Abstract
Sodium channel inhibitor (SCI) insecticides selectively target voltage-gated sodium (Nav) channels in the slow-inactivated state by binding at or near the local anesthetic receptor within the sodium channel pore. Metaflumizone is a new insecticide for the treatment of fleas on domesticated pets and has recently been reported to block insect sodium channels in the slow-inactivated state, thereby implying that it is also a member of the SCI class. Using the two-electrode voltage-clamp technique, we examined metaflumizone inhibition of rat Nav1.4 sodium channels expressed in Xenopus laevis oocytes. Metaflumizone selectively inhibited Nav1.4 channels at potentials that promoted slow inactivation and shifted the voltage dependence of slow inactivation in the direction of hyperpolarization. Metaflumizone perfusion at a hyperpolarized holding potential also shifted the conductance-voltage curve for activation in the direction of depolarization and antagonized use-dependent lidocaine inhibition of fast-inactivated sodium channels, actions not previously observed with other SCI insecticides. We expressed mutated Nav1.4/F1579A and Nav1.4/Y1586A channels to investigate whether metaflumizone shares the domain IV segment S6 (DIV-S6) binding determinants identified for other SCI insecticides. Consistent with previous investigations of SCI insecticides on rat Nav1.4 channels, the F1579A mutation reduced sensitivity to block by metaflumizone, whereas the Y1586A mutation paradoxically increased the sensitivity to metaflumizone. We conclude that metaflumizone selectively inhibits slow-inactivated Nav1.4 channels and shares DIV-S6 binding determinants with other SCI insecticides and therapeutic drugs. However, our results suggest that metaflumizone interacts with resting and fast-inactivated channels in a manner that is distinct from other compounds in this insecticide class.
Introduction
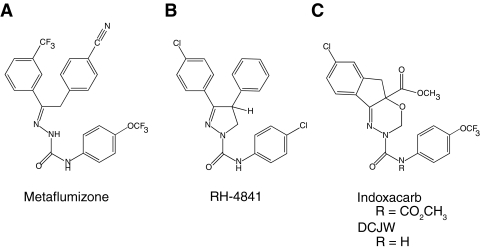
Sodium channel inhibitor (SCI) drugs, an important group of therapeutic agents that include local anesthetics, class I anticonvulsants, and class I antiarrhythmics, have been in clinical use for more than a century. Elucidating the molecular details of their action on sodium channels remains relevant to modern drug discovery efforts (Scholz, 2002). Voltage-gated sodium channels are also the molecular targets for SCI insecticides, a structurally diverse group of insect control agents of increasing importance (Silver and Soderlund, 2005b; Wing et al., 2005). Metaflumizone (Fig. 1A), a new agent to control fleas on domesticated animals, is identified as a novel type of SCI insecticide based on its state-dependent inhibition of insect sodium channels (Salgado and Hayashi, 2007; Silver et al., 2009). Indoxacarb (Fig. 1C), the first and only other commercialized SCI insecticide, is a proinsecticide that is converted in insects to its active metabolite, DCJW (Fig. 1C) (Wing et al., 2005). Other SCI insecticides include dihydropyrazoles [e.g., N,3-bis(4-chlorophenyl)-4,5-dihydro-4-phenyl-1H-pyrazole-1-carboxamide (RH-4841); Fig. 1B] and arylalkylbenzhydrolpiperidines that were not commercialized because of their high toxicity to mammals (Silver and Soderlund, 2005b).
Fig. 1.
Structures of SCI insecticides. A, metaflumizone. B, RH-4841. C, indoxacarb and DCJW.
SCI insecticides exhibit little or no effect on sodium channels under experimental conditions that promote resting, activated, or fast-inactivated channel conformations, but they cause a slowly developing inhibition of sodium currents under experimental conditions that promote slow inactivation of channels (Salgado, 1992; Zhao et al., 2003; Silver and Soderlund, 2005a, 2007). Moreover, SCI insecticides typically cause hyperpolarizing shifts in the voltage dependence of slow inactivation and impede recovery from slow inactivation. Thus, SCI insecticides seem to selectively bind to and stabilize sodium channels in a slow-inactivated state. The state-dependent action of SCI insecticides is analogous to that of SCI drugs that typically exhibit a preferential interaction with activated or fast-inactivated channels (Ragsdale et al., 1996). Unlike therapeutic drugs, the apparent association kinetics of SCI insecticides are very slow, on the order of several minutes. Such slow kinetics preclude the direct inhibition of channels in transient conformations, such as the activated or fast-inactivated states. Therefore, SCI insecticides exhibit no frequency- or use-dependent inhibition in response to repetitive stimulation (Silver and Soderlund, 2005a; Salgado and Hayashi, 2007), a hallmark of many SCI drugs.
SCI insecticides share common molecular determinants for binding to mammalian sodium channels with SCI drugs. SCI drugs bind at the “local anesthetic (LA) receptor” in the inner pore, involving residues in the S6 segments of homology domains I, III, and IV (for review, see Mike and Lukacs, 2010). Two residues (Phe1579 and Tyr1586) in DIV-S6 of rat Nav1.4 channels, identified previously as critical determinants of state-dependent SCI drug block, are also important determinants of SCI insecticide action (Silver and Soderlund, 2007). Alanine substitution at Phe1579 significantly reduces the extent of channel inhibition by both SCI drugs (Ragsdale et al., 1996; Wang et al., 1998; Wagner et al., 2001) and insecticides (Silver and Soderlund, 2007). Mutations analogous to Y1586A in rat Nav1.4 also significantly reduce the affinity of inactivated rat Nav1.2 (Ragsdale et al., 1996) and Nav1.3 channels (Li et al., 1999) for some classes of therapeutic SCI drugs. However, the Y1586A mutation paradoxically enhances the extent of Nav1.4 channel inhibition by SCI insecticides (Silver and Soderlund, 2007). It is noteworthy that the F1817A mutation in the German cockroach BgNav1-1a sodium channel (analogous to F1579A in rat Nav1.4) apparently enhances DCJW and metaflumizone inhibition, whereas the Y1824A mutation in BgNav1-1a (analogous to Y1586A in Nav1.4) selectively decreases inhibition by metaflumizone (Silver et al., 2009). Together, these data suggest that SCI insecticides have different binding determinants on mammalian and insect channels.
SCI insecticides such as indoxacarb and RH-4841 are conformationally restricted, nearly planar molecules (Wellinga et al., 1977; Grosscurt et al., 1979; Hasan et al., 1996). Metaflumizone, which lacks the central heterocyclic moiety of other SCI insecticides, is a flexible molecule that may assume binding conformations that are not available to other SCI insecticides. The present study was designed to characterize the action of metaflumizone on Nav1.4 sodium channels expressed in Xenopus laevis oocytes and test the hypothesis that metaflumizone shares the DIV-S6 binding determinants identified for other SCI insecticides on Nav1.4 channels. We report here that metaflumizone is an effective state-dependent inhibitor of Nav1.4 channels and show that substituting alanine for Phe1579 or Tyr1586 modulates metaflumizone sensitivity in a manner that is consistent with other SCI insecticides (Silver and Soderlund, 2007). However, our data suggest that metaflumizone interacts with mammalian sodium channels in multiple conformational states and may bind to channels without inhibiting them in a manner that is distinct from other compounds in this insecticide class.
Materials and Methods
Molecular Biology.
We used the rat Nav1.4 channel to enable us to place our findings in the context of previous studies (Silver and Soderlund, 2005a, 2006, 2007). We coexpressed the Nav1.4 α subunit with the rat β1 subunit because these channels exhibit aberrant inactivation gating when expressed alone in X. laevis oocytes (Balser et al., 1996). The cloned rat Nav1.4 cDNA was obtained from R. G. Kallen (University of Pennsylvania, Philadelphia, PA), and the rat β1 subunit cDNA was obtained from W.A. Catterall (University of Washington, Seattle, WA). The F1579A and Y1586A mutations were introduced by site-directed mutagenesis as described previously (Silver and Soderlund, 2007) using a commercial kit (QuikChange XL, Stratagene, La Jolla, CA). Plasmid cDNAs were digested with restriction enzymes to provide linear templates for Nav1.4 parental and mutated cRNA synthesis in vitro using a commercial kit (mMessage mMachine; Ambion, Austin, TX). The integrity of the cRNAs was determined by electrophoresis in 1% agarose-formaldehyde gels.
Oocyte Preparation and Injection.
Oocytes from female X. laevis frogs (Nasco, Ft. Atkinson, WI) were washed with Ca2+-free OR-2+ (containing 82.5 mM NaCl, 2 mM KCl, 1 mM MgCl2, and 5 mM HEPES, pH adjusted to 7.6 with NaOH) before being digested in 1 mg/ml collagenase (type 1A; Sigma-Aldrich, St. Louis, MO) for approximately 60 min to loosen the surrounding follicle cells. Healthy stage V and VI oocytes were isolated and manually defolliculated with a pair of fine-tipped surgical forceps before being incubated overnight at 16°C in ND-96 (containing 96 mM NaCl, 2 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, and 5 mM HEPES, pH adjusted to 7.6 with NaOH) solution supplemented with 6% horse serum (Sigma-Aldrich), 0.5% penicillin/streptomycin, and 2.5 mM sodium pyruvate (Goldin, 1992). Oocytes were pressure-injected with 50 nl of a 2:1 (mass ratio) mixture of β1 and parental or mutated rat Nav1.4 channel α subunit cRNA. The α-subunit concentration ranged from 10 to 50 ng/ml, which yielded sodium current amplitudes between −10 and −30 μA. The injected oocytes were maintained at 16°C in supplemented ND-96 media for 2 to 5 days before use.
Electrophysiology.
Electrophysiological experiments were conducted in disposable chambers fabricated from 0.25 in. Plexiglas and a glass coverslip sealed together with ethyl cyanoacrylate as described elsewhere (Smith and Soderlund, 1998). Recording microelectrodes were pulled from borosilicate glass capillaries (1.0 mm o.d., 0.78 mm i.d.; World Precision Instruments Inc., Sarasota, FL) on a P-87 Flaming Brown puller (Sutter Instruments, Novato, CA) and filled with 3 M KCl. Filled electrodes had resistances that ranged between 0.5 and 0.7 MΩ when submerged in ND-96 saline. The two-microelectrode voltage-clamp technique was used to record macroscopic sodium currents at room temperature (22–24°C) using a Geneclamp 500B amplifier and pClamp 10.2 software (Molecular Devices, Sunnyvale, CA). Currents were filtered at 5 kHz with a low-pass four-pole Bessel filter and digitized at 50 kHz with a Digidata 1320A (Molecular Devices). Capacitive transient currents were subtracted using the pulse/number (P/N) protocol (N = 4) (Bezanilla and Armstrong, 1977). Electrophysiological recordings were initiated at least 5 min after clamping oocytes at a hyperpolarized membrane potential of −120 mV. Oocytes were perfused with ND-96 saline at approximately 0.45 ml/min using a custom-fabricated disposable passive capillary perfusion system similar to that described previously (Tatebayashi and Narahashi, 1994). The entire bath volume could be replaced in ∼1 min. All perfusion capillaries, recording chambers, and microelectrodes were used only once to prevent potential cross-contamination between experiments.
The voltage dependence of activation was determined by clamping oocytes at a membrane potential of −120 mV and recording sodium currents during 20-ms depolarizing test pulses ranging from −80 to +40 mV in 10-mV increments. To determine the voltage dependence of steady-state fast inactivation, oocytes were clamped at a holding potential of −120 mV and stimulated with a 200-ms conditioning prepulse to potentials ranging from −100 to 0 mV in 10-mV increments followed by a 20-ms test pulse to −10 mV. The voltage dependence of slow inactivation was determined by clamping oocytes at −120 mV and giving a 100-s conditioning prepulse to potentials ranging from −90 to 0 mV in 10-mV increments followed by a 50-ms hyperpolarization to −120 mV to completely recover fast-inactivated channels and then a 20-ms test pulse to −10 mV (Nav1.4 and Nav1.4/F1579A) or 0 mV (Nav1.4/Y1586A). To avoid potential time-dependent cumulative effects, oocytes were held at −120 mV for >2 min between conditioning pulses. The effects of metaflumizone on activation and inactivation gating was assessed after 15 min of metaflumizone perfusion at a holding potential of −120 mV while sodium currents were measured every minute with a 20-ms test depolarization to −10 mV. The time course of inhibition by the insecticides was studied by clamping oocytes at a holding potential of −30 mV (Nav1.4), −35 mV (Nav1.4/F1579A), or −50 mV (Nav1.4/Y1586A) and giving a 20-ms test depolarization to −10 mV preceded by a 2-s hyperpolarization to −120 mV that recovered a fraction (∼45%) of sodium channels to the resting state, thus enabling a sodium current of adequate amplitude to be recorded (Salgado, 1992; Silver and Soderlund, 2005a, 2007). For determinations of use dependence, oocytes were clamped at a holding potential of −120 mV and stimulated at 20 Hz with trains of 0 to 20 brief (25-ms) conditioning depolarizing pulses to −50 mV, separated by 25-ms interpulse intervals, followed by a 20-ms test pulse to −10 mV. Pulse trains were separated by a 20-s interval at −120 mV to avoid possible time-dependent cumulative effects (Ragsdale et al., 1996). Experiments in the presence of lidocaine were initiated after 10 min of lidocaine perfusion at a holding potential of −120 mV. For experiments characterizing the effects of SCI insecticides on use-dependent lidocaine inhibition, oocytes were clamped at −120 mV and perfused with a given insecticide for 15 min before changing the bath perfusate to one containing both the insecticide and lidocaine together for 10 min before pulse protocols were initiated. Additional details of voltage protocols used are provided in the figure legends.
Chemicals.
Metaflumizone and lidocaine were purchased from Sigma-Aldrich. RH-4841 was provided by G. Carlson (Rohm and Haas Company, Spring House, PA), and DCJW was provided by K. Wing (DuPont Agricultural Products, Newark, DE). Stock solutions of insecticides (10 mM) and lidocaine (200 mM) were prepared in dimethyl sulfoxide (DMSO) and diluted in ND-96 just before use to a final concentration of 10 μM (insecticides) and 200 μM (lidocaine). The final DMSO concentration never exceeded 0.2% (v/v). Initial time-matched vehicle control experiments verified that 0.2% (v/v) DMSO has no effect on sodium channel gating.
Data Analysis and Statistics.
Data analysis was performed using Origin 8.1 (OriginLab Corp., Northampton, MA). Conductance-voltage and sodium channel inactivation data were fitted using the Boltzmann equation [y = (A1 − A2)/{1 + exp[(V − V1/2)/k]} + A2], where V1/2 is the midpoint of the curve, k is the slope factor, and A1 and A2 are the maximum and minimum values in the fit, respectively. Time constants for sodium-current inhibition were obtained by fitting the mean data with a single-exponential decay function [y = A1 × exp(−x/τ1) + y0], where x is time, τ1 is the time constant, and y0 is the noninactivating component. All fitted data are expressed as means and S.E.
Statistical analyses were performed using Prism 5.0 (GraphPad Software, San Diego, CA). Statistical significance between two means was assessed using either paired or unpaired Student's t test, depending on the design of the experiment. Statistical significance of two or more mean values from a single control data set was assessed using one-way analysis of variance followed by Dunnett's post hoc analysis. Differences were considered statistically significant at P < 0.05.
Results
Effects of Metaflumizone on Gating Properties of Wild-Type Nav1.4 Sodium Channels.
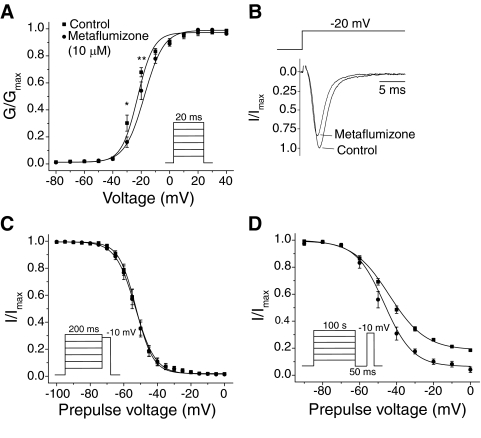
We first characterized the effects of 10 μM metaflumizone, the highest concentration achievable in ND-96 saline, on the voltage-dependent gating of Nav1.4 sodium channels (Fig. 2). Statistical analyses of these data are summarized in Table 1. Perfusion of metaflumizone for 15 min at a holding potential of −120 mV caused a 4.7-mV depolarizing shift in the midpoint potential (V1/2) of activation but did not affect the slope factor of the voltage response curve for activation fitted with the Boltzmann equation (Fig. 2A; Table 1). Consequently, the amplitude of sodium currents measured at submaximal activation potentials were reduced in the presence of metaflumizone compared with the controls (Fig. 2B). At test potentials of −30 and −20 mV, the reduction in the amplitude of the sodium current by metaflumizone was statistically significant. Metaflumizone had no effect on the voltage dependence of steady-state fast inactivation (Fig. 2C). By contrast, metaflumizone increased the extent of maximal slow inactivation at depolarized potentials, shifted the midpoint potential for slow inactivation by 3.5 mV in the direction of hyperpolarization, and increased the slope of the voltage response compared with the control (Fig. 2D).
Fig. 2.
Effects of metaflumizone (10 μM) on the voltage dependence of activation, steady-state fast inactivation and slow inactivation. A, conductance-voltage plots of activation measured upon depolarization from −120 mV to test potentials ranging from −80 to +40 mV. Peak sodium currents were transformed to conductances (G) using the equation G = INa/(Vtest − Vrev), where INa is the peak sodium current during test depolarization (V), and Vrev is the sodium current reversal potential. Data were normalized to maximum peak conductance (GMax) and fitted using the Boltzmann equation; values are means ± S.E. from five oocytes. The asterisks indicate test potentials of submaximal activation at which the measured sodium current amplitude in the presence of metaflumizone was significantly (*, P < 0.05; **, P < 0.001; paired t test) different from the control. B, representative normalized sodium current traces recorded from an oocyte before or after metaflumizone exposure upon depolarization from −120 to −20 mV. C, voltage dependence of steady-state fast inactivation; conditioning pulses (200 ms) from −120 mV to potentials ranging from −100 to 0 mV were followed immediately by 20-ms test pulses to −10 mV. Peak sodium currents were normalized to the maximum current obtained during the inactivation protocol for that oocyte and plotted versus the conditioning potential; curves were fitted using the Boltzmann equation; values are means ± S.E. from five oocytes. D, voltage dependence of slow inactivation; amplitudes of peak transient currents measured during a 20-ms depolarization to −10 mV after a 100-s conditioning prepulse from a holding potential (Vhold) of −120 mV to potentials ranging from −90 to 0 mV and a 50-ms hyperpolarization to −120 mV were normalized to the maximum current obtained during the inactivation protocol for that oocyte and plotted as a function of the conditioning potential. Values are means ± S.E. from six (control) or five (metaflumizone) separate experiments in different oocytes; curves are fitted to the Boltzmann equation.
TABLE 1.
Activation and inactivation gating parameters of Nav1.4 channels in the absence or presence of metaflumizone
Values calculated from fits of data obtained in the absence or presence of 10 μM metaflumizone (Fig. 2) to the Boltzmann equation.
| Condition | Activation |
Fast Inactivation |
Slow Inactivation |
||||||
|---|---|---|---|---|---|---|---|---|---|
| V1/2 | k | n | V1/2 | k | n | V1/2 | k | n | |
| mV | mV | mV | |||||||
| Control | −24.9 ± 0.7 | 5.9 ± 0.6 | 5 | −53.3 ± 0.2 | 5.0 ± 0.2 | 5 | −44.9 ± 0.7 | 9.3 ± 0.6 | 6 |
| Metaflumizone | −20.2 ± 0.7* | 6.4 ± 0.6 | 5 | −53.5 ± 0.2 | 5.2 ± 0.2 | 5 | −48.4 ± 0.9† | 7.5 ± 0.8† | 5 |
V1/2, midpoint potential for voltage-dependent activation or inactivation; k, slope factor.
Significantly different from control (paired t test, P < 0.05).
Significantly different from control (unpaired t test, P < 0.05).
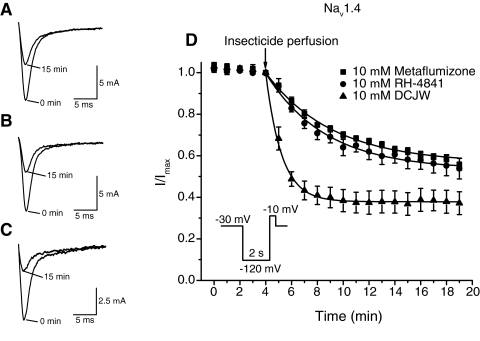
Other SCI insecticides selectively inhibit voltage-gated sodium channels at depolarized membrane potentials that promote slow inactivation (Salgado, 1990, 1992; Silver and Soderlund, 2005a). Figure 3 illustrates the inhibition of sodium currents through Nav1.4 sodium channels by 10 μM metaflumizone (Fig. 3A), RH-4841 (Fig. 3B), and DCJW (Fig. 3C) after 15 min at a holding potential of −30 mV. Figure 3D shows the time course of sodium current inhibition by these compounds. Inhibition by metaflumizone and RH-4841 increased progressively over the 15 min of exposure, whereas inhibition by DCJW reached an apparent steady state after ∼6 min of perfusion. Inhibition by metaflumizone and RH-4841 was slower in onset and less extensive after 15 min than that caused by DCJW.
Fig. 3.
Inhibition of Nav1.4 sodium currents by SCI insecticides. A to C, representative traces of sodium currents through Nav1.4 channels recorded before and after 15 min of perfusion with 10 μM metaflumizone (A), RH-4841 (B), or DCJW (C). D, time course of sodium current inhibition caused by metaflumizone, RH-4841, and DCJW. Oocytes were held at a holding potential of −30 mV and stimulated once every minute with a test pulse (20 ms) to −10 mV that was preceded by a 2-s hyperpolarization to −120 mV. After 4 min of stable control recordings at −30 mV, insecticides were perfused into the bath for 15 min; peak sodium currents were normalized to the mean sodium current amplitude obtained during the 4 min of stable control currents before insecticide perfusion. Data were fitted using a first-order exponential decay function that yielded a single time constant (τ1); values are means ± S.E. of four or more individual experiments in separate oocytes.
Metaflumizone and Other SCI Insecticides Are State-Dependent Inhibitors.
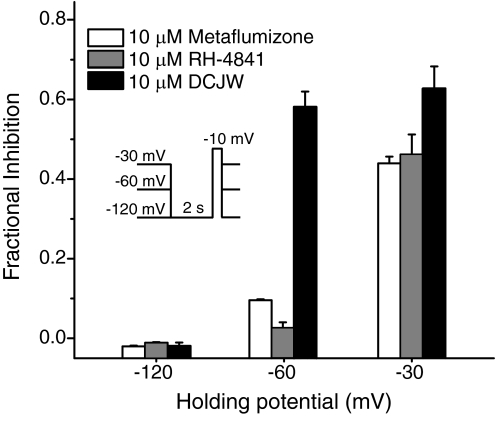
Changes in the transmembrane voltage of the cell determine the conformational state of voltage-gated ion channels, so state-dependent inhibition is often manifested as a voltage-dependent phenomenon. Figure 4 illustrates the voltage-dependent (i.e., state-dependent) inhibition of sodium currents through Nav1.4 channels after 15 min of insecticide exposure. Insecticides had little or no effect on the peak current amplitude when oocytes were clamped at a holding potential of −120 mV, where channels were almost exclusively in the resting state. However, after 15 min of exposure at a holding potential of −60 mV, peak sodium currents were reduced to variable extents by all insecticides tested. Holding the membrane at −30 mV significantly increased the extent of inhibition by metaflumizone and RH-4841, whereas the extent of inhibition at −30 mV by DCJW was not significantly different from that at −60 mV.
Fig. 4.
Voltage-dependent inhibition of Nav1.4 sodium currents by SCI insecticides. Oocytes were clamped at a membrane potential of −120, −60, or −30 mV and stimulated every minute with a test pulse (20 ms) to −10 mV during 15 min of insecticide perfusion; a 2-s hyperpolarization to −120 mV preceded each test pulse in experiments at holding potentials −60 and −30 mV. Fractional inhibition was derived from the amplitude of the peak transient sodium current at the end of the perfusion period normalized to the mean sodium current amplitude in that oocyte obtained after 4 min of stable control recordings before insecticide application.
Metaflumizone Interacts with the LA Receptor on Nav1.4 Channels.
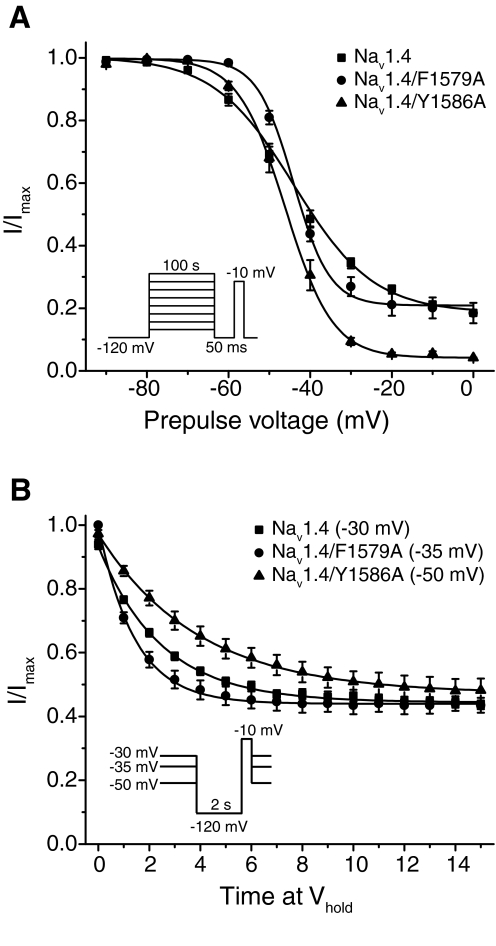
Previous studies (O'Reilly et al., 2000; Bai et al., 2003) have demonstrated that mutations in DIV-S6 that alter local anesthetic binding also modulate slow inactivation gating in a residue-specific manner. Therefore, we initially characterized the effects of the F1579A and Y1586A mutations on the voltage dependence of slow inactivation in the absence of insecticides (Fig. 5A). At depolarized potentials, slow inactivation was more complete in Nav1.4/Y1586A channels (∼95% inactivation at 0 mV) than in Nav1.4/F1579A (∼80% inactivation at 0 mV) or Nav1.4 channels (∼82% inactivation at 0 mV). Neither mutation significantly affected the midpoint potential for the voltage dependence of slow inactivation curve, although the slope was significantly steeper for both Nav1.4/F1579A (V1/2 = −44.1 ± 0.5 mV, k = 5.0 ± 0.4) and Nav1.4/Y1586A (V1/2 = −45.7 ± 0.4 mV, k = 5.6 ± 0.3) channels compared with Nav1.4 (V1/2 = −44.9 ± 0.7 mV, k = 9.3 ± 0.6). To avoid possible effects on insecticide potency caused indirectly by mutation-induced changes in the propensity of channels to occupy high-affinity slow-inactivated states, oocytes were clamped at holding potentials of −35 mV (Nav1.4/F1579A) or −50 mV (Nav1.4/Y1586A) for studies of insecticide action. These holding potentials were empirically determined to produce fractional slow inactivation (∼55%) equivalent to that in Nav1.4 channels clamped at −30 mV (Fig. 5B).
Fig. 5.
Voltage dependence and development of steady-state slow inactivation for Nav1.4, Nav1.4/F1579A and Nav1.4/Y1586A sodium channels. A, the voltage dependence of slow inactivation was examined using the standard protocol described in Fig. 2C and shown in the inset. Amplitudes of peak transient currents measured during a 20-ms depolarization to −10 mV (Nav1.4 and Nav1.4/F1579A) or 0 mV (Nav1.4/Y1586A) after a 100-s conditioning prepulse from a holding potential (Vhold) of −120 mV to potentials ranging from −90 to 0 mV and a 50-ms hyperpolarization to −120 mV were normalized to the maximum current obtained during the inactivation protocol for that oocyte and plotted as a function of the conditioning potential. Values are means ± S.E. from four to six experiments in separate oocytes; curves are fitted to the Boltzmann equation. B, the development of steady-state slow inactivation was assessed using a two-pulse protocol (see inset) in which oocytes were clamped at a Vhold of −30 mV (Nav1.4), −35 mV (Nav1.4/F1579A), or −50 mV (Nav1.4/Y1586A) and stimulated every minute with a 20-ms test pulse to −10 mV (Nav1.4 and Nav1.4/F1579A) or 0 mV (Nav1.4/Y1586A) that was preceded by a 2-s repolarization to −120 mV. Currents were normalized to peak test pulses elicited from a Vhold of −120 mV before depolarization; values are means ± S.E. from nine experiments in separate oocytes. Curves were fitted with a single-exponential decay function.
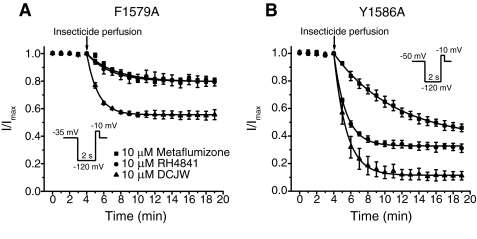
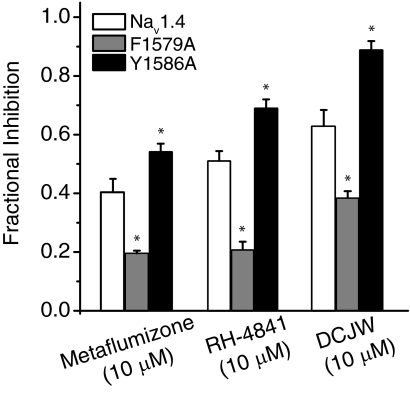
We used the F1579A and Y1586A mutations to determine whether metaflumizone shares common molecular determinants on mammalian sodium channels with other SCI insecticides. Figure 6 shows the time course of inhibition of Nav1.4/F1579A (Fig. 6A) and Nav1.4/Y1586A (Fig. 6B) channels by metaflumizone, RH-4841, and DCJW, and Fig. 7 compares the extent of inhibition at 15 min for Nav1.4, Nav1.4/F1579A, and Nav1.4/Y1586A by these compounds. Nav1.4/F1579A channels were significantly less sensitive to inhibition than Nav1.4 channels, whereas Nav1.4/Y1586A channels were significantly more sensitive to inhibition than Nav1.4 channels.
Fig. 6.
Effects of the F1579A and Y1586A mutations on the inhibition of Nav1.4 sodium channels by SCI insecticides. A, time course of sodium current inhibition in oocytes expressing Nav1.4/F1579A channels by metaflumizone, RH-4841, and DCJW. Oocytes were held at a holding potential of −35 mV and stimulated once every minute with a test pulse (20 ms) to −10 mV that was preceded by a 2-s hyperpolarization to −120 mV. After 4 min of stable control recordings at −35 mV, insecticides were perfused into the bath for 15 min; peak sodium currents were normalized to the mean sodium current amplitude obtained during the 4 min of stable control recordings before insecticide perfusion. Data were fitted using a first-order exponential decay function that yielded a single time constant (τ1); values are means ± S.E. of four to five individual experiments in separate oocytes. B, time course of sodium current inhibition in oocytes expressing Nav1.4/Y1586A channels by metaflumizone, RH-4841, and DCJW. Oocytes were held at a holding potential of −50 mV and stimulated once every minute with a 20-ms test pulse to 0 mV that was preceded by a 2-s hyperpolarization to −120 mV. After 4 min of stable control recordings at −50 mV, insecticides were perfused into the bath for 15 min; peak sodium currents were normalized to the mean sodium current amplitude during the 4 min of stable control recordings before insecticide perfusion. Data were fitted using a first-order exponential decay function that yielded a single time constant (τ1); values are means ± S.E. of three or more individual experiments in separate oocytes.
Fig. 7.
Relative sensitivity of Nav1.4, Nav1.4/F1579A, and Nav1.4/Y1586A sodium channels to inhibition by SCI insecticides. Values are fractional inhibition after 15 min of insecticide perfusion and are derived from data in Figs. 3 and 6. Values for Nav1.4/F1579A and Nav1.4/Y1586A channels marked with asterisks were significantly different from values for inhibition of Nav1.4 channels by the same compound (one-way analysis of variance with Dunnett' post hoc analysis, P < 0.05).
Metaflumizone Binding in the Absence of Inhibition Selectively Attenuates Lidocaine Inhibition of Fast-Inactivated Nav1.4 Channels.
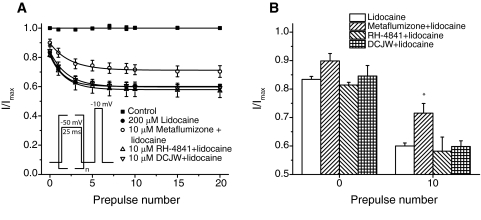
SCI drugs have been shown previously to interfere with the ability of SCI insecticides to inhibit mammalian voltage-gated sodium channels under experimental conditions in which no inhibition by the drugs is observed (Silver and Soderlund, 2005a). However, whether SCI insecticides are reciprocally capable of modulating the extent of inhibition by SCI drugs remains unclear. We therefore assessed the effects of SCI insecticides on Nav1.4 sodium channel inhibition by lidocaine to elucidate whether these insecticides bind to non–slow-inactivated channel states without inhibiting them. Figure 8A shows the effects of high-frequency (20 Hz) stimulation on the extent of sodium channel inhibition by 200 μM lidocaine alone and in the presence of 10 μM metaflumizone, RH-4841, or DCJW. Figure 8B summarizes the statistical analyses of steady-state use-dependent inhibition. Repeated depolarization did not affect the stability of the peak transient sodium current in the absence of SCI drugs and insecticides. Lidocaine reduced peak currents by 16.6 ± 1.1 and 40.0 ± 1.0% after 0 and 10 depolarizing prepulses, respectively. None of the SCI insecticides exhibited any detectable use-dependent inhibition of sodium channels under these conditions (data not shown). However, metaflumizone significantly antagonized the extent of lidocaine inhibition after 10 depolarizing prepulses. Metaflumizone also slightly reduced the extent of tonic sodium channel inhibition by lidocaine after zero prepulses, but this effect was not statistically significant. Neither RH-4841 nor DCJW interfered with the tonic or use-dependent inhibition of sodium currents by lidocaine.
Fig. 8.
Use-dependent lidocaine inhibition of sodium channels in the absence and presence of SCI insecticides. A, effects of 10 μM metaflumizone, RH-4841, or DCJW on the extent of inhibition by lidocaine (200 μM) after 0 to 20 depolarizing prepulses (25 ms) from −120 to −50 mV at 20 Hz. Values were normalized to the mean peak sodium current amplitude measured from the same oocyte before the perfusion of lidocaine. Data points are means ± S.E. of five (lidocaine, metaflumizone + lidocaine, RH-4841 + lidocaine) or four (DCJW + lidocaine) separate experiments with different oocytes; curves were fitted to mean data points using a single-exponential decay function. B, comparison of the extent of resting (after 0 prepulses) and fast-inactivated (after 10 prepulses) sodium channel inhibition by lidocaine in the absence or presence of metaflumizone, RH-4841, or DCJW. Values for use-dependent inhibition marked with an asterisk (*) are significantly (P < 0.05) different from use-dependent inhibition by lidocaine alone.
Discussion
This is the first report of the action of metaflumizone on mammalian voltage-gated sodium channels. The gating properties of Nav1.4+β1 channel complexes that we describe here are in good agreement with the properties of these channels reported in previous studies. Minor differences between our data and previously published data can likely be explained by differences in experimental conditions, such as the choice of expression system or the details of the pulse protocols employed.
Molecules that modify ion channel function often interact preferentially with channels in one conformational state. Negative holding potentials placed Nav1.4 channels predominantly in the resting state, making them virtually insensitive to inhibition by the highest soluble concentration of metaflumizone or any other SCI insecticide tested. Holding the membrane potential at −60 mV caused partial inactivation of channels and facilitated insecticide inhibition. In contrast to DCJW, the extent of inhibition by metaflumizone and RH-4841 was significantly greater at −30 mV, where channel inactivation was more complete, than at −60 mV. These findings are consistent with previous studies of metaflumizone and other SCI insecticides (Silver and Soderlund, 2005a; Salgado and Hayashi, 2007) and have been interpreted as evidence that SCI insecticides preferentially interact with slow-inactivated channels.
Fast inactivation in sodium channels terminates sodium conductance within milliseconds after depolarization and involves occlusion of the inner pore of the channel by the highly conserved inactivation gate in the intracellular linker between domains III and IV (Goldin, 2003). However, channel opening is not a prerequisite for fast inactivation, because channels can enter the fast-inactivated state from the resting state after brief depolarization to potentials below the activation threshold (Ragsdale et al., 1996). Under our experimental conditions, metaflumizone had no effect on the voltage dependence of fast inactivation that was measured with standard 200-ms prepulses. This result is consistent with data from recent studies with metaflumizone on cloned cockroach sodium channels expressed in X. laevis oocytes (Silver et al., 2009) and the current hypothesis that SCI insecticides inhibition occurs selectively only under experimental conditions that promote slow inactivation.
Slow inactivation of sodium channels occurs after sustained depolarization or prolonged high-frequency stimulation and exhibits development and recovery kinetics on the order of seconds to minutes (for reviews, see Vilin and Ruben, 2001; Ulbricht, 2005). The molecular basis of slow inactivation is poorly understood, but it is different from fast inactivation because internal protease perfusion or amino acid substitutions that disable the fast-inactivation gate do not interfere with slow inactivation (Goldin, 2003). Slow inactivation of Nav1.4 channels in our assays developed at potentials negative to channel opening but positive to fast inactivation, as previously reported (O'Reilly et al., 2001; McNulty and Hanck, 2004). Metaflumizone enhanced the extent of slow inactivation and caused a modest hyperpolarizing shift in the voltage dependence of slow inactivation. These effects provide further evidence that SCI insecticides preferentially target and stabilize channels in a slow-inactivated state (Salgado, 1992). However, the 100-s conditioning pulses used to measure slow inactivation were too short to allow the slow inhibition by metaflumizone to develop completely. Therefore, our data are likely to underestimate the magnitude of the effects of metaflumizone on the voltage dependence of slow inactivation. In contrast to our data, Silver et al. (2009) reported that metaflumizone caused a modest depolarizing shift in the voltage dependence of slow inactivation of cloned cockroach sodium channels expressed in X. laevis oocytes. This discrepancy may reflect differences in the pulse protocols used to characterize the voltage dependence of slow inactivation in the two studies.
Substituting alanine at Phe1579 or Tyr1586 in the rat Nav1.4 channel produced marked effects on metaflumizone and RH-4841 sensitivity that are consistent with previous studies in Nav1.4 with other SCI insecticides (Silver and Soderlund, 2007). The F1579A mutation reduced the sensitivity to metaflumizone, RH-4841, and DCJW, an effect that is also consistent with the impact of this mutation on the state-dependent inhibition of SCI drugs (Wang et al., 1998). These data indicate that metaflumizone and RH-4841 share common molecular determinants on mammalian sodium channels with other SCI insecticides and therapeutic drugs acting at the LA receptor. The Y1586A mutation significantly increased the sensitivity of Nav1.4 to metaflumizone and RH-4841, which is consistent with results from previous studies with this sodium channel isoform and other compounds in this insecticide class. Mutations at this position seem to strongly influence the state-dependent inhibition of local anesthetics but not of other therapeutic SCI drugs (for review, see Mike and Lukacs, 2010). Studies of the impact of different amino acid substitutions at Tyr1717 in rat Nav1.3 channels (analogous to Tyr1586 in Nav1.4) expressed in X. laecis oocytes suggest that this residue does not directly contribute to the LA receptor (Li et al., 1999). A molecular model of the LA receptor in Nav1.4 channels indicates that the convergence of S6 segments constrains space in the inner pore, especially in the region of Tyr1586 (Lipkind and Fozzard, 2005). We hypothesize that the much smaller alanine side chain in the Y1586A mutation indirectly increases SCI insecticide inhibition of rat Nav1.4 channels by reducing steric hindrance, thereby enhancing binding interaction between the insecticide and other inner-pore residues, as suggested previously (Silver and Soderlund, 2007).
Perfusion of metaflumizone at a hyperpolarized holding potential reduced the voltage-dependent probability of sodium channel activation, as indicated by the depolarizing shift in the midpoint potential of the conductance-voltage curve. Consequently, the amplitude of sodium currents measured during test pulses to submaximal activation potentials (i.e., −30 mV and −20 mV) were significantly reduced in the presence of metaflumizone compared with the control. These results imply that metaflumizone interacts with Nav1.4 channels in the resting state. Likewise, Salgado and Hayashi (2007) reported a slight decrease in the maximal amplitude of sodium currents through Drosophila melanogaster Para/TipE channels expressed in X. laevis oocytes after perfusion of 10 μM metaflumizone at a holding potential where channels were predominantly in the resting state. However, effects of other SCI insecticides on activation gating are not observed in assays with rat Nav1.4 channels expressed in oocytes (Silver and Soderlund, 2005a), tetrodotoxin-sensitive or -resistant sodium channels in rat dorsal root ganglion neurons (Tsurubuchi et al., 2001; Tsurubuchi and Kono, 2003; Zhao et al., 2003) or sodium channels in cockroach dorsal unpaired median neurons (Lapied et al., 2001). Taken together, these results imply that metaflumizone, unlike other SCI insecticides, interacts with Nav1.4 channels in the resting state. Additional experiments are needed to determine how the F1579A and Y1586A mutations influence the effect of metaflumizone on the voltage dependence of activation. The results of these experiments may help to clarify whether the effects of metaflumizone on activation and slow inactivation involve a single binding site.
The effects of metaflumizone on activation gating led us to examine whether metaflumizone or other SCI insecticides interact with resting and fast-inactivated channels despite the lack of evidence for block from these states. We used lidocaine as a model SCI to establish conditions for block from the resting state and the use-dependent enhancement of block of fast-inactivated channels. Although none of the insecticides alone exhibited any use dependence in response to high-frequency pulse trains that caused significant fast inactivation from the resting state and enhancement of lidocaine inhibition, metaflumizone was unique in its ability to interfere with use-dependent inhibition by lidocaine. These results offer further evidence that binding of SCI insecticides to channels is not always accompanied by channel inhibition (Silver and Soderlund, 2005a, 2007) and suggest that metaflumizone, unlike other rigid, nearly planar SCI insecticides, is able to bind to the LA receptor in multiple conformational states. It is noteworthy that metaflumizone did not significantly affect the level of resting inhibition by lidocaine that was evident upon depolarization after zero prepulses. Resting and inactivated sodium channels may possess fundamentally distinct drug binding sites (Hille, 1977; Lenkey et al., 2011). Thus, the ability of metaflumizone to antagonize lidocaine inhibition of fast-inactivated but not resting sodium channels offers further evidence for different drug receptor conformations on resting and inactivated sodium channels.
Selective binding to sodium channels in slow-inactivated states may also produce therapeutically useful effects. The antihypertensive drug mibefradil (McNulty and Hanck, 2004), the anticonvulsant lacosamide (Errington et al., 2008), and the investigational compound for neuropathic pain Z123212 (Hildebrand et al., 2011) preferentially target sodium channels in the slow-inactivated state. However, the properties that distinguish between neurotoxic and therapeutic SCIs are presently unknown. Therefore, SCI insecticides represent additional novel and unique chemical probes that may be useful for understanding the molecular details of ligand-receptor interactions that underlie the toxic or therapeutic effects of agents that target slow-inactivated channels.
Acknowledgments
We thank P. Adams and S. Kopatz for technical assistance and R. Araújo and S. McCavera for critical review of the manuscript.
This work was supported by the National Institutes of Health National Institute of Environmental Health Sciences [Grant R01-ES014591].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
- SCI
- sodium channel inhibitor
- Nav
- voltage-gated sodium
- DCJW
- decarbomethoxylated (S)-methyl 7-chloro-2,5-dihydro-2-[[(methoxycarbonyl)[4-(trifluoromethoxy)phenyl]amino]carbonyl]indeno]1,2-e][1,3,4]oxadiazine-4a(3H)-carboxylate
- RH-4841
- N,3-bis(4-chlorophenyl)-4,5-dihydro-4-phenyl-1H-pyrazole-1-carboxamide
- LA
- local anesthetic
- DIV-S6
- domain IV segment S6
- DMSO
- dimethyl sulfoxide.
Authorship Contributions
Participated in research design: von Stein and Soderlund.
Conducted experiments: von Stein.
Performed data analysis: von Stein.
Wrote or contributed to the writing of the manuscript: von Stein and Soderlund.
References
- Bai CX, Glaaser IW, Sawanobori T, Sunami A. (2003) Involvement of local anesthetic binding sites on IVS6 of sodium channels in fast and slow inactivation. Neurosci Lett 337:41–45 [DOI] [PubMed] [Google Scholar]
- Balser JR, Nuss HB, Orias DW, Johns DC, Marban E, Tomaselli GF, Lawrence JH. (1996) Local anesthetics as effectors of allosteric gating. Lidocaine effects on inactivation-deficient rat skeletal muscle Na channels. J Clin Invest 98:2874–2886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezanilla F, Armstrong CM. (1977) Inactivation of the sodium channel. I. Sodium current experiments. J Gen Physiol 70:549–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Errington AC, Stöhr T, Heers C, Lees G. (2008) The investigational anticonvulsant lacosamide selectively enhances slow inactivation of voltage-gated sodium channels. Mol Pharmacol 73:157–169 [DOI] [PubMed] [Google Scholar]
- Goldin AL. (1992) Maintenance of Xenopus laevis and oocyte injection. Methods Enzymol 207:266–279 [DOI] [PubMed] [Google Scholar]
- Goldin AL. (2003) Mechanisms of sodium channel inactivation. Curr Opin Neurobiol 13:284–290 [DOI] [PubMed] [Google Scholar]
- Grosscurt AC, van Hes R, Wellinga K. (1979) 1-Phenylcarbamoyl-2-pyrazolines, a new class of insecticides. 3. Synthesis and insecticidal properties of 3,4-diphenyl-1-phenylcarbamoyl-2-pyrazolines. J Agric Food Chem 27:406–409 [Google Scholar]
- Hasan R, Nishimura K, Okada M, Akamatsu M, Inoue M, Ueno T. (1996) Stereochemical basis for the insecticidal activity of carbamoylated and acylated pyrazoline. Pestic Sci 46:105–112 [Google Scholar]
- Hildebrand ME, Smith PL, Bladen C, Eduljee C, Xie JY, Chen L, Fee-Maki M, Doering CJ, Mezeyova J, Zhu Y, et al. (2011) A novel slow-inactivation-specific ion channel modulator attenuates neuropathic pain. Pain 152:833–843 [DOI] [PubMed] [Google Scholar]
- Hille B. (1977) Local anesthetics: hydrophilic and hydrophobic pathways for the drug-receptor interaction. J Gen Physiol 69:497–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapied B, Grolleau F, Sattelle DB. (2001) Indoxacarb, an oxadiazine insecticide, block insect neuronal sodium channels. Br J Pharmacol 132:587–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenkey N, Karoly R, Epresi N, Vizi E, Mike A. (2011) Binding of sodium channel inhibitors to hyperpolarized and depolarized conformations of the channel. Neuropharmacology 60:191–200 [DOI] [PubMed] [Google Scholar]
- Li HL, Galue A, Meadows L, Ragsdale DS. (1999) A molecular basis for the different local anesthetic affinities of resting versus open and inactivated states of the sodium channel. Mol Pharmacol 55:134–141 [DOI] [PubMed] [Google Scholar]
- Lipkind GM, Fozzard HA. (2005) Molecular modeling of local anesthetic drug binding by voltage-gated sodium channels. Mol Pharmacol 68:1611–1622 [DOI] [PubMed] [Google Scholar]
- McNulty MM, Hanck DA. (2004) State-dependent mibefradil block of Na+ channels. Mol Pharmacol 66:1652–1661 [DOI] [PubMed] [Google Scholar]
- Mike A, Lukacs P. (2010) The enigmatic drug binding site for sodium channel inhibitors. Curr Mol Pharmacol 3:129–144 [DOI] [PubMed] [Google Scholar]
- O'Reilly JP, Wang SY, Wang GK. (2000) A point mutation in domain 4-segment 6 of the skeletal muscle sodium channel produces an atypical inactivation state. Biophys J 78:773–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Reilly JP, Wang SY, Wang GK. (2001) Residue-specific effects on slow inactivation at V787 in D2–S6 of Nav1.4 sodium channels. Biophys J 81:2100–2111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragsdale DS, McPhee JC, Scheuer T, Catterall WA. (1996) Common molecular determinants of local anesthetic, antiarrhythmic, and anticonvulsant block of voltage-gated Na+ channels. Proc Natl Acad Sci USA 93:9270–9275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salgado VL. (1990) Mode of action of insecticidal dihydropyrazoles: selective block of impulse generation in sensory nerves. Pestic Sci 28:389–411 [Google Scholar]
- Salgado VL. (1992) Slow voltage-dependent block of sodium channels in crayfish nerve by dihydropyrazole insecticides. Mol Pharmacol 41:120–126 [PubMed] [Google Scholar]
- Salgado VL, Hayashi JH. (2007) Metaflumizone is a novel sodium channel blocker insecticide. Vet Parasitol 150:182–189 [DOI] [PubMed] [Google Scholar]
- Scholz A. (2002) Mechanism of (local) anesthetics on voltage-gated sodium and other ion channels. Br J Anaesth 89:52–61 [DOI] [PubMed] [Google Scholar]
- Silver K, Soderlund DM. (2005a) State-dependent block of rat Nav1.4 sodium channels expressed in Xenopus oocytes by pyrazoline-type insecticides. Neurotoxicology 26:397–406 [DOI] [PubMed] [Google Scholar]
- Silver KS, Soderlund DM. (2005b) Action of pyrazoline-type insecticides at neuronal target sites. Pestic Biochem Physiol 81:136–143 [Google Scholar]
- Silver KS, Soderlund DM. (2006) Differential sensitivity of rat voltage-sensitive sodium channel isoforms to pyrazoline-type insecticides. Toxicol Appl Pharmacol 214:209–217 [DOI] [PubMed] [Google Scholar]
- Silver KS, Soderlund DM. (2007) Point mutations at the local anesthetic receptor site modulate the state-dependent block of rat Nav1.4 sodium channels by pyrazoline-type insecticides. Neurotoxicology 28:655–663 [DOI] [PubMed] [Google Scholar]
- Silver KS, Nomura Y, Salgado VL, Dong K. (2009) Role of the sixth transmembrane segment of domain IV of the cockroach sodium channel in the action of sodium channel blocker insecticides. Neurotoxicology 30:613–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TJ, Soderlund DM. (1998) Action of the pyrethroid insecticide cypermethrin on rat brain IIa sodium channels expressed in Xenopus oocytes. Neurotoxicology 19:823–832 [PubMed] [Google Scholar]
- Tatebayashi H, Narahashi T. (1994) Differential mechanism of action of the pyrethroid tetramethrin on tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels. J Pharmacol Exp Ther 270:595–603 [PubMed] [Google Scholar]
- Tsurubuchi Y, Zhao X, Nagata K, Kono Y, Nishimura K, Yeh JZ, Narahashi T. (2001) Modulation of tetrodotoxin-resistant sodium channels by dihydropyrazole insecticide RH-3421 in rat dorsal root ganglion neurons. Neurotoxicology 22:743–753 [DOI] [PubMed] [Google Scholar]
- Tsurubuchi Y, Kono Y. (2003) Modulation of sodium channels by the oxadiazine insecticide indoxacarb and its N-decarbomethoxylated metabolite in rat dorsal root ganglion neurons. Pest Manag Sci 59:999–1006 [DOI] [PubMed] [Google Scholar]
- Ulbricht W. (2005) Sodium channel inactivation: molecular determinants and modulation. Physiol Rev 85:1271–1301 [DOI] [PubMed] [Google Scholar]
- Vilin YY, Ruben PC. (2001) Slow inactivation in voltage-gated sodium channels: molecular substrates and contributions to channelopathies. Cell Biochem Biophys 35:171–190 [DOI] [PubMed] [Google Scholar]
- Wagner LE, 2nd, Gingrich KJ, Kulli JC, Yang J. (2001) Ketamine blockade of voltage-gated sodium channels: evidence for a shared receptor site with local anesthetics. Anesthesiology 95:1406–1413 [DOI] [PubMed] [Google Scholar]
- Wang GK, Quan C, Wang S. (1998) A common local anesthetic receptor for benzocaine and etidocaine in voltage-gated μ1 Na+ channels. Pflugers Arch 435:293–302 [DOI] [PubMed] [Google Scholar]
- Wellinga K, Grosscurt AC, van Hes R. (1977) 1-Phenylcarbamoyl-2-pyrazoline: a new class of insecticides. 1. Synthesis and insecticidal properties of 3-phenyl-1-phenylcarbamoyl-2-pyrazoline. J Agric Food Chem 25:987–992 [Google Scholar]
- Wing KD, Andaloro JT, McCann SF, Salgado VL. (2005) Indoxacarb and the sodium channel blocker insecticides: chemistry, physiology, and biology in insects, in Comprehensive Molecular Insect Science (Gilbert LI, Iatrou K, Gill SS. eds) pp 30–53, Elsevier, New York [Google Scholar]
- Zhao X, Ikeda T, Yeh JZ, Narahashi T. (2003) Voltage-dependent block of sodium channels in mammalian neurons by the oxadiazine insecticide indoxacarb and its metabolite DCJW. Neurotoxicology 24:83–96 [DOI] [PubMed] [Google Scholar]