Abstract
Background and Aims
Self-pollination dominates in wheat, with a small level of out-crossing due to flowering asynchrony and male sterility. However, the timing and synchrony of male and female flowering in wheat is a crucial determinant of seed set and may be an important factor affecting gene flow and resilience to climate change. Here, a methodology is presented for assessing the timing and synchrony of flowering in wheat, Triticum aestivum.
Methods
From the onset of flowering until the end of anthesis, the anther and stigma activity of each floret was assessed on the first five developing ears in potted plants grown under ambient conditions and originating from ‘Paragon’ or ‘Spark-Rialto’ backgrounds. At harvest maturity, seed presence, size and weight was recorded for each floret scored.
Key Results and Conclusions
The synchrony between pollen dehiscence and stigma collapse within a flower was dependent on its relative position in a spike and within a floret. Determined on the basis of synchrony within each flower, the level of pollination by pollen originating from other flowers reached approx. 30 % and did not change throughout the duration of flowering. A modelling exercise parameterized by flowering observations indicated that the temporal and spatial variability of anther activity within and between spikes may influence the relative resilience of wheat to sudden, extreme climatic events which has direct relevance to predicted future climate scenarios in the UK.
Keywords: Wheat, Triticum aestivum, flowering synchrony, pollination, climate change, heat resistance
INTRODUCTION
The time of flowering in cereals is usually stated as when 50 % of ears are in flower, which is adequate for differentiating gross differences in flowering time between genotypes, such as for photoperiod sensitivity (Marcello and Single, 1971), or between environments (Griffiths et al., 2009). This type of characterization, however, ignores differences in the temporal and spatial variation in flowering that occurs between spikes, amongst spikelets, and within florets of a single spikelet (Percival, 1921). Further fluctuations in timing may include diurnal effects, as demonstrated in rice to avoid high midday light intensities and temperature (Virmani and Edwards, 1983), but also in wheat (De Vries, 1974). Hence, florets of a single cereal genotype do not flower simultaneously. This variability in the temporal and spatial pattern of flowering has potential implications for (a) gene flow between plant populations (Demotes-Mainard et al., 1996; Willenborg et al., 2009); (b) plant breeding; and (c) physiological escape of those florets which are active before or after a major abiotic stress event (Saini and Aspinall, 1982; Zhao et al., 2010).
Wheat flowering biology was first studied in detail by Russian and Hungarian scientists when hybrid wheat production came to the fore (for review, see Pickett, 1993). The stages of floral development which have been identified as sensitive to climatic stress include pollen formation (Saini and Aspinall, 1982; Craufurd and Peacock, 1993; Lalonde et al., 1997), anthesis (Bidinger et al., 1987; Ferris et al., 1998; Zhao et al., 2010) and seed set (Tashiro and Wardlaw, 1990). Experimentally, such vulnerable stages have been identified through applying extended durations of heat stress, usually lasting for a number of days. High temperature stress seems increasingly relevant in UK conditions; maximum daily temperatures in major wheat-growing areas of the UK reached between 28 °C and 30 °C on 2 d in 2010 (23 May and 4 June), shortly before and during flowering. At these growth stages, prolonged exposure to such temperatures can be associated with significant reductions in seed set in UK wheat (Ferris et al., 1998). These heat-stress events commonly only last for a few hours around midday and in the early afternoon for consecutive days. For this reason, it is the number of florets at vulnerable stages during each of these successive, short-duration stress events that is likely to be directly relevant to the success or otherwise of seed set of the crop.
In bread wheat, out-crossing is estimated to be around 6 % in the absence of stress (Hucl, 1996). However, most estimates of cross-pollination in wheat have been obtained during a total loss of pollen from one vulnerable genotype. For example, cross-pollination in field conditions was found to reach 32 % in cold and dark-sensitive Moulin, compared with 7 % in Aubaine – a less-sensitive cultivar (Demotes-Mainard et al., 1996). Hence there is little understanding of within-plant pollen movement, and of the potential scope of individual genotypes buffering the loss of pollen in one floret by pollination with pollen from another floret on the same spike or between spikes of the same or different plants. In this paper, the terminology ‘same-floret’ pollination will refer to pollination of the stigma by the pollen formed in the same flower, ‘cross-floret’ pollination will refer to a stigma pollinated by the pollen from a different floret (either within the same spikelet, on the same spike or between spikes). Within-plant pollen movement cannot be quantified genetically, but accurate analyses of the developmental stages of the stigma and the stamen of each flower can provide an estimation of the diversity of flowering behaviour within a single genotype. A quantitative scale for flower development in wheat and barley was created by Waddington et al. (1983). However, the classification only characterizes the development of the stigma, with no reference to anther development or pollen release. The intrinsic variability between anther and stigma activity, driven by the flowering phenology of each genotype, interacts with stress factors of differing type, severity and duration, thus limiting our understanding of stress responses at various physiological stages leading to, and following on from, anthesis.
Analyses of temporal and spatial diversity of developmental stages of both male and female flower parts can indicate the scope for individual plants of a single genotype to buffer loss of pollen from a proportion of florets at any one time. Making use of several near-isogenic lines (NILs) from ‘Paragon’ and ‘Spark-Rialto’ backgrounds, the objectives of this study were (a) to develop a methodology to quantify phenological variation between and within wheat backgrounds; (b) to determine the timing of flowering and level of synchrony within individual flowers; and (c) to estimate the effects of stress on same-floret pollination.
MATERIALS AND METHODS
Plant material
Plants of Triticum aestivum originating from varieties ‘Paragon’ and ‘Spark-Rialto’ were grown in 12·5-cm-diameter pots filled with a 4 : 4 : 2 : 1 mixture of steam-sterilized 6-mm gravel, medium vermiculite, 3-mm sharp sand and peat-based potting compost. To supplement plant nutrition, 2 kg of Osmocote Pro 3–4 months (Scotts, UK) were added per cubic metre of planting mixture. Pots filled with planting medium were soaked with water for a minimum of 5 h before being planted with single seeds to a depth of 2–2·5 cm. In total, six ‘Paragon’ and nine ‘Spark-Rialto’ NILs were used in this experiment (Table 1). All pots were placed in a cold room at 4 °C for 42 d, at which point the majority of coleoptiles had emerged. Pots were then transferred outside to a fenced off area and raised to a height of approx. 10 cm on bricks to allow free drainage. Plants were irrigated automatically through a drip system and treated against powdery mildew once on the 25 June with Flexity [300 g L−1 (25·2 % w/w) metrafenone (BASF Plc, UK) in 0·5 L ha−1]. From stem extension to maturity, the whole area was protected by bird-proof netting.
Table 1.
Details of ‘Paragon’ and ‘Spark-Rialto’ lines used for flowering and seed assessment
| Line | Region of interest | No. of ears scored |
|---|---|---|
| ‘Paragon’ | ||
| ‘Paragon’ (GS-100 Ppd-A1a) | 2A | 10 |
| ‘Paragon’ (Sonora 64 Ppd-D1a) | 2D | 10 |
| ‘Paragon’ (Sonora 64 Ppd-B1a) | 2B | 10 |
| ‘Paragon’ (Chinese Spring Ppd-B1a) | 2B | 10 |
| ‘Paragon’ | n.a. | 5 |
| ‘Spark-Rialto’ | ||
| SR13-418-2 | 3B | 5 |
| SR20-388-4 | 3B | 15 |
| SR20-387-1 | 3B | 15 |
| SR13-417-3 | 3B | 20 |
| SR13-418-2 | 3B | 15 |
| SR23-441-1 | 1D | 25 |
| SR23-413-2 | 1D | 20 |
| SR9-413-2 | 1D | 25 |
| SR9-411-2 | 1D | 20 |
Flowering and yield assessment
A total of 43 plants from ‘Paragon’ (Bentley et al., 2011) and ‘Spark-Rialto’ (currently being developed at John Innes Centre, Norwich, UK) NILs were used to assess the progress of flowering in both male and female parts. All inflorescences to develop on the first five ear-bearing tillers (main stem = tiller 1) were observed every day from the initial signs of anthesis until the cessation of flowering activity. The spikelets of an ear were numbered from the collar upwards, the lowest being ‘1’ and the subsequent numbers alternating between sides such that one side of the ear is ‘odd’ and the other ‘even’. All spikelets were assessed in ‘Paragon’ NILs, while only odd numbered spikelets were assessed in ‘Spark-Rialto’. The lowest potential grain site frequently lacked both stigma and anthers, but this was consistently labelled as spikelet 1. Floret labelling followed the scheme of Kirby and Appleyard (1984), with the first floret from the lower glume labelled as ‘A’, subsequent florets up the spikelet alternated between sides such that florets ‘B’ and ‘D’ were on the same side. Assessments continued until all flowering stopped on tiller 5.
Observations started at 10·00 h and were consistently completed within 2·5 h. Emerging ears were first inspected at growth stage 57 (Zadoks et al., 1974). Scoring of a spikelet was initiated when the lower glume could be opened easily with a thumbnail. In the early stages of flowering, usually during days 1–3 after first inflorescence on an ear, not all glumes could be opened as some flowers were lagging in development. Care was taken not to force such flowers, but to observe them on the subsequent day. Subsequent florets within the same spikelet were observed by easing open each lemma. Each floret which could be opened was scored for the developmental stage of both male and female parts according to the scheme outlined in Table 2. Male flowers were assessed on a four-point developmental scale, while female flowers were assigned three stages. Anthers were defined as ‘active’ when showing signs of pollen dehiscence (stage 3) whereas stigmas were considered receptive to pollination when graded as F (fluffy). Active stigma duration was defined as the number of consecutive days for which a stigma was graded as F. Flowering synchrony was defined as the day of pollen dehiscence minus the day of observed stigma collapse within an individual flower. Negative flowering synchrony values indicate pollen release before stigma collapse. All plants were left to mature outside and were harvested to assess grain yield. Weight of each individual seed was established, together with the position within an ear.
Table 2.
Definition of scoring terminology for male and female parts of a wheat ear (the female scoring system is compared with the equivalent scale developed by Waddington et al., 1983)
| Score | Flowering stage description | Waddington et al. (1983) |
|---|---|---|
| Female | ||
| HF | Styles and stigmatic branches erect or spreading. | 9·25–9·5 |
| F | Stigmatic branches spread widely, stigma curved and fully spread. | 10 |
| PF | Stigmatic branches curled or collapsed. Stigma showing partial or total collapse. | n.a. |
| Male | ||
| 1 | Immature anthers green and alongside immature styles. | n.a. |
| 2 | Anthers pale green to pale yellow and filaments extending. Anthers no longer parallel with style. | n.a. |
| 3 | Pollen extrusion, anthers bright yellow. Anthers inside or outside the floret lemma. | n.a. |
| 4 | Anthers pale yellow, collapsed or missing. Anthers inside or outside the floret lemma. | n.a. |
Temporal development of flowering activity was analysed by fitting a Gaussian distribution to the aggregate numbers of active flowers in all five tillers under observation and by testing for differences in the timing of peak flowering. Linear regressions were fitted to flowering synchrony and active-stigma duration data, the slope of the regression was then used to assess the significance of temporal and spatial trends. Seed weight and number data were compared by a t-test. A reverse cumulative Gaussian function was used to model the number of surviving male flowers (Fig. 1) and a Gaussian distribution to model anther flowering activity (Fig. 1). All analyses were performed in Statistica 8·0 (Statsoft Inc.) considering individual plants as true replicates; any effects were deemed significant at P < 0·05.
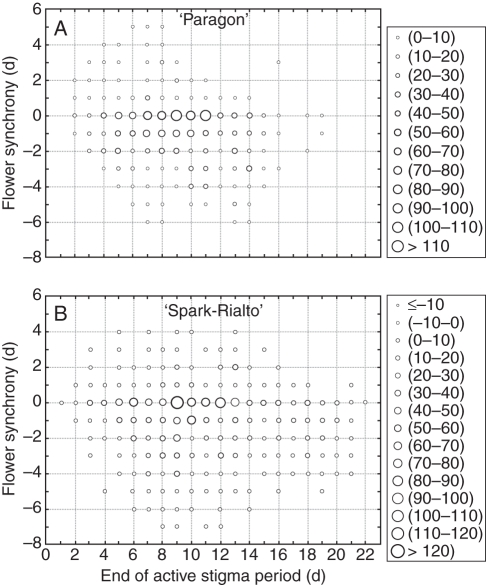
Fig. 1.
Frequency scatterplots of flowering synchrony calculated as the day of pollen dehiscence minus day of stigma collapse for ‘Paragon’ (A) and ‘Spark-Rialto’ lines (B). Negative synchrony values imply dehiscence before stigma collapse; zero on x-axis indicates the first day of flowering in a plant (n = 11 for ‘Paragon’ and n = 32 for ‘Spark-Rialto’).
RESULTS
The majority of flowering activity takes place in mid-morning (De Vries, 1974), all plants were assessed within a relatively short time window (2–3 h). In order to increase the number of assessed plants, it was possible to score only one side of an ear (i.e. only odd or only even spikelets). This did not affect the mean flower score of male (P = 0·335) or female flowers of ‘Paragon’ NILs (P = 0·620; data not shown).
No differences were observed in the timing of male and female peak flowering at the plant level, across all tillers in ‘Paragon’ NILs (see also Supplementary data Figs S1 and S2) and in the majority of ‘Spark-Rialto’ NILs, apart from: SR74-2 SR20 (P = 0·009) and SR75-1 SR13 (P = 0·006). Comparing ‘Paragon’ NILs with each other, there was no difference in the timing of peak flowering of male (P = 0·104) or female parts (P = 0·210). Peak flowering in ‘Spark-Rialto’ NILs, however, took place at different times both for male (P = 0·001) and female flowers (P = 0·016).
To account for observed differences between wheat lines and to allow for an analysis of flowering development in time, the activity of the stigma relative to the anthers was expressed on a relative time scale where time 1 is defined as the day when the first active flower was observed on a plant. This flowering synchrony was calculated as the difference between the day pollen dehiscence was observed and the day the stigma showed signs of pollination.
After aligning the flower synchrony data on this time-scale, the synchrony between male and female parts within individual flowers decreased with the temporal progress of flowering both in ‘Paragon’ (Fig. 1A) and in ‘Spark-Rialto’ (Fig. 1B) NILs. Negative values of synchrony indicate the pollen release took place before stigma collapse. The majority of flowers which had both active stigma and pollen-releasing anthers were concentrated at or close to the 0 line, indicating a high likelihood of same-floret self-pollination. Defining the window of opportunity for such self-pollination as synchrony between the male and female parts from –1 to 1 day (De Vries, 1971; Saini and Aspinall, 1982; Flint and Caldwell, 1984), an estimate could be made of the proportion of flowers where same-floret self-pollination took place (Fig. 2A, B). There was no difference between the ‘Paragon’ and ‘Spark-Rialto’ backgrounds in the overall level of same-floret self-pollination potential (70 % in ‘Paragon’, 68 % in ‘Spark-Rialto’; P = 0·619). Similarly, there was no difference in spatial distribution of the potential self-pollinated flowers between the two backgrounds. The highest proportion of self-pollinated florets was found in the outermost florets in the middle of the ear in both ‘Paragon’ and ‘Spark-Rialto’ NILs (see Supplementary data Fig. S2).
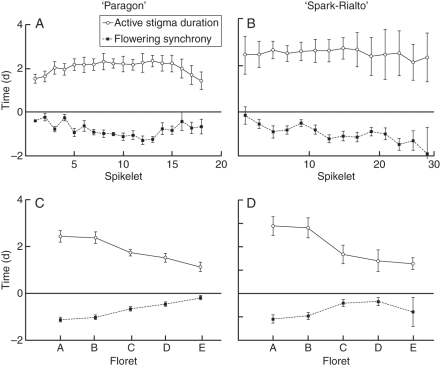
Fig. 2.
Average duration of active stigmas and mean flowering synchrony between male and female flowers of ‘Paragon’ (n = 11) and ‘Spark-Rialto’ (n = 26) NILs. Figures show duration and synchrony within spikelets (Fig. 2A, B) and florets (Fig. 2C, D; mean ± s.e.m.).
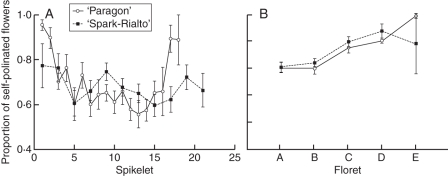
The relationship between the position of a flower and its flowering behaviour was significantly influenced by both the spikelet and floret designation. ‘Paragon’ NILs were characterized by the longest duration of active stigmas in the middle spikelets, with the average duration significantly decreasing towards the top and the base of the ear. The observed pattern of stigma longevity may be related to the fact that the most-asynchronous flowers are found in the middle of the ear (Fig. 2A). In contrast to the ‘Paragon’ NILs, ‘Spark-Rialto’ showed no significant difference in the mean length of active stigma duration but a significant trend (P = 0·0002) was identified in flowering synchrony, which decreased from the base of the ear towards the top (Fig. 2B). Floret position significantly influenced active stigma duration and flower synchrony both in ‘Paragon’ and ‘Spark-Rialto’ backgrounds. The mean length of time during which a stigma is receptive is the longest in the outermost and the shortest in the innermost florets (P < 0·001 both for ‘Paragon’ and ‘Spark-Rialto’ NILs). Similarly, flowering synchrony is lowest in floret A and highest in floret E (P < 0·001 and P = 0·001 for ‘Paragon’ and ‘Spark-Rialto’, respectively; Fig. 2C, D), which is reflected in the level of self-pollination (Fig. 3).
Fig. 3.
Ratio between the number of self-pollinated flowers and the total number of flowers where active stages of male and female flowers were observed. Self-pollinated flowers were defined as those where the stigma collapsed within ±1 d from pollen dehiscence. Mean values (± s.e.m.) for ‘Paragon’ lines (Fig. 3A, n = 11) and ‘Spark-Rialto’ lines (Fig. 3B, n = 26) are shown.
Finally, at harvest, the presence or absence of seed in each position was determined within ears which had been manually assessed at anthesis. In ‘Paragon’, repeated handling of flowers during scoring significantly reduced seed yield (P = 0·040), with an apparent affect on seed number (P = 0·104) per individual ear. In ‘Spark-Rialto’, on the other hand, there was no observed effect of scoring on yield (P = 0·402) or seed number (P = 0·612).
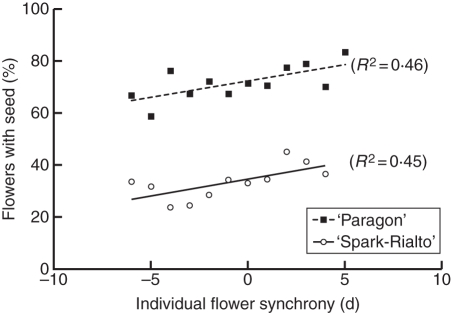
Figure 4 shows the relationship between individual flower synchrony and successful seed set. There was a significant relationship between flower synchrony and the proportion of flowers with seed (Fig. 4; P = 0·015 in ‘Paragon’ and P = 0·024 in ‘Spark-Rialto’). The probability of successful seed set decreases linearly between positive and negative values of synchrony. There was no difference between the slope of the decrease between the two wheat backgrounds (P = 0·930). The position of an individual flower within an ear also appears to influence the probability of seed set. Both in ‘Paragon’ and ‘Spark-Rialto’ NILs, the relative number of filled grain sites where active stigmas had been recorded decreased with increasing spikelet number (P < 0·0001 and P = 0·001 in ‘Paragon’ and ‘Spark-Rialto’, respectively). The flower position within a spikelet did not influence success of seed set in ‘Paragon’ (P = 0·776) or in ‘Spark-Rialto’ NILs (P = 0·697).
Fig. 4.
Individual flower synchrony affects the probability of successful seed set (P for trend equal to zero 0·015 in ‘Paragon’ and 0·024 in ‘Spark-Rialto’). Positive values of flower synchrony indicate stigma collapse before pollen dehiscence, negative after dehiscence.
The timing of peak flowering differed both within and between spikes in ‘Paragon’ and ‘Spark-Rialto’ NILs. There was a significant difference between the date of peak flowering on the spike of tiller 1 and tiller 3 (P = 0·004) and tiller 4 (P = 0·011) in ‘Paragon’, while spikes of tillers 2 and 5 flowered at the same time as tiller 1. ‘Spark-Rialto’ lines showed a significant difference between peak flowering between tillers 1, 2 and 3 (P < 0·015).
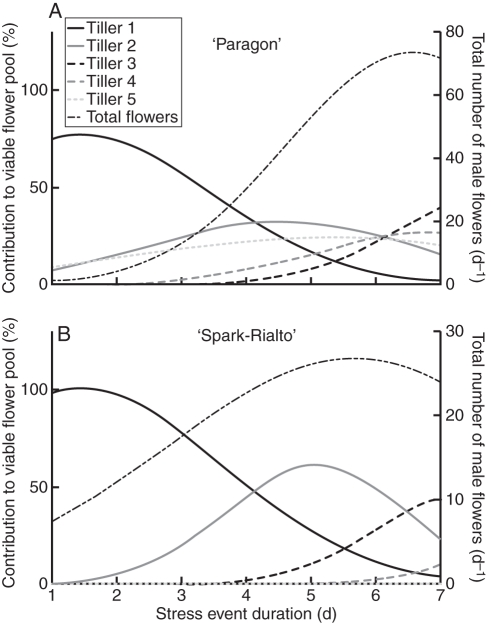
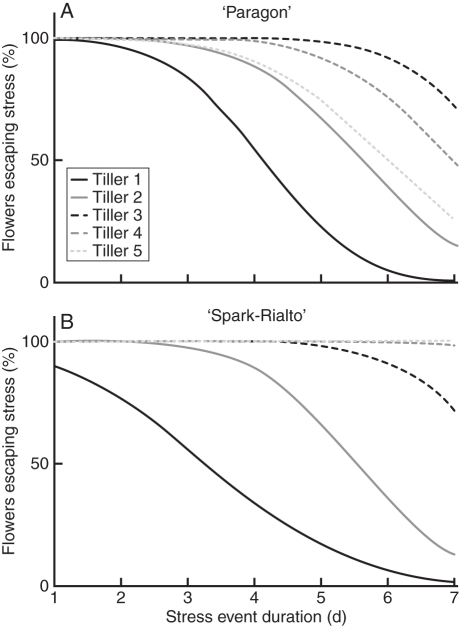
The temporal spread of anther activity between the spikes of tillers 1–5 (Fig. 5) and the interaction with a potential sequence of short-term extreme stress events lasting 1–8 d was estimated. A conceptual model of pollen loss was constructed to identify the potential number of active anthers (i.e. releasing pollen) for a given stress duration. Figure 6 shows decreasing male flower survival with increasing stress-event duration in ‘Paragon’ and ‘Spark-Rialto’ NILs. The model assumes 100 % mortality of active anthers within a floret at the time of heat stress. Overall pollen availability started to decline in ‘Paragon’ after approx. 5 d due to tiller clustering, while in ‘Spark-Rialto’ later tillers lost very few of their male flowers even after a 7-d stress event (Table 3). Maximum observed stigma duration was 7–8 d in both backgrounds (Fig. 2A, B).
Fig. 5.
Relative contribution of surviving male flowers on each tiller to the total number of surviving flowers on a plant (left axis) and the total number of active male flowers per day (right axis), while the x-axis represents the duration of a hypothetical heat-stress event. See Supplementary Data Table S2 for model description and parameter estimates.
Fig. 6.
Effect of a hypothetical heat-stress event on the number of surviving male flowers on five tillers of ‘Paragon’ (A) and ‘Spark-Rialto’ (B) lines. Active male flower numbers are determined by observed flower distribution patterns; heat-stress event starts 1 s.d. prior to mean peak flowering on tiller 1. See Supplementary data Table S1 for model description and parameter estimates.
Table 3.
Interactive effect of heat-stress duration and number of tillers on the percentage of killed pollen in ‘Paragon’ and ‘Spark-Rialto’
| Heat duration (d) | No. of tillers |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ‘Paragon’ |
‘Spark-Rialto’ |
|||||||||
| 1 | 1–2 | 1–3 | 1–4 | 1–5 | 1 | 1–2 | 1–3 | 1–4 | 1–5 | |
| 1 | 0 % | 0 % | 0 % | 0 % | 0 % | 8 % | 5 % | 3 % | 3 % | 2 % |
| 2 | 3 % | 2 % | 1 % | 1 % | 1 % | 23 % | 14 % | 10 % | 8 % | 7 % |
| 3 | 16 % | 9 % | 6 % | 4 % | 4 % | 45 % | 28 % | 21 % | 16 % | 14 % |
| 4 | 46 % | 27 % | 17 % | 14 % | 13 % | 67 % | 45 % | 33 % | 26 % | 22 % |
| 5 | 78 % | 53 % | 34 % | 29 % | 28 % | 84 % | 64 % | 47 % | 37 % | 32 % |
| 6 | 95 % | 76 % | 52 % | 46 % | 47 % | 92 % | 81 % | 62 % | 49 % | 42 % |
| 7 | 99 % | 91 % | 69 % | 65 % | 67 % | 95 % | 92 % | 75 % | 60 % | 51 % |
DISCUSSION
The first signs of anthesis in wheat appear in the middle of an ear and flowering then proceeds in both directions towards the top and bottom spikelets (Waines and Hegde, 2003; Supplementary data Fig. S1). Similarly, the outermost florets in a spikelet flower first, followed by the florets found on the inside of a spikelet (Percival, 1921). Furthermore, the spike of tiller 1 flowers before subsequent spikes (De Vries, 1973). The results presented in this paper agree with previous literature, but further insights have been achieved with regard to the relative temporal and spatial activity of the anthers and the stamens based on findings from the novel flower-scoring methodology. Wheat is generally considered to be a self-pollinating plant with fertilization likely to happen within florets, between florets at the spike and plant level, but also between plants. There are recorded instances of cross-pollination in wheat (e.g. summary in Waines and Hegde, 2003), where the highest observed levels reach 10–14 % and relate mostly to loss of pollen viability due to stress or were recorded in male-sterile plants (Khan et al., 1973; Virmani and Edwards, 1983; Demotes-Mainard et al., 1996). Previous literature may have under-estimated cross-floret pollination because the activity within a spike would remain undetected. In this research, 10 % of active stigmas in both the ‘Paragon’ and ‘Spark-Rialto’ NILs had collapsed before pollen extrusion in the same floret. Waines and Hegde (2003) speculate that the large amount of pollen shed by wheat outside the florets (in turn influenced by the relative extrusion of anthers and the angle of floret opening) is indicative of a higher amount of cross-pollination in wheat than has previously been predicted. There remains, however, a need to identify the interaction between this cross-floret self-pollination and the cross-pollination as defined by previous authors. The timing of stigma receptivity and pollen dehiscence define floret synchrony, which may have a direct bearing on the level of self-pollination. Timar et al. (1997) found developmental asynchrony of male and female gametes in wheat which increases with ploidy level, thus creating an opportunity for cross-floret pollination. The position of a floret within a spikelet and on a spike significantly affected the level of synchrony in ‘Paragon’ and ‘Spark-Rialto’. Flowers in the middle of the ear and on the outer edges of a spikelet tend to have larger asynchrony between pollen release and stigma collapse. It has also been shown that the stigmas of these flowers remain receptive for longer periods of time, which may result from a delay in the supply of same-floret pollen.
Within a spikelet, the innermost florets D and E were the least likely to open both in ‘Paragon’ and ‘Spark-Rialto’ NILs, which agrees with previous findings that the inner florets are frequently cleistogamous (closed), while the outer florets are chasmogamous (open) (Waines and Hegde, 2003). Florets D and E had the highest level of synchrony between pollen dehiscence and stigma collapse, which supports the hypothesis that closed florets are largely same-floret pollinated compared with the greater chance of cross-floret pollination in positions A, B and C. Florets with longest-lasting active stigmas in the middle of the spike, which are also the most asynchronous, would appear to have the greatest probability of being pollinated by pollen from other florets.
Flower fertility was calculated in all wheat lines used in this experiment by comparing the number of flowers where viable stigma was observed with the number of seeds. The probability of a viable flower delivering a seed decreased from the bottom of the ear towards the top, but was not affected by the position of the florets within a spikelet. Interestingly, individual flower synchrony may be one of the determinants of successful seed set. Both in ‘Paragon’ and in ‘Spark-Rialto’, the probability of seed set was strongly related to synchrony (Fig. 5). The probability of successful seed set was the highest for the most positively asynchronous florets. Since the stigma collapsed several days before same-floret pollen release, these florets had the greatest likelihood of out-pollination. However, further evidence is needed to differentiate the effect of floret asynchrony and assimilate supply (Slafer and Savin, 1994).
Wheat pollen has very short viability in open environments, thus decreasing the probability of viable pollen transfer over longer distances. As a result, flowers with high levels of asynchrony (< 1 d) or total absence of viable pollen are likely to receive pollen from elsewhere. This is most likely in florets which are active during peak flowering, when a large amount of pollen is present around the ear. Based on the findings in this paper, the earliest flowers – those in the middle of an ear and in florets A and B – tend to flower during a period of low ‘background’ pollen availability, have the greatest asynchrony and show the greatest potential for out-crossing as a result. A study of wind-pollinated hybrids of Fraxinus excelsior and F. angustifolia has confirmed that female flowers receive pollen from more distant individuals when flowering early, rather than during peak flowering (Gerard et al., 2006). Both ‘Paragon’ and ‘Spark-Rialto’ NILs exhibit similar patterns of flowering behaviour; the stigmas of early flowers are receptive for longer periods, increasing the chance of out-crossing. Due to the spatial arrangement of florets, and possibly due to their earliness, they tend to produce the largest seeds (data not shown).
Increased ambient temperature at mid-anthesis has been shown to induce sterility in barley by anther degeneration (Sakata and Higashitani, 2008) and low pollen dehiscence in rice (Matsui and Omasa, 2002). In addition, wheat pollen remains viable for an estimated 30 min (De Vries, 1971), with significant decreases in viability during heat events (Saini and Aspinall, 1982), and with high sunlight intensity (Flint and Caldwell, 1984). Even if viable pollen successfully evades unfavourable environmental conditions and reaches the stigma, there are indications that high temperature at anthesis limits pollen germination and pollen-tube elongation (Salem et al., 2007). Based on these findings, a period of 1 d either side of stigma pollination as the time window favourable to self-pollination was selected for the conceptual model. Stigma activity was not considered in the modelling exercise, since the female flower is much less sensitive to heat stress at anthesis in cereals (Cárcova and Otegui, 2001; Sakata and Higashitani, 2008). This distinction is based on the frequency of anthesis assessments in this research; a higher resolution could be achieved with multiple assessments per day with concomitant evaluation of ultra-violet levels, temperature and humidity. The model provides an indication of potential anther activity, and therefore volume of viable pollen on the assumption that anther activity is the determinant of pollen number for each wheat NIL. Joppa (1968) demonstrated that the relative volume of pollen could be estimated for different varieties by assessing the number of pollen grains per anther, and the number of extruding anthers.
The model produced in this paper was developed to predict the interaction between the number of active anthers (and therefore the volume of viable pollen) and the effect of a sudden, extreme stress event which causes a total loss of viability at the specific time of the stress event. Lalonde et al. (1997) identified sporogenesis as one of the most vulnerable stages in pollen formation; if a heat-stress event occurs at, for example, over 30 °C for 1 d, a scenario increasingly probable in the UK (UKCP09, 2009), the pollen at the most vulnerable stage of development will be killed on that day. If the heat stress occurs on three successive days, pollen viability is likely to be lost for 3 d running. Based on the flowering model, a difference in effects of heat stress on potential pollen viability was identified between the ‘Paragon’ and the ‘Spark-Rialto’ NILs for a given stress duration (Table 3). The genotypes with a wider spread of anthesis lost a smaller proportion of pollen for a given stress duration, compared with a rapid, short-duration genotype. Furthermore, the model can be used to hypothesize that crops with a higher number of tillers per plant flower longer and therefore are more resilient to a short and intensive heat-stress event. Significant variation was observed in the duration and the timing of flowering activity within ‘Paragon’ and ‘Spark-Rialto’ NILs, hinting at the existence of temporal and spatial flowering diversity in northern European wheat varieties. Further work is needed to understand the interaction between flowering phenology and predicted climate variability to understand how physiological escape from effects of abiotic stress can be exploited, and indeed be a component of increasing the relative stability of yield in UK wheat.
Conclusions
This paper demonstrates that the presented methodology is sufficiently sensitive to study the timing and the synchrony of individual flowers. The level of synchrony and consequent potential for out-crossing is determined by the position of the flower within an ear. The first florets to flower in a wheat ear have the largest negative asynchrony and their stigmas are receptive for the longest period of time. Comparing several wheat NILs originating from ‘Paragon’ and ‘Spark-Rialto’ backgrounds, significant differences were identified in the timing and the distribution of flowering even among plants with the same background. These findings indicate that there is a significant potential for out-crossing, especially in the early flowers. Climate change, and the consequent plant stress, is likely to have a major impact on flower phenology, underlying the need to create wheat varieties with diverse flowering habits.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
This work was funded by a research grant from the Biotechnology and Biological Sciences Research Council (BBSRC) as part of project BB/H012176/1. We would like to thank Caroline Hadley and Laurence Hansen for their excellent technical support, and Frederick Steinmeyer and Rajneet Uppal for their help in data collection and the scoring of flowering. We thank Luzie U. Wingen for commenting on the manuscript.
LITERATURE CITED
- Bentley AR, Turner AS, Gosman N, et al. Frequency of photoperiod-insensitive Ppd-A1a alleles in tetraploid, hexaploid and synthetic hexaploid wheat germplasm. Plant Breeding. 2011;130:10–15. [Google Scholar]
- Bidinger FR, Mahalakshmi V, Rao GDP. Assessment of drought resistance in pearl millet [Pennisetum americanum (L.) Leeke]. I. Factors affecting yields under stress. Australian Journal of Agricultural Research. 1987;38:37–48. [Google Scholar]
- Cárcova J, Otegui ME. Pollination asynchrony and kernel abortion in maize. Crop Science. 2001;41:1809–1815. [Google Scholar]
- Craufurd PQ, Peacock JM. Effect of heat and drought stress on sorgum. Experimental Agriculture. 1993;29:77–86. [Google Scholar]
- Demotes-Mainard S, Doussinault G, Meynard JM. Abnormalities in the male developmental programme of winter wheat induced by climatic stress at meiosis. Agronomie. 1996;16:505–515. [Google Scholar]
- De Vries AP. Flowering biology of wheat—particularly in view of hybrid seed production: a review. Euphytica. 1971;20:152–170. [Google Scholar]
- De Vries AP. Some aspects of cross pollination in wheat (Triticum aestivum L.). 2 Anther extrusion and ear and plant flowering pattern and duration. Euphytica. 1973;22:445–456. [Google Scholar]
- De Vries AP. Some aspects of cross-pollination in wheat (Triticum aestivum L.). 3 Anther length and number of pollen grains per anther. Euphytica. 1974;23:11–19. [Google Scholar]
- Ferris R, Wheeler TR, Hadley P, Ellis RH. Recovery of photosynthesis after environmental stress in soybean grown under elevated CO2. Crop Science. 1998;38:948–955. [Google Scholar]
- Flint SD, Caldwell MM. Partial inhibition of in vitro pollen germination by simulated solar ultraviolet-B radiation. Ecology. 1984;65:792–795. [Google Scholar]
- Gerard PR, Klein EK, Austerlitz F, Fernandez-Manjarres JF, Frascaria-Lacoste N. Assortative mating and differential male mating success in an ash hybrid zone population. BMC Evolutionary Biology. 2006;6:96. doi: 10.1186/1471-2148-6-96. http://dx.doi.org/10.1186/1471-2148-6-96 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths S, Simmonds J, Leverington M, et al. Meta-QTL analysis of the genetic control of ear emergence in elite European winter wheat germplasm. Theoretical and Applied Genetics. 2009;119:383–395. doi: 10.1007/s00122-009-1046-x. [DOI] [PubMed] [Google Scholar]
- Hucl P. Out-crossing rates for 10 Canadian spring wheat cultivars. Canadian Journal of Plant Science. 1996;76:423–427. [Google Scholar]
- Joppa LR. Pollen production and pollen shedding of hard red spring (Triticum aestivum L. em. Thell.) and durum (T. durum Desf.) wheats. Crop Science. 1968;8:487–490. [Google Scholar]
- Khan MN, Heyne EG, Arp AL. Pollen distribution and seedset on Triticum aestivum L. Euphytica. 1973;13:223–226. [Google Scholar]
- Kirby EJM, Appleyard M. Cereal development guide. Stoneleigh, UK: Arable Unit, National Agricultural Centre; 1984. [Google Scholar]
- Lalonde S, Beebe DU, Saini HS. Early signs of disruption of wheat anther development associated with the induction of male sterility by meiotic-stage water deficit. Sexual Plant Reproduction. 1997;10:40–48. [Google Scholar]
- Marcello H, Single WV. Quantitative responses of wheat to photoperiod and temperature in the field. Australian Journal of Agricultural Research. 1971;22:343–351. [Google Scholar]
- Matsui T, Omasa K. Rice (Oryza sativa L.) cultivars tolerant to high temperature at flowering: anther characteristics. Annals of Botany. 2002;89:683–687. doi: 10.1093/aob/mcf112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percival J. The wheat plant. London: Duckworth; 1921. [Google Scholar]
- Pickett AA. Hybrid wheat: results and problems. Berlin: Paul Parey Scientific Publishers; 1993. [Google Scholar]
- Saini HS, Aspinall D. Abnormal sporogenesis in wheat (Triticum aestivum L.) induced by short periods of high-temperature. Annals of Botany. 1982;49:835–846. [Google Scholar]
- Sakata T, Higashitani A. Male sterility accompanied with abnormal anther development in plants – genes and environmental stresses with special reference to high temperature injury. International Journal of Plant Developmental Biology. 2008;2:42–51. [Google Scholar]
- Salem MA, Kakani VG, Koti S, Reddy KR. Pollen-based screening of soybean germplasm for high temperatures. Crop Science. 2007;47:219–231. [Google Scholar]
- Slafer GA, Savin R. Sink–source relationships and grain mass at different positions within the spike of wheat. Field Crops Research. 1994;37:39–49. [Google Scholar]
- Tashiro T, Wardlaw IF. The effect of high temperature at different stages of ripening on grain set, grain weight, grain dimensions in the semi-dwarf wheat ‘Banks’. Annals of Botany. 1990;65:51–61. [Google Scholar]
- Timar I, Kristof Z, Barnabas B. Comparative studies on the male and female gametophyte development in three different Triticum species. Plant Science. 1997;126:97–104. [Google Scholar]
- UKCP09. UK climate predictions. London: Department for Environment, Food and Rural Affairs; 2009. [Google Scholar]
- Virmani SS, Edwards IB. Current status and future-prospects for breeding hybrid rice and wheat. Advances in Agronomy. 1983;36:145–214. [Google Scholar]
- Waddington SR, Cartwright PM, Wall PC. A quantitative scale of spike initial and pistil development in barley and wheat. Annals of Botany. 1983;51:119–130. [Google Scholar]
- Waines JG, Hegde SG. Intraspecific gene flow in bread wheat as affected by reproductive biology and pollination ecology of wheat flowers. Crop Science. 2003;43:451–463. [Google Scholar]
- Willenborg CJ, Luschei EC, Brule-Babel AL, Van Acker RC. Crop genotype and plant population density impact flowering phenology and synchrony between cropped and volunteer spring wheat. Agronomy Journal. 2009;101:1311–1321. [Google Scholar]
- Zadoks JC, Chang TT, Konzak CF. Decimal code for growth stages of cereals. Weed Research. 1974;14:415–421. [Google Scholar]
- Zhao L, Kobayasi K, Hasegawa T, et al. Traits responsible for variation in pollination and seed set among six rice cultivars grown in a miniature paddy field with free air at a hot, humid spot in China. Agriculture, Ecosystems & Environment. 2010;139:110–115. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.