Abstract
Previous studies have shown that female rats exhibit enhanced cocaine seeking during multiple phases of cocaine addiction compared with males. The orexin/hypocretin system recently has been implicated in drug addiction in male rats. Based on the known sex differences in cocaine addiction, in the current study we examined orexin-mediated cocaine seeking during self-administration, extinction, and reinstatement in age-matched male (initial weight 250–300 g) and female (initial weight 175–225 g) Sprague-Dawley rats by using the orexin-1 receptor (OX1R) antagonist 1-(2-methylbenzoxazol-6-yl)-3-[1,5]naphthyridin-4-yl urea (SB-334867) (10–30 mg/kg). OX1R blockade had no effect on established cocaine self-administration, but attenuated cocaine seeking during extinction in both male and female rats. It is noteworthy that OX1R blockade potently attenuated cue-induced reinstatement in males but had no effect on females. SB-334867 also reduced cocaine seeking during pharmacological stress-induced (yohimbine, 2.5 mg/kg) and yohimbine + cue-induced reinstatement in both sexes. SB-334867 failed to affect reinstatement induced by cocaine (10 mg/kg) in either male or female rats, but selectively reduced cocaine + cue-induced reinstatement only in males. In separate experiments examining basal and cocaine-induced locomotion, SB-334867 attenuated locomotion in both male and female rats. Finally, assessment of plasma and brain levels of SB-334867 showed that estrus females had slightly higher plasma levels than diestrus females, but no overall sex differences or estrous cycle differences were observed in plasma or brain SB-334867 concentrations. These results show that OX1R signaling plays a role in mediating cocaine seeking, but differs between the sexes for cue-induced reinstatement.
Introduction
Cocaine addiction is a chronic illness with high rates of relapse and limited treatment options. Addicts have a persistent vulnerability to relapse to drug use even after months or years of abstinence (O'Brien, 2005). Similar to human addicts, rodent models of addiction have been developed, whereby relapse to drug seeking can be triggered by the drug itself, re-exposure to the drug-associated context or discrete cues, and physical or pharmacological stressors after a period of abstinence or extinction of responding (Epstein et al., 2006). With the lack of successful pharmacotherapies for reducing craving and relapse, cocaine addiction continues to exert enormous economic and medical burdens on society.
Studies have implicated the involvement of the orexin system in drug addiction, suggesting that orexin receptors may be highly useful targets for antirelapse therapeutic interventions. The orexins (also known as hypocretins) are peptide neurotransmitters expressed in neurons exclusively in hypothalamic nuclei (Sakurai et al., 1998; de Lecea et al., 1998). Orexin occurs in two forms, orexin-A and orexin-B, which bind to two G protein-coupled orexin receptors, orexin-1 receptor (OX1R) and orexin-2 receptor (OX2R), with different affinity (Sakurai et al., 1998). OX1R, which is coupled exclusively to Gq protein, has 10-fold higher affinity for orexin-A; whereas OX2R, which is coupled to both Gq and Gi/o proteins, has equal affinity for both peptides (Sakurai et al., 1998). Both the orexin peptides and orexin receptors are widely distributed throughout the entire brain, including brain regions involved in drug reward and addiction, with somewhat different patterns of expression (Sakurai, 2003). 1-(2-Methylbenzoxazol-6-yl)-3-[1,5]naphthyridin-4-yl urea (SB-334867) is a selective antagonist of OX1R (Porter et al., 2001). Blockade of orexin signaling at OX1R via SB-334867 has been shown to significantly reduce cocaine self-administration under both a fixed ratio (FR) 5 schedule of reinforcement (J. A. Hollander and P. J. Kenny, unpublished observations) and a progressive ratio (PR) schedule (Borgland et al., 2009). Although SB-334867 failed to affect cocaine-induced reinstatement of cocaine seeking, it dose-dependently attenuated context-, cue-, and foot shock stress-induced reinstatement of cocaine seeking (Boutrel et al., 2005; Smith et al., 2009, 2010) and attenuated alcohol seeking induced by the pharmacological stressor yohimbine (Lawrence et al., 2006).
Previous studies on the orexin system in addiction have used only male subjects, although extensive evidence in both human and animal models has shown a number of sex differences across a wide variety of drugs of abuse. Significant gender differences exist in cocaine addiction, in that women differ from men on several measures of addictive behavior, ranging from temporal development of dependence to susceptibility to relapse (Fattore et al., 2008). In rodent models, compared with males, female rats acquire cocaine self-administration faster and exhibit greater resistance to extinction (Lynch and Carroll, 1999; Fuchs et al., 2005). Female rats also show enhanced cocaine-primed reinstatement (Kippin et al., 2005), but fairly similar cue-induced or yohimbine stress-induced reinstatement compared with males (Fuchs et al., 2005; Feltenstein et al., 2011). In addition, female rats demonstrate higher sensitivity to the locomotor-stimulating effects of cocaine than males (Festa et al., 2004). Although the exact mechanisms underlying these sex differences remain unclear, gonadal hormones play a critical role, because estrogen facilitates drug seeking and locomotor sensitization, whereas progesterone counteracts the effects of estrogen (Quinones-Jenab and Jenab, 2010). It is noteworthy that sex and estrous cycle differences in the orexin system have also been reported. Female rats express higher levels of orexin-A and OX1R in the hypothalamus compared with male rats (Taheri et al., 1999; Jöhren et al., 2001). Within the same brain area, the expression of OX1R and OX2R increases selectively in female rats during proestrus compared with rats in other estrous stages (Silveyra et al., 2010). These findings suggest a potential biological basis for behavioral differences between males and females in response to drugs of abuse.
In the current study, we compared the effects of OX1R blockade on established cocaine self-administration, early extinction responding, and reinstatement to cocaine seeking induced by cocaine, cues, yohimbine stress, and the combination of cocaine + cues and yohimbine + cues in male and freely cycling female rats. We also examined basal and cocaine-induced locomotion after acute OX1R blockade and measured SB-334867 concentrations in the plasma and brains of male and female rats.
Materials and Methods
Subjects.
Age-matched male (initial weight 250–300 g) and female (initial weight 175–225 g) Sprague-Dawley rats (Charles River Laboratories, Raleigh, NC) were single-housed in a temperature- and humidity-controlled animal facility on a reversed 12-h light-dark cycle (lights off at 6:00 AM). All experimental procedures occurred during the dark cycle between 7:00 AM and 3:00 PM. Animals were given ad libitum access to water and maintained on a diet of standard rat chow (Harlan, Indianapolis, IN). All experimental procedures were approved by the Institutional Animal Care and Use Committee of the Medical University of South Carolina and complied with federal guidelines in the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996).
Drugs.
Cocaine hydrochloride (National Institute on Drug Abuse, Research Triangle Park, NC) was dissolved in 0.9% saline. SB-334867 (RTI International, Research Triangle Park, NC; generously provided by the National Institute on Drug Abuse Drug Supply) was suspended in 2% dimethylsulfoxide and 10% 2-hydroxypropyl-β-cyclodextrin (Sigma-Aldrich, St. Louis, MO) in double-distilled water and intraperitoneally injected in a volume of 4 ml/kg 30 min before testing. Yohimbine hydrochloride (Sigma-Aldrich) was dissolved in double-distilled water and given in a volume of 1 ml/kg i.p. 15 min before testing. Drugs used for anesthesia included ketamine (Vedco Inc., St. Joseph, MO), xylazine (Lloyd Laboratories, Shenandoah, IA), and equithesin (4 mg/kg sodium pentobarbital, 17 mg/kg chloral hydrate, and 21.3 mg/kg magnesium sulfate heptahydrate dissolved in 44% propylene glycol and 10% ethanol solution). Ketorolac (Sigma-Aldrich) was administered before surgery for analgesia. Catheter patency was maintained with heparin (Elkins-Sinn, Cherry Hill, NJ) and cephazolin (Schein Pharmaceuticals, Florham Park, NJ) and periodically verified with methohexital sodium (Eli Lilly & Co., Indianapolis, IN).
Surgery.
Rats were anesthetized by using a mixture of ketamine hydrochloride and xylazine (66 and 1.33 mg/kg i.p., respectively), followed by equithesin (0.5 ml/kg i.p.). Ketorolac (2 mg/kg i.p.) was administered as a preoperative analgesic. One end of a silastic catheter was implanted into the right jugular vein, and the other end exited on the back where it was attached to an infusion harness (Instech Laboratories, Plymouth Meeting, PA) for intravenous infusions. To maintain catheter patency, daily intravenous infusion of 0.1 ml of antibiotic cefazolin (10 mg/ml) and 0.1 ml of heparinized saline (100 U/ml) was given during surgery recovery and maintained throughout self-administration (given after each session). An additional 0.1 ml of heparinized saline (10 U/ml) was infused immediately before each self-administration session. Catheter patency was periodically verified with an intravenous infusion of 0.1 ml of methohexital sodium (10 mg/ml), a short-acting barbiturate that produces a rapid loss of muscle tone. If a catheter failed this test, another catheter was implanted in the left jugular vein.
Cocaine Self-Administration.
Self-administration chambers (30 × 20 × 20 cm; MED Associates, St. Albans, VT) were housed inside sound-attenuating cubicles fitted with a fan for airflow. Each chamber contained two retractable levers, two stimulus lights, a speaker for tone delivery, and a house light to provide general illumination. In addition, each chamber was equipped with a balanced metal arm and spring leash attached to a swivel (Instech Laboratories). Tygon tubing extended through the leash and was connected to a 10-ml syringe mounted on an infusion pump located outside of the sound-attenuating cubicle. For each session, the catheter was connected to the tubing, and the house light signaled the initiation of the session and remained on throughout the entire session. Presses on the active (i.e., cocaine-paired) lever resulted in a 2-s activation of the infusion pump for a 50-μl infusion of cocaine at 0.20 mg (males) or 0.15 mg (females), resulting in approximately 0.6 mg/kg/infusion. However, daily cocaine intake was individually calculated for each animal based on actual body weight. Cocaine infusions were paired with a 5-s presentation of a stimulus light and tone (4.5 kHz, 78 dB) complex, followed by a 20-s timeout period, during which responses on the active lever were recorded but resulted in no programmed consequences. Presses on the inactive (i.e., noncocaine-paired) lever had no consequences. Rats self-administered cocaine during daily 2-h sessions under an FR1 schedule of reinforcement for 10 (experiments 2 and 3) or 13 (experiment 1) sessions, in which they earned ≥10 infusions per session. An additional one to three sessions were allowed to reach this criterion.
Extinction and Reinstatement of Cocaine Seeking.
After self-administration and between reinstatement tests, animals underwent daily 2-h extinction sessions, during which responses on either lever had no consequences. Extinction sessions continued until animals met the criteria of two consecutive sessions with <25 active lever presses (minimum of seven sessions before the first reinstatement test and minimum of two sessions between reinstatement tests). Reinstatement tests consisted of the light + tone cues, yohimbine stress (2.5 mg/kg i.p.), cocaine prime (10 mg/kg i.p.), or the combination of cues + yohimbine or cues + cocaine. During tests that included cues, responses on the active lever resulted in 5-s presentation of the previously cocaine-paired light + tone cues. For all other tests, lever presses had no programmed consequences.
Estrous Cycle Monitoring.
To determine estrous cycle stages, vaginal lumen samples were collected immediately before and after each test. To habituate female rats to the vaginal cytology procedure, samples were taken daily throughout each experiment. Vaginal lumen samples were collected by gently flushing 30 μl of double-distilled water and extracting the sample by using a micropipette and 100-μl pipette tips. Samples were placed on glass slides, dried, and stained by using Quick-Dip Hematology Stain (Mercedes Medical, Sarasota, FL). Slides were then examined under a light microscope at 10× magnification, and estrous cycle was classified according to previously published criteria (Marcondes et al., 2002).
Experiment 1: Self-Administration and Extinction.
To test the effects of SB-334867 on established cocaine self-administration, male (n = 19) and female (n = 18) rats were pretreated with vehicle or SB-334867 (30 mg/kg) 30 min before the 10th self-administration session. Animals underwent three additional self-administration sessions before they began daily extinction sessions. To test the effects of SB-334867 on early extinction, animals received vehicle or SB-334867 (30 mg/kg) 30 min before the first extinction session. Injections were counterbalanced based on prior drug treatment history during self-administration. We used a single high dose of SB-334867, because previous studies in males (Smith et al., 2009, 2010) demonstrated that 30 mg/kg SB-334867 failed to attenuate cocaine self-administration, although it effectively reduced cue- and context-induced cocaine seeking.
Experiment 2: Cue- and Yohimbine Stress-Induced Reinstatement.
Animals underwent cocaine self-administration and extinction as described above before reinstatement tests. Because a previous study (Smith et al., 2009) revealed that SB-334867 dose-dependently attenuated cue-induced reinstatement to cocaine seeking in male rats, we tested multiple doses of SB-334867 in this experiment. First, to test the effects of the OX1R antagonist SB-334867 (10, 20, or 30 mg/kg i.p.) on cue-induced reinstatement of cocaine seeking, the same animal received four test sessions: two late extinction sessions (no cues) with separate vehicle and SB-334867 pretreatment and two cue-induced reinstatement sessions with separate vehicle and SB-334867 pretreatment in a counterbalanced order. After these tests were done, the same four tests were repeated in the same order, but with an injection of yohimbine (2.5 mg/kg i.p.) 15 min before each test for induction of stress. Thus, the same rat received an additional two stress-induced reinstatement sessions (no cues) and two stress + cue-induced reinstatement sessions 30 min after pretreatment with separate vehicle and SB-334867. Each animal received only one dose of SB-334867 (10, 20, or 30 mg/kg) for all tests (n = 12–16 per group).
Experiment 3: Cocaine- and Cue-Induced Reinstatement.
Animals underwent cocaine self-administration and extinction as described above before reinstatement tests. To test the effects of SB-334867 on cocaine- and cocaine + cue-induced reinstatement, male (n = 9) and female (n = 9) rats received vehicle or SB-334867 (30 mg/kg i.p.) treatment 30 min before testing and a cocaine priming injection (10 mg/kg i.p.) immediately before each test session. In total, rats received four tests in a counterbalanced order: two sessions under extinction conditions for cocaine-induced reinstatement and two sessions with the presentation of cues for cocaine + cue-induced reinstatement. We limited this experiment to one SB-334867 dose, because a previous study (Smith et al., 2010) showed that SB-334867 at 30 mg/kg failed to attenuate cocaine-induced reinstatement in male rats.
Experiment 4: Locomotor Activity.
Drug-naive animals (n = 9–12 per group) were tested for the effects of SB-334867 on basal and cocaine-induced locomotor activity in clear acrylic chambers (approximately 40 × 40 × 30 cm) equipped with Digiscan monitors (AccuScan Instruments Inc., Columbus, OH). Each chamber contained a 16 × 16 photobeam array for the x/y-axes (horizontal activity) and 16 photobeams for the z-axis (vertical activity). Photobeam breaks were detected by a Digiscan analyzer and recorded by Versamax (version 1.82; Accuscan Instruments Inc., Columbus, OH). Thirty minutes before the start of testing, male and female rats were pretreated with vehicle or SB-334867 (10, 20, or 30 mg/kg i.p.). Basal locomotor activity was recorded for 90 min. Each rat then received a saline injection and was returned to the test chamber for 30 min, at which time it received a cocaine injection (10 mg/kg i.p.). Cocaine-induced locomotor activity was recorded for the next 120 min.
Experiment 5: Plasma and Brain Levels of SB-334867.
For repeated blood collection, naive male (n = 7) and female (n = 17) rats underwent catheter implantation followed by 5 days to recover from the surgery. Before testing, estrous cycle stage in the females was determined by cytology. Blood samples (0.2 ml) were collected into tubes prefilled with 20 μl of heparinized saline (1000 U/ml) at 5, 15, 30, 60, 90, and 120 min after SB-334867 injection (30 mg/kg i.p.). Whole blood samples were centrifuged for 20 min at 10,000g at 4°C. Plasma was separated and stored at −80°C until further analysis. Animals were rapidly decapitated, and their brains were removed after the last blood draw, flash-frozen in 2-methobutane, and stored at −80°C before being homogenized. SB-334867 concentrations in plasma and brain were quantified by liquid chromatography/tandem mass spectrometry. Peak areas of the transition m/z 320 →146 (declustering potential = 40, collision energy = 24) were measured against the peak areas of the internal standard (i.e., sunitinib) m/z 399 → 283 (declustering potential = 86, collision energy = 41). Quantitation was against a standard curve made in blank rat plasma or brain tissue.
Data Analysis.
To evaluate SB-334867 effects on cocaine self-administration and extinction in male and female rats, mixed-factors, repeated-measures analyses of variance (ANOVA) were used to analyze active lever responses or cocaine intake with treatment and session as the between- and within-subjects factors, respectively. Planned comparisons (paired t tests) between vehicle and SB-334867 treatment were used to evaluate the effects of SB-334867 treatment under different reinstatement conditions. Locomotor data were analyzed by one-way ANOVA with SB-334867 dose as the between-groups factor. For SB-334867 plasma levels, one-way ANOVA on area under the curve (SB-334867 concentration plotted against time) was used for comparison between groups. For brain SB-334867 levels, one-way ANOVA was used. All post hoc analyses were conducted by using Bonferroni tests. All data were presented as mean ± S.E.M., and α was set at p < 0.05. Data points were eliminated if Grubbs' test (Grubbs, 1969) revealed them as significant outliers. Based on consistently very low responses in all groups, data for inactive lever presses were not presented.
Results
Experiment 1: Self-Administration and Extinction.
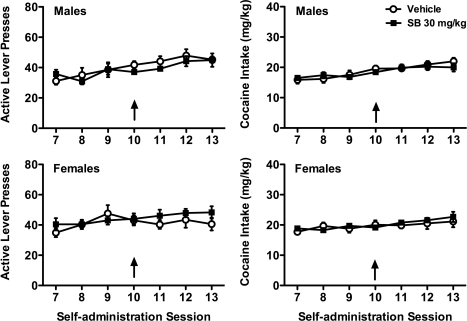
Mixed-model ANOVAs for active lever pressing and cocaine intake across self-administration sessions 7 to 13 revealed a significant main effect for sessions in male rats (F6,102 = 9.46, p < 0.001 and F6,102 = 12.80, p < 0.001, respectively; Fig. 1, top), reflecting a significant increase in cocaine self-administration over time. In female rats (Fig. 1, bottom), a significant session main effect was observed for cocaine intake (F6,96 = 5.64; p < 0.001), but not for active lever pressing. However, no significant treatment or interaction effects were observed in either males or females. Comparison (t tests) between vehicle- and SB-334867-treated groups on the day of injection (session 10) confirmed that acute SB-334867 administration failed to affect cocaine self-administration in both sexes.
Fig. 1.
OX1R blockade fails to affect cocaine seeking or cocaine intake during self-administration in either male or female rats. Male and female rats received vehicle or SB-334867 (SB; 30 mg/kg) 30 min before the 10th self-administration session (indicated by arrows). Active lever presses (left) and cocaine intake (right) are shown for males (top) and females (bottom).
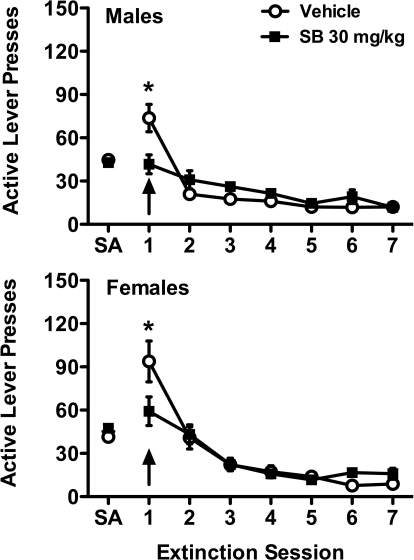
In male rats (Fig. 2, top), mixed-model ANOVA for active lever pressing across all seven extinction sessions revealed no significant effect for treatment, but a significant overall effect for session (F6,102 = 28.64; p < 0.001), as seen by a steady decrease in cocaine seeking across extinction sessions. There was a significant treatment × session interaction (F6,102 = 6.16; p < 0.001), and post hoc analyses revealed that acute SB-334867 injection before the first session significantly attenuated responding on that session, but not on subsequent sessions. Similar effects were observed in female rats (Fig. 2, bottom), with a significant main effect of session (F6,96 = 47.69; p < 0.001) and a treatment × session interaction (F6,96 = 4.45; p < 0.001). Post hoc analysis of the first session showed reduced cocaine seeking after acute SB-334867 injection. Whereas female rats showed slightly higher responding than males after vehicle treatment on the first extinction session, it was not significant (p > 0.05).
Fig. 2.
SB-334867 attenuates cocaine seeking during extinction in both male and female rats. Male and female rats received vehicle or SB-334867 (SB; 30 mg/kg) before the first extinction session (indicated by arrows). Average active lever responding for the last 3 days of self-administration (SA) and active lever responding over the 7 days of extinction is shown for males (top) and females (bottom). Significant differences between vehicle and SB-334867 pretreatment in the first session are indicated; *, p < 0.05.
Experiment 2: Cue-, Yohimbine-, and Yohimbine + Cue-Induced Reinstatement.
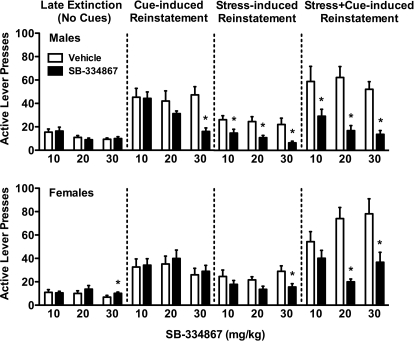
As shown in Fig. 3, top, acute SB-334867 in male rats at any dose failed to affect extinction responding (which was uniformly low by design) during the reinstatement test phase. High doses of SB-334867 (30 mg/kg), but not the other two doses of SB-334867, reduced cocaine seeking during cue-induced reinstatement (t12 = 4.43; p < 0.01). All three doses of SB-334867 in males attenuated yohimbine-induced reinstatement (10 mg/kg: t10 = 3.95, p < 0.01; 20 mg/kg: t11 = 2.74, p < 0.05; and 30 mg/kg: t12 = 3.37, p < 0.01) and yohimbine + cue-induced reinstatement (10 mg/kg: t10 = 2.76, p < 0.05; 20 mg/kg: t11 = 4.94, p < 0.001; and 30 mg/kg: t12 = 6.10, p < 0.001).
Fig. 3.
SB-334867 effects in late extinction and cue-, yohimbine-, and yohimbine + cue-induced reinstatement in male and female rats. Rats received vehicle or one of three SB-334867 doses (10, 20, or 30 mg/kg) 30 min before testing. Active lever presses are shown for males (top) and females (bottom). Significant attenuation by SB-334867 treatment compared with the corresponding vehicle treatment under the same test condition is indicated; *, p < 0.05.
In female rats (Fig. 3, bottom), a slight increase in responding was observed during extinction after the high dose of SB-334867 (30 mg/kg: t15 = −2.75; p < 0.05). However, in marked contrast to males, SB-334867 at any dose failed to affect cue-induced reinstatement in females. For yohimbine stress-induced reinstatement, only the high dose of SB-334867 (30 mg/kg) significantly reduced cocaine seeking (t12 = 2.37; p < 0.05), whereas both the 20 and 30 mg/kg doses of SB-334867 reduced yohimbine + cue-induced reinstatement (t14 = 6.24, p < 0.001; t12 = 2.85, p < 0.05, respectively) in females.
Comparisons of responding between late extinction and reinstatement responding in vehicle-treated rats confirmed that cues, yohimbine stress, and yohimbine + cues all significantly reinstated cocaine seeking in both male and female rats (p < 0.05). There were no differences in cocaine seeking during yohimbine stress- or yohimbine + cue-induced reinstatement in vehicle-treated male and female rats. However, cue-induced reinstatement was elevated in males compared with females (F1,73 = 5.66; p < 0.05).
Experiment 3: Cocaine- and Cocaine + Cue-Induced Reinstatement.
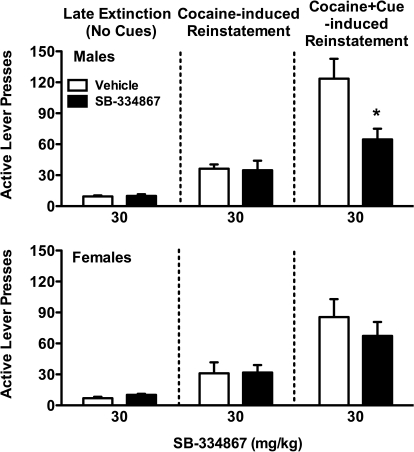
In male rats (Fig. 4, top), SB-334867 (30 mg/kg) failed to affect cocaine-induced reinstatement, but did reduce cocaine seeking during the combination of a cocaine prime and conditioned cues (t8 = 3.77; p < 0.01). In contrast, SB-334867 failed to affect either cocaine- or cocaine + cue-induced reinstatement in females (Fig. 4, bottom). Cocaine and cocaine + cues significantly reinstated cocaine seeking in both male and female rats treated with vehicle compared with extinction responding (p < 0.05), with similar baseline levels in males and females.
Fig. 4.
SB-334867 effects in cocaine- and cocaine + cue-induced reinstatement in male and female rats. Male and female rats received vehicle or SB-334867 (30 mg/kg) 30 min before reinstatement tests. Active lever responding is shown for males (top) and females (bottom). Significant attenuation by SB-334867 compared with the corresponding vehicle control is indicated; *, p < 0.05.
Experiment 4: Basal and Cocaine-Induced Locomotor Activity.
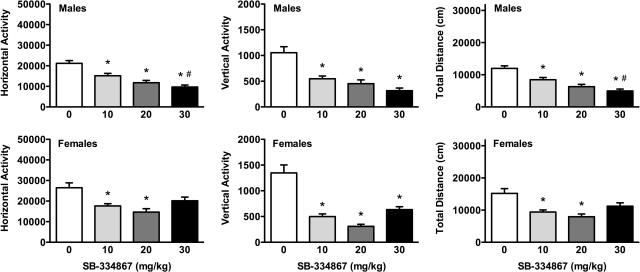
SB-334867 attenuated basal locomotor activity (i.e., response to the novel test environment) in both male and female rats (Fig. 5). In males (Fig. 5, top), SB-334867 treatment decreased horizontal (F3,39 = 17.99; p < 0.001), vertical (F3,39 = 16.06; p < 0.001), and total (F3,39 = 18.65; p < 0.001) locomotor activity at all three doses compared with vehicle treatment. In female rats (Fig. 5, bottom), similar effects of SB-334867 were observed for horizontal (F3,41 = 7.52; p < 0.001), vertical (F3,41 = 26.54; p < 0.001), and total (F3,41 = 8.52; p < 0.001) locomotor activity. It is noteworthy that only the 10 and 20 mg/kg doses of SB-334867 significantly lowered all three locomotor measures compared with vehicle, whereas the highest dose of 30 mg/kg attenuated vertical activity only in females.
Fig. 5.
SB-334867 attenuation in basal locomotion in male and female rats. Male and female rats received vehicle or SB-334867 (10, 20, or 30 mg/kg) 30 min before the basal locomotion measurement. Horizontal activity (left), vertical activity (center), and total distance traveled (right) are shown for males (top) and females (bottom). Significant attenuation by SB-334867 compared with vehicle treatment (*, p < 0.05) and significant differences between the 10 and 30 mg/kg SB-334867 doses (#, p < 0.05) are indicated.
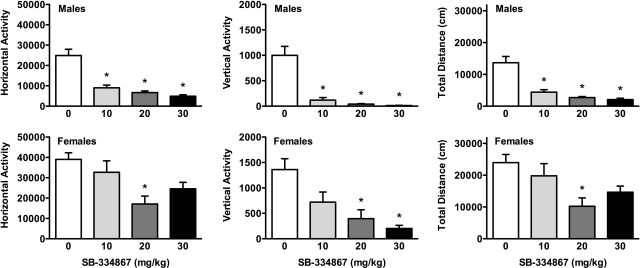
SB-334867 also reduced cocaine-induced locomotor activity in both sexes (Fig. 6). Male rats (Fig. 6, top) showed lower horizontal (F3,39 = 23.80; p < 0.001), vertical (F3,39 = 22.51; p < 0.001), and total (F3,39 = 21.36; p < 0.001) cocaine-induced locomotor activity after all three doses of SB-334867. In female rats (Fig. 6, bottom), SB-334867 also attenuated cocaine-induced locomotion for horizontal (F3,41 = 5.43; p < 0.01), vertical (F3,41 = 9.45; p < 0.001), and total (F3,41 = 4.54; p < 0.01) locomotor activity. However, only the 20 mg/kg dose was effective for horizontal and total locomotion, and the 20 and 30 mg/kg doses were effective for vertical activity.
Fig. 6.
SB-334867 attenuation in cocaine-induced locomotion in male and female rats. Horizontal activity (left), vertical activity (center), and total distance traveled (right) after acute cocaine (10 mg/kg) treatment and vehicle or different SB-334867 doses (10, 20, or 30 mg/kg) are shown for males (top) and females (bottom). Significant attenuation by SB-334867 compared with vehicle treatment is shown; *, p < 0.05.
Experiment 5: Plasma and Brain Levels of SB-334867.
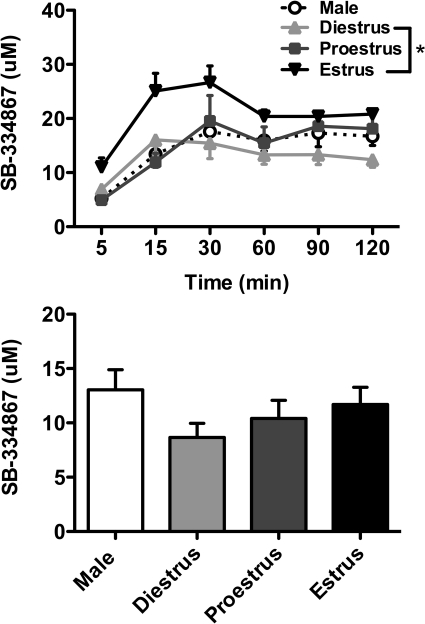
All rats showed stable plasma levels of SB-334867 over the 2-h test period after the initial rise between 5 and 15 min (Fig. 7, top). One-way ANOVA revealed a significant main effect of group (F3,23 = 3.95; p < 0.05) for plasma SB-334867 levels, with post hoc analysis showing significantly higher SB-334867 levels in estrus female rats compared with females in diestrus. Brain levels of SB-334867 were similar across subjects; however, no significant differences were observed between groups for brain SB-334867 concentrations (Fig. 7, bottom).
Fig. 7.
Plasma and brain SB-334867 levels in male and female rats. Male and female rats in diestrus, proestrus, and estrus received acute SB-334867 (30 mg/kg). Plasma (top) and brain (bottom) SB-334867 levels are shown. Significant differences in plasma SB-334867 levels are shown between estrus and diestrus females; *, p < 0.05.
Discussion
In the current study, several key findings revealed that OX1R mediated cocaine seeking in male and female rats. As reported previously (Smith et al., 2009), SB-334867 attenuated cocaine seeking during conditioned cued reinstatement in males, as well as during the combination of cocaine and cues. Strikingly, females showed no changes in cue- or cocaine + cue-induced cocaine seeking during OX1R blockade at any dose of SB-334867. Furthermore, males were sensitive to the effects of SB-334867 on reinstatement induced by yohimbine stress and the combination of yohimbine and cues, because all three SB-334867 doses significantly attenuated reinstatement to cocaine seeking. On the other hand, female rats showed attenuation only to the two higher doses of SB-334867. Our data also suggest that the noted patterns in males and females are not likely caused by nonspecific effects or drug disposition. Although male and female rats showed similar patterns after SB-334867 treatment during cocaine self-administration, early extinction, and cocaine-primed reinstatement, the observed sex differences in response to OX1R blockade suggest that sexually dimorphic OX1R-mediated activity occurs in cocaine addiction, particularly in regard to the processing of cue-driven motivation for cocaine.
Consistent with previous findings (Smith et al., 2009), OX1R blockade failed to affect lever pressing or cocaine intake during established cocaine self-administration under an FR1 schedule of reinforcement in both sexes. However, when male rats were tested under a PR schedule, SB-334867 (10 mg/kg) significantly reduced the breakpoint of cocaine self-administration, indicating a reduction in motivated drug seeking (Borgland et al., 2009). The lack of effectiveness of SB-334867 on cocaine self-administration under an FR1 schedule suggests that OX1R-mediated signaling may not be necessary for cocaine taking under certain conditions of lower motivation, a point supported by the observations that SB-334867 dose-dependently decreased cocaine self-administration in male rats under a higher demand FR5 schedule (J. A. Hollander and P. J. Kenny, unpublished observations). However, it should be noted that the effects of OX1R blockade on cocaine self-administration need to be further explored under varied schedules of reinforcement, different bolus doses of cocaine, and across different SB-334867 doses. To date, the effects of SB-334867 under PR or higher FR schedules in female rats have not been investigated.
Our results show that SB-334867 (30 mg/kg) on the first day of extinction attenuated the burst of increased cocaine seeking in both male and female rats. This attenuation occurred only on the SB-334867 treatment day with no carryover effects, because the extinction responding was similar to vehicle-treated animals on subsequent sessions. Cocaine seeking produced by a return to the self-administration context is assumed to reflect habitual and compulsive responses that engage a somewhat different circuitry than that relied on for contingent discrete cue-induced responding (Gerdeman et al., 2003; Everitt and Robbins, 2005). Thus, the role of orexin mediation of context-induced cocaine seeking might be mediated by signaling via pathways that do not show sexual dimorphism in this regard (e.g., caudate-putamen or substantia nigra) (Fuchs et al., 2006; See et al., 2007).
In agreement with a previous study (Smith et al., 2009), SB-334867 dose-dependently attenuated cue-induced reinstatement, but failed to affect cocaine-primed reinstatement of cocaine seeking in male rats. In the present study, we further demonstrated that SB-334867 (30 mg/kg) attenuated cocaine + cue-induced reinstatement in males, an effect presumably caused by the impact of OX1R blockade on cue processing and not the primary rewarding effects of the drug itself (i.e., cocaine-primed reinstatement alone). A possible explanation for the differential role of OX1R on cue-induced versus cocaine-primed reinstatement is the distinct neuronal circuitries involved in these two forms of reinstatement, particularly as cue-induced reinstatement relies on certain limbic structures (e.g., amygdala) that are not necessary for cocaine-primed reinstatement. Inhibition of orexin signaling in the amygdala and/or extended amygdala may account for the selectivity of SB-334867 effects. The assumption is further supported by the involvement of extended amygdala structures in stress-induced reinstatement (Buffalari and See, 2011). Because SB-334867 blocked reinstatement of cocaine seeking induced by foot shock (Boutrel et al., 2005) and yohimbine (present study) stress, stress-induced reinstatement may rely on orexin signaling in the extended amygdala. On the other hand, OX1R blockade failed to affect cue-induced or cocaine + cue-primed cocaine seeking in female rats. This difference in cue-induced reinstatement may be caused by different orexin-mediated circuitry in male and female rats, perhaps in limbic regions, because morphological and volumetric studies have indicated sex differences in the amygdala and the bed nucleus of the stria terminalis (Hines et al., 1992; Stefanova and Ovtscharoff, 2000). However, the relationship of these and other potential neurocircuitry differences to the observed differences in reinstatement to cocaine seeking in male and female rats remains unknown.
Although OX1R blockade attenuated yohimbine and yohimbine + cue-induced reinstatement in both sexes, SB-334867 was effective at lower doses in males. Systemic yohimbine treatment activates hypothalamic pathways via its noradrenergic actions (Myers et al., 2005). In the hypothalamus, female rats express higher levels of orexin-A and OX1R than males (Taheri et al., 1999; Jöhren et al., 2001), a difference that may affect response to yohimbine. On the other hand, stress-induced cocaine seeking probably is influenced by interactions between orexin, corticotropin-releasing factor, and norepinephrine (Ida et al., 2000; Walling et al., 2004; Boutrel et al., 2005), possibly at the locus coeruleus (Abercrombie et al., 1988; Britton et al., 1992), a sexually dimorphic area (Bangasser et al., 2011) with the highest OX1R expression in the brain (Hervieu et al., 2001). Because females show different patterns of locus coeruleus activity than males upon stress stimulation (Curtis et al., 2006; Bangasser et al., 2010), this may account for somewhat different orexin-mediated changes during yohimbine-induced stress.
We also found that OX1R blockade by SB-334867 attenuated basal and cocaine-induced locomotor activities in both male and female rats, with somewhat more pronounced effects of SB-334867 in males. The effects of SB-334867 on general locomotion are unlikely to fully account for SB-334867 reductions of some forms of cocaine seeking. First, in both male and female rats, SB-334867 doses that effectively attenuated basal locomotion did not always result in the inhibition of cue-induced reinstatement, suggesting that simple motor inhibition cannot account for attenuated cocaine seeking. Second, although cocaine-induced locomotion was attenuated, cocaine-primed reinstatement induced by the same cocaine treatment was unaffected by OX1R blockade. Finally, in both males and females, decreased basal locomotion did not impair the ability to seek cocaine, because “basal” cocaine seeking during late extinction was generally not affected by OX1R antagonism. These findings indicate that the inhibitory effects of SB-334867 on cocaine seeking cannot be primarily attributed to nonspecific decreases in locomotion by OX1R blockade.
SB-334867 pharmacokinetics are also unlikely to account for SB-334867 effects on reinstatement because male and female rats had similar SB-334867 levels in brain and plasma. It is noteworthy that, although no sex differences were observed, estrous cycle differences were seen in plasma SB-334867 levels, in that female rats in estrus exhibited higher SB-334867 levels in the plasma than those in diestrus. Sex and estrous cycle differences in drug pharmacokinetics have been reported, with the fluctuation of gonadal hormones proposed to be the primary cause (Kashuba and Nafziger, 1998). In addition to the central nervous system, orexin peptides and orexin receptors are expressed in peripheral tissues, such as the adrenal gland and gonads (Sakurai, 2003). The peripheral orexin system might also contribute to cocaine seeking, a hypothesis supported by the observed sex and estrous cycle differences in orexin receptor expression in these areas (Silveyra et al., 2010). Because of the limited number of female rats in each of the cycle phases, the present study did not explore estrous cycle differences in OX1R-mediated cocaine seeking.
In conclusion, the present study demonstrates unique patterns of OX1R mediation of cocaine seeking, particularly for cue-driven reinstatement in male and female rats. The sexual dimorphisms in the orexin system that underlie these behavioral differences will need to be characterized in the future. Further assessment of SB-334867 pharmacokinetics in specific brain regions (e.g., hypothalamus) is also warranted, because whole brain tissue analysis does not provide information on potential variance in SB-334867 distribution in specific brain areas. In addition, the distribution of orexin peptides and orexin receptors, as well as the pharmacodynamics of orexin receptor binding, may reveal some of the mechanisms that account for different behavioral effects in males and females. Site-selective inhibition of orexin signaling in discrete brain areas will help to determine their role in orexin-mediated reinstatement. In addition, further clarification of the interactions between orexin and noradrenergic systems may better elucidate the roles of the orexin system in mediating cocaine seeking. Finally, as noted, the influence of gonadal hormones on the orexin system is probably a major contributor to these sex differences. Further investigation of the interaction between gonadal hormones and orexin on cocaine seeking may provide information on cycle-dependent treatment and the usage of oral contraceptives or hormone replacement. Increased understanding of the orexin system in models of cocaine addiction will help facilitate gender-specific treatment approaches in the future.
Acknowledgments
We thank Dr. Wei-Lun Sun for comments on the manuscript; Drs. Joyce Nicholas and Nathaniel Baker for statistical consultation; and Melza Van Roijen, Phong Do, Bernard Smalls, and John Yang for technical assistance.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grants P50 DA16511, DA023915]; and the National Institutes of Health National Center for Research Resources [Grant C06 RR015455].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- OX1R
- orexin-1 receptor
- OX2R
- orexin-2 receptor
- SB-334867
- 1-(2-methylbenzoxazol-6-yl)-3-[1,5]naphthyridin-4-yl urea
- FR
- fixed ratio
- PR
- progressive ratio
- ANOVA
- analysis of variance.
Authorship Contributions
Participated in research design: Zhou, Kenny, and See.
Conducted experiments: Zhou, Ghee, Chan, Lin, and Cameron.
Performed data analysis: Zhou and See.
Wrote or contributed to the writing of the manuscript: Zhou, Kenny, and See.
References
- Abercrombie ED, Keller RW, Jr, Zigmond MJ. (1988) Characterization of hippocampal norepinephrine release as measured by microdialysis perfusion: pharmacological and behavioral studies. Neuroscience 27:897–904 [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Curtis A, Reyes BA, Bethea TT, Parastatidis I, Ischiropoulos H, Van Bockstaele EJ, Valentino RJ. (2010) Sex differences in corticotropin-releasing factor receptor signaling and trafficking: potential role in female vulnerability to stress-related psychopathology. Mol Psychiatry 15:877, 896–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangasser DA, Zhang X, Garachh V, Hanhauser E, Valentino RJ. (2011) Sexual dimorphism in locus coeruleus dendritic morphology: a structural basis for sex differences in emotional arousal. Physiol Behav 103:342–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, Chou J, Chen BT, Bonci A. (2009) Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci 29:11215–11225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L. (2005) Role for hypocretin in mediating stress-induced reinstatement of cocaine seeking behavior. Proc Natl Acad Sci U S A 102:19168–19173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton KT, Segal DS, Kuczenski R, Hauger R. (1992) Dissociation between in vivo hippocampal norepinephrine response and behavioral/neuroendocrine responses to noise stress in rats. Brain Res 574:125–130 [DOI] [PubMed] [Google Scholar]
- Buffalari DM, See RE. (2011) Inactivation of the bed nucleus of the stria terminalis in an animal model of relapse: effects on conditioned cue-induced reinstatement and its enhancement by yohimbine. Psychopharmacology (Berl) 213:19–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis AL, Bethea T, Valentino RJ. (2006) Sexually dimorphic responses of the brain norepinephrine system to stress and corticotropin-releasing factor. Neuropsychopharmacology 31:544–554 [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, et al. (1998) The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A 95:322–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. (2006) Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 189:1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. (2005) Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci 8:1481–1489 [DOI] [PubMed] [Google Scholar]
- Fattore L, Altea S, Fratta W. (2008) Sex differences in drug addiction: a review of animal and human studies. Womens Health (Lond Engl) 4:51–65 [DOI] [PubMed] [Google Scholar]
- Feltenstein MW, Henderson AR, See RE. (2011) Enhancement of cue-induced reinstatement of cocaine seeking in rats by yohimbine: sex differences and the role of the estrous cycle. Psychopharmacology (Berl) 216:53–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Festa ED, Russo SJ, Gazi FM, Niyomchai T, Kemen LM, Lin SN, Foltz R, Jenab S, Quinones-Jenab V. (2004) Sex differences in cocaine-induced behavioral responses, pharmacokinetics, and monoamine levels. Neuropharmacology 46:672–687 [DOI] [PubMed] [Google Scholar]
- Fuchs RA, Branham RK, See RE. (2006) Different neural substrates mediate cocaine seeking after abstinence versus extinction training: a critical role for the dorsolateral caudate-putamen. J Neurosci 26:3584–3588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs RA, Evans KA, Mehta RH, Case JM, See RE. (2005) Influence of sex and estrous cyclicity on conditioned cue-induced reinstatement of cocaine seeking behavior in rats. Psychopharmacology (Berl) 179:662–672 [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Partridge JG, Lupica CR, Lovinger DM. (2003) It could be habit forming: drugs of abuse and striatal synaptic plasticity. Trends Neurosci 26:184–192 [DOI] [PubMed] [Google Scholar]
- Grubbs FE. (1969) Procedures for detecting outlying observations in samples. Technometrics 11:1–21 [Google Scholar]
- Hervieu GJ, Cluderay JE, Harrison DC, Roberts JC, Leslie RA. (2001) Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience 103:777–797 [DOI] [PubMed] [Google Scholar]
- Hines M, Allen LS, Gorski RA. (1992) Sex differences in subregions of the medial nucleus of the amygdala and the bed nucleus of the stria terminalis of the rat. Brain Res 579:321–326 [DOI] [PubMed] [Google Scholar]
- Ida T, Nakahara K, Murakami T, Hanada R, Nakazato M, Murakami N. (2000) Possible involvement of orexin in the stress reaction in rats. Biochem Biophys Res Commun 270:318–323 [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC [Google Scholar]
- Jöhren O, Neidert SJ, Kummer M, Dendorfer A, Dominiak P. (2001) Prepro-orexin and orexin receptor mRNAs are differentially expressed in peripheral tissues of male and female rats. Endocrinology 142:3324–3331 [DOI] [PubMed] [Google Scholar]
- Kashuba AD, Nafziger AN. (1998) Physiological changes during the menstrual cycle and their effects on the pharmacokinetics and pharmacodynamics of drugs. Clin Pharmacokinet 34:203–218 [DOI] [PubMed] [Google Scholar]
- Kippin TE, Fuchs RA, Mehta RH, Case JM, Parker MP, Bimonte-Nelson HA, See RE. (2005) Potentiation of cocaine-primed reinstatement of drug seeking in female rats during estrus. Psychopharmacology (Berl) 182:245–252 [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. (2006) The orexin system regulates alcohol-seeking in rats. Br J Pharmacol 148:752–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Carroll ME. (1999) Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology (Berl) 144:77–82 [DOI] [PubMed] [Google Scholar]
- Marcondes FK, Bianchi FJ, Tanno AP. (2002) Determination of the estrous cycle phases of rats: some helpful considerations. Braz J Biol 62:609–614 [DOI] [PubMed] [Google Scholar]
- Myers EA, Banihashemi L, Rinaman L. (2005) The anxiogenic drug yohimbine activates central viscerosensory circuits in rats. J Comp Neurol 492:426–441 [DOI] [PubMed] [Google Scholar]
- O'Brien CP. (2005) Anticraving medications for relapse prevention: a possible new class of psychoactive medications. Am J Psychiatry 162:1423–1431 [DOI] [PubMed] [Google Scholar]
- Porter RA, Chan WN, Coulton S, Johns A, Hadley MS, Widdowson K, Jerman JC, Brough SJ, Coldwell M, Smart D, et al. (2001) 1,3-Biarylureas as selective non-peptide antagonists of the orexin-1 receptor. Bioorg Med Chem Lett 11:1907–1910 [DOI] [PubMed] [Google Scholar]
- Quinones-Jenab V, Jenab S. (2010) Progesterone attenuates cocaine-induced responses. Horm Behav 58:22–32 [DOI] [PubMed] [Google Scholar]
- Sakurai T. (2003) Orexin: a link between energy homeostasis and adaptive behaviour. Curr Opin Clin Nutr Metab Care 6:353–360 [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, et al. (1998) Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92:573–585 [DOI] [PubMed] [Google Scholar]
- See RE, Elliott JC, Feltenstein MW. (2007) The role of dorsal vs ventral striatal pathways in cocaine seeking behavior after prolonged abstinence in rats. Psychopharmacology (Berl) 194:321–331 [DOI] [PubMed] [Google Scholar]
- Silveyra P, Cataldi NI, Lux-Lantos VA, Libertun C. (2010) Role of orexins in the hypothalamic-pituitary-ovarian relationships. Acta Physiol (Oxf) 198:355–360 [DOI] [PubMed] [Google Scholar]
- Smith RJ, See RE, Aston-Jones G. (2009) Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine seeking. Eur J Neurosci 30:493–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Tahsili-Fahadan P, Aston-Jones G. (2010) Orexin/hypocretin is necessary for context-driven cocaine seeking. Neuropharmacology 58:179–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanova N, Ovtscharoff W. (2000) Sexual dimorphism of the bed nucleus of the stria terminalis and the amygdala. Adv Anat Embryol Cell Biol 158:III-X, 1–78 [DOI] [PubMed] [Google Scholar]
- Taheri S, Mahmoodi M, Opacka-Juffry J, Ghatei MA, Bloom SR. (1999) Distribution and quantification of immunoreactive orexin A in rat tissues. FEBS Lett 457:157–161 [DOI] [PubMed] [Google Scholar]
- Walling SG, Nutt DJ, Lalies MD, Harley CW. (2004) Orexin-A infusion in the locus ceruleus triggers norepinephrine (NE) release and NE-induced long-term potentiation in the dentate gyrus. J Neurosci 24:7421–7426 [DOI] [PMC free article] [PubMed] [Google Scholar]