Abstract
ABCG2 is an ATP-binding-cassette (ABC) transporter that confers multidrug resistance (MDR) to tumor cells by extruding a broad variety of chemotherapeutic agents, ultimately leading to failure of cancer therapy. Thus, the down-regulation of ABCG2 expression and/or function has been proposed as part of a regimen to improve cancer therapeutic efficacy. In this study, we found that a group of xanthines including caffeine, theophylline, and dyphylline can dramatically decrease ABCG2 protein in cells that have either moderate (BeWo, a placental choriocarcinoma cell line) or high (MCF-7/MX100, a breast cancer drug-resistant cell subline) levels of ABCG2 expression. This down-regulation is time-dependent, dose-dependent, and reversible. Using lysosomal inhibitors, we found that xanthines decreased ABCG2 by inducing its rapid internalization and lysosome-mediated degradation. As a consequence, caffeine treatment significantly increased the retention of an established ABCG2 substrate in MCF-7/MX100 cells but not in parental MCF-7 cells and sensitized the MDR cells to the chemotherapeutic agent mitoxantrone (MX); combination treatment with MX and caffeine decreased the IC50 of MX ∼10-fold and induced a greater degree of apoptotic cell death than MX treatment alone. Taken together, our results describe a novel function for this large class of therapeutically relevant compounds and suggest that a subset of xanthines could be developed as combination therapy to improve the efficacy of anticancer drugs that are ABCG2 substrates.
Introduction
The ABCG2 (breast cancer resistance protein) protein was first identified as a result of its overexpression in breast cancer cells exhibiting a MDR phenotype in the absence of other “classic” drug transporters (Doyle et al., 1998). Although a member of the same ABC family of membrane transporters as P-glycoprotein and multidrug resistance proteins, ABCG2 belongs to a unique subclass whose genes encode a half transporter with one classic nucleotide-binding domain and six transmembrane segments. Initially believed to exist as a homodimer, recent evidence suggests that ABCG2 may function as an oligomer consisting of 8 to 12 identical subunits (McDevitt et al., 2006) and transports a broad spectrum of natural and synthetic substrates including some cancer chemotherapeutic agents.
Given the role of ABCG2 in conferring MDR, there has been considerable focus on the expression of this transporter in human tumors and its correlation with clinical outcome. Elevated expression of ABCG2 was first reported in patients with acute myeloid leukemia (AML) (Ross et al., 2000) and subsequently confirmed in patients with AML and those with acute lymphocytic leukemia (van den Heuvel-Eibrink et al., 2002; Plasschaert et al., 2003; Wilson et al., 2006). ABCG2 conferred resistance to flavopiridol in patients with AML (Nakanishi et al., 2003), and its overexpression was higher after relapse of AML; hence, ABCG2 was suggested to act as a prognostic indicator for this disease (Benderra et al., 2004; Uggla et al., 2005). The role of ABCG2 in the resistant phenotype of solid tumors is less clear, although ABCG2 overexpression has been described in several tumor types (Diestra et al., 2002; Turner et al., 2006). Of interest, ABCG2 expression was rapidly increased in hepatoblastoma in response to certain chemotherapeutic drugs (Vander Borght et al., 2008), reminiscent of the induction of P-glycoprotein observed after treatment of metastatic sarcoma with doxorubicin (Abolhoda et al., 1999). ABCG2 is also expressed in adult normal tissues, including the intestine, colon, placenta, and blood-brain barrier, where it plays a role in restricting the oral bioavailability and pharmacokinetics of its substrates and probably plays a critical role in the homeostasis of endogenous compounds such as heme, porphyrins, riboflavin, and estrogens (Grube et al., 2007; Krishnamurthy et al., 2007; van Herwaarden et al., 2007; Ni et al., 2010).
Of interest is the observation that ABCG2 appears to play a critical role in both normal cells and cancer stem cells (CSCs); indeed, the observation that ABCG2 is often enriched in normal stem cells and CSCs relative to their more mature progeny has led to the proposed use of ABCG2 as a universal stem cell marker (Zhou et al., 2001; Bunting, 2002). The function of ABCG2 in CSCs is not well understood; however, some studies suggest that it is required for maintaining the “stemness” of the population, perhaps by enhancing proliferation and/or decreasing differentiation potential. Although this requires further investigation, there is ample evidence supporting a role for ABCG2 in protecting both normal and cancer stem cells from various stressors, including cancer chemotherapeutic drugs (Dean et al., 2005; Krishnamurthy and Schuetz, 2006).
Given the pleiotropic roles of ABCG2 in drug disposition, drug resistance, and CSC survival, inhibition or down-regulation of ABCG2 may be a valid approach to reverse ABCG2-mediated drug resistance and to increase oral absorption of certain chemotherapeutic drugs. Moreover, the putative role of ABCG2 in maintenance of CSCs suggests that ABCG2 inhibitors, unlike other drug transporter inhibitors, may function to reduce or eliminate the CSC population.
In the current study, we have investigated the use of xanthine derivatives as ABCG2 inhibitors. Xanthines are common in the human diet, where they are consumed in the form of nutrients, stimulants, and drugs. Caffeine (1,3,7-trimethylxanthine), the best known, best-studied, and most widely consumed xanthine, is a purine alkaloid, with a variety of pharmacological effects. It is used in the treatment of migraines and postprandial hypotension and obesity and as a respiratory stimulant in neonates (Sawynok, 1995). The effect of caffeine on cell cycle transition is sensitization of some human tumors to ionizing radiation and alkylating agents such as nitrogen mustard and cisplatin (Sarkaria et al., 1999). Analogous to caffeine, many other xanthine-related heterocyclins with enhanced potency and selectivity toward specific biological targets have provided powerful tools for research and potential therapeutic agents for intervention in a variety of diseases (Daly, 2007). For example, theophylline, a caffeine metabolite found in tea, is used for the treatment of asthma and has anti-inflammatory properties. Dyphylline [7-(2,3-dihydroxypropyl)-theophylline] is used as a bronchodilator in the treatment of asthma, chronic bronchitis, and emphysema. Additional xanthine derivatives are currently in preclinical and clinical studies (McCarty et al., 2002).
We now show that caffeine and several related xanthines can dramatically decrease ABCG2 protein in multiple cell lines by targeting this cell surface transporter for internalization and lysosomal degradation. This down-regulation has functional consequences. Exposure to caffeine increased intracellular accumulation of the ABCG2 substrate mitoxantrone (MX), resulting in a 10-fold increased sensitivity of tumor cells to the cytotoxic effects of this agent. These findings suggest that xanthines can be developed as combination therapy to improve the efficacy of drugs that are ABCG2 substrates.
Materials and Methods
Cell Culture and Chemicals.
BeWo cells were purchased from American Type Culture Collection (Manassas, VA) and maintained in F-12K medium (American Type Culture Collection) supplemented with 10% heat-inactivated fetal bovine serum (Atlanta Biologicals, Norcross, GA) at 37°C in 5% (v/v) CO2. MCF-7 parental cells and the MX-resistant subline MCF-7/MX100 were kindly provided by Dr. Susan Bates (National Institutes of Health, Bethesda, MD). Both cell lines were grown in improved minimum essential medium (Invitrogen, Carlsbad, CA) containing 2 g/l glucose, 2 mM l-glutamine,1 mM sodium pyruvate, and 10% fetal bovine serum at 37°C in 5% (v/v) CO2. MCF-7/MX100 cells were maintained in the presence of 100 nM MX (Honjo et al., 2001). Caffeine and caffeine analogs [theophylline, pentoxifylline, dyphylline, paraxanthine, theobromine, 7-(β-hydroxyethyl)theophylline, and 7-methlxanthine], ammonium chloride, and leupeptin were purchased from Sigma-Aldrich (St. Louis, MO).
Protein Analysis.
For Western blot analysis, cells were washed twice with ice-cold phosphate-buffered saline and lysed in radioimmunoprecipitation assay buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 0.1% Triton X-100, 0.1% SDS, and 1% deoxycholate, sodium salt) plus protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN) and 100 mg/ml phenylmethylsulfonyl fluoride. Protein concentrations were determined using the BCA Protein Assay (Thermo Fisher Scientific, Waltham, MA). Equal amounts of total protein (5–15 μg) were analyzed by 8% SDS-polyacrylamide gel electrophoresis followed by immunoblotting using mouse monoclonal antibody (clone BXP-21) against ABCG2 (1:1000; Kamiya Biomedical, Thousand Oaks, CA) or rabbit monoclonal antibody against GAPDH (1:1000; Cell Signaling). The secondary antibody was either horseradish peroxidase-conjugated goat anti-mouse IgG (1:2500; GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK) or horseradish peroxidase-conjugated goat anti-rabbit IgG (Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Immunoreactive bands were visualized using an enhanced chemiluminescent system (Thermo Fisher Scientific) according to the manufacturer's recommendations.
For immunocytochemical analysis, cells grown on glass coverslips were washed three times with PBS, fixed in 4% paraformaldehyde solution, and permeabilized in 0.2% Triton X-100 solution for 10 min. Cells were washed with PBS three times at each interval. Coverslips were incubated with 2% bovine serum albumin in 0.1% Triton X-100 PBS buffer at room temperature for 1 h, followed by incubation with monoclonal ABCG2 antibody BXP-21 (1:250) in 0.1% Triton X-100 in a humid chamber, washed three times with PBS, and then incubated with Alexa Fluor 488-conjugated goat anti-mouse IgG at 37°C for 1 h. Coverslips were mounted, sealed on glass slides with 4,6-diamidino-2-phenylindole mounting medium, and observed using an Eclipse TE2000-U confocal microscope (Nikon, Melville, NY).
Efflux Assays.
Efflux assays were performed as described previously (Robey et al., 2001) with minor modifications. In brief, cells were collected and suspended in complete medium alone or complete medium containing 500 nM BODIPY-prazosin (an ABCG2 substrate; Invitrogen) with or without 10 μM fumitremorgin C (FTC), a known ABCG2 inhibitor (Rabindran et al., 2000), and incubated at 37°C in 5% CO2 for 30 min. The incubations were stopped with the addition of 4 ml of ice-cold PBS, and cells were washed three times with ice-cold PBS and incubated for 1 h at 37°C in 5% CO2 in complete medium with or without 10 μM FTC. Cells incubated in blank medium were used as the control for cell autofluorescence. Dead cells and debris were gated out for each treatment by propidium iodide staining. Fluorescence was visualized using a flow cytometer (FC500; Beckman Coulter, Fullerton, CA) with a 488-nm argon laser and 530-nm band-pass filter.
Cytotoxicity and Cell Death Assays.
MX cytotoxicity was assayed as follows. BeWo or MCF-7/MX100 cells were plated in 96-well plates at a density of 5000 cells/well. Twenty-four hours after plating, cells were treated with or without caffeine for another 24 h. After caffeine pretreatment, the medium was replaced with medium containing increasing concentrations of MX, and cells were incubated for 72 h at 37°C. Cell death was assayed using CellTiter 96 AQueous One solution according to the manufacturer's instructions (Promega, Madison, WI). Cytotoxicity was assessed by monitoring the absorbance at 490 nm using a Multiskan Spectrum microplate reader (Thermo Fisher Scientific). The IC50 value was defined as the drug concentration resulting in 50% cell death. Both the fitted sigmoidal dose-response curve and IC50 were calculated using Prism 4.
The Guava EasyCyte flow cytometry analysis system (Guava Technologies, Hayward, CA) was used to determine percentage of apoptotic cells. Assays were conducted according to the manufacturer's instructions. In brief, total cells were collected and washed with ice-cold PBS; then 5 μl of annexin V-phycoerythrin, a marker for early apoptosis, and 5 μl of 7-amino-actinomycin, a cell-impermeant dye indicating late apoptosis or dead cells (PCA-96 Nexin Kit; Guava Technologies), were added to cell suspensions. After a 20-min incubation and thorough mixing, samples were analyzed on a Guava flow cytometer.
Results
Xanthines Down-Regulated ABCG2 Expression in Cancer Cells.
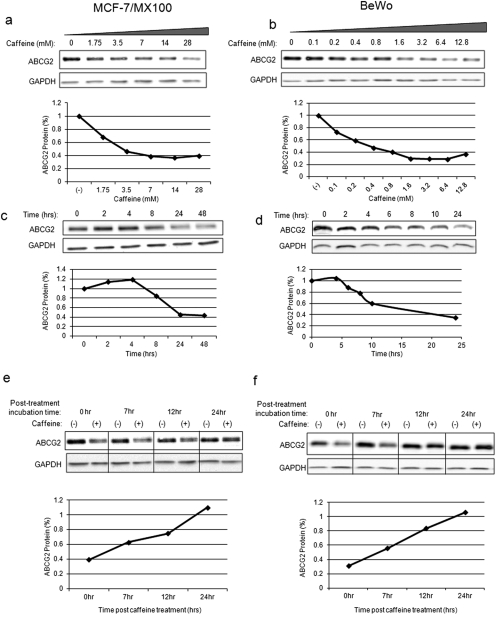
Xanthines, particularly methylxanthines, are found in more than 60 plant species and are among the most widely consumed substances in our diet. To evaluate the effect of xanthines on the expression of the drug transporter ABCG2 in tumor cells, we used the MX-resistant human breast cancer cell line MCF-7/MX100, which is resistant to MX relative to its MCF-7 parental cells by virtue of its overexpression of ABCG2 (Fig. 1, a and c) and the human placental choriocarcinoma cell line BeWo (Fig. 1, b and d), which has intrinsic expression of functional ABCG2. Exposure to methylxanthine caffeine reduced ABCG2 protein levels in a dose-dependent (Fig. 1, a and b) and time-dependent (Fig. 1, c and d) manner. Effects could be seen as early as 6 h after treatment with the maximum decrease (∼60–75%) achieved by 24 h [IC50 = 0.4 mM (BeWo) and 2.5 mM (MCF-7/MX100)]. This effect was reversible; when caffeine was removed and replaced with fresh medium 24 h after treatment, ABCG2 levels gradually recovered, reverting to normal levels within the next 24 h (Fig. 1, e and f). Of note, although some caffeine-induced cell cycle arrest was observed in MCF-7/MX100 cells, this arrest was not observed in BeWo cells (data not shown), yet the degree of down-regulation of ABCG2 by caffeine in both cell lines was similar. Therefore, it does not appear that caffeine-induced cell cycle inhibition plays a role in the regulation of ABCG2 levels by xanthines. Moreover, caffeine did not have a general effect on membrane protein stability, because there was minimal impact on the expression of epidermal growth factor receptor and no apparent effect on Na+/K+-ATPase (data not shown).
Fig. 1.
Caffeine down-regulated ABCG2 expression in cancer cell lines. a, ABCG2 protein levels were decreased by caffeine in a dose-dependent manner in ABCG2-overexpressing MCF-7/MX100 cells. Cells were treated with increasing concentrations of caffeine for 24 h, and Western blot analysis was performed using antibody BXP-21. b, similar results were obtained in placental choriocarcinoma BeWo cells. c and d, caffeine (14 mM) reduced ABCG2 protein levels in a time-dependent manner, with maximum reduction achieved by 24 h in MCF-7/MX100 cells (c) and BeWo cells (d). e and f, caffeine-mediated down-regulation of ABCG2 protein was reversible. MCF-7/MX100 cells (e) and BeWo cells (f) were treated with caffeine for 24 h and then were washed with drug-free medium and incubated for the times indicated before lysate preparation and Western blot analysis. Graphs display quantifications of the Western blot using Quantity One software (Bio-Rad Laboratories, Hercules, CA). In all cases, levels of ABCG2 protein were normalized to a GAPDH loading control and to the untreated sample.
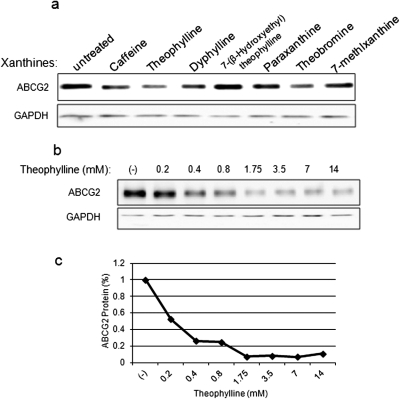
A number of naturally occurring xanthines are structurally similar to caffeine and share some of its pharmacological effects. To determine the generality of the effect of caffeine on ABCG2 expression, we analyzed ABCG2 protein levels after exposure to several of these caffeine analogs. As shown in Fig. 2a, among the analogs we tested, theophylline, a direct metabolite of caffeine, was the most potent compound in terms of down-regulating ABCG2 protein levels (Fig. 2b); 0.2 mM of theophylline was sufficient to reduce ABCG2 protein levels by 50%, and the maximum decrease (90%) was achieved by 1.75 mM. Other compounds, such as paraxanthine and 7-(β-hydroxyethyl)theophylline, did not significantly affect ABCG2 expression levels. The structure-activity relationship of a large group of xanthines is currently under investigation.
Fig. 2.
Methylxanthines and caffeine analogs down-regulated ABCG2 expression. a, BeWo cells were treated with caffeine (14 mM), theophylline (14 mM), dyphylline (14 mM), theobromine (2.5 mM), paraxanthine (1.5 mM), 7-(β-hydroxyethyl)theophylline (14 mM), and 7-methylxanthine (1 mM) for 24 h, and ABCG2 protein levels were analyzed by Western blot. GAPDH was used as a loading control. b, theophylline decreased ABCG2 protein levels potently and dose dependently. BeWo cells were treated with theophylline at concentrations for 24 h. GAPDH was used as a loading control. c, quantification of the effect of theophylline on ABCG2. ABCG2 protein levels were normalized to the GAPDH loading control, and the percentage of protein was determined relative to the levels in untreated cells.
Caffeine Induces Internalization and Lysosomal Degradation of ABCG2.
To determine the molecular basis for the down-regulation of ABCG2, we first considered the possibility that caffeine affected the synthesis or processing of ABCG2 mRNA. Indeed, we have previously shown that caffeine can induce the alternative splicing of a subset of genes (Shi et al., 2008). However, quantitative polymerase chain reaction analysis of ABCG2 RNA from untreated and caffeine-treated cells revealed no impact on either ABCG2 RNA levels or splicing in either MCF-7/MX100 or BeWo cells (data not shown), suggesting that the effect of caffeine occurred at the level of protein synthesis or degradation.
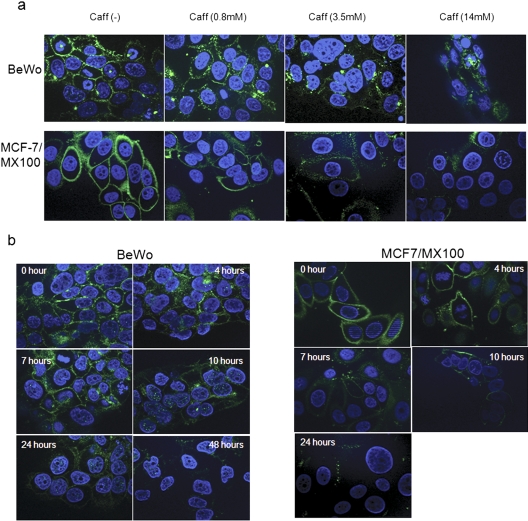
To examine the fate of ABCG2 protein in the presence of caffeine, cells were subjected to immunofluorescence staining after caffeine treatment. As shown in Fig. 3a, both MCF-7/MX100 and BeWo cells exhibited intense membrane staining and some cytoplasmic staining in the presence of an ABCG2-specific antibody. After treatment with high levels of caffeine, there was a significant decrease in the total fluorescence intensity in both cell lines, and membrane-localized ABCG2 was largely attenuated. At lower concentrations (0.8 mM), more intracellular staining was observed compared with that in the untreated cells, suggesting internalization of ABCG2 protein in the presence of caffeine. Again, this effect was time-dependent (Fig. 3b), with internalization apparent after 4 h of caffeine treatment and peaking between 10 and 24 h after caffeine exposure. By 24 h, little ABCG2-related staining was observed in the cell, suggesting that caffeine had induced the internalization and subsequent degradation of ABCG2 protein.
Fig. 3.
Caffeine (Caff) decreased membrane-localized ABCG2 and altered its cellular localization. a, both MCF-7/MX100 and BeWo cells were treated with increasing concentrations of caffeine as indicated and subjected to immunocytochemical analysis using BXP-21 antibody. Caffeine decreased plasma membrane-bound ABCG2 in a dose-dependent manner. b, caffeine-induced alterations of the cellular localization of ABCG2. MCF-7/MX100 and BeWo cells were treated with 7 mM caffeine for different time periods as indicated.
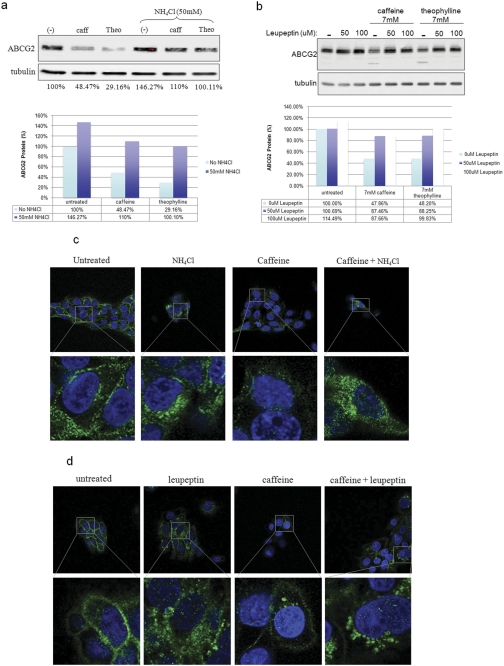
It has been reported previously that wild-type ABCG2 is primarily degraded through the lysosome-mediated pathway, although misfolded ABCG2 is degraded by ubiquitin-mediated proteasomal machinery (Nakagawa et al., 2009). To determine whether caffeine or its analog, theophylline, enhanced ABCG2 lysosomal degradation, we examined the effect of lysosome inhibitors on caffeine-mediated ABCG2 down-regulation. Figure 4 shows a Western blot analysis of ABCG2 expression after methylxanthine exposure in the presence or absence of the lysosomal inhibitors ammonium chloride (NH4Cl) (Fig. 4a) or leupeptin (Fig. 4b). Ammonium chloride, which inactivates lysosomal enzymes by neutralizing the luminal pH of the lysosome, prevented caffeine and theophylline from decreasing ABCG2 protein levels (Fig. 4a). This effect could be seen as early as 4 h after treatment (data not shown). A similar result was observed when the lysosomal inhibitor leupeptin was used (Fig. 4b).
Fig. 4.
Caffeine (caff)- and theophylline (Theo)-induced lysosomal-mediated degradation of ABCG2. a, the lysosomal inhibitor NH4Cl prevented caffeine and theophylline from reducing ABCG2 protein levels. BeWo cells were treated with caffeine (7 mM), theophylline (7 mM), and/or NH4Cl for 24 h, and ABCG2 protein levels were determined. Graph shows the quantification of the Western blot image. b, the lysosomal protease inhibitor leupeptin also blocked the effect of caffeine on ABCG2. Treatment combinations similar to those for NH4Cl were used. Quantification is shown below the figure. c, immunofluorescence analysis demonstrates that caffeine promotes the internalization of membrane-localized ABCG2 (green). d, single-cell view of the caffeine- and NH4Cl-treated cells.
To confirm this result, immunofluorescence was determined in cells treated with caffeine in the absence or presence of either NH4Cl or leupeptin (Fig. 4, c and d). As shown previously, caffeine caused an overall decrease in the ABCG2 fluorescence signal, and the ratio between the membrane and cytosol signals remained similar to that in the untreated samples. In the presence of NH4Cl, a decrease in ABCG2 signal intensity was still observed after caffeine treatment, but there was a marked increase in cytosol staining, particularly around the perinuclear region. This intracellular accumulation of ABCG2 was even more prominent after treatment with the specific lysosomal protease inhibitor leupeptin. These results suggest that caffeine induced the internalization of ABCG2 protein, probably through endocytosis, and directed it to the lysosomal degradation pathway, which was blocked by NH4Cl or leupeptin treatment.
Caffeine Potentiates Cytotoxicity of the ABCG2 Substrate Mitoxantrone.
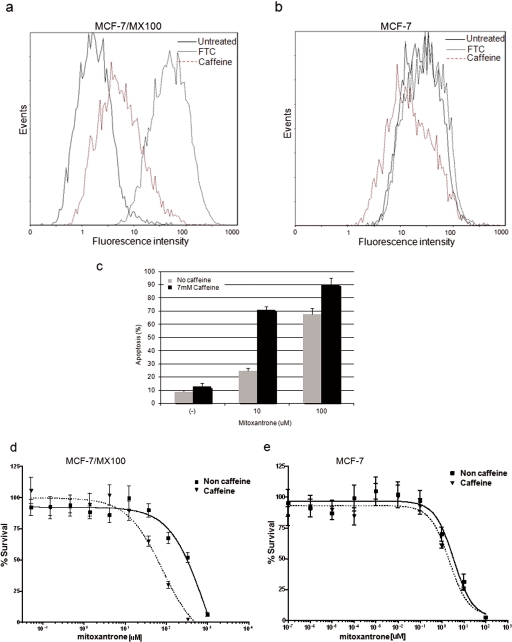
To determine the functional impact of the down-regulation of ABCG2 by methylxanthines, we determined whether caffeine could affect the accumulation of an ABCG2 substrate, using a flow cytometry assay (Robey et al., 2001). MCF-7/MX100 (a high ABCG2 expresser) and its parental cell line MCF-7 (a low ABCG2 expresser) were treated with caffeine for 24 h, and then cells were collected and incubated with the ABCG2-specific fluorescence substrate Bodipy-prazosin (BP). After incubation, cells were grown in fresh media without substrate, and the intracellular concentration of BP was determined as a measure of ABCG2 expression/function. The highly potent but toxic ABCG2 inhibitor, FTC, was used as a positive control. As shown in Fig. 5a, accumulation of BP in the highly expressing MCF-7/MX100 cells was markedly increased in the presence of caffeine, suggesting that caffeine inhibited transport of this substrate by ABCG2. In contrast, there was little effect of caffeine on BP accumulation in the low-expressing MCF-7 parental cell line (Fig. 5b), confirming that the effect of caffeine on BP uptake was specific for ABCG2.
Fig. 5.
Caffeine inhibited efflux of an ABCG2 substrate and sensitized cells to the chemotherapeutic agent mitoxantrone. a, MCF-7/MX100 cells were treated with either 14 mM caffeine or 10 μM FTC 24 h before assay of cells for their ability to efflux the ABCG2-specific substrate BP. Both caffeine (red dashed line) and FTC treatments (black dotted line) increased the mean fluorescence intensity compared with that for untreated cells (black solid line). b, parental MCF-7 cells have very low levels of ABCG2 expression. As expected, neither caffeine (red dashed line) nor FTC (black dotted line) had a significant impact on the uptake of Bodipy-prazosin in these cells. c, caffeine potentiated mitoxantrone-mediated apoptosis in MCF-7/MX100 cells. Cells were treated sequentially with 7 mM caffeine and MX at either 10 or 100 μM for 24 h, and the percentage of apoptotic cells was determined. Results are expressed as means ± S.D.; n = 3. d and e, caffeine increased the cytotoxicity of mitoxantrone in MCF-7/MX100 cells (d) but not in MCF-7 parental cells (e). Cells were pretreated with 7 mM caffeine for 24 h followed by increasing concentrations of mitoxantrone for 72 h before the CellTiter 96 AQueous nonradioactive cell proliferation assay. Data are expressed as means ± S.D.; n = 8.
MX is used in the treatment of certain cancers, including metastatic breast cancer and acute myeloid leukemia, for which it has been shown to induce programmed cell death or apoptosis (Kluza et al., 2004). Therefore, to further confirm the functional effect of caffeine on ABCG2 and to investigate the potential of using methylxanthines as chemosensitizing agents to treat ABCG2-mediated multidrug resistance, we analyzed the effect of caffeine on MX-induced apoptosis in MCF7/MX100 cells. Cells were pretreated with caffeine for 24 h to achieve maximum down-regulation of ABCG2 protein and then were treated with 10 or 100 μM MX for an additional 24 h. The apoptotic status of the cells was determined using annexin staining and flow cytometry. As shown in Fig. 5c, compared with untreated cells, treatment with caffeine alone (7 mM) induced relatively little apoptosis (1.6%), whereas MX alone caused a dose-dependent apoptotic effect. However, pretreatment of cells with caffeine before MX exposure (10 μM) increased MX-induced apoptosis ∼3-fold and shifted the IC50 by ∼10-fold (IC50 = 946.5 ± 1.71 μM for MX alone and 81.4 ± 1.31 μM for caffeine and MX) (Fig. 5d). Parental MCF-7 cells were intrinsically more sensitive to MX (IC50 = 3.233 ± 1.84 μM) because of their relatively low levels of ABCG2; as expected, caffeine had little effect on MX cytotoxicity in MCF-7 cells (IC50 = 2.174 ± 1.44 μM for caffeine-treated cells), again supporting the hypothesis that caffeine is sensitizing cells to MX by down-regulating ABCG2 expression.
Discussion
In the present study, we show that xanthines, including the widely consumed methylxanthine caffeine and the respiratory therapeutic drug theophylline, can down-regulate the MDR membrane protein ABCG2 by inducing its translocation and subsequent lysosomal degradation. As a consequence of this down-regulation, xanthines inhibited efflux of an ABCG2 substrate and sensitized drug resistance breast cancer cells to mitoxantrone, an antineoplastic agent commonly used to treat acute myeloid leukemia, metastatic breast cancer, and non-Hodgkin's lymphoma.
Previous studies have suggested that caffeine can modulate cancer drug sensitivity. The many cellular effects attributed to caffeine have implicated a number of pathways/targets in this sensitization. The primary mechanism that has been suggested is caffeine-mediated inhibition of ATM/ATR kinases, resulting in cell cycle arrest and sensitization of certain tumor cells to DNA-damaging agents and irradiation (Sarkaria et al., 1999; Lu et al., 2008). We can achieve ABCG2 down-regulation in the absence of a cell cycle block in the BeWo cell line, and induction of cell cycle arrest with different agents did not lead to the down-regulation of ABCG2 (data not shown), suggesting that this is not the mechanism by which caffeine down-regulates ABCG2. Another mechanism by which caffeine has been proposed to potentiate tumor cell death is through direct DNA binding to prevent repair of drug-induced DNA damage (Tornaletti et al., 1989), although there is little information to support this hypothesis. Of note, early studies suggested that methylxanthines could promote the antitumor activities of chemotherapeutic agents such as doxorubicin (Adriamycin) by increasing the intracellular accumulation of this drug both in vitro and in vivo (Sadzuka et al., 1995, 1999), although the mechanism by which this interference with drug transport was accomplished had not been investigated. Our finding that methylxanthines alleviated multidrug resistance by down-regulating ABCG2 expression reveals a novel molecular action of this class of compounds.
Xanthines join a growing list of potential ABCG2 inhibitors; to date, none of these inhibitors are in clinical use. Some ABCG2 inhibitors bind directly to the transporter, hindering its efflux activity; certain tyrosine kinase inhibitors fall into this class. The observation that xanthines reversed ABCG2-mediated multidrug resistance by inducing its degradation places it in a second class of compounds, those that affect ABCG2 expression. In a recent study, a novel inhibitor was identified that falls into both classes; the binding of this agent inhibits ABCG2 function and expression by targeting the transporter for lysosomal degradation (Peng et al., 2010). Our studies now show that xanthines, including those already in clinical use, also induce the lysosomal degradation of ABCG2. Indeed, early studies tested xanthines as part of a combinational therapeutic regimen to achieve better efficacy of anticancer drugs; caffeine was shown to potentiate etoposide and doxorubicin and prolong the overall survival of patients with high-grade soft tissue sarcoma, osteosarcoma, lymphoma of bone, and metastatic carcinoma (Hayashi et al., 2005; Takeuchi et al., 2007; Kimura et al., 2009). Although the mechanism of this potentiation by caffeine was not determined in these studies and it may involve the role of caffeine in regulation of the DNA repair process, it is notable that both etoposide and doxorubicin are substrates of ABCG2 and interesting to speculate that down-regulation of ABCG2 by caffeine may have mediated the increased tumor sensitivity to these drugs. That said, given the high concentrations of caffeine required to down-regulate ABCG2 in vitro, coupled with the narrow therapeutic window and multiple mechanisms of action of this agent, it is unlikely that caffeine itself will be clinically useful for modulation of ABCG2. Instead, we are currently using caffeine and caffeine analogs as 1) tools to further dissect the mechanism underlying regulation of degradation of ABCG2 protein and 2) as lead compounds for the identification/development of more potent/less toxic agents. Regarding this latter point, we have recently investigated the effect of a synthetic xanthine, 1,3-dipropyl-8-cyclopentylxanthine (DPCPX), on ABCG2 levels. DCPCX is in clinical trials for the treatment of cystic fibrosis; single doses up to 1000 mg were tested with no apparent toxicity (McCarty et al., 2002). Of importance, DPCPX was able to down-regulate ABCG2 levels to the same degree as caffeine, but at concentrations (5–50 μM, data not shown) that are more likely to be achievable in vivo. Animal studies are planned to evaluate the antitumor effect of this agent in combination with chemotherapeutic drugs that are ABCG2 substrates.
One complication of most of the chemotherapeutic regimens currently in use is that their targets are also expressed in normal cells, which is the case with ABCG2, which is found in normal stem cells, endothelial barriers, and excretory cells of the liver, kidney, and intestines (Robey et al., 2009). This situation presents both advantages and disadvantages. On the one hand, down-regulation of ABCG2 in secretory tissues may enhance drug bioavailability; in contrast, decreased ABCG2 levels may also lead to greater toxicity of chemotherapeutic agents in some cell types. Thus, as is the case with most cancer-directed agents, unwanted effects of candidate xanthines on normal cells/tissues will need to be considered as in vivo studies progress. It is also important to note that ABCG2 is expressed in cancer stem cells, in which it may play a role in stemness maintenance and proliferation. Thus, down-regulation of ABCG2 may prove useful for the elimination of this oftentimes drug-resistant subpopulation. Studies addressing these questions are underway.
It has been reported previously that ABCG2 can be degraded through both proteasome- and lysosome-mediated pathways. Mutated or misfolded newly synthesized ABCG2 protein is rapidly eliminated via ubiquitin-mediated proteasome degradation before reaching the membrane (Nakagawa et al., 2009). In contrast, membrane-associated wild-type ABCG2 is normally degraded through the lysosome (Wakabayashi et al., 2007). How xanthines induce the translocation and lysosomal degradation of ABCG2 is not yet clear. One possibility is that xanthines directly interact with ABCG2 and negatively regulate its stability; indeed, it has been reported that some nucleosides and nucleoside analogs can bind to the drug pocket of ABCG2 (de Wolf et al., 2008), although there is no evidence that nucleobases can do the same. Of note, we have observed that some of the xanthines tested did not mimic the effect of caffeine on ABCG2. For example, theophylline, dyphylline, and theobromine actively reduced the protein level of ABCG2, whereas 7-(β-hydroxyethyl)theophylline, paraxanthine, and 7-methylxanthine did not have a significant impact on its expression. The facts that the size of the side chain modification on the xanthine ring did not correlate with activity and that caffeine is closely related to a nucleobase rather than a nucleoside reduce the likelihood that xanthines act by direct binding to ABCG2. Although we have not yet ruled out this possibility, our current hypothesis favors a model whereby active xanthines affect/induce a signaling event that triggers the translocation and degradation of ABCG2, perhaps via a regulated process such as ubiquitin-mediated endocytosis. Recent studies have demonstrated the importance of monoubiquitination as a critical signal for selective degradation (d'Azzo et al., 2005). For example, the ion transporter epithelial Na+ channel can be recognized by the E3 ligase Nedd4-2 for ubiquitination in response to metabolic stress, triggering its subsequent endocytosis and lysosomal degradation (Bhalla et al., 2006). Although we have yet to determine whether a caffeine-induced signaling pathway regulates ABCG2 degradation or which pathway is involved, a previous study demonstrating that inhibition of phosphatidylinositol 3-kinase activity led to the intracellular translocation of ABCG2 and subsequent decrease in the efflux of ABCG2 substrates (Bleau et al., 2009) supports this hypothesis and suggests an avenue for further investigation. Clarifying the mechanisms by which xanthines induce lysosomal degradation of ABCG2 will inform the development of more specific ABCG2 modulators.
Taken together, our data demonstrate that a class of xanthine derivatives, including many already in clinical use, can modulate the expression of ABCG2 and sensitize cells to chemotherapeutic agents that are substrates for this transporter. This result defines a new function for this large family of compounds and identifies a new class of agents that may be useful in sensitizing tumors to the cytotoxic effects of chemotherapeutic drugs. As we further dissect the mechanism/pathway through which xanthines induce the degradation of ABCG2, it is likely that more specific and efficacious inhibitors will be identified.
Acknowledgments
We thank Drs. Joseph Bertino, Barton Kamen, Nancy Walworth, and Arnold Rabson for valuable discussions. We thank Dr. Susan Bates for generously providing the MCF-7/MX100 cell line. R.D. is a graduate student in the University of Medicine and Dentistry of New Jersey Graduate School of Biomedical Sciences.
This study was supported by the National Institutes of Health National Cancer Institute [Grants P30-CA072720, R01-CA122573] and the University of Medicine and Dentistry of New Jersey Foundation Grant program.
This work was previously presented in part: Ding R, Shi R, Scotto KW, Methylxanthines antagonize multidrug resistance by downregulating ABCG2, Proceedings of the 101st Annual Meeting of the American Association for Cancer Research; 2010 Apr 17–21; Washington, DC. American Association for Cancer Research, Philadelphia, PA.
Article, publication date, and citation information can be found at http://molpharm.aspetjournals.org.
- ABC
- ATP-binding cassette
- MDR
- multidrug resistance
- CSC
- cancer stem cell
- MX
- mitoxantrone
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- PBS
- phosphate-buffered saline
- FTC
- fumitremorgin C
- BP
- Bodipy-prazosin
- DPCPX
- 1,3-dipropyl-8-cyclopentylxanthine.
Authorship Contributions
Participated in research design: Ding, Shi, and Scotto.
Conducted experiments: Ding and Pabon.
Performed data analysis: Ding, Shi, and Scotto.
Wrote or contributed to the writing of the manuscript: Ding and Scotto.
References
- Abolhoda A, Wilson AE, Ross H, Danenberg PV, Burt M, Scotto KW. (1999) Rapid activation of MDR1 gene expression in human metastatic sarcoma after in vivo exposure to doxorubicin. Clin Cancer Res 5:3352–3356 [PubMed] [Google Scholar]
- Benderra Z, Faussat AM, Sayada L, Perrot JY, Chaoui D, Marie JP, Legrand O. (2004) Breast cancer resistance protein and P-glycoprotein in 149 adult acute myeloid leukemias. Clin Cancer Res 10:7896–7902 [DOI] [PubMed] [Google Scholar]
- Bhalla V, Oyster NM, Fitch AC, Wijngaarden MA, Neumann D, Schlattner U, Pearce D, Hallows KR. (2006) AMP-activated kinase inhibits the epithelial Na+ channel through functional regulation of the ubiquitin ligase Nedd4–2. J Biol Chem 281:26159–26169 [DOI] [PubMed] [Google Scholar]
- Bleau AM, Hambardzumyan D, Ozawa T, Fomchenko EI, Huse JT, Brennan CW, Holland EC. (2009) PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell 4:226–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunting KD. (2002) ABC transporters as phenotypic markers and functional regulators of stem cells. Stem Cells 20:11–20 [DOI] [PubMed] [Google Scholar]
- d'Azzo A, Bongiovanni A, Nastasi T. (2005) E3 ubiquitin ligases as regulators of membrane protein trafficking and degradation. Traffic 6:429–441 [DOI] [PubMed] [Google Scholar]
- Daly JW. (2007) Caffeine analogs: biomedical impact. Cell Mol Life Sci 64:2153–2169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wolf C, Jansen R, Yamaguchi H, de Haas M, van de Wetering K, Wijnholds J, Beijnen J, Borst P. (2008) Contribution of the drug transporter ABCG2 (breast cancer resistance protein) to resistance against anticancer nucleosides. Mol Cancer Ther 7:3092–3102 [DOI] [PubMed] [Google Scholar]
- Dean M, Fojo T, Bates S. (2005) Tumour stem cells and drug resistance. Nat Rev Cancer 5:275–284 [DOI] [PubMed] [Google Scholar]
- Diestra JE, Scheffer GL, Català I, Maliepaard M, Schellens JH, Scheper RJ, Germà-Lluch JR, Izquierdo MA. (2002) Frequent expression of the multi-drug resistance-associated protein BCRP/MXR/ABCP/ABCG2 in human tumours detected by the BXP-21 monoclonal antibody in paraffin-embedded material. J Pathol 198:213–219 [DOI] [PubMed] [Google Scholar]
- Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, Rishi AK, Ross DD. (1998) A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci USA 95:15665–15670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grube M, Reuther S, Meyer Zu Schwabedissen H, Köck K, Draber K, Ritter CA, Fusch C, Jedlitschky G, Kroemer HK. (2007) Organic anion transporting polypeptide 2B1 and breast cancer resistance protein interact in the transepithelial transport of steroid sulfates in human placenta. Drug Metab Dispos 35:30–35 [DOI] [PubMed] [Google Scholar]
- Hayashi M, Tsuchiya H, Yamamoto N, Karita M, Shirai T, Nishida H, Takeuchi A, Tomita K. (2005) Caffeine-potentiated chemotherapy for metastatic carcinoma and lymphoma of bone and soft tissue. Anticancer Res 25:2399–2405 [PubMed] [Google Scholar]
- Honjo Y, Hrycyna CA, Yan QW, Medina-Pérez WY, Robey RW, van de Laar A, Litman T, Dean M, Bates SE. (2001) Acquired mutations in the MXR/BCRP/ABCP gene alter substrate specificity in MXR/BCRP/ABCP-overexpressing cells. Cancer Res 61:6635–6639 [PubMed] [Google Scholar]
- Kimura H, Tsuchiya H, Shirai T, Nishida H, Hayashi K, Takeuchi A, Ohnari I, Tomita K. (2009) Caffeine-potentiated chemotherapy for metastatic osteosarcoma. J Orthop Sci 14:556–565 [DOI] [PubMed] [Google Scholar]
- Kluza J, Marchetti P, Gallego MA, Lancel S, Fournier C, Loyens A, Beauvillain JC, Bailly C. (2004) Mitochondrial proliferation during apoptosis induced by anticancer agents: effects of doxorubicin and mitoxantrone on cancer and cardiac cells. Oncogene 23:7018–7030 [DOI] [PubMed] [Google Scholar]
- Krishnamurthy P, Schuetz JD. (2006) Role of ABCG2/BCRP in biology and medicine. Annu Rev Pharmacol Toxicol 46:381–410 [DOI] [PubMed] [Google Scholar]
- Krishnamurthy P, Xie T, Schuetz JD. (2007) The role of transporters in cellular heme and porphyrin homeostasis. Pharmacol Ther 114:345–358 [DOI] [PubMed] [Google Scholar]
- Lu YP, Lou YR, Peng QY, Xie JG, Nghiem P, Conney AH. (2008) Effect of caffeine on the ATR/Chk1 pathway in the epidermis of UVB-irradiated mice. Cancer Res 68:2523–2529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty NA, Standaert TA, Teresi M, Tuthill C, Launspach J, Kelley TJ, Milgram LJ, Hilliard KA, Regelmann WE, Weatherly MR, et al. (2002) A phase I randomized, multicenter trial of CPX in adult subjects with mild cystic fibrosis. Pediatr Pulmonol 33:90–98 [DOI] [PubMed] [Google Scholar]
- McDevitt CA, Collins RF, Conway M, Modok S, Storm J, Kerr ID, Ford RC, Callaghan R. (2006) Purification and 3D structural analysis of oligomeric human multidrug transporter ABCG2. Structure 14:1623–1632 [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Wakabayashi-Nakao K, Tamura A, Toyoda Y, Koshiba S, Ishikawa T. (2009) Disruption of N-linked glycosylation enhances ubiquitin-mediated proteasomal degradation of the human ATP-binding cassette transporter ABCG2. FEBS J 276:7237–7252 [DOI] [PubMed] [Google Scholar]
- Nakanishi T, Karp JE, Tan M, Doyle LA, Peters T, Yang W, Wei D, Ross DD. (2003) Quantitative analysis of breast cancer resistance protein and cellular resistance to flavopiridol in acute leukemia patients. Clin Cancer Res 9:3320–3328 [PubMed] [Google Scholar]
- Ni Z, Bikadi Z, Rosenberg MF, Mao Q. (2010) Structure and function of the human breast cancer resistance protein (BCRP/ABCG2). Curr Drug Metab 11:603–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng H, Qi J, Dong Z, Zhang JT. (2010) Dynamic vs static ABCG2 inhibitors to sensitize drug resistant cancer cells. PLoS ONE 5:e15276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plasschaert SL, van der Kolk DM, de Bont ES, Kamps WA, Morisaki K, Bates SE, Scheffer GL, Scheper RJ, Vellenga E, de Vries EG. (2003) The role of breast cancer resistance protein in acute lymphoblastic leukemia. Clin Cancer Res 9:5171–5177 [PubMed] [Google Scholar]
- Rabindran SK, Ross DD, Doyle LA, Yang W, Greenberger LM. (2000) Fumitremorgin C reverses multidrug resistance in cells transfected with the breast cancer resistance protein. Cancer Res 60:47–50 [PubMed] [Google Scholar]
- Robey RW, Honjo Y, van de Laar A, Miyake K, Regis JT, Litman T, Bates SE. (2001) A functional assay for detection of the mitoxantrone resistance protein, MXR (ABCG2). Biochim Biophys Acta 1512:171–182 [DOI] [PubMed] [Google Scholar]
- Robey RW, To KK, Polgar O, Dohse M, Fetsch P, Dean M, Bates SE. (2009) ABCG2: a perspective. Adv Drug Deliv Rev 61:3–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross DD, Karp JE, Chen TT, Doyle LA. (2000) Expression of breast cancer resistance protein in blast cells from patients with acute leukemia. Blood 96:365–368 [PubMed] [Google Scholar]
- Sadzuka Y, Mochizuki E, Takino Y. (1995) Mechanism of caffeine modulation of the antitumor activity of Adriamycin. Toxicol Lett 75:39–49 [DOI] [PubMed] [Google Scholar]
- Sadzuka Y, Sugiyama T, Sawanishi H, Miyamoto K. (1999) Enhanced efficacy of 1-methyl-3-propyl-7-butylxanthine on the antitumor activity of doxorubicin against doxorubicin-resistant P388 leukemia. Cancer Lett 138:5–11 [DOI] [PubMed] [Google Scholar]
- Sarkaria JN, Busby EC, Tibbetts RS, Roos P, Taya Y, Karnitz LM, Abraham RT. (1999) Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res 59:4375–4382 [PubMed] [Google Scholar]
- Sawynok J. (1995) Pharmacological rationale for the clinical use of caffeine. Drugs 49:37–50 [DOI] [PubMed] [Google Scholar]
- Shi J, Hu Z, Pabon K, Scotto KW. (2008) Caffeine regulates alternative splicing in a subset of cancer-associated genes: a role for SC35. Mol Cell Biol 28:883–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A, Tsuchiya H, Yamamoto N, Hayashi K, Yamauchi K, Kawahara M, Miyamoto K, Tomita K. (2007) Caffeine-potentiated chemotherapy for patients with high-grade soft tissue sarcoma: long-term clinical outcome. Anticancer Res 27:3489–3495 [PubMed] [Google Scholar]
- Tornaletti S, Russo P, Parodi S, Pedrini AM. (1989) Studies on DNA binding of caffeine and derivatives: evidence of intercalation by DNA-unwinding experiments. Biochim Biophys Acta 1007:112–115 [DOI] [PubMed] [Google Scholar]
- Turner JG, Gump JL, Zhang C, Cook JM, Marchion D, Hazlehurst L, Munster P, Schell MJ, Dalton WS, Sullivan DM. (2006) ABCG2 expression, function, and promoter methylation in human multiple myeloma. Blood 108:3881–3889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uggla B, Ståhl E, Wågsäter D, Paul C, Karlsson MG, Sirsjö A, Tidefelt U. (2005) BCRP mRNA expression v. clinical outcome in 40 adult AML patients. Leuk Res 29:141–146 [DOI] [PubMed] [Google Scholar]
- van den Heuvel-Eibrink MM, Wiemer EA, Prins A, Meijerink JP, Vossebeld PJ, van der Holt B, Pieters R, Sonneveld P. (2002) Increased expression of the breast cancer resistance protein (BCRP) in relapsed or refractory acute myeloid leukemia (AML). Leukemia 16:833–839 [DOI] [PubMed] [Google Scholar]
- van Herwaarden AE, Wagenaar E, Merino G, Jonker JW, Rosing H, Beijnen JH, Schinkel AH. (2007) Multidrug transporter ABCG2/breast cancer resistance protein secretes riboflavin (vitamin B2) into milk. Mol Cell Biol 27:1247–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Borght S, van Pelt J, van Malenstein H, Cassiman D, Renard M, Verslype C, Libbrecht L, Roskams TA. (2008) Up-regulation of breast cancer resistance protein expression in hepatoblastoma following chemotherapy: a study in patients and in vitro. Hepatol Res 38:1112–1121 [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Nakagawa H, Tamura A, Koshiba S, Hoshijima K, Komada M, Ishikawa T. (2007) Intramolecular disulfide bond is a critical check point determining degradative fates of ATP-binding cassette (ABC) transporter ABCG2 protein. J Biol Chem 282:27841–27846 [DOI] [PubMed] [Google Scholar]
- Wilson CS, Davidson GS, Martin SB, Andries E, Potter J, Harvey R, Ar K, Xu Y, Kopecky KJ, Ankerst DP, et al. (2006) Gene expression profiling of adult acute myeloid leukemia identifies novel biologic clusters for risk classification and outcome prediction. Blood 108:685–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Schuetz JD, Bunting KD, Colapietro AM, Sampath J, Morris JJ, Lagutina I, Grosveld GC, Osawa M, Nakauchi H, et al. (2001) The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nat Med 7:1028–1034 [DOI] [PubMed] [Google Scholar]