Abstract
Aldosterone synthase (AS) regulates blood volume by synthesizing the mineralocorticoid aldosterone. Overproduction of aldosterone in the adrenal gland can lead to hypertension, a major cause of heart disease and stroke. Aldosterone production depends upon stimulation of AS expression by the renin-angiotensin system, which takes 12 h to reach full effect, and then 24 h to subside. However, this promoter-dependent regulation of aldosterone production fails to explain phenomena such as rapid-onset hypertension that occurs quickly and then subsides. Here, we investigate the fate of AS after expression and how these events relate to aldosterone production. Using isolated mitochondria from steroidogenic cells and cell-free synthesized AS, we first showed that the precursor form of AS translocated into the matrix of the mitochondria, where it underwent cleavage by mitochondrial processing peptidase to a mature form approximately 54 kDa in size. Mature AS seemed to translocate across the inner mitochondrial membrane a second time to finally reside in the intermembrane space. Unprocessed N-terminal AS has 2-fold more activity than physiological levels. These results show how the subcellular mechanisms of AS localization relate to production of aldosterone and reveal a rapid, promoter-independent regulation of aldosterone production.
Introduction
Aldosterone excess can lead to hypertension, a leading factor in cardiovascular disease. Aldosterone, a mineralocorticoid, controls salt balance and blood volume by binding to the mineralocorticoid receptor and activating genes involved in sodium and water reabsorption as well as potassium release in the kidney. The mineralocorticoid receptor is expressed in the kidney, heart, brain, vascular smooth muscle, and other tissues (Walker et al., 1991; Lombès et al., 1995; Slight et al., 1996; Hatakeyama et al., 2000). Activation of the mineralocorticoid receptor by aldosterone in these tissues can trigger responses such as increased vascular resistance and cardiac output (Wehling et al., 1998; Schmidt et al., 1999). Primary hypertension results from overproduction of aldosterone that is independent of excess renin-angiotensin stimulation and affects a significant portion of patients with hypertension (Gonzaga and Calhoun, 2008). Prolonged exposure to excess aldosterone can result in an inflammatory response, followed by damage to vascular and myocardial tissues and hypertensive effects (Rocha et al., 2002). Thus, overproduction of aldosterone leads to deterioration of target organs, enhancing risk for circulatory diseases.
Aldosterone synthase (AS) is the sole producer of aldosterone in multicellular organisms. AS is expressed in the zona glomerulosa of the adrenal gland, an organ that releases steroids to regulate stress response, sexual development, and blood volume (Ogishima et al., 1989; Curnow et al., 1991). Expression of human AS in the cytosol leads to increased production of aldosterone (Mornet et al., 1989). AS expression is very low in vivo (Mornet et al., 1989). Depletion of sodium levels causes an increase in both the synthesis of aldosterone and the binding of substrate with AS (Kramer et al., 1979). Deoxycorticosterone (DOC) is synthesized from other precursors in the steroidogenic pathway, starting with conversion of cholesterol to pregnenolone by cytochrome P450 side-chain cleavage enzyme (SCC) (Fig. 1A). AS converts DOC to aldosterone in three distinct reactions (Fig. 1A).
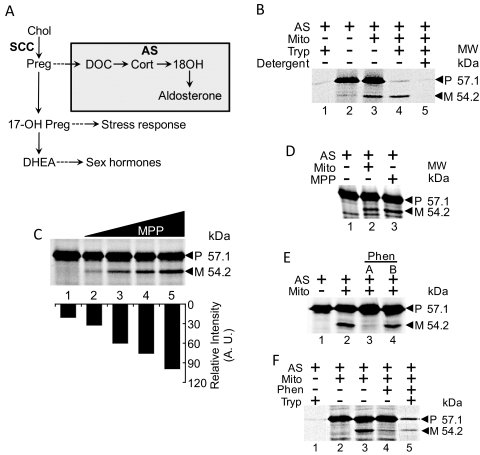
Fig. 1.
Translocation, cleavage, and residence of AS in mitochondria, uncleaved form of AS is more active. A, steroidogenic pathway. Reactions catalyzed by AS are contained within the colored box. B, translocation of 35S-labeled AS into mitochondria isolated from NCI-H295 cells. Lane 1, AS is fully digested by trypsin. Lane 2, AS. Lane 3, incubation of AS with mitochondria results in cleavage of precursor AS [P] to mature AS [M]. Lane 4, addition of trypsin digests precursor AS [P], whereas mature AS [M] is protected. Lane 5, permeabilization of membranes with Triton X-100 before protease treatment results in digestion of both precursor [P] AS and mature [M] AS. C, cleavage of AS by rMPP. Lane 1, AS only. Lanes 2 to 5, increasing amounts of rMPP incubated with AS, resulting in increasing cleavage of AS. Bottom, quantitative estimation of the shorter cleavage band from the high-molecular-weight AS precursor. The x-axis shows the respective bands after addition of increasing concentrations of MPP and y-axis represents the intensity of bands as measured by a PhosphorImager (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK). D, mitochondria cleave AS at the same position as rMPP. Lane 1, AS only. Lane 2, AS incubated with mitochondria, resulting in cleavage of precursor AS to form mature AS. Lane 3, AS incubated with rMPP, resulting in cleavage of precursor AS to form mature AS. E, mitochondrial cleavage of precursor AS is blocked by the MPP inhibitor phenanthroline. The effect of phenanthroline is reversible, as seen when mitochondria treated with phenanthroline are resuspended in phenanthroline-free buffer before incubation with precursor AS (lane 4). F, uncleaved precursor AS resides in mitochondria treated with phenanthroline. Addition of trypsin with the mitochondria pretreated with phenanthroline results in a protease-protected precursor AS (lane 5) but identical concentration of trypsin proteolyses AS (lane 1).
Aldosterone release is regulated by the renin-angiotensin system through control of expression of AS and SCC (Bird et al., 1993; Denner et al., 1996). Renin is released in response to drops either in blood pressure or circulating sodium levels. It goes on to stimulate angiotensin II (ANGII) release. ANGII stimulates aldosterone synthesis by binding the AT-1 receptor of zona glomerulosa cells, which stimulates release of calcium (Baukal et al., 1988; Bird et al., 1993; Denner et al., 1996). The renin-angiotensin system controls expression of AS and SCC. This is a relatively slow process, with a full response in AS and SCC expression from ANGII stimulation taking 12 h in cultured adrenal cells and then lasting for 24 h (Denner et al., 1996). Promoter-dependent regulation of aldosterone production fails to explain phenomena such as rapid-onset hypertension, which occurs and then subsides within minutes. A more rapid physiological regulation of aldosterone production would allow for greater short-term control over blood pressure, although disorders in that regulation could explain rapid onset hypertension.
AS probably localizes into the mitochondria. Because of the extremely low abundance, however, only microscopy studies suggest that AS localizes to the mitochondria of the zona glomerulosa (Mitani et al., 1982). Aldosterone production was found specifically in the mitochondrial fraction of adrenal extracts, suggesting that AS must reside in the mitochondria before aldosterone synthesis (Miller and Auchus, 2011), but its localization to the mitochondria and its mechanism of action is unknown. Here, we investigated what happens to AS after expression and whether these events relate to the production of aldosterone. We found that AS is targeted to the mitochondria by an N-terminal targeting sequence. AS is translocated into the mitochondria, where it is cleaved by mitochondrial processing peptidase (MPP), removing the targeting sequence. Mature AS then relocates to the intermembrane space (IMS). We found that AS produces 3-fold more aldosterone when residing within the mitochondria. These results reveal how the mechanisms of AS localization relate to production of aldosterone and identify a rapid, promoter-independent regulation of aldosterone production.
Materials and Methods
Cell Culture.
Waymouth and Dulbecco's/F12 (1:1) media were purchased from Sigma. MA-10 cells were cultured in Waymouth medium supplemented with 10% fetal bovine serum. NCI-H295 cells were cultured in Dulbecco's/F12 (1:1) medium supplemented with 10% donor equine serum. Cells were grown at 37°C at 5% CO2.
Isolation of Mitochondria.
NCI-H295 cells were harvested from Petri dishes by first removing the growth medium, washing with room temperature phosphate-buffered saline, and then adding 10 ml of 10 mM HEPES, pH 7.4, at 4°C per 14.5-cm plate. The cells were gently scraped using a cell lifter, and then the mixture was incubated for 30 min on ice. The cells were then pelleted by centrifugation at 3500g for 5 min at 4°C. The supernatant was removed, and the pellet was resuspended in 2 ml of mitochondrial isolation buffer (10 mM HEPES, 250 mM sucrose, and 1 mM EGTA, pH 7.4). The suspension was then homogenized using a Dounce homogenizer by adding the suspension and moving glass stopper up and down 42 times. The homogenate was then centrifuged at 3500g for 10 min at 4°C. The supernatant was removed and then centrifuged at 13,000g at 4°C. The supernatant was removed, and the mitochondrial pellet was resuspended in energy regeneration buffer (125 mM sucrose, 80 mM KCl, 5 mM MgCl2, 10 mM NaH2PO4, 10 mM isocitrate, 1.0 mM ATP, 1.0 mM NADP, 0.1 mM ADP, and 25 mM HEPES, pH 7.4) and then used in assay.
Cell-Free Transcription/Translation.
A cell-free transcription/translation system was used to express proteins for in vitro experiments. The kit, purchased from Promega, uses rabbit reticulocyte lysate to provide ribosomes and cofactors for protein expression. Amino acids (provided with the kit) were added. For nonradioactive expression, all 20 amino acids were added. For radioactive expression, a mixture of 19 amino acids without unlabeled methionine were added along with [35S]methionine, obtained from MP Biomedicals (Solon, OH). The protein of interest coupled to an SP6 promoter was added, along with SP6 polymerase provided with the kit. The mixture was incubated at 26°C for 2 h. Expression was verified using SDS-polyacrylamide gel electrophoresis.
Translocation Assay.
Mitochondria were suspended in buffer consisting of 20 mM HEPES, pH 7.5, 125 mM sucrose, 10 mM dithiothreitol, 1 mM ATP, 0.08 mM ADP, 2 mM potassium phosphate, 5 mM sodium succinate, 80 mM KCl, and 2 mM magnesium acetate. After addition of the protein of interest, mitochondria were incubated at 26°C. Mitochondria were pretreated with valinomycin, phenanthroline, or in “low-energy” buffer (lacking ATP, ADP, and succinate) for 10 min at room temperature before addition of protein of interest.
Aldosterone Bioassay.
The radioimmunoassay (RIA) kit was purchased from MP Biomedicals. The assay buffer consisted of the previously described import buffer with the following additions: 4 μM MgCl2, 0.5 mM NADPH, and 8 mM fumaric acid. These are known direct and indirect cofactors for enhancing AS activity (Psychoyos et al., 1966). The substrate, DOC, was dissolved in ethanol at a concentration of 10 mM, and the concentration used in the assay was 100 nM to 1 μM. For extraction of aldosterone, dichloromethane/polyethylene glycol (50 mg/l) was added at 40 times the reaction volume, and the samples were vortexed for 2 min at low speed, and then the organic and inorganic phases were allowed to separate. Half of the total organic volume was removed from the mixture and left to evaporate overnight. The dried samples were resuspended in phosphate-buffered saline containing 5% methanol. The RIA was conducted according to the provided protocol. Resulting pellets were resuspended in 1 M NaOH, and counted with a gamma counter (5500B; Beckman Coulter, Fullerton, CA).
Mitochondrial Fractionation.
The mitochondrial compartments were purified after established procedures with slight modifications. A stock of 0.6% digitonin was prepared by suspension in mitochondrial import buffer (as mentioned above) with gentle heating to dissolve the digitonin. Initial fractionation with AS and controls was performed with a range from 0.05 to 0.3% digitonin. After resuspension of mitochondrial pellet, the mitochondria were incubated at 4°C for 5 min, followed by centrifugation at 13,000g for 10 min. Because 0.2% digitonin was found to solubilize outer mitochondrial membrane (OMM) controls, and inner mitochondrial membrane (IMM) controls remained in the pellet fraction, this concentration was then used for subsequent experiments.
Generation of AS Antiserum.
Amino acid sequence analysis of AS protein showed that amino acids 28 through 197 were highly antigenic. Thus, the cDNA encoding this region was amplified by polymerase chain reaction and subcloned into the BamHI/SalI site of pQE30 (QIAGEN GmbH, Hilden, Germany). The His-tag AS protein was expressed in Escherichia coli and purified by nitrilotriacetic acid-Sepharose affinity chromatography in denaturing conditions. The high-purity protein was used to immunize rabbits to raise polyclonal antibodies commercially (Lampire Biologicals, Pipersville, PA). When the antibody titer was raised to 1:5000 dilution, the rabbits were exsanguinated and the whole serum was collected. In specific experiments, the AS-specific antiserum was purified using protein A Sepharose CL-4B. The purified antiserum was stored under 20% glycerol at −70°C.
Results
AS Translocates to the Mitochondrial Matrix and Undergoes Cleavage, Resulting in Lower Activity.
We set our first goal to identify the specific localization of AS in the mitochondria using translocation assays. Because only steroidogenic cells express AS, we isolated mitochondria from human NCI-H295 adrenal cells (Bird et al., 1993). [35S]Methionine-labeled AS was generated by a cell-free transcription/translation system, and then incubated with the isolated mitochondria. We observed AS cleavage as indicated by formation of a smaller, mature form of AS from the larger precursor form (Fig. 1B, lanes 2 and 3). The difference in molecular weight between precursor and mature forms suggests cleavage of a polypeptide between 20 and 30 amino acids in size. The unimported fraction, but not the imported fraction, was easily proteolyzed with trypsin because the mitochondrial compartment does not allow easy access of protease (Fig. 1B, lane 4). Permeabilization of mitochondrial membranes with nonionic detergent (Triton X-100) along with addition of protease resulted in digestion of both precursor and mature AS, indicating that mature AS resisted proteolysis because of its residence inside mitochondria (Fig. 1B, lane 5).
Mitochondrial processing peptidase (MPP) frequently cleaves proteins targeted to mitochondria at their N termini (Schneider et al., 1990). Analyzing the AS sequence revealed the presence of two arginine residues at amino acids 20 and 22, residues typically found upstream of MPP cleavage sites (von Heijne et al., 1989). This suggests that MPP may be responsible for cleavage of AS. To test this, we purified recombinantly expressed MPP from E. coli and then incubated it with [35S]AS. The results show a direct correlation between rMPP levels and generation of a 54.2-kDa mature form of AS (Fig. 1C), which migrated in a manner identical to that of AS cleaved by mitochondrial import (Fig. 1D). To confirm specificity of this cleavage, we inhibited native MPP in the mitochondria with phenanthroline, a metalloprotease inhibitor that chelates zinc needed for MPP activity (Böhni et al., 1983). We found that presence of phenanthroline prevented cleavage of precursor AS by isolated mitochondria (Fig. 1E, lane 3). However, washing phenanthroline-pretreated mitochondria with import buffer before addition of AS restored cleavage (Fig. 1E, lane 4), suggesting that phenanthroline has a reversible effect. To find protease resistance of AS, we added trypsin after incubation of AS with mitochondria and phenanthroline. We found that a portion of precursor AS was protected from proteolysis (Fig. 1F, lane 5), confirming that AS was translocated into phenanthroline-treated mitochondria, but not cleaved.
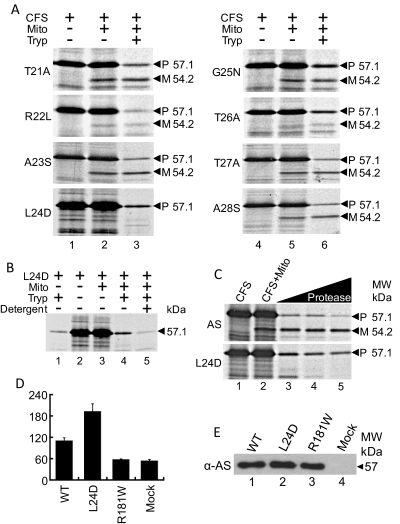
We next focused on identifying the exact site of MPP cleavage in AS. Residues spanning amino acids 23 to 26 represented a likely cleavage site for MPP based on the presence of upstream arginine residues and molecular weight of mature AS. We performed mutational analysis of amino acids 21 to 28 by substituting polar for nonpolar residues or acidic for basic residues, as described previously (Fig. 2A). Incubation with isolated mitochondria resulted in appropriate cleavage of all of mutated forms of AS, except for L24D-AS (Fig. 2A). To rule out the possibility that the L24D mutation altered cleavage by impeding import, we incubated L24D-AS with mitochondria and then treated with protease. The result showed that a portion of L24D-AS translocated into mitochondria as some of the uncleaved precursor was protected from protease digestion (Fig. 2B, lane 4). Permeabilization of mitochondrial membranes with nonionic detergent (Triton X-100) removed the protective effect (Fig. 2B, lane 5). We next compared translocation of L24D-AS with wild-type AS, using increasing concentration of protease. Precursor AS was completely digested, whereas a portion of L24D-AS was protected, further indicating translocation of L24D-AS into mitochondria (Fig. 2C).
Fig. 2.
Leucine 24 critical for proper cleavage of AS in the mitochondria; L24D mutant is more active than wt AS. A, translocation of mutant constructs into mitochondria. L24D is not cleaved when incubated with mitochondria, whereas the other specific point mutations (T21A, R22L, A23S, G25N, T26A, T27A, and A28S) are cleaved, with the mature form migrating at 54.2 kDa. B, translocation of mutant L24D AS into mitochondria. Lane 1, L24D alone is fully digested by trypsin (2 μg/ml). Lane 2, L24D only. Lane 3, incubation with mitochondria does not cleave L24D. Lane 4, addition of trypsin (2 μg/ml) digests most of the L24D, with a portion protected from digestion. Lane 5, permeabilizing of membranes with 0.5% Triton X-100 (0.5%) before trypsin treatment results in digestion of the protected portion of L24D. C, comparison of AS versus L24D-AS proteolysis after import into the isolated mitochondria. Increasing amounts of protease were used to compare digestion of precursor AS and uncleaved L24D-AS. L24D-AS was partially protected, whereas precursor AS was fully digested, indicating translocation of L24D-AS into mitochondria. D, biological activity, as measured by the accumulated aldosterone synthesis, after cotransfection of the nonsteroidogenic COS-1 cells with F2 and the indicated constructs. Data presented in this panel are the mean of a minimum of three transfections (± S.D.) performed independently. Wild-type AS, 110.6 ± 7.8 pg/ml; L24D, 193.0 ± 21.2 pg/ml; R181W, 58.0 ± 1.1 pg/ml; Mock, 54.2 ± 3.2. E, immunoblot of cell extracts of transfected COS-1 cells, normalized for overall protein concentration. Membrane probed with anti-AS polyclonal antibody.
We next addressed the fate of unprocessed AS using L24D-AS and COS-1 cells. COS-1 cells do not express SCC, ferredoxin, and ferrodoxin reductase. Thus, we cotransfected cells with L24D-AS and F2, a construct that fuses SCC, ferrodoxin, and ferrodoxin reductase, resulting in a single chimeric protein (Harikrishna et al., 1993). Wild-type AS served as a positive control, whereas mutant R181W-AS served as a negative control. We used radioimmunoassays to measure accumulated aldosterone. Figure 2D shows that wild-type AS synthesized 120 ng/ml aldosterone, two times more than empty vector control and mutant R181W-AS. However, L24D-AS produced two times more aldosterone than the wild-type AS, without any difference in protein expression as determined by Western blotting (Fig. 2E). In the absence of cleavage of the N-terminal sequence, the possibility exists that the protein is partially unfolded as it had to pass through the import channel during its journey from the OMM to the matrix. However, the N-terminal sequence of mutant R181W was cleaved, but possibly folded differently from wild-type, and thus became inactive.
Mature AS Resides in the Intermembrane Space after Cleavage in the Matrix.
The above experiments suggested that translocation of AS is necessary for activity at physiological levels, whereas processing of AS decreases the production of aldosterone. Changes in the rate that AS translocates into the matrix could disturb normal production of aldosterone. These observations emphasize the importance of where AS localizes in the mitochondria and how moving from one location to another could affect aldosterone synthesis. For example, slower translocation to the matrix from the OMM would cause AS to remain in the more active precursor form for a longer period of time. Most proteins targeted to the matrix by their N-termini remain there (Neupert and Herrmann, 2007). However, there are examples of proteins that are targeted to other areas of the mitochondria after translocation to the matrix, including the IMS (Hahne et al., 1994). Thus, we next addressed where AS resides in mitochondria.
Protein translocation across the IMM is initiated by the electrical potential generated by the electron transport chain (Koehler, 2004) and driven by the import motor consisting of Tim44 and Hsp70 (Ungermann et al., 1994). We did translocation assays in the presence of valinomycin, which blocks translocation across the IMM by dissipating the electrochemical gradient of the inner membrane (Gasser et al., 1982) and found that valinomycin prevented AS cleavage (Fig. 3A, lane 3). Translocation across the inner membrane also requires Hsp70 and Tim44, which make up the import motor that uses ATP to actively pull proteins across the IMM (Ungermann et al., 1994). To study the role of Hsp70 and Tim44 in AS translocation across the IMM, we conducted import assays in the absence of ATP, ADP, and succinate in the buffer, generating conditions of very low energy. This low energy resulted in reduced cleavage of AS (Fig. 3A, lane 4). Quantitation of the 54-kDa band showed that cleaved band intensity was reduced to 55 ± 5.6% compared with the precursor's 100 ± 0.3% (Fig. 3A, bar graph). These results support the view that blocking translocation across the IMM prevents processing of AS.
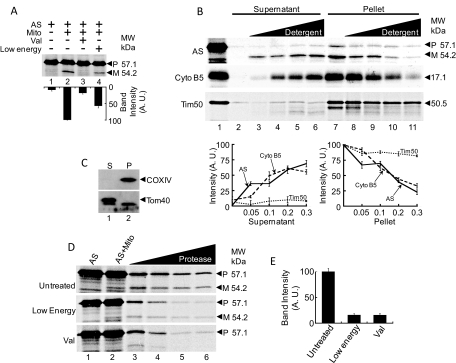
Fig. 3.
Mature AS translocates directly to the matrix then translocates back across the inner membrane to the intermembrane space. A, blocking translocation across the IMM prevents cleavage of precursor AS by MPP. Lane 1, AS only. Lane 2, cleavage of precursor AS by mitochondria to form mature AS. Lane 3, mitochondria pretreated with valinomycin do not cleave precursor AS. Lane 4, removal of ATP, ADP, and succinate from the buffer system results in reduced cleavage of precursor AS by mitochondria. Bottom, bar graph showing intensities of the 54-kDa band representing mature AS. Arbitrary values for band intensity taken as percentages of highest value, which is taken as 100% (± S.D.). AS only, 8.9 ± 3.2%; AS/Mito, 100.0 ± 0.3%; AS/Mito/Val, 18.4 ± 3.0%; and AS/Mito/Low Energy, 57.5 ± 5.9%. B, digitonin fractionation of mitochondria to determine localization of mature AS. Lane 1, CFS only. Lanes 2 to 6, supernatant after CFS incubation with mitochondria. Lane 2, no digitonin. Lanes 3 to 6, increasing amounts of digitonin (3, 0.05%; 4, 0.1%; 5, 0.2%; and 6, 0.3%). Lanes 7 to 11, pellet after CFS incubation with mitochondria. Lane 7, no digitonin. Lanes 8 to 11, increasing amounts of digitonin (8, 0.05%; 9, 0.1%; 10, 0.2%; and 11, 0.3%). Mature AS shifts from the pellet to the supernatant with increasing amounts of digitonin, as does cytochrome b5, an OMM resident protein. Tim50, an IMM resident, remains in the pellet while increasing digitonin. These results indicate that mature AS resides in the IMS. The bottom two panels represent the quantitative estimation of the imported 54.2-kDa band intensity and its comparison with B5 and Tim50 import. C, immunoblot of digitonin fractions to detect COX IV and Tom40. Lane 1, supernatant (OMM/ IMS). Lane 2, pellet (IMM/ matrix). IMM resident COX IV is found entirely in pellet (lane 2). OMM resident Tom40 is found mostly in the supernatant (lane 1). These results confirm successful fractionation of mitochondria. D, blocking translocation across the IMM results in exposure of precursor AS to the outside of mitochondria. Lane 1, AS only. Lane 2, AS incubated with mitochondria. For untreated mitochondria, precursor AS is cleaved to form mature AS. For low-energy and valinomycin-treated mitochondria, cleavage is reduced and blocked, respectively. Lanes 3 to 6, increasing amounts of protease results in full digestion of precursor AS under low-energy and valinomycin conditions, whereas precursor AS is partially protected after incubation with untreated mitochondria. E, relative intensities of bands corresponding to precursor AS in lane 6 of Fig. 2E. Arbitrary values for band intensity taken as percentages of highest value, which is taken as 100% (± S.D.). Untreated, 100.0 ± 6.0%; Low-energy, 16.0 ± 2.4%; Val, 15.9 ± 3.1%.
Our next goal was to identify the mitochondrial compartment in which the mature form of AS resides. Digitonin is a nondenaturing, nonionic detergent that removes the OMM at low concentrations, leaving the mitoplast intact (Rickwood et al., 1987). These phases can be separated by differential centrifugation, leaving the OMM and soluble contents of the IMS in the supernatant, and the mitoplast in the pellet. After mitochondrial import, increasing the concentration of digitonin caused mature AS to shift from the pellet, which contained the mitoplast, to the supernatant (Fig. 3B). As a control, we performed the import assay with cell-free synthesized cytochrome b5, an OMM-resident protein (Neupert and Herrmann, 2007). Cytochrome b5 also shifted from pellet to supernatant in a fashion similar to that of AS (Fig. 2C, middle). In contrast, IMM-resident Tim50 (Neupert and Herrmann, 2007) predominantly localized to the pellet fraction (Fig. 3B). Immunoblots of the same mitochondrial fractions showed IMM-resident COX IV associated with the mitoplast fraction, whereas OMM-resident Tom40 was associated with the OMM/IMS fraction (Fig. 3C), demonstrating successful mitochondrial fractionation. These results suggest that mature AS resides outside of the mitoplast, either in the IMS or associated with the OMM facing the IMM. However, given that mature AS is protected from proteosomal degradation, the protein probably resides in the IMS.
Translocation into the matrix typically occurs in one step, as Tom40 and Tim23 complexes associate via contact sites, allowing translocated protein to proceed directly from cytosol to the matrix (Habib et al., 2007). Alternatively, proteins targeted to the matrix may require interaction with the Tim23-Tim50 complex before entering into the matrix (Tamura et al., 2009). If AS follows the first model, then blocking translocation of AS across the IMM would result in a stalled, partially translocated AS exposed to the cytosol. This exposed AS would then face proteosomal degradation. Increase in protease concentration after translocation of AS into mitochondria resulted in protection of a portion of precursor AS in addition to mature AS (Fig. 3D, top [Untreated]). On the other hand, when translocation across the IMM is blocked, precursor AS is fully proteolyzed, indicating exposure to protease under otherwise identical conditions (Fig. 3D, middle [Low Energy] and bottom [Val]). Quantitation of band intensity (Fig. 3E) shows that bands corresponding to precursor AS in low-energy conditions and in valinomycin-treated mitochondria are both 15% of the intensity of the bands corresponding to untreated precursor AS (Fig. 3E, lane 6).
AS Is Active in the Mitochondria.
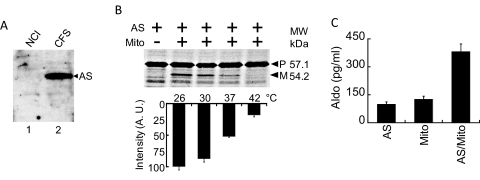
We next investigated whether AS localization to the mitochondria is critical for production of aldosterone. Detection of aldosterone in the mitochondrial fraction of adrenal gland extracts suggests a link between aldosterone production and the mitochondria (Miller and Auchus, 2011). We hypothesized that this link results from localization of AS to the mitochondria. We sought to test this by designing a bioassay to measure production of aldosterone by translocated, mature AS generated from CFS. First, we investigated the amount of endogenous AS present in mitochondria isolated from NCI cells to determine background activity. It is noteworthy that AS is expressed at very low levels in vivo (Ogishima et al., 1991). Western blotting with our anti-AS antibody showed no expression of endogenous AS, whereas CFS expressed AS was clearly detectable (Fig. 4A). We next examined mitochondrial import and processing of [35S]AS at 37°C, 30°C and 26°C. As a negative control, we also incubated the mixture at 42°C, a temperature that destroys the metabolic activity of the mitochondria. The highest level of AS cleavage occurred at 26°C, two times greater than cleavage at 37°C (Fig. 4B). Thus, to evaluate AS activity, we assayed at 26°C in the presence of magnesium, fumaric acid, and NADPH, cofactors that enhance production of aldosterone in adrenal extracts (Psychoyos et al., 1966). Aldosterone production was measured using a radioimmunoassay. Exogenous AS alone generated 99.5 ± 12.0 pg/ml aldosterone, whereas purified mitochondria from NCI cells, which probably contained endogenous AS, produced 126.0 ± 16.1 pg/ml (Fig. 4C). Combining AS with mitochondria resulted in synthesis of 381.9 ± 40.7 pg/ml aldosterone, more than 3-fold the amount produced by AS alone (Fig. 4C). These results show that AS activity is enhanced by mitochondria.
Fig. 4.
Aldosterone production by AS is enhanced by the mitochondria. A, comparison of endogenous AS versus CFS-expressed AS. Immunoblot of NCI cell extract (lane 1) and CFS-expressed AS (lane 2) using rabbit anti-AS polyclonal antibody. The amount of CFS-expressed AS added to the sample is the equivalent of the amount used for the bioassay. B, increasing temperature reduces translocation and cleavage of precursor AS in vitro. Lane 1, CFS-expressed AS only. Lanes 2 to 5, AS and mitochondria incubated under increasing temperature. Bottom, quantitative estimation of the 54.2-kDa cleaved band from the precursor at different temperatures. Arbitrary values for mature AS band intensity taken as percentages of highest value, which is taken as 100%. 26°C, 100.0 ± 5.3%; 30°C, 87.6 ± 5.1%; 37°C, 52.3 ± 1.1%; and 42°C, 18.2 ± 2.8%. C, measurement of aldosterone production by RIA. All activities show data for a minimum of four independent experiments, with error in SD. CFS-expressed AS plus mitochondria has over 3-fold increase in activity compared with mitochondria or AS alone. CFS-expressed AS alone produces aldosterone to a similar level as mitochondria alone. AS alone, 99.5 ± 12.0 pg/ml; mito alone, 126.0 ± 16.1 pg/ml; and AS/mito, 381.9 ± 40.7 pg/ml.
N-Terminal Leader Sequence of AS Has Role in Targeting to the Mitochondria and Folding Proteins.
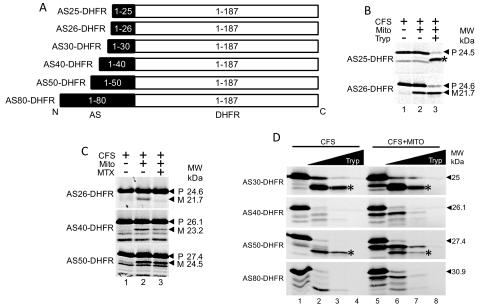
We next investigated the matrix-targeting mechanism of the N-terminal sequence. To do so, we built constructs that fused different lengths of the AS N terminus to dihydrofolate reductase (DHFR) (Fig. 5A). We chose DHFR as the cargo protein, because it is a soluble protein found in the cytosol, and it stabilizes when bound to methotrexate (Eilers et al., 1988). Chimeric proteins were synthesized in the cell-free system and then incubated with mitochondria isolated from NCI cells. Import assays showed that AS26-DHFR underwent cleavage, but AS25-DHFR did not, indicating that successful targeting of DHFR to the mitochondria required at least the first 26 residues of AS (Fig. 5B). Limited proteolysis resulted in cleavage of unimported precursor but not the imported mature form of AS26-DHFR, indicating that the fusion protein resides in mitochondria (Fig. 5B).
Fig. 5.
N-terminal 26 amino acids of AS target cargo protein DHFR to the mitochondria. A, the diagram shows the different AS-DHFR fusion constructs, where the full-length DHFR was fused to the varying length of N-terminal AS, as indicated. The black indicates AS, whereas the empty white boxes indicate DHFR. B, translocation of AS-DHFR fusions into mitochondria. Lane 1, CFS only. Lane 2, CFS incubated with mitochondria. AS25-DHFR is not cleaved when incubated with mitochondria, whereas AS26-DHFR is cleaved. Lane 3, mature AS26-DHFR is protected from protease, whereas precursor AS25-DHFR is digested. A fragment of AS25-DHFR is protected from proteolysis; however, this fragment is a proteolytic breakdown product that forms in the absence of mitochondria, indicated by a star. C, halting of translocation of AS-DHFR fusions by methotrexate indicates that between 41 and 50 amino acids are required to span the OMM and IMM, allowing cleavage of the AS-targeting sequence by MPP. Lane 1, CFS only. Lane 2, CFS incubated with mitochondria. All three constructs are cleaved to mature forms. Lane 3, addition of methotrexate (MTX) prevents cleavage of AS26-DHFR and AS40-DHFR, whereas AS50-DHFR is cleaved. D, comparison of protease digestion of AS30-DHFR, AS40-DHFR, AS50-DHFR, and AS60-DHFR shows changes in folding of the proteins. Lane 1, CFS only. Lanes 2 to 4, increasing concentrations of protease incubated with CFS. Lane 5, CFS incubated with mitochondria, with cleavage of each fusion protein. Lanes 6 to 8, increasing concentrations of protease incubated with CFS and mitochondria. Protected fragments from AS30-DHFR and AS50-DHFR form with CFS only and CFS with mitochondria (indicated by star). Occurrence of these bands depends on the size of the N-terminal AS sequence fused to DHFR.
We next investigated the minimum number of amino acids needed for AS to reach the matrix. Methotrexate binds to DHFR, and thus addition of methotrexate will hold the N-terminal chain in the cytosol, blocking translocation. Indeed, we found that the addition of methotrexate during import of AS26-DHFR into the mitochondria prevented the formation of a smaller, cleaved fragment (Fig. 5C), confirming that methotrexate impeded translocation. However, AS50-DHFR was processed by mitochondria in the presence of methotrexate, whereas AS40-DHFR underwent minimal processing (Fig. 5C). This suggests that the N terminus can enter the matrix, allowing for MPP cleavage, even if the bulk of the protein remains in the cytosol. In general, spanning both the outer and inner membranes requires 30 to 40 amino acids (Ungermann et al., 1994), but the results here indicate that approximately 40 to 50 amino acids are required for the N terminus of the fusion protein to reach the matrix and be cleaved by MPP. Although the N-terminal 26 amino acids of AS can act as an N-terminal targeting sequence, we found that the folding of DHFR changed depending of the length of AS N terminus. AS30-DHFR and AS50-DHFR had a large fragment protected from protease digestion (Fig. 5D, lanes 3 and 7, star). AS40-DHFR or AS80-DHFR, however, did not form this fragment under identical conditions (Fig. 5D). AS25-DHFR formed a similar fragment upon protease treatment, whereas AS26-DHFR did not (Fig. 5B, star). These results suggest that the AS N terminus influences tertiary structure of cargo proteins in addition to its role in mitochondrial targeting.
Rate of AS Translocation Is Independent of the Targeting Sequence.
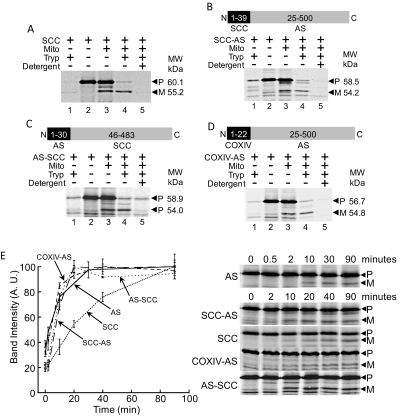
Because the N-terminal sequence targets AS to the mitochondria and seems to affect folding of the overall protein, we next investigated whether the targeting sequence modulates rate of translocation of protein into the mitochondria. We compared AS with SCC, which is also targeted to the mitochondria (Chung et al., 1986). SCC converts cholesterol, the steroid precursor, to pregnenolone at the start of the steroidogenic pathway (Fig. 1A). Import of cell-free synthesized SCC into isolated mitochondria from NCI cells resulted in cleavage and protection from protease unless mitochondrial membranes were permeabilized with nonionic detergent (Fig. 6A).
Fig. 6.
Rate of translocation of AS is not dependent on the targeting sequence. A, translocation of SCC into mitochondria. Lane 1, SCC is fully digested by protease. Lane 2, SCC. Lane 3, incubation of SCC with mitochondria results in cleavage of precursor SCC [P] to mature SCC [M]. Lane 4, addition of protease digests precursor SCC [P], whereas mature SCC [M] is protected. Lane 5, permeabilization of membranes before protease treatment results in digestion of both precursor [P] SCC and mature [M] SCC. B, translocation of SCC-AS into mitochondria. Lane 1, SCC-AS is fully digested by protease. Lane 2, SCC-AS. Lane 3, incubation of SCC-AS with mitochondria results in cleavage of precursor SCC-AS [P] to mature SCC-AS [M]. Lane 4, addition of trypsin digests precursor SCC-AS [P], whereas mature SCC-AS [M] is protected. Lane 5, permeabilization of membranes before protease treatment results in digestion of both precursor [P] SCC-AS and mature [M] SCC-AS. C, translocation of AS-SCC into mitochondria. Lane 1, AS-SCC is fully digested by protease. Lane 2, AS-SCC. Lane 3, incubation of AS-SCC with mitochondria results in cleavage of precursor AS-SCC [P] to mature AS-SCC [M]. Lane 4, addition of protease digests precursor AS-SCC [P], whereas mature AS-SCC [M] is protected. Lane 5, permeabilization of membranes before protease treatment results in digestion of both precursor [P] AS-SCC and mature [M] AS-SCC. D, translocation of COX IV-AS into mitochondria. Lane 1, COX IV-AS is fully digested by protease. Lane 2, COX IV-AS. Lane 3, incubation of COX IV-AS with mitochondria results in cleavage of precursor COX IV-AS [P] to mature COX IV-AS [M]. Lane 4, addition of trypsin digests precursor COX IV-AS [P], whereas mature COX IV-AS [M] is protected. Lane 5, permeabilization of membranes before protease treatment results in digestion of both precursor [P] COX IV-AS and mature [M] COX IV-AS. E, translocation kinetics of AS, SCC, SCC-AS, COX IV-AS, and AS-SCC. AS (solid line) translocates at a rapid rate initially but reaches a plateau at which translocation ceases. COX IV-AS (smaller dashed line) and SCC-AS (larger dashed line) match with this translocation pattern, despite MTS of COX IV and SCC, respectively. AS-SCC (looser dotted line) translocation kinetics match with that of AS, and not of SCC (compact dotted line), indicating that the rate of translocation of SCC is influenced by the MTS. Gel images in this panel were cropped from the same original gel image for each experiment.
To understand how the N terminus affects translocation kinetics, we generated a construct in which the 39 N-terminal amino acids of SCC replaced the 24 N-terminal amino acids of AS (SCC-AS). We found that SCC-AS, like SCC, was imported and cleaved in the mitochondria, forming mature SCC-AS that was protected from proteolysis by intact mitochondrial membranes (Fig. 6B). To test whether SCC retained the same translocation kinetics, we replaced the N-terminal 45 amino acids of SCC with the N-terminal 30 amino acids of AS. Import assays demonstrated the translocation and cleavage of AS-SCC as well as membrane-mediated protection from proteolysis (Fig. 6C). To study the effect of replacing the N terminus of AS with that of a nonsteroidogenic protein, we generated a chimeric protein with the N-terminal 22 amino acids of COX IV replacing the N-terminal 24 amino acids of AS. Similar to the other chimeric proteins, COX IV-AS also was imported and cleaved when incubated with mitochondria, and the mature form only proteolyzed after addition of Triton X-100 (Fig. 6D). To evaluate translocation kinetics, we measured MPP-mediated cleavage of both the parent and chimeric proteins over time. The result shows that the import efficiency of AS and SCC differed significantly (Fig. 6E). Initial translocation of AS was very rapid but reached a plateau after 20 min (Fig. 6E, solid line). Translocation of SCC, however, proceeded in a more linear fashion, continuing to translocate over time without reaching a maximum value (Fig. 6E, closely dotted line). COX IV-AS (Fig. 6E, small dashed line) and SCC-AS (Fig. 6E, large dashed line) were translocated at the same rate as wild-type AS, indicating that replacing the targeting sequence of AS with SCC and COX IV targeting sequences did not change AS translocation (Fig. 6E). However, AS-SCC kinetics function differently than wild-type SCC and adopt a translocational rate similar to wild type AS (Fig. 6E, loosely dotted line). These results suggest that the translocation rate into mitochondria is controlled differently depending on the protein and that the N terminus does not necessarily regulate this rate.
Discussion
Thus far, known regulatory mechanisms of aldosterone production have been controlled by angiotensin II by stimulation of AS, SCC, and steroidogenic acute regulatory protein promoters. These effects require 12 h to reach full strength and then last 24 h before subsiding (Denner et al., 1996). We have identified new potential mechanisms to control aldosterone production that are promoter-independent and could provide rapid response to physiological changes. We have shown that AS targets and translocates into mitochondria (Fig. 1B) and that the complex, multistep mechanism is linked to aldosterone production (Fig. 4C). Targeting newly expressed precursor AS to the OMM is the first critical step. Aldosterone production by AS is enhanced by mitochondria, indicating that localization to the organelle is linked with increased synthesis (Fig. 4C). The N-terminal 26 residue sequence of AS is of immense importance to the physiological role of the protein. The sequence targets AS to the mitochondria (Fig. 1B) and is able to target nonmitochondrial proteins to the organelle (Fig. 5B). The AS-targeting sequence also seems to serve a regulatory role, with cleavage of the polypeptide leading to a lower level of aldosterone production.
Mitochondrial translocation of AS was very rapid but was followed by an abrupt halt in the translocation rate. This halt happened with wild-type AS and also with chimera constructs in which the AS targeting sequence was replaced with SCC or COX IV targeting sequences (Fig. 6E). This suggests that there is a regulatory sequence located elsewhere on AS that influences rate of translocation. We find it likely that the rate of translocation depends mostly on folding states of the protein before initiation of translocation, with tighter folding resulting in the protein crossing membranes at a slower rate and vice versa. Although the rate of AS translocation was independent of the targeting sequence, translocation rates of SCC resembled that of AS when the targeting sequence of AS was substituted in place of the targeting sequence of SCC (Fig. 6E). Because specific lengths of the AS N terminus influenced folding when fused to DHFR (Fig. 3I), perhaps these same effects by the AS targeting sequence changed the rate of AS-SCC translocation (Fig. 6E).
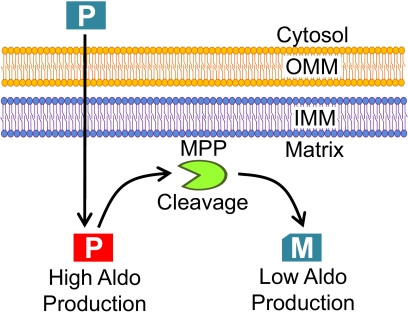
AS translocated directly to the matrix, bypassing the IMS (Fig. 3D). MPP then cleaves precursor AS to form mature AS in the matrix (Fig. 1D). Uncleavable mutant L24D-AS was found to be more active than wild type, suggesting that this processing step acts as a regulatory mechanism by reducing activity (Fig. 2D). Mature AS crosses the IMM again to finally reside in the IMS (Fig. 3B). NADH cytochrome b5 reductase follows a similar translocation mechanism (Hahne et al., 1994). This is an unusual step for a protein targeted to the matrix and may signify an additional regulatory mechanism to block aldosterone production and trigger eventual breakdown of the protein by IMS proteases. We have proposed a model of AS translocation into mitochondria, where the enzyme is more active after initial translocation and then is less active after cleavage of precursor AS to the mature form (Fig. 7). We expect that blockage of the steps before arrival of precursor AS in the matrix would lower aldosterone production, whereas blockage of the cleavage of AS to its mature form and translocation of mature AS to the IMS would increase aldosterone production. Our model provides a strong suggestion that aldosterone production could be regulated in the mitochondria by increasing or decreasing translocation rates and cleavage of AS. These events could be influenced by physiological factors, possibly through not yet known pathways. Disorders in the mechanism of AS localization to the mitochondria could lead to disease states and possibly explain primary hypertensive disorders. Control of these mechanisms could provide rapid, promoter-independent regulation of aldosterone production.
Fig. 7.
Proposed model of AS action into mitochondria. Precursor Protein AS (Blue) reaches to the matrix after translocation through the OMM and IMM. Precursor AS is not active until translocated into mitochondria. Next, AS is active (Red P) until cleavage of 24 amino acid N-terminal sequence at the matrix by MPP, forming a less active mature protein (M).
Acknowledgments
We thank Dr. Sakae Kitada for the generous gift of MPP cDNA.
This work was supported by the National Institutes of Health [Grant HD057876] and the Anderson Cancer Institute (H.S.B.).
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
- AS
- aldosterone synthase
- DOC
- deoxycorticosterone
- SCC
- cytochrome P450 side-chain cleavage enzyme
- ANGII
- angiotensin II
- MPP
- mitochondrial processing peptidase
- IMS
- intermembrane space
- CFS
- cell-free transcription/translation
- RIA
- radioimmunoassay
- OMM
- outer mitochondrial membrane
- MPP
- mitochondrial processing peptidase
- IMM
- inner mitochondrial membrane
- COXIV
- cytochrome c oxidase subunit IV
- DHFR
- dihydrofolate reductase
- Hsp70
- heat shock protein 70
- Tim
- translocase inner membrane
- Tom
- translocase outer membrane.
Authorship Contributions
Participated in research design: Adams and Bose.
Conducted experiments: Adams.
Performed data analysis: Adams and Bose.
Wrote or contributed to the writing of the manuscript: Adams and Bose.
References
- Baukal AJ, Balla T, Hunyady L, Hausdorff W, Guillemette G, Catt KJ. (1988) Angiotensin II and guanine nucleotides stimulate formation of inositol 1,4,5-trisphosphate and its metabolites in permeabilized adrenal glomerulosa cells. J Biol Chem 263:6087–6092 [PubMed] [Google Scholar]
- Bird IM, Hanley NA, Word RA, Mathis JM, McCarthy JL, Mason JI, Rainey WE. (1993) Human NCI-H295 adrenocortical carcinoma cells: a model for angiotensin-II-responsive aldosterone secretion. Endocrinology 133:1555–1561 [DOI] [PubMed] [Google Scholar]
- Böhni PC, Daum G, Schatz G. (1983) Import of proteins into mitochondria. Partial purification of a matrix-located protease involved in cleavage of mitochondrial precursor polypeptides. J Biol Chem 258:4937–4943 [PubMed] [Google Scholar]
- Chung BC, Matteson KJ, Voutilainen R, Mohandas TK, Miller WL. (1986) Human cholesterol side-chain cleavage enzyme, P450scc: cDNA cloning, assignment of the gene to chromosome 15, and expression in the placenta. Proc Natl Acad Sci USA 83:8962–8966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curnow KM, Tusie-Luna MT, Pascoe L, Natarajan R, Gu JL, Nadler JL, White PC. (1991) The product of the CYP11β2 gene is required for aldosterone biosynthesis in the human adrenal cortex. Mol Endocrinol 5:1513–1522 [DOI] [PubMed] [Google Scholar]
- Denner K, Rainey WE, Pezzi V, Bird IM, Bernhardt R, Mathis JM. (1996) Differential regulation of 11β-hydroxylase and aldosterone synthase in human adrenocortical H295R cells. Mol Cell Endocrinol 121:87–91 [DOI] [PubMed] [Google Scholar]
- Eilers M, Hwang S, Schatz G. (1988) Unfolding and refolding of a purified precursor protein during import into isolated mitochondria. EMBO J 7:1139–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser SM, Ohashi A, Daum G, Böhni PC, Gibson J, Reid GA, Yonetani T, Schatz G. (1982) Imported mitochondrial proteins cytochrome b2 and cytochrome c1 are processed in two steps. Proc Natl Acad Sci USA 79:267–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzaga CC, Calhoun DA. (2008) Resistant hypertension and hyperaldosteronism. Curr Hypertens Rep 10:496–503 [DOI] [PubMed] [Google Scholar]
- Habib SJ, Waizenegger T, Niewienda A, Paschen SA, Neupert W, Rapaport D. (2007) The N-terminal domain of Tob55 has a receptor-like function in the biogenesis of mitochondrial β-barrel proteins. J Cell Biol 176:77–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahne K, Haucke V, Ramage L, Schatz G. (1994) Incomplete arrest in the outer membrane sorts NADH-cytochrome b5 reductase to two different submitochondrial compartments. Cell 79:829–839 [DOI] [PubMed] [Google Scholar]
- Harikrishna JA, Black SM, Szklarz GD, Miller WL. (1993) Construction and function of fusion enzymes of the human cytochrome P450scc system. DNA Cell Biol 12:371–379 [DOI] [PubMed] [Google Scholar]
- Hatakeyama H, Inaba S, Takeda R, Miyamori I. (2000) 11β-Hydroxysteroid dehydrogenase in human vascular cells. Kidney International 57:1352–1357 [DOI] [PubMed] [Google Scholar]
- Koehler CM. (2004) New developments in mitochondrial assembly. Annu Rev Cell Dev Biol 20:309–335 [DOI] [PubMed] [Google Scholar]
- Kramer RE, Gallant S, Brownie AC. (1979) The role of cytochrome P-450 in the action of sodium depletion on aldosterone biosynthesis in rats. J Biol Chem 254:3953–3958 [PubMed] [Google Scholar]
- Lombès M, Alfaidy N, Eugene E, Lessana A, Farman N, Bonvalet JP. (1995) Prerequisite for cardiac aldosterone action. Mineralocorticoid receptor and 11 beta-hydroxysteroid dehydrogenase in the human heart. Circulation 92:175–182 [DOI] [PubMed] [Google Scholar]
- Miller WL, Auchus RJ. (2011) The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr Rev 32:81–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani F, Shimizu T, Ueno R, Ishimura Y, Izumi S, Komatsu N, Watanabe K. (1982) Cytochrome P-45011β and P-450scc in adrenal cortex: zonal distribution and intramitochondrial localization by the horseradish peroxidase-labeled antibody method. J Histochem Cytochem 30:1066–1074 [DOI] [PubMed] [Google Scholar]
- Mornet E, Dupont J, Vitek A, White PC. (1989) Characterization of two genes encoding human steroid 11β-hydroxylase (P45011β). J Biol Chem 264:20961–20967 [PubMed] [Google Scholar]
- Neupert W, Herrmann JM. (2007) Translocation of proteins into mitochondria. Annu Rev Biochem 76:723–749 [DOI] [PubMed] [Google Scholar]
- Ogishima T, Mitani F, Ishimura Y. (1989) Isolation of aldosterone synthase cytochrome P-450 from zona glomerulosa mitochondria of rat adrenal cortex. J Biol Chem 264:10935–10938 [PubMed] [Google Scholar]
- Ogishima T, Shibata H, Shimada H, Mitani F, Suzuki H, Saruta T, Ishimura Y. (1991) Aldosterone synthase cytochrome P-450 expressed in the adrenals of patients with primary aldosteronism. J Biol Chem 266:10731–10734 [PubMed] [Google Scholar]
- Psychoyos S, Tallan HH, Greengard P. (1966) Aldosterone synthesis by adrenal mitochondria. J Biol Chem 241:2949–2956 [PubMed] [Google Scholar]
- Rickwood D, Wilson MT, Darly-Usmar VM. (1987) Isolation and characterization of intact mitochondria, in Mitochondria: A Practical Approach (Darly-Usmar VM, Rickwood D, Wilson MT. eds) pp 1–16, IRL Press, Washington D.C [Google Scholar]
- Rocha R, Rudolph AE, Frierdich GE, Nachowiak DA, Kekec BK, Blomme EA, McMahon EG, Delyani JA. (2002) Aldosterone induces a vascular inflammatory phenotype in the rat heart. Am J Physiol Heart Circ Physiol 283:H1802–H1810 [DOI] [PubMed] [Google Scholar]
- Schmidt BM, Montealegre A, Janson CP, Martin N, Stein-Kemmesies C, Scherhag A, Feuring M, Christ M, Wehling M. (1999) Short term cardiovascular effects of aldosterone in healthy male volunteers. J Clin Endocrinol Metab 84:3528–3533 [DOI] [PubMed] [Google Scholar]
- Schneider H, Arretz M, Wachter E, Neupert W. (1990) Matrix processing peptidase of mitochondria. Structure-function relationships. J Biol Chem 265:9881–9887 [PubMed] [Google Scholar]
- Slight SH, Ganjam VK, Gómez-Sánchez CE, Zhou MY, Weber KT. (1996) High affinity NAD(+)-dependent 11 beta-hydroxysteroid dehydrogenase in the human heart. J Mol Cell Cardiol 28:781–787 [DOI] [PubMed] [Google Scholar]
- Tamura Y, Harada Y, Shiota T, Yamano K, Watanabe K, Yokota M, Yamamoto H, Sesaki H, Endo T. (2009) Tim23-Tim50 pair coordinates functions of translocators and motor proteins in mitochondrial protein import. J Cell Biol 184:129–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungermann C, Neupert W, Cyr DM. (1994) The role of Hsp70 in conferring unidirectionality on protein translocation into mitochondria. Science 266:1250–1253 [DOI] [PubMed] [Google Scholar]
- von Heijne G, Steppuhn J, Herrmann RG. (1989) Domain structure of mitochondrial and chloroplast targeting peptides. Eur J Biochem 180:535–545 [DOI] [PubMed] [Google Scholar]
- Walker BR, Yau JL, Brett LP, Seckl JR, Monder C, Williams BC, Edwards CR. (1991) 11β-Hydroxysteroid dehydrogenase in vascular smooth muscle and heart: implications for cardiovascular responses to glucocorticoids. Endocrinology 129:3305–3312 [DOI] [PubMed] [Google Scholar]
- Wehling M, Spes CH, Win N, Janson CP, Schmidt BM, Theisen K, Christ M. (1998) Rapid cardiovascular action of aldosterone in man. J Clin Endocrinol Metab 83:3517–3522 [DOI] [PubMed] [Google Scholar]