Abstract
Furan is a liver toxicant and carcinogen in rodents. It is classified as a possible human carcinogen, but the human health effects of furan exposure remain unknown. The oxidation of furan by cytochrome P450 (P450) enzymes is necessary for furan toxicity. The product of this reaction is the reactive α,β-unsaturated dialdehyde, cis-2-butene-1,4-dial (BDA). To determine whether human liver microsomes metabolize furan to BDA, a liquid chromatography/tandem mass spectrometry method was developed to detect and quantify BDA by trapping this reactive metabolite with N-acetyl-l-cysteine (NAC) and N-acetyl-l-lysine (NAL). Reaction of NAC and NAL with BDA generates N-acetyl-S-[1-(5-acetylamino-5-carboxypentyl)-1H-pyrrol-3-yl]-l-cysteine (NAC-BDA-NAL). Formation of NAC-BDA-NAL was quantified in 21 different human liver microsomal preparations. The levels of metabolism were comparable to that observed in F-344 rat and B6C3F1 mouse liver microsomes, two species known to be sensitive to furan-induced toxicity. Studies with recombinant human liver P450s indicated that CYP2E1 is the most active human liver furan oxidase. The activity of CYP2E1 as measured by p-nitrophenol hydroxylase activity was correlated to the extent of NAC-BDA-NAL formation in human liver microsomes. The formation of NAC-BDA-NAL was blocked by CYP2E1 inhibitors but not other P450 inhibitors. These results suggest that humans are capable of oxidizing furan to its toxic metabolite, BDA, at rates comparable to those of species sensitive to furan exposure. Therefore, humans may be susceptible to furan's toxic effects.
Introduction
Furan is a widely used industrial chemical and is found throughout the environment. It has been detected in foods (Maga, 1979; also see the U.S. Food and Drug Administration, Exploratory Data on Furan in Food: Individual Food Products, http://www.fda.gov/Food/FoodSafety/FoodContaminantsAdulteration/ChemicalContaminants/Furan/ucm078439.htm, 2009), cigarette smoke, and air pollution (Capurro, 1973; International Agency for Research on Cancer, 1995). One calculation indicates that baby food consumption could result in a daily exposure as high as 3.5 μg/kg for an infant (Vranová and Ciesarová, 2009). Others have calculated the mean infant food-derived furan exposure as ∼0.4 μg/kg per day with the 90th percentile being 1 μg/kg per day and the mean adult food-derived furan exposure at ∼0.3 μg/kg per day with the 90th percentile predicted to be 0.6 μg/kg per day (the U.S. Food and Drug Administration, Exploratory Data on Furan in Food: Individual Food Products, http://www.fda.gov/Food/FoodSafety/FoodContaminantsAdulteration/ChemicalContaminants/Furan/ucm078439.htm, 2009). Furan has been found to be toxic and carcinogenic in rats and mice after oral exposure (National Toxicology Program, 1993). Because furan is carcinogenic to rodents and there is a high potential for human exposure, it has been classified as a possible human carcinogen (International Agency for Research on Cancer, 1995; National Toxicology Program, 2011).
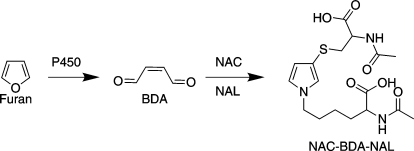
In rodent studies, furan-derived toxicity requires metabolism. Furan is oxidized by cytochrome P450 (P450) enzymes to the reactive metabolite cis-2-butene-1,4-dial [(BDA) Scheme 1] (Chen et al., 1995; Peterson et al., 2005). Its toxicity is reduced by CYP2E1 inhibitors and enhanced by CYP2E1 inducers (Carfagna et al., 1993; Kedderis et al., 1993). BDA is genotoxic (Marinari et al., 1984; Peterson et al., 2000; Kellert et al., 2008a) and forms adducts with various cellular nucleophiles such as DNA, protein, and polyamines (Chen et al., 1997; Gingipalli and Dedon, 2001; Byrns et al., 2002, 2004; Lu et al., 2009; Peterson et al., 2011), which is evidence that BDA may be responsible for furan-induced toxicities. Conjugates of BDA have been detected in the urine and bile of furan-treated rats (Peterson et al., 2006, 2011; Kellert et al., 2008b; Lu et al., 2009; Hamberger et al., 2010; Lu and Peterson, 2010).
Scheme 1.
Metabolism of furan to BDA that is trapped by NAC and NAL.
We hypothesized that if humans can metabolize furan to BDA, humans may be susceptible to the toxic effects of furan exposure. Whereas furan uptake in human hepatocytes is comparable to that in rat and mouse hepatocytes (Kedderis and Held, 1996), it is unknown whether human liver converts furan to its reactive metabolite, BDA. In a first step toward determining whether humans are susceptible to the same toxic effects as F-344 rats and B6C3F1 mice, rates of furan metabolism in humans were compared with those of rats and mice using liver microsomes as an in vitro model for liver metabolism. In addition, recombinant human P450 enzymes and chemical inhibitors were employed to identify which P450 enzymes are involved in the oxidation of furan to BDA in human liver microsomes.
Materials and Methods
Chemicals and Reagents.
Aqueous solutions of BDA were prepared from 2,5-diacetoxy-2,5-dihydrofuran as previously described (Byrns et al., 2004). Unlabeled N-acetyl-S-[1-(5-acetylamino-5-carboxypentyl)-1H-pyrrol-3-yl]-l-cysteine (NAC-BDA-NAL) was synthesized as reported previously (Lu et al., 2009). [13C615N2]l-Lysine was purchased from Cambridge Isotope Laboratories, Inc. (Andover, MA). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO). Insect cell cDNA-expressing human P450s (Supersomes) were purchased from BD Biosciences Discovery Labware (Bedford, MA). Human liver microsomes were obtained as a gift from Dr. Sharon Murphy's laboratory at the University of Minnesota, Minneapolis. Human liver samples were received as a gift from Dr. F. Peter Guengerich's laboratory at Vanderbilt University, Nashville, Tennessee, and were processed into microsomes in our laboratory.
Synthesis of [13C615N2]N-Acetyl-S-[1-(5-acetylamino-5-carboxypentyl)-1H-pyrrol-3-yl]-l-Cysteine.
N-Acetyl cysteine (NAC) (0.22 mM) and BDA (0.22 mM) were combined in 1 M HEPES, pH 7.4, for 5 min before adding [13C615N2]l-lysine (0.22 mM, total volume: 5 ml). After 18 h at room temperature, the reaction mixture was separated on a semipreparative Synergi Hydro RP column (250 × 10 mm; Phenomenex, Torrance, CA). The column was eluted with 50 mM ammonium formate, pH 2.8, containing 12.5% acetonitrile (flow rate = 4 ml/min). Two reaction products were observed. The first eluting peak at 33 min was [13C615N2]S-[1-(5-amino-1-carboxypentyl)-1H-pyrrol-3-yl]-l-cysteine. The second eluting peak at 40 min was [15N2,13C6]S-[1-(5-amino-5-carboxypentyl)-1H-pyrrol-3-yl]-l-cysteine. The latter peak was preparatively purified, concentrated to dryness under reduced pressure, and dissolved in 0.1% formic acid. It was desalted on a semipreparative Synergi 4μ Hydro-RP column with the following gradient (flow = 4 ml/min): 5 min at 100% solvent A (0.1% v/v formic acid) and 10-min gradient to 100% solvent B (50% v/v acetonitrile in water). The desired compound eluted at 11 min. The high-performance liquid chromatography (HPLC) fractions containing this chemical were concentrated under reduced pressure and were dissolved in a saturated bicarbonate solution. Acetic anhydride was added (50 μl, 0.53 mmol). The reaction mixture was stirred at room temperature and then was separated on the semipreparative HPLC column, which was isocratically eluted with 0.05% formic acid containing 22.5% acetonitrile at a flow rate of 4 ml/min. The final product eluted at 4 min. The nuclear magnetic resonance analysis was consistent with literature reports (Chen et al., 1997). Mass spectrometry (MS) m/z (relative intensity) 408 (M + 1, 100). Tandem MS m/z (relative intensity) 172 (100), 271 (91).
[2H6]Dimethyl sulfoxide solutions of unlabeled and NAC-BDA-[13C615N2]NAL were quantified by quantitative 1H nuclear magnetic resonance analysis using toluene as an internal standard. The concentration of the standard solutions was determined by integrating the pyrrole proton signals of NAC-BDA-NAL (6.0, 6.67, and 6.85 ppm) relative to the aromatic proton signals of toluene (7.13 to 7.26 ppm). The T1 time was set to 10 s to allow for complete relaxation of the toluene protons.
Furan Oxidation Assay.
Mouse, rat, and human liver microsomes were prepared as described previously (Guengerich, 1982). Protein concentration was determined as described previously with Bio-Rad Quick Start Bradford 1x Dye Reagent (Bio-Rad Laboratories, Hercules, CA) and bovine serum albumin as a standard (Bradford, 1976). Human liver microsomes from both cited sources were used. Furan (0–400 μM) was incubated in the presence of human liver microsomes (0.5 μg/μl) or recombinant human P450 Supersomes (1 or 2 pmol P450/μl) in 100 mM potassium phosphate, pH 7.4, containing 25 mM glucose 6-phosphate, 2 units/ml glucose-6-phosphate dehydrogenase, 4 mM NADP+, 3 mM MgCl2, 1 mM EDTA, 8 mM NAC, and 8 mM N-acetyl lysine (NAL) for 10 min in a shaking water bath at 37°C in sealed tubes (final volume, 250 μl). Incubations were performed with or without the following P450 inhibitors: 5 or 200 μM 1-phenylimidazole, 50 μM 4-methylpyrazole, 20 μM diethyldithiocarbamic acid (CYP2E1 inhibitors), 5 μM quinidine (CYP2D6 inhibitor), or 2 μM ketoconazole (CYP3A4 inhibitor). Incubations were started by the addition of furan as an aqueous solution. This solution was prepared as described previously (Peterson et al., 2005). The reaction was terminated by adding 25 μl each of 0.3 N ZnSO4 and Ba(OH)2. The samples were centrifuged for 3 min at 13,000g, and the supernatant was drawn off and frozen at −20°C for liquid chromatography/tandem mass spectrometry (LC-MS/MS) analysis.
The recovery of BDA by NAC and NAL in the presence or absence of rat liver microsomes was also investigated. The above incubation method was done with BDA (0–250 μM) instead of furan in the presence or absence of microsomes. Samples were centrifuged for 3 min at 13,000g, and the supernatant stored at −20°C for LC-MS/MS analysis.
LC-MS/MS Analysis.
For MS preparation, microsomal incubation mixtures were diluted 1:400 in water and spiked with 5 pmol NAC-BDA-[13C615N2]NAL (total volume: 200 μl). LC-MS/MS analysis was performed with a 250 × 0.5 mm Synergi Hydro RP 4 μm 80 Å pore size column (Phenomenex) coupled with a Thermo Fisher TSQ Vantage or Quantum Ultra AM mass spectrometer (Thermo Fisher Scientific, Waltham, MA).
Two different LC-MS methods were used:
Method A.
For Supersomes incubation analysis, samples (1 μl) were eluted with 10 mM ammonium formate buffer, pH 2.8 (solvent A), and 75% acetonitrile in water (solvent B) at a flow rate of 10 μl/min. The initial conditions were 87% solvent A and 13% solvent B. From 7 to 8 min, the gradient increased to 54% solvent B and then returned to initial conditions from 9 to17 min. The electrospray ionization source was operated in positive ion mode. NAC-BDA-NAL and the internal standard were detected by monitoring the neutral loss of 129 or 234 from the parent ions (NAC-BDA-NAL: m/z 400 to 271 or 166; NAC-BDA-[13C615N2]NAL: m/z 408 to 279 or 172).
Method B.
For the human liver microsomal incubation analyses, samples (8 μl) were eluted with 15 mM ammonium acetate buffer, pH 6.8 (solvent A) and methanol (solvent B) at a flow rate of 10 μl/min. The initial conditions were 99% solvent A and 1% solvent B. The gradient increased to 70% solvent B from 5 to 18 min and then returned to initial conditions from 21 to 23 min, holding at 1% solvent B until the end of the 35-min run. The electrospray ionization source was operated in negative ion mode. NAC-BDA-NAL and the internal standard were detected by monitoring the neutral loss of 129 or 171 from the parent ions (NAC-BDA-NAL: m/z 398 to 269 or 227; NAC-BDA-[13C615N2]NAL: m/z 406 to 277 or 235).
p-Nitrophenol Assay.
p-Nitrophenol (100 μM) was incubated in the presence of human liver microsomes (0.5 μg protein/μl) in 44 mM potassium phosphate, pH 7.4, containing 2.2 mM glucose 6-phosphate, 0.4 units/ml glucose-6-phosphate dehydrogenase, 2.2 mM NADP+, and 2.2 mM MgCl2, for 30 min in a shaking water bath at 37°C (final volume, 250 μl) (Chang et al., 2006). Incubations were started by the addition of microsomes. The reaction was terminated by adding 25 μl each of 0.3 N ZnSO4 and 0.3 N Ba(OH)2, and the samples were spun for 5 min at 10,000g. The supernatant was immediately analyzed by HPLC with a Waters 600 controller coupled with a Waters 996 Photodiode Array Detector set to 304 nm (Waters, Milford, MA). The samples (200 μl) were isocratically eluted with 25 mM triethylamine, pH 2.1, containing 25% acetonitrile on a 250 x 4.6 mm Phenomenex Synergi Hydro RP 4 μm 80 Å pore size column at 1 ml/min. The metabolite p-nitrocatechol had a retention time of 12 min and was quantified with an external standard curve generated with known concentrations of p-nitrocatechol.
Results and Discussion
Because BDA is so reactive, it must be trapped for quantification. Microsomally generated BDA has been successfully captured with either semicarbazide or glutathione (Chen et al., 1995; Peterson et al., 2005). However, semicarbazide does not completely block the protein binding of furan's reactive metabolite (Parmar and Burka, 1993), and reaction with glutathione yielded multiple products (Peterson et al., 2005). A simpler trapping method for reactive furanyl intermediates was reported by Baer et al. (2005) in which the reactive enedial intermediate of 4-ipomeanol was quenched with NAC and NAL, forming a single substituted pyrrole conjugate in which NAC was cross-linked to NAL. A similar reaction product was reported for BDA in which NAC reacts with BDA via Michael addition followed by condensation of the two aldehydes with NAL to form NAC-BDA-NAL (Scheme 1) (Chen et al., 1997). Therefore, furan was incubated with liver microsomes in the presence of NAC and NAL to convert any BDA generated to NAC-BDA-NAL.
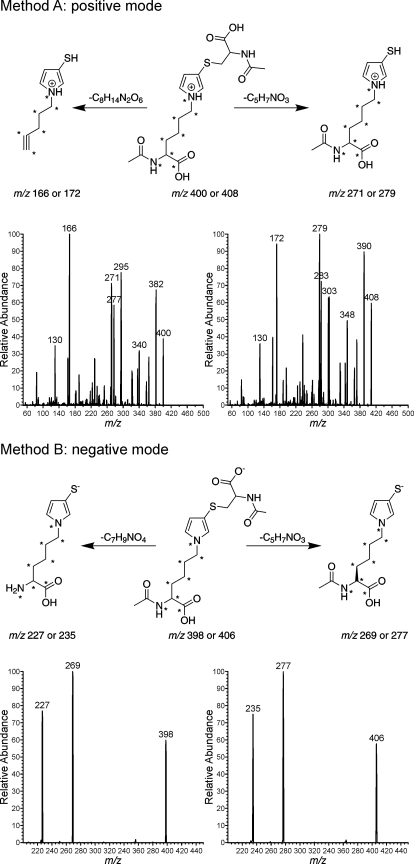
The levels of NAC-BDA-NAL formed were determined by LC-MS/MS analysis with selective reaction monitoring. The positive mass spectrum of NAC-BDA-NAL contained two prominent ions at m/z 271 and m/z 166, representing the loss of 129 Da (−C5H7NO3) and 234 Da (−C8H14N2O6), respectively (Fig. 1, Method A). The negative mass spectrum had two significant ions at m/z 269 and m/z 227, representing the loss of 129 Da (−C5H7NO3) and 171 Da (−C7H9NO4), respectively (Fig. 1, Method B). These fragmentations were targeted for selected reaction monitoring (negative mode: m/z 398 → m/z 227 and m/z 398 → m/z 269; positive mode: m/z 400 → m/z 166 and m/z 400 → m/z 271).
Fig. 1.
Positive ion and negative ion fragmentation of NAC-BDA-NAL and NAC-BDA-[13C615N2]NAL. *, 13C- or 15N-labeled atoms.
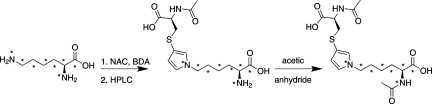
The assay was made quantitative by the addition of an isotopically labeled internal standard, NAC-BDA-[13C615N2]NAL. This compound was prepared by reacting BDA and NAC with [13C615N2] lysine, followed by acetylation of the Nα-amine of lysine with acetic anhydride (Scheme 2). The molecular ion of NAC-BDA-[13C615N2]NAL was shifted by 8 Da relative to the unlabeled standard, occurring at 406 m/z (negative mode) or 408 m/z (positive mode). The selected reaction monitoring for the standard were as follows: for negative mode: m/z 406 → m/z 235 and m/z 406 → m/z 277; for positive mode: m/z 408 → m/z 279 and m/z 408 → m/z 172 (Fig. 1).
Scheme 2.
Synthesis of NAC-BDA-[13C615N2]NAL. *, 13C- or 15N-labeled atoms.
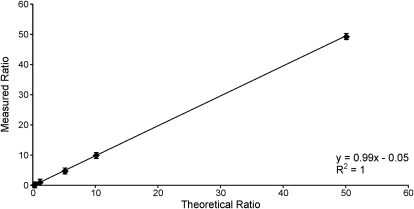
Standard curves were generated with known ratios of unlabeled to labeled standard. Linear standard curves with a slope of ∼1 were observed for all four transitions (ratios ranged from 0.1 to 50). The standard curve obtained when monitoring m/z 398 → m/z 269 and m/z 406 → m/z 277 is displayed in Fig. 2. Limits of detection for NAC-BDA-NAL were 10 fmol when operating in the positive mode and 0.1 fmol when operating in negative ion mode. The difference in sensitivity resulted from reduced background in negative ion mode. Preliminary investigations demonstrated that the presence of microsomal protein reduced the recovery of BDA by 5 to 10% using this trapping scheme (data not shown). Therefore, NAC/NAL was more efficient at trapping BDA than what has been reported for GSH in a related trapping assay; microsomal proteins reduced the ability of GSH to react with BDA by 20 to 25% (Peterson et al., 2005). This increased efficiency results from the greater reactivity of the NAL amino group relative to the GSH amino group (Peterson et al., 2011). Because the influence of microsomal protein on product formation was so small in this current report, the data were not corrected for the slightly reduced recovery of BDA in microsomal mixtures.
Fig. 2.
Standard curve obtained for known ratios of NAC-BDA-NAL and NAC-BDA-[13C615N2]NAL obtained when monitoring m/z 398 → m/z 269 and m/z 406 → m/z 277 in negative ion mode. Error bars represent standard deviation. Similar graphs were obtained for the other transitions monitored: m/z 398 → m/z 227 and m/z 406 → m/z 235 (data not shown).
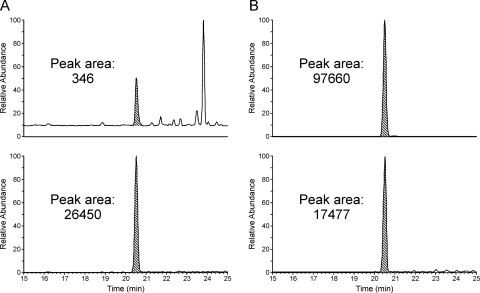
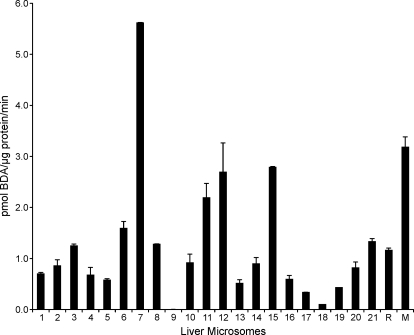
Inclusion of NAC/NAL in liver microsomal incubations of furan led to the formation of a single peak in the LC-MS/MS assay corresponding to the expected mass (Fig. 3). The presence of this peak required NADPH, microsomes, furan and NAC/NAL. Incubations with human, F-344 rat, and B6C3F1 mouse liver microsomes were performed with 50 μM furan because this concentration is just above the Km of furan measured in untreated rat liver microsomes (37.6 μM) (Peterson et al., 2005). The results obtained for liver microsomes from rat, mouse, and 21 different humans are displayed in Fig. 4 and in Supplemental Table 1. A range of NAC-BDA-NAL formation was observed in human liver microsomes, varying from 0.002 to 5.6 pmol BDA/μg protein/min with an average of 1.3 pmol BDA/μg protein/min. Rat and mouse liver microsomes were more active than the average human liver microsomal preparation (1.6 and 3.2 pmol BDA/μg protein/min, respectively). However, several human preparations were significantly more active than the rodent ones. This in vitro model of liver metabolism provides evidence that humans are capable of converting furan to BDA at rates comparable to those of rats and mice, two species susceptible to the toxicity of furan.
Fig. 3.
LC-MS/MS analysis of microsomal incubations with 50 μM furan and NAC/NAL in the absence (A) or presence (B) of an NADPH regenerating system. The top trace was generated by monitoring for the neutral loss of 129 Da from the microsomally generated NAC-BDA-NAL (m/z 398 → m/z 269) in negative ion mode. The bottom trace was generated by monitoring for the neutral loss of 129 Da from the standard, NAC-BDA-[13C615N2]NAL (m/z 406 → m/z 277) in negative ion mode.
Fig. 4.
Activity of liver microsomes (21 humans, R = F-344 rat and M = B6C3F1 mouse) to convert furan to NAC-BDA-NAL. Incubations were performed in triplicate in the presence of NAC and NAL to trap BDA as NAC-BDA-NAL. Furan (50 μM) was incubated with liver microsomes (0.5 mg/ml protein) in the presence of 8 mM NAC, 8 mM NAL, and an NADPH-regenerating system for 10 min at 37°C. Error bars represent S.E. A table displaying the numbers ± S.E. is available in the supplemental data.
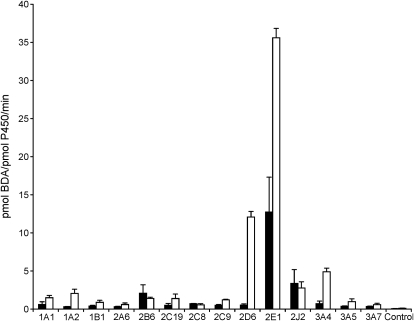
To determine which human P450s were responsible for the oxidation of furan to BDA, recombinant human P450 enzymes were incubated with 20 or 400 μM furan in the presence of NAC/NAL and NADPH. The extent of metabolism was quantified by the LC-MS/MS assay (Fig. 5). CYP2E1 was the most active at oxidizing furan to BDA. The activity of CYP2E1 in this furan oxidase assay was comparable to that observed with GSH trapping (Peterson et al., 2005). At 20 μM furan, the relative activity of the enzymes was CYP2E1 ≫ CYP2J2 > CYP2B6. At 400 μM furan, CYP2D6 and CYP3A4 were also significant catalysts of furan oxidation to BDA. These results demonstrate that CYP2E1 may be the major contributor to furan metabolism in human liver. Although CYP2B6 expression levels can be high in some individuals (Hodgson and Rose, 2007; Wang and Tompkins, 2008), it was not very active in converting furan to BDA. CYP2J2 is primarily expressed extrahepatically (Paine et al., 2006; Bièche et al., 2007). Therefore, this enzyme is not likely to contribute significantly to human liver metabolism of furan. CYP2D6 is also a minor liver P450, but genetic variants of this protein lead to high activity (Neafsey et al., 2009b), indicating that this enzyme could contribute to liver furan metabolism in some individuals. CYP3A4 protein levels are much higher than those of CYP2E1 (Shimada et al., 1994); therefore, CYP3A4 may play a role in furan metabolism in human liver. However, there is a large difference between the Km values determined for CYP3A4 and CYP2E1 (CYP3A4: Km = 234 ± 17 μM, Vmax = 6.2 ± 0.7 pmol BDA/pmol P450/min; CYP2E1: Km = 24 ± 13 μM, Vmax = 34 ± 4 pmol BDA/pmol CYP/min), indicating that CYP2E1 is likely the major enzyme responsible for BDA formation at submicromolar concentrations of furan.
Fig. 5.
Rate of NAC-BDA-NAL formation catalyzed by human recombinant P450s. Furan (black bars, 20 μM; white bars, 400 μM) was incubated with human recombinant P450s (1 or 2 pmol P450/μl) in the presence of 8 mM NAC, 8 mM NAL, and an NADPH-regenerating system for 10 min at 37°C. Control = Supersomes expressing only NADPH reductase. Error bars represent S.E.
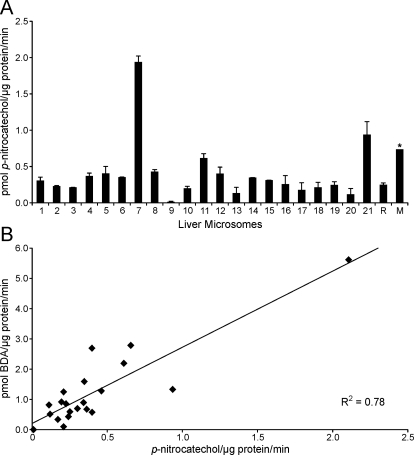
To confirm that CYP2E1 was the major catalyst of furan oxidation in human liver microsomes, the furan oxidase activity was compared with p-nitrophenol hydroxylase activity in each human liver microsomal preparation. The latter activity is primarily catalyzed by CYP2E1. Therefore, the rate of formation of p-nitrocatechol is a measurement for CYP2E1 activity (Chang et al., 2006). If CYP2E1 is the primary enzyme catalyzing the oxidation of furan to BDA, then a correlation between the extent of p-nitrocatechol and BDA generation in the human liver microsomes will be observed. A range of p-nitrophenol hydroxylase activity was observed (0.009–2 pmol p-nitrocatechol/μg protein/min; Fig. 6A and Supplemental Table 1). This activity was strongly correlated to furan oxidase activity (R2 = 0.78) (Fig. 6B), indicating that CYP2E1 is primarily responsible for furan oxidation in human liver microsomes.
Fig. 6.
A, p-nitrophenol hydroxylase activity in human liver microsomes. p-Nitrophenol (100 μM) was incubated with liver microsomes (0.5 mg/ml protein) in the presence of an NADPH-regenerating system for 30 min at 37°C. Error bars represent S.E. B, correlation of p-nitrophenol hydroxylase and furan oxidase in human liver microsomes. A table displaying the numbers ± S.E. is available in the supplemental data.
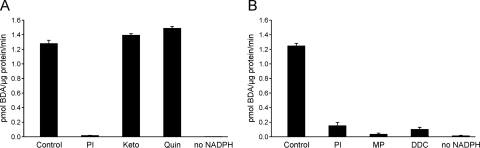
To provide additional support for the role of CYP2E1 in the oxidation of furan to BDA, several human liver microsomes were incubated with 50 μM furan in the presence of inhibitors for CYP2E1 (1-phenylimidazole, 4-methylpyrazole, or diethyldithiocarbamate), CYP2D6 (quinidine), or CYP3A4 (ketoconazole). The CYP2E1 inhibitors were the only chemicals to significantly knock down BDA formation (Fig. 7). Comparable results were obtained with 0.2 and 4 μM furan in human liver microsomal preparations 2 and 3; 20 μM diethyldithiocarbamate inhibited 93% and >95% of BDA formation in these two preparations, respectively (data not shown). These results confirm that CYP2E1 is the major catalyst of furan oxidation to BDA in human liver microsomes at the low furan concentrations expected in human exposure situations.
Fig. 7.
Formation of NAC-BDA-NAL was significantly inhibited by CYP2E1 but not CYP3A4 or CYP2D6 inhibitors. A, results obtained with human liver microsomal preparation 8 in which 50 μM furan was incubated with 200 μM 1-phenylimidazole (PI), 2 μM ketoconazole (Keto), or 5 μM quinidine (Quin) in the presence of NAC, NAL, and an NADPH-regenerating system. B, results obtained with human liver microsomal preparation 3 in which 50 μM furan was incubated with 5 μM 1-phenylimidazole (PI), 50 μM 4-methylpyrazole (MP), or 20 μM diethyldithiocarbamate (DDC) in the presence of NAC, NAL, and an NADPH-regenerating system.
Collectively, these results demonstrate that human P450s, particularly CYP2E1, metabolize furan to its reactive metabolite, BDA, at a rate comparable to that observed in F-344 rats and B6C3F1 mice. This parallels what was reported for furan uptake in human, mouse, and rat hepatocytes (Kedderis and Held, 1996). Studies are currently underway in human hepatocytes to determine whether humans are equally proficient as rats and mice at converting furan to BDA-derived metabolites observed in rat hepatocytes (Lu et al., 2009; Peterson et al., 2011). The observation that human P450s are able to metabolize furan to a toxic metabolite, BDA, suggests that humans may be at risk when exposed to furan.
CYP2E1 was the dominant enzyme responsible for the generation of NAC-BDA-NAL in human liver microsomes. It is not surprising that CYP2E1 is the major enzyme involved in the metabolism of furan because it can metabolize a number of low-molecular-weight compounds, including other carcinogens such as benzene and ethanol (Guengerich et al., 1991). Because a wide variation in NAC-BDA-NAL production was observed in the human liver microsomes, it is likely that there will be individual differences in susceptibility to the harmful effects of furan. Genetic variations are partially responsible for diversity in CYP2E1 activity (Lieber, 1997; Neafsey et al., 2009a). CYP2E1 activity and expression can also be influenced by lifestyle factors such as ethanol consumption and obesity (Lieber, 1997; Emery et al., 2003). Future studies will explore the influence of genetics and environmental factors on furan metabolism in humans.
The LC-MS/MS assay developed can also be applied to determine the levels of NAC-BDA-NAL in the urine of humans exposed to furan. NAC-BDA-NAL is a significant metabolite detected in the urine of furan-treated rats (Kellert et al., 2008b; Lu et al., 2009). Data support the hypothesis that this compound is a downstream metabolite of degraded furan-derived protein adducts (Lu et al., 2009; Hamberger et al., 2010). Therefore, this metabolite can serve as a biomarker for furan exposure, metabolism, and bioactivation. An important next step will be to determine whether furan-exposed humans have this metabolite in their urine. If so, this would suggest that humans are generating metabolites and cellular reaction products similar to those generated in rodents. All of this information is important in the risk assessment of furan as a human health hazard.
Supplementary Material
Acknowledgments
We thank Linda Von Weymarn for helpful discussions and Peter Villalta and Brock Matter for mass spectrometry assistance. We also thank Fred Guengerich and Sharon Murphy for their gifts of human livers and liver microsomes.
This work was supported by the National Institutes of Health National Institute of Environmental Health Sciences [Grant ES-10577]. All liquid chromatography/tandem mass spectrometry work was performed in the Masonic Cancer Center Analytical Biochemical Core facility, which is supported by the National Institutes of Health National Cancer Institute [Grant CA-77598].
Portions of this work were presented as follows: Brus LA, Lu D, and Peterson LA (2011) Oxidation of furan to a reactive metabolite by human cytochrome P450 enzymes. Fall National Meeting of the American Chemical Society; 2011 Aug 28–Sept 1; Denver, CO. American Chemical Society, Washington, DC.
Article, publication date, and citation information can be found at http://dmd.aspetjournals.org.
 The online version of this article (available at http://dmd.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://dmd.aspetjournals.org) contains supplemental material.
- P450
- cytochrome P450
- BDA
- cis-2-butene-1,4-dial
- NAC
- N-acetyl cysteine
- HPLC
- high-performance liquid chromatography
- NAL
- N-acetyl lysine
- MS
- mass spectrometry
- NAC-BDA-NAL
- N-acetyl-S-[1-(5-acetylamino-5-carboxypentyl)-1H-pyrrol-3-yl]-l-cysteine
- NAC-BDA-[13C615N2]NAL
- [13C615N2]N-acetyl-S-[1-(5-acetylamino-5-carboxypentyl)-1H-pyrrol-3-yl]-l-cysteine
- LC-MS/MS
- liquid chromatography/tandem mass spectrometry.
Authorship Contributions
Participated in research design: Gates, Lu, and Peterson.
Conducted experiments: Gates and Lu.
Contributed new reagents or analytic tools: Gates and Lu.
Wrote or contributed to the writing of the manuscript: Gates, Lu, and Peterson.
References
- Baer BR, Rettie AE, Henne KR. (2005) Bioactivation of 4-ipomeanol by CYP4B1: adduct characterization and evidence for an enedial intermediate. Chem Res Toxicol 18:855–864 [DOI] [PubMed] [Google Scholar]
- Bièche I, Narjoz C, Asselah T, Vacher S, Marcellin P, Lidereau R, Beaune P, de Waziers I. (2007) Reverse transcriptase-PCR quantification of mRNA levels from cytochrome (CYP)1, CYP2 and CYP3 families in 22 different human tissues. Pharmacogenet Genomics 17:731–742 [DOI] [PubMed] [Google Scholar]
- Bradford MM. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254 [DOI] [PubMed] [Google Scholar]
- Byrns MC, Predecki DP, Peterson LA. (2002) Characterization of nucleoside adducts of cis-2-butene-1,4-dial, a reactive metabolite of furan. Chem Res Toxicol 15:373–379 [DOI] [PubMed] [Google Scholar]
- Byrns MC, Vu CC, Peterson LA. (2004) The formation of substituted 1,N6-etheno-2′-deoxyadenosine and 1,N2-etheno-2′-deoxyguanosine adducts by cis-2-butene-1,4-dial, a reactive metabolite of furan. Chem Res Toxicol 17:1607–1613 [DOI] [PubMed] [Google Scholar]
- Capurro PU. (1973) Effects of exposure to solvents caused by air pollution with special reference to CCl 4 and its distribution in air. Clin Toxicol 6:109–124 [DOI] [PubMed] [Google Scholar]
- Carfagna MA, Held SD, Kedderis GL. (1993) Furan-induced cytolethality in isolated rat hepatocytes: correspondence with in vivo dosimetry. Toxicol Appl Pharmacol 123:265–273 [DOI] [PubMed] [Google Scholar]
- Chang TK, Crespi CL, Waxman DJ. (2006) Spectrophotometric analysis of human CYP2E1-catalyzed p-nitrophenol hydroxylation. Methods Mol Biol 320:127–131 [DOI] [PubMed] [Google Scholar]
- Chen LJ, Hecht SS, Peterson LA. (1995) Identification of cis-2-butene-1,4-dial as a microsomal metabolite of furan. Chem Res Toxicol 8:903–906 [DOI] [PubMed] [Google Scholar]
- Chen LJ, Hecht SS, Peterson LA. (1997) Characterization of amino acid and glutathione adducts of cis-2-butene-1,4-dial, a reactive metabolite of furan. Chem Res Toxicol 10:866–874 [DOI] [PubMed] [Google Scholar]
- Emery MG, Fisher JM, Chien JY, Kharasch ED, Dellinger EP, Kowdley KV, Thummel KE. (2003) CYP2E1 activity before and after weight loss in morbidly obese subjects with nonalcoholic fatty liver disease. Hepatology 38:428–435 [DOI] [PubMed] [Google Scholar]
- Gingipalli L, Dedon PC. (2001) Reaction of cis- and trans-2-butene-1,4-dial with 2′-deoxycytidine to form stable oxadiazabicyclooctaimine adducts. J Am Chem Soc 123:2664–2665 [DOI] [PubMed] [Google Scholar]
- Guengerich FP. (1982) Microsomal enzymes involved in toxicology-analysis and separation, in Principles and Methods of Toxicology (Hayes AW. ed) pp 609–634, Raven Press, New York [Google Scholar]
- Guengerich FP, Kim DH, Iwasaki M. (1991) Role of human cytochrome P-450 IIE1 in the oxidation of many low molecular weight cancer suspects. Chem Res Toxicol 4:168–179 [DOI] [PubMed] [Google Scholar]
- Hamberger C, Kellert M, Schauer UM, Dekant W, Mally A. (2010) Hepatobiliary toxicity of furan: identification of furan metabolites in bile of male f344/n rats. Drug Metab Dispos 38:1698–1706 [DOI] [PubMed] [Google Scholar]
- Hodgson E, Rose RL. (2007) The importance of cytochrome P450 2B6 in the human metabolism of environmental chemicals. Pharmacol Ther 113:420–428 [DOI] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (1995) Furan, in Dry Cleaning, Some Chlorinated Solvents and Other Industrial Chemicals, p 393, IARC, Lyon, France [Google Scholar]
- Kedderis GL, Carfagna MA, Held SD, Batra R, Murphy JE, Gargas ML. (1993) Kinetic analysis of furan biotransformation by F-344 rats in vivo and in vitro. Toxicol Appl Pharmacol 123:274–282 [DOI] [PubMed] [Google Scholar]
- Kedderis GL, Held SD. (1996) Prediction of furan pharmacokinetics from hepatocyte studies: comparison of bioactivation and hepatic dosimetry in rats, mice, and humans. Toxicol Appl Pharmacol 140:124–130 [DOI] [PubMed] [Google Scholar]
- Kellert M, Brink A, Richter I, Schlatter J, Lutz WK. (2008a) Tests for genotoxicity and mutagenicity of furan and its metabolite cis-2-butene-1,4-dial in L5178Y tk+/− mouse lymphoma cells. Mutat Res 657:127–132 [DOI] [PubMed] [Google Scholar]
- Kellert M, Wagner S, Lutz U, Lutz WK. (2008b) Biomarkers of furan exposure by metabolic profiling of rat urine with liquid chromatography-tandem mass spectrometry and principal component analysis. Chem Res Toxicol 21:761–768 [DOI] [PubMed] [Google Scholar]
- Lieber CS. (1997) Cytochrome P-4502E1: its physiological and pathological role. Physiol Rev 77:517–544 [DOI] [PubMed] [Google Scholar]
- Lu D, Peterson LA. (2010) Identification of furan metabolites derived from cysteine-cis-2-butene-1,4-dial-lysine cross-links. Chem Res Toxicol 23:142–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D, Sullivan MM, Phillips MB, Peterson LA. (2009) Degraded protein adducts of cis-2-butene-1,4-dial are urinary and hepatocyte metabolites of furan. Chem Res Toxicol 22:997–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maga JA. (1979) Furans in foods. CRC Crit Rev Food Sci Nutr 11:355–400 [DOI] [PubMed] [Google Scholar]
- Marinari UM, Ferro M, Sciaba L, Finollo R, Bassi AM, Brambilla G. (1984) DNA-damaging activity of biotic and xenobiotic aldehydes in Chinese hamster ovary cells. Cell Biochem Funct 2:243–248 [DOI] [PubMed] [Google Scholar]
- National Toxicology Program (1993) Toxicology and carcinogenesis studies of furan in F344/N rats and B6C3F1 mice. [NTP Technical Report No. 402]. US Department of Health and Human Services, Public Health Service, National Institutes of Health, Research Triangle Park, NC [Google Scholar]
- National Toxicology Program (2011) 12th Report on Carcinogens. U.S. Department of Health and Human Services, Washington, DC [Google Scholar]
- Neafsey P, Ginsberg G, Hattis D, Johns DO, Guyton KZ, Sonawane B. (2009a) Genetic polymorphism in CYP2E1: population distribution of CYP2E1 activity. J Toxicol Environ Health B Crit Rev 12:362–388 [DOI] [PubMed] [Google Scholar]
- Neafsey P, Ginsberg G, Hattis D, Sonawane B. (2009b) Genetic polymorphism in cytochrome P450 2D6 (CYP2D6): Population distribution of CYP2D6 activity. J Toxicol Environ Health B Crit Rev 12:334–361 [DOI] [PubMed] [Google Scholar]
- Paine MF, Hart HL, Ludington SS, Haining RL, Rettie AE, Zeldin DC. (2006) The human intestinal cytochrome P450 “pie”. Drug Metab Dispos 34:880–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmar D, Burka LT. (1993) Studies on the interaction of furan with hepatic cytochrome P-450. J Biochem Toxicol 8:1–9 [DOI] [PubMed] [Google Scholar]
- Peterson LA, Cummings ME, Chan JY, Vu CC, Matter BA. (2006) Identification of a cis-2-butene-1,4-dial-derived glutathione conjugate in the urine of furan-treated rats. Chem Res Toxicol 19:1138–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson LA, Cummings ME, Vu CC, Matter BA. (2005) Glutathione trapping to measure microsomal oxidation of furan to cis-2-butene-1,4-dial. Drug Metab Dispos 33:1453–1458 [DOI] [PubMed] [Google Scholar]
- Peterson LA, Naruko KC, Predecki DP. (2000) A reactive metabolite of furan, cis-2-butene-1,4-dial, is mutagenic in the Ames assay. Chem Res Toxicol 13:531–534 [DOI] [PubMed] [Google Scholar]
- Peterson LA, Phillips MB, Lu D, Sullivan MM. (2011) Polyamines are traps for reactive intermediates in furan metabolism. Chem Res Toxicol 24:1924–1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Yamazaki H, Mimura M, Inui Y, Guengerich FP. (1994) Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J Pharmacol Exp Ther 270:414–423 [PubMed] [Google Scholar]
- Vranová J, Ciesarová Z. (2009) Furan in food - a review. Czech J Food Sci 27:1–10 [Google Scholar]
- Wang H, Tompkins LM. (2008) CYP2B6: new insights into a historically overlooked cytochrome P450 isozyme. Curr Drug Metab 9:598–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.