compared to controls. Covarying for depressed mood rendered group differences in the ventral cingulate cortex non-significant, but had less effect on differences in medial temporal cortex. Covarying for history of aggression (LHA) had relatively little effect on group differences, while covarying for impulsivity (BIS), rendered all previously noted voxel-level group differences non-significant. Co-variation does not prove statistical mediation; however, these results suggest a more robust effect for impulsivity on gray matter concentrations in BPD compared to aggression. These exploratory VBM studies suggest that structural differences between BPD and HC subjects may be related, in part, to gender, depressed mood, and trait impulsivity, which should be assessed in confirmatory studies.
We are aware of only one prior VBM study in subjects with BPD. Rusch et al. (2003) compared 20 female BPD subjects to 21 controls and found loss of gray matter concentration in the left basolateral amygdala among BPD subjects, but not in hippocampus, or PFC. Subjects were excluded for current major depression, substance use or eating disorders. This study followed and extended a small ROI morphometry study by Tebartz van Elst et al. (2003), which found large volume reductions in 8 female BPD subjects compared to 8 healthy controls in hippocampus (20 – 21% decrease) and amygdala bilaterally (23–25%), but also in left orbitofrontal cortex (24%), and anterior cingulate (26%). Although we also found diminished gray matter concentration in Lt. amygdala in female BPD subjects relative to controls, our VBM results more closely resemble Tebarz van Elst’s (2003) ROI morphometry study. i.e. We found diminished gray matter concentrations in BPD subjects in cingulate cortex, hippocampus and amygdala bilaterally in our combined gender sample, and in hippocampus and amygdala bilaterally in our female-only sample. Rusch et al. (2003) also reported bilateral loss of gray matter concentration in the fusiform gyrus (occipitotemporal cortex), which proved non-significant after correction for multiple comparisons. Gray matter was diminished bilaterally in the fusiform gyrus in our combined gender sample, and in left fusiform gyrus among our female BPD subjects. The fusiform gyrus is involved in recognition of facial expression, and is modulated by projections from the amygdala. Studies using fMRI suggest that this function is abnormal in BPD, and may be related to the borderline patients’ affective instability (Herpertz et al., 2001). Differences in sample selection between our study and that of Rusch et al. (2003), especially in regard to co-morbidity, may contribute to the differing results.
In our combined gender sample, we noted decreased gray matter concentrations in BPD subjects in ventral cingulate, and, among male BPD subjects, in anterior cingulate, bilaterally. Diminished volumes in anterior cingulate among BPD subjects have been previously reported in ROI morphometry studies (Tebartz van Elst et al., 2003; Hazlett et al., 2005) and related to increased impulsivity (Hazlett et al., 2005). Gender differences in impulsivity may explain our finding decreased gray matter concentration in anterior cingulate among male, but not female BPD subjects in our study.
Contrary to expectation, we did not find structural abnormalities in the orbital frontal or ventromedial PFC among BPD subjects. Some, but not all, ROI morphometry studies in BPD have noted decreased volumes in anterior prefrontal and orbital frontal cortex (Tebartz van Elst et al., 2003; Hazlett et al., 2005). Hazlett et al. (2005) found a relationship between decreased gray matter volume in anterior prefrontal cortex (BA 10) and increased impulsiveness (BIS) in BPD subjects compared to controls. This large sample study (n=50) is noteworthy for a predominance of male subjects (60%) and co-morbidity with schizotypal personality disorder (26%). In contrast, our BPD subjects are predominately female, with only one subject meeting Axis II criteria for schizotypal personality disorder. Although we found no structural abnormalities in these areas, PET studies in BPD subjects and other impulsive PDs have demonstrated functional abnormalities in orbital frontal and ventromedial PFC which may contribute to impulsive-aggression, behavioral disinhibition, and affective instability in BPD.
PET studies in BPD subjects have demonstrated hypometabolism in anterior cingulate cortex, orbital and ventromedial PFC (De La Fuenta et al., 1997; Soloff et al., 2003). Orbital frontal and ventromedial PFC are known to be important in response inhibition, regulation of impulsivity and reactive aggression (Blair, 2004; Weinberger et al., 1993). Impulsive BPD subjects, and other impulsive PDs, have diminished metabolic responses to serotonergic activation (by fenfluramine (FEN), or meta-chlorophenylpiperazine (m-CPP)) in these same prefrontal cortical areas (Siever et al., 1999; New et al., 2002; Soloff et al., 2000; Soloff et al., 2005). Impulsivity and impulsive-aggression may be mediated, in part, by diminished serotonergic regulation or loss of connectivity in these prefrontal areas and related brain circuits.
Our VBM results are consistent with ROI morphometry studies which report associations between childhood abuse, hippocampal and amygdala volume loss in BPD compared to control subjects (Driessen et al., 2000; Schmahl et al., 2003; Brambilla et al., 2004; Irle et al., 2005). In the context of BPD, duration and severity of childhood traumatization have been related to degree of hippocampal volume loss (Driessen et al., 2000; Irle et al., 2005). These studies contrasted BPD and healthy control subjects, but not abused and non-abused BPD subjects. Since half of research subjects ascertained for a BPD diagnosis have no reported history of childhood physical or sexual abuse (Soloff et al., 2002), contrasting abused and non-abused BPD subjects controls for the effect of diagnosis alone. Although our sample sizes for this contrast were small, we found significantly decreased concentrations of gray matter in Rt. amygdala and Rt. parahippocampal gyrus in abused compared to non-abused BPD females.
Hippocampal volume loss among female BPD inpatients has also been directly correlated with histories of multiple hospitalizations (Zetsche et al., 2007). Zetsche et al. (2006, 2007) reported an ROI morphometry study of 25 female inpatients with extensive co-morbid psychiatric diagnoses (e.g. 68% current MDD, 32% PTSD), and current psychotropic drug use (80%). In this sample, hippocampal volume was not related to sexual abuse, or impulsivity (BIS), but was inversely related to aggression (LHA). In contrast, we reported a robust statistical effect on VBM results for impulsivity (BIS) but not for aggression (LHA). The two studies differ greatly in sample characteristics, especially in regard to degree of illness severity and comorbidity. (e.g. Our subjects were all living in the community and were free of psychotropic medications a minimum of 2 – 6 weeks prior to the scan.) Within our total sample, we found a relationship between depressed mood (HamD) and both impulsivity (BIS) (rho=0.65, P<0.001, n=54) and aggression (LHA) (rho = 0.44, P=0.001, n=55), suggesting the need to control for interaction effects of depressed mood when examining the relationship of aggression and impulsivity on brain volumes.
In this same sample, Zetsche et al. (2006) also reported an increase in amygdala volumes bilaterally among BPD subjects with comorbid MDE compared to non-depressed BPD subjects, and a significant positive correlation between depression scores (HamD-21) and Lt. amygdala volumes. Although contrary to prior reports in subjects with BPD, increased amygdala volumes have been reported in some studies of patients with MDE (Frodl et al., 2003; Lange and Irle, 2004), though not all (Sheline et al., 1999, Mervaala et al., 2000). Zetsche et al. (2006) caution that comorbidity with MDE can lead to discrepant findings between studies with regard to amygdala measurements in BPD.
We found increased gray matter concentration in the Rt. putamen in male, but not female, BPD subjects compared to controls. In an earlier ROI morphometry study conducted in our laboratory, Brambilla et al. (2004) reported increased bilateral putamen volumes associated with co-morbid substance use disorder in a mixed gender sample of 10 BPD subjects compared to healthy controls. Because our sample overlaps with that of Brambilla et al. (2004) (i.e., 4 BPD males were in both studies), finding increased gray matter concentration in the putamen in BPD subjects in our study suggests concurrence between methods. Our sample had too few subjects with Axis I substance use disorder to allow a meaningful test of the association with putamen volumes.
In our combined gender sample, increased gray matter concentrations were also found in BPD subjects in a very large area of the Rt. cerebrum extending from the Rt. superior frontal gyrus posteriorly and across the parietal lobe to the precuneus. Similar results were obtained in the female-only analysis, but not among male subjects. In contrast, an ROI morphometry study by Irle et al. (2005) reported diminished volumes of Rt. parietal cortex in BPD compared to control subjects, and a significant direct relationship between Rt. parietal cortex volumes, psychotic symptoms and schizoid personality traits among BPD patients. Patients studied by Irle et al. (2005) were co-morbid for current depression (60%), lifetime or current PTSD (37%), depersonalization disorder (77%) and other Axis I comorbid disorders, and had significantly lower IQ and marked neuropsychological test performance deficits compared to normal controls. Our sample does not reflect this degree of clinical severity or comorbidity, which may confound results
4.1. PFC, the hippocampal-amygdala complex, and BPD
The medial temporal cortex (including the hippocampal-amygdala complex) has extensive connections to the orbital and ventral medial PFC, and contributes to emotion regulation and impulse control. The amygdala assesses facial expressions and emotions in social situations (especially negative emotions), and generates fear and anxiety in response to perceived threat. At times of stress, the hippocampal-amygdala complex modulates and regulates expression of fear responses (Davidsonet al., 1999; Davis, 2001; Philips and LeDoux, 1992). Loss of inhibitory regulation in these limbic circuits results in disinhibited fear responding, anger and impulsive-aggressive behavior, core characteristics of BPD (Philips and LeDoux, 1992). The hippocampus is also involved in attentional monitoring of emotional states, addressing discrepancies between current perception of reality and past representations in associative working memory. The integrity of the hippocampal-orbitomedial PFC circuit is important in maintaining cognitive-emotional control of behavior (Wall and Messier, 2001).
4.2. Limitations
Structural studies of subjects with BPD may be confounded by Axis I co-morbidity, especially MDD, PTSD, and alcohol use disorders (AUD), each of which may be associated with structural abnormalities. In addition, childhood ADHD may persist into adulthood and confound studies of BPD with overlap in symptoms relative to impulsivity and affect regulation. ADHD has also been associated with structural brain abnormalities (Faraone et al.,2000).
ROI morphometry studies in MDD have described diminished volumes in the subgenual prefrontal cortex (Drevets, 1997), medial orbitofrontal cortex (gyrus rectus) (Bremner et al., 2002), hippocampus and amygdala (Sheline et al., 1996, 1999; Bremner et al., 2000; Mervaala et al.,2000; Frodl et al., 2002). Volume loss is found in post-depressed patients in remission (Bremner et al.,2002), and correlates with total lifetime duration of depression (Sheline et al.,1999), introducing a potential confounding effect on interpretation of structural studies (Sheline et al., 2000). In our sample, 16 subjects (47.1%) had past histories of MDD.
Diminished hippocampal volumes are also reported in subjects with PTSD (Stein et al.,1997; Bremner et al., 1997). In our study, only two BPD subjects (5.9%) met SCID criteria for current PTSD, and 1 for a lifetime diagnosis (3%). Our results appear at variance with the findings of Bremner et al. (2003), who asserted that “abuse per se in the absence of PTSD does not lead to deficits in hippocampal structure or function.” (op.cit. p. 929). In some subjects, though not all, BPD may represent a characterologic resolution of childhood PTSD (Gunderson and Sabo, 1993, for review, Zanarini M., 2000). As adults, these patients no longer have acute PTSD symptoms or meet SCID criteria for PTSD. Hippocampal atrophy may be related to childhood PTSD in these BPD adults. This view is consistent with preclinical evidence of the effects of early adverse experiences on brain development (Kaufman et al., 2000).
A current diagnosis of AUD was found in 3 BPD subjects (8.8%), and 4 with a lifetime diagnosis (11.8%). In adults, AUD has been associated with volume loss in the frontal lobes, including the PFC (Pfefferbaum et al., 1997). In adolescents, early onset AUD has been associated with volume loss in both PFC and hippocampus (De Bellis et al., 2000, De Bellis et al., 2005).
There is great overlap in clinical features of adult ADHD and BPD, especially in regard to impulsivity and affective instability (Davids and Gastpar, 2005; Dowson et al., 2004). In one study of adult subjects with BPD, 60% scored above a diagnostic threshold for ADHD symptoms on a retrospective, self rated scale (the Wender Utah Rating Scale) (Fossati et al., 2002). Among adults with ADHD (diagnosed retrospectively), 20.3% also meet criteria for co-morbid BPD (Miller et al., 2007). Despite areas of symptom overlap, adult ADHD can be discriminated from BPD using scales specifically designed for adult ADHD (e.g. the Attention-Deficit Scales for Adults (Dowson et al., 2004). It remains unclear whether the co-occurrence of ADHD and BPD represent two independent disorders or whether childhood ADHD is a risk factor for the development of BPD in adulthood (Thatcher et al., 2005). ADHD has been associated with structural abnormalities on neuroimaging, especially in prefrontal and anterior cingulate volumes (Seidman et al., 2006). Subjects in our protocol were diagnosed using the SCID I and IPDE, which do not screen for ADHD. However, using all available clinical data, including medical records, only one subject was found to have a co-morbid diagnosis of ADHD.
Gender differences found in structural studies of BPD may be related to clinical characteristics or co-morbid conditions which are differentially distributed by gender, and have their own effects on structure (e.g. sexual abuse in females, antisocial personality disorder (ASPD) in males). In the current sample, we found no significant differences by gender in suicide attempter status, number of hospital admissions, incidence of sexual or physical abuse, or pooled Axis I depressive diagnoses; however, co-morbid ASPD was more frequently diagnosed among male BPD subjects (58.3%) compared to BPD females (9.1%, p.004, Fisher’s exact, 2 sided). Male, but not female, BPD subjects have decreased gray matter concentrations in anterior cingulate bilaterally, which may be related to the neurobiology of impulsivity and aggression. Large sample studies are needed to control for gender, clinical characteristics, Axis I and Axis II co-morbidities, suggesting an important indication for VBM as an exploratory method.
Acknowledgments
The research reported was supported by NIMH grants MH 48463 (PS) and MH068680 (VD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashburner J, Friston KJ. Why voxel-based morphometry should be used. Neuroimage. 2001;14(6):1238–1243. doi: 10.1006/nimg.2001.0961. [DOI] [PubMed] [Google Scholar]
- Barratt ES, Slaughter L. Defining, measuring and predicting impulsive-aggression: A heuristic model. Behavioral Science and Law. 1998;16:285–302. doi: 10.1002/(sici)1099-0798(199822)16:3<285::aid-bsl308>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Blair RJR. The roles of orbital frontal cortex in the modulation of antisocial behavior. Brain and Cognition. 2004;55:198–208. doi: 10.1016/S0278-2626(03)00276-8. [DOI] [PubMed] [Google Scholar]
- Brambilla P, Soloff PH, Sala M, Nicoletti MA, Keshavan MS, Soares JC. Anatomical MRI study of borderline personality disorder patients. Psychiatry Research: Neuroimaging. 2004;131:125–133. doi: 10.1016/j.pscychresns.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Anderson ER, Staib LH, Miller HL, Charney DS. Hippocampal volume reduction in major depression. American Journal of Psychiatry. 2000;157:115–117. doi: 10.1176/ajp.157.1.115. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Narayan M, Staib LH, Southwick SM, McGlashan T, Charney DS. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. American Journal of Psychiatry. 1999;156:1787–1795. doi: 10.1176/ajp.156.11.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Vermetten E, Staib L, Bronen RA, Mazure C, Capelli S, McCarthy G, Innis R, Charney D. Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse- A preliminary report. Biological Psychiatry. 1997;41:23–32. doi: 10.1016/s0006-3223(96)00162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Nazeer A, Adil J, Kahn S, Staib LH, Charney DS. Reduced volume of orbitofrontal cortex in major depression. Biological Psychiatry. 2002;51:273–279. doi: 10.1016/s0006-3223(01)01336-1. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vythilingam M, Vermetten E, Southwick SM, McGlashan T, Nazeer A, Khan S, Vaccarino LV, Soufer R, Garg PK, Ng CK, Staib LH, Ducan JS, Charney DS. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. American Journal of Psychiatry. 2003;160:924–932. doi: 10.1176/appi.ajp.160.5.924. [DOI] [PubMed] [Google Scholar]
- Brown GL, Ebert MH, Goyer PF, Jimerson DC, Klein WJ, Bunney WE, Goodwin FK. Aggression, suicide, and serotonin: Relationships to CSF amine metabolites. American Journal of Psychiatry. 1982;139:741–746. doi: 10.1176/ajp.139.6.741. [DOI] [PubMed] [Google Scholar]
- Davids E, Gastpar M. Attention deficit hyperactivity disorder and borderline personality disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2005;29:865–877. doi: 10.1016/j.pnpbp.2005.04.033. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Abercrombie H, Nitschke JB, Putnam K. Regional brain function, emotion and disorders of emotion. Current Opinion in Neurobiology. 1999;9:228–234. doi: 10.1016/s0959-4388(99)80032-4. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- De La Fuenta JM, Goldman S, Stanus E, Vizuete C, Morlan I, Bobes J, Mendlewicz J. Brain glucose metabolism in borderline personality disorder. Journal Psychiatric Research. 1997;31:531–541. doi: 10.1016/s0022-3956(97)00001-0. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, Kersh A, Keshavan MS. Hippocampal volume in adolescent-onset alcohol use disorders. American Journal of Psychiatry. 2000;157:737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Narasimhan A, Thatcher DL, Keshavan MS, Soloff PH, Clark DB. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and co-morbid mental disorders. Alcoholism, Clinical and Experimental Research. 2005;29:1590–1600. doi: 10.1097/01.alc.0000179368.87886.76. [DOI] [PubMed] [Google Scholar]
- Diwadkar VA, Lacerda AL, Keshavan MS, Hardan AY, Soares JC. Voxel-based and region-of-interest morphometry convergently reveal regional gray matter abnormalities in unipolar depression. Proceedings of the International Society of Magnetic Resonance Medicine. 2003;11:182. [Google Scholar]
- Donegan NH, Sanislow CA, Blumberg HP, Fulbright RK, Lacadie C, Skudlarski P, Gore JC, Olson IR, McGlashen TH, Wexler B. Amygdala hyperreactivity in borderline personality disorder: implications for emotional dysregulation. Biological Psychiatry. 2003;54:1284–1293. doi: 10.1016/s0006-3223(03)00636-x. [DOI] [PubMed] [Google Scholar]
- Dowson JH, McLean A, Bazanis E, Toone B, Young S, Robbins TW, Sahakian B. The specificity of clinical characteristics in adults with attention-deficit/hyperactivity disorder: a comparison with patients with borderline personality disorder. European Psychiatry. 2004;19:72–78. doi: 10.1016/j.eurpsy.2003.07.010. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386(6627):824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Driessen M, Herrmann J, Stahl K, Zwaan M, Meier S, Hill A, Osterheider M, Peterson D. Magnetic resonance imaging volumes of the hippocampus and the amygdala in women with borderline personality disorder and early traumatization. Archives of General Psychiatry. 2000;57:1115–1122. doi: 10.1001/archpsyc.57.12.1115. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Biederman J, Spencer T, Wilens T, Seidman LJ, Mick E, Doyle AE. Attention deficit/hyperactivity disorder in adults: An overview. Biological Psychiatry. 2000;48:9–20. doi: 10.1016/s0006-3223(00)00889-1. [DOI] [PubMed] [Google Scholar]
- Fossati A, Novella L, Donati D, Donini M, Maffei C. History of childhood attention deficit/hyperactivity disorder symptoms and borderline personality disorder: A controlled study. Comprehensive Psychiatry. 2002;43 (5):369–377. doi: 10.1053/comp.2002.34634. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: A general linear approach. Human Brian Mapping. 1995;2(4):189–210. [Google Scholar]
- Frodl T, Meisenzahl EM, Zetzsche T, Born C, Jager M, Groll C, Bottlender R, Leinsinger G, Moller HJ. Larger amygdala volumes in first depressive episode as compared to recurrent major depression and healthy controls. Biological Psychiatry. 2003;53:338–344. doi: 10.1016/s0006-3223(02)01474-9. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Goyer PF, Andreason PJ, Semple WE, Clayton AH, King AC, Compton-Toth BA, Schultz SC, Cohen RM. Positron-emission tomography and personality disorders. Neuropsychopharmacology. 1994;10:21–28. doi: 10.1038/npp.1994.3. [DOI] [PubMed] [Google Scholar]
- Gunderson JG, Kolb JE, Austin V. The diagnostic interview for borderlines. American Journal of Psychiatry. 1981;138:896–903. doi: 10.1176/ajp.138.7.896. [DOI] [PubMed] [Google Scholar]
- Gunderson JG, Sabo AN. The phenomenologic and conceptual interface between borderline personality disorder and PTSD. American Journal of Psychiatry. 1993;150:19–27. doi: 10.1176/ajp.150.1.19. [DOI] [PubMed] [Google Scholar]
- Guy W. ECDEU Assessment Manual of Psychopharmacology-Revised. (DHEW Publication No.ADM) MINH; Rockville, Maryland: 1976. pp. 76–338. [Google Scholar]
- Hazlett EA, New AS, Newmark R, Haznedar MM, Lo JN, Speiser LJ, Chen A, Mitropoulou V, Minzenburg M, Siever L. Reduced anterior and posterior cingulate gray matter in borderline personality disorder. Biological Psychiatry. 2005;58:614–623. doi: 10.1016/j.biopsych.2005.04.029. [DOI] [PubMed] [Google Scholar]
- Herpertz SC, Dietrich TM, Wenning B, Krings T, Eberich SG, Willmes K, Thron A, Sass H. Evidence of abnormal amygdala functioning in borderline personality disorder: A functional MRI study. Biological Psychiatry. 2001;50:292–298. doi: 10.1016/s0006-3223(01)01075-7. [DOI] [PubMed] [Google Scholar]
- Irle E, Lange C, Sachsse U. Reduced size and abnormal asymmetry of parietal cortex in women with borderline personality disorder. Biological Psychiatry. 2005;57:173–182. doi: 10.1016/j.biopsych.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Plotsky PM, Nemeroff CB, Charney DS. Effects of early adverse experiences on brain structure and function: Clinical implications. Biological Psychiatry. 2000;48:778–790. doi: 10.1016/s0006-3223(00)00998-7. [DOI] [PubMed] [Google Scholar]
- Lange C, Irle E. Enlarged amygdala volume and reduced hippocampal volume in young women with major depression. Psychological Medicine. 2004;34:1059–1064. doi: 10.1017/s0033291703001806. [DOI] [PubMed] [Google Scholar]
- Loranger AW, Janca A, Sartorius N. Assessment and diagnosis of personality disorder: The ICD-10 International Personality Disorder Examination (IPDE) Cambridge University Press; Cambridge: 1997. [Google Scholar]
- Lyoo K, Han MH, Cho DY. A brain MRI study in subjects with borderline personality disorder. Journal of Affective Disorders. 1998;50:235–243. doi: 10.1016/s0165-0327(98)00104-9. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Mann JJ, Waternaux C, Haas GL, Malone KM. Toward a clinical model of suicidal behavior in psychiatric patients. American Journal of Psychiatry. 1999;156 (2):181–189. doi: 10.1176/ajp.156.2.181. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT. Reciprocal limbic-cortical function and negative mood: Converging PET findings in depression and normal sadness. American Journal of Psychiatry. 1999;156:675–682. doi: 10.1176/ajp.156.5.675. [DOI] [PubMed] [Google Scholar]
- Mervaala E, Fohr J, Kononen M, Valkonen-Korhonen M, Vainio P, Partanen K, Partanen J, Tiihonen J, Viinamaki H, Karjalainen AK, Lehtonen J. Quantitative MRI of the hippocampus and amygdala in severe depression. Psychological Medicine. 2000;30:117–125. doi: 10.1017/s0033291799001567. [DOI] [PubMed] [Google Scholar]
- Miller TW, Nigg JT, Faraone SV. Axis I and II comorbidity in adults with ADHD. Journal of Abnormal Psychology. 2007;116:519–528. doi: 10.1037/0021-843X.116.3.519. [DOI] [PubMed] [Google Scholar]
- New AS, Hazlett EA, Buchsbaum MS, Goodman M, Reynolds D, Mitropoulou V, Sprung L, Shaw RB, Koenigsberg H, Platholi J, Silverman J, Siever LJ. Blunted prefrontal cortical 18-Flurodeoxyglucose positron emission tomography response to meta-chlorophenylpiperazine in impulsive aggression. Archives of General Psychiatry. 2002;59:621–629. doi: 10.1001/archpsyc.59.7.621. [DOI] [PubMed] [Google Scholar]
- Oquendo MA, Halberstam B, Mann JJ. Risk factors for suicidal behavior: the utility and limitations of research instruments, in standardized assessment for the clinician. In: First MB, editor. Standardized Evaluation in Clinical Practice. Review of Psychiatry. American Psychiatric Press; Washington, DC: 2003. pp. 103–129. [Google Scholar]
- Oquendo MA, Mann JJ. The biology of impulsivity and suicidality. Psychiatric Clinics of North America. 2000;23 (1):11–25. doi: 10.1016/s0193-953x(05)70140-4. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Lim KO. Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcoholism: Clinicial and Experimental Research. 1997;21:521–529. doi: 10.1111/j.1530-0277.1997.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Ross CA, Heber S, Norton GR, Anderson G, Anderson D, Barchet P. The dissociative disorders interview schedule: A structured interview. Dissociation. 1989;2(3):169–189. [Google Scholar]
- Rusch N, Tebartz van Elst L, Ludaescher P, Wilke M, Huppertz HJ, Thiel T, Schmahl C, Bohus M, Lieb K, Heblinger B, Hennin J, Ebert D. A voxel-based morphometric MRI study in female patients with borderline personality disorder. Neuroimage. 2003;20:385–392. doi: 10.1016/s1053-8119(03)00297-0. [DOI] [PubMed] [Google Scholar]
- Schmahl CG, Vermetten E, Elzinga BM, Bremner JD. Magnetic resonance imaging of hippocampal and amygdala volume in women with childhood abuse and borderline personality disorder. Psychiatry Research: Neuroimaging. 2003;122:193–198. doi: 10.1016/s0925-4927(03)00023-4. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Valera EM, Makris N, Monuteaux MC, Boriel DL, Kelkar K, Kennedy DN, Caviness VS, Bush G, Aleardi M, Faraone SV, Biedermna J. Dorsolateral prefrontal and anterior cingulate cortex volumetric abnormalities in adults with attention-deficit/hyperactivity disorder identified by magnetic resonance imaging. Biological Psychiatry. 2006;60:1071–1080. doi: 10.1016/j.biopsych.2006.04.031. [DOI] [PubMed] [Google Scholar]
- Sheline YI. 3D MRI studies of neuroanatomic changes in unipolar major depression: The role of stress and medical co-morbidity. Biological Psychiatry. 2000;48:791–800. doi: 10.1016/s0006-3223(00)00994-x. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Sanghavi M, Mintun MA, Gado MH. Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. Journal of Neuroscience. 1999;19:5034–5043. doi: 10.1523/JNEUROSCI.19-12-05034.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Wang PO, Mokhtar GH, Csernansky JG, Vannier MW. Hippocampal atrophy in recurrent major depression. Proceedings of the National Academy of Science. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siever LJ, Buchsbaum MS, New AS, Spiegel-Cohen J, Wei T, Hazlett E, Sevin E, Nunn M, Mitropoulou M. d,l-Fenfluramine response in impulsive personality disorder assessed with [18-F] fluorodeoxyglucose positron emission tomography. Neuropsychopharmacology. 1999;20:413–423. doi: 10.1016/S0893-133X(98)00111-0. [DOI] [PubMed] [Google Scholar]
- Soloff PH. Risk factors for suicidal behavior in borderline personality disorder: A review and update. In: Zanarini MC, editor. Borderline Personality Disorder. Taylor and Francis Group LLC; Boca Raton, FL: 2005. pp. 333–365. [Google Scholar]
- Soloff PH, Lynch KG, Kelly TM. Childhood abuse as a risk factor for suicidal behavior in borderline personality disorder. Journal of Personality Disorders. 2002;16(3):201–214. doi: 10.1521/pedi.16.3.201.22542. [DOI] [PubMed] [Google Scholar]
- Soloff PH, Lynch KG, Kelly TM, Malone KM, Mann JJ. Characteristics of suicide attempts of patients with major depressive episode and borderline personality disorder: A comparative study. American Journal of Psychiatry. 2000a;157:601–608. doi: 10.1176/appi.ajp.157.4.601. [DOI] [PubMed] [Google Scholar]
- Soloff PH, Meltzer CC, Becker C, Greer PJ, Constantine D. Gender differences in a fenfluramine-activated FDG-PET study of borderline personality disorder. Psychiatry Research: Neuroimaging. 2005;138:183–195. doi: 10.1016/j.pscychresns.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Soloff PH, Meltzer CC, Becker C, Greer PJ, Kelly TM, Constantine D. Impulsivity and prefrontal hypometabolism in borderline personality disorder. Psychiatric Research: Neuroimaging. 2003;123:153–163. doi: 10.1016/s0925-4927(03)00064-7. [DOI] [PubMed] [Google Scholar]
- Soloff PH, Meltzer CC, Greer PJ, Constantine D, Kelly TM. A fenfluramine-activated FDG-PET study of borderline personality disorder. Biological Psychiatry. 2000b;47:540–547. doi: 10.1016/s0006-3223(99)00202-4. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, First MB. Instruction Manual for the Structured Clinical Interview for DSM III-R (SCID) New York State Psychiatric Institute, Biometrics Research; New York: 1988. [Google Scholar]
- Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B. Hippocampal volume in women victimized by childhood sexual abuse. Psychological Medicine. 1997;27:951–959. doi: 10.1017/s0033291797005242. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-Planar Stereotactic Atlas of the Human Brain: 3-Dimensional Proportional System: An approach to cerebral imaging. New York: Thieme Medical Publishers; 1988. [Google Scholar]
- Tebartz, van Elst L, Hesslinger B, Thiel T, Geiger E, Haegele K, Lemieux L, Lieb K, Bohus M, Henning J, Ebert D. Frontolimbic brain abnormalities in patients with borderline personality disorder: A volumetric magnetic resonance imaging study. Biological Psychiatry. 2003;54:163–171. doi: 10.1016/s0006-3223(02)01743-2. [DOI] [PubMed] [Google Scholar]
- Thatcher DL, Cornelius JR, Clark DB. Adolescent alcohol use disorders predict adult borderline personality. Addictive behaviors. 2005;30:1709–1724. doi: 10.1016/j.addbeh.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Wall PM, Messier C. The hippocampal formation – orbitomedial prefrontal cortex circuit in the attentional control of active memory. Behavioural Brain Research. 2001;127:99–117. doi: 10.1016/s0166-4328(01)00355-2. [DOI] [PubMed] [Google Scholar]
- Weinberger DR. A connectionist approach to the prefrontal cortex. Journal of Neuropsychiatry and Clinical Neurosciences. 1993;5:241–253. doi: 10.1176/jnp.5.3.241. [DOI] [PubMed] [Google Scholar]
- Zanarini MC. Childhood experiences associated with the development of borderline personality disorder. Psychiatric Clinics of North America. 2000;23(1):89–101. doi: 10.1016/s0193-953x(05)70145-3. [DOI] [PubMed] [Google Scholar]
- Zetzsche T, Frodl T, Preuss UW, Schmitt G, Seifert D, Leinsinger G, Born C, Reiser M, Moller H-J, Meisenzahl EM. Amygdala volume and depressive symptoms in patients with borderline personality disorder. Biological Psychiatry. 2006;60:302–310. doi: 10.1016/j.biopsych.2005.11.020. [DOI] [PubMed] [Google Scholar]
- Zetsche T, Preuss UW, Frodl T, Schmitt G, Seifert D, Munchhausen E, Tabrizi S, Leinsinger G, Born C, Reiser M, Moller H-J, Meisenzahl EM. Hippocampal volume reduction and history of aggressive behavior in patients with borderline personality disorder. Psychiatry Research Neuroimaging. 2007;154:157–170. doi: 10.1016/j.pscychresns.2006.05.010. [DOI] [PubMed] [Google Scholar]


