Abstract
As part of a larger clinical drug-drug interaction (DDI) study aimed at in vitro to in vivo prediction of HIV protease inhibitor metabolic and transporter-based DDIs, we measured the inductive (staggered administration) and inductive plus inhibitory (simultaneously administered) effect of multiple dose ritonavir (RTV), nelfinavir (NFV), or rifampin (RIF) on the pharmacokinetics of the P-glycoprotein probe, digoxin (DIG), when administered simultaneously or staggered with the protease inhibitors or RIF. In both cases, NFV did not significantly affect DIG disposition. RTV decreased DIG renal clearance (Clrenal) when administered simultaneously or staggered but significantly increased DIG area under the curve from time zero to 24 h (AUC0–24 h) only when administered simultaneously. RIF decreased DIG AUC0–24 h only when RIF and DIG administration was staggered. When RIF and DIG were administered simultaneously, DIG maximal observed plasma concentration and area under the curve from time zero to 4 h were significantly increased, and DIG Clrenal was decreased. An unexpected and potentially clinically significant DDI was observed between DIG and the CYP2B6 probe, bupropion, which decreased DIG AUC0–24 h 1.6-fold and increased Clrenal 1.8-fold. Because this was an unexpected DDI and our studies were not specifically designed to quantify this interaction, further studies are required to confirm the interaction and understand the mechanistic basis of the DDI. In summary, RTV or NFV do not induce P-glycoprotein activity measured with DIG, and RIF does so only under staggered administration.
Introduction
Clinical use of the HIV protease inhibitors (PIs) is complicated by the profound, paradoxical, and unpredictable nature of drug-drug interactions (DDIs) with the PIs (Unadkat and Wang, 2000). Many of these DDIs arise from potent inhibition or inactivation of CYP3A by the PIs (Josephson, 2010). In addition, the PIs are known to be in vitro inducers or inhibitors of many cytochrome P450 (P450) enzymes including CYP3A and the drug efflux pump P-glycoprotein (P-gp) (Dixit et al., 2007; Hsiao et al., 2008). These multiple modes of interaction are likely the cause of the unpredictable and paradoxical nature of DDIs with the PIs. For example, many of the PIs are believed to be predominantly cleared in vivo by CYP3A and/or P-gp, but they are capable of inducing their own clearance [ritonavir (RTV) and nelfinavir (NFV)] or the clearance of other PIs. In addition, multiple-dose RTV has no effect on the clearance of the CYP3A probe drug alprazolam where on acute dosing of RTV, the clearance of alprazolam is decreased. These DDIs have been hypothesized to be the result of net induction of CYP3A in vivo but may also be the result of induction of other P450 enzymes or drug transporters significantly contributing to the clearance of the PIs or alprazolam. In an effort to understand these paradoxical DDIs with the PIs, we designed two DDI studies in healthy volunteers to determine whether RTV or NFV and the induction positive control rifampin (RIF) are net inducers of CYP3A, inducers of other P450 enzymes and/or inducers of P-gp. In our first manuscript from these studies, we showed that multiple-dose treatment with RTV or NFV does not result in net induction of CYP3A, rather CYP3A activity is substantially decreased (Kirby et al., 2011b). In our second manuscript, we showed that RTV or NFV does in fact induce CYP1A2, CYP2B6, and CYP2C9, but the magnitude of induction is not substantial enough to explain the induced clearance of the PIs or alprazolam (Kirby et al., 2011a). Therefore, in this manuscript, we determine whether induction of P-gp by RTV or NFV would provide explanations for these paradoxical DDIs.
P-gp is highly expressed in the intestine and is thought to play a role in the absorption of P-gp substrates such as DIG, the PIs, as well as other drugs (Endres et al., 2006). After oral administration of P-gp inhibitors, the inhibitor concentrations in the intestinal lumen and portal vein are expected to be high and therefore can potentially produce profound inhibition and/or induction of intestinal and/or hepatic P-gp and CYP3A activity. Because of this potential for simultaneous inhibition and induction of P-gp or P450 enzymes, the design of clinical DDI induction studies is critical for accurate interpretation of study outcomes from a mechanistic perspective (e.g., induction of P-gp). Therefore, in our study, we administered the P-gp probe drug DIG in a staggered and simultaneous manner with RTV, NFV, or the induction positive control RIF.
Herein, we describe the effect of multiple-dose treatment of RTV, NFV, or RIF administered in a staggered or simultaneous fashion on the pharmacokinetics of DIG as a marker for P-gp activity. In addition, we describe an unexpected DDI between DIG and bupropion (BUP; a CYP2B6 probe) that may be clinically significant.
Materials and Methods
Study Design.
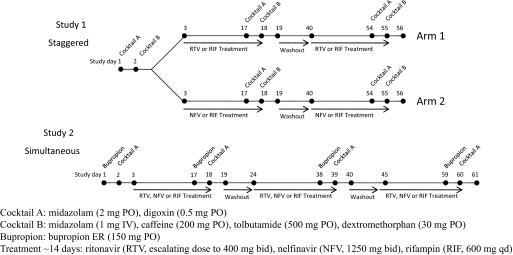
The general study design, subject selection criteria, and subject safety monitoring have been described in detail in our previous manuscript (Kirby et al., 2011b) (Fig. 1). The design of the study with respect to the P-gp-mediated DDIs is described herein. In brief, DIG (0.5 mg p.o.) was used to probe P-gp activity in the intestine, liver, and kidney as part of a larger DDI study to determine the inductive effect of ∼14 days of treatment with RTV, NFV, or RIF in healthy volunteers. In study 1, we administered the probe drug cocktails staggered by ∼12 h after the dose of the inducers, RTV, NFV, or RIF. This staggered design allowed a more accurate assessment of induction because the likelihood of reversible inhibition by RTV, NFV, or RIF was minimized. In study 2, we measured the combined effect (induction and inhibition) by simultaneous administration of RTV, NFV, or RIF with the probe drugs. In study 2, to measure the effect of RTV, NFV, or RIF on CYP2B6 activity, we also administered BUP in a staggered fashion similar to administration of P450 probe drugs in study 1 (the results are presented in Kirby et al. (2011a). BUP was not included in study 1, because a validated phenotyping cocktail containing BUP was not available. BUP was administered on the first of two consecutive study days and DIG and midazolam (P-gp and CYP3A probes) were administered on the second day (∼24 h after BUP) (Kirby et al., 2006). Blood and urine samples were collected before and up to 48 h after probe drug administration. Although desirable, we were unable to sample blood and urine for longer periods because of difficulty in recruiting subjects willing to collect over longer periods. Plasma and urine samples were stored at −20°C until analysis.
Fig. 1.
Study design showing administration of probe drug cocktails before (control) and after RTV, NFV, or RIF treatment. In study 1 (staggered), the probe drug cocktails were staggered ∼12 h after the last dose of RTV, NFV, or RIF. In study 2 (simultaneous), a dose of RTV, NFV, or RIF was simultaneously administered with MDZ and digoxin.
Study Drugs, Chemicals, and Reagents.
All study drugs were supplied by the University of Washington Investigational Drug Services (Seattle, WA). Study drugs were purchased from the following suppliers: DIG (Lanoxin, 0.25-mg tablets; GlaxoSmithKline, Philadelphia, PA), NFV (625-mg tablets; Agouron Pharmaceuticals, La Jolla, CA), RTV (100-mg tablets; Abbott Laboratories, Abbott Park, IL), and RIF (300-mg capsules; Novartis, Basel, Switzerland).
Digoxin Analysis.
Reference standards of DIG and digitoxin (internal standard for DIG analysis) were purchased from MP Biomedicals (Solon, OH). Optima grade water, methanol, and methyl t-butyl ether were purchased from Thermo Fisher Scientific (Waltham, MA). All other chemicals used were reagent grade or higher. Plasma and urine samples were assayed for DIG concentration following a previously published method using a liquid/liquid extraction and liquid chromatography/mass spectrometry detection (Kirby et al., 2008).
Pharmacokinetic Analysis.
Noncompartmental analysis of the plasma concentration-time profiles of DIG was performed using WinNonlin Professional version 5.0 (Pharsight, Mountain View, CA). Parameters estimated included area under the plasma concentration-time curve (AUC0-t) with t = 4 and 24 h, maximal plasma concentration (Cmax), time of maximal plasma concentration (Tmax). Renal clearance (Clrenal) of DIG was estimated by the ratio of total amount of DIG excreted in the urine over 24 h (Ae,0–24 h) and area under the curve from time zero to 24 h (AUC0–24 h). DIG t1/2β and oral clearance were not estimated because of the limited sampling time (24 h) after DIG administration.
Statistical Analyses.
Statistical analysis was conducted on log-transformed pharmacokinetic parameters. This was performed by calculating the geometric mean ratio by exponentiation of the average difference of log transformed pharmacokinetic parameters. If the 90% confidence interval (CI) of this geometric mean ratio included the value of unity, the treatment was considered to not have significantly altered the pharmacokinetic parameter. Because of an unexpected DDI between DIG and BUP (administered 24 h before DIG in study 2), we compared the pharmacokinetic parameters of DIG before and after treatment with RTV, NFV, or RIF in both studies using an unpaired Student's t test assuming equal variance. A p value of ≤0.05 was considered to be statistically significant.
Using historical data of DIG pharmacokinetics in healthy volunteers, we conducted an a priori power analysis using plasma AUC as the primary outcome measure. Assuming equal variance between control and treatment groups, our analysis indicated that n = 7 would provide 80% power (α < 0.05) to discern a 30% change in plasma AUC of DIG.
Results
Subject demographics, treatment periods for RTV, NFV, RIF, and cocktail administration were described previously (Kirby et al., 2011b). In brief, 16 healthy volunteers (33 ± 9 years, 78 ± 14 kg, 5 males and 11 females) completed study 1 (staggered administration) with n = 16, 7, 8, and 16 completing the control, NFV, RTV, and RIF treatment, respectively (one subject did not complete the NFV treatment period). Nine subjects (29 ± 9 years, 79 ± 14 kg, 3 males and 6 females) completed study 2 (simultaneous administration) (Fig. 1).
Effect of RTV, NFV, or RIF on P-gp Activity (Digoxin).
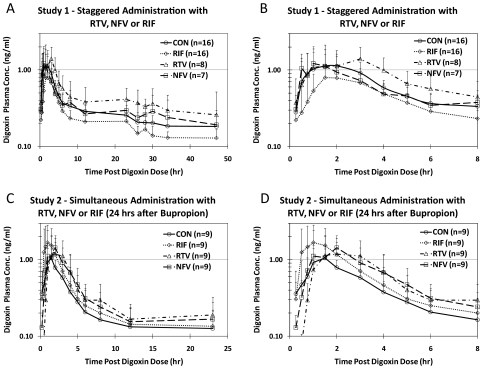
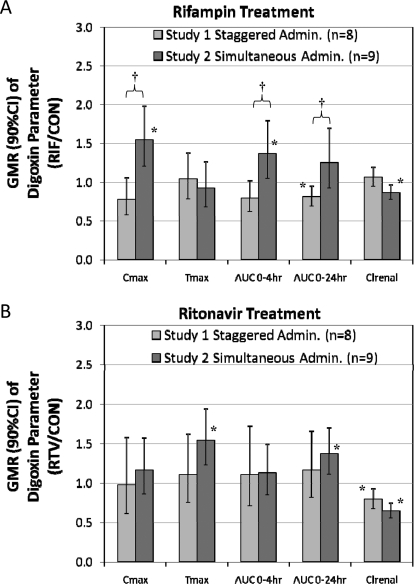
The average plasma concentration-time profiles of DIG before and after NFV, RTV, or RIF treatment in studies 1 and 2 are shown in Fig. 2. Compared with pretreatment, RIF (staggered dosing) significantly but modestly decreased DIG AUC0–24 h (0.81, 90% CI 0.69–0.96), which is a composite of intestinal, hepatic, and renal P-gp activity. Staggered RIF dosing did not affect Clrenal of DIG. No change was observed in DIG AUC0–4 h (0.79, CI 0.62–1.02) or Cmax (0.78, CI 0.57–1.06), indicators of intestinal P-gp activity. In contrast, simultaneous administration of RIF (study 2) significantly increased DIG Cmax (1.55, CI 1.20–1.99), AUC0–4 h (1.37, CI 1.05–1.80), and decreased Clrenal (0.87, CI 0.78–0.97) (Table 1; Fig. 3A). Compared with staggered RIF administration, simultaneous administration of RIF significantly increased DIG Cmax, AUC0–4 h, and AUC0–24 h.
Fig. 2.
Average (±S.D.) plasma concentration-time profiles for DIG before treatment (control) and after RTV, NFV, or RIF treatment for study 1 (staggered administration) (A and B) and study 2 (simultaneous administration) (C and D). A and C show the full profiles, whereas B and D show only the first 8 h after DIG dosing.
TABLE 1.
Pharmacokinetic parameters of digoxin before (control) and after nelfinavir, ritonavir, or rifampin treatment
Bold values are statistically significant (90% CI does not include 1.00).
| Parameter | Controla |
Nelfinavirb |
Ritonavirc |
Rifampind |
|||
|---|---|---|---|---|---|---|---|
| Average ± S.D. | Average ± S.D. | GMR (90% CI) | Average ± S.D. | GMR (90% CI) | Average ± S.D. | GMR (90% CI) | |
| Study 1: staggered administration | |||||||
| Cmax, ng/ml | 1.34 ± 0.53 | 1.44 ± 0.96 | 1.05 (0.63–1.74) | 1.59 ± 0.80 | 0.98 (0.61–1.57) | 1.02 ± 0.38 | 0.78 (0.57–1.06) |
| Tmax, h | 1.66 ± 0.77 | 2.56 ± 2.47 | 1.32 (0.66–2.64) | 2.13 ± 1.03 | 1.10 (0.75–1.62) | 1.82 ± 0.85 | 1.04 (0.78–1.38) |
| AUC0–4 h, h · ng/ml | 2.96 ± 0.97 | 3.08 ± 1.64 | 1.05 (0.63–1.74) | 4.13 ± 2.15 | 1.10 (0.71–1.73) | 2.26 ± 0.67 | 0.79 (0.62–1.02) |
| AUC0–24 h, h · ng/ml | 8.66 ± 2.57 | 9.46 ± 2.93 | 1.23 (1.00–1.48) | 12.7 ± 6.22 | 1.16 (0.81–1.66) | 6.83 ± 1.68 | 0.81 (0.69–0.96) |
| Clrenal, ml/min | 146 ± 43.3 | 141 ± 65.4 | 0.87 (0.65–1.16) | 115 ± 39.1 | 0.79 (0.67–0.93) | 162 ± 51.4 | 1.06 (0.95–1.19) |
| Study 2: simultaneous administration (24 h after BUP) | |||||||
| Cmax, ng/ml | 1.24 ± 0.35 | 1.66 ± 0.81 | 1.23 (0.91–1.68) | 1.55 ± 0.71 | 1.16 (0.86–1.57) | 2.12 ± 1.04 | 1.55* (1.20–1.99) |
| Tmax, h | 1.56 ± 0.64 | 2.21 ± 1.04 | 1.38 (0.93–2.05) | 2.30 ± 0.89 | 1.54 (1.22–1.94) | 1.56 ± 0.89 | 0.93 (0.68–1.26) |
| AUC0–4 h, h · ng/ml | 2.47 ± 0.49 | 3.14 ± 1.68 | 1.02 (0.59–1.74) | 3.06 ± 1.41 | 1.13 (0.85–1.49) | 3.71 ± 1.72 | 1.37* (1.05–1.80) |
| AUC0–24 h, h · ng/ml | 5.45 ± 1.99* | 7.35 ± 3.50 | 1.20 (0.90–1.60) | 7.59 ± 3.14 | 1.37 (1.11–1.70) | 7.08 ± 3.25 | 1.25* (0.93–1.70) |
| Clrenal, ml/min | 258 ± 64.9* | 236 ± 83.0 | 0.92 (0.75–1.13) | 168 ± 46.3 | 0.64 (0.55–0.75) | 225 ± 59.8 | 0.87 (0.78–0.97) |
GMR, geometric mean ratio.
Values are significantly different between Studies 1 and 2 (unpaired t test, p < 0.05).
Study 1 n = 16, Study 2 n = 9;
Study 1 n = 7, Study 2 n = 9;
Study 1 n = 8, Study 2 n = 9;
Study 1 n = 16, Study 2 n = 9.
Fig. 3.
Comparison of staggered versus simultaneous administration of RIF (A) or RTV (B) after ∼14 days of treatment on the pharmacokinetics of DIG. Staggered administration of RIF and DIG did not significantly alter DIG Cmax, Tmax, AUC0–4 h or Clrenal but significantly decreased AUC0–24 h of DIG compared with pretreatment. Upon simultaneous administration of RIF and DIG, these effects were reversed, showing only a statistically significant increase in DIG Cmax, AUC0–4 h, and Clrenal compared with pretreatment. Staggered versus simultaneous administration of RIF and DIG significantly altered the Cmax, AUC0–4 h, and AUC0–24 h, but not the Tmax, or Clrenal of DIG. Simultaneous administration of RIF and DIG masked the apparent induction of intestinal and/or hepatic P-gp by RIF and resulted in an apparent increase in DIG bioavailability. No statistically significant difference in DIG pharmacokinetic parameters was observed between staggered and simultaneous administration for RTV. *, 90% CI does not include unity; therefore, the treatment significantly altered the parameter relative to control. †, p < 0.05 unpaired t test comparison of treatment/control between studies 1 and 2.
Multiple doses of NFV had no significant effect on any measured pharmacokinetic parameters of DIG in either study 1 or study 2 (Table 1). Multiple doses of RTV significantly decreased DIG Clrenal when administered in a staggered (0.79, CI 0.67–0.93) or simultaneous manner (0.64, CI 0.55–0.75) (Table 1; Fig. 3B). RTV did not alter other DIG pharmacokinetic parameters after staggered administration of RTV. In the simultaneous administration study, RTV significantly increased DIG Tmax (1.54, CI 1.22–1.94) and AUC0–24 h (1.37, CI 1.11–1.70). A comparison of staggered versus simultaneous RTV administration showed no significant differences in any of the DIG parameters.
Unexpected Interaction between Digoxin and Bupropion.
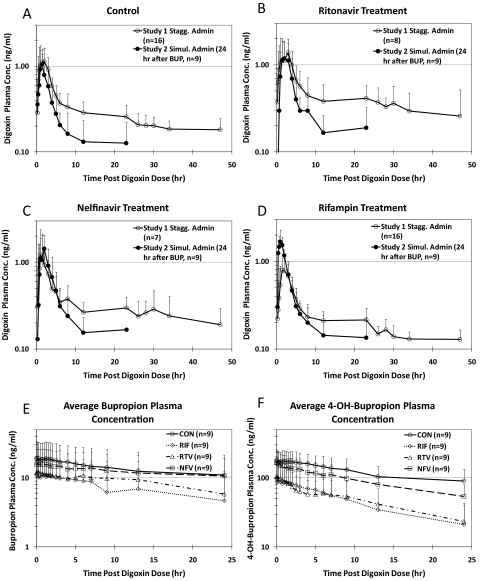
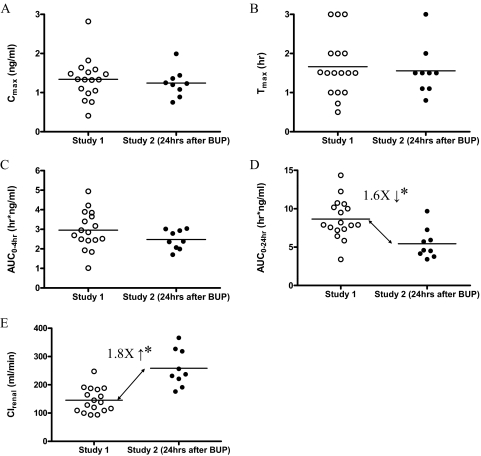
An unexpected DDI was observed when the pharmacokinetics of DIG before any treatment (control) were compared between staggered (study 1) versus simultaneous (study 2) administration (Fig. 4A). In the control phase of study 2 where DIG was given ∼24 h after BUP (extended release, 150 mg), the DIG AUC0–24 h was decreased 1.6-fold, and Clrenal was increased 1.8-fold compared with study 1 (Table 1; Fig. 5). This interaction was also observed during RTV, NFV, or RIF treatment (Fig. 4, B through D). DIG Cmax, Tmax, and AUC0–4 h were not significantly different between studies 1 and 2. BUP and 4-hydroxy-BUP plasma concentration profiles during DIG administration are shown in Fig. 4, E and F, respectively, to show the level of exposure to these drugs over the interval during which the DDI was observed.
Fig. 4.
Comparison of average (±S.D.) plasma concentration-time profiles for DIG in study 1 (staggered administration) and study 2 (simultaneous administration) before (control, A) and after ritonavir (B), nelfinavir (C), or rifampin (D) treatment. Average (±S.D.) plasma concentrations of racemic bupropion (E) and racemic 4-OH-bupropion (F) during DIG administration (24–48 h after BUP administration) before (CON) and after ritonavir, nelfinavir, or rifampin treatment are shown for reference.
Fig. 5.
Comparison of pharmacokinetics of DIG during the pretreatment (control) phase of study 1 (n = 16) and study 2 (n = 9, 24 h after bupropion administration). Administration of bupropion 24 h before DIG did not significantly affect DIG Cmax (A), Tmax (B), or AUC0–4 h (C) but significantly decreased AUC0–24 h (D) and significantly increased DIG Clrenal (E).
Discussion
RIF induces intestinal and hepatic P-gp by pregnane X receptor-mediated transcription, thereby decreasing bioavailability and increasing nonrenal clearance of DIG (Greiner et al., 1999; Drescher et al., 2003). Consistent with these reports, we observed a decrease in the DIG AUC0–24 h and slight but not statistically significant decreases in AUC0–4 h and Cmax when RIF and DIG administration was staggered. In contrast, when RIF and DIG were administered simultaneously, DIG AUC0–24 h was unchanged, but AUC0–4 h and Cmax were increased (Fig. 3A). The different effect of RIF on DIG AUC0–4 h and Cmax between staggered and simultaneous administration indicates the presence of an interaction mechanism other than induction of intestinal/hepatic P-gp. RIF is an inhibitor and substrate of the hepatic transporters, organic anion-transporting polypeptides (OATPs), and P-gp (Tirona et al., 2003; Lau et al., 2007; Reitman et al., 2011). Simultaneously administered RIF could inhibit intestinal P-gp and/or hepatic P-gp/OATPs during hepatic first pass, increasing DIG Cmax and bioavailability. Such an effect on hepatic OATPs has been shown in rats using intravenous DIG and RIF (Lam et al., 2006). Reitman et al. (2011) verified the findings of Lam et al. (2006) in the rat and our results that simultaneous administration of RIF and DIG increased DIG Cmax and AUC0–3 h, masking P-gp induction. The magnitude of increase in DIG Cmax and AUC0–3 h observed by Reitman et al. (2011) is comparable to our observations, implying that the underlying DIG-BUP interaction (described below) did not substantially alter the effect of simultaneous RIF administration on DIG pharmacokinetics. Reitman et al. (2011) attributed this interaction to inhibition of intestinal P-gp, whereas Lam et al. (2006) showed inhibition of hepatic OATPs. Recently, DIG was shown to not be a substrate of the human OATP1A2, OATP1B1, OATP1B3, or OATP2B1 but is a substrate of an unidentified transporter that might be the sodium-dependent uptake transporter expressed in HEK293 cells (Kimoto et al., 2011; Taub et al., 2011).
Mixed inhibition/induction interactions have significant implications for induction DDI study design. The purpose of induction studies may be 2-fold: first, to characterize the effect of an inducer on the object drugs pharmacokinetics, and second, to assess induction of specific enzymes or transporters. To address the first purpose, coadministration of the inducer and the object drug is logical provided the two drugs are usually dosed simultaneously. However, to address the second and mechanistic purpose, our data demonstrate the need for staggered administration of the two drugs to avoid confounding inhibitory interactions from simultaneous administration of the two drugs, which may mask induction of transporters or enzymes. Unfortunately, the current U.S. Food and Drug Administration draft guidance for industry on the conduct of DDI studies (Drug Interaction Studies–tudy Design, Data Analysis, and Implications for Dosing and Labeling, www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM072101.pdf, 2006) does not specify such design considerations. We believe it should.
NFV and RTV are ligands of pregnane X receptor (Dussault et al., 2001) and possibly aryl hydrocarbon receptor (Frötschl et al., 1998), thereby inducing transcription of many P450 enzymes (CYP3A, CYP1A2, CYP2B6, CYP2C9, and CYP2C19) as well as P-gp (Dixit et al., 2007; Gupta et al., 2008). In our study, NFV (1250 mg b.i.d., 14 days) had no effect on intestinal, hepatic, or renal P-gp activity, in agreement with a previous study using fexofenadine to measure intestinal P-gp (Kharasch et al., 2009). Likewise, staggered or simultaneous administration of RTV (400 mg b.i.d., 14 days) did not result in net induction of P-gp activity, in contrast to a previous study using fexofenadine, which indicated slight induction of P-gp by RTV (Kharasch et al., 2008). Upon simultaneous administration of RTV and DIG, DIG Tmax was prolonged, but AUC0–4 h was unchanged, suggesting a slower rate but not extent of absorption, or decreased DIG oral clearance. The latter is supported by the increased DIG AUC0–24 h and decreased Clrenal, suggesting inhibition of hepatic and/or renal P-gp. However, inhibition of intestinal or hepatic uptake transport cannot be ruled out. In fact, digoxin is actively taken up into human hepatocytes by a saturable process other than OATP1B1, OATP1B3, or OATP2B1(Kimoto et al., 2011).
Previously, 200 mg b.i.d. RTV simultaneously administered with DIG increased DIG AUC0–72 h by inhibiting hepatic but not renal P-gp (Penzak et al., 2004). Therefore, in our staggered administration study, DIG AUC0–24 h would be expected to increase when renal P-pg was inhibited by RTV (decreased Clrenal). The reason for this discrepancy is unclear but may include competing inhibition/induction of hepatic P-gp. Collectively, our data suggest that P-gp is not induced by NFV and that 400 mg b.i.d. RTV may slightly induce hepatic P-gp, but the net effect, irrespective of staggered or simultaneous administration, is inhibition of P-gp or no effect, respectively. We do not believe these data are confounded by the underlying BUP-DIG interaction because the observed inhibition of renal P-gp by simultaneous RTV administration in the presence of the BUP-DIG interaction is comparable to that observed with staggered administration when BUP was not present.
When designing our studies, we assumed BUP given 24 h before DIG would have no effect on the pharmacokinetics of either drug. We were surprised by a substantial interaction between these drugs because BUP is extensively metabolized (Lai and Schroeder, 1983), whereas DIG is minimally metabolized, and its excretion is mediated by filtration and net secretion (via transporters). The metabolites of BUP are extensively excreted in the urine (Laizure et al., 1985), but whether they are secreted and/or filtered is unknown. The effect of DIG on BUP and its metabolite 4-hydroxy-BUP was described previously (Kirby et al., 2011a). The effect of BUP and/or its metabolites (BUP/Met) on DIG pharmacokinetics is evident when comparing DIG pharmacokinetics in the absence of BUP (study 1) and in the presence of BUP (study 2) before treatment (control phase). In the presence of BUP/Met, we observed a statistically significant decrease (p < 0.05, with an unpaired t test because subjects were not paired between the studies) in DIG AUC0–24 h (1.6-fold) and increase in DIG Clrenal (1.8-fold) (Fig. 5). Clinically, steady-state DIG plasma concentrations 6 h postdose are maintained between 0.5 and 1.0 ng/ml. Clearly, the 60% decrease in DIG AUC0–24 h in the presence of BUP/Met is clinically significant because it would result in subtherapeutic DIG plasma concentrations. There are multiple possible mechanisms of this DDI. First, BUP/Met may have increased DIG-free fraction in plasma, resulting in increased DIG renal and nonrenal clearances. Because DIG is only 25% bound in plasma (Evered, 1972), complete protein binding displacement cannot explain the increased DIG Clrenal. Second, DIG is actively secreted in the renal proximal tubules by basal uptake by OATPs (likely OATP4C1) and apical efflux by P-gp. For BUP/Met to increase secretion of DIG, activation of the rate-limiting step of these processes would be necessary. Activation of P-gp has been shown in vitro (Soldner et al., 1999) but to date has not been demonstrated in vivo. Because BUP was administered 24 h before DIG, induction of P-gp or OATP4C1 by BUP/Met is unlikely. Therefore, it is more likely that BUP/Met increased DIG renal secretion possibly by inhibiting reabsorption. Currently it is not known whether DIG is actively reabsorbed in the kidney. Least likely is the possibility that BUP/Met increased glomerular filtration by increasing renal blood flow. There are no reports that BUP/Met can alter renal blood flow. BUP/Met did not affect DIG AUC0–4 h, which is used as a measure of intestinal P-gp activity, implying that BUP/met did not affect DIG intestinal bioavailability. Assessing the effect of BUP/Met on hepatic clearance of DIG was not possible from our data because of insufficient plasma sampling to estimate nonrenal clearance.
Irrespective of the underlying mechanism(s) of this DDI, an important question to address is whether this interaction would be greater upon coadministration or multiple dosing. Initiating BUP treatment for a patient stabilized on DIG could result in substantially decreased DIG concentrations, necessitating increasing DIG dose to avoid therapeutic failure. Further dose adjustments may be needed if the DDI is greater after multiple doses of BUP. On the other hand, when BUP therapy is terminated, DIG plasma concentrations could dramatically increase, causing clinically significant toxicity. Because our study was not designed to confirm or quantify this unexpected interaction, a study where DIG and BUP are coadministered to steady state is warranted. To gain insight into the site (hepatic/intestinal versus renal) and mechanisms of this interaction, the study design should include intravenous and oral administration of DIG.
In summary, we have shown that the PIs, NFV or RTV, do not substantially induce hepatic or intestinal P-gp activity measured with DIG. These findings do not provide an explanation for the paradoxical DDIs with the PIs such as autoinduction of PI clearance. Hence, other mechanisms such as induction of enzymes other than CYP3A, CYP1A2, CYP2B6, or CYP2C9 or other drug transporters may explain these paradoxical DDIs. Our contrasting results of RIF induction of P-gp dependent on DIG and RIF dosing exemplify the need for careful clinical DDI study design and attention to both influx and efflux transporters. We were surprised to discover evidence of an unexpected, novel DDI between the CYP2B6 probe drug BUP and DIG that has clinical relevance. The mechanistic basis of this DDI is not clear and warrants further study.
Acknowledgments
We thank Eric Helgeson and Christine Hoffer for clinical study coordination.
This work was supported by the National Institutes of Health National Institute of General Medical Sciences [Grant GM032165]; the National Institutes of Health National Institute on Drug Abuse [Grants K24-DA00417, R01-DA14211]; and the National Institutes of Health National Center for Research Resources [Grant M01-RR00037]. A portion of this work was conducted through the Clinical Research Center Facility at the University of Washington. B.J.K. was supported in part by an ARCS fellowship, a National Institutes of Health National Institute of General Medical Sciences Pharmacological Sciences training grant [Grant GM07550], and a Simcyp-sponsored fellowship.
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
- PI
- protease inhibitor
- DDI
- drug-drug interaction
- P450
- cytochrome P450
- P-gp
- P-glycoprotein
- RTV
- ritonavir
- RIF
- rifampin
- NFV
- nelfinavir
- DIG
- digoxin
- BUP
- bupropion
- Cmax
- maximal observed plasma concentration
- Tmax
- time of maximal observed plasma concentration
- Clrenal
- renal clearance
- OATP
- organic anion-transporting polypeptide
- AUC0-t
- area under the plasma concentration-time curve
- AUC0–24 h
- area under the curve from time zero to 24 h
- AUC0–4 h
- area under the curve from time zero to 4 h
- AUC0–3 h
- area under the curve from time zero to 3 h
- AUC0–72 h
- area under the curve from time zero to 72 h
- AUC
- area under the plasma concentration-time curve
- BUP/Met
- bupropion and/or its metabolites.
Authorship Contributions
Participated in research design: Kirby, Collier, Kharasch, Thummel, and Unadkat.
Conducted experiments: Kirby and Whittington.
Contributed new reagents or analytic tools: Whittington.
Performed data analysis: Kirby and Whittington.
Wrote or contributed to the writing of the manuscript: Kirby, Collier, Kharasch, Whittington, Thummel, and Unadkat.
References
- Dixit V, Hariparsad N, Li F, Desai P, Thummel KE, Unadkat JD. (2007) Cytochrome P450 enzymes and transporters induced by anti-human immunodeficiency virus protease inhibitors in human hepatocytes: implications for predicting clinical drug interactions. Drug Metab Dispos 35:1853–1859 [DOI] [PubMed] [Google Scholar]
- Drescher S, Glaeser H, Mürdter T, Hitzl M, Eichelbaum M, Fromm MF. (2003) P-glycoprotein-mediated intestinal and biliary digoxin transport in humans. Clin Pharmacol Ther 73:223–231 [DOI] [PubMed] [Google Scholar]
- Dussault I, Lin M, Hollister K, Wang EH, Synold TW, Forman BM. (2001) Peptide mimetic HIV protease inhibitors are ligands for the orphan receptor SXR. J Biol Chem 276:33309–33312 [DOI] [PubMed] [Google Scholar]
- Endres CJ, Hsiao P, Chung FS, Unadkat JD. (2006) The role of transporters in drug interactions. Eur J Pharm Sci 27:501–517 [DOI] [PubMed] [Google Scholar]
- Evered DC. (1972) The binding of digoxin by the serum proteins. Eur J Pharmacol 18:236–244 [DOI] [PubMed] [Google Scholar]
- Frötschl R, Chichmanov L, Kleeberg U, Hildebrandt AG, Roots I, Brockmöller J. (1998) Prediction of aryl hydrocarbon receptor-mediated enzyme induction of drugs and chemicals by mRNA quantification. Chem Res Toxicol 11:1447–1452 [DOI] [PubMed] [Google Scholar]
- Greiner B, Eichelbaum M, Fritz P, Kreichgauer HP, von Richter O, Zundler J, Kroemer HK. (1999) The role of intestinal P-glycoprotein in the interaction of digoxin and rifampin. J Clin Invest 104:147–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Mugundu GM, Desai PB, Thummel KE, Unadkat JD. (2008) Intestinal human colon adenocarcinoma cell line LS180 is an excellent model to study pregnane X receptor, but not constitutive androstane receptor, mediated CYP3A4 and multidrug resistance transporter 1 induction: studies with anti-human immunodeficiency virus protease inhibitors. Drug Metab Dispos 36:1172–1180 [DOI] [PubMed] [Google Scholar]
- Hsiao P, Bui T, Ho RJ, Unadkat JD. (2008) In vitro-to-in vivo prediction of P-glycoprotein-based drug interactions at the human and rodent blood-brain barrier. Drug Metab Dispos 36:481–484 [DOI] [PubMed] [Google Scholar]
- Josephson F. (2010) Drug-drug interactions in the treatment of HIV infection: focus on pharmacokinetic enhancement through CYP3A inhibition. J Intern Med 268:530–539 [DOI] [PubMed] [Google Scholar]
- Kharasch ED, Bedynek PS, Walker A, Whittington D, Hoffer C. (2008) Mechanism of ritonavir changes in methadone pharmacokinetics and pharmacodynamics: II. Ritonavir effects on CYP3A and P-glycoprotein activities. Clin Pharmacol Ther 84:506–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharasch ED, Walker A, Whittington D, Hoffer C, Bedynek PS. (2009) Methadone metabolism and clearance are induced by nelfinavir despite inhibition of cytochrome P4503A (CYP3A) activity. Drug Alcohol Depend 101:158–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimoto E, Chupka J, Xiao Y, Bi YA, Duignan DB. (2011) Characterization of digoxin uptake in sandwich-cultured human hepatocytes. Drug Metab Dispos 39:47–53 [DOI] [PubMed] [Google Scholar]
- Kirby B, Kharasch ED, Thummel KT, Narang VS, Hoffer CJ, Unadkat JD. (2006) Simultaneous measurement of in vivo P-glycoprotein and cytochrome P450 3A activities. J Clin Pharmacol 46:1313–1319 [DOI] [PubMed] [Google Scholar]
- Kirby BJ, Collier AC, Kharasch ED, Dixit V, Desai P, Whittington D, Thummel KE, Unadkat JD. (2011a) Complex drug interactions of HIV protease inhibitors 2: in vivo induction and in vitro to in vivo correlation of induction of cytochrome P450 1A2, 2B6, and 2C9 by ritonavir or nelfinavir. Drug Metab Dispos 39:2329–2337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BJ, Collier AC, Kharasch ED, Whittington D, Thummel KE, Unadkat JD. (2011b) Complex drug interactions of HIV protease inhibitors 1: inactivation, induction, and inhibition of cytochrome P450 3A by ritonavir or nelfinavir. Drug Metab Dispos 39:1070–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby BJ, Kalhorn T, Hebert M, Easterling T, Unadkat JD. (2008) Sensitive and specific LC-MS assay for quantification of digoxin in human plasma and urine. Biomed Chromatogr 22:712–718 [DOI] [PubMed] [Google Scholar]
- Lai AA, Schroeder DH. (1983) Clinical pharmacokinetics of bupropion: a review. J Clin Psychiatry 44:82–84 [PubMed] [Google Scholar]
- Laizure SC, DeVane CL, Stewart JT, Dommisse CS, Lai AA. (1985) Pharmacokinetics of bupropion and its major basic metabolites in normal subjects after a single dose. Clin Pharmacol Ther 38:586–589 [DOI] [PubMed] [Google Scholar]
- Lam JL, Shugarts SB, Okochi H, Benet LZ. (2006) Elucidating the effect of final-day dosing of rifampin in induction studies on hepatic drug disposition and metabolism. J Pharmacol Exp Ther 319:864–870 [DOI] [PubMed] [Google Scholar]
- Lau YY, Huang Y, Frassetto L, Benet LZ. (2007) effect of OATP1B transporter inhibition on the pharmacokinetics of atorvastatin in healthy volunteers. Clin Pharmacol Ther 81:194–204 [DOI] [PubMed] [Google Scholar]
- Penzak SR, Shen JM, Alfaro RM, Remaley AT, Natarajan V, Falloon J. (2004) Ritonavir decreases the nonrenal clearance of digoxin in healthy volunteers with known MDR1 genotypes. Ther Drug Monit 26:322–330 [DOI] [PubMed] [Google Scholar]
- Reitman ML, Chu X, Cai X, Yabut J, Venkatasubramanian R, Zajic S, Stone JA, Ding Y, Witter R, Gibson C, et al. (2011) Rifampin's acute inhibitory and chronic inductive drug interactions: experimental and model-based approaches to drug-drug interaction trial design. Clin Pharmacol Ther 89:234–242 [DOI] [PubMed] [Google Scholar]
- Soldner A, Christians U, Susanto M, Wacher VJ, Silverman JA, Benet LZ. (1999) Grapefruit juice activates P-glycoprotein-mediated drug transport. Pharm Res 16:478–485 [DOI] [PubMed] [Google Scholar]
- Taub ME, Mease K, Sane RS, Watson CA, Chen L, Ellens H, Hirakawa B, Reyner EL, Jani M, Lee CA. (2011) Digoxin is not a substrate for organic anion-transporting polypeptide transporters OATP1A2, OATP1B1, OATP1B3, and OATP2B1 but is a substrate for a sodium-dependent transporter expressed in HEK293 cells. Drug Metab Dispos 39:2093–2102 [DOI] [PubMed] [Google Scholar]
- Tirona RG, Leake BF, Wolkoff AW, Kim RB. (2003) Human organic anion transporting polypeptide-C (SLC21A6) is a major determinant of rifampin-mediated pregnane X receptor activation. J Pharmacol Exp Ther 304:223–228 [DOI] [PubMed] [Google Scholar]
- Unadkat JD, Wang Y. (2000) Protease inhibitors, in Metabolic Drug Interactions (Levy RH, et al. eds) pp 647–652, Lippincott Williams & Wilkins, Philadelphia [Google Scholar]