Abstract
κ Opioid receptor agonists that do not readily cross the blood-brain barrier are peripherally restricted and distribute poorly to the central nervous system after systemic administration. Peripherally restricted κ agonists have promise as candidate analgesics, because they may produce antinociception mediated by peripheral κ receptors more potently than they produce undesirable sedative and psychotomimetic effects mediated by central κ receptors. The present study used assays of pain-related stimulation and depression of behavior in rats to compare effects of 1) two peripherally restricted κ agonists [the tetrapeptide d-Phe-d-Phe-d-Ile-d-Arg-NH2 (ffir) and the nonpeptidic compound ((R,S)-N-[2-(N-methyl-3,4-dichlorophenylacetamido)-2-(3-carboxyphenyl)-ethyl]pyrrolidine hydrochloride (ICI204448)], 2) a centrally penetrating κ agonist (salvinorin A), and 3) several reference drugs, including a nonsteroidal anti-inflammatory drug (NSAID; ketoprofen). Intraperitoneal injection of dilute lactic acid served as a noxious stimulus to stimulate a stretching response and depress intracranial self-stimulation (ICSS) maintained by the delivery of electrical brain stimulation to the medial forebrain bundle. Acid-stimulated stretching was blocked by ketoprofen, the peripherally restricted κ agonists, and salvinorin A. However, acid-induced depression of ICSS was blocked only by ketoprofen. The peripherally restricted κ agonists had little effect, and salvinorin A exacerbated acid-induced depression of ICSS. These results suggest that peripherally restricted κ agonists may be safer than centrally penetrating κ agonists but less efficacious than NSAIDS or μ opioid receptor agonists to block pain-related depression of behavior; however, the peripheral selectivity of ffir and ICI204448 is limited, and future studies with κ agonists capable of greater peripheral selectivity are warranted.
Introduction
κ Opioid receptor agonists are one class of medications that has been evaluated as a source of alternatives to existing analgesics for the treatment of pain. Although centrally penetrating κ agonists produce antinociceptive effects in many conventional preclinical assays of basal, inflammatory, and neuropathic pain (e.g., reviewed in Vanderah et al., 2004; Negus et al., 2010b), they have not proven to be viable analgesics in humans in part because of the production of sedative and psychotomimetic effects (Pande et al., 1996a,b; Wadenberg, 2003). One strategy to dissociate analgesic from sedative/psychotomimetic effects has been to develop κ agonists that do not readily cross the blood-brain barrier and remain peripherally restricted after systemic administration (Vadivelu et al., 2011). Theoretically, such compounds would retain an ability to produce analgesia by acting on peripheral κ receptors, but their potency to produce centrally mediated undesirable effects, including sedation and psychotomimesis, would be reduced. Preclinical studies have provided some support for this approach. For example, centrally penetrating κ-selective agonists such as (+)-(5α,7α,8β)-n-methyl-n-[7-(1-pyrrolidinyl)-1-oxaspiro[4.5]dec-8-yl]-benzeneacetamide (U69593) produced antinociception in assays of chemical irritant-induced abdominal stretching in rodents at doses only 2- to 3-fold lower than doses that also produced signs of centrally mediated motor impairment (Seguin et al., 1995; Vanderah et al., 2004). However, peripherally restricted κ agonists such as the nonpeptide small molecule ((R,S)-N-[2-(N-methyl-3,4-dichlorophenylacetamido)-2-(3-carboxyphenyl)-ethyl]pyrrolidine hydrochloride (ICI204448) and the tetrapeptide d-Phe-d-Phe-d-NIe-d-Arg-NH2 [a.k.a. ff(d-Nle)r; FE200041] produced antinociception in the same assays at doses >20-fold lower than doses that produced signs of motor impairment (Barber et al., 1994; Vanderah et al., 2004). The clinical significance of these findings is a topic of active investigation (Machelska et al., 1999; Arendt-Nielsen et al., 2009).
To further assess distinctions in antinociception produced by centrally penetrating versus peripherally restricted κ agonists, the present study used assays of pain-related stimulation and depression of behavior to compare antinociceptive effects of the centrally penetrating κ agonist salvinorin A (Prisinzano, 2005) and two peripherally restricted κ agonists [the tetrapeptide d-Phe-d-Phe-d-Ile-d-Arg-NH2 (a.k.a. ffir) (Dooley et al., 1998) and ICI204448 (Shaw et al., 1989); see Fig. 1]. The advantages of using complementary assays of pain-related stimulation and depression of behavior have been discussed previously (Negus et al., 2006, 2010a; see Discussion). In the present study, intraperitoneal injection of dilute lactic acid served as a noxious visceral stimulus to stimulate an abdominal stretching response and depress intracranial self-stimulation (ICSS) in rats. When tested under similar conditions, the centrally penetrating κ agonist U69593 dose-dependently blocked acid-stimulated stretching (an antinociceptive effect) but only exacerbated acid-induced depression of ICSS at doses that also depressed ICSS in the absence of the noxious stimulus (Negus et al., 2010b). These results were interpreted to suggest that the apparent antinociceptive effects of U69593 in the assay of acid-stimulated stretching resulted from nonselective behavioral depression rather than analgesia. In the present study, we hypothesized that salvinorin A would produce a similar profile of effects. Conversely, we hypothesized that ffir and ICI204448 would produce an antinociceptive blockade of both acid-stimulated stretching and acid-induced depression of ICSS at doses that would have no effect on ICSS in the absence of the noxious stimulus. The effects of these κ agonists were compared with the effects of the analgesic nonsteroidal anti-inflammatory drug (NSAID) ketoprofen (Spofford et al., 2009), which was expected to function as a positive control. The effects of the stimulant cocaine and the neuroleptic flupenthixol were also examined as drugs expected to produce nonselective stimulation and depression of behavior, respectively (Nielsen et al., 1973; Johanson and Fischman, 1989).
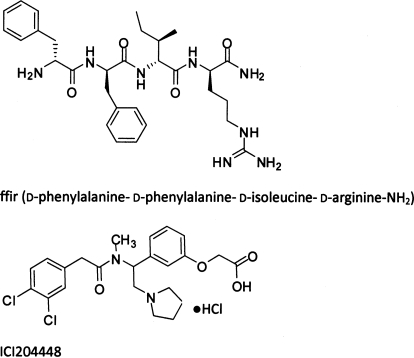
Fig. 1.
Structures of peripherally restricted κ agonists. Top, ffir. Bottom, ICI204448.
Materials and Methods
Subjects
Male Sprague-Dawley rats (Harlan, Frederick MD) with initial weights of 295 to 405 g at the time of surgery were used for studies of ICSS and lactic acid-induced stretching. Rats were individually housed and maintained on a 12-h light/dark cycle with lights on from 6:00 AM to 6:00 PM. Food and water were continuously available except during experimental sessions. Animal maintenance and research were in compliance with National Institutes of Health guidelines on the care and use of animal subjects in research (Institute of Laboratory Animal Resources, 1996) and the guidelines of the Committee for Research and Ethical Issues of the International Association for the Study of Pain. In addition, animal-use protocols were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee.
All animals were anesthetized with isoflurane gas (2.5–3% in oxygen; Webster Veterinary, Phoenix, AZ) for stereotaxic surgery to implant a stainless-steel bipolar electrode (Plastics One, Roanoke, VA) in the left medial forebrain bundle (2.8 mm posterior to bregma, 1.7 mm lateral from midsagittal suture, and 7.8 mm below dura). One pole (the cathode) of each bipolar electrode was 0.25 mm in diameter and covered with polyamide insulation except at the flattened tip. The other pole (the anode) was 0.125 mm in diameter and uninsulated. Three to four screws were implanted in the skull, and the anode was grounded by wrapping it around one of these skull screws. The electrode was secured into place by using orthodontic resin. Rats received ketoprofen (5 mg/kg i.p. for 2 days) as the postoperative analgesic. ICSS training began after 7 days of recovery.
Assay of Intracranial Self-Stimulation
Apparatus.
Experiments were conducted in sound-attenuating boxes that contained modular acrylic test chambers (29.2 × 30.5 × 24.1 cm) equipped with a response lever (4.5 cm wide, extended 2.0 cm through the center of one wall, 3 cm off the floor), stimulus lights (three lights colored red, yellow, and green, positioned 7.6 cm directly above the response lever), a 2-W white house light, and an ICSS stimulator (MED Associates, St. Albans, VT). Electrodes were connected to the stimulator via a swivel connector (model SL2C; Plastics One). The stimulator was controlled by computer software that also controlled all of the programming parameters and data collection (MED Associates).
Behavioral Procedure.
After initial shaping of lever-press responding, rats were trained under a continuous reinforcement schedule of brain stimulation by using procedures similar to those described previously (Negus et al., 2010a,b). During initial training sessions lasting 30 to 60 min, the white house light was illuminated, and responding produced electrical stimulation under a continuous schedule of reinforcement. Under this schedule, each lever press resulted in the delivery of a 0.5-s train of square-wave cathodal pulses (0.1-ms pulse duration) and illumination for 0.5 s of the colored stimulus lights over the lever. Responses during the 0.5-s stimulation period did not earn additional stimulation. Initially, the frequency of stimulation was held constant at 126 Hz, and the stimulation intensity for each rat was adjusted gradually to the lowest value that would sustain a high rate of ICSS (≥30 stimulations/min). Frequency manipulations were then introduced, and the terminal schedule consisted of sequential 10-min components. During each component, a descending series of 10 current frequencies was presented, with a 60-s trial at each frequency. The frequency range extended from 158 to 56 Hz in 0.05-log increments. Each frequency trial began with a 10-s timeout, during which the house light was off and responding had no scheduled consequences. During the last 5 s of this timeout, five noncontingent stimulations were delivered once per second at the frequency available during that trial, and the lever lights were illuminated during each stimulation. This noncontingent stimulation was then followed by a 50-s “response” period, during which the house light was illuminated, and responding produced electrical stimulation under the continuous reinforcement schedule described above. Training continued with presentation of three sequential components per day, and intensity was again adjusted as necessary until rats reliably responded for at least three and no more than six trials of all components for at least 2 consecutive days. In general, rats were trained in groups of 10 to 14 for each drug. The first six rats to meet training criteria were then advanced to ICSS testing. As discussed previously (Pereira Do Carmo et al., 2009; Negus et al., 2010b), the remaining rats from each group were assigned to studies of lactic acid-induced stretching using methods described below.
ICSS test sessions consisted of six sequential components. The first component of each test session was considered to be an acclimation component, and data from this component were discarded. Data from the second and third “baseline” components were used to calculate baseline parameters of frequency-rate curves for that session (see Data Analysis). After these baseline components, a test drug or its vehicle was administered intraperitoneally as a pretreatment to 1.8% lactic acid or its vehicle (intraperitoneally in a volume of 1.0 ml/kg). Drugs, doses, and pretreatment times were as follows: salvinorin A (0.32–3.2 mg/kg; 10 min), ffir (0.32–10 mg/kg i.p.; 30 min), ICI204448 (10–32 mg/kg; 30 min), ketoprofen (1 mg/kg; 30 min), cocaine (1–10 mg/kg; 10 min), and flupenthixol (0.1–1.0 mg/kg; 60 min). Doses and pretreatment times were based on preliminary studies and previous studies in the literature (Corbett, 1990; Barber et al., 1994; McCurdy et al., 2006; Chartoff et al., 2008; Spofford et al., 2009). Three sequential test components were conducted immediately after administration of lactic acid or its vehicle. Testing was conducted twice per week using a schedule under which test drugs were administered twice per week (usually Tuesday and Friday), with the test drug dose being followed by 1.8% lactic acid on one test day and lactic acid vehicle on the other test day. The order of drug doses for a group of rats was arranged according to a within-subject, Latin-square design such that all rats received all doses of a given drug, and training sessions were conducted on other weekdays.
Data Analysis.
The primary dependent variable was the reinforcement rate in stimulations/trial during each frequency trial. To normalize these data, raw reinforcement rates from each trial in each rat were converted to percentage of maximum control rate (%MCR), with the maximum control rate defined as the mean of the maximal rates observed during any frequency trial of the second and third baseline components for that session. Thus, %MCR values for each trial were calculated as (response rate during a frequency trial ÷ maximum control rate) × 100. For each test session, data from the second and third components were averaged to yield a baseline frequency-rate curve, and data from the three test components were averaged to yield a test frequency-rate curve. Baseline and test curves were then averaged across rats to yield mean baseline and test curves for each manipulation. For statistical analysis, results were compared by two-way ANOVA, with treatment and ICSS frequency as the two factors. A significant ANOVA was followed by the Holm-Sidak post hoc test, and the criterion for significance was set at p < 0.05.
To provide an additional summary of ICSS performance, the total number of stimulations per component obtained across all frequencies was determined, and the average number of stimulations per test component was expressed as a percentage of the average number of stimulations per baseline component during each session. These values were then averaged across rats in each experimental condition and compared by one-way ANOVA or t test as appropriate. A significant ANOVA was followed by the Dunnett or Newman-Keuls post hoc test, and the criterion for significance was set a priori at p < 0.05.
Assay of Lactic Acid-Stimulated Stretching
Behavioral Procedure.
Test sessions were conducted once per week. Test drugs were administered intraperitoneally before treatment with 1.8% lactic acid (intraperitoneally in a volume of 1 ml/kg). Immediately after acid injection, rats were placed into acrylic test chambers (31.0 × 20.1 × 20.0 cm) for 30-min observation periods. A stretch was operationally defined as a contraction of the abdomen followed by extension of the hind limbs, and the number of stretches during the observation period was counted. A dose-effect curve for salvinorin A (0.32–3.2 mg/kg) was determined by using a 10-min pretreatment time, and a time course for salvinorin A (10–100 min) was determined by using a dose of 3.2 mg/kg. A dose-effect curve for ffir (0.32–3.2 mg/kg) was determined by using a 30-min pretreatment time, and a time course for ffir (10–300 min) was determined by using a dose of 3.2 mg/kg. In addition, the effects of 3.2 mg/kg salvinorin A (10-min pretreatment) and 3.2 mg/kg ffir (30-min pretreatment) were redetermined 24 h after pretreatment with the κ opioid receptor-selective antagonist norbinaltorphimine (10 and 32 mg/kg) (Negus et al., 2010b). A dose-effect curve for ICI204448 (3.2–32 mg/kg) was determined by using a 30-min pretreatment time. Ketoprofen, cocaine, and flupenthixol were tested by using doses and pretreatment times identical to those used in the ICSS procedure, with the exception that lower ketoprofen doses were added to extend the overall ketoprofen dose range (0.01–1.0 mg/kg). The experiment with cocaine was repeated in two groups of rats (11 rats total). Results did not differ, and data were averaged. The order of drug doses for a group of rats was arranged according to a within-subject, Latin-square design such that all rats received all doses of a given drug.
Data Analysis.
Drug effects on stretching were evaluated by one-way ANOVA. A significant ANOVA was followed by the Dunnett or Newman-Keuls post hoc test, and the criterion for significance was set at p < 0.05.
Drugs
Lactic acid (Sigma, St. Louis, MO) was diluted in sterile water. Salvinorin A (Sigma) was dissolved in a vehicle of 75% dimethyl sulfoxide in sterile water as described previously (Carlezon et al., 2006). ffir (J. McLaughlin and R. Houghten, Torrey Pines Institute of Molecular Studies, Port St. Lucie, FL), ICI204448 and cis(Z)-flupenthixol 2HCl (Sigma), norbinaltorphimine (K. Rice, National Institutes of Health, Bethesda, MD), ketoprofen propionate (Spectrum Chemical, Gardena, CA), and cocaine HCl (National Institute on Drug Abuse Drug Supply Program, Bethesda, MD) were dissolved in sterile water. In accordance with the nomenclature used by Dooley et al. (1998) in their article describing the synthesis and pharmacology of d-Phe-d-Phe-d-Ile-d-Arg-NH2, this compound is referred to as ffir (f = d-phenylalanine, i = d-isoleucine, r = d-arginine). All drugs were administered intraperitoneally in a volume of 1 ml/kg, except for ICI204448, which was administered intraperitoneally in volumes up to 2 ml/kg.
Results
Effects of ffir and Salvinorin A on Acid-Stimulated Stretching.
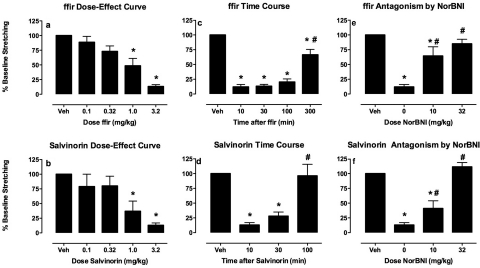
Across all 39 rats used for studies of acid-stimulated stretching, intraperitoneal administration of 1.8% lactic acid after drug vehicle pretreatments elicited a mean ± S.E.M. of 32.0 ± 2.4 stretches. The absolute number of stretches elicited by lactic acid alone in each group is reported in the figure legends. Figure 2 shows the antinociceptive effects of ffir and salvinorin A in this assay of pain-stimulated behavior. Both ffir and salvinorin A produced a dose-dependent, time-dependent, and norbinaltorphimine-reversible blockade of acid-stimulated stretching. ffir and salvinorin A displayed similar potencies and sensitivities to norbinaltorphimine antagonism, and ffir displayed a longer duration of action (≥100 min, off by 300 min) than salvinorin A (≥30 min, off by 100 min).
Fig. 2.
Effects of the κ agonists ffir (a, c, and e) and salvinorin A (b, d, and f) on acid-stimulated stretching in rats. a and b, abscissae, dose in milligram/kilogram of ffir (30 min pretreatment) or salvinorin A (10 min pretreatment). c and d, abscissae, time in minutes after administration of 3.2 mg/kg ffir or 3.2 mg/kg salvinorin A. e and f, abscissae, dose of norBNI in milligram/kilogram administered 24 h before 3.2 mg/kg ffir (30-min pretreatment) or 3.2 mg/kg salvinorin A (10-min pretreatment). Ordinates: percentage of vehicle baseline number of stretches observed after treatment with κ agonist vehicle + lactic acid (baseline ± S.E.M. = 31.0 ± 3.3 for ffir and 28.4 ± 4.2 for salvinorin A). * indicates significantly different from vehicle (Veh), and # indicates significantly different from 10 min (c and d) or 0 mg/kg norBNI (e and f) as determined by a significant one-factor ANOVA followed by Dunnett's (a and b) or Neuman-Keuls (c–f) post hoc test (p < 0.05). All bars show mean + S.E.M. for six rats.
Effects of ffir and Salvinorin A on Acid-Depressed ICSS.
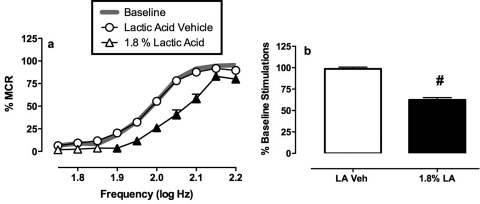
Figure 3 shows the effects of the same acid noxious stimulus (intraperitoneal injection of 1.8% lactic acid) on ICSS. During each test session, a baseline frequency-rate curve was determined before testing to permit determination of the MCR. Over the course of the entire study in all 34 rats tested, the mean ± S.E.M. MCR was 62.9 ± 1.8 stimulations/trial. Reinforcement rates during each frequency trial of a session were then expressed as a percentage of that session's MCR, and the average baseline frequency-rate curve for all rats is shown in Fig. 3a as a gray line. Rats generally did not respond at low stimulation frequencies (1.75–1.85 log Hz), and reinforcement rates increased across a frequency range of 1.9 to 2.1 log Hz. Maximum reinforcement rates were typically maintained by the highest frequencies. Treatment with lactic acid vehicle (sterile water) had no effect on the frequency-rate curve. However, treatment with 1.8% lactic acid depressed ICSS. This depression was manifested as a rightward shift in the frequency-rate curve (Fig. 3a). Figure 3 also shows summary data for the total number of stimulations delivered across all 10 frequencies during each component. The mean ± S.E.M. baseline number of stimulations per component was 309.1 ± 11.9, and lactic acid produced a decrease in the percentage baseline number of stimulations per component (Fig. 3b). This lactic acid-induced depression of ICSS provided a measure of pain-related behavioral depression, and drugs were evaluated for their ability to block acid-induced depression of ICSS.
Fig. 3.
Depression of ICSS by lactic acid. a, abscissa, brain stimulation frequency in log Hz. Ordinate, rate of ICSS expressed as %MCR. Filled points indicate significantly different from lactic acid vehicle as determined by a significant two-factor ANOVA followed by the Holm-Sidak post hoc test (p < 0.05). Average baseline data collected before testing are shown by the gray line, but these data were not included in statistical analysis. b, abscissa, treatment with lactic acid vehicle (LA Veh) or 1.8% lactic acid (1.8% LA). Ordinate, percentage of baseline number of stimulations per component. # indicates significantly different from LA Veh as determined by paired t test (p < 0.05). All data show mean ± S.E.M. from 34 rats used in the study (n = 6 for ffir, salvinorin A, ketoprofen, and flupenthixol; n = 5 for ICI204448 and cocaine).
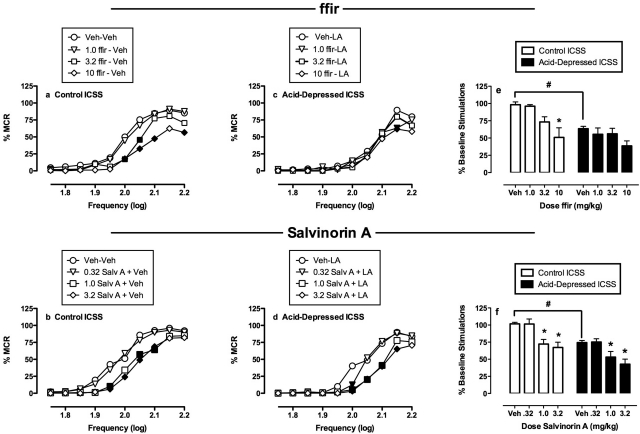
Figure 4 shows the effects of ffir and salvinorin A on control ICSS in the absence of the noxious stimulus (a and b; open bars in e and f) and on acid-depressed ICSS (c and d; filled bars in e and f). Neither ffir nor salvinorin A produced antinociception in the assay of acid-depressed ICSS. Rather, both ffir and salvinorin A significantly decreased control ICSS and only exacerbated acid-induced depression of ICSS. However, there were differences in the relative potencies of ffir and salvinorin A to produce behavioral effects. Thus, salvinorin A was equipotent in decreasing acid-stimulated stretching, control ICSS, and acid-depressed ICSS insofar as a dose of 1.0 mg/kg was sufficient to significantly decrease rates of all three behavioral dependent measures. Conversely, ffir was more potent in decreasing acid-stimulated stretching (significant at 1.0 mg/kg) than control ICSS (significant at 3.2 mg/kg). Moreover, doses of 3.2 to 10 mg/kg ffir produced only small decreases in acid-depressed ICSS that were significant only at a single frequency of the frequency-rate curve (2.15 log Hz) and not on the summary measure of percentage of baseline stimulations.
Fig. 4.
Effects of ffir (a, c, and e) and salvinorin A (b, d, and f) on ICSS. a to d, κ agonist effects on full frequency-rate curves. Abscissae, brain stimulation frequency in log Hz. Ordinates, rate of ICSS expressed as %MCR. a and b, data for κ agonists administered as a pretreatment to lactic acid vehicle (control ICSS). c and d, data for κ agonists administered as a pretreatment to 1.8% lactic acid (acid-depressed ICSS). Filled points indicate significantly different from κ agonist vehicle as determined by a significant two-factor ANOVA followed by the Holm-Sidak post hoc test (p < 0.05). Error bars are not shown for clarity. e and f, summary data for each κ agonist. Abscissae, dose in milligram/kilogram. Ordinates, percentage of baseline number of stimulations per component (baseline ± S.E.M. = 275 ± 14.1 for ffir and 325 ± 23.5 for salvinorin A). * indicates significantly different from Veh as determined by a significant one-factor ANOVA followed by Dunnett's post hoc test (p < 0.05). # indicates significantly different from lactic acid vehicle as determined by a paired t test (p < 0.05). All data show results from six rats, and error bars in e and f show S.E.M.
Effects of ICI204448.
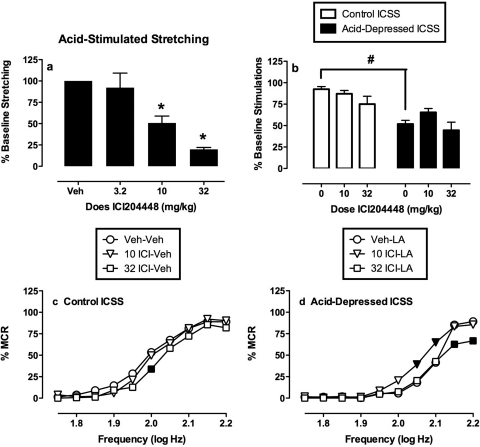
Figure 5 shows the effects of ICI204448 on acid-stimulated stretching and on control and acid-depressed ICSS. Like the other κ agonists, ICI204448 dose-dependently decreased acid-stimulated stretching (Fig. 5a). However, doses of 10 and 32 mg/kg ICI204448, which significantly decreased stretching, did not significantly alter summary measures of control or acid-depressed ICSS (Fig. 5b). Analysis of ICI204448 effects on full ICSS frequency-rate curves is shown in Fig. 5, c and d. The lower dose of 10 mg/kg ICI204448 had no effect on control ICSS, but partially blocked acid-induced depression of ICSS at frequencies of 2.05 and 2.1 log Hz. This was the only evidence in this study of an antinociceptive effect of a κ agonist in the assay of acid-depressed ICSS. The higher dose of 32 mg/kg ICI204448 only depressed control ICSS (2.0 log Hz) and exacerbated acid-induced depression of ICSS (2.15 and 2.2 log Hz).
Fig. 5.
Effects of ICI204448 on acid-induced stimulation of stretching (a) and acid-induced depression of ICSS (b–d). a and b, summary data for stretching and ICSS. Abscissae, dose of ICI204448 (mg/kg). a, ordinate, percentage of vehicle baseline number of stretches (baseline ± S.E.M. = 39.2 ± 2.9 stretches). b, ordinate, percentage of baseline number of stimulations per component (baseline ± S.E.M. = 305.8 ± 29.4). * indicates significantly different from Veh as determined by a significant one-factor ANOVA followed by Dunnett's post hoc test (p < 0.05). # indicates significantly different from lactic acid vehicle as determined by a paired t test (p < 0.05). c and d, full frequency-rate curves. Abscissae, brain stimulation frequency in log Hz. Ordinates, rate of ICSS expressed as %MCR. c, data for ICI204448 administered as a pretreatment to lactic acid vehicle (control ICSS). d, data for ICI204448 administered as a pretreatment to 1.8% lactic acid (acid-depressed ICSS). Filled points indicate significantly different from ICI204448 vehicle as determined by a significant two-factor ANOVA followed by the Holm-Sidak post hoc test (p < 0.05). Error bars are not shown for clarity. All data show mean results from six rats (stretching) or five rats (ICSS), and error bars in a and b show S.E.M.
Effects of Ketoprofen, Cocaine, and Flupenthixol.
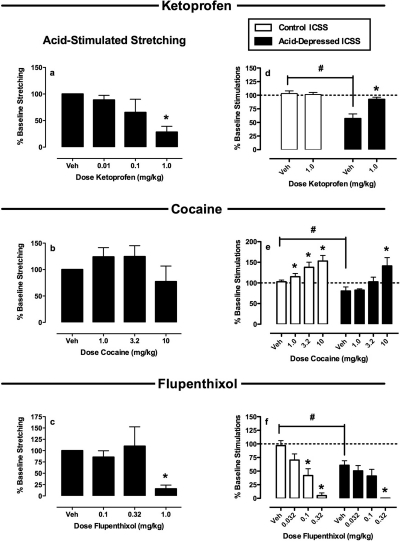
Figure 6 shows summary data for the NSAID ketoprofen, the monoamine uptake inhibitor cocaine, and the dopamine receptor antagonist flupenthixol on acid-stimulated stretching and on control and acid-depressed ICSS. Ketoprofen blocked both acid-stimulated stretching and acid-induced suppression of ICSS without affecting control ICSS. Conversely, cocaine failed to block acid-stimulated stretching, although it blocked acid-induced depression of ICSS at doses that also increased control ICSS in the absence of the noxious stimulus. Flupenthixol blocked acid-stimulated stretching, but only at doses that also decreased both control and acid-depressed ICSS. Thus, only ketoprofen produced antinociception in assays of both pain-stimulated and pain-depressed behavior.
Fig. 6.
Effects of ketoprofen (a and d), cocaine (b and e), and flupenthixol (c and f) on acid-induced stimulation of stretching (a–c) and acid-induced depression of ICSS (d–f). Abscissae, dose drug in milligram/kilogram. a to c, ordinate, percentage of vehicle baseline number of stretches [baseline ± S.E.M. = 29.1 ± 3.1 for ketoprofen (n = 5), 30.0 ± 7.1 for cocaine (n = 11), and 34.4 ± 8.7 for flupenthixol (n = 5)]. d to f, ordinate, percentage of baseline number of stimulations per component [baseline ± S.E.M. = 296.8 ± 25.5 for ketoprofen (n = 6), 347 ± 55.1 for cocaine (n = 5), and 308.6 ± 26.9 for flupenthixol (n = 6)]. * indicates significantly different from Veh as determined by a significant one-factor ANOVA followed by Dunnett's post hoc test (p < 0.05). # indicates significantly different from lactic acid vehicle in the right panels as determined by a paired t test (p < 0.05).
Discussion
The main finding of this study was that the peripherally restricted κ agonists ffir and ICI204448 produced dose-dependent and robust antinociception in the assay of pain-related stimulation of behavior (acid-stimulated stretching) but produced little or no antinociception in the assay of pain-related depression of behavior (acid-depressed ICSS). This profile of effects contrasts with profiles produced by the NSAID ketoprofen (antinociceptive in both assays) and the centrally penetrating κ agonist salvinorin A (antinociceptive in the assay of acid-stimulated stretching but pronociceptive in the assay of acid-depressed ICSS). Taken together with previous findings (Pereira Do Carmo et al., 2009; Negus et al., 2010b), these results demonstrate that complementary assays of pain-related behavioral stimulation and depression can dissociate effects of centrally penetrating κ agonists and peripherally restricted κ agonists on pain-related behaviors in rats. In addition, these results suggest that peripherally restricted κ agonists may be safer than centrally penetrating κ agonists but less efficacious as analgesics than NSAIDs or μ opioid agonists.
Rationale for Studies of Pain-Related Depression of Behavior.
Most preclinical assays of nociception measure “pain-stimulated” behaviors, which can be defined as behaviors that increase in rate or intensity upon presentation of a pain stimulus (Negus et al., 2010a). Common examples of pain-stimulated behaviors include withdrawal responses elicited by thermal and mechanical stimuli or (as in the present study) abdominal stretching responses elicited by intraperitoneal injection with chemical irritants. There are at least two weaknesses associated with exclusive reliance on assays of pain-stimulated behavior in analgesic drug development. First, drugs can reduce pain-stimulated behaviors either by reducing sensitivity to the noxious stimulus (true analgesia) or impairing the ability of the subject to emit the pain-stimulated behavior (resulting in a “false-positive” drug effect). Second, commonly measured pain-stimulated behaviors may be mediated by different neural circuits and respond to different pharmacological treatments than other pain-related behaviors, such as the affective manifestations of pain. These weaknesses may contribute to the imperfect ability of conventional preclinical assays to predict clinical effects of candidate analgesic drugs in humans (Negus et al., 2006; Mogil, 2009). As one strategy for addressing these weaknesses, we have developed assays to measure “pain-depressed” behaviors, which can be defined as behaviors that decrease in rate or intensity upon presentation of a noxious stimulus. Examples of pain-depressed behavior include pain-related reductions in feeding, locomotion, or (as in the present study) positively motivated operant behavior (Stevenson et al., 2006, 2009; Pereira Do Carmo et al., 2009). In assays of pain-depressed behavior, antinociception is expressed as an increase in the target behavior. Consequently, drugs that produce motor impairment do not produce false-positive effects. In addition, assays of pain-depressed behavior may model clinically relevant aspects of pain-related functional impairment and/or depression of mood (Cleeland and Ryan, 1994; Dworkin et al., 2005). In view of these considerations, we have argued that assays of pain-depressed behavior may complement conventional assays of pain-stimulated behavior in the preclinical evaluation of candidate analgesic drugs (Negus et al., 2010a). For the purposes of the present study, results with the reference compounds ketoprofen, cocaine, flupenthixol, and salvinorin A will be discussed first to provide a context for interpretation of results with peripherally restricted κ agonists.
Effects of Ketoprofen, Cocaine, and Flupenthixol.
In agreement with its clinical analgesic efficacy in veterinary and human medicine (Nolan, 2000; Sarzi-Puttini et al., 2010), ketoprofen in the present study blocked both acid-stimulated stretching and acid-induced depression of ICSS at a dose that had no effect on ICSS in the absence of a noxious stimulus. These results are consistent with the interpretation that ketoprofen reduced sensitivity to the noxious stimulus without producing nonselective motor depressant or stimulant effects. Previous studies found that the clinical analgesic and μ opioid receptor agonist morphine also blocked both acid-stimulated stretching and acid-induced depression of ICSS at doses that did not alter ICSS in the absence of a noxious stimulus (Pereira Do Carmo et al., 2009; Negus et al., 2010b). Conversely, the present results with flupenthixol and cocaine illustrate effects of nonanalgesic drugs that nonselectively depress or stimulate behavior. Flupenthixol produced an antinociception-like decrease in acid-stimulated stretching, but only at doses greater than those that also decreased ICSS in the absence of a noxious stimulus and exacerbated acid-induced depression of ICSS. Cocaine produced an antinociception-like blockade of acid-induced depression of ICSS, but only at doses greater than those that also stimulated ICSS in the absence of a noxious stimulus and failed to block acid-stimulated stretching. Taken together, these results provide evidence that complementary assays of acid-stimulated stretching and acid-depressed ICSS are sensitive to known classes of analgesic drugs and able to dissociate analgesics from nonanalgesics that produce nonselective depressant or stimulant behavioral effects.
Effects of Salvinorin A.
Effects of salvinorin A in the present study were similar to results reported previously with U69593, another centrally penetrating κ receptor agonist (Negus et al., 2010b). Both compounds produced dose-dependent and κ receptor-mediated antinociception-like effects in the assay of acid-stimulated stretching, but only at doses that also decreased control ICSS in the absence of a noxious stimulus and exacerbated acid-induced depression of ICSS. This profile of effects is similar to that produced by flupenthixol and consistent with the interpretation that salvinorin A (Ansonoff et al., 2006; McCurdy et al., 2006) and other centrally acting κ agonists produce antinociception in assays of pain-stimulated behavior at least in part by producing nonselective behavioral depression. The similarity in effects between κ agonists and the dopamine receptor antagonist flupenthixol may be more than superficial because both classes of compounds reduce mesolimbic dopaminergic signaling that is critical to motivated behavior (dopamine antagonists by blocking postsynaptic dopamine receptors, κ agonists by reducing presynaptic dopamine release) (Zhang et al., 2005; Carlezon et al., 2006). The failure of centrally penetrating κ agonists to produce antinociception in the assay of acid-depressed ICSS may also be related to their failure to produce clinically viable analgesia. The potential analgesic effects of salvinorin A have not been systematically examined; however, salvinorin A is the principal psychoactive constituent of the plant Salvia divinorum, and analgesia has not been cited as a prominent effect by Salvia users (Baggott et al., 2010). Likewise, synthetic κ agonists examined in clinical laboratory studies failed to produce viable analgesia either because of insufficient efficacy or the emergence of dose-limiting untoward effects (Pande et al., 1996a,b; Wadenberg, 2003).
Effects of ffir and ICI204448.
ffir is a tetrapeptide with high affinity for κ opioid receptors (Dooley et al., 1998). This is the first study to evaluate the in vivo behavioral effects of ffir. Although peripheral selectivity of ffir has not been confirmed in pharmacokinetic studies, the present results are consistent with the conclusion that ffir functioned as a κ-selective and peripherally restricted κ agonist in this study. Thus, ffir produced dose-dependent and norBNI-reversible antinociception in the assay of acid-stimulated stretching, and ffir was approximately 10-fold more potent in producing this antinociceptive effect than in decreasing ICSS in the absence of a noxious stimulus. Moreover, previous studies found that structurally related tetrapeptides also functioned as peripherally restricted κ agonists (Dooley et al., 1998; Vanderah et al., 2004, 2008). ICI204448 is a nonpeptidic small molecule that has also been described as a peripherally restricted κ-selective agonist (Shaw et al., 1989), and previous studies have reported dose-dependent and κ receptor-mediated antinociceptive effects in various assays of pain-stimulated behavior, including assays of acid-stimulated stretching in mice and rats (Barber et al., 1994; Keïta et al., 1995; Caram-Salas et al., 2007). Results of the present study are consistent with this characterization insofar as ICI204448, like ffir, produced dose-dependent antinociception in the assay of acid-stimulated stretching at doses that did not decrease ICSS in the absence of a noxious stimulus. These findings add to a growing body of evidence that stimulation of peripheral κ receptors may block reflexive manifestations of pain-related behavioral stimulation without producing more generalized signs of motor impairment (Barber et al., 1994; Vanderah et al., 2004, 2008). In this regard, peripherally selective κ agonists may be safer than centrally penetrating κ agonists that produce evidence of antinociception/analgesia only at doses that also produce untoward effects including sedation and (in humans) psychotomimesis.
Despite this evidence for their activities as peripherally restricted κ-selective agonists capable of blocking pain-related stimulation of behavior, ffir produced no antinociception and ICI204448 produced only weak antinociception in the assay of acid-depressed ICSS. The failure of these peripherally restricted κ agonists to block acid-induced depression of ICSS suggests that 1) stimulation of peripheral κ receptors may not be sufficient to block prodepressant manifestations of pain, such as pain-related functional impairment or depression of mood, and 2) peripherally restricted κ agonists may consequently be less efficacious as analgesics than NSAIDs or μ opioid agonists. Moreover, this conclusion is consistent with clinical reports that peripherally restricted κ agonists such as the nonpeptide asimadoline often fail to produce significant analgesia and sometimes exacerbate pain (Machelska et al., 1999; Delvaux et al., 2004; Szarka et al., 2007; Mangel et al., 2008). However, as a caveat to this conclusion, it should be noted that peripheral distribution of ffir, ICI204448, and related compounds is not absolute, and high doses can be expected to result in sufficient central nervous system penetration to produce centrally mediated effects that may oppose and limit any peripherally mediated blockade of pain-related behavioral depression. For example, high ffir and ICI204448 doses significantly decreased control ICSS in the present study, and relatively high asimadoline and ICI204448 doses mimicked the discriminative stimulus effects of the centrally penetrating κ agonist enadoline in squirrel monkeys (Carey and Bergman, 2001). These findings raise the possibility that κ agonists with greater peripheral selectivity than ffir, ICI204448, or asimadoline may display reduced propensity to act centrally and correspondingly greater efficacy to produce peripherally mediated blockade of pain-related behavioral depression. In support of this possibility, CR665 (H-d-Phe-d-Phe-d-Nle-d-Arg-NH-4-picolyl amide) has been described as a tetrapeptide κ agonist with very high peripheral selectivity in preclinical studies (Vanderah et al., 2008) and efficacy equivalent to oxycodone in one of two measures of mechanical visceral pain in humans (Arendt-Nielsen et al., 2009). CR665 was inactive on other measures of visceral and muscle pain, and it exacerbated responses to a cutaneous mechanical noxious stimulus, but further evaluation of κ agonists with high peripheral selectivity may be warranted.
This work was supported in part by the National Institutes of Health National Institute on Drug Abuse [Grant R01-DA11460]; the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grant R01-NS070715]; and the Intramural Research Programs of the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism.
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- U69593
- (+)-(5α,7α,8β)-n-methyl-n-[7-(1-pyrrolidinyl)-1-oxaspiro[4.5]dec-8-yl]-benzeneacetamide
- ffir
- d-phenylalanine-d-phenylalanine-d-isoleucine-d-arginine-NH2
- ICI204448
- ((R,S)-N-[2-(N-methyl-3,4-dichlorophenylacetamido)-2-(3-carboxyphenyl)-ethyl]pyrrolidine hydrochloride
- NSAID
- nonsteroidal anti-inflammatory drug
- ICSS
- intracranial self-stimulation
- MCR
- maximum control rate
- ANOVA
- analysis of variance
- norBNI
- norbinaltorphimine
- Veh
- vehicle
- LA
- lactic acid
- CR665
- H-d-Phe-d-Phe-d-Nle-d-Arg-NH-4-picolyl amide.
Authorship Contributions
Participated in research design: Negus, O'Connell, and Morrissey.
Conducted experiments: Negus, O'Connell, and Morrissey.
Contributed new reagents or analytic tools: Cheng and Rice.
Performed data analysis: Negus, O'Connell, and Morrissey.
Wrote or contributed to the writing of the manuscript: Negus, O'Connell, Morrissey, and Rice.
References
- Ansonoff MA, Zhang J, Czyzyk T, Rothman RB, Stewart J, Xu H, Zjwiony J, Siebert DJ, Yang F, Roth BL, et al. (2006) Antinociceptive and hypothermic effects of Salvinorin A are abolished in a novel strain of κ-opioid receptor-1 knockout mice. J Pharmacol Exp Ther 318:641–648 [DOI] [PubMed] [Google Scholar]
- Arendt-Nielsen L, Olesen AE, Staahl C, Menzaghi F, Kell S, Wong GY, Drewes AM. (2009) Analgesic efficacy of peripheral κ-opioid receptor agonist CR665 compared to oxycodone in a multi-modal, multi-tissue experimental human pain model: selective effect on visceral pain. Anesthesiology 111:616–624 [DOI] [PubMed] [Google Scholar]
- Baggott MJ, Erowid E, Erowid F, Galloway GP, Mendelson J. (2010) Use patterns and self-reported effects of Salvia divinorum: an internet-based survey. Drug Alcohol Depend 111:250–256 [DOI] [PubMed] [Google Scholar]
- Barber A, Bartoszyk GD, Greiner HE, Mauler F, Murray RD, Seyfried CA, Simon M, Gottschlich R, Harting J, Lues I. (1994) Central and peripheral actions of the novel κ-opioid receptor agonist, EMD 60400. Br J Pharmacol 111:843–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caram-Salas NL, Reyes-García G, Bartoszyk GD, Araiza-Saldaña CI, Ambriz-Tututi M, Rocha-González HI, Arreola-Espino R, Cruz SL, Granados-Soto V. (2007) Subcutaneous, intrathecal and periaqueductal grey administration of asimadoline and ICI-204448 reduces tactile allodynia in the rat. Eur J Pharmacol 573:75–83 [DOI] [PubMed] [Google Scholar]
- Carey GJ, Bergman J. (2001) Enadoline discrimination in squirrel monkeys: effects of opioid agonists and antagonists. J Pharmacol Exp Ther 297:215–223 [PubMed] [Google Scholar]
- Carlezon WA, Jr, Béguin C, DiNieri JA, Baumann MH, Richards MR, Todtenkopf MS, Rothman RB, Ma Z, Lee DY, Cohen BM. (2006) Depressive-like effects of the κ-opioid receptor agonist salvinorin A on behavior and neurochemistry in rats. J Pharmacol Exp Ther 316:440–447 [DOI] [PubMed] [Google Scholar]
- Chartoff EH, Potter D, Damez-Werno D, Cohen BM, Carlezon WA., Jr (2008) Exposure to the selective κ-opioid receptor agonist salvinorin A modulates the behavioral and molecular effects of cocaine in rats. Neuropsychopharmacology 33:2676–2687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleeland CS, Ryan KM. (1994) Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore 23:129–138 [PubMed] [Google Scholar]
- Corbett D. (1990) Differences in sensitivity to neuroleptic blockade: medial forebrain bundle versus frontal cortex self-stimulation. Behav Brain Res 36:91–96 [DOI] [PubMed] [Google Scholar]
- Delvaux M, Beck A, Jacob J, Bouzamondo H, Weber FT, Frexinos J. (2004) Effect of asimadoline, a κ opioid agonist, on pain induced by colonic distension in patients with irritable bowel syndrome. Aliment Pharmacol Ther 20:237–246 [DOI] [PubMed] [Google Scholar]
- Dooley CT, Ny P, Bidlack JM, Houghten RA. (1998) Selective ligands for the μ, δ, and κ opioid receptors identified from a single mixture based tetrapeptide positional scanning combinatorial library. J Biol Chem 273:18848–18856 [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, et al. (2005) Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 113:9–19 [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington DC [Google Scholar]
- Johanson CE, Fischman MW. (1989) The pharmacology of cocaine related to its abuse. Pharmacol Rev 41:3–52 [PubMed] [Google Scholar]
- Keïta H, Kayser V, Guilbaud G. (1995) Antinociceptive effect of a κa-opioid receptor agonist that minimally crosses the blood-brain barrier (ICI 204448) in a rat model of mononeuropathy. Eur J Pharmacol 277:275–280 [DOI] [PubMed] [Google Scholar]
- Machelska H, Pflüger M, Weber W, Piranvisseh-Völk M, Daubert JD, Dehaven R, Stein C. (1999) Peripheral effects of the κ-opioid agonist EMD 61753 on pain and inflammation in rats and humans. J Pharmacol Exp Ther 290:354–361 [PubMed] [Google Scholar]
- Mangel AW, Bornstein JD, Hamm LR, Buda J, Wang J, Irish W, Urso D. (2008) Clinical trial: asimadoline in the treatment of patients with irritable bowel syndrome. Aliment Pharmacol Ther 28:239–249 [DOI] [PubMed] [Google Scholar]
- McCurdy CR, Sufka KJ, Smith GH, Warnick JE, Nieto MJ. (2006) Antinociceptive profile of salvinorin A, a structurally unique κ opioid receptor agonist. Pharmacol Biochem Behav 83:109–113 [DOI] [PubMed] [Google Scholar]
- Mogil JS. (2009) Animal models of pain: progress and challenges. Nat Rev Neurosci 10:283–294 [DOI] [PubMed] [Google Scholar]
- Negus SS, Bilsky EJ, Pereira Do Carmo G, Stevenson GW. (2010a) Rationale and methods for assessment of pain-depressed behavior in preclinical assays of pain and analgesia, in Methods in Molecular Biology: Analgesia (Szallasi A. ed) pp 79–91, Humana Press, New York: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Morrissey EM, Rosenberg M, Cheng K, Rice KC. (2010b) Effects of κ opioids in an assay of pain-depressed intracranial self-stimulation in rats. Psychopharmacology (Berl) 210:149–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Vanderah TW, Brandt MR, Bilsky EJ, Becerra L, Borsook D. (2006) Preclinical assessment of candidate analgesic drugs: recent advances and future challenges. J Pharmacol Exp Ther 319:507–514 [DOI] [PubMed] [Google Scholar]
- Nielsen IM, Pedersen V, Nymark M, Franck KF, Boeck V, Fjalland B, Christensen AV. (1973) The comparative pharmacology of flupenthixol and some reference neuroleptics. Acta Pharmacol Toxicol 33:353–362 [DOI] [PubMed] [Google Scholar]
- Nolan AM. (2000) Pharmacology of analgesic drugs, in Pain Management in Animals (Flecknell PA, Waterman-Pearson A. eds) pp 21–52, Elsevier, Philadelphia [Google Scholar]
- Pande AC, Pyke RE, Greiner M, Cooper SA, Benjamin R, Pierce MW. (1996a) Analgesic efficacy of the κ-receptor agonist, enadoline, in dental surgery pain. Clin Neuropharmacol 19:92–97 [DOI] [PubMed] [Google Scholar]
- Pande AC, Pyke RE, Greiner M, Wideman GL, Benjamin R, Pierce MW. (1996b) Analgesic efficacy of enadoline versus placebo or morphine in postsurgical pain. Clin Neuropharmacol 19:451–456 [DOI] [PubMed] [Google Scholar]
- Pereira Do Carmo G, Stevenson GW, Carlezon WA, Negus SS. (2009) Effects of pain- and analgesia-related manipulations on intracranial self-stimulation in rats: further studies on pain-depressed behavior. Pain 144:170–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prisinzano TE. (2005) Psychopharmacology of the hallucinogenic sage Salvia divinorum. Life Sci 78:527–531 [DOI] [PubMed] [Google Scholar]
- Sarzi-Puttini P, Atzeni F, Lanata L, Bagnasco M, Colombo M, Fischer F, D'Imporzano M. (2010) Pain and ketoprofen: what is its role in clinical practice? Reumatismo 62:172–188 [DOI] [PubMed] [Google Scholar]
- Seguin L, Le Marouille-Girardon S, Millan MJ. (1995) Antinociceptive profiles of non-peptidergic neurokinin1 and neurokinin2 receptor antagonists: a comparison to other classes of antinociceptive agent. Pain 61:325–343 [DOI] [PubMed] [Google Scholar]
- Shaw JS, Carroll JA, Alcock P, Main BG. (1989) ICI 204448: a κ-opioid agonist with limited access to the CNS. Br J Pharmacol 96:986–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spofford CM, Ashmawi H, Subieta A, Buevich F, Moses A, Baker M, Brennan TJ. (2009) Ketoprofen produces modality-specific inhibition of pain behaviors in rats after plantar incision. Anesth Analg 109:1992–1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson GW, Bilsky EJ, Negus SS. (2006) Targeting pain-suppressed behaviors in preclinical assays of pain and analgesia: effects of morphine on acetic acid-suppressed feeding in C57BL/6J mice. J Pain 7:408–416 [DOI] [PubMed] [Google Scholar]
- Stevenson GW, Cormier J, Mercer H, Adams C, Dunbar C, Negus SS, Bilsky EJ. (2009) Targeting pain-depressed behaviors in preclinical assays of pain and analgesia: drug effects on acetic acid-depressed locomotor activity in ICR mice. Life Sci 85:309–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szarka LA, Camilleri M, Burton D, Fox JC, McKinzie S, Stanislav T, Simonson J, Sullivan N, Zinsmeister AR. (2007) Efficacy of on-demand asimadoline, a peripheral κ-opioid agonist, in females with irritable bowel syndrome. Clin Gastroenterol Hepatol 5:1268–1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadivelu N, Mitra S, Hines RL. (2011) Peripheral opioid receptor agonists for analgesia: a comprehensive review. J Opioid Manag 7:55–68 [DOI] [PubMed] [Google Scholar]
- Vanderah TW, Largent-Milnes T, Lai J, Porreca F, Houghten RA, Menzaghi F, Wisniewski K, Stalewski J, Sueiras-Diaz J, Galyean R, et al. (2008) Novel D-amino acid tetrapeptides produce potent antinociception by selectively acting at peripheral κ-opioid receptors. Eur J Pharmacol 583:62–72 [DOI] [PubMed] [Google Scholar]
- Vanderah TW, Schteingart CD, Trojnar J, Junien JL, Lai J, Riviere PJ. (2004) FE200041 (d-Phe-d-Phe-d-Nle-d-Arg-NH2): A peripheral efficacious κ opioid agonist with unprecedented selectivity. J Pharmacol Exp Ther 310:326–333 [DOI] [PubMed] [Google Scholar]
- Wadenberg ML. (2003) A review of the properties of spiradoline: a potent and selective κ-opioid receptor agonist. CNS Drug Rev 9:187–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ. (2005) Effects of the plant-derived hallucinogen salvinorin A on basal dopamine levels in the caudate putamen and in a conditioned place aversion assay in mice: agonist actions at κ opioid receptors. Psychopharmacology (Berl) 179:551–558 [DOI] [PubMed] [Google Scholar]