Abstract
Objectives:
Neuromyelitis optica (NMO) immunoglobulin G (IgG) (aquaporin-4 [AQP4] IgG) is highly specific for NMO and related disorders, and autoantibody detection has become an essential investigation in patients with demyelinating disease. However, although different techniques are now used, no multicenter comparisons have been performed. This study compares the sensitivity and specificity of different assays, including an in-house flow cytometric assay and 2 commercial assays (ELISA and transfected cell-based assay [CBA]).
Methods:
Six assay methods (in-house or commercial) were performed in 2 international centers using coded serum from patients with NMO (35 patients), NMO spectrum disorders (25 patients), relapsing-remitting multiple sclerosis (39 patients), miscellaneous autoimmune diseases (25 patients), and healthy subjects (22 subjects).
Results:
The highest sensitivities were yielded by assays detecting IgG binding to cells expressing recombinant AQP4 with quantitative flow cytometry (77; 46 of 60) or visual observation (CBA, 73%; 44 of 60). The fluorescence immunoprecipitation assay and tissue-based immunofluorescence assay were least sensitive (48%–53%). The CBA and ELISA commercial assays (100% specific) yielded sensitivities of 68% (41 of 60) and 60% (36 of 60), respectively, and sensitivity of 72% (43 of 60) when used in combination.
Conclusions:
The greater sensitivity and excellent specificity of second-generation recombinant antigen-based assays for detection of NMO-IgG in a clinical setting should enable earlier diagnosis of NMO spectrum disorders and prompt initiation of disease-appropriate therapies.
Neuromyelitis optica (NMO) is a severe relapsing inflammatory CNS demyelinating disease that predominantly affects the optic nerves and spinal cord.1 At presentation, the most common differential diagnosis is multiple sclerosis (MS). However, unlike MS, disability in NMO accrues with each attack. Hence, early diagnosis and treatment are critical.2 The discovery of specific immunoglobulin G (IgG) antibodies binding to CNS astrocytic membranes identified the target as the water channel aquaporin-4 (AQP4), which has aided early recognition of the disease and broadened the clinical spectrum to include patients who have only optic neuritis or transverse myelitis (neuromyelitis optica spectrum disorders [NMOSDs]).3,4 Studies in vitro and in vivo have demonstrated the pathogenic potential of these autoantibodies.5–10 However, different assays for detecting AQP4-IgG in patients' sera differ in their sensitivities for NMO and other NMOSDs. 3,4,11–17 In this international multicenter study, 6 different AQP4-IgG assays were compared on coded samples.
METHODS
Standard protocol approvals, registrations, and patient consents.
This study was approved by all 3 institutional review boards.
Patients.
Serum samples from 146 patients and control subjects were tested in duplicate, and 35 patients fulfilled the Wingerchuck diagnostic criteria for NMO (either 1999 or 2006 [excluding antibody status]).2 Of the patients, 25 were classified by the investigators as having NMOSDs; this group included 14 patients with longitudinally extensive transverse myelitis (9 recurrent) and 8 patients with optic neuritis (5 recurrent; all patients with single attack cases of optic neuritis were seropositive). A total of 86 controls (22 healthy controls and 64 with miscellaneous diseases [fulfilling the McDonald criteria for relapsing-remitting MS, 39; Sjögren syndrome, 1; systemic lupus erythematosus, 4; rheumatoid arthritis, 1; spinal dural arteriovenous fistulae, 4; noninflammatory myelopathy, 3; short segment-longitudinal transverse myelitis, 2; sarcoid longitudinally extensive transverse myelitis, 1; polyclonal hypergammaglobulinemia, 1; and other, 8]) were also tested. The samples were provided by 3 institutions: Mayo Clinic, Rochester MN (93 patients), Neuroimmunology Group, Nuffield Department of Clinical Neurosciences, Oxford, UK (39 patients); and McGill University, Montreal, Canada (14 patients). For longitudinal analysis of antibody titers, 9 serum samples taken over 5 years from a single patient with NMO were analyzed.
All samples were submitted to McGill University, aliquoted, recoded, and returned frozen to the other 2 centers. Testing at Mayo Clinic included a tissue-based indirect immunofluorescence (IIF) assay for NMO-IgG,3 ELISA-R (provided by RSR/Kronus, Ltd.; manufacturer's recommended level for seropositivity, 5 U/mL or greater), and GFP-AQP4 fluorescence immunoprecipitation assay (FIPA-M).13,18 Testing at Oxford included a fluorescence immunoprecipitation assay (FIPA-O),11 visual fluorescence-observation cell-based assay (CBA-O),11,18 and a new quantitative flow cytometry (fluorescence-activated cell sorting [FACS]) assay. The results were sent to the McGill University coauthor (A.B-O.) for decoding. Subsequently, both laboratories performed, according to the manufacturer's instructions (EUROIMMUN), a visual fluorescence-observation cell-based assay (CBA-E) that incorporated fixed HEK293 cells transfected singly with either human AQP4-M1 or M23 isoform.15 Details of published methods are found in appendix e-1 on the Neurology® Web site at www.neurology.org.
Flow cytometry.
Thirty-six hours after HEK293 cells were transfected with a plasmid encoding both human AQP4 and the fluorescent protein dsRed, the cells were trypsinized, resuspended in Dulbecco's modified Eagle's medium, 1% fetal calf serum, and 1 mM EDTA (FACS buffer) at 1.0 × 106 cells/mL, and rotated at 4°C for 2 hours. Patient serum (diluted 1:10 in FACS buffer) was mixed with 1.0 × 105 cells (100 μL). After holding at 4°C for 15 minutes, the cells were washed, and bound IgG was detected with Alexa 488–labeled antihuman IgG (diluted 1:500 in FACS buffer). The cells were resuspended 30 minutes later in 400 μL phosphate-buffered saline/5 mM EDTA and analyzed by FACSCalibur. The level of transfection was determined by measuring dsRed intensity (PE-Texas Red channel) in live cells (figure e-1, y-axis). Two gates were created: the higher gate captured cells expressing high levels of dsRed (labeled R5 in figure e-1); the lower gate captured untransfected or poorly transfected cells (labeled as R7 in figure e-1) and served as a negative control for each sample. Bound IgG was measured in the green channel (a shift to the right on the x-axis). A score for each serum was determined by subtracting the median green fluorescence in the lower gate from the median green fluorescence in the higher gate.
Statistical analyses.
We used receiver operating characteristic curve (ROC) analysis to identify post hoc the optimal cutoff values for the ELISA-R assay to maximize disease sensitivity and specificity, and analyzed all data using SAS, version 9.1. The McNemar test for paired proportions was used to compare the sensitivities of the FACS, CBA-Oxford, and ELISA-R, with Bonferroni correction for multiple comparisons.
RESULTS
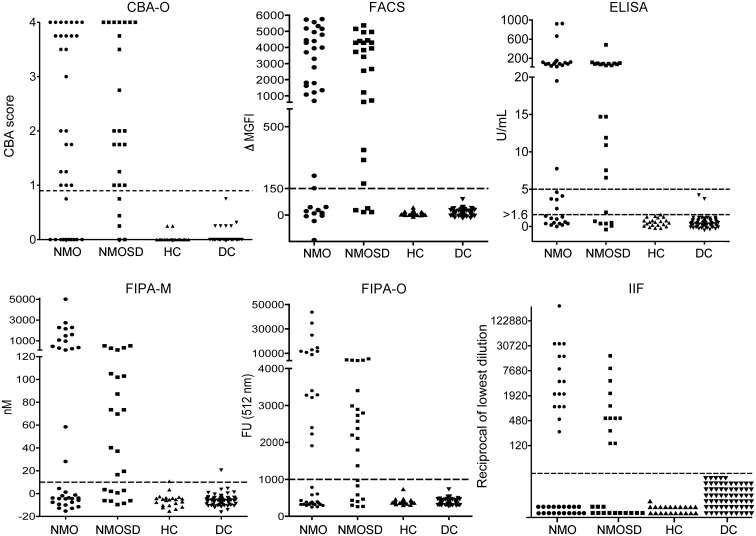
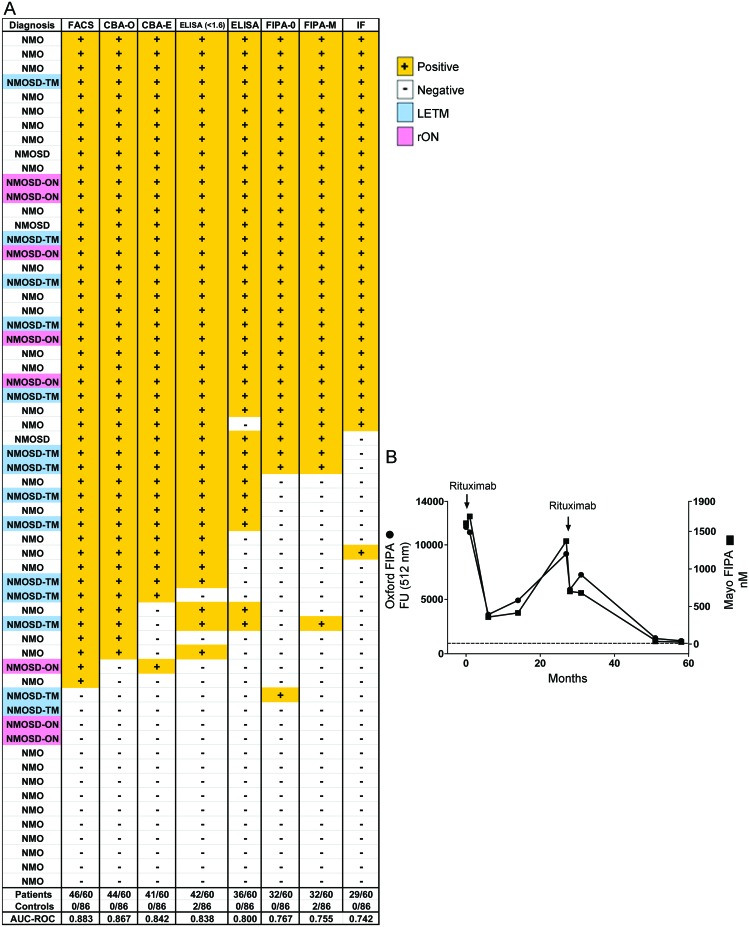
Assays based on binding of IgG to HEK293 cells transfected with AQP4 proved to be the most sensitive (figures 1 and 2A): FACS (77%; 46 of 60), CBA-O (73%; 44 of 60), and CBA-E (68%; 41 of 60). The ELISA-R was the next most sensitive (60%: 36 of 60). FIPAs and the tissue-based IIF assay were least sensitive (48%–53%) (table 1, figure 2A). The 2 commercial assays combined (ELISA-R and CBA-E) identified 72% (43 of 60) of NMO/NMOSD samples as positive and no false positives (table 2). Interassay concordance overall was excellent (figure 2A); 28 of 60 samples were positive in all assays, and only 2 false-positive results were encountered (FIPA-M).
Figure 1. Distribution of neuromyelitis optica (NMO)/aquaporin-4 (AQP4)-immunoglobulin G (IgG) titers by 6 assays in serum samples of patients and controls.
Scatterplots of the 6 assays with cutoffs shown as dotted lines. The ELISA-R has 2 cutoffs, the recommended cutoff (5 U/mL) and a modified cutoff (1.6 U/mL). CBA = cell-based assay; DC = disease control; FACS = fluorescence-activated cell sorting; FIPA = fluorescence immunoprecipitation assay; FU = fluorescence units; HC = healthy controls; IIF = indirect immunofluorescence; M = Mayo Clinic; ΔMGFI = difference in median green fluorescence intensity; NMOSD = neuromyelitis optica spectrum disorder; O = Oxford.
Figure 2. Concordance of assays in neuromyelitis optica (NMO) and NMO spectrum disorder (NMOSD) cohort.
Positive samples are marked + with an orange background. Negative samples are marked – with a white background. Longitudinally extensive transverse myelitis (LETM, TM) samples are highlighted in blue; recurrent optic neuritis (rON, ON) in pink. (B) The results from the fluorescence immunoprecipitation assays (FIPAs), performed in both centers on 9 samples from a single patient plotted against time, demonstrate the consistency of this simple assay to monitor serial samples. AUC = area under the curve; CBA = cell-based assay; E = EUROIMMUN; FACS = fluorescence-activated cell sorting; FU = fluorescence units; IF = immunofluorescence; M = Mayo Clinic; O = Oxford; ROC = receiver operating characteristic curve.
Table 1.
Sensitivity and specificity of 6 aquaporin-4-IgG assaysa
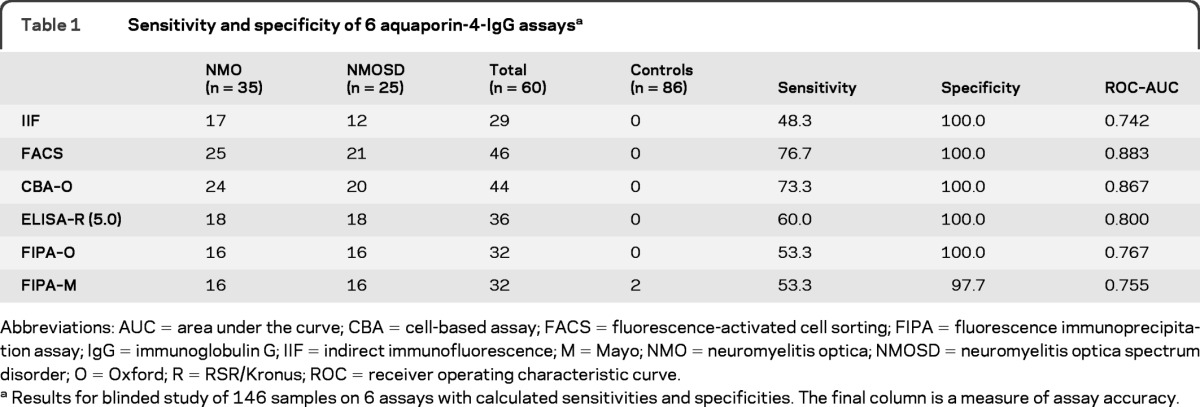
Abbreviations: AUC = area under the curve; CBA = cell-based assay; FACS = fluorescence-activated cell sorting; FIPA = fluorescence immunoprecipitation assay; IgG = immunoglobulin G; IIF = indirect immunofluorescence; M = Mayo; NMO = neuromyelitis optica; NMOSD = neuromyelitis optica spectrum disorder; O = Oxford; R = RSR/Kronus; ROC = receiver operating characteristic curve
Results for blinded study of 146 samples on 6 assays with calculated sensitivities and specificities. The final column is a measure of assay accuracy.
Table 2.
Post hoc assay results: results with CBA-E and ELISA-Ra
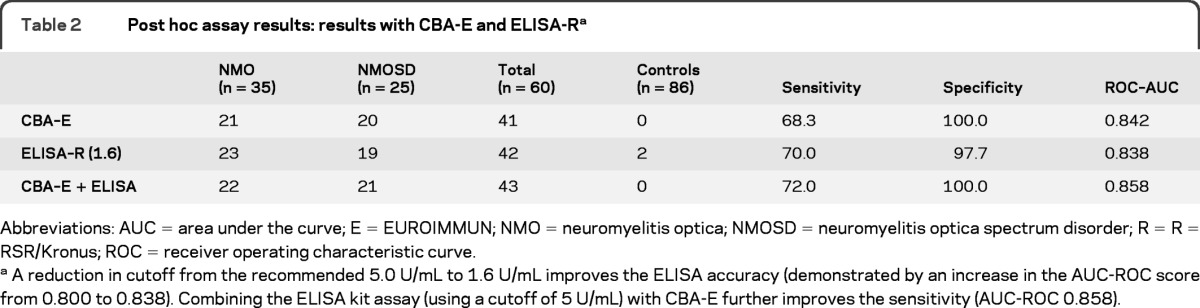
Abbreviations: AUC = area under the curve; E = EUROIMMUN; NMO = neuromyelitis optica; NMOSD = neuromyelitis optica spectrum disorder; R = R = RSR/Kronus; ROC = receiver operating characteristic curve
A reduction in cutoff from the recommended 5.0 U/mL to 1.6 U/mL improves the ELISA accuracy (demonstrated by an increase in the AUC-ROC score from 0.800 to 0.838). Combining the ELISA kit assay (using a cutoff of 5 U/mL) with CBA-E further improves the sensitivity (AUC-ROC 0.858).
A post hoc ROC analysis of ELISA-R raw data revealed that by lowering the cutoff value from 5.0 U/mL to 1.6 U/mL, the sensitivity could be increased from 60% to 70%. An additional 6 of 60 patients with NMO or NMOSD were then scored as positive (figure 2A, table 2). All 6 were positive by FACS and CBA-O with 5 positive by CBA-E; however, there were also 2 false-positive results (1 healthy control and 1 patient with relapsing-remitting MS). Both of these latter false-positive samples tested negative on both cell-based assays.
As a diagnostic assay, the FIPAs were not very sensitive, but they were found to be suitable for serial determinations (figure 2B). Nine sera samples from a single patient taken over 5 years were also tested blinded. The FIPAs showed striking reproducibility between the 2 testing laboratories and a positive correlation between antibody level and treatment response. AQP4-IgG values fell after a single infusion of rituximab (Rituxan) and slowly increased in the following 20 months. A second infusion of rituximab (Rituxan) reduced the AQP4-IgG to an almost undetectable level.
DISCUSSION
Sensitive and specific detection of NMO-IgG (AQP4-IgG) has become an essential laboratory investigation in evaluating patients with inflammatory CNS demyelinating disorders, because seropositivity has diagnostic, prognostic, and therapeutic implications. Several different techniques have been used to detect these autoantibodies, and no previous international multicenter comparative study has been performed. In this fully blinded study, we assessed different assays, including a novel in-house FACS method and 2 recent commercially available kit assays (ELISA-R and CBA-E).
The in-house FACS and CBA-O assay proved to be the most sensitive. There was a significant difference in the sensitivity between these assays and the ELISA-R. However, reducing the recommended cutoff for the ELISA-R from 5.0 U/mL to 1.6 U/mL eliminated these significant differences (table 3) but slightly reduced the ELISA-R specificity. The tissue-based IIF assay and FIPA were not optimal for diagnostic assays because of lower sensitivities (48%–53%).
Table 3.
Improvement in ELISA sensitivitya
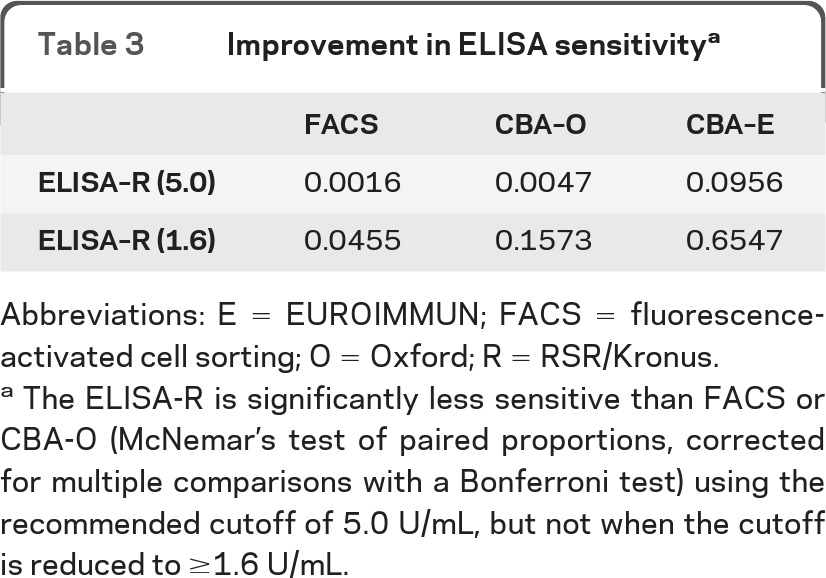
Abbreviations: E = EUROIMMUN; FACS = fluorescence-activated cell sorting; O = Oxford; R = RSR/Kronus
The ELISA-R is significantly less sensitive than FACS or CBA-O (McNemar's test of paired proportions, corrected for multiple comparisons with a Bonferroni test) using the recommended cutoff of 5.0 U/mL, but not when the cutoff is reduced to ≥1.6 U/mL.
The demand for AQP4-IgG testing is increasing globally. In the past 12 months, the Mayo Clinic's Neuroimmunology Laboratory tested 20,334 patients' sera for AQP4-IgG on a service basis and the Oxford laboratory tested 3,500. The expertise and resources required to perform flow cytometry assays preclude its use in small-scale clinical diagnostic laboratories. This study affirms that commercially available kit assays (ELISA-R and CBA-E) are both sensitive and specific for AQP4-IgG detection. Their relative simplicity to perform, minimal reagent requirement, and amenability to currently available automation platforms allow nonspecialized laboratories to offer sensitive and specific AQP4-IgG testing. This study suggests that CBA-E could be used as a convenient routine assay. The ELISA-R, with the lower cutoff value of 1.6 U/mL, may be a sensitive screening tool, but sera yielding values between 1.6 and 4.9 U/mL would require confirmatory specificity testing by CBA-E.
Assay refinement has reduced the frequency of NMO-IgG seronegativity. It is important to determine whether IgG of a different CNS antigen specificity might account for patients with NMOSD who lack detectable AQP4-IgG. An essential prerequisite is to maximize AQP4-IgG assay sensitivity to exclude false negatives. The FACS assay appears to be the most useful in this research context, whereas the FIPA may be more convenient for monitoring patients longitudinally. Functional assays are promising for yielding data to correlate with disease severity and possibly predict relapse.19
Supplementary Material
ACKNOWLEDGMENT
The authors thank John Schmeling for performing Mayo Clinic assays and for assistance with data analysis, Kevin Clark for advice with the flow cytometry (WIMM, Oxford), Dr. Klaus-Peter Wandinger and Dr. Christian Probst (EUROIMMUN) for provision of EUROIMMUN CBA slides, and RSR/Kronus for providing AQP4 ELISA-R kits.
GLOSSARY
- AQP4
aquaporin-4
- CBA
cell-based assay
- E
EUROIMMUN
- FACS
fluorescence-activated cell sorting
- FIPA
fluorescence immunoprecipitation assay
- IgG
immunoglobulin G
- IIF
indirect immunofluorescence
- M
Mayo
- MS
multiple sclerosis
- NMO
neuromyelitis optica
- NMOSD
neuromyelitis optica spectrum disorder
- O
Oxford
- R
RSR/Kronus
- ROC
receiver operating characteristic curve
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Study design and conceptualization: P.J.W., A.B.-O., S.P. Drafting of manuscript: P.J.W., S.J.P. Acquisition, analysis and interpretation of data: P.J.W., A.M., M.I.L., S.R., V.A.L, A.V., J.P., J.N.M., A.Villalobos, A.B.-O., S.J.P. Statistical analysis: P.J.W., J.N.M., S.J.P. Critical revision of the manuscript: P.J.W., A.M., M.I.L., S.R., V.A.L., A.Villalobos, J.P., J.N.M., A.Vincent, A.B.-O., S.J.P.
DISCLOSURE
Dr. Waters is a named inventor on a patent relating to assays for the detection of antibodies to Lgi1, Caspr2, and Contactin2 and may receive royalties for this technology. Dr. Waters receives research support from the Oxford NIHR Biomedical Research Centre. Dr. McKeon receives research support from the Guthy Jackson Charitable Foundation. Dr. Leite receives research support from the Oxford Biomedical Research Centre, the National Commissioning Group, and the Sir Halley Stewart Trust, UK. Dr. Rajasekharan reports no disclosures. Dr. Lennon is a named inventor on a patent relating to aquaporin-4 antibodies for diagnosis of neuromyelitis optica and receives royalties for this technology; is a named inventor on patents that relate to functional AQP4/NMO-IgG assays and NMO-IgG as a cancer marker; and receives research support from the Guthy Jackson Charitable Foundation. A. Villalobos reports no disclosures. Dr. Palace serves on scientific advisory boards for Merck Serono, Bayer Schering Pharma, Biogen Idec, Teva Pharmaceutical Industries Ltd., Novartis, and sanofi-aventis; has received funding for travel from Merck Serono; and receives research support from the Multiple Sclerosis Society and the Department of Health Risk Sharing Scheme (Clinical Coordinator). Dr. Mandrekar reports no disclosures. Dr. Vincent is a named inventor on a patent relating to assays for the detection of antibodies to Lgi1, Caspr2, and Contactin2 and may receive royalties for this technology. Dr. Vincent has served on scientific advisory boards for the Patrick Berthoud Trust and the Myasthenia Gravis Foundation of America; has received funding for travel and a speaker honorarium from Baxter International Inc.; serves as an Associate Editor for Brain; receives royalties from the publication of Clinical Neuroimmunology (Blackwell Publishing, 2005) and Inflammatory and Autoimmune Disorders of the Nervous System in Children (Mac Keith Press, 2010); receives research support from the European Union, the Oxford NIHR Biomedical Research Centre, and Sir Halley Stewart Trust; and has received Musk antibody royalties and consulting fees from Athena Diagnostics, Inc. and Musk antibody royalties from RSR Ltd., Cardiff, UK. The University of Oxford, where A.V. is based, receives royalties and payments for antibody assays in neurologic diseases. Dr. Bar-Or serves on scientific advisory boards for BioMS Medical, DioGenix, Inc., Ono Pharmaceutical Co. Ltd., GlaxoSmithKline, Roche, Guthy Jackson Greater Good Foundation, and NMO Research and Clinical Care Consortium; serves on the editorial boards of Neurology® and Clinical and Experimental Neuroimmunology; has received speaker honoraria from Biogen Idec, Bayhill Therapeutics, Bayer Schering Pharma (Berlex), Eli Lilly and Company, Genentech, Inc., GlaxoSmithKline, Merck Serono, Novartis, Wyeth, sanofi-aventis, and Teva Pharmaceutical Industries Ltd.; and receives/has received research support from BioMS Medical, Merck Serono, Bayhill Therapeutics, Biogen Idec, Genentech, Inc., and Teva Pharmaceutical Industries Ltd. Dr. Pittock is a named inventor on patents that relate to functional AQP4/NMO-IgG assays and NMO-IgG as a cancer marker; receives research support from Alexion Pharmaceuticals, Inc., the Guthy Jackson Charitable Foundation, and the NIH.
REFERENCES
- 1. Wingerchuk DM, Lennon VA, Lucchinetti CF, Pittock SJ, Weinshenker BG. The spectrum of neuromyelitis optica. Lancet Neurol 2007;6:805–815 [DOI] [PubMed] [Google Scholar]
- 2. Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology 2006;66:1485–1489 [DOI] [PubMed] [Google Scholar]
- 3. Lennon VA, Wingerchuk DM, Kryzer TJ, et al. A serum autoantibody marker of neuromyelitis optica: distinction from multiple sclerosis. Lancet 2004;364:2106–2112 [DOI] [PubMed] [Google Scholar]
- 4. Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med 2005;202:473–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hinson SR, Pittock SJ, Lucchinetti CF, et al. Pathogenic potential of IgG binding to water channel extracellular domain in neuromyelitis optica. Neurology 2007;69:2221–2231 [DOI] [PubMed] [Google Scholar]
- 6. Hinson SR, Roemer SF, Lucchinetti CF, et al. Aquaporin-4-binding autoantibodies in patients with neuromyelitis optica impair glutamate transport by down-regulating EAAT2. J Exp Med 2008;205:2473–2481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bradl M, Misu T, Takahashi T, et al. Neuromyelitis optica: pathogenicity of patient immunoglobulin in vivo. Ann Neurol 2009;66:630–643 [DOI] [PubMed] [Google Scholar]
- 8. Saadoun S, Waters P, Bell BA, Vincent A, Verkman AS, Papadopoulos MC. Intra-cerebral injection of NMO-IgG and human complement produces neuromyelitis optica lesions in mice. Brain 2010;133:349–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kinoshita M, Nakatsuji Y, Kimura T, et al. Neuromyelitis optica: passive transfer to rats by human immunoglobulin. Biochem Biophys Res Commun 2009;386:623–627 [DOI] [PubMed] [Google Scholar]
- 10. Bennett JL, Lam C, Kalluri SR, et al. Intrathecal pathogenic anti-aquaporin-4 antibodies in early neuromyelitis optica. Ann Neurol 2009;66:617–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Waters P, Jarius S, Littleton E, et al. Aquaporin-4 antibodies in neuromyelitis optica and longitudinally extensive transverse myelitis. Arch Neurol 2008;65:913–919 [DOI] [PubMed] [Google Scholar]
- 12. Jarius S, Franciotta D, Bergamaschi R, et al. NMO-IgG in the diagnosis of neuromyelitis optica. Neurology 2007;68:1076–1077 [DOI] [PubMed] [Google Scholar]
- 13. McKeon A, Fryer J, Apiwattanakul M, et al. Diagnosis of neuromyelitis spectrum disorders: comparative sensitivities and specificities of immunohistochemical and immunoprecipitation assays. Arch Neurol 2009;66:1134–1138 [DOI] [PubMed] [Google Scholar]
- 14. Takahashi T, Fujihara K, Nakashima I, et al. Anti-aquaporin-4 antibody is involved in the pathogenesis of NMO: a study on antibody titre. Brain 2007;130:1235–1243 [DOI] [PubMed] [Google Scholar]
- 15. Jarius S, Probst C, Borowski K, et al. Standardized method for the detection of antibodies to aquaporin-4 based on a highly sensitive immunofluorescence assay employing recombinant target antigen. J Neurol Sci 2010;291:52–56 [DOI] [PubMed] [Google Scholar]
- 16. Hayakawa S, Mori M, Okuta A, et al. Neuromyelitis optica and anti-aquaporin-4 antibodies measured by an enzyme-linked immunosorbent assay. J Neuroimmunol 2008;196:181–187 [DOI] [PubMed] [Google Scholar]
- 17. De Vidi I, Boursier G, Delouche N, et al. Strategy for anti-aquaporin-4 auto-antibody identification and quantification using a new cell-based assay. Clin Immunol 2011;138:239–246 [DOI] [PubMed] [Google Scholar]
- 18. Takahashi T, Fujihara K, Nakashima I, et al. Establishment of a new sensitive assay for anti-human aquaporin-4 antibody in neuromyelitis optica. Tohoku J Exp Med 2006;210:307–13 [DOI] [PubMed] [Google Scholar]
- 19. Hinson SR, McKeon A, Fryer JP, Apiwattanakul M, Lennon VA, Pittock SJ. Prediction of neuromyelitis optica attack severity by quantitation of complement-mediated injury to aquaporin-4-expressing cells. Arch Neurol 2009. September;66:1164–1167 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.