Abstract
Background and Aims
Fine-scale, spatial heterogeneity in soil nutrient availability can increase the growth of individual plants, the productivity of plant communities and interspecific competition. If this is due to the ability of plants to concentrate their roots where nutrient levels are high, then nutrient heterogeneity should have little effect on intraspecific competition, especially when there are no genotypic differences between individuals in root plasticity. We tested this hypothesis in a widespread, clonal species in which individual plants are known to respond to nutrient heterogeneity.
Methods
Plants derived from a single clone of Alternanthera philoxeroides were grown in the greenhouse at low or high density (four or 16 plants per 27·5 × 27·5-cm container) with homogeneous or heterogeneous availability of soil nutrients, keeping total nutrient availability per container constant. After 9 weeks, measurements of size, dry mass and morphology were taken.
Key Results
Plants grew more in the heterogeneous than in the homogeneous treatment, showing that heterogeneity promoted performance; they grew less in the high- than in the low-density treatment, showing that plants competed. There was no interactive effect of nutrient heterogeneity and plant density, supporting the hypothesis that heterogeneity does not affect intraspecific competition in the absence of genotypic differences in plasticity. Treatments did not affect morphological characteristics such as specific leaf area or root/shoot ratio.
Conclusions
Results indicate that fine-scale, spatial heterogeneity in the availability of soil nutrients does not increase competition when plants are genetically identical, consistent with the suggestion that effects of heterogeneity on competition depend upon differences in plasticity between individuals. Heterogeneity is only likely to increase the spread of monoclonal, invasive populations such as that of A. philoxeroides in China.
Keywords: Alligator weed, Alternanthera philoxeroides, clonal plant, competition, density effect, intraspecific interaction, log response ratio, resource heterogeneity, spatial heterogeneity
INTRODUCTION
Plants in natural habitats often experience spatial heterogeneity in soil nutrients (Gross et al., 1995; Farley and Fitter, 1999; Hutchings et al., 2000; James et al., 2003). Different roots of individual, non-clonal plants frequently grow into patches of soil that differ in nutrient availability, and connected ramets of clonal plants frequently root in microsites with contrasting levels of nutrients. Many species respond by concentrating their roots where nutrient levels are relatively high (Alpert and Stuefer, 1997; Wijesinghe et al., 2001; Hodge, 2004; de Kroon, 2007; de Kroon et al., 2009), and one common effect of nutrient heterogeneity is to increase plant performance as measured by accumulation of dry mass. This has been observed at the levels of individual ramets, groups of connected ramets and whole communities (Huber-Sannwald and Jackson, 2001; Hutchings and Wijesinghe, 2008; Garcia-Palacios et al., 2011), and may be due at least partly to greater efficiency of uptake at higher concentrations of nutrients.
Heterogeneity of soil nutrients can also influence the relationships between plant species (Fransen et al., 2001; Wardle and Peltzer, 2003; Mommer et al., 2011; van der Waal et al., 2011). For example, changing the degree of nutrient heterogeneity can change the relative abundances of species grown in mixtures (Wijesinghe et al., 2005; Maestre and Reynolds, 2007; van der Waal et al., 2011). One might expect that the ability of plants to concentrate their roots where nutrients are high would cause heterogeneity to increase competition between plants, as the roots of neighbouring plants would be concentrated in a smaller area of the soil. Soil nutrient heterogeneity can increase interspecific competition (Fransen et al., 2001; Day et al., 2003), but does not always do so (Rajaniemi, 2007). One suggestion is that heterogeneity increases the relative competitive ability of species that are more able to place their roots where nutrient levels are high (Bliss et al., 2002), and that heterogeneity affects competition only when species differ in this ability.
If so, then soil nutrient heterogeneity should have relatively little effect on intraspecific competition. This has been little studied, but Day et al. (2003) found that heterogeneity did increase intraspecific competition in one of two species. In a study of a different type of resource heterogeneity, Hagiwara et al. (2010) found evidence that temporal variation in water availability increased intraspecific competition in another species. Effect of heterogeneity on intraspecific competition might still be explained by differences between genotypes within a species in ability to concentrate roots where nutrients are high. If this is the case, then heterogeneity should not increase competition between plants of the same genotype propagated in the same environment.
To test the hypothesis that greater heterogeneity of soil nutrients does not affect intraspecific competition in the limiting case when plants are genetically identical, even in clonal species in which heterogeneity strongly increases growth, we conducted a greenhouse experiment on a widespread, well-studied clonal species. We predicted: (1) that growth of plants as measured by total final dry mass, masses of plant parts, leaf area, number of new ramets, and number and length of new stolons would be greater when they were grown in a soil with fine-scale patches of low and high nutrients than when they were in a uniform soil containing the same total amount of soil nutrients (i.e. that heterogeneity would promote plant growth); (2) that growth of separate plants of the same clone would be less at higher plant density (i.e. that intraspecific competition would occur); but (3) that the negative effect of high density on growth would not depend on the heterogeneity of soil nutrients (i.e. that heterogeneity would not affect intraspecific competition). We also measured a number of morphological characteristics (internode length, specific internode length, specific leaf area and ratio of root to shoot mass) to test whether competition or heterogeneity induced plastic responses that might underlie their effects on growth. Because inequality in size often increases with increasing plant density due to asymmetric competition for light (Weiner and Solbrig, 1984; Weiner, 1990), we compared size inequality as measured by coefficients of variation in the growth of plants to see whether greater competition or heterogeneity produced greater differences in size between plants grown together.
MATERIALS AND METHODS
Species and propagation
Alternanthera philoxeroides (Mart.) Griseb., or alligator weed, is a perennial, stoloniferous, amphibious, herbaceous plant in the Amaranthaceae native to South America (Holm et al., 1997; Xu et al., 2010). The species has been introduced to the United States, Australia, New Zealand, India, China and other countries, where it is widely regarded as highly invasive (Julien et al., 1995; Holm et al., 1997; Geng et al., 2007). The species reproduces both by seed and by clonal growth via stolons; along the stolons, even single stem nodes, which function as ramets, can establish and grow (Dong et al., 2010a, b). Physiological integration between connected ramets can modify their individual and combined growth and spread (Wang et al., 2009; Yu et al., 2009; Xu et al., 2010). A. philoxeroides in China has extremely low genetic diversity and may be derived from a single clone (Ye et al., 2003; Wang et al., 2005; Pan et al., 2007).
The experiment was conducted in a greenhouse under natural sunlight at Forest Science Company of Beijing Forestry University in Beijing, China. Plants were propagated vegetatively from a clone collected in Jiangxi Province, China, and maintained at the Institute of Botany of the Chinese Academy of Science in Beijing. Single stem nodes cut from stock plants were planted in plastic boxes filled with a 1 : 1 (v/v) mixture of peat to increase water-holding capacity and a locally collected, riparian, sandy soil similar to soils in which A. philoxeroides often grows so that the nodes rooted and produced an axillary stolon. On 9 August 2010, 320 nodes with stolons that were about 10 cm long and bore four to five nodes, or ramets, were selected for use in the experiment. For simplicity, these clonal fragments (i.e. groups of connected ramets) are referred to hereafter as ‘plants’.
Experimental design
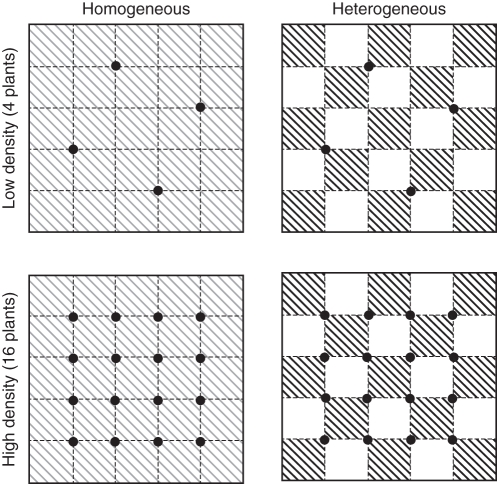
Plants were subjected to two density treatments (four or 16 plants per container) crossed with two soil treatments (homogeneous or heterogeneous; Fig. 1). Each plastic container was 27·5 × 27·5 × 14·2 cm deep. For the heterogeneous soil treatment, alternate 5·5 × 5·5-cm patches in a container were filled with the sand used for propagation, which was low in total N, P and organic matter [mean (s.e.): 0·065 (0·003) mg N g−1 soil, 0·64 (0·02) mg P g−1, 1·40 (0·07) mg organic matter g−1], or with a high-nutrient soil [7·16 (0·28) mg N g−1 soil, 3·13 (0·09) mg P g−1, 116·3 (11·5) mg organic matter g−1] prepared by mixing one part of the sand with two parts of a commercial potting soil (Organic Cultivation Soil, Changchun Flower Soil Company, Changchun, China). For the homogeneous soil treatment, each container was filled in the same way and the soil was then thoroughly mixed. Total amounts of soil nutrients were thus the same in both treatments.
Fig. 1.
Experimental design. Darkly shaded and unshaded squares represent high and low soil nutrient patches, respectively; lightly shaded squares received the mean of the high and low levels. Black circles mark positions where plants of Alternanthera philoxeroides were planted.
To assign plants to containers, plants were first divided into eight groups on the basis of size, first by number of nodes (four or five), then by stolon length and last by stolon thickness. The plants in a group were then randomly assigned to one replicate of each of the four combinations of density and soil heterogeneity treatments to generate an experimental block, giving eight replicate blocks in total. In the heterogeneous treatment, plants were positioned vertically at the corners of nutrient patches to ensure equal access to high- and low-nutrient patches, with the parental node of the plant buried 2 cm deep; in the homogeneous treatment, plants were positioned in similar relative positions (Fig. 1). Containers were arranged by blocks and watered every 2–3 d to minimize limitation of growth by water availability. The mean temperature and relative humidity in the greenhouse during the experiment were 22·8 °C and 76 %.
After 9 weeks, on 10 October 2010, the total numbers of ramets, new stolons and leaves on each plant were recorded, and the total stolon length and leaf area (WinFOLIA Pro 2004a, Regent Instruments, Québec, Canada) were measured. Each plant was then divided into roots, leaves and stolons, dried at 70 °C for 48 h and weighed.
Statistical analysis
We used two-way ANOVA to test the effects of soil nutrient heterogeneity (heterogeneous or homogeneous) and plant density (four or 16 plants per container) on total dry mass, stolon mass, leaf mass, root mass, leaf area, total stolon length, number of new ramets, number of new stolons, internode length, specific internode length (cm g−1), specific leaf area (cm2 g−1), ratio of root to shoot mass, or coefficient of variation (s.d./mean) of total, root or above-ground (leaf plus stolon) mass. In the ANOVA models, soil nutrient heterogeneity and plant density were treated as fixed effects. Mean values for the plants in a container were used in these analyses except for those of coefficient of variation. Data for leaf area and number of new stolons were transformed to the natural log before analysis; other data required no transformation to meet requirements for homoscedasticity and normality. Effect of heterogeneity on competition was also directly tested by comparing the log response ratios of total mass per plant to density in the heterogeneous and homogeneous treatments, using pairs of treatments within blocks as replicates. All analyses were conducted using SPSS 17·0 (SPSS, Chicago, IL, USA). Effects were considered significant at P < 0·05.
RESULTS
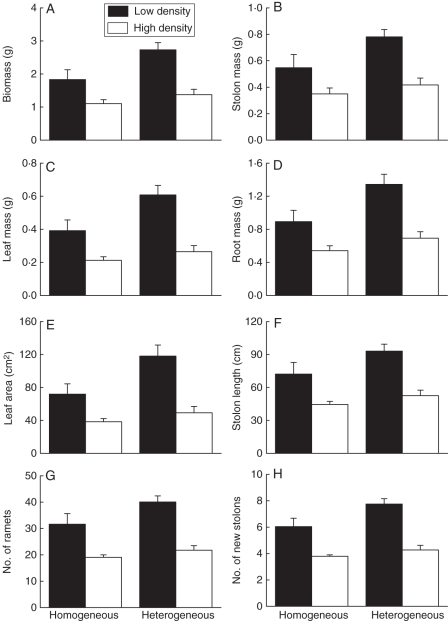
As predicted, plants grew more in the low- than in the high-density treatment, and more in the heterogeneous than in the homogeneous soil treatment (Fig. 2, Table 1). This was true for final total dry mass and for all other measures of growth. Across the density treatments, plants accumulated about 40 % more mass and produced about 22 % more new stolons and ramets in the heterogeneous than in the homogenous treatment.
Fig. 2.
Effects of heterogeneity of soil nutrients and density of plants on (A) total dry biomass, (B) mass of stolons, (C) mass of leaves, (D) mass of roots, (E) total leaf area, (F) total stolon length, (G) number of new ramets and (H) number of new stolons. See Table 1 for ANOVA results.
Table 1.
ANOVA results for effects of heterogeneity of soil nutrients and density of plants on measures of growth and morphology of Alternanthera philoxeroides
| Heterogeneity (H) |
Density (D) |
H × D |
||||
|---|---|---|---|---|---|---|
| F1,28 | P | F1,28 | P | F1,28 | P | |
| Total mass | 7·8 | 0·009 | 24·6 | < 0·001 | 2·2 | 0·14 |
| Root mass | 8·4 | 0·007 | 23·3 | < 0·001 | 2·1 | 0·16 |
| Stolon mass | 5·1 | 0·03 | 17·6 | < 0·001 | 1·5 | 0·2 |
| Leaf mass | 7·7 | 0·01 | 29·2 | < 0·001 | 2·9 | 0·10 |
| Leaf area | 4·6 | 0·04 | 23·4 | < 0·001 | 1·5 | 0·2 |
| Number of ramets | 4·9 | 0·04 | 37·3 | < 0·001 | 1·3 | 0·3 |
| Stolon length | 4·5 | 0·04 | 25·2 | < 0·001 | 0·9 | 0·4 |
| Number of new stolons | 6·4 | 0·02 | 48·5 | < 0·001 | 1·5 | 0·3 |
| Internode length | 0·7 | 0·4 | 0·6 | 0·4 | 0·009 | 0·9 |
| Specific internode length | 3·5 | 0·07 | 0·3 | 0·6 | 1·1 | 0·3 |
| Specific leaf area | 0·9 | 0·4 | 1·1 | 0·3 | 0·9 | 0·3 |
| Root/shoot ratio | 0·2 | 0·6 | 0·3 | 0·6 | 0·3 | 0·6 |
Also as predicted, the negative effect of high density on plant growth did not differ between the heterogeneous and the homogeneous treatments (Fig. 2, Table 1: each P > 0·05 for the interactive effect of density and heterogeneity). For example, both total mass per plant and number of ramets per plant were about 40 % less in the high-density than in the low-density treatment when soils were homogeneous, and about 50 % less in the high- than in the low-density treatment when soils were heterogeneous. Likewise, the log response ratio of total mass per plant at high and low density did not differ (t-test: t14 = 1·56, P = 0·14) between homogeneous [mean (s.e.): –0·21 (0·05)] and heterogeneous treatments [–0·32 (0·05)].
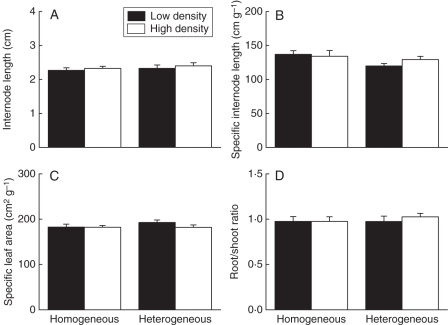
Neither plant density nor soil heterogeneity significantly affected plant morphology (Fig. 3, Table 1: each P > 0·05). Mean internode length, specific internode length, specific leaf area and root/shoot ratio were each very similar in the different treatments. Density and heterogeneity treatments did not significantly affect the difference in performance between the plants within a container, as measured by the coefficients of variance of root, above-ground (leaf plus stolon) or total mass (Table 2).
Fig. 3.
Effects of heterogeneity of soil nutrients and density of plants on (A) internode length, (B) specific internode length, (C) specific leaf area and (D) root to shoot ratio of plants of Alternanthera philoxeroides. Values are means + s.e. See Table 1 for ANOVA results.
Table 2.
Effects of soil nutrient heterogeneity (homogeneous or heterogeneous) and plant density (low or high) on the coefficients of variance in final total, root and above-ground (leaf plus stolon) mass of plants of Alternanthera philoxeroides
| Homogeneous |
Heterogeneous |
Heterogeneity (H) |
Density (D) |
H × D |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Low | High | Low | High | F1,28 | P | F1,28 | P | F1,28 | P | |
| Root | 0·39 (0·06) | 0·40 (0·03) | 0·41 (0·06) | 0·44 (0·04) | 0·35 | 0·6 | 0·11 | 0·7 | 0·09 | 0·8 |
| Above-ground | 0·39 (0·07) | 0·46 (0·02) | 0·41 (0·05) | 0·45 (0·03) | 0·045 | 0·8 | 1·28 | 0·3 | 0·10 | 0·8 |
| Total | 0·35 (0·06) | 0·38 (0·03) | 0·39 (0·05) | 0·41 (0·03) | 0·77 | 0·4 | 0·21 | 0·6 | 0·004 | > 0·9 |
Values are mean coefficients with s.e. in parentheses, with results of ANOVAs.
DISCUSSION
Plants of Alternanthera philoxeroides grew more when soil nutrient availability was spatially heterogeneous than when it was homogeneous. Studies on clonal plants generally show positive effects of heterogeneity on overall growth, although this can depend upon patch size (Hutchings and Wijesinghe, 2008), whereas studies on non-clonal plants show different effects in different species (Bliss et al., 2002; Day et al., 2003). For example, Pinus taeda grew more in conditions with heterogeneous than with homogeneous nutrient supply, whereas Chamaecrista nictitans, Erechtites hieracifolia and Liquidambar styraciflua grew the same in both conditions and Hypericum gentianoides tended to grow less in heterogeneous treatments (Bliss et al., 2002). This could imply that nutrient heterogeneity will tend to favour clonal over non-clonal plants, probably because connected ramets of clonal plants can share resources and thus may make better use of heterogeneously distributed nutrients in the soil (Birch and Hutchings, 1994; Hutchings and Wijesinghe, 2008).
Competition between plants of A. philoxeroides was not greater when soil nutrients were heterogeneous than when they were homogeneous. This was not due to an absence of competition, as plants grew less at high than at low density both when nutrients were homogeneous and when they were heterogeneous. Neither was it due to an absence of response to heterogeneity, as described above. Instead, it supports the hypothesis that heterogeneity does not increase intraspecific competition when individuals are genetically identical. One possible mechanism for this could be self-recognition between ramets of the same clone (e.g. Holzapfel and Alpert, 2003), although this remains controversial (e.g. Semchenko et al., 2007) and might be overcome by somatic mutation. It could be interesting to test whether heterogeneity can increase competition between ramets of A. philoxeroides from different populations in China despite the likely presence of only one clone.
Together with previous reports that resource heterogeneity can increase intraspecific competition when individuals are not genetically identical (Day et al., 2003; Hagiwara et al., 2010), this suggests that promotion of intraspecific competition by resource heterogeneity depends upon differences between individuals. The critical differences seem likely to be those in ability to place roots where nutrient levels are high, as plants can differ greatly in this ability (Wijesinghe et al., 2001; Hodge, 2004), and because effects of nutrient heterogeneity on interspecific competition appear to be linked to interspecific differences in precision of root placement (Bliss et al., 2002; Rajaniemi, 2011). More generally, this work agrees with the intuitive idea that heterogeneity will benefit plastic species more, although plasticity can also lead to avoidance of competition (Schiffers et al., 2011) and increased nutrient availability may decrease intensity of root competition (Schenk, 2006).
In the case of A. philoxeroides, the positive effects of nutrient heterogeneity on the growth of individual clonal fragments do not seem to be counterbalanced by any negative effects due to increased competition between separate fragments of the same clone. As the species may be represented by only one clone in China (Ye et al., 2003; Pan et al., 2007), increased fine-scale heterogeneity in soil nutrients is not likely to decrease the spread of A. philoxeroides due to intraspecific competition but only to increase it due to increased growth of individual fragments. A number of highly invasive, clonal species are represented over wide areas by one or mainly one clone (e.g. Ahmad et al., 2008; Zhang et al., 2010). If a strong, positive reaction to fine-scale nutrient heterogeneity is a general feature of these clones, then factors that increase such heterogeneity could be an important promoter of invasions by clonal plants.
ACKNOWLEDGEMENTS
We thank Yi-Ke Peng, Rui Zhu, Zong-Ji Fan, Bo Guan, Kun Ma and Qian Zhang for assistance with research, and two anonymous reviewers for their comments. This work was supported by the Forestry Commonwealth Project (Grant 201004078), the Fundamental Research Funds for the Central Universities (Grant JC2011-4), the External Cooperation Program of the Chinese Academy of Sciences (Grant GJHZ0904) and the National Science Foundation of China (Grant 31070371). This material was based in part on work supported by the US National Science Foundation, while working at the Foundation, but does not necessarily reflect the views of the Foundation.
LITERATURE CITED
- Ahmad R, Liow P-S, Spencer DF, Jasieniuk M. Molecular evidence for a single genetic clone of invasive Arundo donax in the United States. Aquatic Botany. 2008;88:113–120. [Google Scholar]
- Alpert P, Stuefer JF. Division of labour in clonal plants. In: de Kroon H, van Groenendael J, editors. The ecology and physiology of clonal plants. Leiden: Backhuys Publishers; 1997. pp. 137–154. [Google Scholar]
- Birch CPD, Hutchings MJ. Exploitation of patchily distributed soil resources by the clonal herb Glechoma hederacea. Journal of Ecology. 1994;82:653–664. [Google Scholar]
- Bliss KM, Jones RH, Mitchell RJ, Mou PP. Are competitive interactions influenced by spatial nutrient heterogeneity and root foraging behavior? New Phytologist. 2002;154:409–417. doi: 10.1046/j.1469-8137.2002.00389.x. [DOI] [PubMed] [Google Scholar]
- Day KJ, John EA, Hutchings MJ. The effects of spatially heterogeneous nutrient supply on yield, intensity of competition and root placement patterns in Briza media and Festuca ovina. Functional Ecology. 2003;17:454–463. [Google Scholar]
- Dong B-C, Yu G-L, Guo W, Zhang M-X, Dong M, Yu F-H. How internode length, position and presence of leaves affect survival and growth of Alternanthera philoxeroides after fragmentation? Evolutionary Ecology. 2010a;24:1447–1461. [Google Scholar]
- Dong B-C, Zhang M-X, Alpert P, Lei G-C, Yu F-H. Effects of orientation on survival and growth of small fragments of the invasive, clonal plant Alternanthera philoxeroides. PLoS ONE. 2010b;5 doi: 10.1371/journal.pone.0013631. e13631. http://dx.doi.org/10.1371/journal.pone.0013631 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farley RA, Fitter AH. Temporal and spatial variation in soil resources in a deciduous woodland. Journal of Ecology. 1999;87:688–696. [Google Scholar]
- Fransen B, de Kroon H, Berendse F. Soil nutrient heterogeneity alters competition between two perennial grass species. Ecology. 2001;82:2534–2546. [Google Scholar]
- Garcia-Palacios P, Maestre FT, Gallardo A. Soil nutrient heterogeneity modulates ecosystem responses to changes in the identity and richness of plant functional groups. Journal of Ecology. 2011;99:551–562. doi: 10.1111/j.1365-2745.2010.01765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y-P, Pan X-Y, Xu C-Y, et al. Phenotypic plasticity rather than locally adapted ecotypes allows the invasive alligator weed to colonize a wide range of habitats. Biological Invasions. 2007;9:245–256. [Google Scholar]
- Gross KL, Pregitzer KS, Burton AJ. Spatial variation in nitrogen availability in three successional plant communities. Journal of Ecology. 1995;83:357–368. [Google Scholar]
- Hagiwara Y, Kachi N, Suzuki J-I. Effects of temporal heterogeneity of water supply on the growth of Perilla frutescens depend on plant density. Annals of Botany. 2010;106:173–181. doi: 10.1093/aob/mcq096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge A. The plastic plant: root responses to heterogeneous supplies of nutrients. New Phytologist. 2004;162:9–24. [Google Scholar]
- Holm LG, Doll J, Holm E, Pancho J, Herberger J. World weeds: natural histories and distribution. New York: John Wiley & Sons; 1997. [Google Scholar]
- Holzapfel C, Alpert P. Root cooperation in a clonal plant: connected strawberries segregate roots. Oecologia. 2003;134:72–77. doi: 10.1007/s00442-002-1062-x. [DOI] [PubMed] [Google Scholar]
- Huber-Sannwald E, Jackson RB. Heterogeneous soil-resource distribution and plant responses – from individual-plant growth to ecosystem functioning. Progress in Botany. 2001;62:451–476. [Google Scholar]
- Hutchings MJ, Wijesinghe DK. Performance of a clonal species in patchy environments: effects of environmental context on yield at local and whole-plant scales. Evolutionary Ecology. 2008;22:313–324. [Google Scholar]
- Hutchings MJ, John EA, Stewart AJA, editors. The ecological consequences of environmental heterogeneity. Oxford: Blackwell Science Ltd; 2000. [Google Scholar]
- James SE, Pärtel M, Wilson SD, Peltzer DA. Temporal heterogeneity of soil moisture in grassland and forest. Journal of Ecology. 2003;91:234–239. [Google Scholar]
- Julien M, Skarratt B, Maywald GF. Potential geographical distribution of alligator weed and its biological control by Agasicles hygrophila. Journal of Aquatic Plant Management. 1995;33:55–60. [Google Scholar]
- de Kroon H. How do roots interact? Science. 2007;318:1562–1563. doi: 10.1126/science.1150726. [DOI] [PubMed] [Google Scholar]
- de Kroon H, Visser EJW, Huber H, Mommer L, Hutchings MJ. A modular concept of plant foraging behaviour: the interplay between local responses and systemic control. Plant, Cell and Environment. 2009;32:704–712. doi: 10.1111/j.1365-3040.2009.01936.x. [DOI] [PubMed] [Google Scholar]
- Maestre FT, Reynolds JF. Amount or pattern? Grassland responses to the heterogeneity and availability of two key resources. Ecology. 2007;88:501–511. doi: 10.1890/06-0421. [DOI] [PubMed] [Google Scholar]
- Mommer L, Visser EJW, van Ruijven J, de Caluwe H, Pierik R, de Kroon H. Contrasting root behaviour in two grass species: a test of functionality in dynamic heterogeneous conditions. Plant and Soil. 2011;344:347–360. [Google Scholar]
- Pan X-Y, Geng Y-P, Sosa A-J, Zhang W-J, Li B, Cheng J-K. Invasive Alternanthera philoxeroides: biology, ecology and management. Acta Phytotaxonomica Sinica. 2007;45:884–900. [Google Scholar]
- Rajaniemi TK. Root foraging traits and competitive ability in heterogeneous soils. Oecologia. 2007;153:145–152. doi: 10.1007/s00442-007-0706-2. [DOI] [PubMed] [Google Scholar]
- Rajaniemi TK. Competition for patchy soil resources reduces community evenness. Oecologia. 2011;165:169–174. doi: 10.1007/s00442-010-1710-5. [DOI] [PubMed] [Google Scholar]
- Schenk HJ. Root competition: beyond resource depletion. Journal of Ecology. 2006;94:725–739. [Google Scholar]
- Schiffers K, Tielborger K, Tietjen B, Jeltsch F. Root plasticity buffers competition among plants: theory meets experimental data. Ecology. 2011;92:610–620. doi: 10.1890/10-1086.1. [DOI] [PubMed] [Google Scholar]
- Semchenko M, John EA, Hutchings MJ. Effects of physical connection and genetic identity of neighbouring ramets on root-placement patterns in two clonal species. New Phytologist. 2007;176:644–654. doi: 10.1111/j.1469-8137.2007.02211.x. [DOI] [PubMed] [Google Scholar]
- van der Waal C, de Kroon H, Heitkönig IMA, et al. Scale of nutrient patchiness mediates resource partitioning between trees and grasses in a semi-arid savanna. Journal of Ecology. 2011;99:1124–1133. [Google Scholar]
- Wang B, Li W, Wang J. Genetic diversity of Alternanthera philoxeroides in China. Aquatic Botany. 2005;81:277–283. [Google Scholar]
- Wang N, Yu F-H, Li P-X, et al. Clonal integration supports the expansion from terrestrial to aquatic environments of the amphibious stoloniferous herb Alternanthera philoxeroides. Plant Biology. 2009;11:483–489. doi: 10.1111/j.1438-8677.2008.00133.x. [DOI] [PubMed] [Google Scholar]
- Wardle DA, Peltzer DA. Interspecific interactions and biomass allocation among grassland plant species. Oikos. 2003;100:497–506. [Google Scholar]
- Weiner J. Asymmetric competition in plant populations. Trends in Ecology & Evolution. 1990;5:360–364. doi: 10.1016/0169-5347(90)90095-U. [DOI] [PubMed] [Google Scholar]
- Weiner J, Solbrig OT. The meaning and measurement of size hierarchies in plant populations. Oecologia. 1984;61:334–336. doi: 10.1007/BF00379630. [DOI] [PubMed] [Google Scholar]
- Wijesinghe DK, John EA, Beruskens S, Hutchings MJ. Root system size and precision in nutrient foraging: responses to spatial pattern of nutrient supply in six herbaceous species. Journal of Ecology. 2001;89:972–983. [Google Scholar]
- Wijesinghe DK, John EA, Hutchings MJ. Does pattern of soil resource heterogeneity determine plant community structure? An experimental investigation. Journal of Ecology. 2005;93:99–112. [Google Scholar]
- Xu C-Y, Schooler S-S, van Klinken R-D. Effects of clonal integration and light availability on the growth and physiology of two invasive herbs. Journal of Ecology. 2010;98:833–844. [Google Scholar]
- Ye W-H, Li J, Cao H-L, Ge X-J. Genetic uniformity of Alternanthera philoxeroides in South China. Weed Research. 2003;43:297–302. [Google Scholar]
- Yu F-H, Wang N, Alpert P, He W-M, Dong M. Physiological integration in an introduced, invasive plant increases its spread into experimental communities and modifies their structure. American Journal of Botany. 2009;96:1983–1989. doi: 10.3732/ajb.0800426. [DOI] [PubMed] [Google Scholar]
- Zhang Y-Y, Zhang D-Y, Barrett SCH. Genetic uniformity characterizes the invasive spread of water hyacinth (Eichhornia crassipes), a clonal aquatic plant. Molecular Ecology. 2010;19:1774–1786. doi: 10.1111/j.1365-294X.2010.04609.x. [DOI] [PubMed] [Google Scholar]