Abstract
Soluble guanylyl cyclase (sGC), a ubiquitously expressed heme-containing receptor for nitric oxide (NO), is a key mediator of NO-dependent processes. In addition to NO, a number of synthetic compounds that target the heme-binding region of sGC and activate it in a NO-independent fashion have been described. We report here that dicyanocobinamide (CN2-Cbi), a naturally occurring intermediate of vitamin B12 synthesis, acts as a sGC coactivator both in vitro and in intact cells. Heme depletion or heme oxidation does not affect CN2-Cbi-dependent activation. Deletion mutagenesis demonstrates that CN2-Cbi targets a new regulatory site and functions though a novel mechanism of sGC activation. Unlike all known sGC regulators that target the N-terminal regulatory regions, CN2-Cbi directly targets the catalytic domain of sGC, resembling the effect of forskolin on adenylyl cyclases. CN2-Cbi synergistically enhances sGC activation by NO-independent regulators 3-(4-amino-5-cyclopropylpyrimidine-2-yl)-1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine (BAY41-2272), 4-[((4-carboxybutyl){2-[(4-phenethylbenzyl)oxy]phenethyl}amino) methyl [benzoic]-acid (cinaciguat or BAY58-2667), and 5-chloro-2-(5-chloro-thiophene-2-sulfonylamino-N-(4-(morpholine-4-sulfonyl)-phenyl)-benzamide sodium salt (ataciguat or HMR-1766). BAY41-2272 and CN2-Cbi act reciprocally by decreasing the EC50 values. CN2-Cbi increases intracellular cGMP levels and displays vasorelaxing activity in phenylephrine-constricted rat aortic rings in an endothelium-independent manner. Both effects are synergistically potentiated by BAY41-2272. These studies uncover a new mode of sGC regulation and provide a new tool for understanding the mechanism of sGC activation and function. CN2-Cbi also offers new possibilities for its therapeutic applications in augmenting the effect of other sGC-targeting drugs.
Introduction
Nitric oxide (NO) is an important signaling molecule with key roles in many physiological processes including vasodilation, neurotransmission, and platelet aggregation. Many of these NO functions are mediated by soluble guanylyl cyclase (sGC). sGC is often referred to as an NO receptor because its capacity to convert GTP into the second messenger cGMP is increased several hundred-fold upon binding of the NO molecule (Koesling and Friebe, 1999).
The essential function of sGC in supporting vascular plasticity and blood pressure homeostasis makes it an important therapeutic target. Therapies based on inhaled NO or NO-releasing compounds, such as glyceryl trinitrate, isosorbide nitrates, or molsidomine, are capitalizing on sGC activation for the treatment of persistent pulmonary hypertension of the newborn, respiratory distress syndrome, or angina pectoris and congestive heart failure (Scatena et al., 2010).
sGC is a heterodimeric protein composed of one larger α subunit and one smaller β subunit. The most ubiquitously expressed soluble GC isoform has the α1/β1 composition, whereas the α2/β1 heterodimer is expressed at much lower levels with a higher presence in nervous tissues (Koesling and Friebe, 1999). Nitric oxide binds to the ferrous heme moiety located in the heme nitric oxide and oxygen (HNOX) domain of the β subunit. Binding of the NO molecule triggers cleavage of the coordinating bond between the heme iron and the His105 residue of the β1 subunit (Zhao et al., 1998), resulting in conformational changes that lead to a release of inhibition of the catalytic domain (Martin et al., 2003).
Studies over the last decade identified, in addition to the NO-dependent mode of sGC activation, a number of NO-independent regulators, which activate sGC through different mechanisms. One class of NO-independent sGC stimulators consists of structurally diverse benzylindazole/pyrazolopyridine and acrylamide derivatives, which only moderately activate sGC by themselves (Stasch and Hobbs, 2009). However, these stimulators sensitize sGC to low concentrations of NO and to carbon monoxide, synergistically enhancing sGC activation by these gaseous activators (Friebe et al., 1996). Another class of NO-independent activators revealed an alternative mode of sGC activation. This class, represented by BAY58-2667 (cinaciguat, 4-[((4-carboxybutyl){2-[(4-phenethylbenzyl)oxy]phenethyl}amino) methyl [benzoic]-acid) or HMR1766 (ataciguat, 5-chloro-2-(5-chloro-thiophene-2-sulfonylamino-N-(4-(morpholine-4-sulfonyl)-phenyl)-benzamide), activates sGC by replacing the heme moiety (for extensive reviews, see Schmidt et al., 2009; Stasch and Hobbs, 2009). This mechanism of action resembles the activation of sGC by protoporphyrin IX. Despite the diversity of their structures and different mechanisms of action, all existing NO-independent regulators also capitalize on the activating function of the regulatory domain of sGC. Mutagenesis (Schmidt et al., 2004), spectroscopic (Denninger et al., 2000; Martin et al., 2005), and protein crystallography (Martin et al., 2010) data provide evidence that these regulators bind to the heme-binding domain or their mechanism of action is strictly dependent on its presence (for reviews, see Schmidt et al., 2009; Stasch and Hobbs, 2009).
The catalytic domain of soluble guanylyl cyclase is highly homologous to the catalytic domain of adenylyl cyclases (Sunahara et al., 1998). Although the catalytic region of adenylyl cyclases may be activated through direct binding of Gα protein or forskolin, no sGC-activating mechanisms that would target sites other that the heme-binding domain have been described previously.
In this report, we identified cobinamides, natural precursors of vitamin B12, as sGC regulators with a novel mode of action. We demonstrate that cobinamide-dependent sGC activation is not affected by heme oxidation, and it does not require the presence of a heme moiety. We show that cobinamides activate sGC lacking the N-terminal domain of both subunits, which comprises the known sGC regulatory elements. Thus, sGC activation by cobinamides reveals the existence of a novel regulatory site outside the boundaries of the regulatory domain, which may be targeted for sGC regulation. Cobinamides synergistically enhance the activating effects of existing classes of NO-independent sGC regulators but diminish the maximal effect of NO. These compounds represent a new class of sGC stimulators, which may be a useful tool to elucidate the mechanism of sGC activation. Cobinamides may also be potentially developed into therapeutic sGC-targeting agents used alone or in combination with other sGC regulators.
Materials and Methods
Recombinant Human sGC Enzyme.
Full-length sGC was purified from Sf9 cells as described previously (Martin et al., 2001). The quality of sGC preparations was assessed by the [α-32P]GTP → [32P]cGMP conversion assay (see below) with 10 μM DEA-NO donor. Only preparations with a specific activity >5 μmol · min−1 · mg−1 were used in our studies. To generate truncated sGC variants, the open reading frames coding residues 269 to 690 of the α subunit or residues 200 to 619 of the β subunit were cloned into the transfer vector pBacPak9 (Clontech, Mountain View, CA) to obtain the pBacPak-αΔ269 and the pBacPak-βΔ200 plasmids, respectively. A hexahistidine tag was also inserted at the C terminus of the αΔ269 variant by polymerase chain reaction mutagenesis. Using these plasmids and the linearized baculovirus DNA (BaculoGold; BD Pharmingen, San Diego, CA), baculoviruses expressing the truncated αΔ269 or βΔ200 sGC were generated according to the manufacturer's protocol. These viruses were used to coinfect Sf9 cells to generate the truncated αΔ269βΔ200 sGC variant.
High-Throughput Screening sGC Assay.
The libraries were screened in a 96-well format using a modified sGC screening assay reported previously by Gerber and coworkers (Sousa et al., 2006). In brief, 1 μl of 10 mM pharmacophore dissolved in DMSO was delivered robotically into 30 μl of reaction solution (40 mM TEA, pH 7.4, 0.1 mM dithiothreitol, and 0.2 mM MgCl2) containing 0.1 μg of purified human recombinant sGC and 10 mU of inorganic pyrophosphatase (Sigma-Aldrich, St. Louis, MO). After 5 min of preincubation at 37°C, the reaction was initiated by addition of 10 μl of 400 μM Mg2+-GTP and stopped after 15 min by 60 μl of 0.5 M HCl. The amount of generated phosphate was determined by measuring absorbance at 630 nm using a SpectraMax M5 plate reader (Molecular Devices, Sunnyvale, CA) 20 min after addition of 100 μl of developing reagent [1.05% (w/v) ammonium molybdate, 1 M HCl, 0.034% (w/v) malachite green, and 0.05% Tween 20] and comparing with a phosphate calibration curve built into each screening plate. sGC activities in the absence of additives (basal) or in the presence of 3-(4-amino-5-cyclopropylpyrimidine-2-yl)-1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine (BAY41-2272) (5 μM) or 10 μM ODQ with 100 nM BAY58-2667 were used as positive controls. The pharmacophores displaying OD > 0.1 absorbance unit above the level of basal activity were subjected to secondary screening.
Removal of Cyanide Groups from Dicyanocobinamide.
A 50 mM aqueous solution of dicyanocobinamide was acidified by 1 M HCl. To remove the HCN formed after the decomposition of the cyano group, the solution was continuously bubbled with argon for 20 min. UV-visible spectroscopy using the 8453 diode array spectrophotometer (Agilent Technologies, Santa Clara, CA) confirmed a complete transformation of dicyanocobinamide into hydroxocobinamide, which was judged by the full conversion of the CN2-Cbi-specific 367-nm peak into the 360-nm peak (Supplemental Fig. 1). The sample was then neutralized by addition of 1 M NaOH and diluted 10 times in 100 mM TEA, pH 7.4, without any observable changes in spectra. For subsequent reactions with sGC, stock solution was further diluted in 50 mM TEA, pH 7.4.
Assay of sGC Activity In Vitro.
Enzymatic activity was assayed using the [α-32P]GTP → [32P] cGMP conversion assay as described previously (Martin et al., 2001) in a 100-μl assay. The reaction is initiated by the addition of 1 mM GTP/[α-32P]GTP (∼100,000 cpm) to 0.1 μg of sGC in 25 mM TEA, pH 7.5, 1 mg/ml bovine serum albumin, 1 mM 3-isobutyl-1-methylxanthine, 1 mM dithiothreitol, 1 mM cGMP, 3 mM MgCl2, 0.05 mg/ml creatine phosphokinase, and 5 mM creatine phosphate and incubated at 37°C for 10 min. To measure NO-induced sGC activity, 10 ng of sGC were used. To evaluate the effect of CN2-Cbi or BAY41-2272 on sGC activity, the enzyme was preincubated for 10 min at room temperature with the indicated concentration of the compound before the reaction was initiated. For the experiments with heme-depleted sGC, we used a previously described protocol (Foerster et al., 1996) with minor modifications. In short, purified sGC was incubated with 0.2% Tween 20 for 20 min at room temperature before it was added to the reaction mixture. The final concentration of Tween 20 was no more than 0.005% and does not affect sGC activity (data not shown). For the experiments with ferric sGC, the enzyme was first incubated for 20 min at room temperature with indicated concentrations of ODQ or ferricyanide and then supplemented with CN2-Cbi and, after 10 min, added to the reaction containing the same concentration of ODQ or ferricyanide. The reaction was stopped by 400 μl of 100 mM zinc acetate followed by 500 μl of 120 mM sodium carbonate. Unreacted GTP was precipitated by centrifugation, and the supernatant containing cGMP was loaded onto 2 ml of Al2O3. cGMP was eluted with 10 ml of 50 mM Tris (pH 7.5), and the amount of generated cGMP was calculated on the basis of the Cherenkov counts in a beta scintillation counter.
Measurement of Intracellular cGMP.
Human breast cancer MDA468 cells (American Type Culture Collection, Manassas, VA) were harvested by trypsinization and washed with Dulbecco's modified phosphate-buffered saline. The cells were then resuspended to a density of 2 × 107 cells/ml in Dulbecco's modified phosphate-buffered saline containing 1 mM 3-isobutyl-1-methylxanthine and incubated for 10 min at 37°C in a CO2 incubator. Then 100-μl aliquots of cell suspension were treated with one of the following, vehicle (0.1% DMSO), 5 μM BAY41-2272, 100 μM DEA-NO, 100 μM CN2-Cbi, or 5 μM BAY41-2272 and 100 μM CN2-Cbi, for 5 min at 37°C and mixed with 100 μl of ice-cold 1 M perchloric acid. cGMP was extracted on ice for 30 min. Supernatant fractions were neutralized with potassium carbonate (25 μl of 2 M) and used for cGMP quantification using a cGMP enzyme immunoassay (Cayman Chemical, Ann Arbor, MI) according to the manufacturer's protocol. Pellets were dissolved in 0.5 ml of 0.1 M NaOH and used to measure protein by a dye-binding assay (Bio-Rad Laboratories, Hercules, CA). Data are expressed in picomoles of cGMP per 5 min per milligram of lysate protein.
Aortic Ring Relaxation.
Male Sprague-Dawley rats (14–16 weeks old, 300–350 g; Harlan, Indianapolis, IN) were sacrificed while under isoflurane anesthesia, and after thoracotomy the descending thoracic aorta was dissected, cut into 3- to 5-mm-long segments, and mounted on a four-channel wire Myograph 610 (DMT, Copenhagen, Denmark) under 1.5 g of passive tension. For endothelium-denuded vessels, the endothelium was removed by gently rubbing the vessel interior with a toothpick with a wet cotton swab. Removal of the endothelium was confirmed by the absence of acetylcholine relaxation in vascular strips precontracted with submaximal concentrations of phenylephrine. The rings were equilibrated for 80 min in Krebs-Henseleit solution (pH 7.4) and oxygenated with carbogen (95% O2 and 5% CO2) with at least three buffer changes every 20 min. All force measurements were recorded using a PowerLab/400 data acquisition system and LabChart software. After equilibration, the rings were preconstricted with 60 mM K+ to determine the 100% contractile response. The buffer was then replaced, and the rings were treated with 100 nM phenylephrine to achieve submaximal contraction. After stabilization, the agonists (CN2-Cbi or vitamin B12 and BAY41-2272) were added cumulatively, and changes in isometric tension were recorded and used to determine percentage of relaxation of maximal phenylephrine-induced contraction.
Statistical Analysis.
Results are expressed as means ± S.D., unless indicated otherwise. Nonlinear regression, calculation of EC50, and statistical analysis were performed using GraphPad Prism (GraphPad Software Inc., San Diego, CA). One-way analysis of variance followed by Turkey's post hoc test was used for multiple comparisons. Two-way analysis of variance followed by a Bonferroni post hoc test was used for comparison of the dose-response curves. p < 0.05 was considered significant.
Results
Screening for sGC Activators.
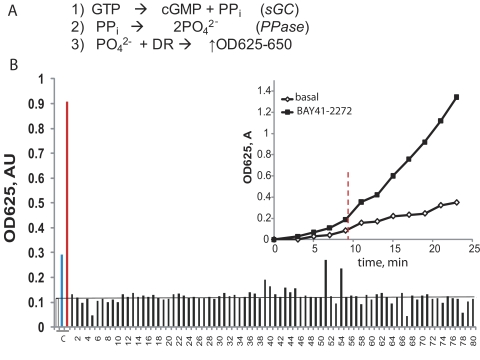
To identify new potential NO-independent regulators of sGC, we established a high-throughput assay that evaluated the activity of sGC preincubated with a library of marketed drugs and natural compounds. The assay quantifies the amount of pyrophosphate released in a cGMP-forming reaction. The principle of this assay has been described previously (Sousa et al., 2006) and is presented in Fig. 1A. In short, using a mixture of recombinant sGC and inorganic pyrophosphatase, the inorganic pyrophosphatase molecule generated by sGC is converted into a phosphate ion, which is then detected using the established Fiske-SubbaRow colorimetric assay. The protocol was optimized to detect the amount of phosphate generated by sGC in the absence of activators (basal activity) and to observe the increase in response to the treatment with the sGC stimulator BAY41-2272 (Fig. 1B, inset) or sGC activator BAY58-2667 in the presence of ODQ. A library of “off-patent” drugs from the Prestwick Chemical Library (ChemBridge Corporation, San Diego, CA) containing 1120 marketed drugs, as well as a selected collection of commercially available vitamins and their derivatives, was screened for sGC activation. The results from one representative 96-well plate are shown in Fig. 1B. In the primary screen, 18 compounds were found to be positive. This colorimetric assay may be affected by a number of factors, e.g., the presence of phosphate ion in the tested compound (e.g., compounds 51 and 54 in Fig. 1B), intrinsic absorbance of tested pharmacophores, or their reaction with the developing reagent. Thus, we validated the activation of sGC by the initial 18 compounds in a secondary screen using the [α-32P]GTP → [32P]cGMP conversion assay, which is not affected by the factors mentioned above.
Fig. 1.
Screening for sGC activators. A, the principle of the coupled sGC/pyrophosphatase assay: PO42− ion produced after the conversion of GTP into cGMP and phosphate (reactions 1 and 2) is quantified by the reaction with a malachite green/molybdate mixture (DR) and detected by absorbance at 630 nm (reaction 3). B, colorimetric detection of PO42− produced in the assay after incubation with different library pharmacophores. The reaction mixture was pretreated with 100 μM pharmacophores from the ChemBridge library (■) before addition of 200 μM GTP. Representative data from one screening plate with 80 pharmacophores are shown. The values were compared with control samples (C) in which sGC was treated with the vehicle (□), 5 μM BAY41-2272 (blue bar), or a mixture of 10 μM ODQ and 100 nM BAY58-2667 (red bar). Inset, the assay is linear over a broad time range, and the difference between basal and BAY41-2272-stimulated sGC activity is significant after 9 min (vertical line). Data are means ± S.D. of two independent measurements performed in duplicate. PPi, inorganic pyrophosphatase; AU, absorbance unit.
Activation of Purified sGC by Vitamin B12 and Related Cobinamides.
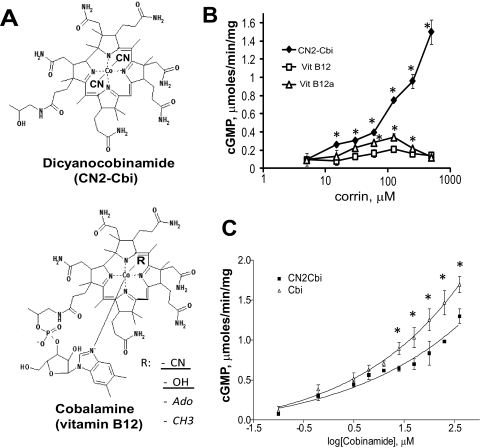
The secondary screening confirmed two of the initial candidates. This screening revealed that the corrinoid CN2-Cbi consistently activates sGC. Cobinamides are the penultimate step in the synthesis of vitamin B12 (cobalamin) (Kräutler, 2005), constituting less than 1% of the total corrin synthesized by bacteria. As shown in Fig. 2A, dicyanocobinamide contains four pyrrole rings connected into a corrin macrocycle typical for vitamin B12. Unlike all forms of vitamin B12, cobinamides do not contain the dimethylbenzimidazole moiety coordinating the cobalt ion in the center of the corrin macrocycle (Fig. 2A). We found that CN2-Cbi activated sGC over a broad range of concentrations, although no saturation was observed even at 500 μM (Fig. 2B). In addition, we tested several forms of vitamin B12. Neither 5′-deoxyadenosylcobalamin, the cofactor for methylmalonyl CoA mutase, nor methylcobalamin, the cofactor for 5-methyltetrahydrofolate-homocysteine methyltransferase, showed any sGC-activating properties (data not shown). However, both B12 vitamers, the cyanocobalamin and the hydroxylcobalamin (see structure in Fig. 2A, bottom panel) showed modest sGC activation with a bell-shaped response curve. A maximal 3- and 5-fold activation, respectively, for cyanocobalamin and hydroxocobalamin was observed (Fig. 2B).
Fig. 2.
Effect of CN2-Cbi and vitamin B12 on sGC activity. A, structural formulae of dicyanocobinamide (top panel) and vitamin B12 or B12-containing cofactors (bottom panel). Underlined ligands are for the B12 vitamer, and italicized ligands are for the cofactors. B, concentration-response curve to dicyanocobinamide (♦), cyanocobalamin (Vit B12, □), and hydroxycobalamin (Vit B12a, ▵) determined using the [α-32P]GTP assay. Data are means ± S.D. of three independent experiments performed in triplicate. *, p < 0.05 versus vitamin B12. C, comparison of concentration-response curve to dicyanocobinamide (♦) and cobalamine (▵). Data are means ± S.D. of two independent experiments performed in triplicate. *, p < 0.05.
Because cobalt ion in the corrin macrocycle of CN2-Cbi is coordinated by two cyano moieties, we tested how these moieties affect sGC activation. The cyano groups are labile under acidic conditions, whereas argon purging of the reaction evacuates the HCN formed in solution. Using this approach, we generated the cobinamide form (Cbi) lacking the cyano groups. UV-visible spectra of the sample before and after treatment show a complete conversion of CN2-Cbi, with three characteristic peaks at 367, 540, and 578 nm, into a new cobinamide, with characteristic peaks at 360, 515, and 549 nm (Supplemental Fig. 1). We then compared the dose-dependent activation of sGC by Cbi or CN2-Cbi and found that Cbi compound lacking cyano groups is a slightly better sGC activator (Fig. 2C). However, in the absence of a bound ligand, the cobalt(II) ion scavenges NO (Broderick et al., 2005), limiting its application as an effective sGC regulator. To avoid the possible artifacts associated with NO scavenging, we focused our work on dicyanocobinamide, which binds NO poorly because all ligating positions of the cobalt(II) ion are occupied.
CN2-Cbi-Dependent Activation Is Not Affected by sGC Heme Status.
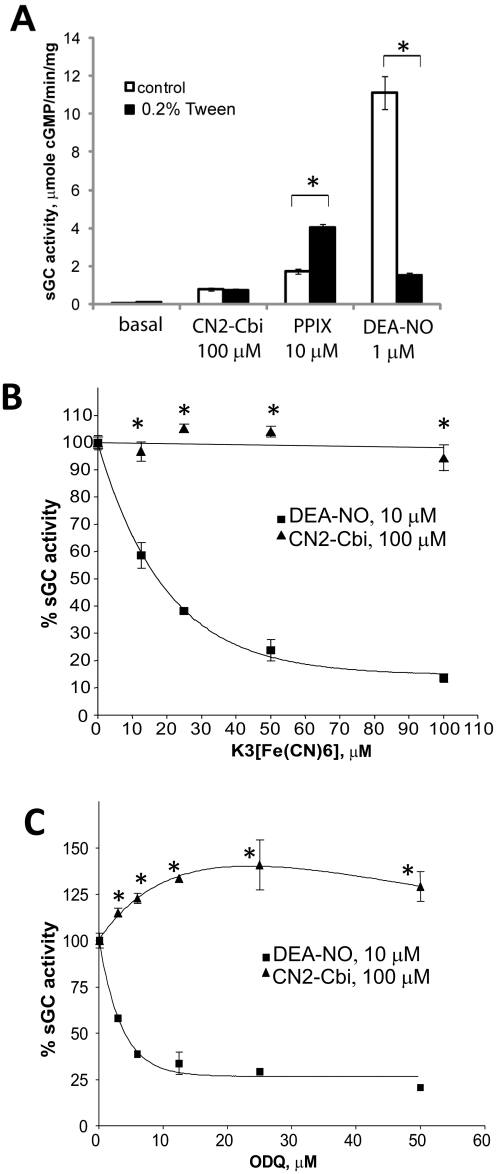
The structure of the corrin macrocycle of CN2-Cbi is reminiscent of that of the porphyrin macrocycle of the heme moiety. We thus tested how changes in sGC heme status affect the activation of sGC by CN2-Cbi. We first tested whether the removal of heme from the enzyme affects the activation by CN2-Cbi. We facilitated the depletion of heme by treating sGC enzyme with 0.2% Tween 20, according to previously described protocols (Foerster et al., 1996; Martin et al., 2003). This treatment resulted in significant heme depletion, reflected in the substantial decrease in sGC activation by NO donor DEA-NO and enhancement of protoporphyrin IX-dependent activation (Fig. 3A). However, CN2-Cbi-dependent activation was not affected by the treatment with Tween 20, suggesting a heme-independent mechanism of sGC regulation. We also tested how oxidation of sGC heme affects CN2-Cbi-dependent activation. We evaluated sGC activity in the presence of 100 μM CN2-Cbi and increasing concentrations of two different heme-oxidizing agents. To convert sGC heme into the ferric form, we used a nonspecific heme oxidizer, potassium ferricyanide, or ODQ, a sGC-specific inhibitor, which also acts by oxidizing sGC heme (Garthwaite et al., 1995). As expected, both agents dose dependently inhibited sGC activation by NO (Fig. 3, B and C, ■). On the contrary, CN2-Cbi-dependent activation was not affected even at high concentrations of these agents (Fig. 3,B and C, ▴), and even a slight activation (∼25%) by ODQ was observed in the presence of CN2-Cbi.
Fig. 3.
Heme depletion or oxidation does not affect CN2-Cbi-dependent activation of sGC. A, human recombinant sGC was incubated 0.2% Tween 20 for 20 min at room temperature and then tested on sGC activation by CN2-Cbi, protoporphyrin IX (PPIX), and DEA-NO (*, p < 0.05). In a separate experiment, sGC was incubated with the indicated concentrations of heme-oxidizing agents potassium ferricyanide (B) or ODQ (C) before measurement of activation by 10 μM DEA-NO (■) or 100 μM CN2-Cbi (▴). Data are means ± S.D. of three (ODQ) or two (K3[Fe(CN)6]) independent experiments performed in duplicate. Activity in the absence of oxidizing agents was assumed to be 100% (15.9 ± 0.2 and 0.8 ± 0.1 μmol · min−1 · mg−1 for NO and CN2-Cbi, respectively).
CN2-Cbi Activation Is Not Mediated by the Regulatory Region of sGC.
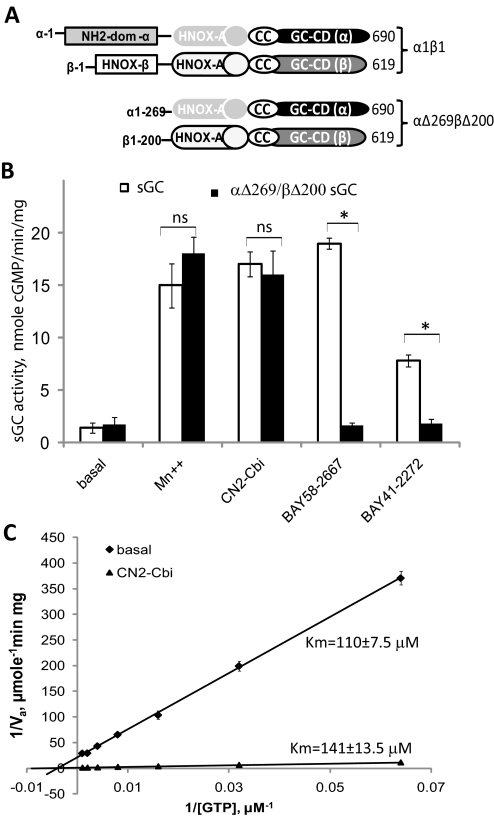
To further understand the mechanism of CN2-Cbi action, we tested whether the N-terminal regions of the α and β subunits are required for activation. We generated a recombinant enzyme that lacks both the N-terminal 269 residues of the α1 subunit and the N-terminal 200 residues of the β1 subunit (Fig. 4A). The lysate of Sf9 cells expressing truncated α1Δ269 and β1Δ200 subunits exhibits the same level of cGMP-forming activity as the lysate with full-length subunits. Moreover, the activity of truncated sGC was enhanced by Mn2+ cofactor to the same extent as for the wild-type enzyme (Fig. 4B). We also found that the activity of the αΔ269/βΔ200 sGC was not affected by BAY58-2667 activator or BAY41-2272 stimulator, confirming that N-terminal elements of sGC are necessary for regulation by these activators. However, the αΔ269/βΔ200 sGC was activated by CN2-Cbi to the same extent as the wild-type sGC (Fig. 4B). Thus, unlike for all known sGC activators, the truncated sGC lacking the N-terminal regulatory regions contains the structural determinants both necessary and sufficient for activation by CN2-Cbi.
Fig. 4.
CN2-Cbi does not require the sGC regulatory region and does not decrease GTP Km. A, schematic representation of the domain structure of the wild-type α1β1 and the truncated αΔ269βΔ200 sGC. The domain structure is adapted from Ma et al. (2008). Coil-coil elements (CC) and the HNOX-associated PAS-like domain (HNOX-A) are involved in dimerization. The heme-NO/oxygen-binding domain of the β subunit (HNOX-β) binds heme. GC-CD, guanylyl cyclase catalytic domains; NH2-dom-α, amino-terminal domain of the α subunit. B, cGMP-forming activity in 100,000g supernatants of Sf9 cells expressing wild-type (□) or truncated αΔ269/βΔ200 (■) sGC was tested under basal conditions in the presence of 4 mM Mn2+, 100 μM CN2-Cbi, 40 nM BAY58-2667, or 2 μM BAY41-2272. Data are presented as means ± S.D. of two independent preparations assayed in triplicate. ns, difference is not statistically significant; *, p < 0.05. C, effect of CN2-Cbi on the GTP Km. Lineweaver-Burk double-reciprocal plots of the basal (♦) and 100 μM CN2-Cbi (▴)-stimulated sGC activity (Va) are shown. sGC activity was determined using the 7.25 to 2000 μM range of GTP concentrations. Summarized data of two independent experiments performed in triplicate are shown.
Effect of CN2-Cbi on Km for GTP.
Previous studies demonstrated that activation by NO and some allosteric sGC stimulators decreases the Km for GTP as part of their activation mechanism (Denninger et al., 2000; Schmidt et al., 2003). We thus tested whether a similar effect is observed for CN2-Cbi. We found that CN2-Cbi, in fact, slightly increased the Km for GTP from 110 ± 7.5 to 141 ± 13.7 μM (Fig. 4C), suggesting that CN2-Cbi activates sGC primarily through increased Vmax.
CN2-Cbi Enhances sGC Activation by NO-Independent Regulators but Not Nitric Oxide.
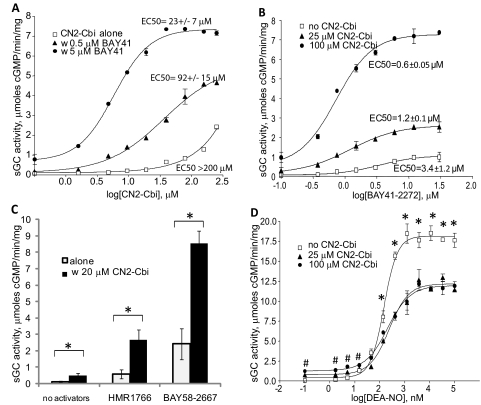
Because CN2-Cbi targets a site different from that for all other sGC regulators, we investigated whether CN2-Cbi displays any cooperative activation with other sGC regulators. As shown in Fig. 5A, CN2-Cbi activation was strongly enhanced by BAY41-2272. Some combinations of CN2-Cbi and BAY41-2272 resulted in sGC activity, which was only 2-fold lower than maximal sGC activation observed with concentrations of the NO donor DEA-NO (7.5 μmol of cGMP · min−1 · mg−1 versus 18.1 μmol of cGMP · min−1 · mg−1, respectively). Both CN2-Cbi and BAY41-2272 reciprocally improved their EC50 values. Even at a 0.5 μM concentration, BAY41-2272 shifted the CN2-Cbi activation curve to the left, suggesting a synergistic enhancement of the CN2-Cbi effect (Fig. 5A). In contrast, CN2-Cbi enhanced the activation of sGC by various concentrations of BAY41-2272 and decreased the EC50 for BAY41-2272 (Fig. 5B). A similar enhancement was observed with the Cbi cobinamide lacking the cyano moieties (Supplemental Fig. 2). However, when the B12 vitamers (methyl- and adenosylcobalamin) were used instead of CN2-Cbi, no significant synergy with BAY41-2272 was observed (data not shown).
Fig. 5.
CN2-Cbi synergistically enhances sGC activation by NO-independent regulators, but not by nitric oxide. A, concentration-response curve to CN2-Cbi alone (□) or in combination with 0.5 (▴) or 5 μM (●) BAY41-2272. Data are means ± S.D. from four independent measurements performed in triplicate. B, concentration-response curve to BAY41-2272 alone (□) or in the presence of 25 (▴) or 100 μM (●) CN2-Cbi. Data are means ± S.D. of two independent measurements performed in triplicate. C, 20 μM CN2-Cbi synergistically enhances the stimulatory effect of submaximal concentration of heme-independent regulator HMR1766 (200 nM) and BAY58-2667 (20 nM). *, p < 0.05. D, concentration-response curve to DEA-NO alone (□) or in combination with 25 (●) or 100 (▴) μM CN2-Cbi. Data are means ± S.D. of two independent experiments performed in triplicate. *, p < 0.05, control versus CN2-Cbi; #, p < 0.05, 100 μM CN2-Cbi versus control.
CN2-Cbi interacted synergistically not only with the heme-dependent BAY41-2272 but also with heme-independent sGC activators. As shown in Fig. 5C, coadministration of CN2-Cbi with submaximal doses of BAY58-2667 or HMR1766 synergistically potentiated the effect of these activators.
The synergistic enhancement of sGC by CN2-Cbi was not observed, however, when sGC was activated by NO. As shown in Fig. 5D, CN2-Cbi slightly increased the observed EC50 for the NO donor DEA-NO (EC50 of 0.15 ± 0.001 μM versus 0.22 ± 0.01 μM), and decreased the maximal sGC activity at saturating NO concentrations from 18.1 ± 0.3 to 12.1 ± 0.38 μmol · min−1 · mg−1.
Synergistic Effect of CN2-Cbi and BAY41-2272 in Intact Cells and Isolated Aorta.
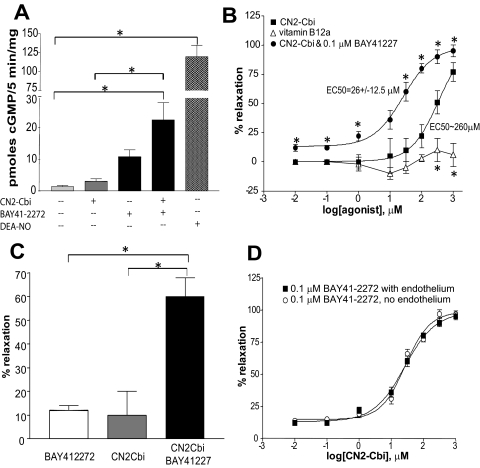
We tested whether CN2-Cbi activates sGC in intact breast cancer MDA468 cells, previously shown to express functional sGC (Mujoo et al., 2010). An increased intracellular cGMP level was observed when these cells were exposed to 100 μM CN2-Cbi (Fig. 5A). The effect of CN2-Cbi was synergistically potentiated by BAY41-2272, suggesting that elevated cGMP is mediated by sGC.
We also tested whether CN2-Cbi-dependent activation has any vasodilatory effects on preconstricted isolated rat aortic rings. As shown in Fig. 5B, CN2-Cbi elicited a concentration-dependent relaxation of rat aortic rings precontracted with 0.1 μM phenylephrine, whereas vitamin B12 (hydroxylcobalamin) did not have any significant vasodilatory effects at tested concentrations. Consistent with in vitro data, cotreatment with 0.1 μM BAY 41-2272 significantly enhanced the vasodilatory effect of CN2-Cbi and decreased the observed EC50 for CN2-Cbi (264 ± 40.5 μM versus 26 ± 12.5 μM). As shown in Fig. 5C, combined treatment with submaximal doses of BAY41-2272 (0.1 μM) and CN2-Cbi (30 μM) induced a much stronger vasodilation than either of the compounds alone at the same dose. Combined treatment with hydroxylcobalamin or B12 cofactors and BAY41-2272 did not show any synergistic effect (data not shown).
Previous studies demonstrated that vitamin B12 and its derivatives react efficiently with superoxide, carrying out a superoxide dismutase-like reaction (Suarez-Moreira et al., 2009). This report suggests that the observed vasodilatory effect of CN2-Cbi may be due to increased bioavailability of NO as a result of a decreased level of superoxide, which is an effective NO scavenger. To test whether the effect of CN2-Cbi may be due to a changed level of NO, we denuded the endothelium, which is the primary source of NO in the vessel. As demonstrated in Fig. 6D, the vasoactive effect of CN2-Cbi was identical in endothelium-competent and endothelium-denuded rings, consistent with direct NO-independent activation of sGC.
Fig. 6.
CN2-Cbi-dependent effect in intact cells and isolated aortic rings. A, levels of intracellular cGMP in MDA468 breast cancer cells after a 5-min exposure to the vehicle (0.1% DMSO), 100 μM CN2-Cbi, 2 μM BAY41-2272, a combination of 100 μM CN2-Cbi and 2 μM BAY41-2272, or 100 μM DEA-NO. B, concentration-dependent relaxation of phenylephrine-contracted rat aortic rings in response to vitamin B12 (▵) or CN2-Cbi alone (■) or in combination with 0.1 μM BAY41-2272 (▾). Data are means ± S.E.M. of four aortic rings obtained from two rats per each treatment. *, p < 0.05 versus CN2-Cbi. C, combined treatment with submaximal doses of BAY41-2272 (0.1 μM) and CN2-Cbi (30 μM CN2-Cbi) shows a synergistic effect in comparison to individual treatments with the same doses. *, p < 0.05. D, relaxation of endothelium-competent (■) and endothelium-denuded (○) precontracted aortic rings in response to various concentrations of CN2-Cbi in combination with 0.1 μM BAY41-2272. Data are means ± S.E.M. of three aortic rings obtained from three different rats.
Discussion
In this report, we determined that cobinamides have sGC-stimulating properties and act through a novel mechanism of sGC activation. Because ligand-free cobinamides may also act as NO scavengers (Broderick et al., 2005), we focused our studies on dicyanocobinamide, which does not bind NO.
We found that CN2-Cbi dose dependently activates sGC, whereas the removal of the cobalt-coordinating cyano groups results in slightly better potency, although in both cases no saturation was achieved when substances were used alone (Fig. 2C). Testing the effect of naturally occurring corrinoids on sGC activity provided some additional structural information. The B12 vitamins show low sGC activation, most likely due to the bulky dimethylbenzimidazole moiety, which hinders the interaction with sGC (Fig. 2B). Moreover, vitamin B12-based cofactors, adenosylcobalamin and methylcobalamin, with larger or less labile ligands, have no sGC activating properties (data not shown). These data indicate that sGC activation is stronger when one coordinating position is not occupied or the ligand is labile, suggesting that sGC coordinates the cobalt ion as part of the activation process. Although NO scavenging by ligand-free cobinamides limits their use as sGC regulators, future structure-activity studies may identify labile ligands offering sGC activation characteristics superior to those of the cyano groups of CN2-Cbi.
We demonstrate that CN2-Cbi is an NO-independent sGC activator with an activation mechanism different from that of all known sGC regulators. The corrin macrocycle resembles the macrocycle of protoporphyrin IX, which activates sGC by heme substitution. However, the heme depletion of sGC does not affect CN2-Cbi-dependent activation (Fig. 3A), opposite to protoporphyrin IX-dependent sGC activation. The lack of a significant functional effect of heme oxidation on CN2-Cbi-dependent activation is also informative. Oxidation of sGC heme significantly decreases sGC activation by YC-1 (benzylindazole), pyrazolopyridines [BAY41-2272 and BAY41-8543 (2[1(2fluorobenzyl]1Hpyrazolo [3,4b] pyridin3yl]5morpholin4yl pyrimidine4,6diamine)] or aryl-acrylamide stimulators or by NO (Hoenicka et al., 1999; Martin et al., 2001; Stasch et al., 2001). On the contrary, the effect of NO-independent sGC regulators, such as cinaciguat (BAY58-2667) and ataciguat (HMR1766), is robustly enhanced by sGC heme oxidation (Stasch et al., 2002; Schindler et al., 2006). In contrast to these sGC regulators, oxidation of sGC heme by ODQ or ferricyanide does not affect the extent of CN2-Cbi-dependent activation of sGC (Fig. 3), even at concentrations shown to fully oxidize sGC heme (Garthwaite et al., 1995) and abolish sGC activation by the NO donor (Fig. 3). These data point to a different mechanism of sGC activation.
Functional analysis of truncated sGC (Fig. 4) confirmed this conclusion. The N-terminal regions of sGC compose the regulatory domain of the enzyme. The heme moiety binds to the β HNOX domain (Derbyshire and Marletta, 2009). Mechanistic and structural studies demonstrate that both stimulators (Denninger et al., 2000; Derbyshire et al., 2009) and activators (Schmidt et al., 2004; Martin et al., 2010) target the β HNOX domain. Functional and cross-linking evidence also suggests that the N-terminal region of the α subunit may be involved in the binding or mediation of activation by stimulators YC-1 and BAY41-2272 (Stasch et al., 2001; Koglin and Behrends, 2003). To test whether the CN2-Cbi-dependent activation is also mediated by the N-terminal domains, we deleted these regulatory regions, leaving intact the HNOX-A and CC regions responsible for dimerization (Zhou et al., 2004; Ma et al., 2008) and both C-terminal regions that constitute the sGC catalytic domain (Wedel et al., 1995). As demonstrated in Fig. 4B, the αΔ269/βΔ200 sGC was activated by CN2-Cbi to the same extent as the full-length sGC. Because the αΔ269/βΔ200 truncated sGC contains only structural elements required for heterodimerization and catalysis, it is most likely that CN2-Cbi directly affects the function of the catalytic domain, although it does not decrease Km for GTP (Fig. 4C). The catalytic domain of sGC is homologous to that of adenylyl cyclase, which is also composed of two catalytic regions. Adenylyl cyclase is potently activated by the small molecule forskolin, which binds to one of the two catalytic regions and facilitates their interaction (Whisnant et al., 1996). Although the existence of a similar small molecule, which may activate sGC by binding to the catalytic domain, has been postulated previously (Friebe et al., 1999), CN2-Cbi is the first molecule that matches this criterion. It remains to be determined whether CN2-Cbi-dependent activation is indeed mediated by the region of the catalytic domain orthologous to the forskolin-binding site.
Our data also demonstrate that, by engaging the new regulatory site, CN2-Cbi synergistically enhances the effect of NO-independent sGC regulators acting through the heme-binding domain. A synergistic interaction is observed between CN2-Cbi and stimulator BAY41-2272, because EC50 values for both CN2-Cbi and BAY41-2272 decrease when the compounds are combined to stimulate sGC (Figs. 5, A and B, and 6C). Likewise, submaximal activation of sGC by the heme-replacing activators BAY58-2667 and HMR1766 is substantially enhanced by CN2-Cbi (Fig. 5C). Moreover, the binding of ODQ to the heme domain not only oxidizes sGC heme but probably induces minor conformational changes weakly activating the basal state (Fig. 3C). This activation is probably enhanced by CN2-Cbi bound to the catalytic region. However, NO-dependent activation was moderately inhibited by CN2-Cbi, which slightly increased the EC50 for DEA-NO and decreased maximal NO-dependent sGC activation (Fig. 5D). Although the cyano moieties are labile at low pH, a small percentage of Cbi lacking the cyano groups may form and persist even at neutral pH. The NO-scavenging properties of Cbi may explain the slight increase in DEA-NO EC50 and the diminished response to NO in the presence of CN2-Cbi. As an alternative, by binding to the catalytic region, CN2-Cbi may partially obstruct the optimal interaction between the regulatory and catalytic regions induced by NO. Future experiments will test these hypotheses.
CN2-Cbi-dependent stimulation of sGC is not only limited to purified sGC but also occurs in intact cells (Fig. 6A). Moreover, we demonstrated that CN2-Cbi acts as a vasorelaxant for phenylephrine-preconstricted rat aortic rings both in the presence and absence of endothelium (Fig. 6, B–D). Consistent with in vitro data, coadministration of BAY41-2272 significantly enhanced the effect of CN2-Cbi both in cells and on isolated rings. These data indicate that CN2-Cbi may be a prototype for a novel type of sGC-regulating therapeutic agent. It is unlikely that physiological concentrations of circulating cobinamides (<30 pM) or B12 vitamins (∼300 pM) (Hardlei and Nexo, 2009) have any effects on sGC function. Because of high affinity to cyano groups, cobinamides are used for cyanide detoxification (Broderick et al., 2006) and doses of 800 mg/kg are deemed safe for laboratory animals (Brenner et al., 2010). Previous studies reported that nitrosyl-cobinamide functions as an NO donor (Broderick et al., 2007), increasing vasodilation and vasodilator-stimulated phosphoproteinphosphorylation, presumably through NO-dependent activation of sGC. Data presented in the current report suggest that some of these effects may be, in fact, due to the direct NO-independent activation of sGC by the cobinamide macrocycle itself.
Existing NO-independent activators were shown to be effective in a variety of experimental animal models and have therapeutic potential in a range of cardiovascular and noncardiovascular disorders, such as arterial and pulmonary hypertension, peripheral arterial disease, heart failure, renal fibrosis, erectile dysfunction, peripheral arterial occlusive disease, atherosclerosis, restenosis and thrombosis, and others (for reviews, see Schmidt et al., 2009; Stasch and Hobbs, 2009; Stasch et al., 2011). However, the mechanisms of action of these sGC regulators impose some limitations for their use in pathological conditions associated with increased oxidative stress. Previous studies reported that even under normal conditions, some sGC molecules are insensitive to NO because of heme oxidation or loss, and the fraction of oxidized sGC increases in disease conditions associated with oxidative stress (Stasch et al., 2006). Thus, NO-independent stimulators (pyrazolopiridines or acrylamides) are less effective under oxidative conditions because of the strict requirement for ferrous heme. On the contrary, the NO-independent activators are more effective with heme-oxidized or heme-free sGC but are less potent with heme-competent sGC. Because CN2-Cbi does not require a heme-binding domain, it is likely that its pharmaceutical effectiveness will be less susceptible to changes in sGC heme content or oxidation status triggered by oxidative stress. Our in vitro measurements demonstrate that CN2-Cbi synergistically enhanced the effects of all tested NO-independent sGC regulators and may be used in combination with these agents. Future studies of acute and chronic administration of CN2-Cbi alone or in combination with other sGC regulators will test whether the observed in vitro effects of CN2-Cbi translate into vasoactive in vivo effects in intact animals.
Relatively high effective concentrations of CN2-Cbi in our studies with intact cells and isolated aortic rings indicate that in its present form CN2-Cbi is of limited therapeutic application. However, CN2-Cbi may serve as a prototype that can be modified in the future to improve its effective concentrations for sGC activation in vitro and regulation of its function in vivo.
In summary, we identified cobinamides as novel NO-independent sGC coactivators targeting the catalytic region. CN2-Cbi may be a useful tool to understand the processes leading to the activation of sGC catalytic function. The unique mechanism of action, which does not overlap with that of other sGC regulators, offers possibilities to develop CN2-Cbi or its derivatives into sGC-targeting therapeutic agents used alone or in combination with other sGC regulators to enhance their therapeutic benefits.
Supplementary Material
Acknowledgments
We thank Dr. Elena Bogatenkova for technical help with Sf9 cultures and lysate preparation.
This work was supported by the National Institutes of Health National Heart, Lung, and Blood Institute [Grants HL088128, 3R01-HL088128]; and the American Heart Association, South Central Affiliate [Grant-in-Aid 09GRNT2060182].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
 The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
The online version of this article (available at http://jpet.aspetjournals.org) contains supplemental material.
- NO
- nitric oxide
- sGC
- soluble guanylyl cyclase
- HNOX
- heme nitric oxide and oxygen
- BAY58-2667
- cinaciguat
- HMR1766
- ataciguat
- DEA
- 2,2-diethyl-1-nitroso-oxthydrazine
- DMSO
- dimethyl sulfoxide
- TEA
- tetraethylammonium
- BAY41-2272
- 3-(4-amino-5-cyclopropylpyrimidine-2-yl)-1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine
- ODQ
- 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- CN2-Cbi
- dicyanocobinamide
- Cbi
- cobinamide
- YC-1
- benzylindazole
- BAY41-8543
- 2[1(2fluorobenzyl]1Hpyrazolo [3,4b] pyridin3yl]5morpholin4yl pyrimidine4,6diamine.
Authorship Contributions
Participated in research design: Sharina, Gryko, and Martin.
Conducted experiments: Sharina, Sobolevsky, Doursout, and Martin.
Performed data analysis: Sharina, Gryko, and Martin.
Wrote or contributed to the writing of the manuscript: Sharina and Martin.
References
- Brenner M, Kim JG, Mahon SB, Lee J, Kreuter KA, Blackledge W, Mukai D, Patterson S, Mohammad O, Sharma VS, et al. (2010) Intramuscular cobinamide sulfite in a rabbit model of sublethal cyanide toxicity. Ann Emerg Med 55:352–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick KE, Alvarez L, Balasubramanian M, Belke DD, Makino A, Chan A, Woods VL, Jr, Dillmann WH, Sharma VS, Pilz RB, et al. (2007) Nitrosyl-cobinamide, a new and direct nitric oxide releasing drug effective in vivo. Exp Biol Med (Maywood) 232:1432–1440 [DOI] [PubMed] [Google Scholar]
- Broderick KE, Potluri P, Zhuang S, Scheffler IE, Sharma VS, Pilz RB, Boss GR. (2006) Cyanide detoxification by the cobalamin precursor cobinamide. Exp Biol Med (Maywood) 231:641–649 [DOI] [PubMed] [Google Scholar]
- Broderick KE, Singh V, Zhuang S, Kambo A, Chen JC, Sharma VS, Pilz RB, Boss GR. (2005) Nitric oxide scavenging by the cobalamin precursor cobinamide. J Biol Chem 280:8678–8685 [DOI] [PubMed] [Google Scholar]
- Denninger JW, Schelvis JP, Brandish PE, Zhao Y, Babcock GT, Marletta MA. (2000) Interaction of soluble guanylate cyclase with YC-1: kinetic and resonance Raman studies. Biochemistry 39:4191–4198 [DOI] [PubMed] [Google Scholar]
- Derbyshire ER, Fernhoff NB, Deng S, Marletta MA. (2009) Nucleotide regulation of soluble guanylate cyclase substrate specificity. Biochemistry 48:7519–7524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derbyshire ER, Marletta MA. (2009) Biochemistry of soluble guanylate cyclase. Handb Exp Pharmacol 191:17–31 [DOI] [PubMed] [Google Scholar]
- Foerster J, Harteneck C, Malkewitz J, Schultz G, Koesling D. (1996) A functional heme-binding site of soluble guanylyl cyclase requires intact N-termini of α1 and β1 subunits. Eur J Biochem 240:380–386 [DOI] [PubMed] [Google Scholar]
- Friebe A, Russwurm M, Mergia E, Koesling D. (1999) A point-mutated guanylyl cyclase with features of the YC-1-stimulated enzyme: implications for the YC-1 binding site? Biochemistry 38:15253–15257 [DOI] [PubMed] [Google Scholar]
- Friebe A, Schultz G, Koesling D. (1996) Sensitizing soluble guanylyl cyclase to become a highly CO-sensitive enzyme. EMBO J 15:6863–6868 [PMC free article] [PubMed] [Google Scholar]
- Garthwaite J, Southam E, Boulton CL, Nielsen EB, Schmidt K, Mayer B. (1995) Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. Mol Pharmacol 48:184–188 [PubMed] [Google Scholar]
- Hardlei TF, Nexo E. (2009) A new principle for measurement of cobalamin and corrinoids, used for studies of cobalamin analogs on serum haptocorrin. Clin Chem 55:1002–1010 [DOI] [PubMed] [Google Scholar]
- Hoenicka M, Becker EM, Apeler H, Sirichoke T, Schröder H, Gerzer R, Stasch JP. (1999) Purified soluble guanylyl cyclase expressed in a baculovirus/Sf9 system: stimulation by YC-1, nitric oxide, and carbon monoxide. J Mol Med 77:14–23 [DOI] [PubMed] [Google Scholar]
- Koesling D, Friebe A. (1999) Soluble guanylyl cyclase: structure and regulation. Rev Physiol Biochem Pharmacol 135:41–65 [DOI] [PubMed] [Google Scholar]
- Koglin M, Behrends S. (2003) A functional domain of the alpha1 subunit of soluble guanylyl cyclase is necessary for activation of the enzyme by nitric oxide and YC-1 but is not involved in heme binding. J Biol Chem 278:12590–12597 [DOI] [PubMed] [Google Scholar]
- Kräutler B. (2005) Vitamin B12: chemistry and biochemistry. Biochem Soc Trans 33:806–810 [DOI] [PubMed] [Google Scholar]
- Ma X, Sayed N, Baskaran P, Beuve A, van den Akker F. (2008) PAS-mediated dimerization of soluble guanylyl cyclase revealed by signal transduction histidine kinase domain crystal structure. J Biol Chem 283:1167–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E, Czarnecki K, Jayaraman V, Murad F, Kincaid J. (2005) Resonance Raman and infrared spectroscopic studies of high-output forms of human soluble guanylyl cyclase. J Am Chem Soc 127:4625–4631 [DOI] [PubMed] [Google Scholar]
- Martin E, Lee YC, Murad F. (2001) YC-1 activation of human soluble guanylyl cyclase has both heme-dependent and heme-independent components. Proc Natl Acad Sci USA 98:12938–12942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin E, Sharina I, Kots A, Murad F. (2003) A constitutively activated mutant of human soluble guanylyl cyclase (sGC): implication for the mechanism of sGC activation. Proc Natl Acad Sci USA 100:9208–9213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin F, Baskaran P, Ma X, Dunten PW, Schaefer M, Stasch JP, Beuve A, van den Akker F. (2010) Structure of cinaciguat (BAY 58-2667) bound to Nostoc H-NOX domain reveals insights into heme-mimetic activation of the soluble guanylyl cyclase. J Biol Chem 285:22651–22657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujoo K, Sharin VG, Martin E, Choi BK, Sloan C, Nikonoff LE, Kots AY, Murad F. (2010) Role of soluble guanylyl cyclase-cyclic GMP signaling in tumor cell proliferation. Nitric Oxide 22:43–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scatena R, Bottoni P, Pontoglio A, Giardina B. (2010) Pharmacological modulation of nitric oxide release: new pharmacological perspectives, potential benefits and risks. Curr Med Chem 17:61–73 [DOI] [PubMed] [Google Scholar]
- Schindler U, Strobel H, Schönafinger K, Linz W, Löhn M, Martorana PA, Rütten H, Schindler PW, Busch AE, Sohn M, et al. (2006) Biochemistry and pharmacology of novel anthranilic acid derivatives activating heme-oxidized soluble guanylyl cyclase. Mol Pharmacol 69:1260–1268 [DOI] [PubMed] [Google Scholar]
- Schmidt HH, Schmidt PM, Stasch JP. (2009) NO- and haem-independent soluble guanylate cyclase activators. Handb Exp Pharmacol 191:309–339 [DOI] [PubMed] [Google Scholar]
- Schmidt P, Schramm M, Schröder H, Stasch JP. (2003) Mechanisms of nitric oxide independent activation of soluble guanylyl cyclase. Eur J Pharmacol 468:167–174 [DOI] [PubMed] [Google Scholar]
- Schmidt PM, Schramm M, Schröder H, Wunder F, Stasch JP. (2004) Identification of residues crucially involved in the binding of the heme moiety of soluble guanylate cyclase. J Biol Chem 279:3025–3032 [DOI] [PubMed] [Google Scholar]
- Sousa EH, Garay PA, Tinianow JN, Gerber NC. (2006) Development of a spectrophotometric assay for cyclase activity. Anal Biochem 348:57–63 [DOI] [PubMed] [Google Scholar]
- Stasch JP, Becker EM, Alonso-Alija C, Apeler H, Dembowsky K, Feurer A, Gerzer R, Minuth T, Perzborn E, Pleiss U, et al. (2001) NO-independent regulatory site on soluble guanylate cyclase. Nature 410:212–215 [DOI] [PubMed] [Google Scholar]
- Stasch JP, Dembowsky K, Perzborn E, Stahl E, Schramm M. (2002) Cardiovascular actions of a novel NO-independent guanylyl cyclase stimulator, BAY 41–8543: in vivo studies. Br J Pharmacol 135:344–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasch JP, Hobbs AJ. (2009) NO-independent, haem-dependent soluble guanylate cyclase stimulators. Handb Exp Pharmacol 191:277–308 [DOI] [PubMed] [Google Scholar]
- Stasch JP, Pacher P, Evgenov OV. (2011) Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation 123:2263–2273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasch JP, Schmidt PM, Nedvetsky PI, Nedvetskaya TY, H S AK, Meurer S, Deile M, Taye A, Knorr A, Lapp H, et al. (2006) Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J Clin Invest 116:2552–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Moreira E, Yun J, Birch CS, Williams JH, McCaddon A, Brasch NE. (2009) Vitamin B12 and redox homeostasis: cob(II)alamin reacts with superoxide at rates approaching superoxide dismutase (SOD). J Am Chem Soc 131:15078–15079 [DOI] [PubMed] [Google Scholar]
- Sunahara RK, Beuve A, Tesmer JJ, Sprang SR, Garbers DL, Gilman AG. (1998) Exchange of substrate and inhibitor specificities between adenylyl and guanylyl cyclases. J Biol Chem 273:16332–16338 [DOI] [PubMed] [Google Scholar]
- Wedel B, Harteneck C, Foerster J, Friebe A, Schultz G, Koesling D. (1995) Functional domains of soluble guanylyl cyclase. J Biol Chem 270:24871–24875 [DOI] [PubMed] [Google Scholar]
- Whisnant RE, Gilman AG, Dessauer CW. (1996) Interaction of the two cytosolic domains of mammalian adenylyl cyclase. Proc Natl Acad Sci USA 93:6621–6625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Schelvis JP, Babcock GT, Marletta MA. (1998) Identification of histidine 105 in the beta1 subunit of soluble guanylate cyclase as the heme proximal ligand. Biochemistry 37:4502–4509 [DOI] [PubMed] [Google Scholar]
- Zhou Z, Gross S, Roussos C, Meurer S, Müller-Esterl W, Papapetropoulos A. (2004) Structural and functional characterization of the dimerization region of soluble guanylyl cyclase. J Biol Chem 279:24935–24943 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.