Abstract
Despite tremendous technological and therapeutic advances, bronchopulmonary dysplasia (BPD) remains a leading cause of respiratory morbidity in very low birth weight infants, and there are no effective preventive and/or therapeutic options. We have previously reported that hyperoxia-induced neonatal rat lung injury might be prevented by rosiglitazone (RGZ). Here, we characterize 1) perturbations in wingless/Int (Wnt) and transforming growth factor (TGF)-β signaling, and 2) structural aberrations in lung morphology following 7-day continuous in vivo hyperoxia exposure to neonatal rats. We also tested whether treatment of neonatal pups with RGZ, concomitant to hyperoxia, could prevent such aberrations. Our study revealed that hyperoxia caused significant upregulation of Wnt signaling protein markers lymphoid enhancer factor 1 (Lef-1) and β-catenin and TGF-β pathway transducers phosphorylated Smad3 and Smad7 proteins in whole rat lung extracts. These changes were also accompanied by upregulation of myogenic marker proteins α-smooth muscle actin (α-SMA) and calponin but significant downregulation of the lipogenic marker peroxisome proliferator-activated receptor-γ (PPARγ) expression. These molecular perturbations were associated with reduction in alveolar septal thickness, radial alveolar count, and larger alveoli in the hyperoxia-exposed lung. These hyperoxia-induced molecular and morphological changes were prevented by systemic administration of RGZ, with lung sections appearing near normal. This is the first evidence that in vivo hyperoxia induces activation of both Wnt and TGF-β signal transduction pathways in lung and of its near complete prevention by RGZ. Hyperoxia-induced arrest in alveolar development, a hallmark of BPD, along with these molecular changes strongly implicates these proteins in hyperoxia-induced lung injury. Administration of PPARγ agonists may thus be a potential strategy to attenuate hyperoxia-induced lung injury and subsequent BPD.
Keywords: bronchopulmonary dysplasia, peroxisome proliferator-activated receptor-γ, lung development, lung fibroblast
despite several technological and therapeutic advances over the past several years, bronchopulmonary dysplasia (BPD) remains a leading cause of respiratory morbidity in very low birth weight infants. The affected infants have a complicated initial neonatal intensive care course, poor growth, neurodevelopmental delays, and repeated hospitalizations during the first few years of life (8, 39). The “new” BPD, a chronic lung disorder of extremely premature infants, is characterized by lung injury that results in abnormal lung architecture hallmarked by failed alveolarization with minimal large and small airway disease and relatively mild inflammation and fibrosis. Although multiple pathophysiological mechanisms contribute to lung injury in the extremely preterm infant, the new BPD is primarily an aberration of lung development. This is in contrast to the “old” BPD, which occurred primarily in larger preterm infants as a consequence of severe lung injury resulting from mechanical ventilation and oxygen exposure and was characterized by inflammation, fibrosis, and smooth muscle hypertrophy in the airways. Although the most important predisposing conditions for BPD include exposure of the premature lung to hyperoxia, volutrauma, inflammation, and/or atelectrauma, the underlying molecular mechanisms involved in its pathogenesis remain incompletely understood.
Lung alveolar interstitial fibroblasts, namely lipofibroblasts, and their communications with adjacent epithelial cells play an important role in lung development and injury/repair (1, 10, 29, 38). We have previously shown that exposure to 24 h of hyperoxia (95% O2) in a neonatal rat model disrupts the critical alveolar epithelial-mesenchymal paracrine homeostatic signaling pathway, leading to downregulation of the lipofibroblastic phenotype of the mesenchyme (34). This was accompanied by upregulation of the myogenic phenotype along with pulmonary morphological changes that are consistent with BPD. Furthermore, we have demonstrated that concomitant treatment with a peroxisome proliferator-activated receptor-γ (PPARγ) agonist markedly attenuates the molecular and morphological changes secondary to 24-h exposure of neonatal pups to hyperoxia (95% O2), highlighting the critical significance of the pulmonary lipogenic phenotype in protecting against hyperoxia-induced neonatal lung injury (34). However, whether this approach would be effective against prolonged exposure to hyperoxia is not known. Furthermore, the effect of hyperoxia on the transforming growth factor (TGF)-β and wingless/Int (Wnt) signaling pathways, the pathways known to be involved in lung perinatal lung development, is not known. To address these questions, we hypothesized that exposure to prolonged hyperoxia would activate the TGF-β and Wnt signaling pathways and that this effect would be blocked by PPARγ agonists. The involvement of the Wnt and TGF-β signaling pathways in various chronic lung diseases has been described by others either in experimental models or in clinical conditions (2, 7, 16, 27, 45).
MATERIALS AND METHODS
Hyperoxia exposure system and animal protocol.
Adult and neonatal Sprague-Dawley rats were housed in humidity- and temperature-controlled rooms on a 12:12-h light-dark cycle and were allowed food and water ad libitum. On day 22 of pregnancy, dams were allowed to deliver naturally. Next, pups were pooled, randomized, and delivered back to nursing dams. One set of pups was maintained in 95% (vol/vol) O2 while the other set was maintained in room air, 21% (vol/vol) O2. Nursing dams were rotated between hyperoxia and room air-exposed litters every 24 h to prevent oxygen toxicity in the dams. Continuous 95% O2 exposure was achieved in the Plexiglas chamber (77 × 64 × 37 cm) by a flow-through system. The oxygen level inside the Plexiglas chamber was monitored continuously with a Ceramatec (MAXO2) oxygen analyzer. Experimental pups were grouped into control (room air for 7 days + placebo intraperitoneal saline administration), hyperoxia only (95% O2 for 7 days + placebo), and hyperoxia with rosiglitazone (RGZ; Cayman) (95% O2 for 7 days + RGZ: 1 or 3 mg/kg). A 100-μl RGZ solution was administered to each animal intraperitoneally with a microsyringe once a day. After the 7-day experimental period, pups were killed using 0.1 ml Euthasol (sodium pentobarbital 390 + 50 mg/ml phenytoin; Virbac Animal Health) per pup. For some experiments, TOPGAL mice, kindly provided by Saverio Bellusci, PhD (University of Southern California), were subjected to normoxia or hyperoxia following the above-described protocol. All animal procedures were performed following National Institutes of Health (NIH) guidelines for the care and use of laboratory animals and approved by the Los Angeles Biomedical Research Institute Animal Care and Use Committee.
Preparation of lung tissue for histological analysis.
Following euthanasia, pup lungs were fixed in situ by perfusing 4% (wt/vol) paraformaldehyde (PFA) in PBS solution (Boston Bioproducts). During perfusion, a constant inflation pressure of 5 cmH2O was maintained via a tracheal catheter. On completion of perfusion, trachea was ligated with Ethicon surgical suture, and the lungs were incubated in fresh 4% PFA-PBS solution on ice for 4–5 h. Following this incubation, PFA-PBS solution was replaced with two quick changes of cold PBS to remove exterior debris. Finally, the lungs were transferred to a filtered sterile PBS/30% sucrose solution (wt/vol) and stored at 4°C until fully equilibrated. The lungs were paraffin-embedded, and 5-μm transverse sections from a single lung of the pup studied were cut, laid on Superfrost microscope glass slides (Fisher Scientific), and processed according to standard procedures. Processed paraffin sections were stained 1) with hematoxylin-eosin to examine tissue morphology and measure morphometric parameters, or 2) with antibody for in situ immunohistochemistry (IHC).
Lung morphometry.
An investigator unaware of the treatment group for each animal sample performed lung morphometry. Fifty randomly selected, nonoverlapping fields from sections obtained from 12 blocks from each treatment group were included for measurement. Each field was viewed at 100-fold magnification, and the image was digitized and projected on a video monitor. For each field, the number of alveoli was counted visually. The objective assessment of the extent of alveolarization was determined by the radial alveolar count (RAC) and mean linear intercept (MLI) methods. Briefly, RAC were performed by identifying respiratory bronchioles, as described by Randell et al. (33). The number of distal air sacs that were transected by a line drawn from a terminal respiratory bronchiole to the nearest pleural surface was counted. No counts were made if the respiratory bronchiole was nearer to the edge of the slide than to the nearest connective tissue septum. All terminal bronchioles for each of the two sections from each pup were used for radial alveolar counts. MLI, which represents the average alveolar diameter, alveolar septal thickness (AST), and tissue density, which is the proportion of the field occupied by tissue (area occupied by tissue/area occupied by lung tissue + alveoli), were analyzed with Image-Pro Plus image analysis software (Zeiss). Slides were examined at 100-fold magnification, and the septal thickness and tissue density of ≥50 alveoli for each section were measured. At least two sections from each pup were used, and the average septal thickness and tissue density were then calculated for each treatment group.
Immunohistochemical staining of lung sections.
In situ protein expression was assessed by IHC performed with UniTect ABC Kit (Calbiochem-EMD Biosciences). Five-micrometer paraffin sections were deparaffinized in xylene and rehydrated by a sequential ethanol wash. Endogenous avidin and biotin activities and nonspecific antibody binding to tissue were inhibited as per manufacturer's instructions. Tissue was incubated with primary antibody overnight at 4°C in a humidified chamber. For lymphoid enhancer factor 1 (Lef-1) and lipoprotein receptor-related protein 6 (LRP6) IHC, rabbit anti-Lef-1 antibody and mouse monoclonal anti-LRP6 (Santa Cruz Biotechnology), respectively, were used as primary antibodies at 1:400 dilutions. For myeloperoxidase (MPO) immunostaining, polyclonal rabbit anti-MPO (DAKO) was used as primary antibody at 1:600 dilution. On the next day, following PBS washes at room temperature, the tissues were incubated sequentially with appropriate biotinylated secondary antibody, ABC reagent, diaminobenzidine substrate (DAB; Sigma- Aldrich, St. Louis, MO), in the dark. Sections were rinsed with water and counterstained with hematoxylin. Finally, tissue slices were dehydrated in xylene and permanently mounted with VectaMount (Vector Laboratories). Immunostained sections were examined under a microscope (Axioskop 40; Zeiss) at ×400, ×600, or ×1,000 magnifications.
Fibroblast culture.
Neonatal lung fibroblasts were isolated and cultured following the previously described method (34).
Protein extraction and Western blotting.
Liquid nitrogen flash-frozen lung tissue was homogenized with a tissue grinder in lysis buffer (RIPA buffer) containing 1 mM EDTA and EGTA each (Boston Bioproducts), supplemented with 1 mM PMSF, phosphatase inhibitor (Sigma-Aldrich), and complete proteinase inhibitor cocktail (Roche Diagnostics). Fifty micrograms of total protein for each sample was denatured by SDS-PAGE sample buffer and electrophoresed in a 10% SDS polyacrylamide gel. Resolved samples were then transferred onto 0.45-μm nitrocellulose or a PVDF membrane, which, after blocking with TBS-Tween (TBST) + 5% milk, were probed with primary antibodies [PPARγ, 1:500; Lef-1, 1:400; activin receptor-like kinase 5 (ALK-5) or TGF-β receptor I (TGF-β-RI), 1:400; and β-tubulin, 1:1,000, all from Santa Cruz Biotechnology; β-catenin, 1:500, and Smad3 and phosphorylated Smad3 (pSmad3), 1:200, from Cell Signaling Technology; and Smad7, 1:500, from Zymed-Invitrogen]; α-smooth muscle actin (α-SMA), 1:100,000; calponin, 1:5,000, from Sigma-Aldrich; and GAPDH, 1:10,000, from Millipore, overnight at 4°C, followed by appropriate secondary antibody and SuperSignal Chemiluminescent substrate (Pierce). Photographic film (Denville Scientific, Metuchen, NJ) was used to capture protein bands, which were quantified by densitometry. Protein band intensities were normalized for loading using the corresponding GAPDH signals and expressed as arbitrary units (AU). Since GAPDH expression has been described to change altered redox stress (14), we checked whether GAPDH changed in hyperoxia in our experimental setup by using a second control β-tubulin. In our experimental model, neither GAPDH nor β-tubulin change in hyperoxia (P > 0.05; n = 6; data not shown). These data are in accordance with other recent publications (4).
Immunoprecipitation.
Cell extracts were prepared in lysis buffer (20 mM HEPES, 2 mM EGTA, 50 mM β-glycerophosphate, 10% glycerol, 1% Triton X-100, 1 mM dithiothreitol, 1 mM vanadate, and 0.04 mM PMSF) containing a cocktail of protease inhibitors (Sigma-Aldrich). Immunoprecipitation was performed on equal amounts of cell extracts (400 μg of protein) from different treatment conditions using anti-β-catenin antibody (Santa Cruz Biotechnology) preabsorbed on protein G-Sepharose beads for 1 h at 4°C followed by overnight incubation at 4°C and purification of the antibody-protein complex beads by centrifugation. The samples were then resolved by SDS-PAGE followed by Western blotting. The signal was detected using SuperSignal Chemiluminescent substrate (Pierce) in accordance with the manufacturer's protocol.
Statistical analysis.
Experiments were done at least 3 independent times. Differences between the groups were evaluated by one-way ANOVA followed by Newman-Keuls post hoc test and unpaired Student's t-test as needed. A P value of <0.05 was considered to be statistically significant. Data are expressed as means ± SE.
RESULTS
Morphometric analysis of hyperoxia-exposed lungs.
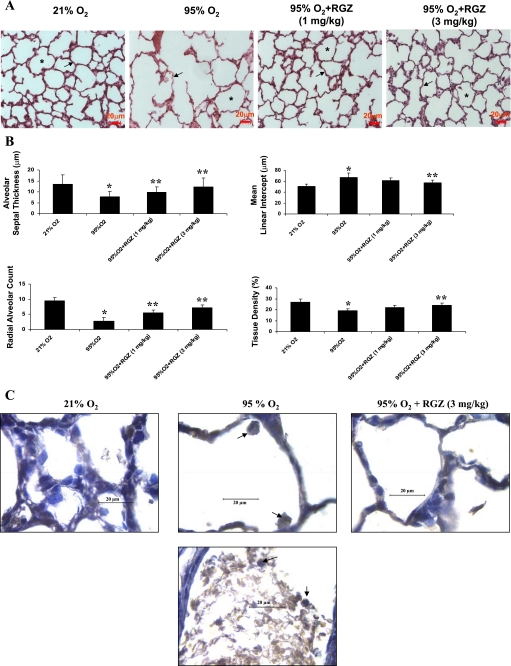
Exposure of neonatal rats to 95% O2 continuously for 7 days resulted in significant arrest in their postnatal lung development. This was reflected by their lung morphology (Fig. 1A). Lungs from the animals exposed to hyperoxia caused failure of secondary septation of distal lung air sacs that resulted in larger than normal alveoli (indicated by asterisks) and marked decrease in the interstitial thickness (indicated by arrows) compared with their normoxic counterparts. On average, compared with the normoxic animals, in the hyperoxic lungs, AST of lungs was reduced by ∼40% (P < 0.05; 95 vs. 21% O2 group), and the RAC was reduced by ∼70% (P < 0.05; 95 vs. 21% O2 group) (Fig. 1B). In accordance with these data, with hyperoxia exposure, tissue density decreased by 35%, and MLI increased by 30% (P < 0.05; 95 vs. 21% O2 group; Fig. 1B). Therefore, hyperoxia of rat lungs caused 1) significant decrease in the thickness of interstitium, and 2) arrested alveolarization compared with the normoxic counterparts. Intraperitoneal administration of RGZ at 1 and 3 mg/kg body wt daily during the course of the hyperoxic exposure significantly reduced these lung aberrations. Thus coadministration of RGZ during hyperoxia significantly prevented the aberrations in principal morphological parameters of the lung cytoarchitecture. Hence, RGZ can mediate protection against oxygen-induced lung injury.
Fig. 1.
A: rosiglitazone (RGZ) prevented hyperoxia-induced aberrations in lung architecture. Seven-day continuous hyperoxia (95% O2) exposure alone caused failure of secondary septation of distal lung air sacs resulting in larger than normal alveoli denoted by asterisks, with marked decrease in interstitial thickness (arrows). These changes were prevented by administration of RGZ (1 and 3 mg/kg ip) concomitant to hyperoxia exposure. Representative hematoxylin-eosin-stained lung sections are shown (magnification, ×40; bar, 20 μm; n = 6). B: morphometric changes of lungs in hyperoxia without and with RGZ treatment. RGZ prevented 7-day hyperoxia (95% O2)-induced decrease in lung alveolar septal thickness, radial alveolar count, alveolar tissue density, and the accompanying increase in mean linear intercept. Significant changes in all of these parameters were observed on exposure to 95% hyperoxia alone, which were prevented by RGZ treatment (*P < 0.05, 95 vs. 21% O2 and **P < 0.05, 95% O2 + RGZ vs. 95% O2 only; n = 6). C: visualization of neutrophils influx in lung of rat pups exposed to 95% O2. Representative myeloperoxidase antibody-stained lung sections (5 μm) are presented. Myeloperoxidase-reactive neutrophils (with characteristic polylobulated nucleus) are seen in hyperoxic alveoli, which are absent in normoxic and RGZ treated lungs. Magnification, ×1,000; arrows point to the myeloperoxidase reactive neutrophils; calibration bar = 20 μm; n = 4.
RGZ inhibits neutrophil influx caused by hyperoxia.
Hyperoxia-induced lung injury is characterized by neutrophil influx as well as by arrested alveolarization. Hence, by MPO immunostaining, we determined neutrophil influx in neonatal lungs exposed to hyperoxia and whether RGZ administration affected this process. MPO immunostaining identified neutrophils in the lungs of hyperoxia-exposed animals. Figure 1C, top, shows neutrophil infiltration in the lung air spaces of hyperoxia-exposed animal, whereas Fig. 1C, bottom, shows influx of neutrophil and other blood cells in the interstitium. This was blocked by RGZ treatment during hyperoxia exposure.
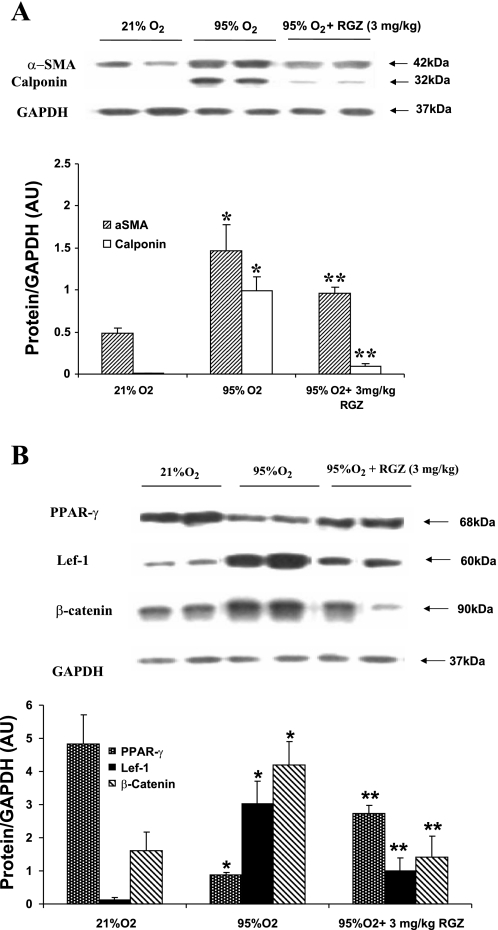
RGZ inhibits the modulation of α-SMA, calponin, PPARγ, Lef-1, and β-catenin by hyperoxia.
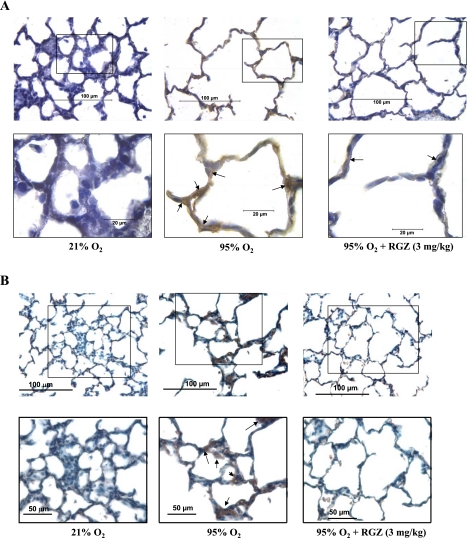
We next examined the protein expression of key markers of lung myogenic and adipogenic differentiation. Two prominent myogenic protein markers, α-SMA and calponin, were upregulated in hyperoxia (P < 0.05 vs. control; Fig. 2A), and RGZ administration significantly inhibited the hyperoxia-induced upregulation of these proteins (P < 0.05 vs. 95% O2 group). Figure 2B shows that, compared with 21% O2 group, 7-day hyperoxia (95% O2) caused a significant decrease in PPARγ expression in whole lung (∼80%; P < 0.05; 95 vs. 21% O2 group). As expected, intraperitoneal injection of RGZ during hyperoxia significantly bolstered PPARγ expression (P < 0.05 vs. 95% O2 group). The downregulation of PPARγ expression was accompanied by a significant increase (P < 0.05 vs. control) in the expression of the canonical Wnt pathway proteins Lef-1 (>10-fold) and β-catenin (∼2.5-fold). These changes were prevented by concomitant administration of RGZ. With RGZ treatment, hyperoxia-induced upregulation of both Lef-1 and β-catenin expression were significantly attenuated (P < 0.05 vs. 95% O2 group). The hyperoxia-induced upregulation of Wnt signaling was further supported by IHC as evidenced by the upregulation of Lef-1 and LRP6 expression in the lung parenchyma (Fig. 3, A and B). The increased expression of these proteins was blocked by intraperitoneal injection of 3 mg/kg RGZ.
Fig. 2.
A: concomitant administration of 3 mg/kg RGZ inhibited hyperoxia (95% O2 for 7 days)-induced changes in α-smooth muscle actin (α-SMA) and calponin expression. α-SMA and calponin were upregulated in hyperoxia, and RGZ administration inhibited the hyperoxia-induced upregulation of these proteins. Representative protein bands in duplicate and densitometric histogram of each group are shown (*P < 0.05, 95 vs. 21% O2, and **P < 0.05, 95% O2 + 3 mg/kg RGZ vs. 95% O2 only; n = 8). B: concomitant administration of 3 mg/kg RGZ inhibited hyperoxia (95% O2 for 7 days)-induced changes in peroxisome proliferator-activated receptor-γ (PPARγ), lymphoid enhancer factor 1 (Lef-1), and β-catenin in whole lung protein extract of 7-day-old rat pups. Seven-day hyperoxia exposure resulted in a significant decrease in PPARγ and a significant increase in Lef-1 and β-catenin protein expression, and concomitant RGZ administration prevented all of these changes. Representative protein bands in duplicate and densitometric histogram of each group are shown (*P < 0.05, 95 vs. 21% O2, and **P < 0.05, 95% O2 + 3 mg/kg RGZ vs. 95% O2 only; n = 8). AU, arbitrary units.
Fig. 3.
A and B: immunostaining for lung Lef-1 and lipoprotein receptor-related protein 6 (LRP6) in neonatal rat pups exposed to 21% O2, 95% O2, or 95% O2 + 3 mg/kg RGZ for 7 days. Representative Lef-1 and LRP6 antibody-stained lung sections (5 μm) are shown. Top: magnification, ×400, with insets. Bottom pictures are inset regions of top; magnification, ×1,000 for Lef-1 and ×600 for LRP6. Arrows point to the lung regions showing Lef-1 and LRP6 staining. At 21% O2 exposure, lung sections have weak base line Lef-1 and LRP6 expression, which is robustly upregulated with 95% O2 exposure. RGZ (3 mg/kg) administration during 95% O2 exposure prevented this upregulation for both Lef-1 and LRP6 (n = 4).
Activation of TGF-β signaling in hyperoxia and inhibition by RGZ.
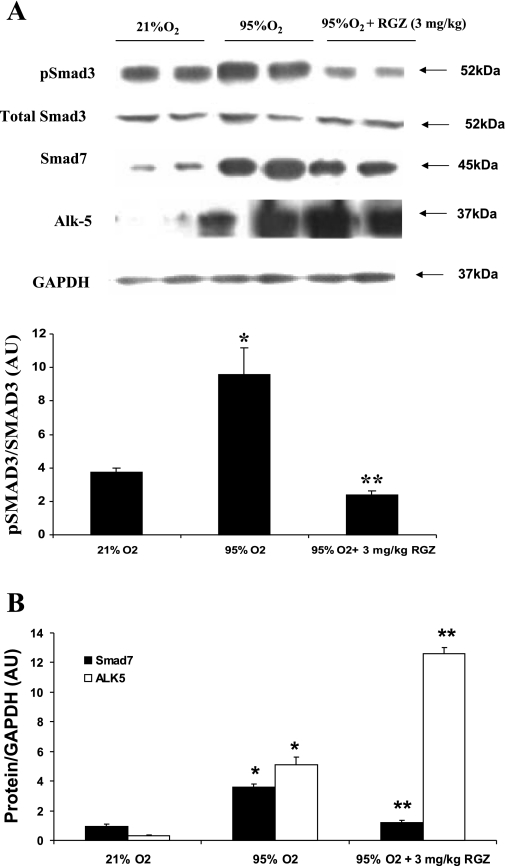
Western blot analysis indicated activation of the TGF-β pathway in hyperoxia as indicated by the significant upregulation of pSmad3 and Smad7 protein levels in hyperoxia (Fig. 4, A and B). Although we did not see any significant perturbation in the total Smad3 level by hyperoxia (95 vs. 21% O2; P > 0.05) or by RGZ (RGZ + 95% O2 group vs. 95% O2 group; P > 0.05), hyperoxia elicited a trend toward downregulation of total Smad3 expression at day 7. In contrast, phosphorylation of Smad3 increased above baseline by ∼2-fold at day 7 in hyperoxia (95 vs. 21% O2; P < 0.05). This change was completely blocked with RGZ administration (RGZ + 95 vs. 95% O2; P < 0.05) as shown by the densitometric analysis of pSmad3 without and with RGZ with respect to total Smad3 (Fig. 4A). The Smad7 expression level in hyperoxia, with or without RGZ, followed the same trend as that of the pSmad3. The Smad7 level also increased significantly following 7-day hyperoxia exposure (95 vs. 21% O2, ∼4-fold; P < 0.05) and was reduced to about one-third by RGZ treatment (95% + RGZ vs. 95% O2; P < 0.05) (Fig. 4B). We then examined the expression of TGF-β-RI (ALK-5), which is upstream in the signal transduction cascade of the TGF-β pathway (24). We noticed robust upregulation (>10-fold, 95 vs. 21% O2; P < 0.05) of ALK-5 expression in hyperoxia (Fig. 4B). However, RGZ did not block the hyperoxia-induced increase in ALK-5 expression (95% O2 + RGZ vs. 95% O2; P > 0.05), indicating that the RGZ-mediated protection against hyperoxia may operate by a mechanism(s) that is downstream from ALK-5.
Fig. 4.
A and B: administration of RGZ inhibited hyperoxia-induced changes in phosphorylated Smad3 (pSmad3) and Smad7 protein levels in whole lung protein extract of 7-day-old rat pups. Although there were no significant perturbations in the total Smad3 levels on exposure to hyperoxia, pSmad3 and Smad7 levels increased significantly. Activin receptor-like kinase 5 (ALK-5) protein level also increased significantly on with hyperoxia (B). Concomitant RGZ treatment prevented hyperoxia-induced increases in pSmad3 and Smad7 protein levels but not that of ALK-5. Representative protein bands in duplicate and densitometric histogram of each group are shown (*P < 0.05, 95 vs. 21% O2, and **P < 0.05, 95% O2 + 3 mg/kg RGZ vs. 95% O2 only; n = 8).
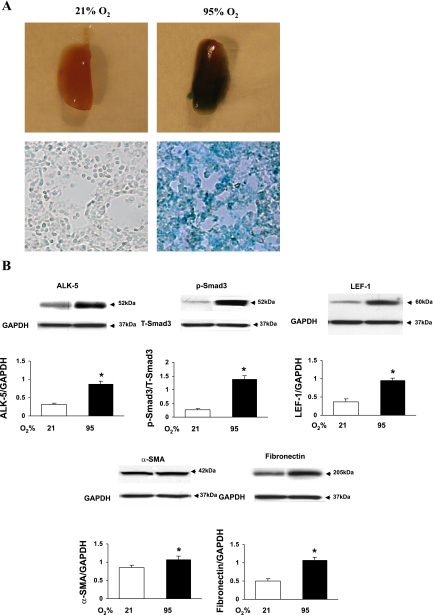
Confirmation of hyperoxia-induced activation of pulmonary TGF-β and Wnt signaling using a genetic model.
For this, we have utilized TOPGAL mice, in which the LacZ-reporter gene is under the control of Wnt signaling-responsive T cell factor (TCF)/Lef binding sites, providing an excellent in vivo model to study Wnt signaling. Exposure of 1-day-old TOPGAL neonatal pups to either 21 or 95% O2 for 7 days resulted in clear activation of Wnt and TGF-β signaling. Hyperoxia-exposed lungs were bluish stained (LacZ positivity) both at the macroscopic and microscopic levels (Fig. 5A). The expression of markers of TGF-β (ALK-5 and pSmad3) and Wnt signaling (Lef-1) and their targets (α-SMA and fibronectin) (Fig. 5B) were significantly increased on hyperoxic exposure compared with controls (P < 0.05 vs. 21% O2).
Fig. 5.
A and B: confirmation of hyperoxia-induced activation of pulmonary transforming growth factor (TGF)-β and wingless/Int (Wnt) signaling in TOPGAL mice. Seven-day exposure to 95% O2 to TOPGAL mice starting on postnatal day 1 resulted in clear evidence of Wnt and TGF-β signaling activation. A shows bluish staining (LacZ positivity) of lung tissue at both macroscopic and microscopic levels. Hyperoxia exposure resulted in increased expression of ALK-5, pSmad3/total Smad3 (T-Smad3), Lef-1, α-SMA, and fibronectin protein levels, clearly indicating activations of TGF-β and Wnt signaling pathways (B; P < 0.05 vs. 21% O2; n = 4).
Evidence for hyperoxia-induced activation of TGF-β and Wnt signaling in alveolar interstitial fibroblasts.
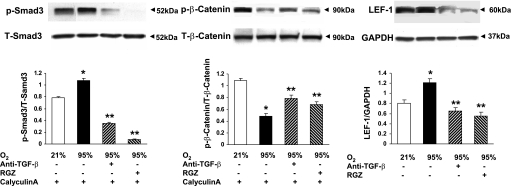
Having proven the activation of the TGF-β and Wnt signaling pathways in whole lung following exposure to hyperoxia and its attenuation by a potent PPAR agonist, RGZ, we next confirmed these findings in cultured alveolar interstitial fibroblasts. Cultured fibroblasts at 80–90% confluence were exposed to 24 h of hyperoxia (95% O2) with or without RGZ pretreatment (10 μM) for 1 h. Furthermore, since lung fibroblasts are a known source of TGF-β, which has been previously suggested to play a prominent role in lung injury/repair (49), in this series of experiments we also examined whether pretreatment with an anti-TGF-β antibody (10 μg/ml; cat. no. 1835; R&D Systems) would block hyperoxia-induced activation of the TGF-β and Wnt signaling pathways. Twenty-four-hour exposure of cultured alveolar interstitial fibroblasts to hyperoxia resulted in significant increases in pSmad3 and Lef-1 accompanied by a significant decrease in phosphorylated β-catenin (p-β-catenin) (P < 0.05 vs. 21% O2), indicating hyperoxia-induced activation of the TGF-β and Wnt signaling pathways (Fig. 6). Interestingly, pretreatment with either anti-TGF-β antibody or RGZ blocked hyperoxia-induced increases in pSmad3 and Lef-1 and a decrease in p-β-catenin (P < 0.05 vs. 95% O2), although this protective effect appeared to be more robust with RGZ (Fig. 6).
Fig. 6.
Evidence for hyperoxia-induced activation of TGF-β and Wnt signaling in alveolar interstitial fibroblasts. Exposure of cultured rat lung alveolar interstitial fibroblasts to 24-h hyperoxia (95% O2) with or without RGZ (10 μM) or anti-TGF-β (10 μg/ml medium) pretreatment resulted in significant increases in pSmad3 (30 min) and Lef-1 (24 h) protein levels, accompanied by a significant decrease in phosphorylated (p-) β-catenin (30 min) protein level. Pretreatment with either anti-TGF-β antibody or RGZ blocked hyperoxia-induced increases in pSmad3 and Lef-1 and a decrease in p-β-catenin (*P < 0.05, 95 vs. 21% O2, and **P < 0.05, 95% O2 + RGZ or 95% O2 + anti-TGF-β vs. 95% O2 only; n = 4). For pSmad3 and p-β-catenin analysis, cells were concomitantly also treated with calyculin A (50 nM).
Evidence for interactions between the TGF-β, Wnt, and PPARγ signaling pathways in alveolar interstitial fibroblasts in response to hyperoxia.
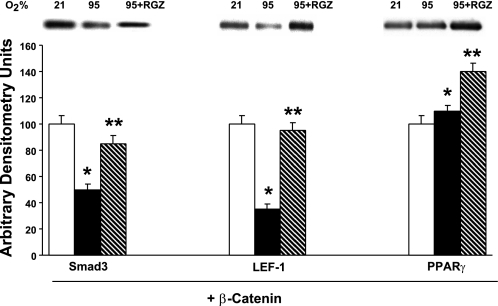
To obtain insights to the mechanism of protection again hyperoxia by PPARγ agonist RGZ, whole cell lysates of cultured alveolar interstitial fibroblasts following 24-h exposure to either normoxia, hyperoxia, or hyperoxia + RGZ (10 μM) were immunoprecipitated with anti-β-catenin antibody. Subjecting these complexes to Western blot analysis using anti-Smad3, anti-Lef-1, and anti-PPARγ antibodies showed that 24-h exposure to hyperoxia resulted in significant decreases in β-catenin-bound Smad3 and Lef-1, whereas levels of β-catenin-bound PPARγ increased (Fig. 7). Furthermore, treatment with RGZ both before and during hyperoxia exposure resulted in attenuation against hyperoxia-induced decreases in Smad3 and Lef-1 bound to β-catenin, whereas the level of β-catenin-bound PPARγ increased further (Fig. 7), providing potential key insights to the mechanism of PPARγ agonist-mediated protection against hyperoxia-induced activations of TGF-β and Wnt signaling pathways.
Fig. 7.
Evidence for interactions between the TGF-β, Wnt, and PPARγ signaling pathways in alveolar interstitial fibroblasts in response to hyperoxia. Immunoprecipitation of whole cell lysates of alveolar interstitial fibroblasts following 24-h exposure to either normoxia or hyperoxia (95% O2), with or without RGZ (10 μM) with anti-β-catenin antibody, and subjecting these complexes to Western blot analysis using anti-Smad3, anti-Lef-1, and anti-PPARγ antibodies showed that hyperoxia resulted in significant decreases in β-catenin-bound Smad3 and Lef-1, whereas levels of β-catenin-bound PPARγ increased. Treatment with RGZ both before and during hyperoxia exposure resulted in attenuation against hyperoxia-induced decreases in Smad3 and Lef-1 bound to β-catenin, whereas the level of β-catenin-bound PPARγ increased further (*P < 0.05, 95 vs. 21% O2, and **P < 0.05, 95% O2 + RGZ vs. 95% O2 only; n = 4).
DISCUSSION
Recently, hyperoxia-induced pulmonary molecular changes in neonatal mouse BPD model have been described (2, 31). In both of these studies, exposure to 85% O2 caused modulation of TGF-β pathway and decreased maturation of the neonatal lung. In our hyperoxia-induced model of neonatal rat lung injury, we found that a 7-day exposure to 95% O2 resulted in significant aberrations in postnatal lung development, characterized by significant arrest of alveolarization, i.e., reduced alveolar count, larger alveolar size, and decreased AST, the structural hallmarks of the new BPD (9, 10, 15, 44a). These structural changes were accompanied by the upregulated expression of TGF-β pathway transducers ALK-5, pSmad3 and Smad7, and two major canonical Wnt signal transducers, namely β-catenin and Lef-1, along with concomitant upregulation of α-SMA and calponin. On the other hand, these changes were accompanied by the downregulation of PPARγ, a key nuclear transcription factor in the lung parenchyma. Hyperoxia also resulted in marked neutrophil influx in both alveolar lumen and interstitium. These hyperoxia-induced morphological and molecular changes were prevented by the concomitant administration of a PPARγ agonist, RGZ. Hyperoxia-induced activation of the TGF-β and Wnt signaling pathways was also confirmed using TOPGAL mice. Mechanistic studies clearly point to physical interactions between key intermediates of the TGF-β, Wnt, and PPARγ signaling pathways, providing a possible mechanism for RGZ-mediated protection against hyperoxia-induced activation of the TGF-β and Wnt signaling pathways. To our knowledge, these data provide the first evidence for the concomitant activation of both TGF-β and Wnt signaling in a neonatal lung injury model and its near complete prevention by PPARγ agonist administration, suggesting a potential role for PPARγ agonists in preventing BPD.
The TGF-β pathway involves binding of the ligand to TGF-β-RII, which, in turn, phosphorylates TGF-β-RI (ALK-5) after forming a heteromeric complex with it. Once phosphorylated, ALK-5 is activated to signal downstream targets, the members of the Smad family of signal transducers, which are critical for transmitting the TGF-β signal to the nucleus. Of these, the receptor-linked, i.e., (R-) Smad3 is particularly notable. Another Smad, the common Smad mediator Smad4, heteromerizes with activated R-Smad, e.g., Smad3, and this Smad3-Smad4 complex translocates to the nucleus to activate TGF-β pathway genes. In addition to these positively activated Smads, the inhibitory, i.e., (I-) Smads, e.g., Smad7, block TGF-β signals. Once activated, the TGF-β pathway may or may not interact with the Wnt pathway. Normally, in the absence of Wnt activation, GSK-3 phosphorylates β-catenin, inducing its degradation by the ubiquitin-proteasome pathway. On activation of Wnt signaling, GSK-3 activity is inhibited, preventing β-catenin degradation, which, in turn, accumulates and translocates to the nucleus, where it binds with Lef-1/TCF, thereby activating the Wnt target genes. Cooperation between TGF-β and Wnt signaling is well-documented since physical interaction between Smad3 and Lef-1 has been shown in a number of studies (13, 20, 22). Furthermore, TGF-β-dependent activation of the target promoter has been shown to require- exposed-1 expression (20, 22). Therefore, it is not surprising that, in response to hyperoxia, we observed activation of both the TGF-β and Wnt pathways. However, in this study, we have not determined whether activation of both pathways was dependent or independent of the activation of each other.
In fact, complex interactions between the Wnt and TGF-β signaling pathways maintain homeostasis and are critical during the early phases of lung development (3, 6, 15, 19, 32). Therefore, it is not unexpected that we observed recapitulation of these pathways during exposure to hyperoxia postnatally, probably in an attempt to limit lung damage and/or to regenerate lung cytoarchitecture following the hyperoxic lung injury (2, 43, 44). This is consistent with the findings of 1) Chilosi et al. (7), who have reported elevated Lef-1 and β-catenin expression in the lungs of patients with idiopathic pulmonary fibrosis, and 2) Nakanishi et al. (28), who have demonstrated increased phosphorylation of Smad2 on chronic 85% O2 inhalation. In addition to the evidence for the activations of both the Wnt and TGF-β signaling pathways in whole lung, on exposure to hyperoxia, our data also specifically demonstrate activation and interactions between these pathways in alveolar interstitial fibroblasts. Taken together, our data indicate an important role of the Wnt and TGF-β pathways in modulating the lung injury response. Furthermore, besides the lung, activation of Wnt and TGF-β signaling has also been implicated in fibrotic diseases of other organs as well such as the kidney (40) and liver (37, 41).
Although there were no significant differences in the levels of total Smad3 between the experimental groups, the pSmad3 level significantly increased in the hyperoxia-exposed group, and this hyperoxia-induced increase was completely blocked in the hyperoxia + RGZ group. The total Smad7 level also increased significantly with hyperoxia, with almost complete abrogation of this increase in the hyperoxia + RGZ group. We view the increase in pSmad3 as a profibrotic response to hyperoxia-mediated activation of TGF-β signaling and the Smad7 increase as an autoinhibitory response to TGF-β activation. The TGF-β-Smad signaling pathway contains an autoinhibitory feedback control, central to which is Smad7, which interacts with ligand-activated ALK-5, interfering with receptor binding and subsequent phosphorylation of receptor-linked Smad3 (15, 24). Smad7 activation is highly regulated. It is induced by TGF-β stimulation and has been shown to completely abrogate the transdifferentiation of hepatic stellate cells to myofibroblasts in a well-established bile duct ligation model of liver fibrosis in rats (11, 30). Tissue repair involving coordinated regulation of cellular proliferation, differentiation, extracellular matrix deposition, and, where appropriate, epithelialization has been shown to be regulated by balanced expression of Smad3 and Smad7 (5, 6, 26).
We attempted to block hyperoxia-induced lung injury by augmenting PPARγ expression in vivo. PPARγ, a member of the steroid hormone receptor superfamily of ligand-activated transcription factors (18), in addition to being the key regulator of adipocyte differentiation, has a myriad of other effects, demonstrated both in vitro and in animal models (21, 36). We (34) have shown that it is a critical modulator of the lung alveolar interstitial fibroblast phenotype. The thiazolidinedione group of drugs, such as RGZ, are high-affinity synthetic ligands that activate PPARγ (47). Following ligand binding to its receptor, PPARγ heterodimerizes with the retinoic X receptor, activating the PPARγ-response elements in the promoters for PPARγ target genes, e.g., the adipogenic differentiation-related genes in lung alveolar interstitial lipofibroblasts. Furthermore, since reciprocating interactions between the Wnt and adipogenesis signaling pathways have previously been reported (35), we hypothesized that upregulation of PPARγ by the exogenous administration of the PPARγ agonist RGZ would mitigate hyperoxia-mediated upregulation of Wnt signaling. As expected, concomitant administration of RGZ blocked the hyperoxia-induced upregulation of β-catenin, Lef-1, and α-SMA, all of which are Wnt pathway-related intermediates. Although the exact mechanism of how RGZ might have prevented the hyperoxia-induced upregulation of the Wnt signaling pathway is not clear, both functional and physical interactions between β-catenin and PPARγ involving the Lef-1 binding domain of β-catenin and the β-catenin binding domain within PPARγ have been reported (23). The immunoprecipitation data obtained from cultured fibroblasts exposed to various experimental conditions provide further key insights to the mechanism of hyperoxia-induced activation of the TGF-β and Wnt signaling pathways and their prevention with RGZ treatment. The observation that significant decreases in β-catenin-bound pSmad3 and Lef-1 allows for the increased transcriptional activities of Smad3 and Lef-1 is consistent with the activation of the TGF-β and Wnt signaling pathways, respectively. We propose that the increased PPARγ binding to β-catenin on exposure to hyperoxia is a protective response, and treatment with a PPARγ agonist greatly bolsters this response, preventing Smad3 and Lef-1 transcriptional activities, key components of TGF-β and Wnt signaling pathway activation.
Furthermore, prevention of hyperoxia-induced activation of Wnt signaling and the consequent increase in expression of α-SMA is also consistent with other reports that have shown that PPARγ agonists can have a potent ameliorating effect against fibrotic diseases in many other organs such as the heart (14), kidney (31), and liver (48, 50). PPARγ-mediated suppression of Wnt/β-catenin signaling during adipogenesis further lends credence to our findings (25). Finally, it is noteworthy that RGZ treatment inhibited the hyperoxia-induced neutrophil alveolar influx, possibly by modulating neutrophil PPARγ expression, which is an important regulator of neutrophil function (46).
In summary, treatment with RGZ during exposure of neonatal rats to hyperoxia continuously for 7 days not only prevented the hyperoxia-induced activation of TGF-β and Wnt signaling pathways in the whole lung, but also the associated lung morphological and immunocytochemical changes, suggesting a potential therapeutic usefulness of this class of drugs in ameliorating hyperoxia-induced neonatal lung injury that leads to BPD. Although further work needs to be performed to establish a safe and effective PPARγ agonist for clinical use in neonates, the work presented here clearly shows promise of this class of drugs in combating neonatal lung injury.
GRANTS
This study was supported by grants from the NIH (HL-55268, HL-075405, and HD-051857), Tobacco-Related Disease Research Program (14RT-0073, 15IT-0250, and 17RT-0170), and American Heart Association (10868-02).
Acknowledgments
We are grateful to Rachelle Bugtong for help with this manuscript.
REFERENCES
- 1.Adamson IY, Young L, King GM. Reciprocal epithelial: fibroblast interactions in the control of fetal and adult rat lung cells in culture. Exp Lung Res 17: 821–835, 1991. [DOI] [PubMed] [Google Scholar]
- 2.Alejandre-Alcázar MA, Kwapiszewska G, Reiss I, Amarie OV, Marsh LM, Sevilla-Pérez J, Wygrecka M, Eul B, Köbrich S, Hesse M, Schermuly RT, Seeger W, Eickelberg O, Morty RE. Hyperoxia modulates TGF-β/BMP signaling in a mouse model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol 292: L537–L549, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Ask K, Bonniaud P, Maass K, Eickelberg O, Margetts PJ, Warburton D, Groffen J, Gauldie J, Kolb M. Progressive pulmonary fibrosis is mediated by TGF-beta isoform 1 but not TGF-beta3. Int J Biochem Cell Biol 40: 484–495, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bixby CE, Ibe BO, Abdallah MF, Zhou W, Hislop AA, Longo LD, Raj JU. Role of platelet activating factor in pulmonary vascular remodeling associated with chronic high altitude hypoxia in ovine fetal lambs. Am J Physiol Lung Cell Mol Physiol 293: L1475–L1482, 2007. [DOI] [PubMed] [Google Scholar]
- 5.Bonniaud P, Kolb M, Galt T, Robertson J, Robbins C, Stampfli M, Lavery C, Margetts PJ, Roberts AB, Gauldie J. Smad3 null mice develop airspace enlargement and are resistant to TGF-beta-mediated pulmonary fibrosis. J Immunol 173: 2099–2108, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Chen H, Sun J, Buckley S, Chen C, Warburton D, Wang XF, Shi W. Abnormal mouse lung alveolarization caused by Smad3 deficiency is a developmental antecedent of centrilobular emphysema. Am J Physiol Lung Cell Mol Physiol 288: L683–L691, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Chilosi M, Poletti V, Zamò A, Lestani M, Montagna L, Piccoli P, Pedron S, Bertaso M, Scarpa A, Murer B, Cancellieri A, Maestro R, Semenzato G, Doglioni C. Aberrant Wnt/β-Catenin pathway activation in idiopathic pulmonary fibrosis. Am J Pathol 162: 1495–1502, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chye JK, Gray PH. Rehospitalization and growth of infants with bronchopulmonary dysplasia: a matched control study. J Paediatr Child Health 31: 105–111, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Coalson JJ, Winter V, deLmos RA. Decreased alveolarization in baboon survivors with bronchopulmonary dysplasia. Am J Respir Crit Care Med 152: 640–646, 1995. [DOI] [PubMed] [Google Scholar]
- 10.Demayo F, Minoo P, Plopper CG, Schuger L, Shannon J, Torday JS. Mesenchymal-epithelial interactions in lung development and repair: are modeling and remodeling the same process? Am J Physiol Lung Cell Mol Physiol 283: L510–L517, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Dooley S, Hamzavi J, Breitkopf K, Wiercinska E, Said HM, Lorenzen J, Ten Dijke P, Gressner AM. Smad7 prevents activation of hepatic stellate cells and liver fibrosis in rats. Gastroenterology 125: 178–191, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Escoubet B, Planès C, Clerici C. Hypoxia increases glyceraldehyde-3-phosphate dehydrogenase transcription in rat alveolar epithelial cells. Biochem Biophys Res Commun 266: 156–161, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Furuhashi M, Yagi K, Yamamoto H, Furukawa Y, Shimada S, Nakamura Y, Kikuchi A, Miyazono K, Kato M. Axin facilitates Smad3 activation in the transforming growth factor beta signaling pathway. Mol Cell Biol 21: 5132–5141, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao DF, Niu XL, Hao GH, Peng N, Wei J, Ning N, Wang NP. Rosiglitazone inhibits angiotensin II-induced CTGF expression in vascular smooth muscle cells-role of PPAR-gamma in vascular fibrosis. Biochem Pharmacol 73: 185–197, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Gauldie J, Kolb M, Ask K, Martin G, Bonniaud P, Warburton D. Smad3 signaling involved in pulmonary fibrosis and emphysema. Proc Am Thorac Soc 3: 696–702, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gonzalez AV, Le Bellego F, Ludwig MS. Imbalance of receptor-regulated and inhibitory Smads in lung fibroblasts from bleomycin-exposed rats. Am J Respir Cell Mol Biol 36: 206–212, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Husain NA, Siddiqui NH, Stocker JR. Pathology of arrested acinar development in postsurfactant bronchopulmonary dysplasia. Hum Pathol 29: 710–717, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Issemann I, Green S. Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature 347: 645–650, 1990. [DOI] [PubMed] [Google Scholar]
- 19.Kim K, Lu Z, Hay ED. Direct evidence for a role of beta-catenin/LEF-1 signaling pathway in induction of EMT. Cell Biol Int 26: 463–476, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Labbe E, Lock L, Letamendia A, Gorska AE, Gryfe R, Gallinger S, Moses HL, Attisano L. Transcriptional cooperation between the transforming growth factor-beta and Wnt pathways in mammary and intestinal tumorigenesis. Cancer Res 67: 75–84, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Lehrke M, Lazar MA. The many faces of PPARγ. Cell 123: 993–999, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Letamendia A, Labbe E, Attisano L. Transcriptional regulation by Smads: crosstalk between the TGF-beta and Wnt pathways. J Bone Joint Surg Am 83: 31–39, 2001. [PubMed] [Google Scholar]
- 23.Liu J, Wang H, Zuo Y, Farmer SR. Functional interaction between peroxisome proliferator-activated receptor gamma and beta-catenin. Mol Cell Biol 26: 5827–5837, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Massagué J TGF-beta signal transduction. Annu Rev Biochem 67: 753–791, 1998. [DOI] [PubMed] [Google Scholar]
- 25.Moldes M, Zuo Y, Morrison RF, Silva D, Park BH, Liu J, Farmer SR. Peroxisome-proliferator-activated receptor gamma suppresses Wnt/beta-catenin signaling during adipogenesis. Biochem J 376: 607–613, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morris DG, Huang X, Kaminski N, Wang Y, Shapiro SD, Dolganov G, Glick A, Sheppard D. Loss of integrin alpha(v)beta6-mediated TGF-beta activation causes Mmp12-dependent emphysema. Nature 422: 169–173, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Morrisey EE Wnt signaling and pulmonary fibrosis. Am J Pathol 162: 1393–1397, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakanishi H, Sugiura T, Streisand JB, Lonning SM, Roberts JD Jr. TGF-β neutralizing antibodies improve pulmonary alveologenesis and vasculogenesis in the injured newborn lung. Am J Physiol Lung Cell Mol Physiol 293: L151–L161, 2007. [DOI] [PubMed] [Google Scholar]
- 29.O'Reilly MA, Stripp BR, Pryhuber GS. Epithelial-mesenchymal interactions in the alteration of gene expression and morphology following lung injury. Microsc Res Tech 38: 473–479, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Osawa Y, Seki E, Adachi M, Taura K, Kodama Y, Siegmund SV, Schwabe RF, Brenner DA. Systemic mediators induce fibrogenic effects in normal liver after partial bile duct ligation. Liver Int 26: 1138–1147, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Panchapakesan U, Sumual S, Pollock CA, Chen X. PPARγ agonists exert antifibrotic effects in renal tubular cells exposed to high glucose. Am J Physiol Renal Physiol 289: F1153–F1158, 2005. [DOI] [PubMed] [Google Scholar]
- 32.Pongracz JE, Stockley RA. Wnt signaling in lung development and diseases. Respir Res 7: 15, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Randell SH, Mercer RR, Young SL. Postnatal growth of pulmonary acini and alveoli in normal and oxygen-exposed rats studied by serial section reconstructions. Am J Anat 186: 55–68, 1989. [DOI] [PubMed] [Google Scholar]
- 34.Rehan VK, Wang Y, Patel S, Santos J, Torday JS. Rosiglitazone, a peroxisome proliferators-activated receptor-γ agonist, prevents hyperoxia-induced neonatal rat lung injury in vivo. Pediatr Pulmonol 41: 558–569, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA. Inhibition of adipogenesis by Wnt signaling. Science 289: 950–953, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Scheen AJ, Paquot N. PPAR-gamma receptors, new therapeutic target in metabolic and cardiovascular diseases. Rev Med Liege 60: 89–95, 2005. [PubMed] [Google Scholar]
- 37.Seyhan H, Hamzavi J, Wiercinska E, Gressner AM, Mertens PR, Kopp J, Horch RE, Breitkopf K, Dooley S. Liver fibrogenesis due to cholestasis is associated with increased Smad7 expression and Smad3 signaling. J Cell Mol Med 10: 922–932, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shannon JM, Pan T, Nielsen LD, Edeen KE, Mason RJ. Lung fibroblasts improve differentiation of rat type II cells in primary culture. Am J Respir Cell Mol Biol 24: 235–244, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Short EJ, Klein NK, Lewis BA, Fulton S, Eisengart S, Kercsmar C, Baley J, Singer LT. Cognitive and academic consequences of bronchopulmonary dysplasia and very low birth weight: 8-year-old outcomes. Pediatrics 112: e359–e366, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Surendran K, Schiavi S, Hruska KA. Wnt-dependent beta-catenin signaling is activated after unilateral ureteral obstruction, and recombinant secreted frizzled-related protein 4 alters the progression of renal fibrosis. J Am Soc Nephrol 16: 2373–2384, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Thompson MD, Monga SP. WNT/beta-catenin signaling in liver health and disease. Hepatology 45: 1298–1305, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Torday JS, Rehan VK. The evolutionary continuum from lung development to homeostasis and repair. Am J Physiol Lung Cell Mol Physiol 292: L608–L611, 2007. [DOI] [PubMed] [Google Scholar]
- 44.Torday JS, Rehan VK. Deconvoluting lung evolution using functional/comparative genomics. Am J Respir Cell Mol Biol 31: 8–12, 2004. [DOI] [PubMed] [Google Scholar]
- 44a.Torday JS, Torres E, Rehan VK. The role of fibroblast transdifferentiation in lung epithelial cell proliferation, differentiation, and repair in vitro. Pediatr Pathol Mol Med 22: 189–207, 2003. [DOI] [PubMed] [Google Scholar]
- 45.Venkatesan N, Pinni L, Ludwig MS. Changes in Smad expression and subcellular localization in bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 287: L1342–L1347, 2004. [DOI] [PubMed] [Google Scholar]
- 46.Weinberger B, Quizon C, Vetrano AM, Archer F, Laskin JD, Laskin DL. Mechanisms mediating reduced responsiveness of neonatal neutrophils to lipoxin A4. Pediatr Res 64: 393–398, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wilson TM, Brown PJ, Sternbach DD, Henke BR. The PPARs: from orphan receptors to drug discovery. J Med Chem 43: 527–550, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Yang L, Chan CC, Kwon OS, Liu S, McGhee J, Stimpson SA, Chen LZ, Harrington WW, Symonds WT, Rockey DC. Regulation of peroxisome proliferator-activated receptor-γ in liver fibrosis. Am J Physiol Gastrointest Liver Physiol 291: G902–G911, 2006. [DOI] [PubMed] [Google Scholar]
- 49.Zhang K, Flanders KC, Phan SH. Cellular localization of transforming growth factor-beta expression in bleomycin-induced pulmonary fibrosis. Am J Pathol 147: 352–361, 1995. [PMC free article] [PubMed] [Google Scholar]
- 50.Zhao C, Chen W, Yang L, Chen L, Stimpson SA, Diehl AM. PPARgamma agonists prevent TGFbeta1/Smad3-signaling in human hepatic stellate cells. Biochem Biophys Res Commun 350: 385–391, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]