Abstract
Background and Aims
To date, most floral nectarins (nectar proteins) are reported to function in nectar defence, particularly for insect-pollinated outcrossing species. We compared nectarin composition and abundance in selfing common tobacco (Nicotiana tobaccum) with outcrossing ornamental tobacco plants to elucidate the functional difference of nectarins in different reproductive systems.
Methods
Common tobacco (CT) nectarins were separated by SDS-PAGE and the N terminus of the most abundant nectarin was sequenced via Edman degradation. The full-length nectarin gene was amplified and cloned from genomic DNA and mRNA with hiTail-PCR and RACE (rapid amplification of cDNA ends), and expression patterns were then investigated in different tissues using semi-quantitative reverse transcriptase PCR. Additionally, high-performance liquid chromatography and enzymatic analyses of nectar sugar composition, and other biochemical traits and functions of the novel nectarin were studied.
Key Results
The most abundant nectarin in CT nectar is an acidic α-galactosidase, here designated NTα-Gal. This compound has a molecular mass of 40 013 Da and a theoretical pI of 5·33. NTα-Gal has a conserved α-Gal characteristic signature, encodes a mature protein of 364 amino acids and is expressed in different organs. Compared with 27 other melliferous plant species from different families, CT floral nectar demonstrated the highest α-Gal activity, which is inhibited by d-galactose. Raffinose family oligosaccharides were not detected in CT nectar, indicating that NTα-Gal does not function in post-secretory hydrolysis. Moreover, tobacco plant fruits did not develop intact skin with galactose inhibition of NTα-Gal activity in nectar, suggesting that NTα-Gal induces cell-wall surface restructuring during the initial stages of fruit development.
Conclusions
α-Gal was the most abundant nectarin in selfing CT plants, but was not detected in the nectar of strictly outcrossing sister tobacco species. No function was demonstrated in antimicrobial defence. Therefore, floral nectarins in selfing species maintain their functional significance in reproductive organ development.
Keywords: α-galactosidase, Nicotiana tobacum, floral nectar, enzymatic activity, selfing syndrome
INTRODUCTION
Most angiosperms are pollinated by insects, which are rewarded with nectar during visits to flowers. Nectar mediates the interactions of plants with pollinators and defenders and also increases the fecundity of plants that produce nectar (Baker and Baker, 1983; Heil, 2011). Carbohydrates and amino acids are the main components in nectar and serve in attraction and nutrition of pollinators. However, nectars also contain other compounds, for example proteins and several classes of secondary metabolites (Nicolson and Thornburg, 2007; Heil, 2011). Surprisingly, the physiological function of nectar proteins (nectarins) remains poorly investigated with the preponderance of botanical research focusing on model plants that either do not produce nectar (i.e. wind-pollinated crops such as rice and maize) or are highly selfing (i.e. Arabidopsis thaliana) and therefore not dependent on nectar for pollination.
When comparing nectarins with other major components of floral nectar such as carbohydrates and free amino acids, nectar proteins have received only collateral attention for their physiological roles. The presence of proteins in nectar has been previously reported, but only a limited number of nectarins from a few plant species have been studied (Nicolson and Thornburg, 2007; Nepi et al., 2009). Floral nectar proteomes appear to be small (Heil, 2011). To date, five proteins have been identified in ornamental tobacco (Carter et al., 1999), and eight proteins have been identified in the nectar of the tropical tree Jacaranda mimosifolia (Kram et al., 2008); and biological activity has been characterized in only a few nectarins (Nepi et al., 2009; Heil, 2011).
Floral nectarins of an outcrossing ornamental tobacco plant derived from an interspecies cross between Nicotiana langsdorffii and Nicotiana sanderae have been studied extensively. Most of the floral nectarins have been considered likely to protect floral nectar from microbial colonization through the nectar redox cycle (Carter et al., 1999, 2007; Carter and Thornburg, 2000, 2004a, b). The putative protective function of other nectarins identified in angiosperm nectars such as phosphatase, mannose binding lectin, allinase and lipase have been previously described in the innate immunological defences for different melliferous species (Nepi et al., 2009). The rich milieu of nutrients found in floral nectar, and the occasion for visiting pollinators to transfer micro-organisms between flowers, provide a positive environment for microbial growth (Thornburg et al., 2003; Herrera et al., 2008). Specifically, there is increased risk for highly outcrossing species to attract pollinators, which might introduce pathogens to the delicate reproductive organ. Notably, floral nectarins have been reported to present a route of infection for some plant pathogens (Keitt and Ivanoff, 1941; Kram et al., 2008). Therefore, it is not difficult to comprehend why so many types of pathogenesis-related (PR) proteins have been identified in nectar (Thornburg, et al., 2003; Park and Thornburg, 2009; González-Teuber et al., 2009, 2010). Notably, some micro-organisms can dramatically change nectar composition, which is closely linked to the function of nectar in flowering plants (Herrera et al., 2008; Heil, 2011). Also noteworthy is that the common tobacco plant used in this study is a highly selfing species which secretes considerable amounts of nectar (approx. 10–40 µL per flower; Zelitch and Day, 1973). This nectar production rate per flower was very similar to the rate of floral nectar secretion observed in the outcrossing ornamental tobacco plant (Carter et al., 1999). These observations appear to be inconsistent with ‘selfing syndrome’ theory, which states that selfing plants tend to produce less nectar (Sicard and Lenhard, 2011). In addition, it is reasonable to deduce that in comparing highly selfing plant species to outcrossing species, the floral nectar in highly selfing plant species is less likely to be infected by pathogens introduced by pollinators. Collectively, these observations evoke compelling arguments for the further characterization of the composition and function of floral nectar and nectarins in selfing species. Additionally, comparing the floral nectar proteomes between closely related selfing and outcrossing plant species might elucidate the different functional significance of nectar proteins in the selfing reproductive system. In this study, we compared floral nectarin composition profiles of closely related selfing and outcrossing tobacco plants. We identified α-galactosidase (NTα-Gal, α-Gal, α-d-galactoside galactohydrolase: EC 3·2·1·22) first in floral nectar and observed it to be the most abundant nectarin in selfing tobacco species. Moreover, we suggest that NTα-Gal in floral nectar functions in the early stages of fruit development.
MATERIALS AND METHODS
Plant floral nectar collection and protein content determination
Raw floral nectar samples from 28 different melliferous plant species (Table 1) were taken for α-Gal activity assays. The samples were collected from the Kunming Botanical Garden, Yunnan province, China, with pipettes and autoclaved tips from June 2010 to April 2011 as described by Carter et al. (1999). The floral nectar samples were pooled, filtered through 0·22-μm syringe filters (Millipore, Bedford, MA, USA) to remove dirt and pollen granules, and stored at –80 °C prior to use. Tobacco floral nectar was collected from 20 common tobacco (Nicotiana tobaccum L.), plants grown in a greenhouse at 25 °C with a 16-h photoperiod. The protein content in the pooled nectar samples was determined according to Bradford (1976), using bovine serum albumin as the standard. The average of triplicate total protein content measurements was calculated for each sample.
Table 1.
Floral nectar from different species for α-galactosidase activity assay
| Name | Abbreviation | Family | Order | Date of collection | Total protein concentration (μg mL−1) |
|---|---|---|---|---|---|
| Tropaeolum majus L. | TM | Tropaeolaceae | Brassicales | 07·2010 | 65·9 |
| Rhododendron delavayi Franch | RD | Ericaceae | Ericales | 03·2011 | 12·7 |
| Rhododendron irroratum Franch | RI | Ericaceae | Ericales | 03·2011 | 68·2 |
| Erythrina variegata L. | EV | Fabaceae | Fabales | 07·2010 | 10·5 |
| Mucuna sempervirens Hemsl. | MS | Fabaceae | Fabales | 04·2011 | 370·9 |
| Alstonia yunnanensis Diels | AY | Apocynaceae | Geraniales | 07·2010 | 29·1 |
| Justicia brandegeeana Wassh. and L.B.Sm. | JB | Acanthaceae | Lamiales | 07·2010 | 6·8 |
| Campsis radicans (L.) Seem. | CR | Bignoniaceae | Lamiales | 06·2010 | 47·1 |
| Aeschynanthus bracteatus Wall. | AB | Gesneriaceae | Lamiales | 06·2010 | 58·7 |
| Salvia coccinea Juss. ex Murr. | SC | Lamiaceae | Lamiales | 07·2010 | 16·2 |
| Antirrhinum majus L. | AnM | Plantaginaceae | Lamiales | 06·2010 | 19·4 |
| Abutilon striatum Dicks. | AS | Malvoideae | Malvales | 10·2010 | 7·6 |
| Hibiscus rosa-sinensis L. | HR | Malvoideae | Malvales | 06·2010 | 18·7 |
| Fuchsia hybrida Hort. | FH | Onagraceae | Myrtales | 06·2010 | 18·5 |
| Kalanchoe fedtschenkoi Raym.-Hamet and H.Perrier | KF | Crassulaceae | Saxifragales | 03·2011 | 7·0 |
| Cestrum purpureum Standl. | CP | Solanaceae | Solanales | 04·2011 | 25·7 |
| Datura aurea Safford | DA | Solanaceae | Solanales | 07·2010 | 20·5 |
| Nicotiana tabacum L. | NT | Solanaceae | Solanales | 03·2011 | 42·2 |
| Hosta plantaginea Asch. | HP | Asparagaceae | Asparagales | 06·2010 | 57·9 |
| Polygonatum kingianum Coll. et Hemsl | PK | Asparagaceae | Asparagales | 04·2011 | 82·8 |
| Kniphofia uvaria (L.) Hook. | KU | Xanthorrhoeaceae | Asparagales | 04·2011 | 18·4 |
| Iris pseudacorus L. | IP | Iridaceae | Asparagales | 03·2011 | 8·5 |
| Lycoris radiata Herb. | LR | Amaryllidaceae | Asparagales | 08·2010 | 71·5 |
| Anigozanthos manglesii D.Don | AM | Haemodoraceae | Commelinales | 06·2010 | 23·0 |
| Stemona tuberosa Lour. | ST | Stemonaceae | Pandanales | 10·2010 | 117·2 |
| Musella lasiocarpa (Franch.) H.W.Li | ML | Musaceae | Zingiberales | 03·2011 | 18·1 |
| Canna generalis L.H.Bailey | CG | Cannaceae | Zingiberales | 06·2010 | 12·2 |
| Hedychium forrestii Diel. | HF | Zingiberaceae | Zingiberales | 10·2010 | 23·1 |
Analysis of tobacco floral nectar basic properties
The pH of fresh tobacco nectar from randomly selected flowers was tested by wide- and narrow-range pH test strips (Sigma). Total sugar content in the nectar samples was determined using phenol-sulphuric acid and d-glucose as standard (Dubois et al., 1956). Sugar composition in the nectar samples was analysed by high-pressure liquid chromatography (HPLC; Agilent 1100, Agilent Technologies) with Cosmosil Sugar-D column (4·6 × 250 mm, Nacalai Tesque Inc., Kyoto, Japan) under the following conditions: solvent, 75 % (v/v) acetonitrile; flow rate, 1 mL min−1; column temperature, 30 °C. Monosaccharides and oligosaccharides eluted from the column were quantified with an evaporative light scattering detector (ELSD; Varian 380-LC, Darmstadt, Germany). Simple and complex sugars, including d-glucose, d-fructose, d-galactose, d-xylose, sucrose and raffinose, generally detected in floral nectar, were used as external standards for determination of peaks. Enzymatic based methods were used to quantify the content of the raffinose family of oligosaccharides (RFOs) with a commercial kit, raffinose/d-galactose assay kit (K-RAFGA; Megazyme, Ireland) according to the manufacturer's instructions. Average measurements were obtained from triplicate reactions for each sample.
Tobacco nectarin concentration and electrophoresis
Fresh tobacco nectarins were concentrated by ultracentrifugal filtering with Amicon YM-3 centrifugal filter units (cut-off 3 kDa; Millipore), boiled in sample buffer (with 0·1 m dithiothreitol) for 5 min, and then analysed using 12 % (w/v) self-poured sodium dodecyl sulfate-polyacrylamine gel electrophoresis (SDS-PAGE) as described by Laemmli (1970). Molecular weight protein markers were used as standards. Proteins were visualized by staining with Coomassie Brilliant Blue (CBB) R-250.
Amino-terminal sequencing
Immediately following SDS-PAGE, the proteins were transferred onto Immobilon PSQ polyvinylidene difluoride (PVDF) membranes (Millipore), then visualized using CBB. The most abundant protein band (Band 5, named NTB5) was excised from the membrane and analysed using a PPSQ-31A automated protein/peptide sequencer (Shimadzu, Kyoto, Japan) according to the manufacturer's instructions. A similarity search of amino acid sequence was applied using the blast program on the National Center for Biotechnology Information (NCBI) protein database.
Nucleic acid extraction
Tobacco genomic DNA (gDNA) was extracted from young fresh leaves using a DNeasy Plant Mini Kit (Qiagen). Total RNA samples were isolated separately from fresh tobacco flowers, leaves and stems from three individual plants using an RNeasy Plant Mini Kit (Qiagen). The quality and concentration of extracted DNA and RNA were assessed using a Nanodrop Spectrophotometer (ND-1000; Thermo Fisher Scientific, USA).
Rapid amplification of cDNA ends (RACE)
The full-length cDNA of tobacco α-Gal was determined from the analysis of stage 11 tobacco flower RNA extracts using a 3′-Full RACE Core Set kit and a 5′-Full RACE Kit (Takara, Dalian, China) according to the manufacturer's instructions (Koltunow et al., 1990). All primers used in this study are listed in Table 2 and their DNA locations are outlined in Fig. 3A. Tobacco α-Gal gene-specific primers (GS1F) for 3′ RACE were designed according to the identified N-terminal sequence, and the primers (GS1R and GS2R) for 5′ RACE were designed according to the sequence data from 3′ RACE. All RACE products were separated by electrophoresis on 1·5 % agarose gels. The amplified fragments were purified and sub-cloned into pMD18-T Vector (TaKaRa) followed by sequencing. The verified full-length α-Gal gene was designated NTα-Gal.
Table 2.
Oligonucleotide primers used in this study
| Primer name | Sequence* (5′ → 3′) | Application |
|---|---|---|
| GS1F | GGCCGIACICCKCARATGGG | 3′ RACE |
| GS1R | CCTTTCTCTTGGACTACGA | 5′ RACE |
| GS2R | GCATCTTGTTCTTCGTGTCC | 5′ RACE |
| F0 | GGTTGTGATTTACGTTCGATGGACCA | hiTAIL-PCR |
| F1 | ACGATGGACTCCAGTCCGGCCTGGACCAAACTGCCCATGAAATC | hiTAIL-PCR |
| F2 | GTCAAGCAGAATGGAGACTTGGAGG | hiTAIL-PCR |
| R0 | CTCTGTTGAGTTCGGCCCAGCAGTC | hiTAIL-PCR |
| R1 | ACGATGGACTCCAGTCCGGCCCCAAGAGAAGCAAGTCCAGTAGATACC | hiTAIL-PCR |
| R2 | GTCCAGTAGATACCATTGCATCAGC | hiTAIL-PCR |
| intF1 | CAGATGGAGCAGCTGGAATC | Nest-PCR |
| intF2 | GCTGATGCAATGGTATCTAC | Nest-PCR |
| intR1 | CATTTCTACCTCACACAAGGT | Nest-PCR |
| intR2 | CCTCCAAGTCTCCATTCTG | Nest-PCR |
| RTF | GCTGATGCAATGGTATCTAC | Semi-qPCR |
| RTR | CCTTTCTCTTGGACTACGA | Semi-qPCR |
| TAC9F | CCCTCCCACATGCTATTCT | Semi qPCR |
| TAC9R | AGAGCCTCCAATCCAGACA | Semi qPCR |
* IUPAC code for mixed bases: R, A/G; K, G/T; V, A/C/G; D, A/G/T; B, C/G/T; N, A/C/G/T.
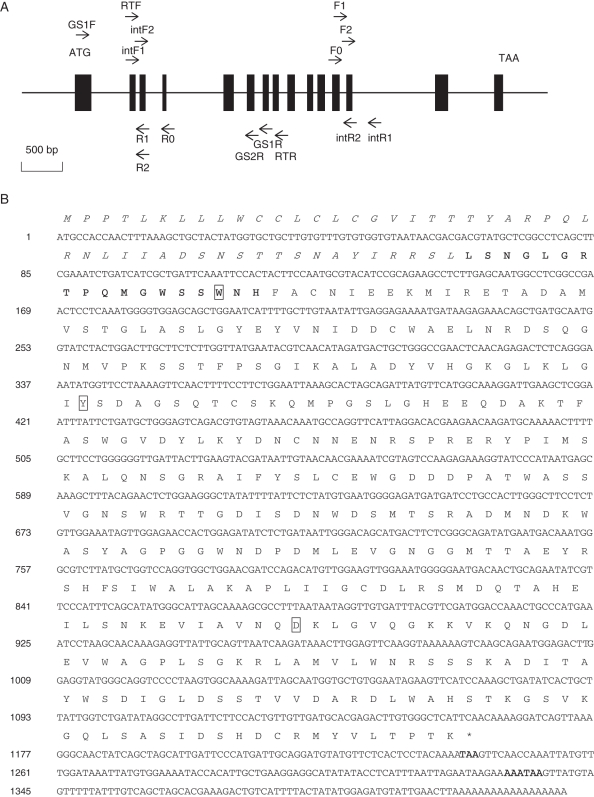
Fig. 3.
NTα-Gal gene. (A) Schematic representation of the genomic organization of NTα-Gal. The genomic structure of NTα-Gal is characterized by 15 exons (indicated by a rectangular box) and 14 introns. An initiation codon and a termination codon are shown. Binding sites and primer directions are indicated by arrows and labelled as in Table 2. All primers for NTα-Gal amplification were located on exons except primer intR1. (B) Nucleotide (GenBank accession no. HQ877671) and deduced amino acid sequences of NTα-Gal. The nucleotide sequence is numbered to the right. The deduced amino acids are shown in a one-letter code above the corresponding codons. Signal peptide sequence data are shown in italics. The N-terminal sequence determined by Edman degradation, stop codon and poly(A) addition signal are indicated in bold. Conserved amino acids acting in NTα-Gal enzymatic activity are boxed.
PCR of genomic DNA
The full-length α-Gal cDNA sequence was analysed using in-genome walking techniques in the 5′ and 3′ directions in conjunction with a high-efficiency thermal asymmetric interlaced PCR (hiTAIL-PCR) methodology (Liu and Chen, 2007). Gene-specific primers (F0, F1, F2, R0, R1 and R2) were designed to the cDNA sequence obtained by RACE. Arbitrary degenerate primers (LAD1–4, AC1) were designed and the cycling conditions of Liu and Chen (2007) were strictly adhered to. The hiTAIL-PCR products of 5′ and 3′ ends of NTα-Gal on gDNA were purified, cloned and sequenced as previously described, and the internal sequence of NTα-Gal was amplified using nested PCR primers (intF1, intF2, intR1 and intR2) designed to anneal at the end flanking sequences of NTα-Gal gDNA obtained by hiTAIL-PCR. Nested PCR detection was performed by: pre-heating the reactions for 4 min at 94 °C (one cycle); dissociation for 1 min at 94 °C; annealing for 1 min at 55 °C; extension for 2 min at 72 °C (33 cycles); and a final extension for 10 min at 72 °C (one cycle). Two microlitres of a ten-fold diluted first-round PCR product was used as template for the second-round PCR in a 50-μL reaction volume. The final product was cloned and sequenced as previously described. Overlapping DNA sequences were assembled to make a full-length NTα-Gal construct. The sequences generated in this study have been deposited in GenBank (accession nos. HQ877670 and HQ877671).
Semi-quantitative RT-PCR
To investigate the relative expression of NTα-Gal in different organs, cDNA was synthesized from total RNA extracted from the tobacco leaves, stems and flower corollas (at stage 11) using a PrimeScript II 1st Strand cDNA Synthesis Kit (Takara, Dalian, China). Gene-specific primers (RTF and RTR) based on the cloned full-length NTα-Gal sequence were used in semi-quantitative reverse transcriptase PCR (RT-PCR). The optimal amounts of cDNA and the number of PCR cycles corresponding to the exponential phase of the reaction were determined. The PCR programme was: 4 min at 94 °C (one cycle); 30 s at 94 °C, 45 s at 55 °C and 45 s at 72 °C (30 cycles); and 10 min at 72 °C (one cycle). The abundance of RT-PCR products was normalized to the level of constitutively expressed tobacco actin, Tac9 (GenBank: X69885). Primers for Tac9 are listed in Table 2. The PCR for Tac9 fragments was performed using the same samples and procedures described above. Average values from triplicate reactions for each sample were calculated and gDNA contamination control reactions lacking RT were run concurrently with the samples. PCR products were separated by electrophoresis in a 1·5 % agarose gel and visualized after staining with ethidium bromide.
Computer analysis
Protein identification searches were performed in databases using software tools found at http://www.Expasy.org/, and sequence alignments were constructed using clustal x software (Thompson et al., 1997).
α-Galactosidase activity assay in floral nectar of tobacco and 27 other plant species
Pooled floral nectar samples from more than ten flowers and collected from 28 different plant species (Table 1) were analysed for α-Gal activity based on the method of Borooah et al. (1961). The reaction mixtures consisted of 100 µL of 10 mm p-nitrophenyl α-d-galactopyranoside (PNPG, sigma), 80 µL of 100 mm sodium citrate buffer (pH 4·0) and either 20 µL of raw nectar sample, 20 µL of a 20 % sucrose solution or protein-free raw nectar samples. Tobacco nectar proteins were removed from the samples using ultracentrifugal filtering with a Microcon YM-3 unit. After incubation for 10 min at 25 °C, the reaction was stopped by the addition of 0·6 mL of 0·2 m sodium borate buffer (pH 9·8) and the quantity of liberated p-nitrophenol was measured at 410 nm. One unit of enzyme activity was defined as the amount of enzyme that released 1 µmol of p-nitrophenol from PNPG per minute at pH 4·0 and 25 °C. The specific activity of the enzyme was expressed as units (U) of α-Gal activity per millilitre of fresh nectar. The data presented for all α-Gal activity determinations are mean values of triplicate assays.
The effect of pH on α-Gal activity in tobacco nectar was determined using a 0·1 m citrate-phosphate buffer (pH 4·0–7·0) and 0·1 m Tris-HCl buffer (pH 8·0–9·0). The optimum temperature was determined over a range of 25 to 80 °C and the thermal stability was derived by pre-incubating nectar aliquots within the above temperature range for 10 min then applied for α-Gal assay as previously described.
Inhibition of α-Gal activity in tobacco nectar was determined by adding monosaccharides and disaccharides to the nectar aliquots during analysis. d-Galactose, d-glucose, d-fructose, d-mannose, d-xylose, lactose and sucrose were added to tobacco nectar aliquots to obtain different final concentrations for each carbohydrate condition varying from 0·5 to 8 %, and then assayed for residual enzyme activity under standard conditions. Each set of experiments was carried out in triplicate.
Determination of the effect on early fruit development after α-Gal activity inhibited in nectar
Thirty ovaries at developmental stage 11 were separated with forceps from flowers and weighed. In total, 103 stage 11 tobacco flowers were randomly selected and divided into three groups for different treatments (30 for galactose treatment, 35 for lactose and 38 for control). Forty microlitres of 15 % d-galactose and lactose were added, respectively, to the bottom of each corolla using geloader tips each having a 15-mm capillary and a defined diameter of less than 0·3 mm (Eppendorf) to avoid injury to the ovary surface for all test groups. Sugars were not added to the flowers in the control group. All flowers used in this assay were hand pollinated. Eight days after the treatment, fruits were harvested, phenotyped and weighed. Differences in fruit fresh weight between groups were tested by one-way analysis of variance (ANOVA) followed by a Bonferroni post-hoc test. Statistical significance was set at P < 0·05.
RESULTS
pH and sugars of tobacco nectar
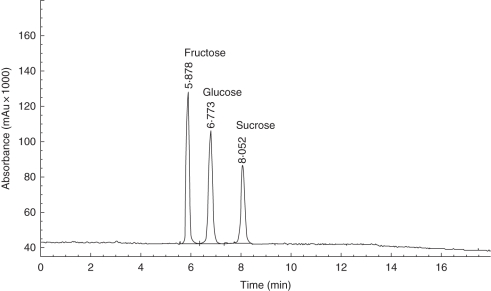
Tobacco floral nectar was acidic with a pH of 4·51 ± 0·35 (mean ± s.d., n = 30). Each flower produced 10–40 µL of nectar, similar to the amount produced by outcrossing ornamental tobacco plants (Carter et al., 1999). Phenol-sulphuric acid analysis yielded the estimates for total sugar content in these tobacco nectar samples of 195·0 ± 34·5 mg mL−1 (mean ± s.d., n = 10). HPLC showed that tobacco nectar in this study contained only glucose, fructose and sucrose at a ratio of 1 : 1 : 0·7 (Fig. 1). Neither RFOs nor d-galactose was detected by HPLC or enzyme-based analysis (data not shown).
Fig. 1.
HPLC-ELSD chromatogram of tobacco floral nectar. HPLC analysis was performed using an Agilent 1100 Elite P230 series HPLC-ELSD system equipped with a Cosmosil Sugar-D column (25 × 4·6 mm, i.d. 5 µm).
Tobacco floral nectarins
Total protein content in raw pooled tobacco nectar samples was approx. 42·2 µg mL−1. Subsequently, the total pooled nectarins were concentrated 20-fold by ultrafiltration to a final concentration of approx. 800 µg mL−1. The concentrated tobacco nectarins were separated by SDS-PAGE, which yielded six distinct bands as visualized by CBB G-250 staining under reducing conditions. The six distinct bands ranged in size from approx. 25 kDa to 100 kDa, with one major band (NTB5) separated at approximately 40 kDa (Fig. 2). The amino-terminal sequence of NTB5 was identified using Edman degradation (ED).
Fig. 2.
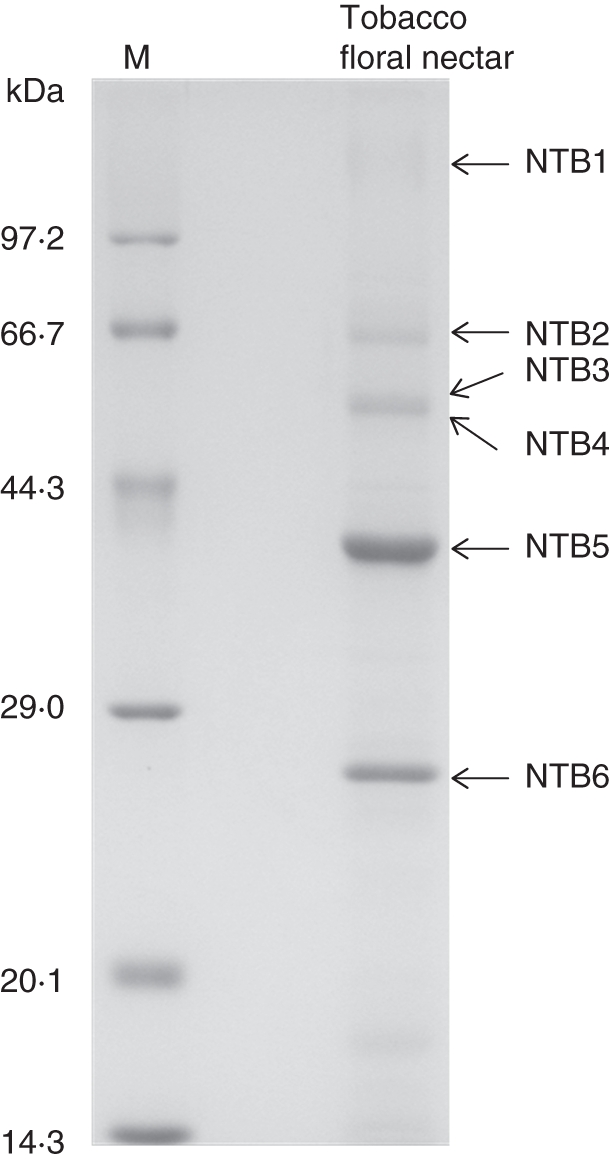
SDS-PAGE of tobacco nectar proteins. Lane 1 shows different ranges of reference proteins with the molecular masses at the standards indicated; lane 2 contains tobacco nectar proteins under reducing and denaturing conditions. The six distinguishable protein bands are labelled NTB1 to NTB6. NTB5 was selected for N-terminal protein sequence analysis.
Amino-terminal sequence of NTB5
One single N-terminal sequence was found for NTB5, confirming its purity. Analysis via blast of the ED-determined amino-terminal sequence for NTB5 (LSNGLGRTPQMGWSSWNH) revealed that the protein displayed high identity to members of the plant α-Gal gene in glycoside hydrolase family 27. Table 3 presents the alignments of the NTB5 N-terminal sequence with other plant α-Gals. The highest sequence identity (88 %) was observed at the N terminus of Solanum lycopersicum α-Gal, another plant in the Solanaceae family.
Table 3.
Alignment of the N-terminal amino acid sequence of NTB5 with other plant α-Gal sequences
| Accession no. | Species | Alignment | I (%) | P (%) |
|---|---|---|---|---|
| NTB5 | Nicotiana tabacum | 50 LSNGLGRTPQMGWSSWNH 67 | ||
| Q9SP05 | Solanum lycopersicum | 46 LGNGLGQTPQMGWSSWNH 63 | 88 | 94 |
| Q5DUH8 | Coffea arabica | 58 LANGLGLTPPMGWNSWNH 75 | 77 | 88 |
| D7TXW5 | Vitis vinifera | 43 LDNGPGQTPQMGWNSYNH 60 | 72 | 83 |
| Q9FXT4 | Oryza sativa | 56 FENGLGRTPQMGWNSWNH 73 | 83 | 94 |
| D5ADT9 | Picea sitchensis | 31 SSNGLGKTPQMGWNSWNH 48 | 83 | 94 |
| Q8RX86 | Arabidopsis thaliana | 32 MNNGLALSPQMGWNSWNH 49 | 66 | 88 |
| **.:* *****.*:** |
Completely conserved amino acids are marked with asterisks, and highly conserved amino acids are marked with dots or double dots. Gaps were not introduced to this alignment. I, the percentage of identical amino acids; P, the percentage of positive amino acids. The numbers flanking sequences are the amino acid positions in the sequence of the peptides.
NTα-Gal gene cloning and amino acid sequence analysis
The full-length coding region of the NTα-Gal gene was amplified from cDNA and gDNA using the RACE and hiTAIL-PCR methods (GenBank accession nos. HQ877670 and HQ877671; Fig. 3). Relative to the cDNA sequence, the genomic sequence of the NTα-Gal gene contained 14 introns ranging from 75 to 1041 bp (Fig. 3A). The full-length open reading frame (ORF) of NTα-Gal consisted of 1242 bp encoding 413 amino acids including a characteristic α-Gal signature (G-[LIVMFY]-x(2)-[LIVMFY]-x-[LIVM]-D-[DF]-x(1,2)-W-x(3,7)-[RV]- [DNSF]; Hofmann et al., 1999). The crucial α-Gal activity in coffee bean plants was demonstrated through site-directed mutagenesis of Trp-31 and Tyr-108 (65 and 142 in tobacco; Zhu et al., 1995, 1996). It has also been suggested that Asp-319 (321 in tobacco) plays a role in the active site of the guar plant α-Gal enzyme (Overbeeke et al., 1989). All three of the amino acids discussed above are conserved in NTα-Gal.
The deduced NTα-Gal protein sequence including the sequence obtained from direct experimental N-terminal sequencing is shown in bold in Fig. 3B. The first 49 amino acids, as determined by N-terminal sequencing, function as a signal peptide required for NTα-Gal entry into the endoplasmic reticulum and later secretion via the endo-membrane system (Vitale and Chrispeels, 1992). Homologues to this unusually long signal peptide of plant α-Gals were also identified in Phaseolus vulgaris and tomato plants, comprising 62 and 45 amino acids, respectively (Davis et al., 1997; Feurtado et al., 2001). The predicted molecular mass for mature NTα-Gal protein is 40016·57 Da, at an acidic isoelectric point of 5·33. This predicted molecular mass was consistent with the sizes observed in our SDS-PAGE data (Fig. 2) and with that of other reported plant α-Gals (Kim et al., 2002; Marraccini et al., 2005; Chrost et al., 2007; Chien et al., 2008).
Searches via the blast program of the entire predicted NTα-Gal amino acid sequence revealed significant affinity to other plant α-Gals. The highest sequence identity, 83 %, was with tomato plant α-Gal (accession no. Q9SP05) cloned and characterized from seeds (Feurtado et al., 2001) (see Supplementary Data Fig. S1, available online). These observations are consistent for all dicot α-Gals displaying very high homology in the mature protein but with apparent variation in signal peptides (Feurtado et al., 2001).
NTα-Gal expression in leaves, stems and flowers
Expression of NTα-Gal in flowers, stems and leaves was examined using semi-quantitative RT-PCR with gene-specific primers. Constitutively expressed actin was used as a reference (Fig. 4). Control reactions with no reverse transcriptase did not yield amplified PCR products (results not shown). NTα-Gal showed a similar pattern of expression in flowers, stems and leaves. This is consistent with the observation that α-Gal functions widely by hydrolysing terminal galactose residues in various tissues with different spatial and temporal expression patterns (Feurtado et al., 2001; Blöchl et al., 2008).
Fig. 4.
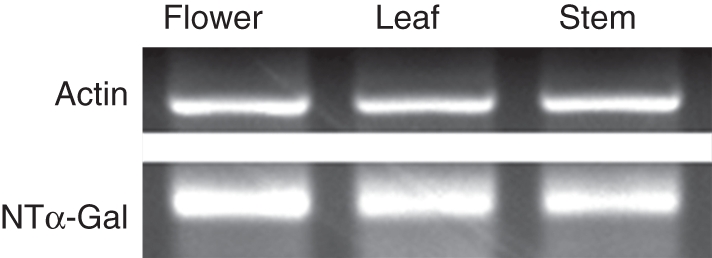
Semi-quantitative RT-PCR analysis of NTα-Gal expression in flower, stem and leaf. The housekeeping gene, actin, was used as the control.
α-Gal activity in nectar of tobacco and 27 other plant species
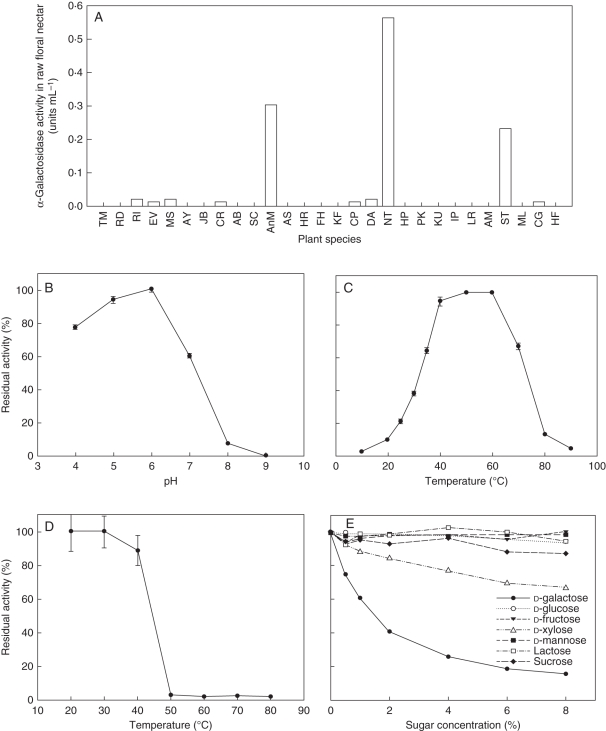
Floral nectar samples from 28 plant species (Table 1), 22 families and 13 orders were tested for α-Gal activity to determinate total nectarin concentration. As shown in Fig. 5A, ten of the 28 nectar samples showed α-Gal activity. All three species (Cestrum purpureum, Datura aurea and tobacco) from Solanaceae demonstrated α-Gal activity in nectar. Notably, tobacco nectar α-Gal activity was the highest of the ten α-Gal active species, at 0·56 U mL−1. No positive correlation between total protein concentration and α-Gal activity was detected (Table 1). Protein-free tobacco nectar samples did not demonstrate any α-Gal activity, which suggests that the observed hydrolysis activity was caused by nectarin. β-Galactosidase activity was not detected in the tobacco nectar samples (data not shown).
Fig. 5.
α—Gal activity in nectar. (A) α—Gal activity in floral nectar of 28 plant species; (B) effects of pH on NTα—Gal activity; (C) effects of temperature on NTα—Gal activity; (D) effects of temperature on NTα—Gal stability; (E) inhibitory effects of monosaccharides and disaccharides on NTα—Gal activity.
α-Gal proteins are generally classified as either acidic or alkaline, according to the optimal pH for their activities (Keller and Pharr, 1996). We observed α-Gal activity in the nectar of the common tobacco plant at various pH levels (Fig. 5B). The optimum pH for α-Gal activity was observed within the range 5·0–6·0, which was near to the natural pH range of raw tobacco nectar. This characterizes NTα-Gal as an acidic α-Gal. The effect of temperature on the enzyme activity of NTα-Gal is shown in Fig. 5C. Thermo-reactivity of α-Gal is shown in Fig. 5D, where the enzyme remained thermo-stable after pre-incubation at 40 °C for 10 min. Notably, the enzyme almost completely lost its activity after pre-incubation at 50 °C for 10 min. The optimum temperature observed to increase NTα-Gal enzymatic activity was 60 °C. Above this temperature, enzyme activity decreased rapidly. The effects of various monosaccharides and disaccharides on α-Gal activity in tobacco nectar are shown in Fig. 5E. d-Galactose is a known product and competitive inhibitor of α-Gal activity (Kachurin et al., 1995). In this study, d-galactose strongly inhibited α-Gal activity in tobacco nectar. More than half NTα-Gal activity was lost in the presence of 2 % d-galactose in the nectar aliquots. d-Xylose is a highly anomer-specific, powerful and competitive inhibitor of plant α-Gals (Sharma and Sharma, 1976). In this study, d-xylose inhibited α-Gal activity but not as effectively as d-galactose. d-Glucose, d-fructose, d-mannose, lactose and sucrose had no effect on α-Gal activity in tobacco nectar.
α-Gal activity and early tobacco fruit development
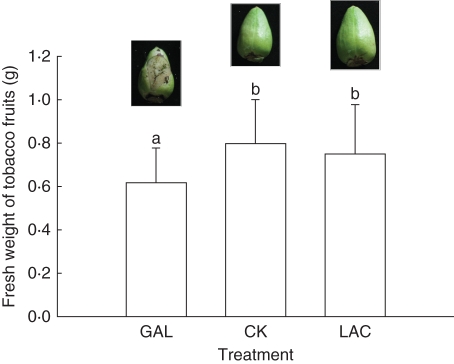
Our results showed that α-Gal activity in tobacco nectar could be inhibited by galactose and xylose but not by lactose or other tested monosaccharides and disaccharides. Also, the common tobacco flower we used began to secret nectar at developmental stage 11, which lasts less than 1 d (Koltunow et al., 1990). At stage 12, the ovary was immersed in nectar at the base of the corolla for 3–4 d (H. G. Zha, pers. observ.). d-Galactose was used to inhibit NTα-Gal activity in nectar. In the galactose-treated group, 20 of 31 fruits did not develop intact skin, resulting in partial seed exposure (Fig. 6). The average weight of the fresh fruit from the galactose-treated group (0·61 ± 0·16 g, mean ± s.d., n = 30) was significantly lower than the averages observed in the lactose-treated (0·75 ± 0·23 g, n = 35) and control groups (0·80 ± 0·20 g, n = 38) (P < 0·05) (Fig. 6). There was no significant developmental difference observed in the fresh fruit from the lactose-treated and control groups. The average weight of the young ovaries at development stage 11 was 0·05 ± 0·01 g (n = 30). Therefore, the weight of the treated ovaries increased more than ten-fold during the 8 d of analysis.
Fig. 6.
Fresh weights of common tobacco plant fruits treated with d-galactose and d-lactose. Photos of typical fruits treated with galactose, lactose and control are shown above each bar. GAL, d-galactose; CK, control; LAC, lactose. Standard errors are given and different letters indicate significant differences by Bonferroni post-hoc test at P < 0·05.
DISCUSSION
α-Gal catalyses cleavage of the terminal α-galactose residues from a wide range of substrates, including linear and branched oligosaccharides, polysaccharides and various synthetic substrates, including PNPG (Shabalin et al., 2002). This enzyme has been widely detected in micro-organisms, plants and animals. Animal α-Gal studies abound, particularly with respect to Fabry's disease, caused by a deficiency in functional α-Gal production (Peters et al., 2001); however, plant α-Gals have been less well studied. Prior to these studies, α-Gal, in higher plants, was purified and characterized mainly in seeds during and following germination (Dey and Pridham, 1972; Carchon and Bruyne, 1975; Dey and Campillo, 1984; Feurtado et al., 2001). It was generally assumed that α-Gal in endosperm is involved in the hydrolysis of wall-associated galactomannan (Bewley, 1997; Minic, 2008). In the present study, α-Gal activity was detected in the floral nectar of ten species of 28 plants, from 20 families including monocots and dicots. Of the ten species displaying α-Gal activity, raw common tobacco nectar displayed the highest α-Gal activity, although the total protein content of common tobacco nectar was only at moderate levels relative to the nectarin levels of other plants in this study. SDS-PAGE results showed that NTα-Gal was the most abundant nectarin in raw tobacco nectar at a purity exceeding 95 %, and also that NTα-Gal was the most abundant nectarin in raw tobacco nectar. Enzyme analysis established that tobacco α-Gal is an acidic α-Gal with an optimum pH of 5–6, close to the pH of raw tobacco nectar. Other relevant traits of NTα-Gal were also determined. To our knowledge, this is the first report of secreted NTα-Gal detection, cloning and characterization in floral nectar.
To date, nearly all nectarins identified appear to function in plant defence (Carter and Thornburg, 2004b; Nepi et al., 2009; Heil, 2011). But to our knowledge, to date, no reports have demonstrated that α-Gals including α-Gals of plant origin have any antimicrobial activity. Plant α-Gals could be easily expressed by bacteria or yeast with enzyme activity but showed no deleterious effects on the host (Carmi et al., 2003; Chien et al., 2008). Alternatively, plant pathogens can secret α-Gal with other polysaccharide-degrading enzymes to degrade plant cell walls during host–pathogen interactions (English et al., 1971). We had hypothesized that α-Gal played similar roles in the nectar post-secretory hydrolysis process as an invertase, as in Acacia extra floral nectar (Heil et al., 2005), but this hypothesis was rejected as trace amounts of galactose or RFOs was not detected in tobacco nectar and it is known that common tobacco is a non-RFO-accumulating species (Ayre et al., 2003). Remarkably, β-galactosidase was identified in the pollination drops of some gymnosperms, and it may function in the processes of pollen selection and development (Poulis et al., 2005). In our study, no β-galactosidase activity was detected with the p-nitrophenyl β-d-galactopyranoside substrate (data not shown) in tobacco nectar. Notably, the common tobacco flower in this study has an approx. 5-cm funnel-like corolla with partly exserted stamens, and its nectar is stored at the bottom of the corolla, making it difficult for pollinators to contaminate.
We were interested in discerning the physiological role of α-Gal, the most abundant proteinaceous component of selfing tobacco nectar, and organized our initial analysis towards that end. As the main physiological function of α-Gal is in cell-wall restructuring, our observation that the tobacco ovary is immersed in nectar during its early developmental stages and undergoes rapid volume expansion post-pollination is quite novel. Prior to these studies, it was strongly suggested that α-Gal functions in ovary development especially in the initiation of surface cell-wall changes. In our study, the d-galactose inhibition of α-Gal activity occurred at the base of the tobacco flower corolla. Remarkably, 8 d after treatment with d-galactose, 20 of 30 treated fruits showed incomplete skin development and partial seed exposure, and 20 % of the fruits were deformed. This phenotype was not observed in lactose-treated fruits nor in the control group. d-Dalactose-treated fruit fresh weights were significantly lower than those of the lactose and control groups. It has been implied that tobacco α-Gal probably acts in softening ovary skin to meet the requirement of rapid volume expansion during the early developmental stages. At this point in our analyses, we are not able to exclude the possibility that galactose acts on fruit development by other means.
Undoubtedly, the reward of floral nectar in attracting animal pollinators, particularly for strictly outcrossing plants, is to increase fecundity for the species. However, the risk of pollinator contamination promotes the production of the many PR proteins observed in floral nectar (Thornburg et al., 2003; Park and Thornburg, 2009). In many ways, the long funnel-like corolla of the common tobacco flower in our study plant functionally recapitulates the famous Pasteur's gooseneck flask, in that we observed that floral nectar like the broth in Pasteur's studies lies at the base of the flower. In this study, we observed that NTα-Gal is the most abundant nectarin in selfing common tobacco plants and that NTα-Gal is not a member of the PR proteins that might act in fruit surface cell-wall differentiation. Furthermore, we deduced that nectarin profiles of selfing and outcrossing tobacco nectar were similar (Fig. 2) but that the abundance of the corresponding proteins varied dramatically (Carter and Thornburg, 2004b, fig. 1). Remarkably, the major nectarins (Nectarin I and V) in the strictly outcrossing tobacco were PR proteins of similar molecular mass to NTB2 and NTB6 (Fig. 2). But both NTB2 and NTB6 are minor nectarins in our study. This observation may be indicative of what distinguishes nectar function in strictly selfing species. When compared with closely related outcrossing species, floral nectarin in selfing species maintain their functional significance in reproductive organ development.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We thank two anonymous reviewers for their constructive and insightful comments. This study was supported by West Light Foundation of the Chinese Academy of Sciences to H.G.Z., National Science Foundation of China (31170216 to H.G.Z.; 30625004, 40930209 to H.S.), and National 973 project (2003CB415103 to H.S.).
LITERATURE CITED
- Ayre BG, Keller F, Turgeon R. Symplastic continuity between companion cells and the translocation stream: long-distance transport is controlled by retention and retrieval mechanisms in the phloem. Plant Physiology. 2003;131:1518–1528. doi: 10.1104/pp.012054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker HG, Baker I. A brief historical review of the chemistry of floral nectar. In: Bentley B, Elias T, editors. The biology of nectaries. New York: Columbia University Press; 1983. pp. 126–152. [Google Scholar]
- Bewley JD. Breaking down the walls – a role for endo-beta-mannanase in release from seed dormancy? Trends in Plant Science. 1997;2:464–469. [Google Scholar]
- Blöchl A, Peterbauer T, Hofmann J, et al. Enzymatic breakdown of raffinose oligosaccharides in pea seeds. Planta. 2008;228:99–110. doi: 10.1007/s00425-008-0722-4. [DOI] [PubMed] [Google Scholar]
- Borooah J, Leaback DH, Walker PG. Studies on glucosamini-dase and substrates for N-acetyl-β-d-glucosaminidase. Biochemical Journal. 1961;78:106–110. doi: 10.1042/bj0780106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Carchon H, Bruyne CKD. Purification and properties of coffee-bean α-d-galactosidase. Carbohydrate Research. 1975;41:175–189. doi: 10.1016/s0008-6215(00)87017-2. [DOI] [PubMed] [Google Scholar]
- Carmi N, Zhang G, Petreikov M, et al. Cloning and functional expression of alkaline α-galactosidase from melon fruit: similarity to plant SIP proteins uncovers a novel family of plant glycosyl hydrolases. The Plant Journal. 2003;33:97–106. doi: 10.1046/j.1365-313x.2003.01609.x. [DOI] [PubMed] [Google Scholar]
- Carter C, Thornburg RW. Tobacco nectarin I: purification and characterization as a germin-like, manganese superoxide dismutase implicated in the defense of floral reproductive tissues. Journal of Biological Chemistry. 2000;275:36726–36733. doi: 10.1074/jbc.M006461200. [DOI] [PubMed] [Google Scholar]
- Carter C, Thornburg RW. Is the nectar redox cycle a floral defense against microbial attack? Trends in Plant Science. 2004a;9:320–324. doi: 10.1016/j.tplants.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Carter CJ, Thornburg RW. Tobacco Nectarin III is a bifunctional enzyme with monodehydroascorbate reductase and carbonic anhydrase activities. Plant Molecular Biology. 2004b;54:415–425. doi: 10.1023/B:PLAN.0000036373.84579.13. [DOI] [PubMed] [Google Scholar]
- Carter C, Graham RA, Thornburg RW. Nectarin I is a novel, soluble germin-like protein expressed in the nectar of Nicotiana sp. Plant Molecular Biology. 1999;41:207–216. doi: 10.1023/a:1006363508648. [DOI] [PubMed] [Google Scholar]
- Carter C, Healy R, O'Tool NM, et al. Tobacco nectaries express a novel NADPH oxidase implicated in the defense of floral reproductive tissues against microorganisms. Plant Physiology. 2007;143:389–399. doi: 10.1104/pp.106.089326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien SF, Chen SH, Chien MY. Cloning, expression, and characterization of rice α-galactosidase. Plant Molecular Biology Reporter. 2008;26:213–224. [Google Scholar]
- Chrost B, Kolukisaoglu U, Schulz B, Krupinska K. An α-galactosidase with an essential function during leaf development. Planta. 2007;225:311–320. doi: 10.1007/s00425-006-0350-9. [DOI] [PubMed] [Google Scholar]
- Davis MO, Hata DJ, Johnson SA, et al. Cloning, sequence, and expression of a blood group B active recombinant α-D-galactosidase from pinto bean (Phaseolus vulgaris) Biochemistry and Molecular Biology International. 1997;42:453–467. doi: 10.1080/15216549700202861. [DOI] [PubMed] [Google Scholar]
- Dey PM, Campillo ED. Biochemistry of the multiple forms of glycosidases in plants. Advances in Enzymology and Related Areas of Molecular Biology. 1984;56:141–249. doi: 10.1002/9780470123027.ch3. [DOI] [PubMed] [Google Scholar]
- Dey PM, Pridham JB. Biochemistry of a-galactosidases. Advanced Enzymology. 1972;36:91–130. doi: 10.1002/9780470122815.ch3. [DOI] [PubMed] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JR, Rebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Analytical Chemistry. 1956;28:350–356. [Google Scholar]
- English PD, Jurale JB, Albersheim P. Host–pathogen interactions: II. Parameters affecting polysaccharide-degrading enzyme secretion by Colletotrichum lindemuthianum grown in culture. Plant Physiology. 1971;47:1–6. doi: 10.1104/pp.47.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feurtado JA, Banik M, Bewley JD. The cloning and characterization of alpha-galactosidase present during and following germination of tomato (Lycopersicon esculentum Mill.) seed. Journal of Experimental Botany. 2001;52:1239–1249. [PubMed] [Google Scholar]
- González-Teuber M, Eilmus S, Muck A, Svatos A, Heil M. Pathogenesis-related proteins protect extrafloral nectar from microbial infestation. Plant Journal. 2009;58:464–473. doi: 10.1111/j.1365-313X.2009.03790.x. [DOI] [PubMed] [Google Scholar]
- González-Teuber M, Pozo MJ, Muck A, et al. Glucanases and chitinases as causal agents in the protection of acacia extrafloral nectar from infestation by phytopathogens. Plant Physiology. 2010;152:1705–1715. doi: 10.1104/pp.109.148478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil M. Nectar: generation, regulation and ecological functions. Trends in Plant Science. 2011;16:191–200. doi: 10.1016/j.tplants.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Heil M, Rattke J, Boland W. Postsecretory hydrolysis of nectar sucrose and specialization in ant/plant mutualism. Science. 2005;308:560–563. doi: 10.1126/science.1107536. [DOI] [PubMed] [Google Scholar]
- Herrera CM, Garcia IM, Perez R. Invisible floral larcenies: microbial communities degrade floral nectar of bumble bee-pollinated plants. Ecology. 2008;89:2369–2376. doi: 10.1890/08-0241.1. [DOI] [PubMed] [Google Scholar]
- Hofmann K, Bucher P, Falquet L. The PROSITE database, its status in 1999. Nucleic Acids Research. 1999;27:215–219. doi: 10.1093/nar/27.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachurin AM, Golubev AM, Geisow MM, Veselkina OS, Isaeva-Ivanova LS, Neustroev KN. Role of methionine in the active site of alpha-galactosidase from Trichoderma reesei. Biochemical Journal. 1995;308:955–964. doi: 10.1042/bj3080955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keitt GW, Ivanoff SS. Transmission of fire blight by bees and its relation to nectar concentration of apple and pear blossoms. Journal of Agriculture Research. 1941;62:745–753. [Google Scholar]
- Keller F, Pharr DM. Metabolism of carbohydrates in sinks and sources: galactosyl-sucrose oligosaccharides. In: Zamski E, Schaffer AA, editors. Photoassimilate distribution in plants and crops. New York: Marcel Dekker; 1996. pp. 157–183. [Google Scholar]
- Kim WD, Kobayashi O, Kaneko S, et al. alpha-Galactosidase from cultured rice (Oryza sativa L. var. Nipponbare) cells. Phytochemistry. 2002;61:621–630. doi: 10.1016/s0031-9422(02)00368-0. [DOI] [PubMed] [Google Scholar]
- Koltunow AM, Truettner J, Cox KH, Wallroth M, Goldberg RB. Different temporal and spatial gene-expression patterns occur during anther development. Plant Cell. 1990;2:1201–1224. doi: 10.1105/tpc.2.12.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kram B, Bainbridge E, Perera MA, Carter C. Identification, cloning and characterization of a GDSL lipase secreted into the nectar of Jacaranda mimosifolia. Plant Molecular Biology. 2008;68:173–183. doi: 10.1007/s11103-008-9361-1. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Liu YG, Chen Y. High-efficiency thermal asymmetric interlaced PCR for amplification of unknown flanking sequences. Biotechniques. 2007;43:649–656. doi: 10.2144/000112601. [DOI] [PubMed] [Google Scholar]
- Marraccini P, Rogers WJ, Caillet V, et al. Biochemical and molecular characterization of alpha-D-galactosidase from coffee beans. Plant Physiology and Biochemistry. 2005;43:909–920. doi: 10.1016/j.plaphy.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Minic Z. Physiological roles of plant glycoside hydrolases. Planta. 2008;227:723–740. doi: 10.1007/s00425-007-0668-y. [DOI] [PubMed] [Google Scholar]
- Nepi M, von Aderkas P, Wagner R, Mugnaini S, Coulter A, Pacini E. Nectar and pollination drops: how different are they? Annals of Botany. 2009;104:205–219. doi: 10.1093/aob/mcp124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson S, Thornburg RW. Nectar chemistry. In: Pacini E, Nepi M, Nicolson S, editors. Nectary and nectar: a modern treatise. Amsterdam: Springer; 2007. pp. 215–263. [Google Scholar]
- Overbeeke N, Fellinger AJ, Toonen MY, D van Wassenaar, Verrips CT. Cloning and nucleotide sequence of the α-galactosidase cDNA from Cyamopsis tetragonoloba (guar) Plant Molecular Biology. 1989;13:541–550. doi: 10.1007/BF00027314. [DOI] [PubMed] [Google Scholar]
- Park S, Thornburg R. Biochemistry of nectar proteins. Journal of Plant Biology. 2009;52:27–34. [Google Scholar]
- Peters FPJ, Vermeulen A, Kho TL. Anderson–Fabry's disease: α galactosidase deficiency. The Lancet. 2001;357:138–140. doi: 10.1016/s0140-6736(00)03554-6. [DOI] [PubMed] [Google Scholar]
- Poulis BAD, O'Leary SJB, Haddow JD, von Aderkas P. Identification of proteins present in the Douglas fir ovular secretion: an insight into conifer pollen selection and development. International Journal of Plant Sciences. 2005;166:733–739. [Google Scholar]
- Shabalin KA, Kulminskaya AA, Savel'ev AN, Shishlyannikov SM, Neustroev KN. Enzymatic properties of α-d-galactosidase from Trichoderma reesei in the hydrolysis of galactooligosaccharides. Enzyme and Microbial Technology. 2002;30:231–239. [Google Scholar]
- Sharma TN, Sharma CB. d-Xylose, an anomer-specific inhibitor of alpha-galactosidase. Phytochemistry. 1976;15:643–646. [Google Scholar]
- Sicard A, Lenhard M. The selfing syndrome: a model for studying the genetic and evolutionary basis of morphological adaptation in plants. Annals of Botany. 2011;107:1433–1443. doi: 10.1093/aob/mcr023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG. The Clustal X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornburg RW, Carter C, Powell A, Mittler R, Rizhsky L, Horner HT. A major function of the tobacco floral nectary is defense against microbial attack. Plant Systematics and Evolution. 2003;238:211–218. [Google Scholar]
- Vitale A, Chrispeels MJ. Sorting of proteins to the vacuoles of plant cells. BioEssays. 1992;14:151–160. doi: 10.1002/bies.950140303. [DOI] [PubMed] [Google Scholar]
- Zelitch I, Day PR. The effect on net photosynthesis of pedigree selection for low and high rates of photorespiration in tobacco. Plant Physiology. 1973;52:33–37. doi: 10.1104/pp.52.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu A, Wang ZK, Goldstein J. Identification of tyrosine-108 in coffee-bean alpha-galactosidase as an essential residue for the enzyme-activity. Biochimica et Biophysica Acta. 1995;1247:260–264. doi: 10.1016/0167-4838(94)00228-9. [DOI] [PubMed] [Google Scholar]
- Zhu A, Monahan C, Wang ZK. Trp-16 is essential for the activity of alpha-galactosidase and alpha-n-acetylgalactosaminidase. Biochimica et Biophysica Acta. 1996;1297:99–104. doi: 10.1016/0167-4838(96)00108-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.