Abstract
Background and Aims
The analysis of variability in mineral allocation to seeds has rarely been considered in relation to allometric patterns and deviations from the allometric trajectory. Here, I examine the scaling of carbon (C), nitrogen (N), phosphorus (P) and sulfur (S) with seed mass in field-collected seeds of Hedera helix, taking into account that brood size might influence the allocation patterns.
Methods
C, N and S contents of 56 individual seeds of different sizes were analysed by combustion using a standard automated CNHS procedure. P content was determined for 200 individual seeds using spectrophotometry after acid digestion of ashed samples. This sample included seeds from different brood sizes.
Key Results
C and N content both varied isometrically with seed mass and this variable explained most of the variation in their content in seeds. P and S, however, varied allometrically with seed mass. Additionally, seed mass only explained 37·3 % and 37·6 % of the total variance in P and S content, respectively. Seeds with higher mass contained proportionately more P and, furthermore, the seeds from small broods contained proportionally more P than those from large broods.
Conclusions
Although seed mass in this species can be used as a surrogate of investment in C and N, it does not account for variability in allocation of nutrients such as P and S. The fact that larger seeds increased their P concentration when found in smaller broods might be a consequence of intense competition for this nutrient among developing seeds. Then, brood size may influence the mineral nutrient concentration of seeds.
Keywords: Allocation, phosphorus, nitrogen, stoichiometry, seed mass, brood size, sibling rivalry
INTRODUCTION
Allocation of resources to seeds is a key phase in a plant's life history due to its influence on the likelihood of seedling emergence, establishment and survival, and its subsequent effects on maternal fitness. The dependence of offspring fitness on seed size has been extensively supported by many studies documenting the fact that larger seeds confer benefits to seedlings against nutrient limitation, drought, shading, herbivory and competition [e.g. Bond et al. (1999), Jakobsson and Eriksson (2000) and Leishman et al. (2000) for across species comparisons; Seiwa (2000), Seiwa et al. (2002) and Gómez (2004) for within species comparisons].
Probably as a result of this ecological importance, seed size variability is well-documented. Within an individual species, most variability occurs among the seeds produced by an individual plant (Michaels et al., 1988; Obeso and Herrera, 1994), suggesting that seed size is a character which shows certain phenotypic plasticity. In fact, an increase in the nutrient concentration of the growing environment often leads to the production of heavier seeds that contain higher levels of mineral nutrients (reviewed in Fenner, 1992). The main sources of within-plant variation in seed size are paternal genetic effects (Sundaresan, 2005), timing of flowering and fecundation, position effects (within plant and within fruit; Diggle, 2005), brood size (Obeso, 1993) and sibling rivalry (Obeso, 2004).
However, despite the considerable information available about seed-mass variation, and the fact that most studies consider seed mass to be an integrated measure of allocation, little is known about the allocation of different resources. Furthermore, the importance of seed mineral content on seedling performance has mainly been documented for cultivated species (Stoddard, 1999; Bramble et al., 2002; Calderini and Ortiz-Monasterio, 2003). Although positive effects of size on fitness are difficult to separate from those of mineral nutrient content due to the obvious correlation between both variables some studies have been able to clarify certain effects. It is known, for example, that phosphorus (P) concentration affects seedling growth rate in Rorippa nasturtium aquaticum in P-deficient growth conditions, independent of seed size (Austin, 1966). Similarly, seeds of Trifolium subterraneum with higher P concentrations but of the same average size have been found to produce plants of greater biomass (Bolland and Paynter, 1990). In Medicago polymorpha, the P concentration of seed was significantly associated with final adult size and seed production, while seed size was only associated with greater biomass (Bolland and Paynter, 1990).
Despite that allometric growth may explain a great amount of the variability in allocation patterns at the plant level (Weiner, 2004), there is relatively little information on stoichiometry at seed level or the consequences of the allometric trajectory on the distribution of resources within seed. In this respect, Krannitz (1997) reported that larger seeds of the shrub Purshia tridentata not only had greater absolute quantities of nitrogen (N) and magnesium but also a higher concentration of N, suggesting a clear advantage for heavier seeds. On the contrary, in wheat grains, N concentration was constant across the entire spike despite position effects on grain mass (Stoddard, 1999).
Due to the well-known trade-off between size and number (Smith and Fretwell, 1974), the number of seeds per fruit (brood size) is an important source of variability in seed mass. Then brood size might potentially explain deviations in allocation from those predicted by the allometric relationships. Furthermore, differences in seed provisioning due to within-fruit position effects, embryo genotype and sibling rivalry can also contribute to within-fruit variability in seed mass (Uma Shaanker et al., 1988; Domínguez, 1995; Obeso, 2004). In spite of its importance, there is a lack of information about mineral nutrient allocation in relation to the number of seeds per fruit and sibling rivalry within fruit. Several theoretical models predict the distribution of resources among sibs in terms of seed mass (e.g. Parker et al., 1989; Mock and Parker, 1997, 1998) and these predictions were empirically examined for seed mass (Obeso, 2004), but to my knowledge there is no information about distribution of other important compounds such as P, which is essential to plants in regard to energy transfer, activation of proteins and regulation of metabolic processes (Ågren, 2004). Furthermore, seed P concentration influences seedling growth rate (Austin, 1966; Parrish and Bazzaz, 1985; DeMarco, 1990); however, the information about factors controlling P concentration on individual seeds is amazingly scarce.
The objective of this study was to examine these relationships using field-collected seeds of Hedera helix. The first objective was to describe the patterns of carbon (C), N, sulfur (S) and P allocation in relation to seed mass and to estimate the percentage of variance in mineral nutrient content that is accounted for by the allometric relationship. When an important proportion of the variance is not explained by the allometric function, like it was in the case of P, the second objective was to determine to what extent the variance in P allocation, not explained by seed mass, can be explained by brood size. Specifically it can be predicted that as a result of resource limitation, sibs from larger broods would face more intense competition and thus contain relatively less P than those from smaller broods.
MATERIALS AND METHODS
Plant material and chemical analyses
Ivy, Hedera helix, is a woody climber native to Europe, western Asia and North Africa that is also very invasive as an exotic in North America (Thomas, 1998). It produces hermaphrodite flowers on globose umbels. The ovary is 5-lobed and contains five ovules (Tutin et al., 1968). The fruit is a black berry containing one to four seeds (five-seeded fruits are extremely rare; 5 in 700 fruits). The seeds are radially oriented in relation to the peduncle; therefore there are no position effects in relation to their access to maternal resources. This species is not pollen limited (Jacobs et al., 2009). The seeds are dispersed by frugivorous birds and martens that fed on fruits during winter. As the droppings contain several seeds, clusters of seedlings are frequent and seedling recruitment frequently forms a dense ground cover in woodland habitats (Metcalfe, 2005).
Samples of >100 fruits were collected from each of 14 individual ivy plants in a natural population living on hedgerow trees located in the Puerto San Lorenzo valley, Teverga, Asturias (northern Spain). Fruits were picked from as many infrutescences and plant positions as possible to reduce possible bias derived from position effects. Fruit samples were oven-dried at 60 °C for a minimum of 7 d. The dry pulp was manually removed and each individual seed was weighed to the nearest 0·1 mg and the seeds within the same fruit were ranked by seed mass. Sib ranks were assigned within fruit from the heaviest seed (A-sib) to the lightest one (D-sib in the case of four-seeded fruits). B-sib is the second in the hierarchy of masses and C-sib the third.
A subsample of 56 seeds, which included four seeds from each of the 14 plants, was separated for chemical analyses. The sample included seeds of different sizes in order to examine the allometric relationships, and the seeds of all plants were combined. Each of these seeds was weighed individually and analysed for C, N and S content by combustion using a standard automated CNHS procedure (PE 2400 Series II, CNHS/O).
Taking into account that P cannot be analysed in individual seeds at the same time as CNHS analyses, a different subsample of 201 seeds, was used for P analyses. This subsample consisted of seeds from 81 fruits (six fruits per plant in 11 plants and five fruits per plant in three plants): 16 one-seeded, 24 two-seeded, 27 three-seeded and 14 four-seeded fruits. The sample included seeds from all different brood sizes (one-, two-, three- and four-seeded fruits) from each of the 14 plants and 12–16 seeds per plant. P content (total content of P per seed) was determined by spectrophotometer after acid digestion of ashed samples (Niklas and Cobb, 2005).
Statistical analyses
Type II models [reduced major axis (RMA), the slopes denoted as αRMA] were used to determine the scaling exponents of C, N, P and S versus seed mass and N versus C since functional (rather than predictive) relationships were sought between variables that are biologically interdependent and subject to measurement error (Niklas, 1994). As standard error for αRMA the same standard error as the ordinary least squares estimate was used (Quinn and Keough, 2002). To determine whether the slopes differed significantly from 1·0 (isometry), t-tests were used. The coefficients of regression (r2) were used as a measure of the proportion of the total variation in Y that is explained by its linear relationship with X. In the case of P, to consider the hierarchical sampling design, the identity of the mother plant was also included as a random factor using linear mixed-effect models. The random slope and random intercept model showed with the lowest AIC (Akaike Information Criterion) estimated the same slope (0·818 ± 0·062) as the linear model (0·819 ± 0·064; Table 1). One-way ANOVAs were performed to examine the effect of brood size and seed rank on seed mass, P content and P concentration. When F-tests were significant, Tukey tests were performed to separate different means. ANCOVA using the seed mass as a covariate was performed to test differences among plants in allocation to P. The statistical analyses were performed with R (R Development Core Team, 2009).
Table 1.
Summary of reduced major axis (RMA) regression analyses of log10-transformed data
| Log Y vs log X | r | αRMA ± s.e. | t-Test | Significance |
|---|---|---|---|---|
| C vs seed mass | 0·994 | 0·993 ± 0·014 | 0·500 | 0·309 |
| N vs seed mass | 0·916 | 1·020 ± 0·056 | 0·357 | 0·361 |
| S vs seed mass | 0·613 | 0·819 ± 0·088 | 2·057 | 0·021 |
| P vs seed mass | 0·611 | 1·191 ± 0·061 | 3·131 | 0·001 |
| N vs C | 0·900 | 1·027 ± 0·061 | 0·442 | 0·330 |
t-Tests examine whether αRMA significantly differs from 1 (isometry).
d.f. = 54, except for P versus dry mass where d.f. = 199.
RESULTS
C and N allocation varied isometrically with seed mass (Table 1 and Fig. 1) and this variable explained 98·8 % and 83·9 % of the total variation in C and N content of seeds, respectively. In addition, N allocation varied isometrically with C content and this relationship explained 81·0 % of the total variance (Table 1).
Fig. 1.
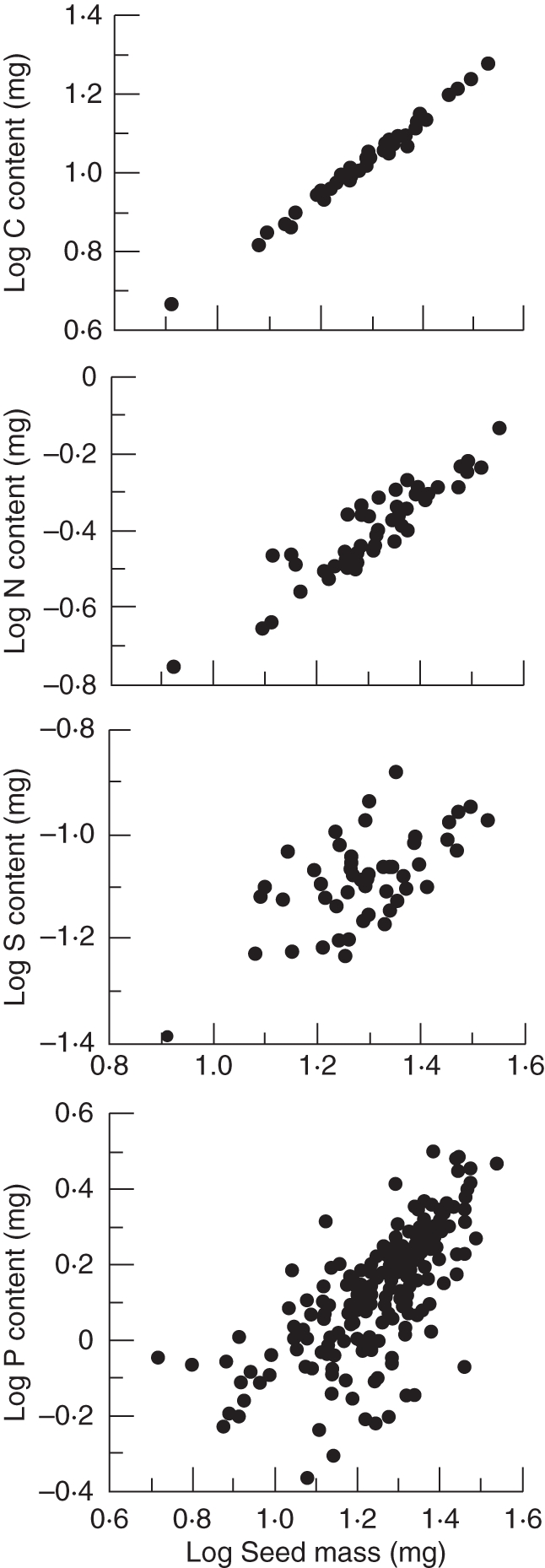
Total amount of carbon, nitrogen, sulfur and phosphorous in seeds of Hedera helix with different seed masses.
However, P and S varied allometrically with seed mass, with the power of the relationship being slightly greater than 1·0 in the case of P and lower than 1·0 in the case of S content (Table 1 and Fig. 1) and seed mass only explained 37·5 % and 37·3 % of the total variance in P and S allocation, respectively.
Brood size had a significant effect on mean seed mass per fruit (F3,197 = 4·404, P = 0·005), and mean P content per seed (F3,197 = 10·547, P < 0·001). Seeds from four-seeded fruits showed a significantly lower mean seed mass and P content than seeds from smaller brood sizes (Fig. 2). P concentration (mg P mg−1 dry matter) varied significantly with brood size (F3,197 = 5·276, P = 0·002) and showed significantly lower values for seeds from four-seeded fruits (Fig. 2).
Fig. 2.
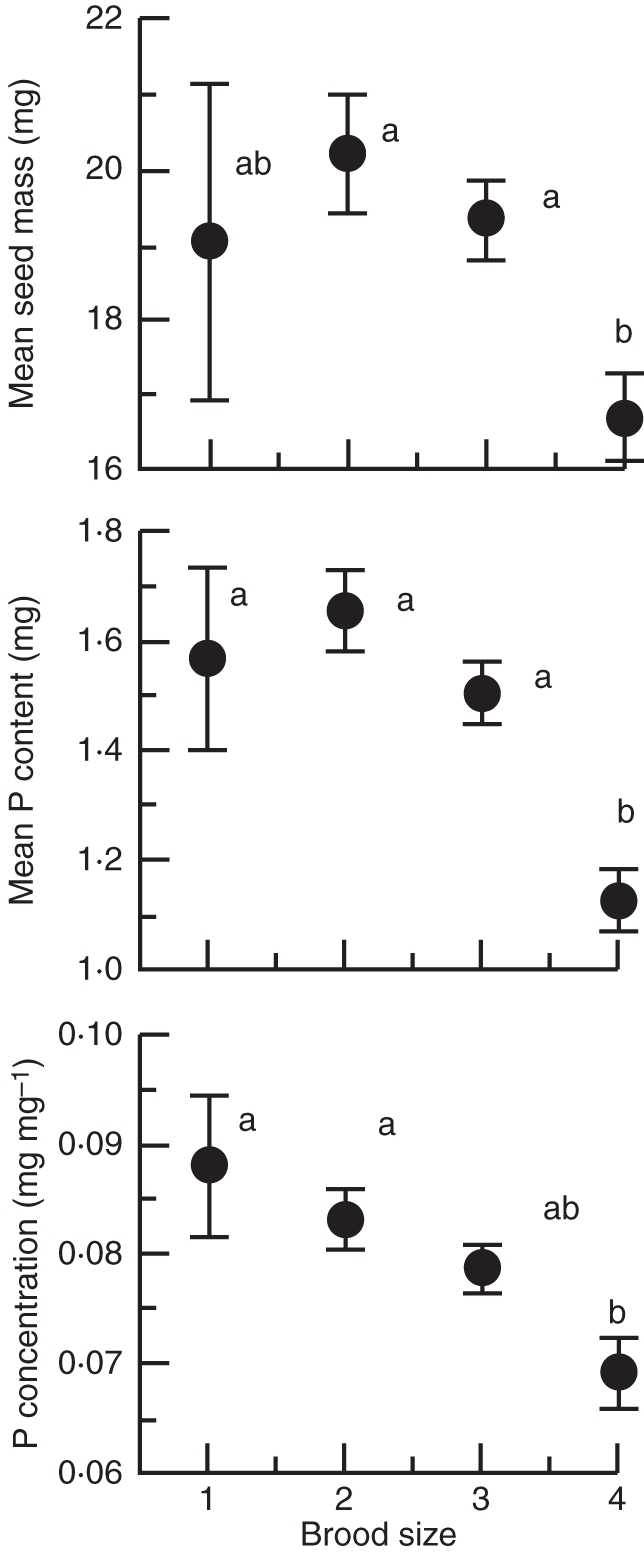
Mean seed mass, mean P content and mean P concentration for seeds from different brood sizes (one- to four-seeded fruits). Bars indicate ± s.e. and significantly different means (P < 0·05, Tukey test) are indicated by different letters.
When seed mass was included as a covariate, neither individual plants (F13,173 = 1·161, P = 0·312) nor differences between plant slopes (interaction plant × seed mass; F13,173 = 0·946, P = 0·507) accounted significantly for variability in P allocation. After removing the non-significant interaction, differences among mother plants became significant (F13,186 = 12·718, P < 0·001) and explained 28 % of total variance in P allocation.
When examining, for all fruits combined, different brood sizes separately, P concentration of seeds (mg P mg−1 dry matter) did not vary with sib rank within fruit (for two-seeded F1,48 = 0·454, P = 0·504; for three-seeded F2,81 = 0·544, P = 0·582; for four-seeded fruits F3,48 = 2·422, P = 0·077). However, A-sib P concentration varied with brood size (F3,77 = 5·479, P = 0·0018), significantly decreasing within four-seeded fruits (Fig. 3). Following a similar pattern, the P concentration of B-sibs also decreased within four-seeded fruits (F2,63 = 3·851, P = 0·026; Fig. 3) but, C-sib P concentration did not vary with brood size (three- versus four-seeded fruits, F1,39 = 3·179, P = 0·082; Fig. 3).
Fig. 3.
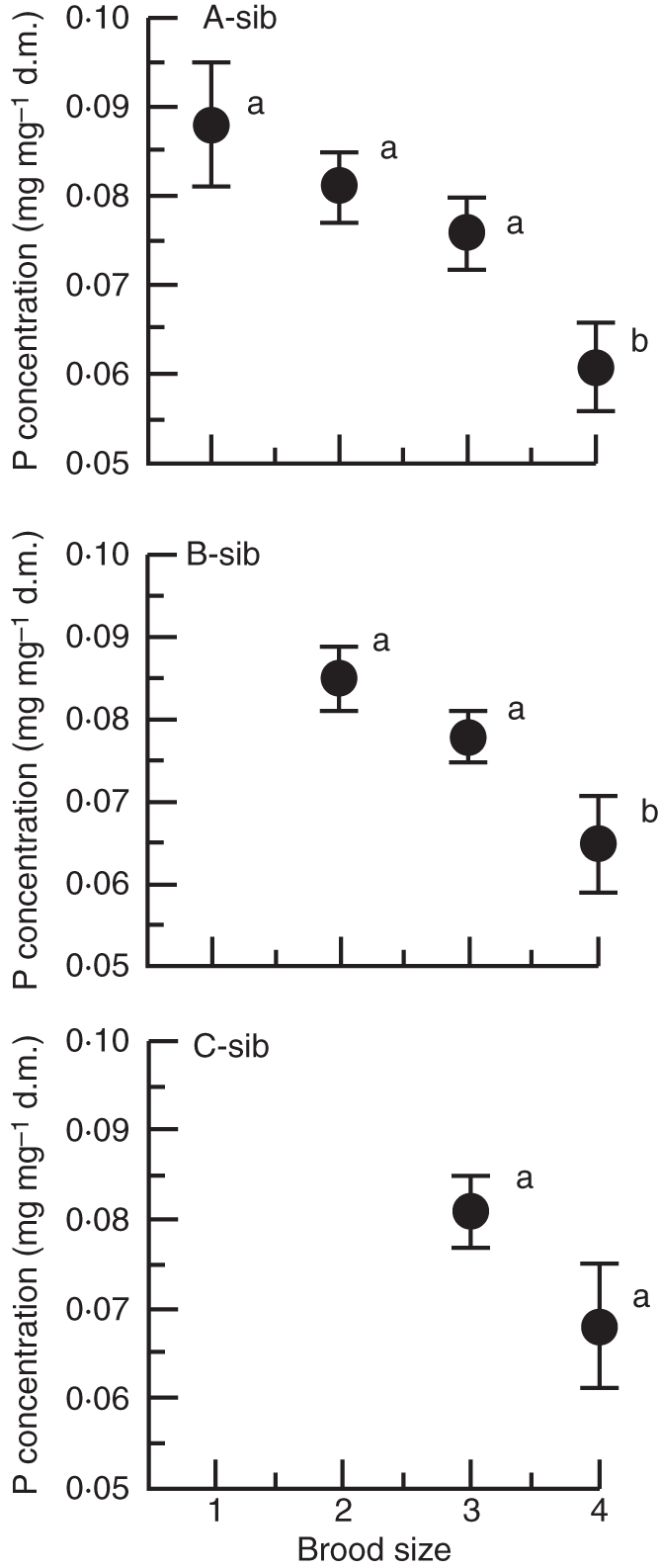
Mean seed P concentration (mg mg−1 dry matter) for sibs of the same rank in fruits of different brood sizes (one- to four-seeded fruits). A-sib (the heaviest seed within fruit) in one- to four-seeded fruits, B-sib (the second seed in the hierarchy of masses within fruit) in two- to four seeded fruits and C-sib (the third seed in the hierarchy of masses within fruit) in three- and four-seeded fruits. Bars indicate ± s.e. and significantly different means (P < 0·05, Tukey test) are indicated by different letters.
DISCUSSION
This study documents that variability in allocation of different essential elements (N, C) to seeds may be almost exclusively attributed to allometric growth and that deviations from the allometric trajectory, like the case of P, can be explained by brood size.
Seed mass is extensively used as an estimate of offspring fitness and is usually used as an integrated measure of allocation under the assumption that it represents an accurate estimate of the allocation of different essential elements. However, present results showed that there are substantial differences in the patterns of scaling of different mineral nutrients in Hedera helix seeds. Whereas the allocation of C and N scales isometrically with increasing seed mass, the allocation of P and S scales allometrically. Whereas most of the variation in allocation of C and N may be explained by variation in seed mass, an important portion of the variation in allocation of S and P still remains unexplained after removing this effect. N and C follow an allometric trajectory that allows us to predict allocation of these elements to seeds after considering any factor that affects seed mass. However, we need an alternative explanation for variability in P allocation. The P concentration of seeds from large broods was reduced and the sibs occupying the highest ranks in the hierarchy of masses (sibs A and B; Fig. 3) were found to obtain an additional advantage in small broods in terms of P concentration.
The balance of N and P in plant tissues plays a pivotal role in plant biology. N allocation reflects investment in proteins, which are particularly rich in N. It is P allocation, however, that represents the capacity to produce proteins. The amount of P should correlate with the amount of ribosomes (a metabolically important sink of P), which represent the machinery to produce proteins and is thought to be related to plant growth rate (Kerkhoff et al., 2006; Niklas, 2006). Specifically, growth rates should correlate positively with increasing ribosomal RNA (and P) investments relative to protein (and N) investments (Niklas, 2006). The positive effect of seed P concentration on seedling and plant growth and even on plant fecundity has in fact been documented in several species (e.g. Austin, 1966; Bolland and Paynter, 1990). Furthermore, P content was found to be more important than seed mass in determining final plant mass of Trifolium subterraneum (Bolland and Paynter, 1990) although, when comparing plants of the same species growing at different rates, Matzek and Vitousek (2009) found that faster-growing plants did not have consistently lower N : P ratios.
Given that N content varied isometrically whilst P content varied allometrically with seed mass it can be concluded that a significant proportion of the variance in P allocation is not explained by N allocation. It therefore follows that there may be variability in P content for a fixed amount of N which might be indicative of differences between seeds in the potential growth rate of their future seedlings. In the case of H. helix, the potential growth rate of seedlings seems to favour larger seeds from small broods, which exhibit higher P : N ratios and probably faster growth.
Following across-species comparisons, it has been established that total leaf P content scales as the 4/3 power of the total plant mass for non-woody plants. Similarly, plant growth rate should scale across all species as the three-quarters power of total leaf P per plant (Niklas, 2006). If we assume that across species allometrical relationship can be translated to within-species comparisons (for Eranthis hyemalis, see Niklas and Cobb, 2006)) and from the whole plant to different plant tissues (Vrede et al., 2004; Niklas, 2006), P content within seed tissues of H. helix should scale as the 4/3 power of seed mass. However, results presented here show that the exponent was significantly >1 and <4/3 in accordance with the different stoichiometric scaling reported for reproductive tissues (Kerkhoff et al., 2006) and the suggestions of Ågren (2004)
S content has been related to the synthesis of proteins containing sulfur amino acids. The allometric allocation of this element to H. helix seeds did not follow the same pattern as P allocation, and a slight proportional advantage seems to be conferred on smaller seeds in terms of S content. The complex interaction between S and N content makes it difficult to interpret the patterns of allocation to S (Zhao et al., 1997; Scherer, 2001).
After removing the effect of seed mass and mother plant identity, 34 % of variance in P allocation remains unexplained. Hence, some of the variability in P allocation that is independent of seed mass or mother plant must be explained at the fruit level. Patterns of seed mass, and consequently, N and C allocation can be interpreted as a physiological constraint leading to sibling competition. Seed P content increases as the brood size increases from one to four, hence larger fruits are stronger sinks of P, but this P has to be shared between more seeds. Furthermore, resources are not equally distributed within broods (for instance, in four-seeded fruits D-sib achieve on average 67 % of A-sib weight) which can be interpreted as the consequence of competition among developing sibs. As a result of the physiological constraint and subsequent competition, smaller broods have proportionally more nutrients per sib and the optimum for an individual seed is a fruit containing two seeds.
The fact that A-sibs increased their P concentration in small broods might open the ground for fierce sibling rivalry, which may result in brood reduction, which would result in an advantage for A- and B-sibs from small broods in terms of P concentration and probably in terms of seedling growth rate (Parrish and Bazzaz, 1985; DeMarco, 1990). This phenomenon probably entails two ecological implications. First, seed development is a stage of the life cycle with great mortality for many angiosperms and only a small proportion of the embryos develop into seeds (57·5 % of the ovules developed into seeds in the mature fruits of ivy). Thus natural selection operating very early in the sporophytic phase might be driven, at least partially, by competition for P. Secondly, brood reduction increases the P : N ratio of the surviving seeds which may additionally enhance the growth rate and competitiveness of the seedlings.
In summary, although in this species seed mass can be used as a surrogate of investment in C and N, it is not an adequate estimate of investment in P and S. P allocation to seeds is not only determined by the allometric trajectory but also the consequence of competition for this nutrient among developing seeds as confirmed by the fact that larger seeds increased their P concentration when found in smaller broods.
ACKNOWLEDGEMENTS
The author thanks Amalia Segura and Jorge Sostres for their expertise and assistance with phosphorus and CNH analyses. The comments by anonymous reviewers helped to improve the original version of the manuscript. Financial support was provided by project MICINN-09-CGL2009-11302 (Spanish Ministry of Science and Innovation)
LITERATURE CITED
- Ågren GI. The C : N : P stoichiometry of autotrophs – theory and observations. Ecology Letters. 2004;7:185–191. [Google Scholar]
- Austin RB. The growth of watercress (Rorippa nasturtium aquaticum (L) Hayek) from seed as affected by the phosphorus nutrition of the parent plant. Plant and Soil. 1966;24:113–120. [Google Scholar]
- Bolland MDA, Paynter BH. Increasing phosphorus concentration in seed of annual pasture legume species increases herbage and seed yields. Plant and Soil. 1990;125:197–205. [Google Scholar]
- Bond WJ, Honig M, Maze KE. Seed size and seedling emergence: an allometric relationship and some ecological implications. Oecologia. 1999;120:132–136. doi: 10.1007/s004420050841. [DOI] [PubMed] [Google Scholar]
- Bramble T, Herrman TJ, Loughin T, Dowell F. Single kernel protein variance structure in commercial wheat fields in western Kansas. Crop Science. 2002;42:1488–1492. [Google Scholar]
- Calderini DF, Ortiz-Monasterio I. Grain position affects grain macronutrient and micronutrient concentrations in wheat. Crop Science. 2003;43:141–151. [Google Scholar]
- DeMarco DG. Effect of seed weight, and seed phosphorous and nitrogen concentrations on the early growth of wheat seedlings. Australian Journal of Experimental Agriculture. 1990;30:545–549. [Google Scholar]
- Diggle PK. Architectural effects and the interpretation of patterns of fruit and seed development. Annual Review of Ecology and Systematics. 1995;26:531–552. [Google Scholar]
- Domínguez CA. Genetic conflicts of interest in plants. Trends in Ecology and Evolution. 1995;10:412–416. doi: 10.1016/s0169-5347(00)89158-2. [DOI] [PubMed] [Google Scholar]
- Fenner M. Environmental influences on seed size and composition. Horticultural Review. 1992;74:385–392. [Google Scholar]
- Gómez JM. Bigger is not always better: conflicting selective pressures on seed size in Quercus ilex. Evolution. 2004;58:71–80. doi: 10.1111/j.0014-3820.2004.tb01574.x. [DOI] [PubMed] [Google Scholar]
- Jacobs JH, Clark SJ, Denholm I, Goulson D, Stoate C, Osborne JL. Pollination biology of fruit-bearing hedgerow plants and the role of flower-visiting insects in fruit-set. Annals of Botany. 2009;104:1397–1404. doi: 10.1093/aob/mcp236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobsson A, Eriksson O. A comparative study of seed number, seed size, seedling size and recruitment in grassland plants. Oikos. 2000;88:494–502. [Google Scholar]
- Kerkhoff AJ, Fagan WF, Elser JJ, Enquist BJ. Phylogenetic and growth form variation in the scaling of nitrogen and phosphorus in the seed plants. American Naturalist. 2006;168:E103–E122. doi: 10.1086/507879. [DOI] [PubMed] [Google Scholar]
- Krannitz PG. Variation in magnesium and nitrogen content in seeds of the Antelope bitterbrush (Purshia tridentata, Rosaceae) American Journal of Botany. 1997;84:1738–1742. [PubMed] [Google Scholar]
- Leishman MR, Wright IJ, Moles AT, Westoby M. The evolutionary ecology of seed size. In: Fenner M, editor. Seeds: the ecology of regeneration in plant communities. Wallingford, UK: CABI Publishing; 2000. pp. 31–57. [Google Scholar]
- Matzek V, Vitousek PM. N:P stoichiometry and protein:RNA ratios in vascular plants: an evaluation of the growth rate hypothesis. Ecology Letters. 2009;12:765–771. doi: 10.1111/j.1461-0248.2009.01310.x. [DOI] [PubMed] [Google Scholar]
- Metcalfe DJ. Biological flora of the British Isles. Hedera helix L. Journal of Ecology. 2005;93:632–648. [Google Scholar]
- Michaels HJ, Benner B, Hartgerink AP, et al. Seed size variation: magnitude, distribution, and ecological correlates. Evolutionary Ecology. 1988;2:157–166. [Google Scholar]
- Mock DW, Parker GA. The evolution of sibling rivalry. Oxford: Oxford University Press; 1997. [Google Scholar]
- Mock DW, Parker GA. Siblicide, family conflict and the evolutionary limits of selfishness. Animal Behaviour. 1998;56:1–10. doi: 10.1006/anbe.1998.0842. [DOI] [PubMed] [Google Scholar]
- Niklas KJ. Plant allometry, the scaling of form and process. Chicago, IL: University of Chicago Press; 1994. [Google Scholar]
- Niklas KJ. Plant allometry, leaf nitrogen and phosphorus stoichiometry, and interspecific trends in annual growth rates. Annals of Botany. 2006;97:155–163. doi: 10.1093/aob/mcj021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niklas KJ, Cobb ED. N, P, and C stoichiometry of Eranthis hyemalis (Ranunculaceae) and the allometry of plant growth. American Journal of Botany. 2005;92:1256–1263. doi: 10.3732/ajb.92.8.1256. [DOI] [PubMed] [Google Scholar]
- Obeso JR. Seed mass variation in the perennial herb Asphodelus albus: sources of variation and position effect. Oecologia. 1993;93:571–575. doi: 10.1007/BF00328967. [DOI] [PubMed] [Google Scholar]
- Obeso JR. Seed provisioning within holly fruits: tissue relatedness and test of the hierarchical model. Evolutionary Ecology. 2004;18:133–144. [Google Scholar]
- Obeso JR, Herrera CM. Inter- and intraspecific variation in fruit traits in co-occurring vertebrate-dispersed plants. International Journal of Plant Science. 1994;155:382–387. [Google Scholar]
- Parker GA, Mock DW, Lamey TC. How selfish should stronger sibs be? American Naturalist. 1989;133:846–868. [Google Scholar]
- Parrish JAD, Bazzaz FA. Nutrient content of Abutilon theophrasti seeds and the competitive ability of the resulting plants. Oecologia. 1985;65:247–251. doi: 10.1007/BF00379224. [DOI] [PubMed] [Google Scholar]
- Quinn GP, Keough MJ. Experimental design and data analysis for biologists. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2009. http://www.R-project.org . [Google Scholar]
- Scherer HW. Sulphur in crop production. European Journal of Agronomy. 2001;14:81–111. [Google Scholar]
- Seiwa K. Effects of seed size and emergence time on tree seedling establishment: importance of developmental constraints. Oecologia. 2000;123:208–215. doi: 10.1007/s004420051007. [DOI] [PubMed] [Google Scholar]
- Seiwa K, Watanabe A, Saitoh T, Kanno H, Akasaka S. Effects of burying depth and seed size on seedling establishment of Japanese chestnuts, Castanea crenata. Forest Ecology and Management. 2002;164:149–156. [Google Scholar]
- Smith CC, Fretwell SD. Optimal balance between size and number of offspring. American Naturalist. 1974;108:499–506. [Google Scholar]
- Stoddard FL. Variation in grain mass, grain nitrogen, and starch B-granule content within wheat heads. Cereal Chemistry. 1999;76:139–144. [Google Scholar]
- Sundaresan V. Control of seed size in plants. Proceedings of the National Academy of Sciences of the USA. 2005;102:17887–17888. doi: 10.1073/pnas.0509021102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas LK., Jr Topographic alterations, forest structure, and invasion by English ivy (Hedera helix L.) in the Rock Creek Floodplain, Washington, D.C. Natural Areas Journal. 1998;18:164–168. [Google Scholar]
- Tutin TG, Heywood VH, Burgess NA, et al. Flora Europaea. Vol. 2. Cambridge: Cambrige University Press; 1968. [Google Scholar]
- Uma Shaanker R, Ganeshaiah KN, Bawa KS. Parent–offspring conflict, sibling rivalry, and brood size in plants. Annual Review of Ecology and Systematics. 1988;19:177–205. [Google Scholar]
- Vrede TD, Dobberfuhl SA, Kooijman LM, Elser JJ. Fundamental connections among organism C:N:P stoichiometry, macromolecular composition, and growth. Ecology. 2004;85:1217–1229. [Google Scholar]
- Weiner J. Allocation, plasticity and allometry in plants. Perspectives in Plant Ecology Evolution and Systematics. 2004;6:207–215. [Google Scholar]
- Zhao FJ, Withers PTA, Evans ET, et al. Sulphur nutrition: an important factor for the quality of wheat and rapeseed. Soil Science and Plant Nutrition. 1997;43:1137–1142. [Google Scholar]


