Abstract
Various neurodegenerative diseases and psychiatric disorders are marked by alterations in brain cholinergic function and cognitive deficits. Efforts to alleviate such deficits have been limited by a lack of selective M1 muscarinic agonists. 5-(3-Ethyl-1,2,4-oxadiazol-5-yl)-1,4,5,6-tetrahydropyrimidine hydrochloride (CDD-0102A) is a partial agonist at M1 muscarinic receptors with limited activity at other muscarinic receptor subtypes. The present studies investigated the effects of CDD-0102A on working memory and strategy shifting in rats. CDD-0102A administered intraperitoneally 30 min before testing at 0.1, 0.3, and 1 mg/kg significantly enhanced delayed spontaneous alternation performance in a four-arm cross maze, suggesting improvement in working memory. In separate experiments, CDD-0102A had potent enhancing effects on learning and switching between a place and visual cue discrimination. Treatment with CDD-0102A did not affect acquisition of either a place or visual cue discrimination. In contrast, CDD-0102A at 0.03 and 0.1 mg/kg significantly enhanced a shift between a place and visual cue discrimination. Analysis of the errors in the shift to the place or shift to the visual cue strategy revealed that in both cases CDD-0102A significantly increased the ability to initially inhibit a previously relevant strategy and maintain a new, relevant strategy once selected. In anesthetized rats, the minimum dose required to induce salivation was approximately 0.3 mg/kg i.p. Salivation increased with dose, and the estimated ED50 was 2.0 mg/kg. The data suggest that CDD-0102A has unique memory and cognitive enhancing properties that might be useful in the treatment of neurological disorders at doses that do not produce adverse effects such as salivation.
Introduction
Five subtypes of muscarinic receptors have been identified in mammals. The odd-numbered receptor subtypes (M1, M3, and M5 receptors) stimulate phosphoinositide metabolism through the Gq/11 family of G proteins, whereas the even numbered receptors (M2 and M4 receptors) inhibit adenylyl cyclase activity through the Gi/o family of G proteins. Muscarinic receptors play important roles in regulating peripheral responses to acetylcholine in the parasympathetic nervous system. For example, M2 receptors slow the heart rate and decrease the force of cardiac contractions, whereas M3 receptors stimulate gastrointestinal activity and promote secretion from salivary and lacrimal glands. Although salivation has been attributed to activation of M3 receptors, it should be noted that M1 receptors also may contribute to the effects of muscarinic agonists on the salivary glands (Gautam et al., 2004).
Studies with knockout mice have provided some insight into the potential roles of muscarinic receptor subtypes, as reviewed previously (Wess, 2004; Wess et al., 2007). Studies with knockout mice suggest that both M1 and M2 receptors are involved in memory function. M1 receptors are critical for performance of nonmatching-to-sample working memory tasks, (Anagnostaras et al., 2003), whereas M2 receptors play important roles in both working memory and behavioral flexibility (Seeger et al., 2004). Muscarinic receptors also modulate biochemical signaling pathways including extracellular signal-regulated kinase (Berkeley et al., 2001) and neurophysiological responses associated with memory function such as long-term potentiation and long-term depression (Berkeley et al., 2001; Shinoe et al., 2005; Origlia et al., 2006). The role of muscarinic receptor subtypes in memory and cognitive function probably depends on a number of factors, including localization of receptors in particular brain regions, the signaling pathways activated (or inhibited) by each receptor subtype, and the type of memory being assessed. Overall, however, there is strong evidence that activation of M1 receptors results in enhanced memory function, leading to drug development efforts focused on M1 agonists for the treatment of memory and cognitive deficits associated with neurological disorders including Alzheimer's disease and schizophrenia.
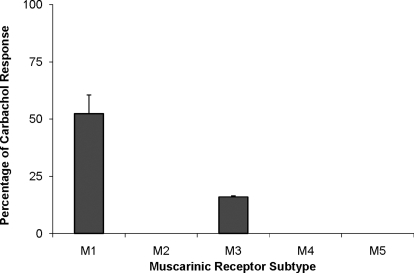
5-(3-Ethyl-1,2,4-oxadiazol-5-yl)-1,4,5,6-tetrahydropyrimidine hydrochloride (CDD-0102A) was synthesized and characterized as a selective muscarinic agonist in a variety of binding and functional assays (Dunbar et al., 1993; Messer et al., 1997a, b). CDD-0102A is a functionally selective agonist with partial agonist activity at M1 muscarinic receptors, weak activity at M3 receptors, and no activity at other muscarinic receptor subtypes (Fig. 1). In the studies described here, the compound was evaluated further for its ability to enhance memory function and behavioral flexibility in rats. Adverse effects were assayed by measuring the effects of CDD-0102A on saliva production in anesthetized rats at comparable doses. The findings suggest that CDD-0102A may be useful in alleviating memory and cognitive flexibility deficits in various neurological disorders.
Fig. 1.
Maximal responses of CDD-0102A at muscarinic receptor subtypes. The data are expressed as a percentage of the maximal response produced by the full agonist carbachol for each receptor subtype. Stimulation of phosphoinositide metabolism provided a measure of agonist activity at M1, M3, and M5 receptors expressed in A9 L cells, whereas inhibition of forskolin-stimulated cAMP formation assessed agonist activity at M2 and M4 receptors expressed in A9 L and Chinese hamster ovary cells, respectively. Data represent the mean ± S.E.M. from at least three experiments, each performed in triplicate. Data for M1 and M3 receptors are summarized from previous publications (Messer et al., 1997b).
Materials and Methods
Animals
For behavioral studies, male Long-Evans rats, weighing between 325 and 375 g, were purchased from Harlan (Indianapolis, IN). Rats were individually housed in plastic cages (20.3 × 20.3 × 41.9 cm) in an environmentally controlled room (23°C, 30% humidity) on a 12-h light/dark cycle (lights on at 7:00 AM). After at least 4 days to acclimate to the colony room, rats were handled for 10 min/day for 4 days to adjust to being handled during training and testing. At the same time rats began to be handled, rats were also food-restricted to reduce their weight to 85 to 90% of their ad libitum weight. Rats had free access to water throughout the experiment. Animal care and use conformed to the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996) and was approved by the institutional laboratory animal care and use committee at the University of Illinois at Chicago (Chicago, IL).
For studies of salivation, male Long-Evans rats, weighing between 250 and 425 g, were purchased from Charles River Laboratories, Inc. (Wilmington, MA). Animals were kept in a vivarium on a 12-h light/dark cycle. Humidity was maintained at 30 to 70%, and temperature was maintained at 20 to 24°C. All procedures were approved by the institutional animal care and use committee at Mithridion, Inc. (Madison, WI).
Behavioral Testing.
All behavioral testing occurred in a cross-maze made of translucent black acrylic. Each arm measured 55 cm long, 10 cm wide, and 15 cm high. A circular food well, with a diameter of 3.2 cm and a depth of 1.6 cm, was placed 3 cm from the end of each arm. The maze was placed on a table with a height of 72 cm.
Delayed Spontaneous Alternation.
In this test, a four-arm cross-maze was used as described above. This test did not contain any food rewards but took advantage of a rat's natural tendency to spontaneously alternate or choose the least recently visited location (Dember and Fowler, 1958). Consistent with the idea that this test has a mnemonic component, increasing the delay between arm choices (Ragozzino et al., 1996; Mohler et al., 2007) or increasing the number of locations to choose (Ragozzino et al., 1995, 1996) reduces alternation scores. For the test, each rat was placed in the testing room 5 min before behavioral testing. At the beginning of a session, a rat was allowed to freely choose an arm, but after making a choice, a block was placed into that arm for 30 s, preventing a rat from entering another arm. The block was a 21.5 × 12-cm piece of plastic. After the 30-s delay, the block was removed, and a rat was allowed to enter another arm. The number and sequence of arm entries was recorded. An arm entry was recorded when all four paws entered an arm. An alternation was defined as entry into four different arms on overlapping quadruple sets of arm entries; e.g., a quadruple set consisting of arm choices A, D, C, B was an alternation, but a quadruple set consisting of arm choices A, D, A, C was not. The percentage alternation score is equal to the ratio of (actual alternations/possible alternations) multiplied by 100; chance performance on this task is 22.2%. The total number of possible alternations was equal to the total number of arm choices minus 3. For example, a rat that made the following sequence of arm choices in a session would have nine possible alternations: A, C, D, A, B, C, B, D, A, B, C, D. In this example, a rat made five alternations (represented in bold), in which there were four different arm entries in consecutive sets of four arm choices. Thus, a rat would have an alternation score of 55.6%. The test session lasted 15 min. Rats that made fewer than 11 arm entries were excluded from the analysis. This criterion is similar to that used in previous experiments (Ragozzino et al., 1996; Mohler et al., 2007). Thirty minutes before testing, a rat received an intraperitoneal injection of one of the following treatments: 1) saline (n = 8); 2) 0.03 mg/kg CDD-0102A (n = 8); 3) 0.1 mg/kg CDD-102A (n = 8); 4) 0.3 mg/kg CDD-0102A (n = 7), or 5) 1 mg/kg CDD-0102A (n = 8). CDD-0102A was mixed in sterile saline.
Place and Visual Cue Discrimination Learning and Strategy Switching.
A group of rats different from that used in the delayed spontaneous alternation test was used for the discrimination learning and strategy switching tests. Before testing, rats were food-restricted to maintain their weight at 85% of their free-feed weight and handled and trained in the maze to obtain a 1/2 piece of Froot Loops cereal in the cross-maze. A training procedure similar to that described previously was used (Ragozzino et al., 2002, 2009).
After training, each rat was tested across 2 consecutive days. In the first experiment, each rat had to learn a place discrimination. Rats were started in a pseudorandom manner from one of two different arms such that any start arm was not used for more than three consecutive trials. The two start arms (“east” and “west”) were always opposite each other. A black plastic block was placed in the entrance of the maze arm opposite to that of the start arm, giving the maze a T-shape. Thus, the same two choice arms were used no matter what start arm was used. A rat was started in the stem arm with only one of the two choice arms baited. In the acquisition phase, one choice arm or place was designated the reinforced arm, which contained a 1/2 piece of cereal reinforcement on each trial. In this phase, a rat was required to enter the reinforced arm containing a 1/2 piece of cereal. If a rat chose the correct arm, the trial was terminated after a rat consumed the cereal piece. If a rat chose the incorrect arm, the trial was terminated after a rat reached the unbaited food well. Black and white visual cues that lined the base and side walls of the choice arms were assigned on a pseudorandom basis to be on the left or right of the start arm so that they occurred in each start arm an equal number of times in blocks of 12 trials. Between trials, the maze, visual cues, and block were wiped down with 2% Quatricide solution to minimize the animals' ability to use odor cues for discriminations. Between trials, a rat was placed in a holding chamber, which was put on a table next to the maze. The maze was then wiped down and rebaited if necessary. The intertrial interval was approximately 15 s. To minimize the use of intramaze cues, the maze was rotated 90° every fourth trial. The criterion for acquisition of the place discrimination was 10 consecutive correct trials.
On the day after the acquisition phase, each rat was tested on the switch to the visual cue strategy. In the switch phase, a rat was required to switch strategies from always entering a choice arm based on spatial location to entering a choice arm based on visual cue (black or white). Again, the visual cues were switched in a pseudorandom manner between choice arms so that a particular cue was associated with the same choice arm for a maximum of three consecutive trials and was equally associated with each turn direction across consecutive blocks of 12 trials. The learning criterion in the switch phase was also 10 consecutive correct arm choices. Thirty minutes before each test phase, rats received an intraperitoneal injection of the designated treatment for that phase. The rats were divided into the following groups: 1) saline-saline (n = 9); 2) saline-0.003 mg/kg CDD-0102A (n = 7); 3) saline-0.03 mg/kg CDD-0102A (n = 9); 4) vehicle-0.01 mg/kg CDD-0102A (n = 8); 5) 0.03 mg/kg CDD-0102A-saline (n = 8); or 6) 0.01 mg/kg CDD-0102A-saline (n = 7).
In a separate experiment, the effect of CDD-0102A on initial learning of a visual cue strategy and switch to a place strategy was determined. All other aspects of the testing procedure were as described above. The treatment groups in this experiment were as follows: 1) saline-saline (n = 8); 2) saline-0.003 mg/kg CDD-0102A (n = 7); 3) saline-0.03 mg/kg CDD-0102A (n = 7); 4) vehicle-0.1 mg/kg CDD-0102A (n = 8); 5) 0.03 mg/kg CDD-0102A-saline (n = 7); or 6) 0.01 mg/kg CDD-0102A-saline (n = 8).
In the switch phase of each experiment, the errors committed were separated into different categories and analyzed. The errors were separated into perseverative, regressive, and never-reinforced errors as described previously (Ragozzino et al., 2002; McCool et al., 2008). In the place acquisition and shift to visual cue experiment, for half the trials a rat had to enter the arm that was in the opposite place as the arm that was reinforced in the place acquisition phase. For example, a rat might have to learn to always enter the “north” arm in the place acquisition phase. In the shift to visual cue phase, a rat now had to always enter the arm with the black visual cue. Thus, when the black visual cue was in the “south” arm, a rat had to switch from choosing the north arm and enter the south arm. These trials were used to analyze the perseverative and regressive error and were initially separated into consecutive blocks of four trials each. Perseveration was defined as initially entering the incorrect arm in three or more trials per block. Thus, if a rat was initially choosing the previously correct choice on the majority of trials, it was considered perseveration. Once a rat made fewer than three errors in a block the first time, all subsequent errors were no longer counted as perseverative errors. When perseveration ended, as defined above, the number of errors was counted when a rat reverted back to the previously correct choice on those trials that required the opposite turn as on the place version. These errors are referred to as regressive errors. During the shift, a third type of error could be made if a rat entered the arm that contained neither the presently correct visual cue nor the previously correct location. For example, in half the trials the presently correct visual cue (e.g., black) was in the previously correct spatial location (e.g., north arm) and if a rat chose the south arm this would count as an error. These errors are referred to as never-reinforced errors because a rat was never reinforced for this choice in either acquisition or switch phases. These same types of errors were calculated in a similar manner for the experiment in which rats first learned a visual cue strategy and then switched to a place strategy.
Statistical Analysis
In the spontaneous alternation task, a one-way analysis of variance was used to identify differences in percent alternation scores and number of arm entries across groups. Newman-Keuls post hoc tests were used to compare treatment and control group measures. In the discrimination learning tests, one-way analyses of variance were used to identify differences in trials to criterion for acquisition and strategy switching. Separate analyses of variance were performed to determine group differences in perseverative, regressive, and never-reinforced errors. Newman-Keuls post hoc tests were used to compare differences between treatment groups.
Salivation Studies
Salivation was quantified in rats after intraperitoneal administration of CDD-0102A. Rats were first anesthetized with isoflurane and then dosed with CDD-0102 by intraperitoneal injection. Anesthesia was maintained by a nose cone that delivered 1.5 to 2.5% isoflurane in 500 ml/min of oxygen. CDD-0102A was dissolved in phosphate-buffered saline, and the injection volume was 2 ml/kg. The hind limbs of anesthetized animals were taped to the top of an incline (25° grade) so that the animal was oriented with its head near the bottom of the slope and its dorsal surface was oriented upward. Body temperature was monitored by a rectal thermometer and maintained at 36 to 38°C by a heating pad. The animal's nose was inserted into a nose cone for maintaining anesthesia while the entire mouth remained outside this nose cone. Preweighed filter paper (approximately 5 × 5 cm) was placed underneath the mouth to absorb the draining saliva. After 5 min, the saliva-wetted filter paper was weighed and replaced with fresh, preweighed filter paper. Total salivary output was calculated by subtracting the mass of the filter paper from the combined mass of paper plus saliva as collected over 60 min.
Results
Delayed Spontaneous Alternation.
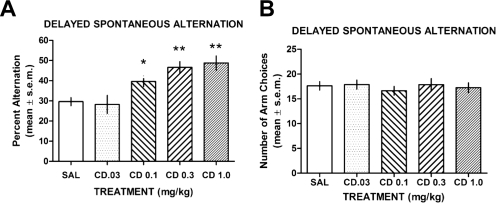
Treatments with CDD-0102A significantly enhanced delayed spontaneous alternation in a dose-dependent manner (Fig. 2A). An ANOVA revealed that there was an overall group effect (F4,34 = 8.59, P < 0.01). Post hoc tests indicated that CDD-0102A at 0.3 and 1.0 mg/kg significantly enhanced alternation performance compared with that of saline controls and CDD-0102A at 0.03 mg/kg (P < 0.01). CDD-0102A at 0.1 mg/kg also significantly enhanced alternation performance compared with that of saline controls and CDD-0102A at 0.03 mg/kg (P < 0.05).
Fig. 2.
The effect of CDD-0102A on delayed spontaneous alternation. Each rat received an intraperitoneal injection of saline (SAL) or one of four doses of CDD-0102A (CD) 30 min before a delayed spontaneous alternation test in a four-arm maze. There was a 30-s delay between each arm choice. A, mean ± S.E.M. percentage alternation scores. CDD-0102A significantly improved alternation scores at 0.1, 0.3, and 1.0 mg/kg. *, P < 0.05 versus saline; **, P < 0.01 versus saline. B, mean ± S.E.M. number of arm choices in the delayed alternation test. CDD-0102A did not affect the number of arm entries.
In contrast to alternation scores, CDD-0102A treatment across all doses led to a number of arm entries similar to that of the control group (Fig. 2B). An ANOVA on the number of arm entries among the groups revealed no significant difference (F4,34 = 0.28, P > 0.05).
Place Discrimination Acquisition and Switch to Visual Cue Discrimination.
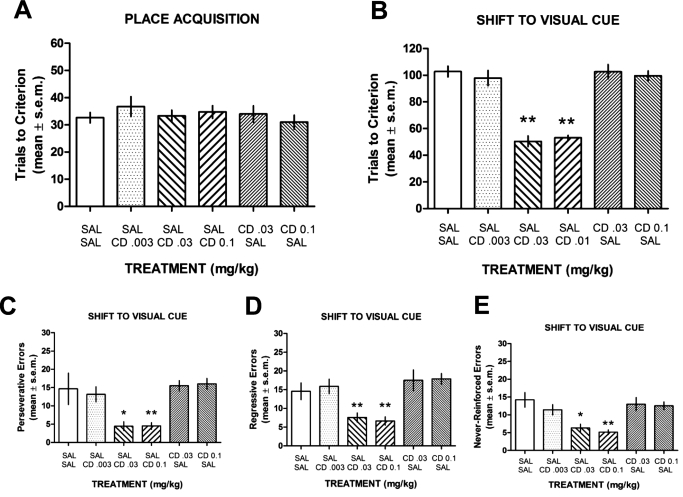
Treatment with CDD-0102A at 0.03 or 0.1 mg/kg 30 min before acquisition of a place discrimination did not affect learning compared with that in saline-treated rats (Fig. 3A). An ANOVA indicated that there was no significant treatment effect on place acquisition (F5,42 = 0.51, P > 0.05). However, there was a significant treatment effect on the switch to the visual cue discrimination (F5,42 = 37.52, P < 0.01). CDD-0102A at doses of 0.03 and 0.1 mg/kg significantly decreased the number of trials to reach criterion compared with those of all other treatment groups (P < 0.01). In contrast, performances of rats treated with CDD-0102A at 0.003 mg/kg were similar to those of saline-treated rats (P > 0.05). Thus, CDD-0102A improved a shift to a visual cue discrimination in a dose-dependent manner (Fig. 3B).
Fig. 3.
The effect of CDD-0102A on place acquisition and switch to a visual cue discrimination. Each rat received an intraperitoneal injection of saline (SAL) or one of three doses of CDD-0102A (CD) 30 min before acquisition and switch phases. The treatments shown represent the treatment received before acquisition (top rows) followed by the treatment received before the switch phase (bottom rows). A, mean ± S.E.M. trials to criterion on acquisition of a place discrimination. Injection of CDD-0102A had no effect on acquisition. B, mean ± S.E.M. trials to criterion on switch to a visual cue strategy. CDD-0102A at 0.03 and 0.1 mg/kg facilitated a shift to a visual cue strategy. **, P < 0.01 versus SAL-SAL. C, mean ± S.E.M. perseverative errors committed in the switch to visual cue discrimination. CDD-0102A at 0.03 and 0.1 mg/kg significantly decreased perseverative errors. *, P < 0.05 versus SAL-SAL; **, P < 0.01 versus SAL-SAL. D, mean ± S.E.M. regressive errors committed in the switch to visual cue discrimination. CDD-0102A at 0.03 and 0.1 mg/kg significantly decreased regressive errors. **, P < 0.01 versus SAL-SAL. E, mean ± S.E.M. never-reinforced errors committed in the switch to visual cue discrimination. CDD-0102A at 0.03 and 0.1 mg/kg significantly decreased never-reinforced errors. *, P < 0.05 versus SAL-SAL; **, P < 0.01 versus SAL-SAL.
The facilitating effects of CDD-0102A on the switch to a visual cue discrimination resulted from a significant decrease in all three of the error measures (Fig. 3, C–E). In particular, CDD-0102A at 0.03 mg/kg significantly reduced perseverative errors compared with that of the lowest dose of CDD-0102A and saline-treated controls (P < 0.05). CDD-0102A at 0.1 mg/kg also significantly reduced perseverative errors compared with saline controls and the lowest dose of CDD-0102A (P < 0.01). CDD-0102A at 0.03 or 0.1 mg/kg also significantly lessened the number of regressive errors compared with those of the other treatment groups (P < 0.01). In addition, the number of never-reinforced errors committed was significantly greater in saline-treated rats and the 0.003 mg/kg CDD-0102A treatment group compared with those for CDD-0102A at 0.03 mg/kg (P < 0.05) and CDD-0102A at 0.1 mg/kg (P < 0.01).
Visual Cue Discrimination Acquisition and Switch to Place Discrimination.
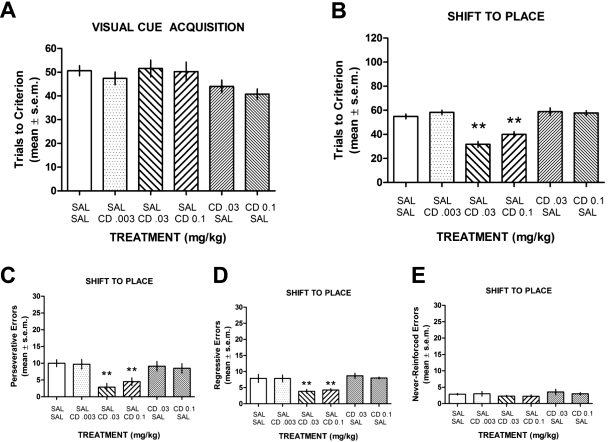
The finding that CDD-0102A treatment enhanced performance on a shift to a visual cue discrimination could reflect a facilitation of strategy switching or a more general enhancement of visual cue learning. To distinguish between these possibilities, an experiment was performed that examined the effects of CDD-0102A treatment on acquisition of a visual cue discrimination and shift to a place discrimination. All groups, including those receiving CDD-0102A before acquisition, performed in a similar manner in learning a visual cue discrimination (Fig. 4A). An ANOVA indicated that there was no significant group effect on visual cue acquisition (F5,39 = 2.10, P > 0.05). CDD-0102A treatment did lead to an overall group effect in the switch to the place discrimination (F5,39 = 24.72, P < 0.01). In particular, CDD-0102A at doses of 0.03 and 0.1 mg/kg significantly enhanced switching to a place strategy compared with all other treatment groups (P < 0.01) (Fig. 4B). CDD-0102A at 0.003 mg/kg had no significant effect on a switch to a place strategy compared with controls (P > 0.05).
Fig. 4.
The effect of CDD-0102A on visual cue acquisition and switch to place discrimination. Each rat received an intraperitoneal injection of saline (SAL) or one of three doses of CDD-0102A (CD) 30 min before acquisition and switch phases. The treatments shown represent the treatment received before acquisition (top rows) followed by the treatment received before the switch phase (bottom rows). A, mean ± S.E.M. trials to criterion on acquisition of a visual cue discrimination. Injection of CDD-0102A had no effect on acquisition. B, mean ± S.E.M. trials to criterion on switch to a place strategy. CDD-0102A at 0.03 and 0.1 mg/kg facilitated a shift to a place strategy. **, P < 0.01 versus SAL-SAL. C, mean ± S.E.M. perseverative errors committed in the switch to visual cue discrimination. CDD-0102A at 0.03 and 0.1 mg/kg significantly decreased perseverative errors. **, P < 0.01 versus SAL-SAL. D, mean ± S.E.M. regressive errors committed in the switch to visual cue discrimination. CDD-0102A at 0.03 and 0.1 mg/kg significantly decreased regressive errors. **, P < 0.01 versus SAL-SAL. E, mean (±S.E.M.) never-reinforced errors committed in the switch to visual cue discrimination. CDD-0102A treatment did not affect never-reinforced errors.
CDD-0102A treatment also affected the errors committed in the switch to the place discrimination (Fig. 4, C–E). Compared with all other treatment groups, CDD-0102A at 0.03 and 0.1 mg/kg significantly decreased the number of perseverative errors (P < 0.01) and regressive errors (P < 0.01). In the switch to the place discrimination, each group made very few never-reinforced errors; thus, there was no overall group effect on the number of never-reinforced errors committed (F5,39 = 0.83, P > 0.05).
Salivation.
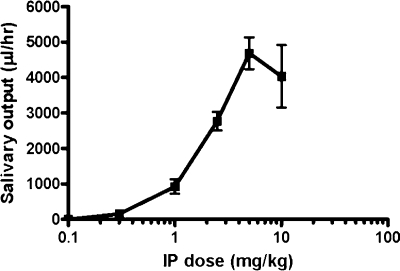
CDD-0102A was administered to adult, male Long-Evans rats at doses of 0.1, 0.3, 1, 2.5, 5, and 10 mg/kg by intraperitoneal injection. Salivary output increased as a function of dose over a 60-min period as shown in Fig. 5. The 0.1 mg/kg dose caused no salivation in rats, whereas the 5 mg/kg dose produced the most salivation (4685 ± 449 μl). Salivation from rats dosed with 10 mg/kg was not significantly different from that for rats given the 5 mg/kg dose (P > 0.5).
Fig. 5.
Salivary output over a 60-min time period after intraperitoneal administration of CDD-0102A. Data represent the mean (±S.E.M.) from three animals for each dose.
Discussion
CDD-0102A is a functionally selective M1 muscarinic agonist with potential utility in treating memory deficits associated with Alzheimer's disease. In the present studies, acute treatment with CDD-0102A enhanced both delayed spontaneous alternation and strategy switching in a dose-dependent fashion. CDD-0102A facilitated delayed spontaneous alternation without affecting the number of arm choices, suggesting that the drug enhanced mnemonic processes without any effect on locomotor activity. This task requires a short-term or working memory for recent arm choices. Consistent with this idea, inserting a delay between arm choices decreases alternation scores (Ragozzino et al., 1996; Mohler et al., 2007). Previous studies have shown that hippocampal acetylcholine output increases during spontaneous alternation performance and facilitating hippocampal acetylcholine output concomitantly enhances spontaneous alternation performance (Ragozzino et al., 1996, 1998; Mohler et al., 2007). Taken together with the in vivo microdialysis experiments, the present findings suggest that increases in brain acetylcholine release may enhance working memory, in part by activating hippocampal M1 muscarinic cholinergic receptors.
The finding that CDD-0102A enhanced delayed spontaneous alternation is consistent with other studies demonstrating that M1-preferring agonists enhance working memory in rodents and nonhuman primates (Wall et al., 2001; Terry et al., 2002). Moreover, CDD-0102A-enhanced delayed alternation is consistent with previous results indicating that CDD-0102A reverses a working memory deficit induced by a 192-IgG saporin lesion of basal forebrain cholinergic neurons (Messer et al., 2002). However, this is the first demonstration that CDD-0102A alone enhances working memory.
CDD-0102A facilitated strategy switching, regardless of whether a rat switched from a place to visual cue strategy or from a visual cue to a place strategy. In contrast, CDD-0102A treatment did not affect the initial learning of a place or visual cue discrimination. This is the first report of an effect of an M1-selective muscarinic agonist on strategy switching. Strategy switching was found to be surprisingly sensitive to CDD-0102A, being maximal at a dose of 0.03 mg/kg i.p., a dose lower than that required to enhance delayed spontaneous alternation and much lower than that required to cause salivation. The data suggest that a low level of muscarinic receptor occupancy is required for this action. Furthermore, the effects of CDD-0102A are consistent with accumulating evidence that increases in striatal acetylcholine output facilitate cognitive flexibility (Ragozzino and Choi, 2004; Ragozzino et al., 2009) and that this might be mediated by M1-like muscarinic receptors, because infusions of M1 muscarinic antagonists into the dorsomedial striatum impair cognitive flexibility (Tzavos et al., 2004; McCool et al., 2008). Because CDD-0102A was administered systemically, the drug effect on strategy switching may be due to activation of multiple brain areas and not limited to the striatum.
CDD-0102A enhanced strategy switching by reducing multiple types of errors. In both experiments, CDD-0102A significantly reduced perseverative errors and regressive errors. Thus, CDD-0102A improved the ability to initially suppress a previously learned strategy and/or generate a new strategy as exhibited by reduced perseveration. CDD-0102A also enhanced the ability to maintain a new strategy once selected as observed by a reduction in the number of regressive errors. Several experiments indicate that different prefrontal cortex subregions are critical for the initial inhibition of a previously learned strategy as measured by perseverative errors, whereas manipulation of the dorsomedial striatum selectively affects the ability to reliably execute a new strategy once selected (Ragozzino et al., 2003; Palencia and Ragozzino, 2004; McCool et al., 2008). The prefrontal cortex and striatum are two brain areas that have moderate to high densities of M1 muscarinic receptors (Levey et al., 1991). Because CDD-0102A treatment reduced both perseverative and regressive errors, the pattern of results suggests that CDD-0102A may act at both prefrontal cortex and striatal regions to facilitate initial inhibition of a previously relevant strategy while reliably executing a new strategy once selected.
CDD-0102A significantly reduced never-reinforced errors in the shift from the place to visual cue strategy, but did not affect never-reinforced errors in the shift from the visual cue to place strategy. The differential effect is probably due to rats making few never-reinforced errors in the shift to the place. A rat may make a never-reinforced error because it is trying an alternative, incorrect strategy such as reversing the place choice or shifting to a response strategy (e.g., always turn right). The finding that CDD-0102A reduced never-reinforced errors in the shift to the visual cue strategy may indicate that it inhibited the selection of alternative, incorrect strategies and facilitated the selection of the new, correct strategy.
Several muscarinic agonists have been developed, although none has been approved, for the treatment of Alzheimer's disease. Liabilities in clinical studies include dose-limiting adverse effects such as nausea, vomiting, and diarrhea. To further assess the adverse effect profile of CDD-0102A, salivation was measured in rats over a range of doses comparable to those used in the behavioral studies. The lowest dose that produced salivation was 0.3 mg/kg i.p. Significant improvement in memory function was observed at doses of 0.1 mg/kg and higher, whereas doses of 0.03 and 0.1 mg/kg were effective in enhancing behavioral flexibility. The data indicate that CDD-0102A has beneficial effects on memory and cognitive function at doses that do not produce salivation. Although salivation has been linked to activation of M3 muscarinic receptors, it may be that the salivation produced by CDD-0102A in the present studies is due to activation of M1 receptors (Gautam et al., 2004). Further studies using, for example, receptor knockout mice are necessary to determine which muscarinic receptor subtypes contribute to the stimulation of salivation by CDD-0102A.
The present findings suggest that CDD-0102A may have a broad range of effects on cognition, facilitating both working memory and cognitive flexibility. Because the present experiment only investigated the effect of short-term treatment with CDD-0102A, future experiments examining the effects of long-term treatment of the drug are crucial in determining whether CDD-0102A can possibly serve as an effective treatment for alleviating cognitive deficits. This is particularly important because of the considerable effort in the development of pharmacological agents that selectively act at the M1 muscarinic acetylcholine receptor, in particular, M1 muscarinic agonists, largely because of studies indicating that M1 muscarinic acetylcholine receptors are altered in Alzheimer's disease and schizophrenia (Langmead et al., 2008). Both of these conditions are marked by cognitive deficits, and thus there has been an interest in developing selective M1 muscarinic agonists to alleviate the cognitive deficits in these conditions.
Acknowledgments
We thank Michael Hendrickson for help in preparing the manuscript for publication.
This work was supported by the National Institutes of Health National Institute on Aging [Grants AG02454, AG027951]; and the National Institutes of Health Eunice Kennedy Shriver National Institute of Child Health and Human Development [Grant P50-HD055751].
W.S.M. serves as Chief Scientific Officer and as a consultant to Mithridion, Inc., is an inventor on several patents covering muscarinic agonists, and holds a financial interest in the company.
T.M.T. is the Chief Executive Officer for Mithridion, Inc. and holds a financial interest in the company.
J.E.B. holds a financial interest in Mithridion, Inc.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- CDD-102A
- 5-(3-ethyl-1,2,4-oxadiazol-5-yl)-1,4,5,6-tetrahydropyrimidine hydrochloride
- ANOVA
- analysis of variance.
Authorship Contributions
Participated in research design: Ragozzino, Twose, Beck, and Messer.
Conducted experiments: Ragozzino, Artis, Singh, and Beck.
Contributed new reagents or analytic tools: Messer.
Performed data analysis: Ragozzino, Artis, Singh, Twose, Beck, and Messer.
Wrote or contributed to the writing of the manuscript: Ragozzino, Twose, Beck, and Messer.
References
- Anagnostaras SG, Murphy GG, Hamilton SE, Mitchell SL, Rahnama NP, Nathanson NM, Silva AJ. (2003) Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nat Neurosci 6:51–58 [DOI] [PubMed] [Google Scholar]
- Berkeley JL, Gomeza J, Wess J, Hamilton SE, Nathanson NM, Levey AI. (2001) M1 muscarinic acetylcholine receptors activate extracellular signal-regulated kinase in CA1 pyramidal neurons in mouse hippocampal slices. Mol Cell Neurosci 18:512–524 [DOI] [PubMed] [Google Scholar]
- Dember WN, Fowler H. (1958) Spontaneous alternation behavior. Psychol Bull 55:412–428 [DOI] [PubMed] [Google Scholar]
- Dunbar PG, Durant GJ, Fang Z, Abuh YF, el-Assadi AA, Ngur DO, Periyasamy S, Hoss WP, Messer WS., Jr (1993) Design, synthesis, and neurochemical evaluation of 5-(3-alkyl-1,2,4-oxadiazol-5-yl)-1,4,5,6-tetrahydropyrimidines as M1 muscarinic receptor agonists. J Med Chem 36:842–847 [DOI] [PubMed] [Google Scholar]
- Gautam D, Heard TS, Cui Y, Miller G, Bloodworth L, Wess J. (2004) Cholinergic stimulation of salivary secretion studied with M1 and M3 muscarinic receptor single- and double-knockout mice. Mol Pharmacol 66:260–267 [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals, 7th ed, Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC [Google Scholar]
- Langmead CJ, Watson J, Reavill C. (2008) Muscarinic acetylcholine receptors as CNS drug targets. Pharmacol Ther 117:232–243 [DOI] [PubMed] [Google Scholar]
- Levey AI, Kitt CA, Simonds WF, Price DL, Brann MR. (1991) Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J Neurosci 11:3218–3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool MF, Patel S, Talati R, Ragozzino ME. (2008) Differential involvement of M1-type and M4-type muscarinic cholinergic receptors in the dorsomedial striatum in task switching. Neurobiol Learn Mem 89:114–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messer WS, Jr, Abuh YF, Liu Y, Periyasamy S, Ngur DO, Edgar MA, El-Assadi AA, Sbeih S, Dunbar PG, Roknich S, et al. (1997a) Synthesis and biological characterization of 1,4,5,6-tetrahydropyrimidine and 2-amino-3,4,5,6-tetrahydropyridine derivatives as selective m1 agonists. J Med Chem 40:1230–1246 [DOI] [PubMed] [Google Scholar]
- Messer WS, Jr, Abuh YF, Ryan K, Shepherd MA, Schroeder M, Abunada S, Sehgal R, El-Assadi AA. (1997b) Tetrahydropyrimidine derivatives display functional selectivity for M1 muscarinic receptors in brain. Drug Dev Res 40:171–184 [Google Scholar]
- Messer WS, Jr, Bachmann KA, Dockery C, El-Assadi AA, Hassoun E, Haupt N, Tang B, Li X. (2002) Development of CDD-0102 as a selective M1 agonist for the treatment of Alzheimer's disease. Drug Dev Res 57:200–213 [Google Scholar]
- Mohler EG, Shacham S, Noiman S, Lezoualc'h F, Robert S, Gastineau M, Rutkowski J, Marantz Y, Dumuis A, Bockaert J, et al. (2007) VRX-03011, a novel 5-HT4 agonist, enhances memory and hippocampal acetylcholine efflux. Neuropharmacology 53:563–573 [DOI] [PubMed] [Google Scholar]
- Origlia N, Kuczewski N, Aztiria E, Gautam D, Wess J, Domenici L. (2006) Muscarinic acetylcholine receptor knockout mice show distinct synaptic plasticity impairments in the visual cortex. J Physiol 577:829–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palencia CA, Ragozzino ME. (2004) The influence of NMDA receptors in the dorsomedial striatum on response reversal learning. Neurobiol Learn Mem 82:81–89 [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Choi D. (2004) Dynamic changes in acetylcholine output in the medial striatum during place reversal learning. Learn Mem 11:70–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Hellems K, Lennartz RC, Gold PE. (1995) Pyruvate infusions into the septal area attenuate spontaneous alternation impairments induced by intraseptal morphine injections. Behav Neurosci 109:1074–1080 [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Kim J, Hassert D, Minniti N, Kiang C. (2003) The contribution of the rat prelimbic-infralimbic areas to different forms of task switching. Behav Neurosci 117:1054–1065 [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Mohler EG, Prior M, Palencia CA, Rozman S. (2009) Acetylcholine activity in selective striatal regions supports behavioral flexibility. Neurobiol Learn Mem 91:13–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Pal SN, Unick K, Stefani MR, Gold PE. (1998) Modulation of hippocampal acetylcholine release and spontaneous alternation scores by intrahippocampal glucose injections. J Neurosci 18:1595–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Ragozzino KE, Mizumori SJ, Kesner RP. (2002) Role of the dorsomedial striatum in behavioral flexibility for response and visual cue discrimination learning. Behav Neurosci 116:105–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragozzino ME, Unick KE, Gold PE. (1996) Hippocampal acetylcholine release during memory testing in rats: augmentation by glucose. Proc Natl Acad Sci USA 93:4693–4698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeger T, Fedorova I, Zheng F, Miyakawa T, Koustova E, Gomeza J, Basile AS, Alzheimer C, Wess J. (2004) M2 muscarinic acetylcholine receptor knock-out mice show deficits in behavioral flexibility, working memory, and hippocampal plasticity. J Neurosci 24:10117–10127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoe T, Matsui M, Taketo MM, Manabe T. (2005) Modulation of synaptic plasticity by physiological activation of M1 muscarinic acetylcholine receptors in the mouse hippocampus. J Neurosci 25:11194–11200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terry AV, Jr, Buccafusco JJ, Borsini F, Leusch A. (2002) Memory-related task performance by aged rhesus monkeys administered the muscarinic M1-preferring agonist, talsaclidine. Psychopharmacology (Berl) 162:292–300 [DOI] [PubMed] [Google Scholar]
- Tzavos A, Jih J, Ragozzino ME. (2004) Differential effects of M1 muscarinic receptor blockade and nicotinic receptor blockade in the dorsomedial striatum on response reversal learning. Behav Brain Res 154:245–253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall PM, Flinn J, Messier C. (2001) Infralimbic muscarinic M1 receptors modulate anxiety-like behaviour and spontaneous working memory in mice. Psychopharmacology (Berl) 155:58–68 [DOI] [PubMed] [Google Scholar]
- Wess J. (2004) Muscarinic acetylcholine receptor knockout mice: novel phenotypes and clinical implications. Annu Rev Pharmacol Toxicol 44:423–450 [DOI] [PubMed] [Google Scholar]
- Wess J, Eglen RM, Gautam D. (2007) Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nat Rev Drug Discov 6:721–733 [DOI] [PubMed] [Google Scholar]