Abstract
Environmental particulate matter (PM) pollutants adversely affect human health, but the molecular basis is poorly understood. The ion channel transient receptor potential vanilloid-1 (TRPV1) has been implicated as a sensor for environmental PM and a mediator of adverse events in the respiratory tract. The objectives of this study were to determine whether TRPV1 can distinguish chemically and physically unique PM that represents important sources of air pollution; to elucidate the molecular basis of TRPV1 activation by PM; and to ascertain the contributions of TRPV1 to human lung cell and mouse lung tissue responses exposed to an insoluble PM agonist, coal fly ash (CFA1). The major findings of this study are that TRPV1 is activated by some, but not all of the prototype PM materials evaluated, with rank-ordered responses of CFA1 > diesel exhaust PM > crystalline silica; TRP melastatin-8 is also robustly activated by CFA1, whereas other TRP channels expressed by airway sensory neurons and lung epithelial cells that may also be activated by CFA1, including TRPs ankyrin 1 (A1), canonical 4α (C4α), M2, V2, V3, and V4, were either slightly (TRPA1) or not activated by CFA1; activation of TRPV1 by CFA1 occurs via cell surface interactions between the solid components of CFA1 and specific amino acid residues of TRPV1 that are localized in the putative pore-loop region; and activation of TRPV1 by CFA1 is not exclusive in mouse lungs but represents a pathway by which CFA1 affects the expression of selected genes in lung epithelial cells and airway tissue.
Introduction
Environmental particulate matter (PM) air pollution is a heterogeneous mixture of geometrically and chemically diverse solids with adsorbed chemicals produced mainly by suspension of geological and road materials, combustion of solid fuels, and secondary atmospheric chemical reactions (Lighty et al., 2000). PM in the size range of 2.5 to 10 μm constitutes the majority of urban air pollution mass, and these materials deposit throughout the respiratory tract, where they can produce serious adverse effects, including tissue damage and local and systemic changes in physiology. Even slight (10 μg/m3) increases in ambient PM can trigger airway inflammation and injury, reflected clinically as airway irritation and cough and epidemiologically as increased rates of hospitalization and premature death, largely attributable to acute cardiopulmonary dysfunction (Bernstein et al., 2004; Ward and Ayres, 2004). Not all people are similarly affected by PM. Developing fetuses (Glinianaia et al., 2004), infants and children (Ward and Ayres, 2004; Chow et al., 2006), elderly persons (Pope et al., 2008), and persons with pre-existing respiratory or heart disease (Bernstein et al., 2004; Pope et al., 2008) are significantly more sensitive. However, the molecular basis for adverse responses to PM remains unclear.
Transient receptor potential vanilloid-1 (TRPV1), a calcium ion channel activated by the botanical irritant capsaicin, endovanilloids, H+, organic acids, and temperature >42°C (Jia et al., 2005; Bessac and Jordt, 2008), has been implicated as a potential mediator of cellular responses to PM in the lung, because inhibition of TRPV1 substantially reduces some of the acute adverse outcomes associated with PM inhalation exposure (Veronesi et al., 1999a,b; Oortgiesen et al., 2000; Agopyan et al., 2003a,b; Wong et al., 2003; Ghelfi et al., 2008). TRPV1 is also expressed by epithelial cells of the major airways and alveoli, and activation of TRPV1 in these cells by PM has been correlated with the production of immunomodulatory cytokines and chemokines including IL-6, IL-8 and TNFα, and cell death in vitro (Veronesi et al., 1999a,b; Oortgiesen et al., 2000; Agopyan et al., 2003a,b; Reilly et al., 2003, 2005; Thomas et al., 2011).
Many acute adverse responses to concentrated urban PM have also been associated with activation of capsaicin-sensitive (i.e., TRPV1-positive) bronchopulmonary C-fiber neurons that initiate pulmonary reflex responses, including cough and reduced lung compliance, as well as edema. These neurons release substance P and neurokinin A, among other substances, that promote abrupt changes in cardiopulmonary function and lung-vascular fluid barrier function and stimulate pro-inflammatory mediator production by non-neuronal cells (Reilly, 2010).
Although existing literature strongly supports a role for TRPV1 as a mediator of several major adverse effects of PM in the lung and on respiratory function, it is not clear how PM interacts with TRPV1 to elicit such responses, whether TRPV1 is a selective or indiscriminate sensor of environmental PM (i.e., do other TRP or calcium channels also detect PM and promote toxicity?), or whether activation of TRPV1 is directly coupled with processes that influence deleterious changes in airway homeostasis. The objectives of this study were to determine whether TRPV1 could distinguish chemically and physically unique forms of PM, to elucidate the molecular basis of TRPV1 activation by such PM, and to ascertain the contributions of TRPV1 to potentially deleterious lung cell and tissue responses elicited by treatment with a moderately selective PM agonist.
Materials and Methods
Chemicals and Reagents.
n-Vanillylnonanamide (nonivamide; a capsaicin analog), (−)-menthol, icilin, allyl isothiocyanate (AITC), hydrogen peroxide (H2O2; 30%), carvacrol, Δ9-tetrahydrocannabinol, N-(1-((4-(2-(((2,4-dichlorophenyl)sulfonyl)amino)-3-hydroxypropanoyl)-1-piperazinyl)carbonyl)-3-methylbutyl)-1-benzothiophene-2-carboxamide (GSK1016790A), carbachol (carbamoyl choline), ionomycin, EGTA, and ruthenium red were purchased from Sigma-Aldrich (St. Louis, MO), and N-(4-tert-butylbenzyl)-N′-(1-[3-fluoro-4-(methylsulfonylamino)phenyl]ethyl)thiourea (LJO-328) was synthesized by author J.L. The structure of LJO-328 is also available in Reilly et al. (2005). H2O2 concentrations were determined spectrophotometrically using the extinction coefficient ε240 = 39.4 · M−1 · cm−1. PCR primers were synthesized by the University of Utah DNA synthesis core facility.
Cells and Cell Culture.
Immortalized human bronchial epithelial cells (BEAS-2B) were purchased from the American Type Culture Collection (Manassas, VA). TRPV1-overexpressing BEAS-2B (TRPV1-OE) cells were generated as described previously (Reilly et al., 2003). BEAS-2B and TRPV1-OE cells were cultured in LHC-9 media (Invitrogen, Carlsbad, CA). Primary normal human bronchial epithelial cells (NHBE), were purchased from Lonza Walkersville, Inc. (Walkersville, MD), and were cultured in bronchial epithelial cell growth medium. Culture flasks for all three cell types were precoated with LHC basal media fortified with 30 μg/ml collagen, 10 μg/ml fibronectin, and 10 μg/ml bovine serum albumin. Cells were maintained between 30 and 90% maximum density in a humidified incubator at 37°C with a 5% CO2/95% air atmosphere, and were subcultured using trypsin.
Particulate Matter.
Six chemically and physically different combustion-derived, soil-derived, and manufactured PM, representative of PM that is found in ambient air, were selected as a test set for identifying TRPV1 agonists, based on their environmental relevance, and variable effects on lung cells and/or tissue, as described by our group and others (Veronesi et al., 1999a,b; Agopyan et al., 2003a,b; Veranth et al., 2004, 2006, 2008; Smith et al., 2006; Deering-Rice et al., 2011). CFA1 is a size-fractionated sample of coal fly ash collected from a power plant in Utah. This material is derived from combustion of low-sulfur bituminous coal and represents “real-world” coal ash emitted directly from combustion of coal and/or from physical breakdown of CFA-fortified pavement and cement. CFA1 is principally insoluble mineral oxides and salts of silicon (12.4%), calcium (5.6%), aluminum (4.9%), and iron (4.2%), partially as sulfates (4%), with ∼3% elemental carbon and ∼1% unspecified organic carbon (percentages by weight) (Smith et al., 2006), presumably polycyclic aromatic hydrocarbons as described for other CFA samples (Cao et al., 2001). CFA2 (20% elemental carbon) was generated in a laboratory-scale research furnace by combustion of coal under low air conditions. The mineral ash component of CFA2 is similar to that of CFA1 in shape and size, but CFA2 also contains agglomerates of submicron soot particles and irregular particles of unburned coal char. Diesel exhaust PM (DEP) was collected from scraping the tailpipe of an in-service 2004 Ford F350 “black smoker” truck and represents a high-soot-content PM from a poorly functioning but environmentally relevant diesel engine. The DEP sample consists of loose agglomerates of submicron primary particles and is comparable with National Institute of Standards and Technology standard reference material 2975 with respect to its ability to activate TRPA1 and other TRP channels (Deering-Rice et al., 2011). Crystalline silica (MUS) is Min-U-Sil 5 (5 μm) (US Silica, Mill Creek, OK). MUS represents silica PM produced by mechanical grinding of mineral material and a redox-active PM. Details on the elemental composition of CFA1 and scanning electron micrographs of CFA1, CFA2, DEP, and MUS can be found in Deering-Rice et al. (2011) and Smith et al. (2006). Desert dust (DD) is a calcium-rich dust that was collected from an arid and sparsely vegetated area south of the salt flats in Tooele County, UT (Veranth et al., 2004). DD PM was size-fractionated to be 2.5 to 10 μm by using a rotary tumbler connected to an Anderson cascade impactor. Details on DD composition can be found in Veranth et al. (2004), and nanosilica (nSiO2; 10 nm, spherical) is a manufactured amorphous material (Nanostructured and Amorphous Materials, Los Alamos NM).
TRP Channel Cloning, TRP Channel Overexpression, and Site-Directed Mutagenesis.
Human TRPA1, -C4α, -M8, the N-terminal truncated TRPM8Δ1–801 variant, -V1, -V2, -V3, and -V4 were cloned as described previously (Reilly et al., 2003; Sabnis et al., 2008; Deering-Rice et al., 2011). Sequence verified plasmid DNA was transfected into HEK-293 cells grown to confluence in 96-well culture dishes precoated for 2 h with 1% gelatin using 175 ng/well plasmid DNA and Lipofectamine 2000 (Invitrogen) at a 2:1 lipid/DNA ratio in 50 μl of Opti-MEM media (Invitrogen) for 4 h upon which 100 μl of Dulbecco's modified Eagle's medium/F12 medium containing 5% fetal bovine serum was added for an additional 20 h. The media were replaced, and cells were assayed 24 h later or selected for stable overexpression by diluting and culturing in media containing 400 μg/ml G418 (Geneticin). Cells stably overexpressing TRPA1, -C4α,- V2-, V3, -V4, and -M8 were generated in our laboratory as described previously (Deering-Rice et al., 2011), whereas TRPM2-overexpressing cells were provided by Dr. Yasuo Mori (Kyoto University, Kyoto, Japan). TRPV1 and the TRPM8Δ1–801 variant were transiently overexpressed in HEK-293 using the methods described above, and TRPV1 was stably overexpressed in BEAS-2B cells as described previously (Reilly et al., 2003). Site-directed mutagenesis of TRPV1 was performed using the QuikChange XL kit (Stratagene, La Jolla, CA), and mutants were compared with wild-type receptors using transient overexpression in HEK-293 cells with normalization of data using nonivamide (25 μM) as the agonist.
Fluorometric Calcium Flux Assays.
Cells were subcultured into 96-well plates, grown to confluence, and loaded with a membrane-permeable fluorogenic Ca2+ indicator, Fluo 4 acetoxymethyl ester, using the Fluo-4 Direct Calcium Assay kit (Invitrogen). TRPV1-overexpressing BEAS-2B cells were loaded for 60 min at room temperature, whereas HEK-293 cells were loaded at 37°C, according to the supplier's protocol. After loading, cells were washed once and incubated in the dark for an additional 15 to 30 min in LHC-9 media fortified with 0.75 mM water-soluble probenecid (Invitrogen) and 0.75 mM trypan red (AAT Bioquest, Sunnyvale, CA). Changes in cellular fluorescence in response to treatments (ΔF) were assessed microscopically at room temperature (∼23°C) as detailed previously (Deering-Rice et al., 2011). In brief, cells were treated with soluble agonists or insoluble particle suspensions by applying a 3× concentrate to achieve the final concentrations listed in the figure legends. Fluorescence images were collected at time = 0 and at 30-s intervals for 3 min. The average value for change in fluorescence at each time point was determined using an image analysis program, and these values were corrected for background and were further normalized to the maximum attainable response elicited by treatment of the cells with ionomycin (10 μM). In some instances, these data were further normalized to the response elicited by the prototypical TRP channel agonist. To be clear, the final PM concentration listed for each figure represents the concentration of the suspension of particles in the treatment well, not the actual dose at the cell surface, which varied over the course of the 3-min treatment as a result of particle settling. Data in the figures represents the change in fluorescence (ΔF) measured between time = 0 and 3 min.
Cell Surface TRPV1 Enrichment, Cell Surface Protein Isolation, and Immunoblotting.
Pretreatment of TRPV1-OE cells for 24 h with the TRPV1 antagonist LJO-328 at 5 μM, as described previously (Johansen et al., 2006), was used to enrich TRPV1 at the cell surface to determine whether CFA1 preferentially activated TRPV1 at the cell surface, as suggested by prior studies of TRPV1-PM interactions (Oortgiesen et al., 2000; Veronesi et al., 2002, 2003; Agopyan et al., 2003a,b, 2004), or if CFA1 uptake or CFA1-derived materials released into the treatment media were the agonists. Cell surface proteins were isolated from two confluent T-75 flasks of cells/group using the Pierce Sulfo-NHS-SS-Biotin [sulfosuccinimidyl 2-(biotinamido)-ethyl-1,3-dithiopropionate] Cell Surface Protein Isolation kit (Thermo Fisher Scientific, Waltham, MA), as instructed by the manufacturer's protocol. Protein concentration was determined using the bicinchoninic acid assay kit (Thermo Fisher Scientific) and equal quantities of protein from control and LJO-328 pretreated cells were resolved by electrophoresis on a 10% NuPAGE gel (Invitrogen) and subsequently transferred to polyvinylidene difluoride for detection of TRPV1. The membranes were blocked for 24 h using 5% (w/v) dry milk and 2.5% goat serum in PBS buffer containing 0.1% (v/v) Tween 20 (PBS-T), then probed using a polyclonal rabbit-anti-TRPV1 antibody raised against amino acid residues 7 to 21 of human TRPV1 (TDLGAAADPLQKDTC) (Abcam, Cambridge, MA) diluted 1:2000 in blocking buffer. The membranes were washed twice with PBS-T and probed overnight at 4°C with a goat-anti-rabbit horseradish peroxidase-conjugated secondary antibody (Abcam) diluted 1:2000 in blocking buffer. After washing an additional two times with PBS-T, the membrane were developed using the ECL Plus chemiluminescent reagent (Thermo Fisher Scientific). Relative band intensities were compared using a GelDoc Image analysis system (Bio-Rad Laboratories, Hercules, CA).
PCR Analysis of Gene Expression in Human Cells.
Cells were subcultured into 6-well (TRPV1-OE) or 12-well (NHBE) cell culture plates, grown to ∼90% density, and treated for 4 h (TRPV1-OE) or 24 h (NHBE) at 37°C. Total RNA was extracted from cells using the RNeasy mini kit (QIAGEN, Valencia, CA), and 2.5 μg of the total RNA was converted to cDNA using SuperScript III (Invitrogen). The resulting cDNA was either assayed for gene expression using multiplex PCR followed by agarose gel electrophoresis and gel densitometry, as described previously (Reilly et al., 2003, 2005; Johansen et al., 2006), or diluted 1:50 for analysis by quantitative real-time PCR (qPCR). qPCR was performed using LightCycler 480 SYBR Green I Master Mix (Roche, Indianapolis, IN) with a LightCycler 480 System. The PCR program consisted of 10 min incubation at 95°C, followed by 40 cycles of 95°C for 15s, 55°C for 30s, then 72°C for 30s. Experiments were performed in triplicate with a copy number standard curve for both the normalization gene (β2 macroglobulin, β2M) and the gene products of interest (GADD153, IL-6, and IL-8). Primer sequences are given in Table 1.
TABLE 1.
Primer sequences
| Primer | Sequence (5′→3′) |
|---|---|
| Human | |
| β2 macroglobulin | |
| Sense | GATGAGTATGCCTGCCGTGTG |
| Antisense | CAATCCAAATGCGGCATCT |
| GADD153 | |
| Sense | AGAACCAGGAAACGGAAACAGA |
| Antisense | TCTCCTTCATGCGCTGCTTT |
| IL-6 | |
| Sense | AACCTGAACCTTCCAAAGATGG |
| Antisense | TCTGGCTTGTTCCTCACTACT |
| IL-8 | |
| Sense | ACTGAGAGTGATTGAGAGTGGAC |
| Antisense | AACCCTCTGCACCCACTTTTC |
| Mouse | |
| CCSP | |
| Sense | ATGAAGATCGCCATCACAATCAC |
| Antisense | GGATGCCACATAACCAGACTCT |
| SP-A | |
| Sense | GAGGAGCTTCAGACTGCACTC |
| Antisense | AGACTTTATCCCCCACTGACAG |
| TRPV1 | |
| Sense | CATCTTCACCACGGCTGCTTAC |
| Antisense | CAGACAGGATCTCTCCAGTGAC |
| GADD153 | |
| Sense | GAACGAGCGGAAAGTGGCA |
| Antisense | CATGCGGTCGATCAGAGCC |
| GAPDH | |
| Sense | AGGTCGGTGTGAACGGATTTG |
| Antisense | TGTAGACCATGTAGTTGAGGTCA |
| CXCL1/KC | |
| Sense | CTGGGATTCACCTCAAGAACATC |
| Antisense | CAGGGTCAAGGCAAGCCTC |
| CXCL2/MIP-2α | |
| Sense | CCAACCACCAGGCTACAGG |
| Antisense | GCGTCACACTCAAGCTCTG |
| IL-6 | |
| Sense | TAGTCCTTCCTACCCCAATTTCC |
| Antisense | TTGGTCCTTAGCCACTCCTTC |
CCSP, clara cell-specific protein; SP-A, surfactant protein A.
PM Instillation.
Animals were maintained at the University of Utah vivarium. Food and water were available ad libitum. All experiments were approved by the University of Utah Institutional Animal Care and Use Committee, in accordance with the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996). Mice (CF1 (Charles River Laboratories, Wilmington, MA), C57BL/6 (The Jackson Laboratory, Bar Harbor, ME), and TRPV1(−/−) (The Jackson Laboratory) weighing ∼20 to 25 g were anesthetized using ketamine (50 mg/kg) + xylazine (10 mg/kg i.p.) and suspended vertically at ∼30° to 45° angle on an immobilizer. The larynx was visualized by gently grasping tongue with tweezers and inserting an otoscope into the mouth; treatment solutions were slowly dispensed into the tracheal opening. Mice were instilled with 25 μl of sterile PBS, nonivamide (0.5 mg/ml in 4% ethanol), or a suspension of 10 mg/ml CFA1 in sterile PBS (250 μg of total PM or ∼10 mg/kg). Mice were maintained in the elevated position for ∼2 min after instillation and returned to their cages for 4 h, at which time they were terminally anesthetized by injection of pentobarbital (100 mg/kg i.p.) and exsanguinated via the abdominal aorta. The lungs and trachea were accessed and processed for gene expression by qPCR and qualitative histological analysis. It is noteworthy that the 250 μg i.t. dose used in this study substantially higher than what occurs through most short-term environmental exposures, but it is within the range used previously for intrathecal instillations of DEP, CFA1-like materials, and other PM materials (Ogugbuaja et al., 2001; Ernst et al., 2002; Takano et al., 2002a,b; Gilmour et al., 2004; Yokota et al., 2008; Costa et al., 2010) and could approximate a total daily dose under some circumstances. Regardless, this exposure should allow a general assessment of whether TRPV1 plays an integral role in mediating select toxicologically relevant outcomes in the mouse lung or not.
Gene Expression Analysis in Mouse Lung Tissue.
Lungs were accessed and inflated with ∼300 μl of RNALater solution (Ambion, Austin, TX) using a syringe. The trachea was tied closed, and the trachea, bronchi, and lungs were removed and placed in RNALater at 4°C overnight and subsequently stored at −80°C. Total RNA was isolated using the TRIzol-Plus Total RNA isolation kit (Invitrogen) and converted to cDNA using the SuperScript III cDNA synthesis kit (Invitrogen). Quantitative PCR was performed using 2.5 μl of cDNA (diluted 1:50) in RT2 SYBR Green qPCR Master Mix (SA Biosciences, Frederick MD) and a Roche LightCycler 480 instrument; a PCR was program used that consisted of 10-min incubation at 95°C, followed by 40 cycles of 95°C for 15s and 63°C for 60s. Copy number standards were used for all genes. Expression of clara cell-specific protein and surfactant protein-A was used to confirm enrichment of bronchioles and alveolar in dissected tissue and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used to normalize expression data. Primers used are given in Table 1.
Histology.
Lungs were accessed and the trachea was cannulated with a blunt-ended needle; the lungs were inflated with 10% neutral-buffered formalin at ∼20 cm fixed pressure. The lungs were fixed for ∼5 min, the trachea was tied off, and the entire respiratory tract was removed and placed in 10% neutral buffered formalin. Cross sections (∼4–5 mm thick) from the bronchial insertion point to the lower portion of the left lobe were taken and used to prepare serial 5-μm sections for staining with hematoxylin and eosin. Tissue sectioning and staining was performed by ARUP Laboratories (Salt Lake City, UT).
Airway Segmentation.
Male CF1 mice were treated with nonivamide [0.5 mg/kg i.t.; PBS containing 4% (v/v) ethanol] or CFA1 (250 μg), and the respiratory tissue was inflated with RNALater, collected as described above, and stored at 4°C. Within 48 h, the lungs were dissected in a bath of RNALater and the trachea, main bronchi, bronchioles from the main bronchus (generation 3) into the parenchyma (as far as possible), and alveolar regions were collected from the left lobe. Whole-lung samples were the right cranial and middle lobes. Tissues were stored at −80°C until mRNA isolation using the TRIzol-Plus kit (Invitrogen), as described above. Before PCR analysis, mRNA from these samples was amplified. In brief, total RNA (1 μg) was amplified using the Message Amp II RNA amplification kit (Ambion), and as above, amplified RNA (1 μg) was converted to cDNA using the SuperScript III cDNA synthesis kit (Invitrogen) and assayed for gene expression by quantitative PCR.
Results
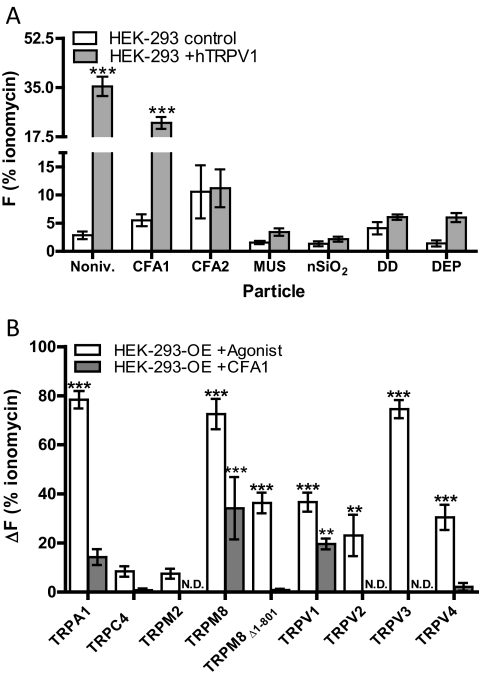
Identification of environmental PM agonists of TRPV1 was achieved by measuring calcium influx into HEK-293 cells transiently transfected with human TRPV1 (Fig. 1A). The known TRPV1 agonist nonivamide was used as positive control, and ionomycin was used to assess maximum cell fluorescence and to normalize the results for the various treatments, as described under Materials and Methods. PM treatments were applied at a concentration of 0.73 mg/ml, which in previous experiments has been shown to produce a maximum TRP-dependent response (i.e., control HEK-293 cells did not exhibit a response). CFA1 produced a significant increase in calcium in TRPV1-expressing HEK-293 cells that was ∼4-fold that observed in the control HEK-293 cells. DEP, as reported previously (Deering-Rice et al., 2011), and MUS also exhibited weak agonist activity, but the responses were not statistically significant. CFA2, DD, and nanosilica (nSiO2) did activate TRPV1.
Fig. 1.
A, nonivamide, CFA1, MUS, and DEP activate TRPV1 and cause calcium influx (ΔF) into TRPV1-expressing HEK-293 cells (gray bars) versus control HEK-293 cells (white bars). Nonivamide (Noniv.; positive control for TRPV1) was applied at 25 μM for 1 min, and PM was applied at 0.73 mg/ml for 3 min. Data are the mean and SEM (n ≥ 3) for ΔF relative to ionomycin (10 μM) and asterisks indicate a statistical difference between control HEK-293 cells and TRPV1-overexpressing HEK-293 cells when treated with PM using two-way ANOVA and post-testing using the Bonferroni multiple comparisons test. B, CFA1 activates TRPV1, TRPM8, and TRPA1. HEK-293 cells transiently (TRPV1 and TRPM8Δ1–801) or stably (all others) overexpressing human TRP channels were treated with a prototype agonist for the specific receptor (white bars) or CFA1 at 0.73 mg/ml for 3 min (gray bars). The prototype agonists were used at a concentration determined to yield a maximum receptor-specific response: TRPA1 (AITC, 150 μM); TRPC4α (carbachol, 0.75 μM); TRPM2 (H2O2, 1 mM); TRPM8 (icilin, 50 μM); TRPM8Δ1–801 [(−)-menthol, 2500 μM]; TRPV1 (nonivamide, 25 μM); TRPV2 (THC, 100 μM); TRPV3 (carvacrol, 250 μM); and TRPV4 (GSK1016790A, 0.0125 μM). Data are the mean and S.E.M. (n ≥ 3) for ΔF relative to ionomycin (10 μM), and asterisks indicate a statistically significant response in overexpressing cells versus HEK-293 cells treated with either a prototypical receptor agonist or CFA1 using 2-way ANOVA and post-testing using the Bonferroni multiple comparisons test. **, p < 0.01; ***, p < 0.001; N.D. = no response detected.
The selectivity of CFA1 for TRPV1 was evaluated by assessing calcium flux in control HEK-293 cells versus HEK-293 cells transfected to overexpress TRPA1, -C4α, -M2, -M8, the TRPM8Δ1–801 variant, -V2, -V3, or -V4. Quantitative results for calcium flux elicited by the various channel-specific positive controls and CFA1 are shown in Fig. 1B. CFA1 was an agonist for human TRPV1, -M8, and to a lesser extent, -A1, a result described previously for TRPA1 (Deering-Rice et al., 2011). Activation of TRPV1 by CFA1 produced a response 54 ± 7% that of the prototype agonist nonivamide, a response 47 ± 16% that of the prototype TRPM8 agonist icilin, and 18 ± 5% that of the prototype TRPA1 agonist AITC. Activation of TRPC4α, -M2, and -V2 to -V4 by CFA1 was not observed.
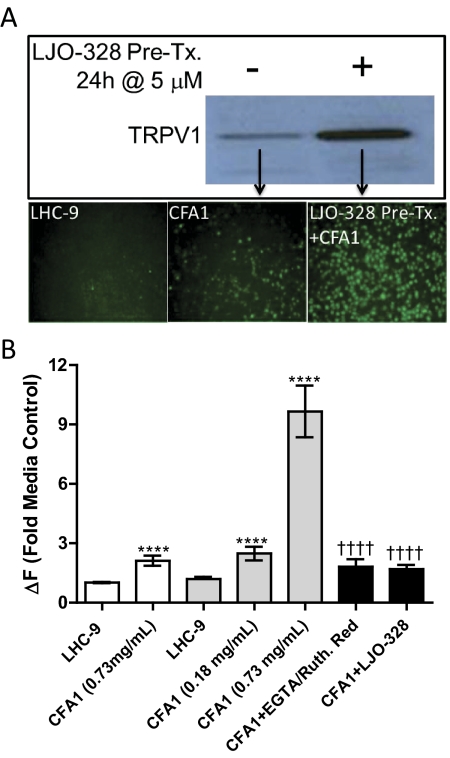
The mechanism of TRPV1 activation by CFA1 was pursued further because of its robust response relative to nonivamide and the postulated role of TRPV1 in mediating respiratory injury by PM. The nature of the agonist responsible for CFA1-induced calcium flux (i.e., extracellular versus intracellular CFA1 or insoluble versus soluble CFA1 components) was compared in TRPV1-OE cells with and without LJO-328 pretreatment to increase the cell-surface abundance of TRPV1 (Johansen et al., 2006). LJO-328-pretreated cells exhibited an increase of approximately 6-fold in immunoreactive TRPV1 protein on the cell surface and were ∼5-fold more responsive to CFA1 than nonpretreated cells (Fig. 2, A and B). CFA1-induced calcium flux in the LJO-328 pretreated TRPV1-OE cells was inhibited by cotreating cells with CFA1 and the TRPV1 antagonist LJO-328 as well as the nonselective cell surface calcium channel blocking agents EGTA and ruthenium red (Fig. 2B). Manipulation of CFA1 by washing and solvent extraction was also used to illustrate that the solid components of CFA1 were the agonist of TRPV1. CFA1 recovered after overnight incubation and repeated (3X) washing with LHC-9 elicited a response that was 117 ± 19% that of CFA1 freshly suspended in LHC-9 (100 ± 23%) (p > 0.05), and the clarified overnight media extract did not have agonist activity. Likewise, CFA1 washed three times for 1 h each with 1 ml of 1:1 chloroform/methanol elicited a response that was 82 ± 12% that of fresh CFA1 (p > 0.05), with no response observed for the dried and reconstituted organic extract residues. It is noteworthy that the residue did contain trace quantities of insoluble CFA1 material, indicating that the reduction in CFA1 potency observed by organic extraction may have been due to particle loss rather than partial removal of a soluble agonist.
Fig. 2.
A, pretreatment of TRPV1-OE BEAS-2B cells with LJO-328 (5 μM; 24 h) stimulates cell surface expression of TRPV1 and CFA1-induced calcium flux. Top, increased recovery and detection of TRPV1 in cell surface protein isolates of LJO-328 pretreated TRPV1-OE (right lane) versus nonpretreated TRPV1-OE cells (left lane) by western blot. Bottom, images showing calcium flux in TRPV1-OE cells treated with LHC-9 media (negative control; left), 0.73 mg/ml CFA1 for 3 min (center), or LJO-pretreated cells (right) treated with 0.73 mg/ml CFA1 for 3 min. Bright green spots represent fluorescent cells where TRPV1-mediated calcium flux was observed. B, quantitation of calcium flux in TRPV1-OE cells treated with CFA1, with or without LJO-328 pretreatment and inhibition of calcium flux by total (LJO-328) or cell-impermeable and nonselective (EGTA and Ruthenium red) TRPV1 inhibitors. The white bars represent calcium flux in normal TRPV1-OE cells treated with LHC-9 (negative control) or CFA1 at 0.73 mg/ml for 3 min, the light gray bars represent LJO-328 pretreated TRPV1-OE cells treated with either LHC-9 or CFA1 at 0.18 or 0.73 mg/ml for 3 min, and the black bars represent LJO-328 pretreated TRPV1-OE cells cotreated with CFA1 at 0.73 mg/ml for 3 min and either EGTA + Ruthenium red (50 + 250 μM) or LJO-328 (20 μM). Data are the mean and SEM (n = 5) and asterisks indicate a statistical difference relative to the media control, or response to CFA1 between nonpretreated and LJO-328 pretreated cells. Daggers indicate a significant reduction in response between LJO-328 pretreated cells treated with CFA1, with and without inhibitor cotreatment using one-way ANOVA with post-testing using the Bonferroni multiple comparisons test. ****, p < 0.0001; ††††, p < 0.0001.
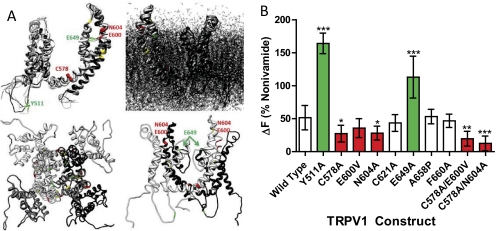
The mechanism of TRPV1 activation by CFA1 was interrogated using site-directed mutagenesis of known functionally important amino acid residues of TRPV1 to identify key particle-protein interactions governing activity (Fig. 3, A and B). Mutation of Tyr511 (Y511A), an intramembrane residue required for TRPV1 activation by capsaicinoids (Jordt and Julius, 2002; Bessac and Jordt, 2008), did not have a negative effect on CFA1 activity. Likewise, mutation of the proton potentiation site (Jordt et al., 2000) Glu649 (E649A) increased the CFA1 response ∼2-fold relative to wild-type TRPV1. Mutation of Cys578 (C578A), the voltage- and cation-sensitive residue Glu600 (E600V) (Jordt et al., 2000; Ahern et al., 2005), the glycosylation site Asn604 (N604A) (Wirkner et al., 2005), and the double mutants C578A+E600V and C578A+N604A all exhibited a diminished response to CFA1, but mutation of the redox-sensitive residue Cys621 (C621A) (Jin et al., 2004) and agonist/antagonist peptide binding residues Ala658 (A658P) and Phe660 (F660A) (Bohlen et al., 2010; Lin et al., 2011) had no effect on TRPV1 activation by CFA1.
Fig. 3.
A, annotated homology model (Fernández-Ballester and Ferrer-Montiel, 2008) of a TRPV1 subunit outside and inside the cell membrane (top images), the TRPV1 tetramer viewed from the outside in (bottom left image), and two TRPV1 subunits (lower right image), highlighting residues effecting TRPV1 activation by CFA1. B, quantitative calcium flux results showing that pore-loop residues of TRPV1 regulate responses to CFA1. The images in Fig. 3, A and B, are color-coded where green represents stimulatory point mutations and red for inhibitory point or double mutants. Data are the mean and S.E.M. (n ≥ 5) and asterisks indicate a statistical difference relative to wild-type TRPV1 using ANOVA with Dunnett's multiple comparison post test. *, p<0.05; **, p < 0.01; ***, p < 0.001.
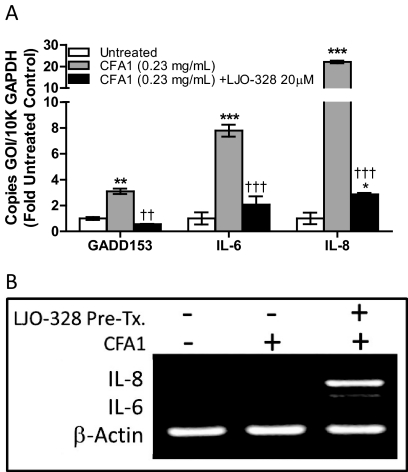
The role of TRPV1 in mediating proapoptotic and immunomodulatory cytokine/chemokine production by lung cells treated with CFA1, a response previously shown for other TRPV1-activating PM and other TRPV1 agonists (Veronesi et al., 1999a,b; Oortgiesen et al., 2000; Agopyan et al., 2003b; Thomas et al., 2007, 2011), was evaluated (Fig. 4). Treatment of NHBE cells with CFA1 increased the expression of mRNA for the proapoptotic gene product GADD153 (3-fold control), the cytokine IL-6 (18-fold control), and the chemokine IL-8 (22-fold control) (Fig. 4A), which were suppressed 82, 73, and 87%, respectively, by LJO-328 cotreatment. In TRPV1-OE cells, induction of IL-6 and IL-8 mRNA, relative to mRNA for β-actin, was also exacerbated by LJO-328 pretreatment (Fig. 4B), consistent with the notion that cell surface TRPV1 acts as the gene product responsible for translating CFA1 contact with the cell surface to changes in the expression of these pro-inflammatory genes.
Fig. 4.
A, CFA1-induced changes in GADD153, IL-6, and IL-8 mRNA in NHBE cells after 24-h treatment at 37°C with 0.23 mg/ml CFA1. Control (white bars), CFA1 (gray bars), and CFA1 plus LJO-328 (20 μM) cotreatment (black bars). Data are the mean and S.E.M. (n = 3), and asterisks indicate a statistical increase in mRNA abundance relative to untreated control cells, whereas daggers indicate a decrease in mRNA induction with LJO-328 cotreatment using two-way ANOVA with a Bonferroni multiple comparisons post test. B, image of PCR-amplified DNA for IL-6, IL-8, and β-actin DNA in an ethidium bromide-stained 2% agarose gel. cDNA was prepared from TRPV1-OE cells treated with LHC-9 (negative control; left lane), 0.43 mg/ml CFA1 (center lane), and LJO-328 pretreated TRPV1-OE cells treated with CFA1 (0.43 mg/ml) for 4h at 37°C. **, p < 0.01; ***, p < 0.001; ††, p < 0.01; †††, p < 0.001.
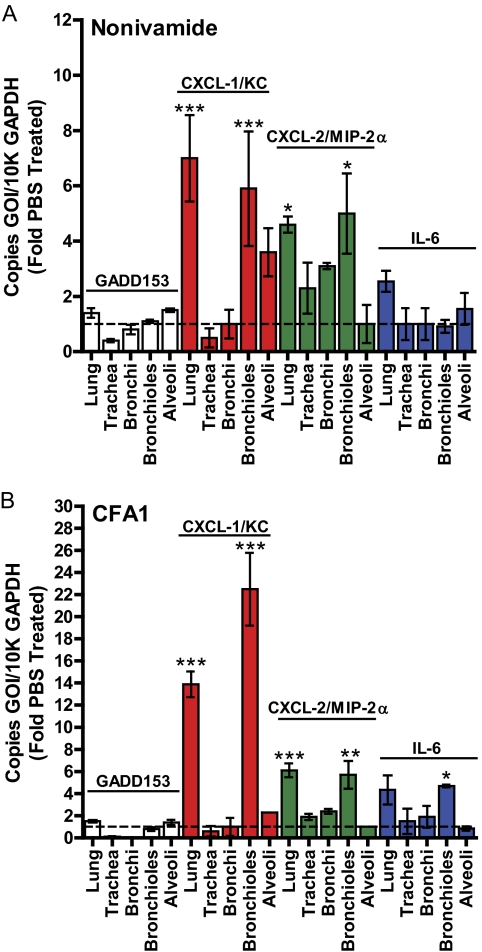
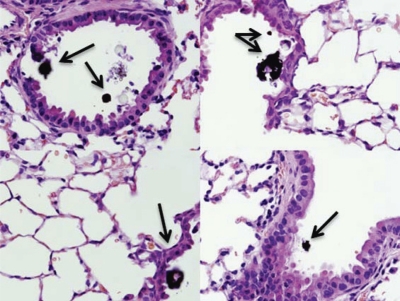
In addition to the cell culture studies, the role of CFA1 in activating TRPV1 was assessed in an animal model. The induction of proapoptotic and proinflammatory responses in the intact mouse lung and airway tissue was assessed using intratracheal administration of nonivamide and CFA1. CF-1 mice were instilled intratracheally with nonivamide (Fig. 5A) or CFA1 (Fig. 5B), and the respiratory tissue was assayed for regional changes in GADD153, CXCL-1/KC, CXCL-2/MIP-2α, and IL-6 mRNA expression, relative to control values obtained from PBS-instilled mice; CXCL-1/KC and CXCL-2/MIP-2α constitute the functional equivalents of human IL-8. Significant induction of CXCL-1/KC, CXCL-2/MIP-2α, and IL-6 were generally observed in whole-lung and bronchiole tissue 4 h after nonivamide (Fig. 5A) and CFA1 (Fig. 5B) treatment, compared with PBS-instilled controls. Decreased expression of GADD153 was observed in the trachea, and to a lesser extent, in the bronchi of nonivamide- and CFA1-treated mice, as reported previously for nonivamide (Thomas et al., 2011), and CXCL-1/KC was elevated in alveolar tissue; however, these responses were not statistically significant. Histological analysis of lung tissue from CFA1-treated mice demonstrated CFA1 was retained in the bronchioles and alveoli (Fig. 6), consistent with the gene expression data in Fig. 5B; no such material was observed in PBS- or nonivamide-treated mice (data not shown).
Fig. 5.
Quantification of GADD153, CXCL-1/KC, CXCL-2/MIP-2α, and IL-6 mRNA, relative to GAPDH mRNA, in CF-1 mouse respiratory tissues by quantitative real-time PCR, 4 h after intratracheal administration of nonivamide (0.5 mg/kg) (A) or CFA1 (250 μg) (B). Data are the mean and S.E.M. (n ≥ 3), and asterisks indicate statistical significance relative to PBS-treated mice using two-way ANOVA with a Bonferroni multiple comparisons post test. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Fig. 6.
Mouse lung histology. Sections of mouse lung were assessed at 160× magnification to localize CFA1 (brown/black materials) retained in the lung 4 h post intrethecal treatment with PM (250 μg). Images are characteristic of results from the analysis of CFA1-treated mouse lungs, and there was no evidence of such materials in lungs of PBS- or nonivamide-treated mice (images not shown).
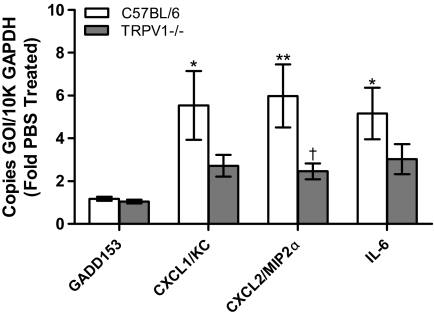
Finally, CFA1-induced changes in GADD153, CXCL-1/KC, CXCL-2/MIP-2α, and IL-6 were compared in wild-type C57BL/6 and TRPV1(−/−) C57BL/6 mice (Fig. 7). No changes in GADD153 expression were detected, but significant increases in CXCL-1/KC, CXCL-2/MIP-2α, and IL-6 were observed in lungs of wild-type mice. CFA1-induced expression of CXCL-1/KC, CXCL-2/MIP-2α, and IL-6 was reduced in TRPV1(−/−) mice, but statistical significance was only achieved for CXCL-2/MIP-2α.
Fig. 7.
Quantification of GADD153, CXCL-1/KC, CXCL-2/MIP-2α, and IL-6 mRNA, relative to GAPDH mRNA, in wild-type C57BL/6 (white bars) and TRPV1(−/−) C57BL/6 (gray bars) mouse lung tissue assayed by quantitative real-time PCR, 4 h after intratracheal administration of CFA1 (250 μg). Data are the mean and S.E.M. (n ≥ 6). Asterisks indicate statistical significance relative to PBS-treated controls and daggers indicate a statistical decrease in expression in TRPV1(−/−) mice relative to wild-type mice using two-way ANOVA with the Bonferroni multiple comparisons post-test. *, p < 0.05; **, p < 0.01; ††, p < 0.01; †, p < 0.05.
Discussion
This study investigated the hypothesis that TRPV1 is differentially activated by chemically and physically unique PM and that activation of TRPV1 by a PM agonist in lung cells and the respiratory tract would promote changes in the expression of proinflammatory and endoplasmic reticulum stress-associated genes that may contribute to pulmonary injury by inhaled PM. TRPV1 was shown to be is activated by some (CFA1, DEP, MUS), but not all PM; TRPM8 was also robustly activated by CFA1, and TRPA1 was slightly activated. Activation of TRPV1 by CFA1 occured via cell surface interactions between the solid components of CFA1 and specific amino acid residues of TRPV1 localized to the putative pore-loop region; and activation of TRPV1 by CFA1 was not exclusive in mouse lungs, but represents a pathway by which CFA1 affects the expression of selected genes in lung epithelial cells and airway tissue.
Previous studies characterizing the activation of TRPV1 in lung cells by PM were limited in that they did not directly evaluate potential contributions of other calcium channels, despite evidence that acid-sensing ion channels also contributed to the responses (Veronesi et al., 1999a,b, 2002, 2003; Oortgiesen et al., 2000; Agopyan et al., 2003a,b; Wong et al., 2003; Ghelfi et al., 2008). Bronchial epithelial and airway sensory neurons cells express numerous other TRP channels that also could be activated by different forms of PM. For example, TRPA1 and -M8 are expressed by airway vagal and trigeminal sensory neurons that respond to PM, and TRPA1 is largely coexpressed with -V1, whereas TRPM8 is expressed independent of -A1 and -V1 (Kobayashi et al., 2005). Likewise, TRPM8 exists as a functional variant, TRPM8Δ1–801 (Sabnis et al., 2008), in lung epithelial cells, which also express high levels of TRPV4 (Li et al., 2011); this may also contribute to lung cell responses to certain forms of PM. Figures 1, 2, and 4 substantiate previous conclusions that TRPV1 mediates the induction of several important proapoptotic and proinflammatory cytokine/chemokine genes in lung epithelial cells to in response to PM. However, although nearly complete inhibition of CFA1-induced calcium flux and gene expression changes were achieved using LJO-328 cotreatment of lung epithelial cells (Figs. 2B and 4A), others have observed, using capsazepine and other forms of PM, only partial attenuation of gene induction was observed in TRPV1(−/−) mice relative to wild-type mice (Fig. 7). These results, in conjunction with Fig. 1B, suggest a possible role for neuronal TRPA1 or -M8 as additional mediators of lung inflammation and toxicity elicited by CFA1. Alternatively, pathways that were not evaluated in these studies may also contribute to the effects of CFA1 in the lung, particularly at the high dose of CFA1 used, where the precise contribution of TRPV1 may be poorly estimated. Ongoing dose-response studies and future research employing more relevant exposure methods (e.g., inhalation of suspended PM for multiple days), combined with additional genetic and/or pharmacological inhibitors should provide a more complete model for TRPV1, -A1, -M8, and other PM sensors collectively contribute to the adverse effects of PM in the lung. Regardless, TRPV1 does play an important role in detecting CFA1 and initiating specific responses that probably contribute to the adverse effects of this and other similar PM in the respiratory tract.
Prior studies have also partially characterized the mechanism of activation of TRPV1 and demonstrated a requirement for cell surface contact and a net-negative surface charge on PM to elicit responses (Oortgiesen et al., 2000; Agopyan et al., 2003a,b). In this study, it is confirmed that TRPV1 activation by CFA1 occurs as a result of interactions between CFA1 and cell surface TRPV1. First, enrichment of TRPV1 at the cell surface enhanced both CFA1-induced calcium flux (Fig. 2, A and B) and IL-6 and IL-8 mRNA expression (Fig. 4B), with the latter serving as a “reporter assay” for cell-surface TRPV1 activation, as supported by previous studies (Reilly et al., 2005; Johansen et al., 2006). Second, chelation of calcium ions in the treatment media with EGTA, and cotreating cells with the cell-impermeable TRPV1 pore blocker ruthenium red, inhibited calcium flux comparable with that achieved using the selective cell-permeable TRPV1 antagonist LJO-328, which inhibits total cellular TRPV1 (Fig. 2B). Finally, the agonist activity of CFA1 was localized to the aqueous- and solvent- (1:1 chloroform/methanol) insoluble CFA1. Thus, unlike the activation of TRPA1 by electrophiles that can be extracted by alcohol and/or solvent treatments and are released from DEP upon cell contact (Deering-Rice et al., 2011), soluble CFA1-derived components do not activate TRPV1, providing a second specific mechanism by which two unique forms of environmental PM activate two specific TRP channels in the airway.
Consistent with prior proposed mechanisms of TRPV1 activation by negatively charged PM (Oortgiesen et al., 2000; Veronesi et al., 2002, 2003; Agopyan et al., 2003a,b), and results in Figs. 2 and 3B, it was demonstrated that mutation of the capsaicin binding site residue Tyr511 (Y511A), located on the intracellular loop between transmembrane segments 3 and 4, did not reduce TRPV1 activation by CFA1, despite completely inhibiting the response to nonivamide. Conversely, neutralization of the cell surface pore-loop residue Glu649 (E649A), which is predicted to reside at the entry to the ion pore domain at the membrane surface, forming a “base” of a cylindrical tetrameric pore region (Fig. 3A), increased TRPV1 activation by CFA1 ∼2-fold, relative to wild-type TRPV1. One interpretation of this result is that interactions between the pore-loop segment of TRPV1 (roughly residues 600–660), specifically Glu600, Asn604 (and/or the N-linked glycan attached at this position) (Wirkner et al., 2005) are facilitated as a result of a reduction in charge-charge repulsive forces that may occur between Glu649 and CFA1 (Fig. 3A). However, this scenario does not fully explain the apparent roles for Cys578, Glu600, and Asn604 in CFA1-mediated activation of TRPV1. As such, from the collective data in Fig. 3, reports that TRPV1, like other TRP channels, can be activated by mechanical mechanisms (Inoue et al., 2009), and identification of a sequence of the extracellular pore loop residues that displaces upon thermal activation (Yang et al., 2010), it is proposed that Cys578, Glu600, and Asn604 may be components of a “charge- and mechanosensitive” domain. Thus, the following model is proposed (refer to Fig. 3A for a visual aid): 1) extracellular components of TRPV1 form a “basket-like structure” above the ion pore opening; 2) Glu600 and Asn604 reside at the membrane interface potentially constituting a flexible “hinge region” that must be displaced in order for CFA1 to activate TRPV1, and the presence of Asn604-linked glycans facilitate this process; 3) the base of the basket-like structure interacts with CFA1 in a charge-dependent manner, and neutralization of Glu649 leads to increased interaction by negatively charged PM; and 4) mechanical movement of the cell surface “turret” or “basket wall” components of TRPV1 are transduced into pore opening through interactions between Cys578 and yet undefined residues of the pore domain.
In summary, this study further illustrates that TRPV1 detects and differentially responds to select forms of environmental PM and that TRPV1 activation contributes to several commonly cited cellular responses to PM, including proapoptotic and proinflammatory gene induction in lung cells and airway tissue. It is anticipated that further elucidation of the contributions of TRPV1 and/or other related TRP channels to PM effects in the respiratory tract will reveal novel therapeutic opportunities to prevent and/or attenuate such effects in humans.
This work was supported by grants from the National Institutes of Health National Institute of Environmental Health Sciences [Grant ES017431], and the 2011 Colgate-Palmolive Postdoctoral Fellowship (to C.E.D.-R.). Development and synthesis of LJO-328 was supported by the National Research Foundation of Korea [R11-2007-107-02001-0] (to J.L.).
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
- PM
- particulate matter
- TRPA1
- transient receptor potential ankyrin-1
- TRPM8
- transient receptor potential melastatin-8
- TRPC4α
- transient receptor potential canonical-4, α variant
- TRPV1
- transient receptor potential vanilloid-1
- TRPV2
- transient receptor potential vanilloid-2
- TRPV3
- transient receptor potential vanilloid-3
- TRPV4
- transient receptor potential vanilloid-4
- IL
- interleukin
- AITC
- allyl isothiocyanate
- GSK1016790A
- N-(1-((4-(2-(((2,4-dichlorophenyl)sulfonyl)amino)-3-hydroxypropanoyl)-1-piperazinyl)carbonyl)-3-methylbutyl)-1-benzothiophene-2-carboxamide
- LJO-328
- N-(4-tert-butylbenzyl)-N′-(1-[3-fluoro-4-(methylsulfonylamino)phenyl]ethyl)thiourea
- BEAS-2B
- human bronchial epithelial cells
- NHBE
- normal human bronchial epithelial cells
- LHC-9
- Lechner and LaVeck media
- CFA1
- power plant coal fly ash
- CFA2
- laboratory-generated-coal fly ash
- DEP
- diesel exhaust particles
- MUS
- Min-U-Sil 5 μM, crystalline silica
- DD
- desert dust particulate
- HEK
- human embryonic kidney
- TRPV1-OE
- TRPV1-overexpressing BEAS-2B cells
- PBS
- phosphate-buffered saline
- PBS-T
- PBS/Tween 20
- PCR
- polymerase chain reaction
- qPCR
- quantitative real-time PCR
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- GADD153
- growth arrest and DNA damage induced gene-153
- MIP
- macrophage inflammatory protein
- ANOVA
- analysis of variance.
Authorship Contributions
Participated in research design: Deering-Rice, Johansen, Yost, Veranth, and Reilly.
Conducted experiments: Deering-Rice, Johansen, Roberts, Thomas, Romero, and Reilly.
Contributed new reagents: Lee.
Performed data analysis: Deering-Rice, Roberts, Romero, and Reilly.
Wrote or contributed to writing of the manuscript: Deering-Rice, Yost, Veranth, and Reilly.
References
- Agopyan N, Bhatti T, Yu S, Simon SA. (2003a) Vanilloid receptor activation by 2- and 10-micron particles induces responses leading to apoptosis in human airway epithelial cells. Toxicol Appl Pharmacol 192:21–35 [DOI] [PubMed] [Google Scholar]
- Agopyan N, Head J, Yu S, Simon SA. (2004) TRPV1 receptors mediate particulate matter-induced apoptosis. Am J Physiol Lung Cell Mol Physiol 286:L563–L572 [DOI] [PubMed] [Google Scholar]
- Agopyan N, Li L, Yu S, Simon SA. (2003b) Negatively charged 2- and 10-microm particles activate vanilloid receptors, increase cAMP, and induce cytokine release. Toxicol Appl Pharmacol 186:63–76 [DOI] [PubMed] [Google Scholar]
- Ahern GP, Brooks IM, Miyares RL, Wang XB. (2005) Extracellular cations sensitize and gate capsaicin receptor TRPV1 modulating pain signaling. J Neurosci 25:5109–5116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein JA, Alexis N, Barnes C, Bernstein IL, Bernstein JA, Nel A, Peden D, Diaz-Sanchez D, Tarlo SM, Williams PB. (2004) Health effects of air pollution. J Allergy Clin Immunol 114:1116–1123 [DOI] [PubMed] [Google Scholar]
- Bessac BF, Jordt SE. (2008) Breathtaking TRP channels: TRPA1 and TRPV1 in airway chemosensation and reflex control. Physiology (Bethesda) 23:360–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlen CJ, Priel A, Zhou S, King D, Siemens J, Julius D. (2010) A bivalent tarantula toxin activates the capsaicin receptor, TRPV1, by targeting the outer pore domain. Cell 141:834–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Xu X, Cui W, Xi Z. (2001) Development and certification of a coal fly ash certified reference material for selected polycyclic aromatic hydrocarbons. Fresenius J Anal Chem 370:1035–1040 [PubMed] [Google Scholar]
- Chow JC, Watson JG, Mauderly JL, Costa DL, Wyzga RE, Vedal S, Hidy GM, Altshuler SL, Marrack D, Heuss JM, et al. (2006) Health effects of fine particulate air pollution: lines that connect. J Air Waste Manag Assoc 56:1368–1380 [DOI] [PubMed] [Google Scholar]
- Costa SK, Teles AM, Kumagai Y, Brain SD, Teixeira SA, Varriano AA, Barreto MA, de Lima WT, Antunes E, Muscará MN, et al. (2010) Involvement of sensory nerves and TRPV1 receptors in the rat airway inflammatory response to two environment pollutants: diesel exhaust particles (DEP) and 1,2-naphthoquinone (1,2-NQ). Arch Toxicol 84:109–117 [DOI] [PubMed] [Google Scholar]
- Deering-Rice CE, Romero EG, Shapiro D, Hughen RW, Light AR, Yost GS, Veranth JM, Reilly CA. (2011) Electrophilic components of diesel exhaust particles (DEP) activate transient receptor potential ankyrin-1 (TRPA1): a probable mechanism of acute pulmonary toxicity for DEP. Chem Res Toxicol 24:950–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst H, Rittinghausen S, Bartsch W, Creutzenberg O, Dasenbrock C, Görlitz BD, Hecht M, Kairies U, Muhle H, Müller M, et al. (2002) Pulmonary inflammation in rats after intratracheal instillation of quartz, amorphous SiO2, carbon black, and coal dust and the influence of poly-2-vinylpyridine-N-oxide (PVNO). Exp Toxicol Pathol 54:109–126 [DOI] [PubMed] [Google Scholar]
- Fernández-Ballester G, Ferrer-Montiel A. (2008) Molecular modeling of the full-length human TRPV1 channel in closed and desensitized states. J Membr Biol 223:161–172 [DOI] [PubMed] [Google Scholar]
- Ghelfi E, Rhoden CR, Wellenius GA, Lawrence J, Gonzalez-Flecha B. (2008) Cardiac oxidative stress and electrophysiological changes in rats exposed to concentrated ambient particles are mediated by TRP-dependent pulmonary reflexes. Toxicol Sci 102:328–336 [DOI] [PubMed] [Google Scholar]
- Gilmour MI, O'Connor S, Dick CA, Miller CA, Linak WP. (2004) Differential pulmonary inflammation and in vitro cytotoxicity of size-fractionated fly ash particles from pulverized coal combustion. J Air Waste Manag Assoc 54:286–295 [DOI] [PubMed] [Google Scholar]
- Glinianaia SV, Rankin J, Bell R, Pless-Mulloli T, Howel D. (2004) Particulate air pollution and fetal health: a systematic review of the epidemiologic evidence. Epidemiology 15:36–45 [DOI] [PubMed] [Google Scholar]
- Inoue R, Jian Z, Kawarabayashi Y. (2009) Mechanosensitive TRP channels in cardiovascular pathophysiology. Pharmacol Ther 123:371–385 [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington DC [Google Scholar]
- Jia Y, McLeod RL, Hey JA. (2005) TRPV1 receptor: a target for the treatment of pain, cough, airway disease and urinary incontinence. Drug News Perspect 18:165–171 [DOI] [PubMed] [Google Scholar]
- Jin Y, Kim DK, Khil LY, Oh U, Kim J, Kwak J. (2004) Thimerosal decreases TRPV1 activity by oxidation of extracellular sulfhydryl residues. Neurosci Lett 369:250–255 [DOI] [PubMed] [Google Scholar]
- Johansen ME, Reilly CA, Yost GS. (2006) TRPV1 antagonists elevate cell surface populations of receptor protein and exacerbate TRPV1-mediated toxicities in human lung epithelial cells. Toxicol Sci 89:278–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordt SE, Julius D. (2002) Molecular basis for species-specific sensitivity to “hot” chili peppers. Cell 108:421–430 [DOI] [PubMed] [Google Scholar]
- Jordt SE, Tominaga M, Julius D. (2000) Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc Natl Acad Sci USA 97:8134–8139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K. (2005) Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol 493:596–606 [DOI] [PubMed] [Google Scholar]
- Li J, Kanju P, Patterson M, Chew WL, Cho SH, Gilmour I, Oliver T, Yasuda R, Ghio A, Simon SA, et al. (2011) TRPV4-mediated calcium influx into human bronchial epithelia upon exposure to diesel exhaust particles. Environ Health Perspect 119:784–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lighty JS, Veranth JM, Sarofim AF. (2000) Combustion aerosols: factors governing their size and composition and implications to human health. J Air Waste Manag Assoc 50:1565–1618; discussion 1619–1622 [DOI] [PubMed] [Google Scholar]
- Lin Z, Reilly CA, Antemano R, Hughen RW, Marett L, Concepcion GP, Haygood MG, Olivera BM, Light A, Schmidt EW. (2011) Nobilamides A-H, Long-Acting Transient Receptor Potential Vanilloid-1 (TRPV1) Antagonists from Mollusk-Associated Bacteria. J Med Chem 54:3746–3755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogugbuaja VO, Onyeyili PA, Moses EA. (2001) Study of effects on haematological parameters of rabbits intratracheally exposed to coal fly ash. J Environ Sci Health A Tox Hazard Subst Environ Eng 36:1411–1418 [DOI] [PubMed] [Google Scholar]
- Oortgiesen M, Veronesi B, Eichenbaum G, Kiser PF, Simon SA. (2000) Residual oil fly ash and charged polymers activate epithelial cells and nociceptive sensory neurons. Am J Physiol Lung Cell Mol Physiol 278:L683–L695 [DOI] [PubMed] [Google Scholar]
- Pope CA, 3rd, Renlund DG, Kfoury AG, May HT, Horne BD. (2008) Relation of heart failure hospitalization to exposure to fine particulate air pollution. Am J Cardiol 102:1230–1234 [DOI] [PubMed] [Google Scholar]
- Reilly CA. (2010) Neurogenic inflammation: the role of TRP channels in the lung, in Comprehensive Toxicology 2nd ed (McQueen CA. ed), Elsevier, Amsterdam [Google Scholar]
- Reilly CA, Johansen ME, Lanza DL, Lee J, Lim JO, Yost GS. (2005) Calcium-dependent and independent mechanisms of capsaicin receptor (TRPV1)-mediated cytokine production and cell death in human bronchial epithelial cells. J Biochem Mol Toxicol 19:266–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly CA, Taylor JL, Lanza DL, Carr BA, Crouch DJ, Yost GS. (2003) Capsaicinoids cause inflammation and epithelial cell death through activation of vanilloid receptors. Toxicol Sci 73:170–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabnis AS, Shadid M, Yost GS, Reilly CA. (2008) Human lung epithelial cells express a functional cold-sensing TRPM8 variant. Am J Respir Cell Mol Biol 39:466–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KR, Veranth JM, Kodavanti UP, Aust AE, Pinkerton KE. (2006) Acute pulmonary and systemic effects of inhaled coal fly ash in rats: comparison to ambient environmental particles. Toxicol Sci 93:390–399 [DOI] [PubMed] [Google Scholar]
- Takano H, Yanagisawa R, Ichinose T, Sadakane K, Inoue K, Yoshida S, Takeda K, Yoshino S, Yoshikawa T, Morita M. (2002a) Lung expression of cytochrome P450 1A1 as a possible biomarker of exposure to diesel exhaust particles. Arch Toxicol 76:146–151 [DOI] [PubMed] [Google Scholar]
- Takano H, Yanagisawa R, Ichinose T, Sadakane K, Yoshino S, Yoshikawa T, Morita M. (2002b) Diesel exhaust particles enhance lung injury related to bacterial endotoxin through expression of proinflammatory cytokines, chemokines, and intercellular adhesion molecule-1. Am J Respir Crit Care Med 165:1329–1335 [DOI] [PubMed] [Google Scholar]
- Thomas KC, Roberts JK, Deering-Rice CE, Romero EG, Dull RO, Lee J, Yost GS, Reilly CA. (2011) Contributions of TRPV1, endovanilloids, and endoplasmic reticulum stress in lung cell death in vitro and lung injury. Am J Physiol Lung Cell Mol Physiol. http://dx.doi.org/10.1152/ajplung.00231.2011 [DOI] [PMC free article] [PubMed]
- Thomas KC, Sabnis AS, Johansen ME, Lanza DL, Moos PJ, Yost GS, Reilly CA. (2007) Transient receptor potential vanilloid 1 agonists cause endoplasmic reticulum stress and cell death in human lung cells. J Pharmacol Exp Ther 321:830–838 [DOI] [PubMed] [Google Scholar]
- Veranth JM, Cutler NS, Kaser EG, Reilly CA, Yost GS. (2008) Effects of cell type and culture media on Interleukin-6 secretion in response to environmental particles. Toxicol In Vitro 22:498–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veranth JM, Moss TA, Chow JC, Labban R, Nichols WK, Walton JC, Watson JG, Yost GS. (2006) Correlation of in vitro cytokine responses with the chemical composition of soil-derived particulate matter. Environ Health Perspect 114:341–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veranth JM, Reilly CA, Veranth MM, Moss TA, Langelier CR, Lanza DL, Yost GS. (2004) Inflammatory cytokines and cell death in BEAS-2B lung cells treated with soil dust, lipopolysaccharide, and surface-modified particles. Toxicol Sci 82:88–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronesi B, Carter JD, Devlin RB, Simon SA, Oortgiesen M. (1999a) Neuropeptides and capsaicin stimulate the release of inflammatory cytokines in a human bronchial epithelial cell line. Neuropeptides 33:447–456 [DOI] [PubMed] [Google Scholar]
- Veronesi B, de Haar C, Lee L, Oortgiesen M. (2002) The surface charge of visible particulate matter predicts biological activation in human bronchial epithelial cells. Toxicol Appl Pharmacol 178:144–154 [DOI] [PubMed] [Google Scholar]
- Veronesi B, Oortgiesen M, Carter JD, Devlin RB. (1999b) Particulate matter initiates inflammatory cytokine release by activation of capsaicin and acid receptors in a human bronchial epithelial cell line. Toxicol Appl Pharmacol 154:106–115 [DOI] [PubMed] [Google Scholar]
- Veronesi B, Wei G, Zeng JQ, Oortgiesen M. (2003) Electrostatic charge activates inflammatory vanilloid (VR1) receptors. Neurotoxicology 24:463–473 [DOI] [PubMed] [Google Scholar]
- Ward DJ, Ayres JG. (2004) Particulate air pollution and panel studies in children: a systematic review. Occup Environ Med 61:e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirkner K, Hognestad H, Jahnel R, Hucho F, Illes P. (2005) Characterization of rat transient receptor potential vanilloid 1 receptors lacking the N-glycosylation site N604. Neuroreport 16:997–1001 [DOI] [PubMed] [Google Scholar]
- Wong SS, Sun NN, Keith I, Kweon CB, Foster DE, Schauer JJ, Witten ML. (2003) Tachykinin substance P signaling involved in diesel exhaust-induced bronchopulmonary neurogenic inflammation in rats. Arch Toxicol 77:638–650 [DOI] [PubMed] [Google Scholar]
- Yang F, Cui Y, Wang K, Zheng J. (2010) Thermosensitive TRP channel pore turret is part of the temperature activation pathway. Proc Natl Acad Sci USA 107:7083–7088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota S, Ohara N, Kobayashi T. (2008) The effects of organic extract of diesel exhaust particles on ischemia/reperfusion-related arrhythmia and on pulmonary inflammation. J Toxicol Sci 33:1–10 [DOI] [PubMed] [Google Scholar]