Abstract
Background and Aims
Seeds can accumulate in the soil or elsewhere, such as on the stems of palms when these are covered by persistent sheaths. These sheaths could act as a safe site for some species. Here, we studied whether persistent sheaths of the palm Attalea phalerata (Arecaceae) are available sites for seed accumulation in the Pantanal wetland of Brazil. We also investigated whether the composition, richness and diversity of species of seeds in the persistent sheaths are determined by habitat (riparian forest and forest patches) and/or season (wet and dry).
Methods
All accumulated material was collected from ten persistent sheaths along the stems of 64 A. phalerata individuals (16 per habitat and 16 per season). The material was then individually inspected under a stereomicroscope to record seed species and number.
Key Results
Of the 640 sheaths sampled, 65 % contained seeds (n = 3468). This seed bank included 75 species belonging to 12 families, and was primarily composed of small, endozoochoric seeds, with a few abundant species (Cecropia pachystachya and Ficus pertusa). Moraceae was the richest family (four species) and Urticaceae the most abundant (1594 seeds). Stems of A. phalerata in the riparian forest had 1·8 times more seeds and 1·3 times more species than those in forest patches. In the wet season we sampled 4·1 times more seeds and 2·2 more species on palm stems than in the dry season. Richness did not differ between habitats, but was higher in the wet season. Abundance was higher in forest patches and in the wet season.
Conclusions
Attalea phalerata stems contain a rich seed bank, comparable to soil seed banks of tropical forests. As most of these seeds are not adapted to grow in flooding conditions, palm stems might be regarded as safe sites for seeds (and seedlings) to escape from the seasonal flooding of the Pantanal.
Keywords: Attalea phalerata, Cecropia pachystachya, Ficus, hemi-epiphyte, palm tree, palm seed bank, phorophyte, seed community, seed dispersal, Pantanal wetland, Brazil
INTRODUCTION
In tropical forests, the forest soils have been the main subject of studies on seed banks and are often regarded as the final place where plant diaspores are deposited (Butler and Chazdon, 1998; Dalling et al., 1998; Grombone-Guaratini and Rodrigues, 2002; Grombone-Guaratini et al., 2004). However, there are other sites of seed deposition that have received only minor attention (Benzing, 1990; Fischer and Araujo, 1995). Considering the great variety of dispersal modes and diaspore traits, it might be expected that seeds may land on plant substrates throughout the vegetation strata (Nadkarni and Haber, 2009). Indeed, epiphytes and parasite plants have some features that facilitate the seeds attachment on plant substrates (phorophytes; Benzing, 1990; Fischer and Araujo, 1995). Therefore, studies of seed banks on different plant substrates can provide more reliable knowledge about forest regeneration (Putz and Holbrook, 1989; Nadkarni and Haber, 2009).
Seed accumulation on vegetation may be high on rough and large trunks, branch forks or dense foliage (Putz and Holbrook, 1989; Bernal and Balslev, 1996; Nadkarni and Haber, 2009). Several palm trees have a canopy architecture resembling a funnel, produced by concentric leaves that intercept falling objects and carry them toward to the stem (Putz and Holbrook, 1989; Bernal and Balslev, 1996). In addition, some palm tree species keep senescent leaf sheaths (persistent sheaths hereafter) covering all the stem. These very rough stems favour accumulation of nutrient-rich sediments that allow plant development and may contain a particular seed bank (Putz and Holbrook, 1989; Bernal and Balslev, 1996; Nadkarni and Haber, 2009). Palm stems can represent safe sites for seed species, providing decreasing mortality risk there than in other sites. Seeds of epiphytic species are expected to be common on palm stems, as they depend on phorophytes to establish seedlings (Benzing, 1990; Fischer and Araujo, 1995). Composition and richness of seeds deposited on palm stems may change according to each palm's neighbouring vegetation, and may vary seasonally due to plant phenologies (Dalling et al., 1998; Grombone-Guaratini et al., 2004; Hopfensperger, 2007). Animal-dispersed seeds commonly show a fruiting peak during the wet season, whereas wind-dispersed seeds do so in the dry season (Howe and Smallwood, 1982; Fischer and Araujo, 1995; Turner, 2001; Ragusa-Netto, 2002).
Here we address the richness and composition of seed banks on persistent sheaths on Attalea phalerata (Arecaceae) stems, by far the most abundant and widely distributed palm tree in the Pantanal floodplain of central South America (Pott and Pott, 1994). At the study site, there are two natural forest habitats, riparian forest and forest patches called capões. In the riparian forest the flooding season is more frequent and extended than in capões. Also, the spatial distribution of A. phalerata individuals differs between habitats, being aggregated in capões but sparse in riparian forests (Damasceno et al., 1999).
We first investigated if A. phalerata stems maintain a seed bank, and then tested the following hypotheses: (1) community parameters (richness of seeds, seed species composition and abundance) are higher on palm stems in capões than in riparian forest because riparian forest palm sheaths could remain longer under water and seeds that had accumulated could be taken out; and (2) community parameters (richness of seeds and abundance) are higher on palm stems in the wet season than in the dry season, as most plant species fruit in the wet season in the Pantanal (Teixeira et al., 2009; Ragusa-Netto, 2004).
MATERIAL AND METHODS
Study site and species
The Pantanal floodplain (approx. 160 000 km2) is part of the upper Paraguay basin in central South America. The climate is tropical (Aw of Köppen), with a dry season from April to September and a wet season from October to March (Cadavid-Garcia, 1984; Teixeira et al., 2009). Mean monthly temperatures vary from 20 to 27 °C and annual rainfall from 800 to 1400 mm (Teixeira et al., 2009). Vegetation is represented by semi-deciduous riparian forests and capões (0·5–5 ha), forest patches surrounded by seasonally flooding fields and water bodies with aquatic plants (Prance and Schaller, 1982; Araujo and Sazima, 2003). Data collection was carried out in capões and riparian forest habitats in the region of Pantanal do Miranda (approx. 19°34′S, 57°01′W), Corumbá, Mato Grosso do Sul, Brazil.
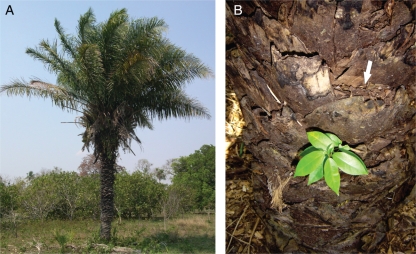
Attalea phalerata (Arecaceae) is the most common palm tree among nine palm species that occur in the Pantanal (Pott and Pott, 1994). Individuals are found either in the understorey, forest borders or open sites. Its leaves are concentrated at the apex with long petioles and broad, invaginant sheaths (Joly, 1991) that remain attached after leaf abscission, covering the entire stem (Fig. 1). The number of persistent sheaths per A. phalerata stem varies from 70 to 500 depending on stem length (0·8–4·0 m). The sediment accumulated on persistent sheaths is acidic (pH = 5·4) with a high proportion of organic matter and a fine clay texture. Cation exchange capacity is 27·6 meq 100 cm−3 and elements found in the sediment are P (42·3 µg cm−3), K (1·38 meq 100 cm−3), Ca (10·7 meq 100 cm−3), Mg (5·8 meq 100 cm−3), Al (0·6 meq 100 cm−3), Fe (7·7 mg dm−3), Mn (70·4 mg dm−3), Cu (0·33 mg dm−3) and Zn (29·2 mg dm−3) (C. E. Corrêa, unpubl. res.).
Fig. 1.
Attalea phalerata individual (A), and the stem showing persistent sheaths with accumulated sediment (arrow) and one epiphyte seedling of Ficus sp. (B), in the Pantanal do Miranda, Brazil.
Data collection
Sixty-four adult individuals of A. phalerata were haphazardly selected for sampling of seeds deposited on persistent sheaths, 32 in the wet season (March) and 32 in the dry season (September). In each season, 16 individuals of A. phalerata were sampled per habitat (capões and riparian forest), with at least 50 m between them. All material deposited on ten persistent sheaths distributed along the stem of each palm was collected. The material from each sheath was individually wrapped in paper bags, weighed and later inspected in the laboratory under a stereomicroscope to separate seeds. We slightly pressed seeds manually to identify rotten ones, which were not included in counts. Only angiosperm seeds larger than 0·5 mm in width or length were included. Seeds were identified through comparisons with herbarium material and identification guides (Lorenzi, 1992, 1998; Lobova and Mori, 2002; Carvalho, 2003); unidentified seeds were classified as morphospecies. To describe the seeds deposited on persistent sheaths we weighed (to ±0·001 g) and classified them according to habit (hemi-epiphyte, herbaceous, shrub or tree) and dispersal mode (zoochoric, anemochoric or autochoric) based on diaspore morphology and the literature (Howe and Smallwood, 1982; Pott and Pott, 1994; Turner, 2001; Carvalho, 2003; Teixeira et al., 2009). Seeds heavier than 0·001 g were individually weighed; otherwise they were weighed in groups of ten seeds per species.
Data analysis
To evaluate whether the species richness and abundance on A. phalerata stems differed between habitats and between seasons, we used a general linear model (GLM) with Poisson distribution, as recommended for count data (Crawley, 2007). In this analysis, abundance (relative abundance) was defined as the number of persistent sheaths in which a given seed species occurred, as it is closer to the number of dispersal events than the number of seeds. As the amount of sediment on each sheath might introduce bias on sampling efforts, we tested for differences of sediment mass on persistent sheaths between habitats and between seasons, using two-way ANOVA. GLM and ANOVA were performed in the R program (R Development Core Team, 2010). To estimate richness of seeds on A. phalerata stems and to evaluate completeness of our sample we computed the Chao 2 estimator based on 100 randomizations using EstimateS 7·5 (Colwell, 1997; Chao, 2005).
To summarize patterns of seed bank communities (species composition and relative abundance) among A. phalerata stems (individuals) we used the hybrid multidimensional scaling (HMDS) technique and the Chord index of dissimilarity, in the PATN program (Faith et al., 1987). To test for effects of habitat and season (independent variables) on similarity of seed banks among A. phalerata stems (values of HMDS axes 1 and 2; dependent variables), we used multivariate analysis of variance (MANOVA) and the Pillai Trace statistic, with Systat 11. Cited means are shown ±s.e.
RESULTS
We recorded 3468 seeds of 75 species/morphospecies on persistent sheaths of A. phalerata in the Pantanal. We found 54·2 ± 10·6 seeds, and 3·6 ± 0·3 species/morphospecies per palm stem (n = 64). These seeds were distributed on 75 ± 8 % persistent sheaths on each palm tree. The 20 seed species (27 %) identified to family level or lower belonged to 12 families and 13 genera (Table 1). Most species/morphospecies (95 %) had small seeds (≤1 g), and were zoochoric (40 %) or anemochoric (34 %). The only large-seeded (approx. 15 g) species on A. phalerata stems was its own seed.
Table 1.
Frequency of occurrence, life forms, mass and dispersal mode of 20 seed species recorded on persistent sheaths in stems of Attalea phalerata (Arecaceae) palm trees in the Pantanal do Miranda, Brazil
| Family/species | Frequency* |
Life form | Seed mass (g) | Dispersal mode | |
|---|---|---|---|---|---|
| Capões | Riparian forest | ||||
| Urticaceae | |||||
| Cecropia pachystachya Trec. | 23 (543) | 31 (1051) | Tree | 0·001 | Zoochory |
| Moraceae | |||||
| Ficus pertusa L. f | 23 (165) | 17 (472) | Hemi-epiphyte | <0·001 | Zoochory |
| Ficus gardneriana (Miq.) Miq | 1 (3) | 0 | Hemi-epiphyte | <0·001 | Zoochory |
| Ficus sp. 1 | 1 (1) | 4 (52) | Hemi-epiphyte† | <0·001 | Zoochory |
| Ficus sp. 2 | 1 (1) | 0 | Hemi-epiphyte† | 0·001 | Zoochory |
| Arecaceae | |||||
| Attalea phalerata Mart. ex. Spreng. | 9 (20) | 4 (13) | Tree | 14·95 | Zoochory |
| Annonaceae | |||||
| Annona sp. | 3 (24) | 2 (11) | Shrub | <0·001 | Zoochory |
| Unonopsis lindmanii R. E. Fries | 0 | 1 (2) | Shrub | 0·153 | Zoochory |
| Fabaceae | |||||
| Aeschynomene sp. | 0 | 1 (1) | Herb | 0·044 | Autochory |
| Deguelia costata (Benth.)A.M.G.Azevedo | 0 | 1 (1) | Tree | 0·001 | Zoochory |
| Pterogyne nitens Tul. | 0 | 1 (2) | Tree | 0·07 | Anemochory |
| Anacardiaceae | |||||
| Astronium fraxinifolium Schott ex Spreng | 1 (1) | 0 | Tree | 0·034 | Anemochory |
| Caryophyllaceae | |||||
| Drymaria cordata (L.) Willd. ex Schult. | 1 (1) | 1 (1) | Herb | 0·002 | Autochory |
| Ulmaceae | |||||
| Trema sp. | 1 (1) | 0 | Shrub | <0·001 | Zoochory |
| Myristicaceae | |||||
| Virola sp. | 0 | 1 (1) | Tree | 0·056 | Zoochory |
| Malpighiaceae | |||||
| Byrsonima sp. | 2 (2) | 0 | Shrub | <0·001 | Zoochory |
| Sapindaceae | |||||
| Morphospecies 1 | 2 (2) | 2 (2) | – | 0·002 | Zoochory |
| Sterculiaceae | |||||
| Morphospecies 2 | 3 (9) | 0 | – | 0·002 | Anemochory |
| Morphospecies 3 | 1 (1) | 0 | – | <0·001 | Zoochory |
* Frequency is the number of palm individuals which contained seeds for each species; the total number of seeds recorded is given in parentheses.
† Two of the six species of Ficus that occur in the study site are not described as hemi-epiphytic.
Moraceae was the richest family (n = 4 species) on stems, but 92 % (637/694) of these seeds belonged to one species, Ficus pertusa. Urticaceae was the most abundant family (n = 1594 seeds), represented only by Cecropia pachystachya. Other families comprised three or fewer species of seeds on A. phalerata stems, and occurred on 23 % (15/64) or less of them. The seed community on persistent sheaths showed a markedly high dominance of two species, C. pachystachya and F. pertusa (Table 1), which occurred on 84 and 63 %, respectively, of the palm individuals. Eighteen species occurred on 3–20 % of the palm stems, and 55 species occurred on 1·5 %.
The number of seed species in the seed bank found on A. phalerata stems did not differ (z = 103·08, P = 0·32) between riparian forests (n = 49) and capões (n = 38). On the other hand, it was higher (z = 69·49, P < 0·01) in the wet season (n = 57) than in the dry season (n = 26). Eight species were common to both seasons and 13 to both habitats. Estimated richness of seeds on palm stems was 171 in riparian forests (95 % CI from 94 to 373) and 96 in capões (95 % CI from 58 to 206), and 137 in the wet season (95 % CI from 89 to 254) and 127 in the dry season (95 % CI from 56 to 365). The number of persistent sheaths containing seeds was higher (z = 287·67, P < 0·01) in riparian forests (n = 277) than in capões (n = 206), and was higher (z = 202·12, P < 0·01) in the wet season (n = 341) than in the dry season (n = 142). Accumulated material per persistent sheath weighed 17·2 ± 1·2 g (n = 32) and did not differ between habitats (F1,28 = 0·78, P = 0·38) or between seasons (F1,28 = 0·02, P = 0·88), and therefore the amount of accumulated material was not a source of bias among samples.
The community structure of seed banks on palm stems, based on species composition and their relative abundances (HMDS: r2 = 0·81; stress = 0·23), differed between habitats (P < 0·01; MANOVA – Pillai Trace = 0·008) but not between seasons (P = 0·198; MANOVA – Pillai Trace = 0·31), and there was no effect of habitat and season interaction (P = 0·38; MANOVA – Pillai Trace = 0·24). Seed species composition was less similar among palm stems in capões than among those in riparian forests.
DISCUSSION
Palm stems as seed banks
Our results indicate that persistent sheaths of A. phalerata stems are accessible sites for arrival of seeds of several forest species in the Pantanal, resulting in a palm seed bank. Most sheaths (65 %) accumulate seeds in amounts comparable to soil seed banks (Butler and Chazdon, 1998; Grombone-Guaratini and Rodrigues, 2002; Grombone-Guaratini et al., 2004), even considering that the limited space between the sheath and stem restricts the number of seeds that can accumulate. The limited space on persistent sheaths is probably a first barrier to retaining large seeds. These are also rare in soil seed banks because they are generally recalcitrant, produced in small quantities and susceptible to predation (Fenner, 1985; Thompson, 1987; Louda, 1989; Gross, 1990; Murdoch and Ellis, 2000; Moles et al., 2004).
Overall, the palm seed banks included 4 % of angiosperm species and 10 % of the angiosperm families already reported in the Pantanal (Pott and Pott, 1994). In addition, the richness and abundance of seeds on A. phalerata persistent sheaths in the Pantanal (75 species and 3468 seeds) are higher than those often found in soil seed banks of tropical forests (<50 species) (e.g. Butler and Chazdon, 1998; Grombone-Guaratini and Rodrigues, 2002; Grombone-Guaratini et al., 2004). This richness may be due to a high accumulation of seeds in a limited space (A. phalerata stems) and low rates of seed movement. Palms are also more frequently used than other trees as feeding perches by seed dispersers.
Seed banks on A. phalerata stems in the Pantanal mainly comprised small endozoochoric and dormant seeds, with a few dominant species, as found for soil seed banks in tropical forests (Thompson, 1987; Gross, 1990; Butler and Chazdon, 1998; Leishman et al., 2000; Murdoch and Ellis, 2000). The two dominant species in palm seed banks – C. pachystachya and F. pertusa – are the two most frequent seeds found in the faeces of Artibeus jamaicensis (Phyllostomidae), by far the most abundant fruit-eating bat in the Pantanal (Teixeira et al., 2009). This indicates that bats are probably the main vectors of seeds to A. phalerata stems (Marinho-Filho, 1992).
The palm sheaths can provide a safe site as they maintain specific conditions of humidity and nutrients (Howe and Smallwood, 1982; Eriksson and Ehrlen, 1992; Bernal and Balslev, 1996), and could provide protection against predation and flooding in the Pantanal. The sandy Pantanal soil is drier (when not flooded) and poorer in nutrients than the palm sheaths (see also Bernal and Balslev, 1996), and thus A. phalerata stems may favour seed germination and seedling establishment. In addition, during the wet season seeds and seedlings in palm stems may stay above inundation level (<1 m deep) so escaping from flooding. Seedlings of several tree species in the Pantanal, including C. pachystachya, are not flood-tolerant (Damasceno et al., 2005).
For epiphyte or hemi-epiphyte species, the arrival of seeds on palm stems is clearly expected to improve their survival and growth; in fact, many epiphyte/hemi-epiphyte species are unable to establish directly on forest ground (Benzing, 1990). Although seeds of epiphytes/hemi-epiphytes represented few of the species recorded, they are among the commonest seed species in palm seed banks. Cecropia pachystachya has been not described as an epiphyte/hemi-epiphyte, but we have found reproductive trees in the Pantanal that germinated on A. phalerata stems and their roots connected to soil after establishment. Therefore, the massive arrival of seeds on palm stems (this study) combined with a negative effect of flooding on recruitment on forest soils (Damasceno et al., 2005; Marques et al., 2009) might favour the hemi-epiphyte habit found for some individuals of C. pachystachya in the Pantanal.
The scarcity of seeds of epiphytes or hemi-epiphytes indicates that most species in the A. phalerata stems are not adapted to grow up on phorophytes. However, their low density in palm seed banks may not be relevant in terms of overall recruitment for their local populations, as only a small proportion of seeds would die on palm stems. In contrast, a massive arrival in palm seed bank could be disadvantageous for species that have no adaptations to grow up on trees. Thus, even though occasional individuals of C. pachystachya were recorded on palm trees, additional data are needed to support the hypothesis that palm stems represent safe sites for its seeds.
Richness, abundance, and composition between habitats and seasons
The estimated richness of seeds in palm seed banks was far higher than the observed number of seed species on A. phalerata sheaths due to the very high number of rare species. Several rare species are expected to be added with increasing number of sampled palm stems at the study site, as deposition of seeds on palm stems occurred only once or twice for many species. Therefore, most diaspores appear to arrive accidentally at A. phalerata stems, probably related to forest community structures that commonly have a large number of rare species (Felfili et al., 2000; Wittmann et al., 2008).
The lower number of sheaths containing seeds among palm trees in riparian forests than for those in capões might be related to differences between habitats in the arrival and/or in retention of seeds on palm stems. In the riparian forest A. phalerata individuals are surrounded by taller (10–20 m) trees with dense canopies, which could be used by the fruit-eating bats as feeding roosts. By contrast, palms in capões commonly have conspecific neighbourhoods and represent the main place suitable as bat feeding roosts. In addition, the high intensity of flooding in riparian forest might flush out the seeds deposited on palm stems, whereas the lower flooding in capões would maintain most of them. The increased richness of seeds and number of sheaths containing seeds during the wet season may be explained by the largely seasonal fruiting seasons of the zoochoric species in the Pantanal (Ragusa-Netto, 2004).
Community structure between habitats and between seasons
Composition and structure of A. phalerata seed banks in forest patches (capões) had lower similarity among them than did palm seed banks in riparian forest. This pattern is probably related to the patchy nature of capões, with a wide range of size, degree of isolation and flooding regime, whereas the riparian forests are continuous formations under a similar flooding regime throughout their extent (Silva et al., 1998; Cunha and Junk, 1999; Damasceno et al., 1999, 2005). The dominant species on palm seed banks, C. pachystachya and F. pertusa, are year-round fruiting plants which occur in capões and riparian forests (Ragusa-Netto, 2004; Teixeira et al., 2009), and thus they contribute to the increased similarity of palm seed banks between seasons. Moreover, the similar community structures between seasons indicate that an accumulation of seeds occurs over time mainly due to the two dominant species.
Conclusions
Attalea phalerata stems maintain seed banks in the Pantanal which have general characteristics similar to those described for the soil seed banks of tropical forests. Therefore, factors which determine overall patterns of soil seed bank structures are probably the same as those shaping the traits of palm seed banks described here. Seeds of fig species and C. pachystachya dominate palm seed banks and they are able to successfully establish seedlings on palms and become adults, but other less abundant seed species would depend on secondary dispersal. Ants are regular visitors of A. phalerata and they commonly carry a wide diversity of small seeds to their nests (Leal et al., 2007), and thus several seed species deposited in A. phalerata stems might be removed to surrounding sites. In this way palm stems could provide seeds with a place to wait for better conditions and ensure the maintenance of seed species in the forest. A. phalerata is a pioneer forest species which contains a source of seeds from the local community, so it could play a key role in successional processes in the Pantanal. Additional studies focusing on seed survival and secondary dispersal after deposition on palm stems are necessary to understand the role of A. phalerata seed banks in forest regeneration. Knowledge of alternative places for seed deposition, other than forest soil, and the traits of such uncommon seed banks might provide new approaches to conservation and restoration.
ACKNOWLEDGEMENTS
We thank to D. Pires, F. Gonçalves, L. C. Corrêa, L. F. Carvalho, M. Delatorre, M. Dias, N. Cunha, N. Penatti and S. Ferreira for help in the field and laboratory; to A. Martini, A. Sartori, F. R. Scarano, J. Raizer and M. Tabarelli for suggestions on previous versions of the manuscript; to D. Hay for a review of the English text; and to Coordenadoria de Estudos do Pantanal staff for logistical support. This work was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES); Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq); Fundação de Apoio ao Desenvolvimento do Ensino, Ciência e Tecnologia do Estado de Mato Grosso do Sul (FUNDECT); and CPNq grants to C.E.C. (130180/2003-3), E.F. (300097/2009-3) and FAMS (308748/2010-7).
LITERATURE CITED
- Araujo AC, Sazima M. The assemblage of flowers visited by hummungbirds in the “capões” of Southern Pantanal, Mato Grosso do Sul, Brazil. Flora. 2003;198:427–435. [Google Scholar]
- Benzing DH. Vascular epiphytes. 1st edn. Cambridge: Cambridge University Press; 1990. [Google Scholar]
- Bernal R, Balslev H. Strangulation of the palm Phytelephas seemannii by the pioneer tree Cecropia obtusifolia: the cost of efficient litter trapping. Ecotropica. 1996;2:177–184. [Google Scholar]
- Butler BJ, Chazdon RL. Species richness, spatial variation, and abundance of the soil seed bank of a secondary tropical rain forest. Biotropica. 1998;30:214–222. [Google Scholar]
- Cadavid-Garcia EA. O clima no Pantanal Mato-Grossense. 1st edn. Corumbá: Embrapa; 1984. [Google Scholar]
- Carvalho PER. Espécies arbóreas brasileiras. 1st edn. Curitiba: Embrapa; 2003. [Google Scholar]
- Chao A. Species richness estimation. In: Balakrishnan N, Read CB, Vidakovic B, editors. Encyclopedia of statistical sciences. New York: Wiley; 2005. pp. 7909–7916. [Google Scholar]
- Colwell RK. EstimateS: statistical estimation of species richness and shared species from samples. 1997 Available from: http://viceroy.eeb.uconn.edu/estimates . (accessed 25 April 2011) [Google Scholar]
- Crawley MJ. The R Book. 1st edn. London: John Wiley & Sons Ltd; 2007. [Google Scholar]
- Cunha CN, Junk WJ. Anais do II Simpósio sobre recursos naturais e sócio-econômicos do Pantanal. Corumbá: Embrapa; 1999. Composição florística de capões e cordilheiras: localização das espécies lenhosas quanto ao gradiente de inundação no Pantanal de Poconé, MT – Brasil; pp. 387–406. November 1999. [Google Scholar]
- Dalling JW, Swaine MD, Garwood NC. Dispersal patterns and seed bank dynamics of pioneer trees in moist tropical forest. Ecology. 1998;79:564–578. [Google Scholar]
- Damasceno GA, Jr, Bezerra MAO, Bortolotto IM, Pott A. Anais do II Simpósio sobre recursos naturais e sócio-econômicos do Pantanal. Corumbá: Embrapa; 1999. Aspectos florísticos e fitofisionômicos dos capões do Pantanal do Abobral; pp. 203–214. November 1999. [Google Scholar]
- Damasceno GA, Jr, Semir J, Santos FAM, Leitão-Filho HF. Structure, distribution of species and inundation in a riparian forest of Rio Paraguai, Pantanal Brazil. Flora. 2005;200:119–135. [Google Scholar]
- Eriksson O, Ehrlen J. Seed and microsite limitation of recruitment in plant populations. Oecologia. 1992;91:360–364. doi: 10.1007/BF00317624. [DOI] [PubMed] [Google Scholar]
- Faith DP, Minchin PR, Belbin L. Compositional dissimilarity as a robust measure of ecological distance. Vegetatio. 1987;69:57–68. [Google Scholar]
- Felfili JM, Rezende AV, Silva MC, Jr, Silva MA. Changes in the floristic composition of cerrado sensu stricto in Brazil over a nine-year period. Journal of Tropical Ecology. 2000;16:579–590. [Google Scholar]
- Fenner M. Seed ecology. 1st edn. London: Chapman and Hall; 1985. [Google Scholar]
- Fischer E, Araujo AC. Spatial organization of a bromeliad community in the Atlantic rainforest, south-eastern Brazil. Journal of Tropical Ecology. 1995;11:559–567. [Google Scholar]
- Grombone-Guaratini MT, Rodrigues RR. Seed bank and seed rain in a seasonal semi-deciduous forest in south-eastern Brazil. Journal of Tropical Ecology. 2002;18:759–774. [Google Scholar]
- Grombone-Guaratini MT, Leitão-Filho HF, Kageyama PY. The seed bank of a gallery forest in southeastern Brazil. Brazilian Archives of Biology and Technology. 2004;47:793–797. [Google Scholar]
- Gross KL. A comparison of methods for estimating seed numbers in the soil. Journal of Tropical Ecology. 1990;78:1079–1093. [Google Scholar]
- Hopfensperger KN. A review of similarity between seed bank and standing vegetation across ecosystems. Oikos. 2007;116:1438–1448. [Google Scholar]
- Howe HF, Smallwood J. Ecology of seed dispersal. Annual Review of Ecology and Systematics. 1982;13:201–228. [Google Scholar]
- Joly AB. Botânica – introdução à taxonomia vegetal. 10th edn. São Paulo: Companhia Editora Nacional; 1991. [Google Scholar]
- Leal IR, Wirth R, Tabarelli M. Seed dispersal by ants in the semi-arid Caatinga of north-east Brazil. Annals of Botany. 2007;99:885–894. doi: 10.1093/aob/mcm017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leishman MR, Wright IJ, Moles AT, Westoby M. The evolutionary ecology of seed size. In: Fenner M, editor. Seeds: the ecology of regeneration in plant communities. New York: CABI Publishing; 2000. pp. 31–57. [Google Scholar]
- Lobova TA, Mori SA. Atlas of seed dispersal by bats in the neotropics. 2002 The New York Botanical Garden. Available from www.botanypages.org/mori/batsplants/batseedatlas/seedatlas_main1.htm. (accessed 20 January 2005) [Google Scholar]
- Lorenzi H. Árvores brasileiras: manual de identificação e cultivo de plantas arbóreas nativas do Brasil. 1st edn. Vol. 1. Nova Odessa: Editora Plantarum; 1992. [Google Scholar]
- Lorenzi H. Árvores brasileiras: manual de identificação e cultivo de plantas arbóreas nativas do Brasil. 1st edn. Vol. 2. Nova Odessa: Editora Plantarum; 1998. [Google Scholar]
- Louda SM. Predation in the dynamics of seed regeneration. In: Leck MA, Parker VT, Simpson RL, editors. Ecology of soil seed bank. London: Academic Press; 1989. pp. 25–51. [Google Scholar]
- Marinho-Filho JS. Ecologia e história natural das interações entre palmeiras, epífitas e frugívoros na região do Pantanal Matogrossense. Campinas: PhD thesis, Universidade Estadual de Campinas; 1992. [Google Scholar]
- Marques MCM, Burslem DFRP, Britez RM, Silva SM. Dynamics and diversity of flooded and unflooded forests in a Brazilian Atlantic rain forest: a 16-year study. Plant Ecology and Diversity. 2009;2:57–64. [Google Scholar]
- Moles AT, Falster DS, Leishman MR, Westoby M. Small-seeded species produce more seeds per square metre of canopy per year, but not per individual per lifetime. Journal of Ecology. 2004;92:384–396. [Google Scholar]
- Murdoch AJ, Ellis RH. Dormancy, viability and longevity. In: Fenner M, editor. Seeds: the ecology of regeneration in plant communities. New York: CABI Publishing; 2000. pp. 183–214. [Google Scholar]
- Nadkarni NM, Haber WA. Canopy seed banks as time capsules of biodiversity in pasture-remnant tree crowns. Conservation Biology. 2009;23:1117–1126. doi: 10.1111/j.1523-1739.2009.01235.x. [DOI] [PubMed] [Google Scholar]
- Pott A, Pott VJ. Plantas do Pantanal. 1st edn. Brasília: Embrapa; 1994. [Google Scholar]
- Prance GT, Schaller GB. Preliminary study of some vegetation types of the Pantanal, Mato Grosso, Brazil. Brittonia. 1982;34:228–251. [Google Scholar]
- Putz FE, Holbrook NM. Strangler fig rooting habits and nutrient relations in the Llanos of Venezuela. American Journal of Botany. 1989;76:781–788. [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2010. http://www.R-project.org . [Google Scholar]
- Ragusa-Netto J. Fruiting phenology and consumption by birds in Ficus calyptroceras (Miq.) Miq. (Moraceae) Brazilian Journal of Biology. 2002;62:339–346. doi: 10.1590/s1519-69842002000200018. [DOI] [PubMed] [Google Scholar]
- Ragusa-Netto J. Flowers, fruits, and the abundance of the Yellow-chevroned Parakeet (Brotogeris chiriri) at a gallery forest in the south Pantanal (Brazil) Brazilian Journal of Biology. 2004;64:867–877. doi: 10.1590/s1519-69842004000500017. [DOI] [PubMed] [Google Scholar]
- Silva MC, Jr, Felfili JM, Nogueira PE, Rezende AV. Análise florística das matas de galeria no Distrito Federal. In: Ribeiro JF, editor. Cerrado: matas de galeria. Planaltina: Embrapa; 1998. pp. 53–82. [Google Scholar]
- Teixeira RC, Corrêa CE, Fischer E. Frugivory by Artibeus jamaicensis (Phyllostomidae) bats in the Pantanal, Brazil. Studies on Neotropical Fauna and Environment. 2009;44:7–15. [Google Scholar]
- Thompson K. Seed and seed banks. New Phytologist. 1987;106:23–34. [Google Scholar]
- Turner IM. The ecology of trees in the tropical rain forest. 1st edn. London: Cambridge University Press; 2001. [Google Scholar]
- Wittmann F, Zorzi BT, Tizianel FAT, et al. Tree species composition, structure, and aboveground wood biomass of a riparian forest of the Lower Miranda River, southern Pantanal, Brazil. Folia Geobotânica. 2008;43:397–411. [Google Scholar]