Abstract
Copper is an essential micronutrient for cell growth but is toxic in excess. Copper transporter (Ctr1) plays an important role in regulating adequate copper levels in mammalian cells. We have shown previously that expression of the human high-affinity copper transporter (hCtr1) was transcriptionally up-regulated under copper-depleted conditions and down-regulated under replete conditions; moreover, elevated hCtr1 levels suppress hCtr1 expression. Specificity protein 1 (Sp1) regulates expression of hCtr1 under copper-stressed conditions. In this study, we made the following important observations: 1) Sp1 expression is down-regulated under copper-replete conditions but up-regulated under copper-depleted conditions. These up- and down-regulations of Sp1 in turn regulate hCtr1 expression to control copper homeostasis. 2) Copper-regulated Sp1 expression involved Sp1 binding to its own promoter as demonstrated by the chromatin immunoprecipitation assay; therefore, Sp1 is also transcriptionally self-regulated via hCtr1/copper intermediation. 3) Both zinc finger and glutamine-rich transactivation domains of Sp1 are involved in the Sp1-mediated hCtr1 and Sp1 regulation by copper stresses. 4) Although Sp3 expression is also regulated by copper availability, Sp3 does not regulate hCtr1 homeostasis. Collectively, our results demonstrated that mammalian cells use Sp1 oscillation in response to copper availability to regulate copper homeostasis through hCtr1 expression in a tripartite inter-regulatory relationship. These findings have important implications in mammalian copper physiology regulation.
Introduction
Copper is a micronutrient that plays an essential role in normal human physiology. Copper deficiency in mammals causes embryonic and neonatal abnormalities in many systems including hematopoietic, cardiovascular, central nervous system, and liver functions. Moreover, copper is a redox-active element, existing in both the Cu+ and Cu2+ states, and excess copper ions cause damages to many vital organs as manifested in Wilson's disease (Collins et al., 2010). An evolutionarily conserved mechanism for maintaining cellular copper homeostasis in response to stresses induced by copper deficiency or excess in eukaryotic cells relies on the interplay among copper transporters (Ctrs) for absorption and storage, chaperones for delivering copper to various compartments, and exporters (ATP7A and ATP7B) for the elimination of excess copper (for review, see Kim et al., 2008). However, the mechanisms that regulate copper homeostasis in mammalian cells are largely unknown.
Many studies have demonstrated that regulation of the copper homeostasis initial response to copper stresses resides on Ctr1 (for reviews, see Kuo et al., 2007; Kim et al., 2008). The human high-affinity Ctr1 (hCtr1) is a single polypeptide of 190 amino acids consisting of three transmembrane motifs—a methionine-rich N terminus, a cysteine-histidine cluster in the C terminu,s and an MXXXM in the second transmembrane domain—that are important for the copper transport function (for reviews, see Kuo et al., 2007; Kim et al., 2008). In addition to its important role in regulating copper physiology, hCtr1 is also known as a transporter for platinum drugs (Ishida et al., 2002); reduced expression of hCtr1 is often associated with platinum drug resistance in cultured cells (Kuo et al., 2007; Howell et al., 2010) and in clinical settings (Ishida et al., 2010; Chen et al., 2011).
Soon after cells are treated with excess copper, Ctr1 protein is internalized and degraded after an endocytic process (Ooi et al., 1996; Petris et al., 2003). Upon removal of copper treatment, the internalized copper recycles back to the membrane (Molloy and Kaplan, 2009), supporting a post-translational mechanism of Ctr1 expression in response to copper stress. Post-translational regulation of Ctr1 in response to copper stress was observed in mice fed copper-deficient diets (Nose et al., 2010).
Transcriptional regulation of hCtr1 in response to copper stresses has also been reported. We demonstrated previously that in cultured cells overexpressing the physiological copper chelator GSH, hCtr1 mRNA and protein levels were increased by transfecting the recombinant DNA encoding γ-glutamylcysteine synthetase, the rate-limiting enzyme of GSH synthesis (Chen et al., 2008). We also demonstrated that expression of hCtr1 mRNA can be induced by treating cells with the copper chelator bathophenanthroline disulfonic acid and down-regulated by treating with CuSO4 (Song et al., 2008). The endogenous hCtr1 (endo-hCtr1) expression levels can be modulated by transfection with recombinant DNA encoding hCtr1 constructs (referred to here as exo-hCtr1). That is, down-regulation of endo-hCtr1 was also observed in cells transfected with the wild-type hCtr1 (hCtr1-wt) plasmid (Song et al., 2008) to increase copper transport activities; up-regulation of endo-hCtr1 mRNA was found in cells overexpressing dominant-negative hCtr1 (hCtr1-DN) by interfering with copper transport activities. These hCtr1-DN recombinants were constructed by site-directed mutagenesis of several critical M residues located at the ecto- or the transmembrane MXXXM domains that are important for copper transport (Liang et al., 2009). Regulation of hCtr1 expression is mediated by the transcription factor Sp1, which interacts with the three GC boxes located at the hCtr1 promoter (Song et al., 2008).
The missing piece of this transcriptional regulation mechanism of mammalian copper homeostasis is how Sp1 itself behaves in response to copper stresses. The current study was initiated to fill in this gap. We observed that Sp1 expression is also regulated by copper stresses, either by changing copper concentrations in cultured cells or by manipulating hCtr1 expression levels by transfection. Our results demonstrated that human cells use Sp1 oscillation in response to copper stresses in regulating hCtr1 expression to maintain copper homeostasis. These results, together with our previous findings (Song et al., 2008; Liang et al., 2009), which were further confirmed here, allowed us to construct a tripartite inter-regulatory model consisting of Sp1, hCtr1, and copper to regulate mammalian copper homeostasis. Our findings thus provide important new insights into mammalian copper physiology regulation. They also have translational implications for the use of copper modulators in regulating the treatment efficacy of platinum-based cancer chemotherapy.
Materials and Methods
Cell Lines, Recombinant DNA, and Reagents.
Small-cell lung cancer (SCLC) cells were obtained from author N.S., and HEK293 cells were obtained from the American Type Culture Collection (Manassas, VA). Establishment of SCLC-hCtr1-wt and SCLC-hCtr1-DN stable cell lines has been described previously (Liang et al., 2009). Expression-recombinant DNA for Sp1-wt, and Sp2, Sp3, and Sp4 were described previously (Tsai et al., 2009). Recombinant LexA-VP16 and reporter pLG-(Gal4)5-(Lex4)2-E1B-Luc (LexA-Luc) were obtained from Mark Ptashne (Memorial Sloan-Kettering Cancer Center, New York, NY). Tetrathiomolybdate (TM) was obtained from Sigma-Aldrich (St. Louis, MO).
Recombinant DNA Constructs and Transient Transfection Assays.
To construct recombinant DNA encoding different domains of Sp1, double-stranded cDNA fragments were synthesized by polymerase chain reaction (PCR) using appropriate primer sets, each tagged with a NotI recognition sequence (available upon request), and full-length Sp1 cDNA as the template. The synthesized DNA was digested by NotI and cloned into the NotI site of the CIN-HA-pcDNA3 vector (Song et al., 2004), and a neomycin-resistance marker was used for transfection selection.
The Sp1-Luc reporter construct was obtained from Dr. C. J. Ciudad (University of Barcelona, Barcelona, Spain) (Nicolás et al., 2001). Site-directed mutations in the Sp1 promoter sequences in the reporter constructs (Sp1-M1 to Sp1-M10) were performed using the QuikChange site-directed mutagenesis kit (Agilent Technologies, Santa Clara, CA). Mutations in the Q-rich2 domain of the Sp1-M3 recombinant were constructed using the same procedure.
For constructing chimerical recombinants encoding the DNA-binding domain (DBD) of LexA or Sp1 fused to transactivating domain (TAD) from VP16 or Sp1, we used the two-step recombinant PCR method using recombinant LexA(1–102)-VP16(410–490) and Sp1 cDNA as templates and appropriate primer sets (available upon request) in the PCRs. The resulting DNA fragments were cloned into the NotI site of the CIN-HA-pcDNA3 vector. All plasmids were confirmed by DNA sequencing. Transient transfection assays were performed using Lipofectamine (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. Luciferase expression was measured by illuminometer.
Measurement of Endo-mRNA and Exo-mRNA Levels Using the RNase Protection Assay.
Plasmid DNA was transfected into cultured cells using lipofectamine. Expression of exo- and endo-mRNA was determined by the RNA protection assay (RPA) using RNase RPA III Assay Kit (Applied Biosystems/Ambion, Austin, TX). The design of RNA probes that can simultaneously detect both endo- and exo-mRNA transcripts in the transfected cells were described previously (Song et al., 2008; Liang et al., 2009).
Chromatin Immunoprecipitation Assay.
The ChIP assay was performed with a kit (Millipore, Billerica, MA) following the manufacturer's instructions. The hCtr1 and Sp1 promoter DNA sequences were determined by PCR using appropriate primer sets (available upon request) according to procedures described previously (Tsai et al., 2009).
Western Blotting and Immunohistochemical Staining.
Procedures for Western blotting using anti-hCtr1 (Kuo et al., 2001) and anti-Sp1 antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) (Song et al., 2008; Liang et al., 2009) and immunocytochemical staining after confocal microscopy (Chen et al., 2008) were described previously.
Results
Mammalian Copper Homeostasis Is Regulated within the Copper-hCtr1-Sp1 Inter-Regulatory Loop.
Regulation of mammalian copper homeostasis is a complex mechanism involving interplay among copper, hCtr1, and Sp1. For clarity, we will present experimental results in sequential order (Fig. 1, A–H) that leads to the development of the copper-hCtr1-Sp1 tripartite inter-regulatory model as depicted in Fig. 1I. To present this model in its entirety, some results presented in this figure were confirmation of our previous studies and will be so indicated.
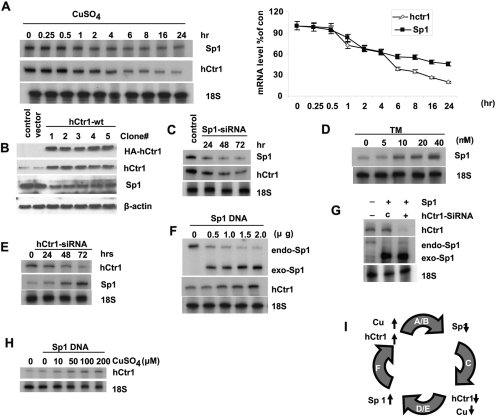
Fig. 1.
Inter-regulatory relationships among copper, hCtr1, and Sp1 in copper homeostasis. All experiments were performed in SCLC cells except where otherwise indicated, in all cases where 18S RNA measurements were used as loading controls for the RPA, and β-actin or α-tubulin measurements were used as loading controls for the Western blot. A, autoradiograph of RNA protection assay showing the time-dependent inhibition of Sp1 and hCtr1 expression (left) and densitometric measurement of the autoradiographs (right). Error bars show S.D. from three experiments. Note that the time was not drawn in scale. B, down-regulation of Sp1 expression by transfection with HA-hCtr1-wt DNA as determined by Western blotting using the indicated antibodies. The numbers 1 to 5 denote the five independent cell lines used. C, down-regulation of hCtr1 expression by Sp1-siRNA. D, concentration-dependent up-regulation of Sp1 by TM. E, time-dependent up-regulation of Sp1 after transfection with hCtr1-siRNA. F, up-regulation of endo-hCtr1 and down-regulation of endo-Sp1 expression by transfecting exo-Sp1 recombinant analyzed by RNP using probe as described below (Fig. 4A). G, down-regulation of endo-Sp1 induced by transfection with exo-Sp1 was partially suppressed by cotransfection with hCtr1 siRNA. c, control siRNA. H, increased hCtr1 expression in CuSO4-treated cells by transfection with Sp1 recombinant. I, schematic diagram showing a summary of the results. The letters inside the curved arrows refer to the results shown in A to F. The upward arrows denote increased levels. Downward arrows denote decreased levels. The thick curved arrows point to the results of the treatments. As an example for A, increased copper concentration down-regulates Sp1 is indicated by (hCtr1↑ → Sp1↓). Note that maintenance of copper homeostasis in human cells is controlled by the cyclic regulatory mechanism as shown.
Figure 1A shows that treating SCLC cells with CuSO4 resulted in time-dependent down-regulation of Sp1 and hCtr1 mRNA as determined by RPA. Quantitative analyses revealed that no apparent changes in Sp1 and hCtr1 mRNA were observed within the first 30 min of treatment. Reduction of Sp1 and hCtr1 mRNA levels began to decrease thereafter and reductions of approximately 40 and 70%, respectively, were observed 24 h after the treatment. Likewise, overexpression of HA-hCtr1-wt by transfection of recombinant plasmid DNA in five independently established clones resulted in down-regulation of Sp1 expression (Fig. 1B), because increased hCtr1-wt expression increases the copper transport activities and thus cellular copper contents. These latter results together with those shown in Fig. 1A indicate that Sp1 expression is down-regulated under copper-replete conditions. Figure 1C shows that knockdown Sp1 by siRNA approach reduces the expression of hCtr1. This result is consistent with our previous report showing that Sp1 functions as a positive regulator for hCtr1 expression (Song et al., 2008). In contrast, Fig. 1D shows that treating SCLC cells with the copper chelator TM up-regulates Sp1 expression as determined by Western blot analysis. Moreover, knockdown of hCtr1 by siRNA resulted in up-regulation of Sp1 (Fig. 1E). These results demonstrated that expression of Sp1 is up-regulated under copper-depleted conditions. Figure 1F shows a concentration-dependent induction of endo-hCtr1 expression by transfection with Sp1-wt recombinant (exo-Sp1), consistent with our previous results showing that Sp1 is a positive regulator for hCtr1 (Song et al., 2008). Here, we show that in these Sp1-wt-transfected cells, the expression of endo-Sp1 is suppressed, as determined by the RPA using a probe that can differentiate between exo- and endo-Sp1 mRNA species (Figs. 1F and 4A, probe design). These results demonstrated that Sp1 expression, like that of hCtr1, is self-regulated, and that the self-regulation mechanism is mediated by hCtr1 up-regulation, because knockdown of hCtr1 by siRNA inhibits Sp1 self-regulation (Fig. 1G). We also demonstrated that suppression of hCtr1 by copper treatment is mediated by down-regulation of Sp1, because replenishment of Sp1 by transfection with Sp1-wt expression recombinant up-regulates hCtr1 (Fig. 1H) instead of down-regulating hCtr1 as when cells were treated with copper alone (Fig. 1A). These results not only support the inter-regulatory relationships among copper, hCtr1, and Sp1 but also demonstrate that each of them is self-regulated within the context of overall mammalian copper homeostasis regulation as depicted in Fig. 1I.
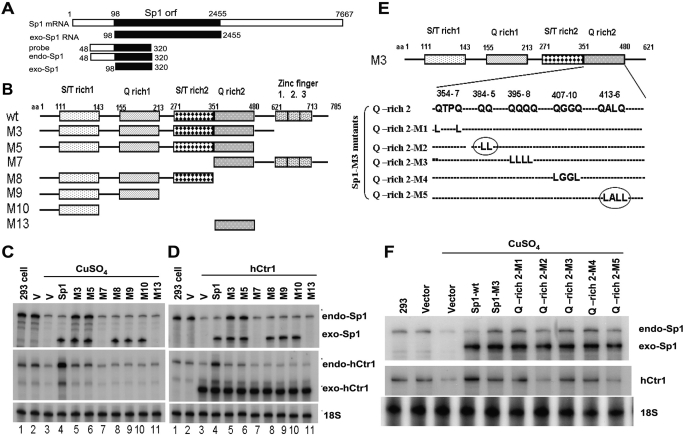
Fig. 4.
The effects of various Sp1 domains and nucleotide residues on the copper-mediated expression of endo-hCtr1 and endo-Sp1. A, a schematic diagram showing the design of the hybridization probe used in RPA for measuring endo- and exo-Sp1 mRNA in transfection experiments. B, a schematic diagram showing the recombinant Sp1 encoding the wild-type (wt) and deleted mutants (M3–M13). Sp1-wt contains two serine/threonine-rich (S/T rich1 and S/T rich2) and two glutamine-rich (Q-rich) domains and three ZF domains. Numbers refer to the amino acid positions from the N terminus. C, effects of various truncated Sp1 on endo-Sp1 and endo-hCtr1 mRNA expression under copper-replete conditions. HEK293 cells were transfected with empty vector alone (V), Sp1-wt, or various Sp1 mutants (M3–M13) as indicated. Cells were treated with 100 μM CuSO4 for 16 h. Endo-hCtr1 mRNA, exo-Sp1 mRNA, and endo-Sp1mRNA levels were determined by the RPA, using 18S RNA as the loading control. D, effects of various truncated Sp1 on endo-Sp1 and endo-hCtr1 mRNA expression under copper-replete conditions. The experimental design was similar to that shown in (C) except that hCtr1-wt recombinant was used in cotransfection into the cells to increase the copper contents. Note that lanes 7 (M7) and 11 (M13) did not show the exo-Sp1 signals because these mutants Sp1 do not contain the N-terminal sequence and therefore cannot be detected by the RPA probe. Instead, their expression was verified by Western blot analysis using anti-HA antibody (data not shown). E, a schematic diagram showing the mutated amino acids (Gln to Leu) in the Q-rich2 domain of Sp1-M3 recombinant. Numbers refer to the positions of amino acids. Circles refer to important amino acid residues for the copper-regulated Sp1 effects. F, effects of Q-rich2 mutations on the expression of endo-Sp1 and endo-hCtr1 under copper treatment. Recombinant constructs as shown in E were transfected into HEK293 cells. Cells were treated with 100 μM CuSO4 for 16 h and the expression levels of endo-Sp1, exo-Sp1, and endo-hCtr1 RNA levels were determined by RPA.
Sp1 Is the Sensor of Copper Stresses.
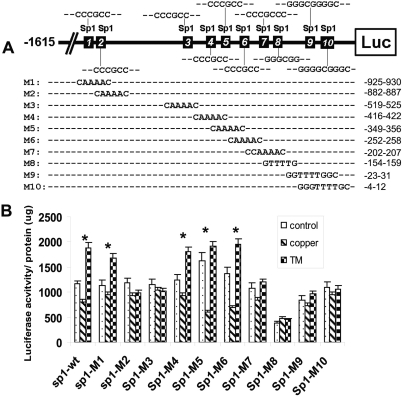
The results shown above strongly suggest that mammalian cells use Sp1 oscillation in response to copper imbalance to drive copper homeostatic regulation. This scenario underscores the role of Sp1 as the sensor of copper stresses. To strengthen these observations, we first determined whether copper-sensing Sp1 expression is mediated through the Sp1-binding sites located at the Sp1 promoter. We used a reporter construct that contains 1615 nucleotides upstream from the transcription start site of Sp1 linked to a bacterial luciferase gene. Ten putative Sp1-binding sites are located within this promoter region. We mutated each of these sites (Fig. 2A). These reporter plasmids were then transfected into HEK293 cells followed by treatment of the cells with CuSO4 or TM. Figure 2B shows that expression of the reporter is down-regulated by copper treatment but up-regulated by TM treatment in the Sp1-wt-reporter–transfected cells. Mutations at sites 2, 3, 7, 8, 9, and 10 abolished the responses of reporter expression to both copper and TM treatments, suggesting that these sites are important for copper-mediated Sp1 regulation. These sites contain the consensus 5′-GGGCGG- or 5′-CCCGCC sequence, which can be recognized by Sp1 transcription factor (Wierstra, 2008). These results demonstrate that regulation of Sp1 by copper stresses, like regulation of hCtr1 [demonstrated previously (Song et al., 2008], is mediated by the Sp1 bindings to their respective promoters. The finding that these copper-responsive sites are located in two clusters (sites 2 and 3 in one cluster and sites 7 to 10 in the other) suggests that regulation of Sp1 expression may involve the simultaneous binding of Sp1 to these two clusters via Sp1-Sp1 interactions, resulting in DNA looping at the Sp1 promoter, as suggested from previous study of modified thymidine kinase promoter (Su et al., 1991).
Fig. 2.
Identification of copper-responsive elements at the Sp1 promoter. A, schematic diagram showing the locations of ten putative Sp1 binding sites and their sequences. Mutated sequences on these sites (M1–M10) are indicated. Numbers on the right refer to positions of these nucleotides upstream from the transcription start site (not drawn in scale). B, transient expression analyses of mutants Sp1 binding sites in response to copper and TM. Each Sp1 mutant was transfected into HEK293 cells and treated with 100 μM CuSO4 or 40 μM TM for 16 h. Expression of luciferase reporter was determined by measuring the luciferase activity per microgram of total cellular protein. *, p < 0.05, statistically significant, as measured by Student t test.
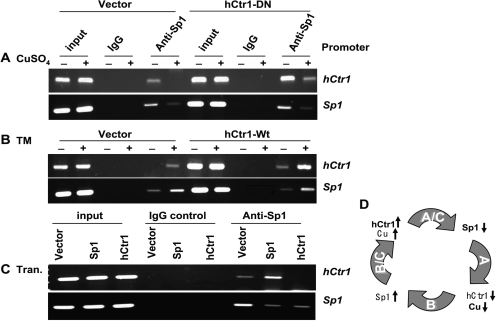
To demonstrate that regulation of hCtr1 and endo-Sp1 by Sp1 in response to copper concentration variations is mediated by promoter binding, we next performed a ChIP assay. HEK293 cells transfected with recombinant plasmid DNA were treated with either CuSO4 or TM. Protein-genomic DNA cross-linking was performed by using formaldehyde. After sonication, the chromatin fragments were precipitated with anti-Sp1 antibody using IgG from nonimmunized rabbit as a control. The hCtr1 and Sp1 promoter sequences in the precipitates were determined by PCR using primer sets specific to their promoters. Figure 3A shows that CuSO4 treatment reduces the bindings of Sp1 to hCtr1 and Sp1 promoters in both the empty vector- and the hCtr1-DN-transfected cells. These results demonstrated that down-regulation of Sp1 and hCtr1 expression by copper treatment as shown in Fig. 1A is due to reduced Sp1 interactions with the hCtr1 and Sp1 promoters. We used two cell lines instead of one to strengthen the results because regulation of hCtr1 expression by copper stresses should occur in all cell lines. Given the complexity of the assay system, we could not be certain as whether there are differences of Sp1 binding capacities to the Sp1 and hCtr1 promoters between these two cell lines. TM treatment enhanced the bindings of Sp1 to the hCtr1 and Sp1 promoters in the empty vector- and hCtr1-wt-transfected cells (Fig. 3B), again demonstrating that up-regulation of hCtr1 and Sp1 expression under copper-depleted conditions is transcriptionally regulated.
Fig. 3.
Determinations of Sp1 Binding to the hCtr1 and Sp1 promoters under various treatments by the ChIP assay. A, vector-transferred SCLC or hCtr1-DN-transfected cells were treated with 100 μM CuSO4 for 16 h followed by the ChIP assay of Sp1 binding to hCtr1 and Sp1 promoters as indicated. Input refers to total chromatin DNA before immunoprecipitation with anti-Sp1 antibody or nonimmune immunoglobulin IgG. B, the ChIP assay was performed in empty-vector- and hCtr1-wt transfected cells treated with or without 40 μM TM for 16 h. C, ChIP assay of Sp1 binding to the Sp1 and hCtr1 promoters in HEK293 cells transfected with the empty vector, Sp1-encoding, and hCtr1-encoding recombinants for 16 h as indicated. D, Schematic diagram showing the summary of results presented in A to C. Details can be found in Fig. 1I.
To substantiate the transcriptional involvement of Sp1 in the regulation of both Sp1 and hCtr1, we next investigated the effects of Sp1 and hCtr1 overexpression on the binding of Sp1 to their promoters in HEK293 cells grown in the regular medium using a transfection approach. We found that Sp1 preferentially bound to the hCtr1 promoter compared with the Sp1 promoter in the Sp1-wt-transfected cells (Fig. 3C). These results are consistent with our previous observation that elevated Sp1 expression up-regulated hCtr1 but down-regulated itself (Fig. 1F). In addition, overexpression of hCtr1 diminished Sp1 binding to both the hCtr1 and Sp1 promoters (Fig. 3C), which is also consistent with the results showing that overexpression of hCtr1 down-regulates both endo-hCtr1 and Sp1 expression (Fig. 1). These results, as summarized in Fig. 3D, not only support the many observations depicted in Fig. 1I but also indicate the transcriptional regulation of copper homeostasis by Sp1 transcriptional regulator.
Both the Zinc Finger Domain and Transactivation Domains of Sp1 Are Important for Sp1 Sensing of Copper Stress.
Sp1 consists of a TAD that contains two serine/threonine-rich and glutamine-rich subdomains and a DNA-binding domain that contains three zinc fingers (ZFs). We previously found that the ZF domain in Sp1 is important for Sp1-mediated hCtr1 expression under copper-depleted conditions (Song et al., 2008. To elucidate whether other domains are also involved in Sp1-mediated hCtr1 expression and whether these domains are important for the hCtr1-mediated Sp1 self-regulation, we constructed several Sp1 deletion mutants encompassing various subdomains (Fig. 4B). These mutants Sp1 were transfected into HEK293 cells, and the transfected cells were treated with CuSO4. Alternatively, these mutants were cotransfected with hCtr1-wt recombinant. We envisioned that cotransfection with hCtr1-wt would increase copper uptake, much like cells treated with CuSO4. We used a probe that can distinguish between exo-Sp1 and endo-Sp1 mRNA signals for the RPA (Fig. 4A).
The following results were obtained from these two sets of experiments: 1) high copper content suppresses the expression of endo-hCtr1 and endo-Sp1 (Fig. 4, C and D, lanes 3), consistent with the results shown earlier (Song et al., 2008). 2) Overexpression of Sp1-wt up-regulates endo-hCtr1 but down-regulates endo-Sp1 expression (Fig. 4, C and D, lanes 4), confirming the results shown in Fig. 1F. 3) Deletion of the three ZF in Sp1 (M3 mutant) suppresses the Sp1-mediated increased endo-hCtr1 expression but decreased endo-Sp1 expression (Fig. 4, C and D, lanes 5). 4) Expression of the intact TAD neutralizes the effects of endo-Sp1 and endo-hCtr1 expression by copper treatment (i.e., their expression levels are similar to those seen in the untransfected or vector-transfected controls) (Fig. 4, C and D, lanes 6). Thus, Sp1-TAD has a dominant-negative function in copper response. Deleting the Q-rich2 subdomain (mutant M8) abolishes the dominant-negative function of TAD; however, the Q-rich2 domain together with Sp1-DBD (M7) cannot confer the dominant-negative function. These results suggest that other subdomains in the TAD are required for the dominant-negative function. 5) Transfection of other mutants with truncated TAD (i.e., M8, M9, M10 and M13) failed to alter the expression of endo-hCtr1 and endo-Sp1 (Fig. 4, C and D, lanes 8–11). We found that mutations that affected endo-hCtr1 expression also affected endo-Sp1 expression, whereas those that did not affect endo-Sp1 expression had no effect on hCtr1 expression. This striking concordance further confirms the inter-regulatory relationship among Sp1, hCtr1, and copper in the maintenance of copper homeostasis and supports the notion that Sp1-regulated hCtr1 and Sp1 expression may involve the same functional domains.
Previous studies have demonstrated that whereas the ZF domain is involved in promoter recognition and the Q-rich2 subdomain of TAD is involved in Sp1-mediated transcription activation by cross-talk with basal transcription machinery (Gill et al., 1994). This Q-rich2 subdomain is also involved in Sp1 oligomerization (Pascal and Tjian, 1991). To further elucidate the amino acid residues in the Q-rich2 subdomain that are involved in copper responsiveness, we introduced several mutations at the Q-rich2 subdomain (Fig. 4E). Transient transfection assay revealed that double-mutations (Gln to Leu) at two amino acid clusters 384/385 (M2 mutant) and 413/416 (M5 mutant) (Fig. 4E, circled) abolished the dominant-negative effects of TAD on the regulation of Sp1 and hCtr1 expression by copper (Fig. 4F). These results identified the roles of the Q-rich2 subdomain in the Sp1-mediated transcriptional regulation of Sp1 and hCtr1 expression by copper.
The Zinc Fingers of Sp1 are the Sensors of Copper Stress.
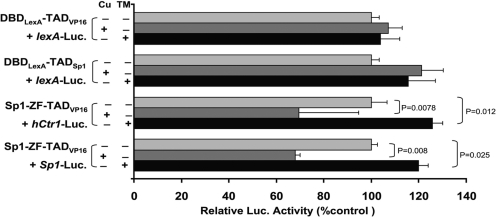
To further characterize the copper- sensing mechanism of Sp1, we performed domain-swapping experiments by constructing fusions composed of the Sp1-ZF and the TAD from herpes simplex virus VP16 (TADvp16), DNA-binding domain from Escherichia coli LexA (DBDlexA), and the TAD from Sp1 (TADSp1). We avoided the commonly used GAL4 DBD because it contains a ZF sequence, whereas DBDlexA contains no metal binding motif. DBDlexA-TADvp16 or DBDlexA-TADSp1 was cotransfected with the Luc reporter recombinant containing eight copies of LexA-binding sequences (LexA-Luc), whereas Sp1-ZF-TADvp16 recombinants were cotransfected with hCtr1-Luc or Sp1-Luc. Neither copper nor TM treatments altered reporter expression levels when DBDlexA-TADvp16 and DBDlexA-TADSp1 recombinants were used (Fig. 5). In contrast, CuSO4 treatment down-regulated reporter expression in cells cotransfecting Sp1-ZF-VP16 with hCtr1-Luc or with Sp1-Luc, and TM treatment up-regulated reporter expression in these similarly transfected cells. These results collectively demonstrated that Sp1-ZF is the sensor of copper stresses in this surrogate assay system.
Fig. 5.
Dissecting the roles of the Sp1 DBD and TAD in the regulation of copper stress-induced hCtr1 reporter Expression using a chimeric fusion approach. Recombinants encoding the chimeric transcription regulators containing DBD either from LexA (DBDLexA) or from Sp1-ZF were fused with either VP16 (TADVP16) or TAD of Sp1 (TADSp1). These recombinant DNA were cotransfected into HEK293 cells with their cognate luciferase reporters as indicated and treated with either 100 μM CuSO4 or 40 μM TM for 18 h. The luciferase activities were assayed and calculated with reference to that in the reference reporter.
Copper Stress Does Not Seem to Induce Cytoplasmic Sequestration of Sp1.
It has been demonstrated that the ZF domain is involved in nuclear targeting of Sp1 (Ito et al., 2010). Because we found that Sp1-ZF plays an important role in copper sensing that regulates Sp1 and hCtr1 expression, we investigated whether copper stresses may affect nuclear localization of Sp1. SCLC cells were treated with CuSO4 or TM followed by immunocytochemical stainings with an anti-Sp1 antibody. We found that, although the overall staining intensities showing Sp1 expression levels were reduced in copper-treated cells but increased in TM-treated cells as consistent with the biochemical results shown in Fig. 1, B and E, respectively, no apparent redistribution of Sp1 between cytoplasmic and nuclear compartments was observed (Supplemental Fig. 1).
Sp3 Expression Is Also Regulated by Copper Stresses, but It Does Not Regulate hCtr1 Expression.
Sp1 was the first described member of the Sp1/Krüppel-like factor (KLF) family, which currently consists of 8 Sp and 15 KLF members. Among the eight Sp proteins, Sp1 to Sp4 form a subgroup and Sp5 to Sp8 form another on the basis of their structural similarities (Kaczynski et al., 2003). This raises an important question about the roles of other Sp members in the regulation of hCtr1 expression, particularly Sp3, which is most closely related to Sp1 in terms of their structural similarities.
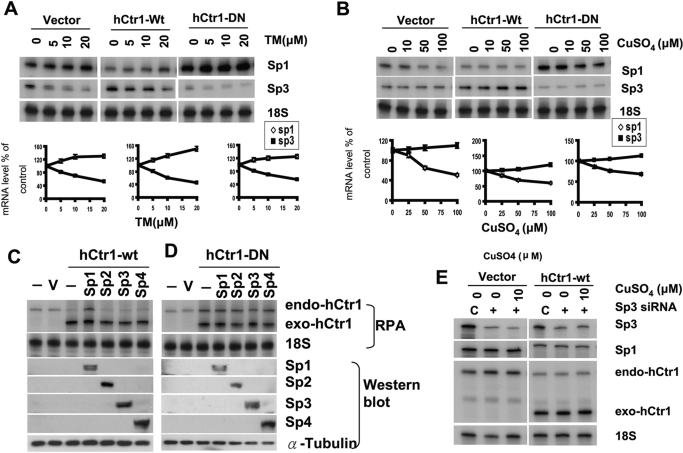
To determine whether Sp3 expression is also regulated by copper and whether its expression also modulates hCtr1 expression, we treated empty vector-, hCtr1-wt-, and hCtr1-DN-transfected cell lines with CuSO4 or TM. Consistent with our previous report (Song et al., 2008) and those shown in Fig. 1, levels of Sp1 expression were increased in the TM-treated cells (Fig. 6A) but down-regulated in the CuSO4-treated cells (Fig. 6B). Although the magnitudes of regulation here that reflected experimental variations were small, the results were very reproducible. In contrast to the expression patterns of Sp1, we found that the expression of Sp3 was down-regulated in these cell lines treated with TM (Fig. 6A) but was slightly up-regulated in cells treated with CuSO4 (Fig. 6B). Again, the magnitudes of Sp3 regulation by copper stresses were not remarkable, but the results were very reproducible in all three cell lines (i.e., vector-, hCtr1-wt-, and hCtr1-DN-transfected cell lines). Moreover, transfections of expression recombinants encoding Sp2, Sp3, and Sp4 failed to up-regulate endo-hCtr1 expression in the hCtr1-wt-transfected cells, whereas transfection with Sp1 recombinant did (Fig. 6C). Transfections of recombinants encoding each of these Sp family members into hCtr1-DN-transfected cells did not alter expression levels of endo-hCtr1 levels, because these cells already express high levels of endo-hCtr1 (Fig. 6D). This is consistent with the interpretation that limited maximal capacity whereby hCtr1 expression can be regulated by copper stresses. Finally, knockdown of Sp3 expression using siRNA did not alter endo-hCtr1 expression in vector- or hCtr1-wt-transfected cells treated with CuSO4 (Fig. 6E). These results demonstrated that although Sp3 expression is regulated by copper stresses in an manner opposite that of Sp1, its expression does not affect hCtr1 expression under copper stress conditions. These results highlight the importance of Sp1 in the KLF family in the regulation of copper mammalian physiology.
Fig. 6.
Regulation of Sp1 family members by copper and TM and the effects of these members on hCtr1 expression. A, regulation of Sp1 and Sp3 expression by the treatments of TM using the concentrations as indicated in the three SCLC cell lines. Densimetric presentations of the levels of expression with reference to the untreated controls (as 100%) are correspondingly below. B, regulation of Sp1 and Sp3 by the treatment of different concentrations of CuSO4 in the three SCLC cell lines. Corresponding quantitative results are shown below. C, up-regulation of endo-hCtr1 in hCtr1-wt-transfected cells by transfection with recombinant encoding Sp1 but not by those encoding Sp2, Sp3, or Sp4. Expression of endo-hCtr1 and exo-hCtr1 was determined by RPA using 18S RNA as a loading control, whereas expression of Sp1- Sp4 was determined by Western blot analysis. Probe design for differentiating between exo- and endo-hCtr1 mRNA was described previously (Liang et al., 2009) using a strategy similar to that described in Fig. 4A. D, overexpression of Sp1, Sp2, Sp3, and Sp4 by transfection did not alteration the expression of endo-hCtr1 mRNA levels in hCtr1-DN-transfected cells. E, no effect on endo-Sp1 and endo-hCtr1 expression by knockdown of Sp3 expression with or without CuSO4 treatments in the vector- and hCtr1-treated cells.
Discussion
The Sp1-hCtr1-Copper Tripartite Model in Regulating Copper Homeostasis in Mammalian Cells.
Extracellular copper exists in the oxidized form (Cu2+), which is reduced to Cu+ by membrane-associated cupric reductases, similar to ferric reductases 1 and 2 found in yeast (Georgatsou et al., 1997), and the reduced Cu+ is primarily transported by the high-affinity Ctr1 to regulate cellular copper bioavailability. Mechanisms for regulation of hCtr1 expression in response to copper bioavailability are complex. In the present communication, we have constructed a tripartite regulation model in which the maintenance of copper homeostasis in human cells is regulated by an inter-regulatory loop consisting of copper, hCtr1, and Sp1 elements. Within this loop, these elements are mutually regulated among themselves, but each is also self-regulated. The demonstration that mammalian cells use Sp1 oscillation in mammalian copper homeostasis regulation is consistent with the multivariant homeostatic regulatory principle: a regulator that regulates a self-regulatory regulator should also be self-regulated. Thus, this study reveals a previously undiscovered homeostatic regulation mechanism for the maintenance of intracellular copper budget in mammalian cells.
Our success in elucidating the inter-regulatory mechanism of mammalian copper homeostasis regulation lies in the use of the RPA, which is sensitive and reliable in measuring the hCtr1 mRNA expression levels. It is noteworthy that by proper probe design, it can simultaneously determine endo-transcript versus exo-transcript levels in the transfection assays. We showed that under copper-adequate conditions, elevated expression of Sp1 by transfection promotes preferential binding of Sp1 to hCtr1 promoter and up-regulates hCtr1 expression. We observed that up-regulation of hCtr1 and Sp1 expression under copper-depleted conditions is mediated by enhanced Sp1 binding to these promoters. The feedback suppression of hCtr1 and Sp1 expression under copper-replete conditions is also mediated by the diminished Sp1 binding to the promoters of these genes. These results strongly suggest that the differential promoter binding of Sp1 between hCtr1 and Sp1 promoters mediates the control mechanism of copper homeostasis in the mammalian system.
Copper homeostasis is evolutionarily conserved from yeasts to humans. Humans have one high-affinity copper transporter (hCtr1), whereas yeast has two (yCtr1 and yCtr3). Like hCtr1, expression of yCtr1 and yCtr3 is transcriptionally up-regulated under copper-depleted conditions but is down-regulated under copper-replete conditions. When copper is deficient, the transcription factor Mac1p binds to the metal-responsive elements located within the promoters of yCtr1 and yCtr3 and turns on the expression of these genes (Labbé et al., 1997; Jensen et al., 1998). Under copper-sufficient conditions, Mac1p dissociates from the promoters, resulting in the shutting down of yCtr1 and yCtr3 expression. The transcription factor Ace1 is then activated to induce the expression of genes encoding copper-chelating proteins (Cup1 and Crs5) and the antioxidant superoxide dismutase (Sod1) (Gralla et al., 1991; Gross et al., 2000) to protect cells from copper toxicities. Moreover, expression of Drosophila melanogaster dCtr1B under copper-deficiency conditions is also regulated by promoter binding of transcription factor MTF1 (Selvaraj et al., 2005). Regulation of plant copper transporters COPT1 and COPT2 in Arabidopsis thaliana under copper-limiting conditions also involves the interactions of the transcription factor SQUAMOSA-promoter like binding protein 7 (SPL-7) with the promoters of these genes (Yamasaki et al., 2009; Penarrubia et al., 2010). In all cases, these transcription fractions bind to the promoters and up-regulate the expression of their target genes under copper-deficient conditions, whereas under copper-replete conditions, these transcription factors dissociate from their promoters. Thus, the tripartite regulatory model may also apply to the regulation of copper homeostasis in these organisms; however, whether these copper-sensing transcription regulators are also regulated within the context of copper homeostasis has not been investigated.
Sp1 is a ubiquitous transcription factor that was previously considered as a regulator for housekeeping genes. The present study reveals a new role of Sp1 in the regulation of copper homeostasis. Of particular intrigue is the finding that Sp1 expression is self-regulated through the hCtr1 intermediate. Several studies have demonstrated that Sp1 may enhance its transcriptional activities through post-translational modifications and/or by cross-talk with coregulators (Wierstra, 2008); we cannot rule out the possible involvements of these post-translational mechanisms in fine-tuning of Sp1 activity in regulating copper homeostasis.
Mechanisms for Copper Sensing by Sp1.
Many transcription factors regulate the expression of copper transporters through their sensing of copper availability. For Ace1, excess copper promotes its DNA binding (Fürst et al., 1988). By contrast, for Mac1p and SPL-7 (Yamasaki et al., 2009), excess copper inhibits their DNA-binding activities, whereas reduced copper promotes binding activities. All these transcriptional regulators contain ZF modules that function as copper sensors in regulating their target genes. These findings suggest that the sensing mechanisms by ZFs in different transcription regulators are complex. In Sp1, each ZF domain consists of two cysteine and two histidine residues that are coordinated by one zinc molecule in a tetrahedral conformation. The finding that excess copper prevents Sp1 from binding to Sp1 and hCtr1 promoters can be readily explained in that excess copper may poison the ZF domain of Sp1 by displacing the bound zinc, resulting in conformational changes that compromise its DNA-binding activities at both promoters, leading to down-regulation of hCtr1 and Sp1 expression. This mechanism is consistent with that describing the effects of cadmium on Sp1's down-regulation of cadmium transporter encoded by Zip8, as reported previously (Aiba et al., 2008). Strikingly, we observed that Sp1 and hCtr1 expression is elevated under copper-depleted conditions through enhanced binding of Sp1 to their promoters. Although these results constitute the important part in the tripartite inter-regulatory mechanism of copper homeostasis regulation, given the complexity of the effects of copper on ZF-DNA interactions as mentioned, the underlying mechanism is currently unknown and requires further in-depth investigation. Although it has been reported that copper stress can affect zinc homeostasis and likewise that zinc can affect copper homeostasis (Hoffman et al., 1988), our preliminary results showed that interactions of Sp1 with its recognition of GC box cannot be simply explained by zinc content, because modulation of zinc levels failed to alter Sp1 binding specificity (Z. Liang and M. Kuo, unpublished data). Currently, we favor the hypothesis that conformational change in the ZF may also occur under copper-limiting conditions that enhance the stabilization of interactions between Sp1 and the Sp1 and hCtr1 promoters.
We observed that despite the fact that Sp3 also contains three ZFs and a TAD and is also regulated by copper availability, Sp3 expression does not regulate hCtr1 expression. These observations reflect promoter selectivity in gene regulation by the Sp1/KLF transcription factor family. How the bioavailable copper affects the transcriptional selectivity of Sp1/hCtr1/Sp3 remains to be investigated.
Our finding that the Q-rich2 subdomain within the Sp1-TAD is involved in Sp1-regulated Sp1 and hCtr1 expression by copper stress has added another aspect of complexity in the Sp1-hCtr1-copper homeostatic regulation. This Q-rich2 domain interacts with TAFII110 in transcription factor IID, a core promoter recognition multiple protein complex in the basal transcriptional machinery of RNA polymerase II (Gill et al., 1994; Liu et al., 2009). How copper concentrations affect interactions between Sp1-TAD and the basal transcriptional assembly, which as a whole contains more than 80 proteins, remains to be investigated. Taken together, our current study provides a new paradigm for the transcriptional regulation of copper homeostasis in the mammalian system.
Implications of Copper Metabolism Regulation by the Sp1-hCtr1-Copper Tripartite Model.
The Sp1-hCtr1-copper inter-regulatory model described in this communication suggests that changes of any one component within this loop would result in either feedback or feed-forward to affect the expression of the other two. This model underscores the dynamic regulation mechanism for the maintenance of copper homeostasis. Accordingly, the magnitudes of Sp1 and hCtr1 regulation by copper imbalance depend upon the intrinsic (basal) expression levels of Sp1 and hCtr1 themselves and are not anticipated to be very high (Z. D. Liang, submitted).
Another example of this copper-related inter-regulatory relationship may be found in the human prion gene regulation. Prion is a copper-binding protein that is intimately associated with transmissible spongiform encephalopathies. Not only does copper regulate prion expression but also prion expression can affect copper metabolism (Kralovicova et al., 2009). Moreover, Bellingham et al. (2009) reported that Sp1 functions as a copper-sensing transcription factor that regulates prion gene expression. Recent bioinformatics from an analysis of 57 different species across the evolutionary tree estimated that the size of the copper proteome is generally less than 1% of the total proteome of an organism in both eukaryotes and prokaryotes (Andreini et al., 2008). It remains to be investigated whether many of these copper-binding proteins, such as prion, Sp1, and hCtr1, may be regulated by copper bioavailability.
Finally, the findings that Sp1 and Sp3 expression are regulated under copper-stressed conditions, although in opposite manners, expand the gene regulation profiling by copper homeostasis beyond the present study, because these transcriptional regulators are known to regulate a vast number of genes involved in cell growth, differentiation, apoptosis, and tumor development (Wierstra, 2008). In the recently developed ChIP-sequencing approach, the entire repertoire of Sp1-regulated genes under various copper stresses can be accessed. These studies eventually should provide a comprehensive picture for the global effect of gene regulation and resulting physiological consequences in mammalian copper physiology.
Supplementary Material
Acknowledgments
We thank Drs. Yien-Ming Kuo (University of California, San Francisco, CA), C. J. Ciudad, and Mark Ptashne for the reagents. We thank Michael Worley (Department of Scientific Publications, The University of Texas MD Anderson Cancer Center) for editing the manuscript.
 The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://molpharm.aspetjournals.org) contains supplemental material.
This work was supported by the National Institutes of Health National Cancer Institute [Grants R01-CA149620, CA16672].
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
- Ctr
- copper transporter
- hCtr1
- human high-affinity copper transporter 1
- DN
- dominant negative
- Sp1
- specificity protein 1
- SCLC
- small-cell lung cancer
- TM
- tetrathiomolybdate
- DBD
- DNA-binding domain
- TAD
- transactivating domain
- PCR
- polymerase chain reaction
- ChIP
- chromatin immunoprecipitation
- RPA
- RNase protection assay
- TM
- tetrathiomolybdate
- siRNA
- small interfering RNA
- wt
- wild type
- endo
- endogenous
- exo
- exogenous
- HEK
- human embryonic kidney
- ZF
- zinc finger
- KLF
- Krüppel-like factor
- HA
- hemagglutinin.
Authorship Contributions
Participated in research design: Liang, Tsai, Savaraj, and Kuo.
Conducted experiments: Liang, Tsai, and Lee.
Performed data analysis: Liang, Tsai, and Kuo.
Wrote or contributed to the writing of the manuscript: Liang and Kuo.
References
- Aiba I, Hossain A, Kuo MT. (2008) Elevated GSH level increases cadmium resistance through down-regulation of Sp1-dependent expression of the cadmium transporter ZIP8. Mol Pharmacol 74:823–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreini C, Banci L, Bertini I, Rosato A. (2008) Occurrence of copper proteins through the three domains of life: a bioinformatic approach. J Proteome Res 7:209–216 [DOI] [PubMed] [Google Scholar]
- Bellingham SA, Coleman LA, Masters CL, Camakaris J, Hill AF. (2009) Regulation of prion gene expression by transcription factors SP1 and metal transcription factor-1. J Biol Chem 284:1291–1301 [DOI] [PubMed] [Google Scholar]
- Chen HH, Song IS, Hossain A, Choi MK, Yamane Y, Liang ZD, Lu J, Wu LY, Siddik ZH, Klomp LW, et al. (2008) Elevated glutathione levels confer cellular sensitization to cisplatin toxicity by up-regulation of copper transporter hCtr1. Mol Pharmacol 74:697–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HH, Yan JJ, Chen WC, Kuo MT, Lai YH, Lai WW, Liu HS, Su WC. (2011) Predictive and prognostic value of human copper transporter 1 (hCtr1) in patients with stage III non-small-cell lung cancer receiving first-line platinum-based doublet chemotherapy. Lung Cancer http://dx.doi.org/10.1016/j.lungcan.2011.06.011 [DOI] [PMC free article] [PubMed]
- Collins JF, Prohaska JR, Knutson MD. (2010) Metabolic crossroads of iron and copper. Nutr Rev 68:133–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürst P, Hu S, Hackett R, Hamer D. (1988) Copper activates metallothionein gene transcription by altering the conformation of a specific DNA binding protein. Cell 55:705–717 [DOI] [PubMed] [Google Scholar]
- Georgatsou E, Mavrogiannis LA, Fragiadakis GS, Alexandraki D. (1997) The yeast Fre1p/Fre2p cupric reductases facilitate copper uptake and are regulated by the copper-modulated Mac1p activator. J Biol Chem 272:13786–13792 [DOI] [PubMed] [Google Scholar]
- Gill G, Pascal E, Tseng ZH, Tjian R. (1994) A glutamine-rich hydrophobic patch in transcription factor Sp1 contacts the dTAFII110 component of the Drosophila TFIID complex and mediates transcriptional activation. Proc Natl Acad Sci USA 91:192–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gralla EB, Thiele DJ, Silar P, Valentine JS. (1991) ACE1, a copper-dependent transcription factor, activates expression of the yeast copper, zinc superoxide dismutase gene. Proc Natl Acad Sci USA 88:8558–8562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross C, Kelleher M, Iyer VR, Brown PO, Winge DR. (2000) Identification of the copper regulon in Saccharomyces cerevisiae by DNA microarrays. J Biol Chem 275:32310–32316 [DOI] [PubMed] [Google Scholar]
- Hoffman HN, 2nd, Phyliky RL, Fleming CR. (1988) Zinc-induced copper deficiency. Gastroenterology 94:508–512 [DOI] [PubMed] [Google Scholar]
- Howell SB, Safaei R, Larson CA, Sailor MJ. (2010) Copper transporters and the cellular pharmacology of the platinum-containing cancer drugs. Mol Pharmacol 77:887–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida S, Lee J, Thiele DJ, Herskowitz I. (2002) Uptake of the anticancer drug cisplatin mediated by the copper transporter Ctr1 in yeast and mammals. Proc Natl Acad Sci USA 99:14298–14302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida S, McCormick F, Smith-McCune K, Hanahan D. (2010) Enhancing tumor-specific uptake of the anticancer drug cisplatin with a copper chelator. Cancer Cell 17:574–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito T, Kitamura H, Uwatoko C, Azumano M, Itoh K, Kuwahara J. (2010) Interaction of Sp1 zinc finger with transport factor in the nuclear localization of transcription factor Sp1. Biochem Biophys Res Commun 403:161–166 [DOI] [PubMed] [Google Scholar]
- Jensen LT, Posewitz MC, Srinivasan C, Winge DR. (1998) Mapping of the DNA binding domain of the copper-responsive transcription factor Mac1 from Saccharomyces cerevisiae. J Biol Chem 273:23805–23811 [DOI] [PubMed] [Google Scholar]
- Kaczynski J, Cook T, Urrutia R. (2003) Sp1- and Krüppel-like transcription factors. Genome Biol 4:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BE, Nevitt T, Thiele DJ. (2008) Mechanisms for copper acquisition, distribution and regulation. Nat Chem Biol 4:176–185 [DOI] [PubMed] [Google Scholar]
- Kralovicova S, Fontaine SN, Alderton A, Alderman J, Ragnarsdottir KV, Collins SJ, Brown DR. (2009) The effects of prion protein expression on metal metabolism. Mol Cell Neurosci 41:135–147 [DOI] [PubMed] [Google Scholar]
- Kuo MT, Chen HH, Song IS, Savaraj N, Ishikawa T. (2007) The roles of copper transporters in cisplatin resistance. Cancer Metastasis Rev 26:71–83 [DOI] [PubMed] [Google Scholar]
- Kuo YM, Zhou B, Cosco D, Gitschier J. (2001) The copper transporter CTR1 provides an essential function in mammalian embryonic development. Proc Natl Acad Sci USA 98:6836–6841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbé S, Zhu Z, Thiele DJ. (1997) Copper-specific transcriptional repression of yeast genes encoding critical components in the copper transport pathway. J Biol Chem 272:15951–15958 [DOI] [PubMed] [Google Scholar]
- Liang ZD, Stockton D, Savaraj N, Tien Kuo M. (2009) Mechanistic comparison of human high-affinity copper transporter 1-mediated transport between copper ion and cisplatin. Mol Pharmacol 76:843–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WL, Coleman RA, Ma E, Grob P, Yang JL, Zhang Y, Dailey G, Nogales E, Tjian R. (2009) Structures of three distinct activator-TFIID complexes. Genes Dev 23:1510–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy SA, Kaplan JH. (2009) Copper-dependent recycling of hCTR1, the human high affinity copper transporter. J Biol Chem 284:29704–29713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolás M, Noé V, Jensen KB, Ciudad CJ. (2001) Cloning and characterization of the 5′-flanking region of the human transcription factor Sp1 gene. J Biol Chem 276:22126–22132 [DOI] [PubMed] [Google Scholar]
- Nose Y, Wood LK, Kim BE, Prohaska JR, Fry RS, Spears JW, Thiele DJ. (2010) Ctr1 is an apical copper transporter in mammalian intestinal epithelial cells in vivo that is controlled at the level of protein stability. J Biol Chem 285:32385–32392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooi CE, Rabinovich E, Dancis A, Bonifacino JS, Klausner RD. (1996) Copper-dependent degradation of the Saccharomyces cerevisiae plasma membrane copper transporter Ctr1p in the apparent absence of endocytosis. EMBO J 15:3515–3523 [PMC free article] [PubMed] [Google Scholar]
- Pascal E, Tjian R. (1991) Different activation domains of Sp1 govern formation of multimers and mediate transcriptional synergism. Genes Dev 5:1646–1656 [DOI] [PubMed] [Google Scholar]
- Peñarrubia L, Andrés-Colás N, Moreno J, Puig S. (2010) Regulation of copper transport in Arabidopsis thaliana: a biochemical oscillator? J Biol Inorg Chem 15:29–36 [DOI] [PubMed] [Google Scholar]
- Petris MJ, Smith K, Lee J, Thiele DJ. (2003) Copper-stimulated endocytosis and degradation of the human copper transporter, hCtr1. J Biol Chem 278:9639–9646 [DOI] [PubMed] [Google Scholar]
- Selvaraj A, Balamurugan K, Yepiskoposyan H, Zhou H, Egli D, Georgiev O, Thiele DJ, Schaffner W. (2005) Metal-responsive transcription factor (MTF-1) handles both extremes, copper load and copper starvation, by activating different genes. Genes Dev 19:891–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song IS, Chen HH, Aiba I, Hossain A, Liang ZD, Klomp LW, Kuo MT. (2008) Transcription factor Sp1 plays an important role in the regulation of copper homeostasis in mammalian cells. Mol Pharmacol 74:705–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song IS, Savaraj N, Siddik ZH, Liu P, Wei Y, Wu CJ, Kuo MT. (2004) Role of human copper transporter Ctr1 in the transport of platinum-based antitumor agents in cisplatin-sensitive and cisplatin-resistant cells. Mol Cancer Ther 3:1543–1549 [PubMed] [Google Scholar]
- Su W, Jackson S, Tjian R, Echols H. (1991) DNA looping between sites for transcriptional activation: self-association of DNA-bound Sp1. Genes Dev 5:820–826 [DOI] [PubMed] [Google Scholar]
- Tsai WB, Aiba I, Lee SY, Feun L, Savaraj N, Kuo MT. (2009) Resistance to arginine deiminase treatment in melanoma cells is associated with induced argininosuccinate synthetase expression involving c-Myc/HIF-1alpha/Sp4. Mol Cancer Ther 8:3223–3233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierstra I. (2008) Sp1: emerging roles—beyond constitutive activation of TATA-less housekeeping genes. Biochem Biophys Res Commun 372:1–13 [DOI] [PubMed] [Google Scholar]
- Yamasaki H, Hayashi M, Fukazawa M, Kobayashi Y, Shikanai T. (2009) SQUAMOSA promoter binding protein-like7 is a central regulator for copper homeostasis in Arabidopsis. Plant Cell 21:347–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.