Summary
Aromatase is expressed in multiple tissues, indicating a crucial role for locally produced oestrogens in the differentiation, regulation and normal function of several organs and processes. This review is an overview of the role of aromatase in different tissues under normal physiological conditions and its contribution to the development of some oestrogen-related pathologies.
Keywords: Aromatase, Oestrogen function
Introduction
Oestradiol, the most active member of the group of hormones known as oestrogens, is crucial for the normal function of a broad range of cells and organs. All oestrogens are synthesised from androgen precursors by a unique enzyme called aromatase, more specifically classified as cytochrome P450 aromatase (CYP19a1). Aromatase uses the androgenic substrates androstenedione, testosterone and 16-hydroxytestosterone with high specificity, and converts them to their respective oestrogens: oestrone, oestradiol and oestriol. Because both the substrates and the products of this enzyme are powerful hormones, alterations in its activity have profound effects on oestrogen and androgen physiology.
The expression of aromatase in the ovary plays an important role in the regulation of the reproductive cycle in females. Aromatase is also expressed in the male gonad; however, in contrast to its key role as an endocrine coordinator in females, in males, the paracrine effects of aromatase products are essential for normal spermatogenesis. In both sexes, aromatase is found in a number of extragonadal sites, including bone, breast, adipose tissue and brain. This tissue-specific expression of aromatase maintains tight local control over the synthesis and action of oestrogens. In this context, aromatase converts circulating ‘pro-hormones’, i.e. androgens, into oestrogens, which then act locally to regulate tissue function. Of course, the expression of aromatase would be meaningful only if the appropriate substrates are provided to/by the tissue where the enzyme is expressed. Similarly, local production of estrogens needs to be coupled with the expression of estrogen receptors to make estradiol actions possible.
The focus of this review is to provide an integrated overview of aromatase expression in different tissues and briefly describe the significance of local oestrogen synthesis. Therefore, the tissue-specific mechanisms that control aromatase expression will not be examined. Several excellent reviews on this subject have recently been published [1-4].
1. Ovary
In the ovaries of sexually mature animals, aromatase expression is limited to differentiated preovulatory granulosa cells and luteal cells. Interestingly, these cells represent two different stages of differentiation of a unique cell, the granulosa cell. Undifferentiated granulosa cells of preantral follicles do not express aromatase. The growth and maturation of preantral follicles to the preovulatory stage is accompanied by the differentiation of granulosa cells and the induction of aromatase (Fig. 1A and 1B), both processes which are stimulated by follicle-stimulating hormone (FSH). After ovulation, however, aromatase is rapidly downregulated as granulosa cells differentiate again, this time into luteal cells. Luteal cells regain aromatase expression during the luteal phase in several species, including primates and rodents (Fig. 1C and 1D).
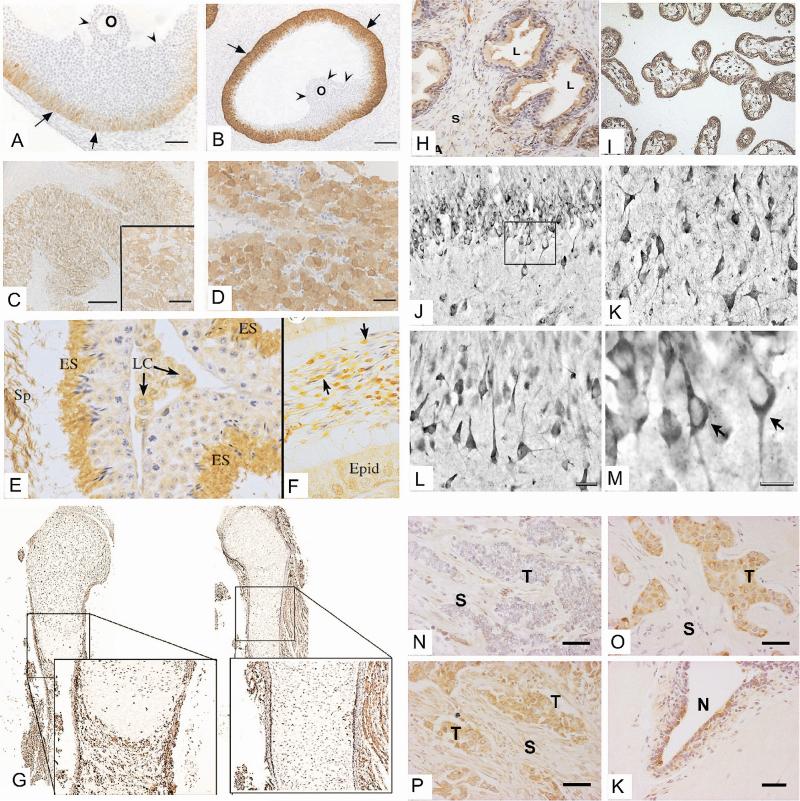
Figure. Tissue expression profile of aromatase.
Ovary: A, B, C, D - [Adapted from 131]. Aromatase is detectable in the ovarian follicles of the rat at dioestrus (A) and pro-oestrus (B). Notice the gradient of expression in the granulosa cell layer. Aromatase is expressed in the human corpus luteum during the later luteal phase (C) and significantly increases after rescue of this gland during early pregnancy by human chorionic gonadotropin (D). O: oocyte. (c) Society for Endocrinology (2002). Reproduced by permission.
Testis: A and F - [Adapted from 40]. Aromatase is expressed in the adult mouse testis (E) and caput epididymis (F). Staining is intense in elongate spermatids (ES) and spermatozoa in the seminiferous tubule lumen (Sp). Germ cells and Sertoli cells show weaker staining. Leydig cells (LC) are also positive in the interstitial space. In the epididymal lumen, aromatase is strongly positive in the cytoplasmic droplet of the sperm tail (arrows). The epididymal epithelium (Epid) may also show some weak staining in some cells. (c) The Royal Society (2010). Reproduced by permission.
Bone: G - [Adapted from 132]. Aromatase expression in the periosteum on tibial sections of control mice at embryonic day 17.5 (Left). Aromatase expression is significantly decreased in mice expressing an inactive truncated form of Runx2 (right). (c) American Society for Microbiology (2010). Reproduced by permission.
Prostate: H - [Adapted from 53]. In the prostate, aromatase immunostaining is found in the cytoplasm of luminal and stromal cells. Strong labelling can be observed at the apex of luminal cells. L, alveolar lumen; S, stromal cells. Copyright (still missing)
Placenta: I - [Adapted from 133]. Immunohistochemical analysis of aromatase protein in human placental tissue sections revealed strong cytoplasmic staining in syncytiotrophoblasts, with adjacent cytotrophoblasts remaining negative. (c) Elsevier (2003). Reproduced by permission.
Brain: J, K, L, M - [Adapted from 66]. Aromatase immunoreactivity in the normal human hippocampus; (J) dentate gyrus showing aromatase immunoreactivity in granule cells. (K) Aromataseimmunoreactive neurons in CA3. (L) Immunoreactivity for aromatase in the stratum pyramidale of CA1. (M) Detail of the boxed area in J showing aromatase-immunoreactive granule cells (arrows) where the immunostaining is restricted to the perikaryon and the initial part of the dendrites. (c) Elsevier (2010). Reproduced by permission.
Breast Cancer: N, O, P, K - [Adapted from 123]. Immunolocalisation of aromatase in breast carcinoma tissues; aromatase immunoreactivity is detectable in the cytoplasm of stromal (S, top left), parenchymal carcinoma cells (T, top right), or both parenchymal and stromal cells (bottom left). Aromatase is also expressed in non-malignant duct epithelial cells (N, bottom right). (c) Elsevier (2007). Reproduced by permission.
In preovulatory follicles, aromatase expression is controlled in a cell-specific, temporal, and spatial manner, resulting in a gradient of expression where aromatase is high in mural cells at the outer edge of healthy follicles, but is absent in cumulus cells [5, 6]. The physiological implication of this differential expression is not yet clear. In cumulus granulosa, aromatase is silenced by oocyte-derived compounds such as bone morphogenetic protein 15 (BMP-15)[7] and growth differentiation factor-9 (GDF9) [8, 9]. Supporting an inhibitory role of the oocyte, GDF9-deficient mice show premature induction of aromatase in preantral follicles [10]. Therefore, the negative effects of oocyte-derived factors and the stimulatory effect of FSH establish the gradient of expression along the granulosa cell layer of preovulatory follicles [reviewed in 11].
Locally produced oestrogens stimulate granulosa cell proliferation and facilitate FSH and LH actions in a paracrine/autocrine manner [12]. One of the main effects of oestradiol is to enhance FSH-induced aromatase expression [13, 14]. This effect of oestradiol was clearly demonstrated in aromatase and oestrogen receptor (ER)β knockout mice. In the aromatase knockout (ArKO), follicle development is arrested at a stage before ovulation and no corpora lutea are formed [15]. Moreover, although ArKO mice contain follicles at all stages of development, antral follicles are abnormal, presenting blood-filled antra and high levels of apoptosis in granulosa cells [16]. In ERβ knockout mice, on the other hand, antral follicles are normal, but aromatase expression is significantly decreased [17]. In the ovary, the substrate for aromatase is produced by the theca cells of the follicle. Theca cell–derived androgens are also directly involved in the regulation of this enzyme. Thus, it has been recently demonstrated that testosterone stimulates aromatase expression in the absence of FSH, an effect that does not depend on aromatisation [18].
On the other hand, the timely expression of aromatase in the ovarian follicle is also responsible for the cyclic changes in serum oestradiol levels. These changes modulate the structure and function of the female reproductive tract, including the oviducts, the uterus and the vagina. These endocrine actions are essential for oocyte survival, fertilisation and implantation [19]. Fluctuations in circulating oestradiol also establish the characteristic female pattern of gonadotropin secretion from the pituitary gland. More importantly, the strong expression of aromatase in preovulatory follicles leads to the generation of the surge of luteinising hormone (LH), which triggers ovulation [20]. Therefore, the well-timed and cell-specific expression of aromatase in the ovary is crucial for the autocrine regulation of folliculogenesis, the endocrine control of the female reproductive tract and the coordination of gonadotropin secretion.
2. Placenta
In addition to the ovary, aromatase is highly expressed in the placenta of humans and non-human primates [21]. In fact, the foetal-placental unit becomes the primary source of oestrogen production during pregnancy making ovarian steroidogenesis unnecessary. The placental precursor cells, i.e. trophoblast and cytotrophoblast cells, do not express aromatase. The syncytiotrophoblast formed from the fusion of cytotrophoblast cells expresses aromatase and becomes a highly active endocrine gland (Fig. 1I). A key and immediate endocrine role of the syncytiotrophoblast is the secretion of human chorionic gonadotropin (hCG), which maintains luteal oestrogen and progesterone production during early pregnancy [22]. Approximately two months into pregnancy, the placenta takes over steroid production. The increase in oestrogen synthesis in the placenta is due to the activation of the promoter I.1 of the aromatase gene [23].
Similar to the ovarian granulosa, oestrogen synthesis by the syncytiotrophoblast requires an external source of androgens, which in this case is provided by the adrenal gland of the foetus. Thus, exclusively in primates, a joint venture is formed between the foetal adrenal gland and the placenta to produce extremely high levels of oestrogens. The foetal adrenal gland produces dehydroepiandrosterone (DHEA) and DHEA-sulphate (DHEAS) abundantly [reviewed in 24]. DHEAS is either 16α-hydroxylated in the foetal liver before being converted to oestriol, or directly converted to oestrone and oestradiol in the placenta [25]. As such, the foetal-placental unit produces 90% of the oestriol and about half of the oestrone and oestradiol present in maternal circulation.
The expression of aromatase seems to be essential for the functional differentiation of the syncytiotrophoblast. Thus, in the baboon, serum oestradiol levels during pregnancy are associated with a corresponding rise in low-density lipoprotein uptake and P450 side chain cleavage expression in the syncytiotrophoblast. These effects of locally produced oestrogens ensure high levels of progesterone biosynthesis, which are needed for the maintenance of pregnancy. Placental oestrogens also stimulate the expression of 11β-hydroxysteroid dehydrogenase, which is necessary for the maturation of the foetal hypothalamic-pituitary-adrenocortical axis. Similar effects have been described in human syncytiotrophoblast cells in vitro [see 22 for review]. Finally, placental oestrogens enhance uteroplacental blood flow and placental neovascularisation to provide the optimal exchange of gases and nutrients required for the rapidly developing foetus [26].
This evidence shows that aromatase expression in the placenta is important for placental growth, implantation and embryo development. In baboons, these effects are evidenced by foetal loss resulting from exposure to aromatase inhibitors [27]. However, despite very low levels of oestrogen, pregnancy is not interrupted in all animals treated with aromatase inhibitors, suggesting that placental aromatase is not essential for the development of the foetus. This conclusion is supported by successful pregnancies in women with unusually low oestrogen levels as a result of sulphatase or aromatase deficiencies [28, 29].
Placental aromatase is critical, however, for the differentiation of the female external genitalia, because it protects the foetus from the virilisating effect of foetal androgens. Placental aromatisation also prevents the build-up of high levels of androgens in maternal circulation. Thus, in mothers carrying foetuses with congenital aromatase deficiency, the overload of androgens causes maternal androgenisation and hirsutism; however, these symptoms rapidly disappear after delivery [30]. In contrast, female foetuses exposed to excessive androgens during development have impaired differentiation and permanent defects in external genitalia. Thus, aromatase-deficient newborn females display various degrees of external genital ambiguity and pseudohermaphroditism [29]. In contrast, aromatase-deficient newborn males do not show obvious defects at birth and are diagnosed much later in life due to skeletal abnormalities (see below).
Although high levels of androgens do not affect ovarian development, ovarian physiology seems to be affected after birth and during adulthood. As early as the first or second year after birth and in the prepubertal years, aromatase-deficient girls develop ovarian cysts similar to those found in polycystic ovarian syndrome [31]. The formation of these cysts seems to be facilitated by the high levels of androgens and gonadotropins, particularly FSH, found in these patients [29].
The presence of aromatase in the placenta seems to be limited to primate and ungulate (hoofed) species [32]. Although, aromatase mRNA has been detected in mouse placenta [33]. Interestingly, aromatase has recently been shown to be expressed in the decidua of mice [34], suggesting that the intrauterine production of oestrogens is also important for normal implantation and foetus development in rodents. Similarly, to primates, aromatase expression in mice decidua seems to be critical for uterine differentiation and angiogenesis during early pregnancy [34].
3. Testis
In males, oestradiol is present at low concentrations in blood, but it is extraordinarily high in semen, where its concentration could be higher than in the serum of females [35]. Testes produce significant quantities of oestradiol attributable to the expression of aromatase in several cell types. In rodents, aromatase activity is high in Sertoli cells before sexual maturity, but becomes more prominent in Leydig cells is adult animals [36]. In humans, both Leydig and Sertoli cells produce oestrogens in vitro [37]. Aromatase activity has also been demonstrated in spermatogenic cells of several species, including humans (Fig. 1E) [38]. Moreover, spermatozoa express aromatase and actively synthesise oestrogens within the lumen of the epididymis [39]. Therefore, the same sperm serve as the source of oestrogens that target oestrogen receptors present in efferent ductules and epididymal epithelia. In aromatase-null males, testicular morphology appears to be normal in young animals, but age-dependent disruption of spermatogenesis, spermatid degeneration, reduction in testis weight, and hypertrophy of Leydig cells have been described, indicating a crucial role of aromatase in normal spermatogenesis [for review see 40].
Several studies have clearly established an association between aromatase and sperm count and motility. Lower sperm concentration and motility have been associated with aromatase polymorphisms causing low enzyme activity in normozoospermic men [41]. Decreased sperm motility is common in aromatase-deficient men and in aromatase knockout mice [42]. Additionally, spermatozoa of asthenospermic, teratospermic and asthenoteratospermic patients exhibit decreased aromatase expression [43]. In aromatase-deficient adult men, spermatogenesis varies from normal to highly reduced (hypospermatogenesis) [44]. In fact, it has been proposed that the quantification of aromatase in ejaculated sperm could be used as an indicative parameter of sperm function and spermiogenesis to evaluate male infertility [37, 43]. More strikingly, in ejaculated spermatozoa, aromatase localises to the midpiece, the tail, and the annular region located at the limit between the acrosomal membrane and the nucleus [38, 43]. More importantly, aromatase in human spermatozoa is still active after ejaculation [45]. The dual localisation of aromatase at the acrosomal membrane and the midpiece, along with the presence of oestrogen receptors in sperm [46], provides evidence for a potential role of oestradiol in energy production, motility, capacitation and acrosomal reaction [40]. Because of the transcriptionally inactive chromatin of the spermatozoa, oestrogens may regulate its function via rapid non-genomic effects [38].
Oestrogens play an important role in epididymal function and sperm maturation [35, 47, 48]. Not surprisingly, aromatase is also expressed in the epithelial cells of efferent ducts and in the proximal caput epididymis (Fig. 1F) [49, 50]. Therefore, in the epididymis there are multiple sources of oestrogen, including the epididymis itself, as well as the sperm, which function as a mobile source of oestrogens.
In conclusion, in contrast to the restricted expression of aromatase in the ovary, where it is found only in granulosa and luteal cells; this enzyme is widely expressed in the testis and accessory glands. The wide distribution of aromatase in the male gonads is essential to maintain the high levels of oestradiol needed for normal spermiogenesis, sperm maturation, sperm motility and possibly acrosomal reaction. More detail on the role of oestrogens in the regulation of the male gonad can be found in recent reviews [38, 40].
4. Prostate
Undoubtedly, androgens play a critical role in normal growth, development and maintenance of the prostate. The prostate, however, also expresses aromatase [51, 52]. In the human prostate, for instance, aromatase immunoreactivity has been found in the cytoplasm of luminal cells as well as stromal cells (Fig. 1H) [53], although there is some controversy regarding its presence in luminal/epithelial cells [54]. These findings indicate that locally produced oestrogens are involved in the regulation of this gland. In fact, it has been demonstrated that adequate testosterone:oestradiol (T:E) ratios are necessary for prostate normal function, whereas high T:E ratios lead to hypertrophy and hyperplasia of the prostate [reviewed in 55]. These findings are supported by the fact that ArKO mice develop non-malignant prostate hyperplasia in adult life [56]. On the other hand, increased local production of oestradiol is common in malignant disorders of the prostate [54, 57]. High levels of oestrogen and aberrant ER signalling promote inflammation, which has been implicated in the development of prostate cancer (PCa), one of the most common diseases affecting males [58].
Normal prostate stromal tissue expresses aromatase via promoter II (PII), but in PCa there is an induction of epithelial expression of aromatase due to the activation of alternative promoters [54]. Aromatase expression in the stroma of the normal prostate results in the autocrine activation of ER and the paracrine activation of ER and ER in epithelial cells. Both actions are needed for the normal function of the prostate. The appearance of aromatase in epithelial cells leads to a disequilibrium of these actions and the development of inflammation and malignancy [for a detailed discussion of the role of oestrogen in development of prostate cancer see 55].
5. Brain
After the identification and isolation of gonadal steroid hormones in the first half of the 20th century, one of the most intriguing findings was the fact that the ‘typical female hormone’ oestradiol could trigger copulatory behaviour in male rats [for a review see 59]. One possible answer to this baffling observation was provided by the discovery of aromatase expression in the brain of rats [60]. These findings led to the hypothesis that, in males, testosterone is a ‘pro-hormone’ that is metabolised in the brain into oestradiol, which in turn regulates male behaviour. This conclusion was further supported by the following findings: i) blocking aromatase activity blocks the male-typical sexual behaviour and ii) administration of oestrogens directly into the brain can set in motion male-typical sexual behaviour in castrated animals [for review see 61]. It is now well recognised that the local production of oestradiol in the brain plays a pivotal roles in the gender-specific brain development of most vertebrates, and participates in numerous functions of the adult central nervous system such as gonadotropin secretion, neural plasticity, response to injury and synapses [62].
Aromatase has been found in the brain in all vertebrates, from fish to primates. Aromatase was first found to be highly expressed in brain regions involved in reproductive functions, such as the hypothalamus [60]. Aromatase activity is highest within specific subregions of the neuroendocrine brain, which include the nucleus of the posteromedial amygdala, encapsulated region of the bed nucleus of the stria terminalis (BNST), ventrolateral portion of the ventromedial hypothalamic nucleus and central component of the medial preoptic nucleus [63]. These endocrine neural circuits contain an overlapping distribution of cells containing androgen- and oestrogen- receptors [64]. In addition, the hippocampus, regions of the cerebral cortex, midbrain, spinal cord and cerebellum also express aromatase [63]. In primates, aromatase has been found in CA1-3 pyramidal cells of the hippocampus, as well as in pyramidal cells in the neocortex (Fig. 1J-M) [65, 66]. A summary of the pattern of aromatase expression in the human brain with particular emphasis on non-primary reproductive areas was recently published [67].
In non-human primates, as well as several other vertebrate species, aromatase protein is located in both neuronal cell bodies and pre-synaptic terminals. This later localisation suggests that aromatase-generated oestrogens can also act in a paracrine manner on adjacent cells. In fact, a putative role for oestradiol as a neurotransmitter has been proposed [see review in 68]. Aromatase has also been shown to be expressed in cortical astrocytes in response to brain injury [69]. The prevalent expression of aromatase in the brain emphasises the importance of the local production of oestrogen in not only the regulation of reproductive processes, but also in other neural functions such as behaviour, cognition, memory and recovery from injury. We will briefly describe some of these functions. We will not describe the complex role that oestrogens have in the regulation brain function: Several reviews in this area have been recently published [62, 63, 70].
Reproductive effects
Oestradiol secretion by ovarian granulosa cells plays a key role in the cyclic and biphasic regulation of gonadotropin levels in females [71]. In males, gonadotropin secretion is controlled by testis-derived testosterone [72]. Interestingly, in primates, inhibition of aromatase activity results in a significant increase in both LH and FSH [72, 73]. Accordingly, aromatase-deficient men [74] and mice [15] exhibit elevated levels of gonadotropins despite high circulating testosterone. Moreover, in monkeys, non-aromatisable androgens are ineffective in decreasing LH after castration [72]. These findings suggest that in some species aromatase is an important component of the negative feedback loop controlling gonadotropin secretion. However, there are significant discrepancies on the relative role of testosterone and oestradiol in the regulation of gonadotropins between species [75 and references therein].
In brain areas involved in the regulation of reproduction, the expression of aromatase is hormonally regulated. In rodents, for instance, aromatase in the hypothalamus decreases after gonadectomy but remains unchanged in the limbic system [76]. This observation suggests that there are at least two populations of aromatase-positive cells in the adult brain: a steroid-dependent and steroid-independent population. In fact, similar to ovarian granulosa cells [18], in the brain, androgens are not only used as raw material for oestrogen synthesis, but also exert stimulatory effects on aromatase expression [72].
Behaviour
Aromatase also participates in the sexual differentiation of the brain. In mammals, sex differences in brain structure and function are programmed by exposure to testosterone during a critical period in perinatal development [77]. In this context, it is known that the brain develops as male when exposed to testosterone produced by the developing testis and as female in the absence of such exposure [78]. Paradoxically, early observations showed that oestradiol is as effective as testosterone in the masculinisation of rat behaviour, whereas non-aromatisable androgens cannot replace testosterone [79] suggesting that oestrogen biosynthesis in the brain is necessary for normal sexual behaviour in males [61, 70]. Accordingly, most sexually dimorphic areas of the brain, especially in the rat, contain substantial levels of both aromatase and oestrogen receptors [80]. Moreover, male copulatory behaviour is severely impaired in male ArKO mice, a defect that cannot be corrected by testosterone alone but the combined treatment with oestradiol and dihydrotestosterone almost completely restored copulatory behaviour [81]. Species differences, however, may exist in the extent to which neural aromatisation is required to maintain male sexual behaviour; for instance, men with congenital aromatase deficiencies are uniformly heterosexual [for review see 80]. On the other hand, estradiol may directly contribute to female-typical sexual differentiation. Hence, ArKO females show less lordosis behaviour and little motivation to investigate olfactory and visual cues from either an oestrous female or an intact male [70, 82].
Estradiol is may also be involved in the regulation of compulsive behaviour in males. For instance, only male ArKO mice develop obsessive-compulsive-like behaviour including excessive barbering, wheel-running and grooming activities [83]. This role of estradiol in supported by evidence showing that male but not female ArKO mice display apoptosis in the medial preoptic area that is involved in the regulation of grooming in mice [84].
Neuroprotection
An increase in aromatase expression is part of the program triggered by neural tissue to cope with neurodegenerative insults. Elevated local oestrogen levels interfere with apoptotic pathways and ultimately decrease the extent of brain damage. There is also evidence suggesting that aromatisation may stimulate cytogenesis. Thus, aromatisation may provide neuroprotection by decreasing degeneration and stimulating repair [for review see 85]. In contrast to the neuroendocrine roles of aromatase in neurons, protective effects are mediated by glial cells (mainly astrocytes), which start expressing aromatase around the site of brain damage [86]. Thus, brain damage stimulates aromatase expression in astrocytes of many brain areas, including the hippocampus, striatum, cortex and corpus callosum [69, 87]. Testosterone also stimulates neuronal survival [88]; however, the nonaromatisable androgen, DHT, has no effect on reactive astrogliosis, suggesting that the role of testosterone in regulating reactive astrogliosis is due to the conversion to oestradiol by aromatase [89]. This conclusion is further supported by the observation that mice null for the aromatase gene are more vulnerable to excitotoxic brain damage than wild-type animals [90].
6. Bones
Androgens as well as oestrogens are important for the maintenance of the skeleton in both sexes [91]. Whereas both androgens and oestrogens can be taken from the circulation, oestrogens are also produced locally in bone due to the expression of aromatase (Fig. 1G) [92]. In fact, aromatisable androgens are more effective in preventing bone loss in gonadectomised rats than non-aromatisable androgens [review in 93]. Therefore, it is accepted now that androgens exert a dual action on bone: a direct one via androgen receptor activation and an indirect one involving the activation of oestrogen receptors after aromatisation [94].
The important role of aromatase in bone physiology is clearly shown in clinical studies of men with aromatase mutation or in aromatase-deficient mice. In both cases, the lack of adequate aromatase activity results in reduced bone density [95-97]. In fact, skeletal abnormalities including tall stature and unfused epiphysis are usually the first symptoms observed in aromatase-deficient men [29]. Moreover, aromatase transcript levels are lower in bone and osteoblast cultures from patients with osteoarthritis than in healthy patients [98]. Interestingly, mice show sexually dimorphic difference by which aromatase deficiency resulted in loss of bone density; in males, aromatase deficiency leads to suppression of bone formation whereas female ArKO mice show and increase in bone turnover [99].
Transgenic mice expressing high levels of aromatase in osteoblast cells further support a role for aromatase in bone growth. In these animals, high local oestrogen concentration increases bone mass without augmenting serum oestradiol levels [100]. These studies suggest that reduced expression of aromatase in bone could facilitate the development of bone pathophysiology, including osteoarthritis.
Increased bone resorption has been observed in postmenopausal women receiving aromatase inhibitors [101], suggesting that low residual oestrogen levels, produced locally or provided by the adipose tissue (see below), are important to maintain normal bone function and structure. Accordingly, aromatase inhibitor therapy of cancer patients represents an important clinical challenge because of the associated reduction in bone mineral density and increased fracture rates [102].
7. Adipose tissue and Metabolism
The main site of oestrogen biosynthesis in the non-pregnant premenopausal woman is the ovarian granulosa cells; however, the adipose tissue becomes a major source of circulating oestradiol in postmenopausal women. After menopause, androstenedione, secreted by the adrenal gland, is converted into oestrone in the adipose tissue [103]. Oestrone is then eventually converted to oestradiol by 17 -HSD enzymes present in peripheral tissues [104]. Pioneering studies in the 1970s showed that conversion of plasma androstenedione to oestrone increases with excess body weight in both pre- and postmenopausal women [105 and reference therein]. Accordingly, aromatase activity has been detected in stromal cells of human subcutaneous adipose tissue [106]. In these cells, aromatase activity is stimulated by glucocorticoids, cytokines and tumour necrosis factor (TNF)-α via activation of a distal promoter I.4 [reviewed in 107]. Recent evidence indicates that aromatase is also expressed in the adipose tissue of mice via activation of promoter I.4 [33, 108].
In humans, it has been clearly shown that the activity of aromatase in subcutaneous adipose stromal cells, as well as aromatase mRNA levels in the adipose tissue of the buttocks, thighs, and abdomen increase with advancing age [109, 110]. These results led researchers to propose that the increase in aromatase expression in fat produces sufficient quantities of oestrogen to prevent bone loss associated with aging (see above) or to contribute to endometrial hyperplasia or breast cancer in women (see below).
Oestrogens play an important role in lipid metabolism. It is well documented that there are sex differences in the pathophysiology of obesity, metabolic disorders, body fat distribution and the prevalence of early insulin resistance and glucose intolerance. The metabolic effects of oestrogen are mostly mediated via the regulation of glucose homeostasis in the skeletal muscle and liver, via modulating insulin production in pancreatic β-cells and via regulation of energy balance at the hypothalamus, where it decreases food consumption and increases activity and energy expenditure [for review see 111]. Not surprisingly, aromatase knockout mice develop an age-dependent increase in intra-abdominal adiposity, which is associated with hyperplasia and hypertrophy of adipocytes and a decrease in lean mass [112, 113]. These animals also develop hypercholesterolaemia, hyperleptinaemia and insulin resistance. Body fat is also increased in ER knockout mice [114]. In humans, aromatase deficiency often leads to impaired lipid metabolism, insulin resistance and type II diabetes; these conditions are usually improved by oestrogen treatment [for review see 115].
Oestradiol plays an important physiological role in regulating lipid metabolism directly in adipose tissue. Thus, oestrogens stimulate adipose tissue lipolysis via the activation of hormone-sensitive lipase and reduction of lipogenesis by decreasing activity of lipoprotein lipase [reviewed in 111]. Oestradiol also increases the proliferation of adipocyte precursors [116], suggesting the anti-lipogenic and prolipolytic action of oestrogens in adipose tissue, which may decrease fat accumulation. However, the role of locally produced oestrogens in the regulation of body fat is not clear. For example, after ovariectomy in rats [117] or menopause in women [109], there is an increase in abdominal fat content despite the increase in aromatase expression in the same tissue. This observation suggests that local production of oestrogen may not be enough to decrease fat accumulation. However, the expression of aromatase makes the adipose tissue an important source of oestrogens, which play a role in cancer initiation. In fact, oestrogens have long been considered to be a major link between body fat and tumour induction and progression [110, 118, 119].
8. Breast Cancer and Endometriosis
Oestrogens are crucial for the normal development and maintenance of the breasts and the uterus. Unfortunately, oestrogens can also be harmful due to their ability to promote cell proliferation, which may also increase a woman's chance of developing breast and uterine cancer [1], as well as endometriosis [120]. Because of the vast literature on oestrogens and cancer biology [for review see 4, 121], concepts suggesting a role for aromatase in cancer development will be described only briefly.
Breast cancer
As in adipose tissue, stromal cells of disease-free breast express low levels of aromatase. However, malignant breast cancers produce large amounts of oestrogens due to high levels of aromatase [4]. This increase in aromatase expression correlates with a switch in the promoter used to drive the aromatase gene. In normal conditions, the low activity of the distal promoter I.4 maintains expression to a minimum; in cancerous tissue the activation of the strong ovarian proximal promoter I.3 and II significantly increases the expression of this gene [122].
Aromatase has been clearly localised to both the malignant epithelial cells and surrounding fibroblasts in breast tumour tissues (Fig. 1N-K) [123]. Although there is some controversy as to whether adipocytes cells also contribute to oestrogen production, the fibroblast layer surrounding breast tumours accounts for the majority of aromatase expression [123]. The activation of the Cyp19 gene in fibroblasts was proposed to be due to the secretion of prostaglandin E2 (PGE2) from malignant epithelial cells [4]; however, proof of this mechanism in vivo has not been provided. Recent evidence suggests that obesity-induced inflammation leads to the local production of proinflammatory mediators such as TNF-α and interleukin-1β, which in turn induce aromatase expression in pre-adipocyte fibroblasts [124]. Independently of the source, oestrogen production in malignant epithelial cells contributes significantly to tumour growth in the breast. The clinical relevance of these findings is exemplified by the successful use of aromatase inhibitors to treat breast cancer [125].
Endometriosis
Endometriosis is also an oestrogen-dependent disease in which local production of oestrogens plays an important role in the growth of the endometriotic tissue [reviewed in 126]. Aromatase is present in endometriotic plaque, enabling this tissue to synthesise its own oestrogens, which in turn stimulates growth [127, 128]. The differential expression of aromatase between normal endometrium and endometriotic plaque seems to be due to the absence or presence, respectively, of the transcription factor steroidogenic factor 1 (SF-1). Methylation of the SF-1 promoter in the normal endometrium seems to silence the expression of this gene [129]. Similarly to breast cancer, aromatase expression in endometriotic plaque is stimulated by PGE2 [130].
Conclusions
In mammals, birds, amphibians, reptiles and fishes, aromatase is expressed in multiple tissues indicating a crucial role for locally produced oestrogens in the differentiation, regulation and normal function of several organs and processes. The importance of the local production of oestradiol is underscored by the evolutionary strategies that lead to the tissue-specific expression of aromatase. This strategy uses local signals to activate alternative promoters in a timely and cell-specific manner. This allows oestrogen actions to be confined to a small group of cells without affecting the concentration of this steroid in circulation. Without these mechanisms, the involvement of oestradiol in such dissimilar processes as the maintenance of bone mass, neuroprotection, sperm production and maturation, neural plasticity and adipogenesis would not be possible.
Understanding these signals is of great importance because they offer invaluable therapeutic opportunities for the treatment of oestrogen-dependent disorders, as well as for the improvement of oestrogen tissue-specific functions. For instance, the development of osteoblast-specific inducers of aromatase might provide a means to stimulate bone mass without systemic adverse effects. Similar strategies could be used to increase oestrogen production in the brain and testis. On the other hand, pharmacological inhibition of the aromatase promoter used in tumours could be of great help to limit the proliferative effect of oestradiol and incite tumour regression without affecting the physiological actions of this steroid in normal tissues.
Highlights.
Aromatase is expressed in multiple tissues.
Tissue-specific expression of aromatase controls local oestrogen actions.
Locally produced oestrogens regulate normal and anomalous processes.
Table I.
Overview of Defects Associated with Decreased Aromatase Expression in Humans and Mice*
| Humans | Mice | |
|---|---|---|
| Bone | Tall stature Unfused epiphysis and delayed bone maturation Eunuchoid proportions of the skeleton Reduce bone density |
Reduce bone density (osteopenia) Males show decreased femur length Differences in bone remodelling activities between males and females |
| Brain | Not reported | Decreased response to brain injuries Behaviour alterations Impaired spatial memory |
| Metabolism and Adipose Tissue | Insulin resistance Abnormal plasma lipids |
Insulin resistance Age dependent intra-abdominal adiposity |
| Female Reproduction | Maternal virilization during gestation Abnormal external genitalia in the female foetus Normal ovarian development but increased polycystic ovary syndrome occurrence and virilization at puberty |
Infertility Disrupted folliculogenesis and failure to ovulate No corpus luteum formation Hemorrhagic follicles |
| Male Reproduction | Decrease spermatogenesis and sperm motility High gonadotropin levels Normal sex differentiation |
Progressive infertility Disrupted spermatogenesis Impaired copulatory behaviour High gonadotrophin levels |
see the text for more details and references
Acknowledgement
Supported by NIH grants R01HD057110 and R21HD066233
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The author declares no conflict of interest.
References
- 1.Bulun SE, Lin Z, Zhao H, Lu M, Amin S, Reierstad S, et al. Regulation of aromatase expression in breast cancer tissue. Ann N Y Acad Sci. 2009;1155:121–31. doi: 10.1111/j.1749-6632.2009.03705.x. [DOI] [PubMed] [Google Scholar]
- 2.Chen D, Reierstad S, Lu M, Lin Z, Ishikawa H, Bulun SE. Regulation of breast cancer-associated aromatase promoters. Cancer Lett. 2009;273:15–27. doi: 10.1016/j.canlet.2008.05.038. [DOI] [PubMed] [Google Scholar]
- 3.Santen RJ, Brodie H, Simpson ER, Siiteri PK, Brodie A. History of aromatase: saga of an important biological mediator and therapeutic target. Endocr Rev. 2009;30:343–75. doi: 10.1210/er.2008-0016. [DOI] [PubMed] [Google Scholar]
- 4.Bulun SE, Lin Z, Imir G, Amin S, Demura M, Yilmaz B, et al. Regulation of aromatase expression in estrogen-responsive breast and uterine disease: from bench to treatment. Pharmacol Rev. 2005;57:359–83. doi: 10.1124/pr.57.3.6. [DOI] [PubMed] [Google Scholar]
- 5.Turner KJ, Macpherson S, Millar MR, McNeilly AS, Williams K, Cranfield M, et al. Development and validation of a new monoclonal antibody to mammalian aromatase. J Endocrinol. 2002;172:21–30. doi: 10.1677/joe.0.1720021. [DOI] [PubMed] [Google Scholar]
- 6.Guigon CJ, Mazaud S, Forest MG, Brailly-Tabard S, Coudouel N, Magre S. Unaltered development of the initial follicular waves and normal pubertal onset in female rats after neonatal deletion of the follicular reserve. Endocrinology. 2003;144:3651–62. doi: 10.1210/en.2003-0072. [DOI] [PubMed] [Google Scholar]
- 7.Otsuka F, Yamamoto S, Erickson GF, Shimasaki S. Bone morphogenetic protein-15 inhibits follicle-stimulating hormone (FSH) action by suppressing FSH receptor expression. J Biol Chem. 2001;276:11387–92. doi: 10.1074/jbc.M010043200. [DOI] [PubMed] [Google Scholar]
- 8.Spicer LJ, Aad PY, Allen D, Mazerbourg S, Hsueh AJ. Growth differentiation factor-9 has divergent effects on proliferation and steroidogenesis of bovine granulosa cells. J Endocrinol. 2006;189:329–39. doi: 10.1677/joe.1.06503. [DOI] [PubMed] [Google Scholar]
- 9.Vitt UA, Hayashi M, Klein C, Hsueh AJ. Growth differentiation factor-9 stimulates proliferation but suppresses the follicle-stimulating hormone-induced differentiation of cultured granulosa cells from small antral and preovulatory rat follicles. Biol Reprod. 2000;62:370–7. doi: 10.1095/biolreprod62.2.370. [DOI] [PubMed] [Google Scholar]
- 10.Elvin JA, Yan C, Wang P, Nishimori K, Matzuk MM. Molecular characterization of the follicle defects in the growth differentiation factor 9-deficient ovary. Mol Endocrinol. 1999;13:1018–34. doi: 10.1210/mend.13.6.0309. [DOI] [PubMed] [Google Scholar]
- 11.Stocco C. Aromatase expression in the ovary: hormonal and molecular regulation. Steroids. 2008;73:473–87. doi: 10.1016/j.steroids.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richards JS, Russell DL, Ochsner S, Hsieh M, Doyle KH, Falender AE, et al. Novel signaling pathways that control ovarian follicular development, ovulation, and luteinization. Recent Prog Horm Res. 2002;57:195–220. doi: 10.1210/rp.57.1.195. [DOI] [PubMed] [Google Scholar]
- 13.Fitzpatrick SL, Richards JS. Regulation of cytochrome P450 aromatase messenger ribonucleic acid and activity by steroids and gonadotropins in rat granulosa cells. Endocrinology. 1991;129:1452–62. doi: 10.1210/endo-129-3-1452. [DOI] [PubMed] [Google Scholar]
- 14.Adashi EY, Hsueh AJ. Estrogens augment the stimulation of ovarian aromatase activity by follicle-stimulating hormone in cultured rat granulosa cells. J Biol Chem. 1982;257:6077–83. [PubMed] [Google Scholar]
- 15.Fisher CR, Graves KH, Parlow AF, Simpson ER. Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proc Natl Acad Sci U S A. 1998;95:6965–70. doi: 10.1073/pnas.95.12.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Britt KL, Drummond AE, Cox VA, Dyson M, Wreford NG, Jones ME, et al. An age-related ovarian phenotype in mice with targeted disruption of the Cyp 19 (aromatase) gene. Endocrinology. 2000;141:2614–23. doi: 10.1210/endo.141.7.7578. [DOI] [PubMed] [Google Scholar]
- 17.Couse JF, Yates MM, Deroo BJ, Korach KS. Estrogen receptor-β is critical to granulosa cell differentiation and the ovulatory response to gonadotropins. Endocrinology. 2005;146:3247–62. doi: 10.1210/en.2005-0213. [DOI] [PubMed] [Google Scholar]
- 18.Wu YG, Bennett J, Talla D, Stocco C. Testosterone, not 5α-dihydrotestosterone, stimulates LRH-1 leading to FSH-independent expression of Cyp19 and P450scc in granulosa cells. Mol Endocrinol. 2011;25:656–68. doi: 10.1210/me.2010-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korach KS, Emmen JM, Walker VR, Hewitt SC, Yates M, Hall JM, et al. Update on animal models developed for analyses of estrogen receptor biological activity. J Steroid Biochem Mol Biol. 2003;86:387–91. doi: 10.1016/s0960-0760(03)00348-0. [DOI] [PubMed] [Google Scholar]
- 20.Naftolin F, Garcia-Segura LM, Horvath TL, Zsarnovszky A, Demir N, Fadiel A, et al. Estrogen-induced hypothalamic synaptic plasticity and pituitary sensitization in the control of the estrogen-induced gonadotrophin surge. Reprod Sci. 2007;14:101–16. doi: 10.1177/1933719107301059. [DOI] [PubMed] [Google Scholar]
- 21.Kragie L. Aromatase in primate pregnancy: a review. Endocr Res. 2002;28:121–8. doi: 10.1081/erc-120015041. [DOI] [PubMed] [Google Scholar]
- 22.Pepe GJ, Albrecht ED. Regulation of functional differentiation of the placental villous syncytiotrophoblast by estrogen during primate pregnancy. Steroids. 1999;64:624–7. doi: 10.1016/s0039-128x(99)00043-4. [DOI] [PubMed] [Google Scholar]
- 23.Mendelson CR, Kamat A. Mechanisms in the regulation of aromatase in developing ovary and placenta. J Steroid Biochem Mol Biol. 2007;106:62–70. doi: 10.1016/j.jsbmb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rainey WE, Rehman KS, Carr BR. The human fetal adrenal: making adrenal androgens for placental estrogens. Semin Reprod Med. 2004;22:327–36. doi: 10.1055/s-2004-861549. [DOI] [PubMed] [Google Scholar]
- 25.Kempna P, Fluck CE. Adrenal gland development and defects. Best Pract Res Clin Endocrinol Metab. 2008;22:77–93. doi: 10.1016/j.beem.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 26.Albrecht ED, Pepe GJ. Estrogen regulation of placental angiogenesis and fetal ovarian development during primate pregnancy. Int J Dev Biol. 2010;54:397–408. doi: 10.1387/ijdb.082758ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albrecht ED, Aberdeen GW, Pepe GJ. The role of estrogen in the maintenance of primate pregnancy. Am J Obstet Gynecol. 2000;182:432–8. doi: 10.1016/s0002-9378(00)70235-3. [DOI] [PubMed] [Google Scholar]
- 28.France JT. Steroid sulphatase deficiency. J Steroid Biochem. 1979;11:647–51. doi: 10.1016/0022-4731(79)90094-3. [DOI] [PubMed] [Google Scholar]
- 29.Belgorosky A, Guercio G, Pepe C, Saraco N, Rivarola MA. Genetic and clinical spectrum of aromatase deficiency in infancy, childhood and adolescence. Horm Res. 2009;72:321–30. doi: 10.1159/000249159. [DOI] [PubMed] [Google Scholar]
- 30.Jones ME, Boon WC, McInnes K, Maffei L, Carani C, Simpson ER. Recognizing rare disorders: aromatase deficiency. Nat Clin Pract Endocrinol Metab. 2007;3:414–21. doi: 10.1038/ncpendmet0477. [DOI] [PubMed] [Google Scholar]
- 31.Mullis PE, Yoshimura N, Kuhlmann B, Lippuner K, Jaeger P, Harada H. Aromatase deficiency in a female who is compound heterozygote for two new point mutations in the P450arom gene: impact of estrogens on hypergonadotropic hypogonadism, multicystic ovaries, and bone densitometry in childhood. J Clin Endocrinol Metab. 1997;82:1739–45. doi: 10.1210/jcem.82.6.3994. [DOI] [PubMed] [Google Scholar]
- 32.Simpson ER, Mahendroo MS, Means GD, Kilgore MW, Hinshelwood MM, Graham-Lorence S, et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr Rev. 1994;15:342–55. doi: 10.1210/edrv-15-3-342. [DOI] [PubMed] [Google Scholar]
- 33.Chow JD, Simpson ER, Boon WC. Alternative 5'-untranslated first exons of the mouse Cyp19A1 (aromatase) gene. J Steroid Biochem Mol Biol. 2009;115:115–25. doi: 10.1016/j.jsbmb.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 34.Das A, Mantena SR, Kannan A, Evans DB, Bagchi MK, Bagchi IC. De novo synthesis of estrogen in pregnant uterus is critical for stromal decidualization and angiogenesis. Proc Natl Acad Sci U S A. 2009;106:12542–7. doi: 10.1073/pnas.0901647106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hess RA, Bunick D, Lee KH, Bahr J, Taylor JA, Korach KS, et al. A role for oestrogens in the male reproductive system. Nature. 1997;390:509–12. doi: 10.1038/37352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carreau S, Silandre D, Bourguiba S, Hamden K, Said L, Lambard S, et al. Estrogens and male reproduction: a new concept. Braz J Med Biol Res. 2007;40:761–8. doi: 10.1590/s0100-879x2007000600003. [DOI] [PubMed] [Google Scholar]
- 37.Carreau S, de Vienne C, Galeraud-Denis I. Aromatase and estrogens in man reproduction: a review and latest advances. Adv Med Sci. 2008;53:139–44. doi: 10.2478/v10039-008-0022-z. [DOI] [PubMed] [Google Scholar]
- 38.Carreau S, Wolczynski S, Galeraud-Denis I. Aromatase, oestrogens and human male reproduction. Philos Trans R Soc Lond B Biol Sci. 2010;365:1571–9. doi: 10.1098/rstb.2009.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lambard S, Galeraud-Denis I, Bouraima H, Bourguiba S, Chocat A, Carreau S. Expression of aromatase in human ejaculated spermatozoa: a putative marker of motility. Mol Hum Reprod. 2003;9:117–24. doi: 10.1093/molehr/gag020. [DOI] [PubMed] [Google Scholar]
- 40.Carreau S, Hess RA. Oestrogens and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1517–35. doi: 10.1098/rstb.2009.0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lazaros L, Xita N, Kaponis A, Hatzi E, Plachouras N, Sofikitis N, et al. The association of aromatase (CYP19) gene variants with sperm concentration and motility. Asian J Androl. 2011;13:292–7. doi: 10.1038/aja.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carreau S, Lambard S, Delalande C, Denis-Galeraud I, Bilinska B, Bourguiba S. Aromatase expression and role of estrogens in male gonad : a review. Reprod Biol Endocrinol. 2003;1:35. doi: 10.1186/1477-7827-1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galeraud-Denis I, Travert C, de Vienne C, Said L, Saad A, Carreau S. New insights about the evaluation of human sperm quality: the aromatase example. Folia Histochem Cytobiol. 2009;47:S13–7. doi: 10.2478/v10042-009-0059-2. [DOI] [PubMed] [Google Scholar]
- 44.Maffei L, Rochira V, Zirilli L, Antunez P, Aranda C, Fabre B, et al. A novel compound heterozygous mutation of the aromatase gene in an adult man: reinforced evidence on the relationship between congenital oestrogen deficiency, adiposity and the metabolic syndrome. Clin Endocrinol (Oxf) 2007;67:218–24. doi: 10.1111/j.1365-2265.2007.02864.x. [DOI] [PubMed] [Google Scholar]
- 45.Aquila S, Sisci D, Gentile M, Middea E, Siciliano L, Ando S. Human ejaculated spermatozoa contain active P450 aromatase. J Clin Endocrinol Metab. 2002;87:3385–90. doi: 10.1210/jcem.87.7.8633. [DOI] [PubMed] [Google Scholar]
- 46.Solakidi S, Psarra AM, Nikolaropoulos S, Sekeris CE. Estrogen receptors α and β (ERα and ERβ) and androgen receptor (AR) in human sperm: localization of ERβ and AR in mitochondria of the midpiece. Hum Reprod. 2005;20:3481–7. doi: 10.1093/humrep/dei267. [DOI] [PubMed] [Google Scholar]
- 47.Shayu D, ChennaKesava CS, Soundarajan R, Rao AJ. Effects of ICI 182780 on estrogen receptor expression, fluid absorption and sperm motility in the epididymis of the bonnet monkey. Reprod Biol Endocrinol. 2005;3:10. doi: 10.1186/1477-7827-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee KH, Hess RA, Bahr JM, Lubahn DB, Taylor J, Bunick D. Estrogen receptor α has a functional role in the mouse rete testis and efferent ductules. Biol Reprod. 2000;63:1873–80. doi: 10.1095/biolreprod63.6.1873. [DOI] [PubMed] [Google Scholar]
- 49.Carpino A, Romeo F, Rago V. Aromatase immunolocalization in human ductuli efferentes and proximal ductus epididymis. J Anat. 2004;204:217–20. doi: 10.1111/j.0021-8782.2004.00272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shayu D, Rao AJ. Expression of functional aromatase in the epididymis: role of androgens and LH in modulation of expression and activity. Mol Cell Endocrinol. 2006;249:40–50. doi: 10.1016/j.mce.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 51.West NB, Roselli CE, Resko JA, Greene GL, Brenner RM. Estrogen and progestin receptors and aromatase activity in rhesus monkey prostate. Endocrinology. 1988;123:2312–22. doi: 10.1210/endo-123-5-2312. [DOI] [PubMed] [Google Scholar]
- 52.Tsugaya M, Harada N, Tozawa K, Yamada Y, Hayashi Y, Tanaka S, et al. Aromatase mRNA levels in benign prostatic hyperplasia and prostate cancer. Int J Urol. 1996;3:292–6. doi: 10.1111/j.1442-2042.1996.tb00537.x. [DOI] [PubMed] [Google Scholar]
- 53.Takase Y, Levesque MH, Luu-The V, El-Alfy M, Labrie F, Pelletier G. Expression of enzymes involved in estrogen metabolism in human prostate. J Histochem Cytochem. 2006;54:911–21. doi: 10.1369/jhc.6A6927.2006. [DOI] [PubMed] [Google Scholar]
- 54.Ellem SJ, Schmitt JF, Pedersen JS, Frydenberg M, Risbridger GP. Local aromatase expression in human prostate is altered in malignancy. J Clin Endocrinol Metab. 2004;89:2434–41. doi: 10.1210/jc.2003-030933. [DOI] [PubMed] [Google Scholar]
- 55.Ellem SJ, Risbridger GP. Aromatase and regulating the estrogen:androgen ratio in the prostate gland. J Steroid Biochem Mol Biol. 2010;118:246–51. doi: 10.1016/j.jsbmb.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 56.McPherson SJ, Wang H, Jones ME, Pedersen J, Iismaa TP, Wreford N, et al. Elevated androgens and prolactin in aromatase-deficient mice cause enlargement, but not malignancy, of the prostate gland. Endocrinology. 2001;142:2458–67. doi: 10.1210/endo.142.6.8079. [DOI] [PubMed] [Google Scholar]
- 57.Ellem SJ, Risbridger GP. Aromatase and prostate cancer. Minerva Endocrinol. 2006;31:1–12. [PubMed] [Google Scholar]
- 58.Palapattu GS, Sutcliffe S, Bastian PJ, Platz EA, De Marzo AM, Isaacs WB, et al. Prostate carcinogenesis and inflammation: emerging insights. Carcinogenesis. 2005;26:1170–81. doi: 10.1093/carcin/bgh317. [DOI] [PubMed] [Google Scholar]
- 59.Beyer C, Morali G, Larsson K, Soderstein P. Steroid regulation of sexual behavior. J Steroid Biochem. 1976;7:1171–6. doi: 10.1016/0022-4731(76)90051-0. [DOI] [PubMed] [Google Scholar]
- 60.Naftolin F, Ryan KJ, Petro Z. Aromatization of androstenedione by the diencephalon. J Clin Endocrinol Metab. 1971;33:368–70. doi: 10.1210/jcem-33-2-368. [DOI] [PubMed] [Google Scholar]
- 61.Ball GF, Balthazart J. Androgen metabolism and the activation of male sexual behavior: it's more complicated than you think! Horm Behav. 2006;49:1–3. doi: 10.1016/j.yhbeh.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 62.Cornil CA, Charlier TD. Rapid behavioural effects of oestrogens and fast regulation of their local synthesis by brain aromatase. J Neuroendocrinol. 2010;22:664–73. doi: 10.1111/j.1365-2826.2010.02023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Roselli CF. Brain aromatase: roles in reproduction and neuroprotection. J Steroid Biochem Mol Biol. 2007;106:143–50. doi: 10.1016/j.jsbmb.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- 65.Yague JG, Wang AC, Janssen WG, Hof PR, Garcia-Segura LM, Azcoitia I, et al. Aromatase distribution in the monkey temporal neocortex and hippocampus. Brain Res. 2008;1209:115–27. doi: 10.1016/j.brainres.2008.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yague JG, Azcoitia I, DeFelipe J, Garcia-Segura LM, Munoz A. Aromatase expression in the normal and epileptic human hippocampus. Brain Res. 2010;1315:41–52. doi: 10.1016/j.brainres.2009.09.111. [DOI] [PubMed] [Google Scholar]
- 67.Azcoitia I, Yague JG, Garcia-Segura LM. Estradiol synthesis within the human brain. Neuroscience. 2011 doi: 10.1016/j.neuroscience.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 68.Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006;29:241–9. doi: 10.1016/j.tins.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 69.Garcia-Segura LM, Wozniak A, Azcoitia I, Rodriguez JR, Hutchison RE, Hutchison JB. Aromatase expression by astrocytes after brain injury: implications for local estrogen formation in brain repair. Neuroscience. 1999;89:567–78. doi: 10.1016/s0306-4522(98)00340-6. [DOI] [PubMed] [Google Scholar]
- 70.Brock O, Baum MJ, Bakker J. The development of female sexual behavior requires prepubertal estradiol. J Neurosci. 2011;31:5574–8. doi: 10.1523/JNEUROSCI.0209-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Christian CA, Moenter SM. The neurobiology of preovulatory and estradiol-induced gonadotropin-releasing hormone surges. Endocr Rev. 2010;31:544–77. doi: 10.1210/er.2009-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roselli CE, Resko JA. Cytochrome P450 aromatase (CYP19) in the non-human primate brain: distribution, regulation, and functional significance. J Steroid Biochem Mol Biol. 2001;79:247–53. doi: 10.1016/s0960-0760(01)00141-8. [DOI] [PubMed] [Google Scholar]
- 73.Hayes FJ, DeCruz S, Seminara SB, Boepple PA, Crowley WF., Jr. Differential regulation of gonadotropin secretion by testosterone in the human male: absence of a negative feedback effect of testosterone on follicle-stimulating hormone secretion. J Clin Endocrinol Metab. 2001;86:53–8. doi: 10.1210/jcem.86.1.7101. [DOI] [PubMed] [Google Scholar]
- 74.Carani C, Qin K, Simoni M, Faustini-Fustini M, Serpente S, Boyd J, et al. Effect of testosterone and estradiol in a man with aromatase deficiency. N Engl J Med. 1997;337:91–5. doi: 10.1056/NEJM199707103370204. [DOI] [PubMed] [Google Scholar]
- 75.Pitteloud N, Dwyer AA, DeCruz S, Lee H, Boepple PA, Crowley WF, Jr., et al. Inhibition of luteinizing hormone secretion by testosterone in men requires aromatization for its pituitary but not its hypothalamic effects: evidence from the tandem study of normal and gonadotropin-releasing hormone-deficient men. J Clin Endocrinol Metab. 2008;93:784–91. doi: 10.1210/jc.2007-2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jakab RL, Horvath TL, Leranth C, Harada N, Naftolin F. Aromatase immunoreactivity in the rat brain: gonadectomy-sensitive hypothalamic neurons and an unresponsive “limbic ring” of the lateral septum-bed nucleus-amygdala complex. J Steroid Biochem Mol Biol. 1993;44:481–98. doi: 10.1016/0960-0760(93)90253-s. [DOI] [PubMed] [Google Scholar]
- 77.Cooke B, Hegstrom CD, Villeneuve LS, Breedlove SM. Sexual differentiation of the vertebrate brain: principles and mechanisms. Front Neuroendocrinol. 1998;19:323–62. doi: 10.1006/frne.1998.0171. [DOI] [PubMed] [Google Scholar]
- 78.Bakker J, Baum MJ. Role for estradiol in female-typical brain and behavioral sexual differentiation. Front Neuroendocrinol. 2008;29:1–16. doi: 10.1016/j.yfrne.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McEwen BS, Lieberburg I, Chaptal C, Krey LC. Aromatization: important for sexual differentiation of the neonatal rat brain. Horm Behav. 1977;9:249–63. doi: 10.1016/0018-506x(77)90060-5. [DOI] [PubMed] [Google Scholar]
- 80.Roselli CE, Liu M, Hurn PD. Brain aromatization: classic roles and new perspectives. Semin Reprod Med. 2009;27:207–17. doi: 10.1055/s-0029-1216274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bakker J, Honda S, Harada N, Balthazart J. Restoration of male sexual behavior by adult exogenous estrogens in male aromatase knockout mice. Horm Behav. 2004;46:1–10. doi: 10.1016/j.yhbeh.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 82.Bakker J, Honda S, Harada N, Balthazart J. The aromatase knock-out mouse provides new evidence that estradiol is required during development in the female for the expression of sociosexual behaviors in adulthood. J Neurosci. 2002;22:9104–12. doi: 10.1523/JNEUROSCI.22-20-09104.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hill RA, McInnes KJ, Gong EC, Jones ME, Simpson ER, Boon WC. Estrogen deficient male mice develop compulsive behavior. Biol Psychiatry. 2007;61:359–66. doi: 10.1016/j.biopsych.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 84.Hill RA, Pompolo S, Jones ME, Simpson ER, Boon WC. Estrogen deficiency leads to apoptosis in dopaminergic neurons in the medial preoptic area and arcuate nucleus of male mice. Mol Cell Neurosci. 2004;27:466–76. doi: 10.1016/j.mcn.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 85.Saldanha CJ, Duncan KA, Walters BJ. Neuroprotective actions of brain aromatase. Front Neuroendocrinol. 2009;30:106–18. doi: 10.1016/j.yfrne.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Azcoitia I, Sierra A, Veiga S, Garcia-Segura LM. Aromatase expression by reactive astroglia is neuroprotective. Ann N Y Acad Sci. 2003;1007:298–305. doi: 10.1196/annals.1286.028. [DOI] [PubMed] [Google Scholar]
- 87.Carswell HV, Dominiczak AF, Garcia-Segura LM, Harada N, Hutchison JB, Macrae IM. Brain aromatase expression after experimental stroke: topography and time course. J Steroid Biochem Mol Biol. 2005;96:89–91. doi: 10.1016/j.jsbmb.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 88.Bialek M, Zaremba P, Borowicz KK, Czuczwar SJ. Neuroprotective role of testosterone in the nervous system. Pol J Pharmacol. 2004;56:509–18. [PubMed] [Google Scholar]
- 89.Barreto G, Veiga S, Azcoitia I, Garcia-Segura LM, Garcia-Ovejero D. Testosterone decreases reactive astroglia and reactive microglia after brain injury in male rats: role of its metabolites, oestradiol and dihydrotestosterone. Eur J Neurosci. 2007;25:3039–46. doi: 10.1111/j.1460-9568.2007.05563.x. [DOI] [PubMed] [Google Scholar]
- 90.McCullough LD, Blizzard K, Simpson ER, Oz OK, Hurn PD. Aromatase cytochrome P450 and extragonadal estrogen play a role in ischemic neuroprotection. J Neurosci. 2003;23:8701–5. doi: 10.1523/JNEUROSCI.23-25-08701.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Riggs BL, Khosla S, Melton LJ., 3rd Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev. 2002;23:279–302. doi: 10.1210/edrv.23.3.0465. [DOI] [PubMed] [Google Scholar]
- 92.Nawata H, Tanaka S, Takayanagi R, Sakai Y, Yanase T, Ikuyama S, et al. Aromatase in bone cell: association with osteoporosis in postmenopausal women. J Steroid Biochem Mol Biol. 1995;53:165–74. doi: 10.1016/0960-0760(95)00031-t. [DOI] [PubMed] [Google Scholar]
- 93.Vanderschueren D, Vandenput L, Boonen S, Lindberg MK, Bouillon R, Ohlsson C. Androgens and bone. Endocr Rev. 2004;25:389–425. doi: 10.1210/er.2003-0003. [DOI] [PubMed] [Google Scholar]
- 94.Clarke BL, Khosla S. Androgens and bone. Steroids. 2009;74:296–305. doi: 10.1016/j.steroids.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bilezikian JP, Morishima A, Bell J, Grumbach MM. Increased bone mass as a result of estrogen therapy in a man with aromatase deficiency. N Engl J Med. 1998;339:599–603. doi: 10.1056/NEJM199808273390905. [DOI] [PubMed] [Google Scholar]
- 96.Oz OK, Hajibeigi A, Howard K, Cummins CL, van Abel M, Bindels RJ, et al. Aromatase deficiency causes altered expression of molecules critical for calcium reabsorption in the kidneys of female mice *. J Bone Miner Res. 2007;22:1893–902. doi: 10.1359/jbmr.070808. [DOI] [PubMed] [Google Scholar]
- 97.Zirilli L, Rochira V, Diazzi C, Caffagni G, Carani C. Human models of aromatase deficiency. J Steroid Biochem Mol Biol. 2008;109:212–8. doi: 10.1016/j.jsbmb.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 98.Hernandez JL, Garces CM, Sumillera M, Fernandez-Aldasoro EV, Garcia-Ibarbia C, Ortiz-Gomez JA, et al. Aromatase expression in osteoarthritic and osteoporotic bone. Arthritis Rheum. 2008;58:1696–700. doi: 10.1002/art.23500. [DOI] [PubMed] [Google Scholar]
- 99.Oz OK, Zerwekh JE, Fisher C, Graves K, Nanu L, Millsaps R, et al. Bone has a sexually dimorphic response to aromatase deficiency. J Bone Miner Res. 2000;15:507–14. doi: 10.1359/jbmr.2000.15.3.507. [DOI] [PubMed] [Google Scholar]
- 100.Sjogren K, Lagerquist M, Moverare-Skrtic S, Andersson N, Windahl SH, Swanson C, et al. Elevated aromatase expression in osteoblasts leads to increased bone mass without systemic adverse effects. J Bone Miner Res. 2009;24:1263–70. doi: 10.1359/jbmr.090208. [DOI] [PubMed] [Google Scholar]
- 101.Heshmati HM, Khosla S, Robins SP, O'Fallon WM, Melton LJ, 3rd, Riggs BL. Role of low levels of endogenous estrogen in regulation of bone resorption in late postmenopausal women. J Bone Miner Res. 2002;17:172–8. doi: 10.1359/jbmr.2002.17.1.172. [DOI] [PubMed] [Google Scholar]
- 102.Barginear M, Clotfelter A, Poznak CV. Markers of bone metabolism in women receiving aromatase inhibitors for early-stage breast cancer. Clin Breast Cancer. 2009;9:72–6. doi: 10.3816/CBC.2009.n.014. [DOI] [PubMed] [Google Scholar]
- 103.Simpson ER, Ackerman GE, Smith ME, Mendelson CR. Estrogen formation in stromal cells of adipose tissue of women: induction by glucocorticosteroids. Proc Natl Acad Sci U S A. 1981;78:5690–4. doi: 10.1073/pnas.78.9.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.MacDonald PC, Madden JD, Brenner PF, Wilson JD, Siiteri PK. Origin of estrogen in normal men and in women with testicular feminization. J Clin Endocrinol Metab. 1979;49:905–16. doi: 10.1210/jcem-49-6-905. [DOI] [PubMed] [Google Scholar]
- 105.Mendelson CR, Simpson ER. Regulation of estrogen biosynthesis by human adipose cells in vitro. Mol Cell Endocrinol. 1987;52:169–76. doi: 10.1016/0303-7207(87)90041-4. [DOI] [PubMed] [Google Scholar]
- 106.Ackerman GE, Smith ME, Mendelson CR, MacDonald PC, Simpson ER. Aromatization of androstenedione by human adipose tissue stromal cells in monolayer culture. J Clin Endocrinol Metab. 1981;53:412–7. doi: 10.1210/jcem-53-2-412. [DOI] [PubMed] [Google Scholar]
- 107.Simpson ER. Aromatase: biologic relevance of tissue-specific expression. Semin Reprod Med. 2004;22:11–23. doi: 10.1055/s-2004-823023. [DOI] [PubMed] [Google Scholar]
- 108.Zhao H, Innes J, Brooks DC, Reierstad S, Yilmaz MB, Lin Z, et al. A novel promoter controls Cyp19a1 gene expression in mouse adipose tissue. Reprod Biol Endocrinol. 2009;7:37. doi: 10.1186/1477-7827-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bulun SE, Simpson ER. Competitive reverse transcription-polymerase chain reaction analysis indicates that levels of aromatase cytochrome P450 transcripts in adipose tissue of buttocks, thighs, and abdomen of women increase with advancing age. J Clin Endocrinol Metab. 1994;78:428–32. doi: 10.1210/jcem.78.2.8106632. [DOI] [PubMed] [Google Scholar]
- 110.Misso ML, Jang C, Adams J, Tran J, Murata Y, Bell R, et al. Adipose aromatase gene expression is greater in older women and is unaffected by postmenopausal estrogen therapy. Menopause. 2005;12:210–5. doi: 10.1097/00042192-200512020-00016. [DOI] [PubMed] [Google Scholar]
- 111.Foryst-Ludwig A, Kintscher U. Metabolic impact of estrogen signalling through ERα and ERβ. J Steroid Biochem Mol Biol. 2010;122:74–81. doi: 10.1016/j.jsbmb.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 112.Jones ME, Thorburn AW, Britt KL, Hewitt KN, Misso ML, Wreford NG, et al. Aromatase-deficient (ArKO) mice accumulate excess adipose tissue. J Steroid Biochem Mol Biol. 2001;79:3–9. doi: 10.1016/s0960-0760(01)00136-4. [DOI] [PubMed] [Google Scholar]
- 113.Jones ME, Thorburn AW, Britt KL, Hewitt KN, Wreford NG, Proietto J, et al. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci U S A. 2000;97:12735–40. doi: 10.1073/pnas.97.23.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ohlsson C, Hellberg N, Parini P, Vidal O, Bohlooly YM, Rudling M, et al. Obesity and disturbed lipoprotein profile in estrogen receptor-α-deficient male mice. Biochem Biophys Res Commun. 2000;278:640–5. doi: 10.1006/bbrc.2000.3827. [DOI] [PubMed] [Google Scholar]
- 115.Jones ME, Boon WC, Proietto J, Simpson ER. Of mice and men: the evolving phenotype of aromatase deficiency. Trends Endocrinol Metab. 2006;17:55–64. doi: 10.1016/j.tem.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 116.Cooke PS, Naaz A. Role of estrogens in adipocyte development and function. Exp Biol Med (Maywood) 2004;229:1127–35. doi: 10.1177/153537020422901107. [DOI] [PubMed] [Google Scholar]
- 117.Gloy V, Langhans W, Hillebrand JJ, Geary N, Asarian L. Ovariectomy and overeating palatable, energy-dense food increase subcutaneous adipose tissue more than intra-abdominal adipose tissue in rats. Biol Sex Differ. 2011;2:6. doi: 10.1186/2042-6410-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cleary MP, Grossmann ME, Ray A. Effect of obesity on breast cancer development. Vet Pathol. 2010;47:202–13. doi: 10.1177/0300985809357753. [DOI] [PubMed] [Google Scholar]
- 119.Cleary MP, Grossmann ME. Minireview: Obesity and breast cancer: the estrogen connection. Endocrinology. 2009;150:2537–42. doi: 10.1210/en.2009-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Maia H, Jr., Casoy J, Valente Filho J. Is aromatase expression in the endometrium the cause of endometriosis and related infertility? Gynecol Endocrinol. 2009;25:253–7. doi: 10.1080/09513590802627647. [DOI] [PubMed] [Google Scholar]
- 121.Folkerd EJ, Dowsett M. Influence of sex hormones on cancer progression. J Clin Oncol. 2010;28:4038–44. doi: 10.1200/JCO.2009.27.4290. [DOI] [PubMed] [Google Scholar]
- 122.Agarwal VR, Bulun SE, Leitch M, Rohrich R, Simpson ER. Use of alternative promoters to express the aromatase cytochrome P450 (CYP19) gene in breast adipose tissues of cancer-free and breast cancer patients. J Clin Endocrinol Metab. 1996;81:3843–9. doi: 10.1210/jcem.81.11.8923826. [DOI] [PubMed] [Google Scholar]
- 123.Miki Y, Suzuki T, Sasano H. Controversies of aromatase localization in human breast cancer--stromal versus parenchymal cells. J Steroid Biochem Mol Biol. 2007;106:97–101. doi: 10.1016/j.jsbmb.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 124.Subbaramaiah K, Howe LR, Bhardwaj P, Du B, Gravaghi C, Yantiss RK, et al. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res (Phila) 2011;4:329–46. doi: 10.1158/1940-6207.CAPR-10-0381. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 125.Lonning PE. The potency and clinical efficacy of aromatase inhibitors across the breast cancer continuum. Annals of Oncology. 2011;22:503–14. doi: 10.1093/annonc/mdq337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–79. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 127.Bulun SE, Yang S, Fang Z, Gurates B, Tamura M, Zhou J, et al. Role of aromatase in endometrial disease. J Steroid Biochem Mol Biol. 2001;79:19–25. doi: 10.1016/s0960-0760(01)00134-0. [DOI] [PubMed] [Google Scholar]
- 128.Bulun SE, Gurates B, Fang Z, Tamura M, Sebastian S, Zhou J, et al. Mechanisms of excessive estrogen formation in endometriosis. J Reprod Immunol. 2002;55:21–33. doi: 10.1016/s0165-0378(01)00132-2. [DOI] [PubMed] [Google Scholar]
- 129.Bulun SE, Utsunomiya H, Lin Z, Yin P, Cheng YH, Pavone ME, et al. Steroidogenic factor-1 and endometriosis. Mol Cell Endocrinol. 2009;300:104–8. doi: 10.1016/j.mce.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 130.Noble LS, Takayama K, Zeitoun KM, Putman JM, Johns DA, Hinshelwood MM, et al. Prostaglandin E2 stimulates aromatase expression in endometriosis-derived stromal cells. J Clin Endocrinol Metab. 1997;82:600–6. doi: 10.1210/jcem.82.2.3783. [DOI] [PubMed] [Google Scholar]
- 131.Turner KJ, Macpherson S, Millar MR, McNeilly AS, Williams K, Cranfield M, et al. Development and validation of a new monoclonal antibody to mammalian aromatase. J Endocrinol. 2002;172:21–30. doi: 10.1677/joe.0.1720021. [DOI] [PubMed] [Google Scholar]
- 132.Jeong JH, Jung YK, Kim HJ, Jin JS, Kim HN, Kang SM, et al. The gene for aromatase, a rate-limiting enzyme for local estrogen biosynthesis, is a downstream target gene of Runx2 in skeletal tissues. Mol Cell Biol. 2010;30:2365–75. doi: 10.1128/MCB.00672-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sasano H, Edwards DP, Anderson TJ, Silverberg SG, Evans DB, Santen RJ, et al. Validation of new aromatase monoclonal antibodies for immunohistochemistry: progress report. J Steroid Biochem Mol Biol. 2003;86:239–44. doi: 10.1016/s0960-0760(03)00363-7. [DOI] [PubMed] [Google Scholar]
- 134.Rochira V, Carani C. Aromatase deficiency in men: a clinical perspective. Nat Rev Endocrinol. 2009;5:559–68. doi: 10.1038/nrendo.2009.176. [DOI] [PubMed] [Google Scholar]