Abstract
Inhibition of insulin-like growth factor-1 receptor (IGF-1R) signaling represents an attractive therapeutic strategy for cancer treatment. A first-generation IGF-1R inhibitor (R)-4-(3-(3-chlorophenyl)-3-hydroxypropyl)-3-(4-methyl-6-morpholino-1H-benzo[d]imidazol-2-yl)pyridin-2(1H)-one (BMS-536924), however, was associated with potent CYP3A4 induction mediated by pregnane X receptor (PXR; NR1I2) transactivation. Structural activity-based modification led to the synthesis of 4-(1-(2-(4-((2-(4-chloro-1H-pyrazol-1-yl)ethyl)amino)-2-oxo-1,2-dihydropyridin-3-yl)-4-methyl-1H-benzo[d]imidazol-6-yl)piperidin-4-yl) piperazine-1-carboxylate (BMS-665351) with no PXR activity while maintaining its ability to inhibit IGF-1R. However, BMS-665351 significantly induces CYP3A4 expression in human primary hepatocytes (HPHs). Here, we report a novel nonclassical constitutive androstane receptor (CAR; NR1I3)-related pathway of BMS-665351-mediated CYP3A4 induction. BMS-665351 treatment resulted in the significant induction of CYP3A4 in HPHs and HepG2 cells, but failed to activate either PXR or CAR in cell-based reporter assays. Moreover, BMS-665351 at concentrations that induce CYP3A4 expression was unable to translocate human CAR from the cytoplasm to the nucleus of HPHs, which represents the initial step of CAR activation. Nevertheless, quantitative polymerase chain reaction analysis demonstrated that BMS-665351 significantly enhanced the expression of CYP3A4 in CAR- but not PXR-transfected HepG2 and Huh7 cells. It is noteworthy that BMS-665351 selectively induced the expression of CAR but not PXR in all tested hepatic cell systems. Synergistic induction of CYP3A4 was observed in HPHs cotreated with BMS-665351 and prototypical activators of CAR but not PXR. In summary, our results indicate that BMS-665351-mediated induction of CYP3A4 is CAR-dependent, but BMS-665351 itself is not a typical activator of either CAR or PXR, rather it functions as a selective inducer of CAR expression and increases CYP3A4 through a noncanonical CAR-related mechanism.
Introduction
Insulin-like growth factor-1 receptor (IGF-1R) signaling has been implicated in the pathophysiology of a variety of human cancers (Samani et al., 2007; Ryan and Goss, 2008). Upon agonistic ligand binding, autophosphorylated IGF-1R triggers the downstream activation of the mitogen-activated protein kinase and phosphatidylinositol 3-kinase cascades, thereby promoting cell proliferation and inhibiting programmed cell death (Baserga et al., 2003; Neudauer and McCarthy, 2003). With evidence demonstrating the overexpression and activation of IGF-1R in many different human cancers (Sarfstein et al., 2006; Tarn et al., 2008), inhibition of IGF-1R activity has been considered an attractive therapeutic strategy that targets a number of IGF-1R-positive tumors. In this effort, a number of benzimidazole-based IGF-1R inhibitors with in vivo antitumor activities have been synthesized; however, a significant limitation of these first-generation IGF-1R inhibitors is the potent induction of CYP3A4 via transactivation of the pregnane X receptor (PXR; NR1I2) (Wittman et al., 2005; Velaparthi et al., 2008). Given that CYP3A4 represents the most abundant hepatic cytochrome P450 enzyme and is responsible for approximately 60% of cytochrome-mediated metabolism of clinically used drugs (Li et al., 1995; Guengerich, 1999), perturbation in the expression and activity of this isozyme is associated with a potentially high risk of drug-drug interactions (DDIs).
Expression of hepatic CYP3A4 is highly inducible upon exposure to many xenobiotic chemicals. Receptor-mediated transcriptional activation represents the most common molecular mechanism of CYP3A4-inductive expression, and the nuclear receptor PXR has been recognized as the predominant mediator of CYP3A induction in multiple species (Blumberg et al., 1998; Kliewer et al., 1998). Transgenic and knockout animal studies firmly established the importance of PXR in chemical-mediated induction of CYP3A genes (Xie et al., 2000; Staudinger et al., 2001). Promoter analysis further demonstrated that maximal CYP3A4 induction by rifampicin (RIF) requires PXR activation of both the proximal everted repeat-6 element (PXRE; bases −172 to −149) and the distal xenobiotic responsive enhancer module (XREM; bases −7836 to −7208) located upstream of the CYP3A4 transcriptional start site (Lehmann et al., 1998; Goodwin et al., 1999). Although PXR plays a leading role in the inductive expression of CYP3A4, other nuclear receptors may also regulate CYP3A4 transcription and contribute to the overall inducible expression (Pascussi et al., 2003). The constitutive androstane receptor (CAR; NR1I3), a sister receptor of PXR, shares several target genes in common with PXR, including CYP3A4, CYP2B6, UDP-glucuronosyltransferase 1A1, and multidrug resistance 1 by recognizing and binding to common response elements identified in the promoters of these genes (Goodwin et al., 2002; Wang and Negishi, 2003; Qatanani and Moore, 2005). Moreover, PXR and CAR also share a number of xenobiotic activators, such as the sedative phenobarbital (PB), the antimalaria artemicinin, and the chemotherapeutic prodrug cyclophosphamide (Wang and LeCluyse, 2003; Wang et al., 2011), making the underlying mechanisms of CYP3A4 induction complicated.
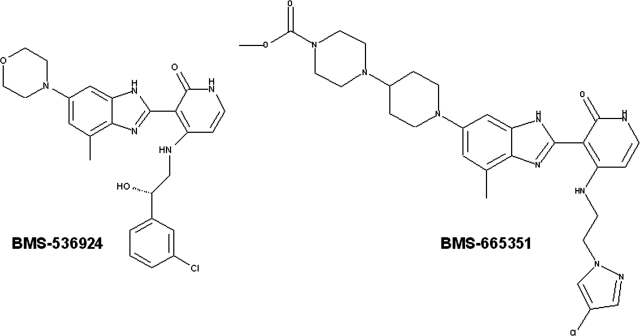
It is noteworthy that an excellent correlation exists between the abilities of drugs to activate human PXR (hPXR) and their induction of CYP3A4 expression. As such, many pharmaceutical companies have used cell-based PXR reporter assays to predict potential CYP3A4 induction at the early stages of drug development (Kim et al., 2010). To improve the absorption, distribution, metabolism, and excretion properties of the first-generation IGF-1R inhibitors and reduce CYP3A4 inducibility, a PXR-based structure-activity relationship strategy led to the synthesis of compounds with no or limited PXR transactivity while not sacrificing their activities toward IGF-1R inhibition (Zimmermann et al., 2010). To our surprise, a notable exception is 4-(1-(2-(4-((2-(4-chloro-1H-pyrazol-1-yl)ethyl)amino)-2-oxo-1,2-dihydropyridin-3-yl)-4-methyl-1H-benzo[d]imidazol-6-yl)piperidin-4-yl)piperazine-1-carboxylate (BMS-665351) (Fig. 1), which exhibits no activation of hPXR but significant induction of CYP3A4 expression in human primary hepatocyte (HPH) cultures.
Fig. 1.
Chemical structures of the first generation of the benzimidazole-based IGF-1R inhibitor BMS-536924 (left) and the modified compound BMS-665351 (right).
The primary objective of this study was to investigate whether other nuclear receptors such as CAR may be involved in the mechanism of this PXR-independent induction of CYP3A4 expression. Using cell-based reporter assays, nuclear receptor transfection assays, quantitative PCR analysis, Western blotting, and CAR-nuclear translocation assays, we provide here experimental evidence to show that BMS-665351 induction of CYP3A4 expression is CAR-related; although BMS-665351 is not a typical CAR activator at concentrations that resulted in significant induction of CYP3A4, it selectively induces the expression of CAR and exhibits synergistic induction of CYP3A4 in the presence of CAR but not PXR activators. Together, these results uncover a unique feature of BMS-665351-type compounds that induce the expression of CYP3A4 through a PXR-independent, noncanonical CAR-related mechanism.
Materials and Methods
Chemicals and Biological Reagents.
BMS-665351 was obtained from Bristol-Myers Squibb (Wallingford, CT). RIF, PB, 1-(2-chlorophenyl-N-methylpropyl)-3-isoquinoline-carboxamide (PK11195), cycloheximide (CHX), and actinomycin D (Act.D) were purchased from Sigma-Aldrich (St. Louis, MO). 6-(4-Chlorophenyl)imidazo[2,1-b][1,3]thiazole-5-carbaldehyde-O-(3,4- dichlorobenzyl) oxime (CITCO) was from Enzo Life Sciences, Inc. (Farmingdale, NY). Oligonucleotide primers were synthesized by Integrated DNA Technologies, Inc. (Coralville, IA). The Dual-Luciferase Reporter Assay System was purchased from Promega (Madison, WI). CYP3A4 antibody (AB1254) was from Millipore Corporation (Billerica, MA). β-Actin antibody (A3854) was from Sigma-Aldrich. Matrigel, insulin, and insulin/transferrin/selenium were obtained from BD Biosciences Discovery Labware (Bedford, MA). Other cell culture reagents were purchased from Invitrogen (Carlsbad, CA) or Sigma-Aldrich.
Plasmid Constructions.
The CYP3A4-PXRE/XREM reporter vector was provided by Dr. Bryan Goodwin (GlaxoSmithKline, Research Triangle Park, NC). The pSG5-hPXR expression vector was obtained from Dr. Steven Kliewer (University of Texas Southwestern Medical Center, Dallas, TX). The pCR3-hCAR expression vector was from Dr. Masahiko Negishi (National Institute of Environmental Health Sciences, National Institutes of Health, Research Triangle Park, NC). The CMV2-hCAR3 vector was from Dr. Curtis Omiecinski (Pennsylvania State University, University Park, PA). The pCR3-hCAR1+A expression vector and CYP2B6-2.2kb reporter construct, containing both PB-responsive enhancer module and the distal XREM, were generated as described previously (Wang et al., 2003; Chen et al., 2010). The pRL-TK renilla luciferase plasmid used to normalize firefly luciferase activities was from Promega.
Human Primary Hepatocyte Cultures and Treatments.
Liver tissues were obtained by qualified medical staff after donor consent and prior approval from the Institutional Review Board at the University of Maryland, School of Medicine. Hepatocytes were isolated from human liver specimens by a modification of the two-step collagenase digestion method as described previously (LeCluyse et al., 2005). Hepatocytes were seeded at 1.5 × 106 cells/well in six-well BioCoat plates in Dulbecco's modified Eagle's medium supplemented with 5% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 4 μg/ml insulin, and 1 μM dexamethasone. After 4 to 6 h of attachment at 37°C in a humidified atmosphere of 5% CO2, cells were overlaid with Matrigel (0.25 mg/ml) in Williams' E medium supplemented with insulin, transferrin, selenium, 0.1 μM dexamethasone, 100 U/ml penicillin, and 100 μg/ml streptomycin. The hepatocytes were maintained for 36 h before treatment with RIF (10 μM), CITCO (1 μM), or BMS-665351 (1 and 5 μM) for another 24 or 72 h for detection of mRNA and protein expression, respectively. In separate experiments, cultured hepatocytes were exposed to CHX (5 μg/ml), Act.D (1 μM), or vehicle 1 h before BMS-665351 treatment (5 μM, 24 h) for detection of mRNA expression.
Quantitative PCR Analysis.
Total RNA was isolated from treated hepatocytes by using the RNeasy Mini Kit (QIAGEN, Valencia, CA) and reverse-transcribed by using a High-Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA) following the manufacturers' instructions. CYP3A4, hCAR, and hPXR mRNA expressions were normalized against that of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Quantitative real-time PCR assays were performed in 96-well optical plates on an ABI Prism 7000 Sequence Detection System with SYBR Green PCR Master Mix (Applied Biosystems). Primers for CYP3A4, CYP2B6, hCAR, hPXR, and GAPDH mRNA detection were as follows: CYP3A4, 5′-GTGGGGCTTTTATGATGGTCA-3′ (forward) and 5′-GCCTCAGATTTCTCACCAACACA-3′ (reverse); CYP2B6, 5′-AGACGCCTTCAATCCTGACC-3′ (forward) and 5′-CCTTCACCAAGACAAATCCGC-3′ (reverse); hCAR, 5′- GAGCTGAGGAACTGTGTGGTA-3′ (forward) and 5′-CTTTTGCTGACTGTTCTCCTGAA-3′ (reverse); hPXR, 5′- AAGCCCAGTGTCAACGCAG-3′ (forward) and 5′- GGGTCTTCCGGGTGATCTC-3′ (reverse); and GAPDH, 5′-CCCATCACCATCTTCCAGGAG-3′ (forward) and 5′-GTTGTCATGGATGACCTTGGC-3′ (reverse). Induction values were calculated according to the equation: fold over control = 2ΔΔCt, where ΔCt represents the differences in cycle threshold numbers between the target gene and GAPDH and ΔΔCt represents the relative change in these differences between control and treatment groups.
Transient Transfection in Hepatoma Cells and Human Primary Hepatocytes.
HepG2 cells in 24-well plates were transfected with CYP3A4-PXRE/XREM reporter or CYP2B6-2.2kb reporter (60 ng/well) in the presence of a hPXR, hCAR3, or hCAR1+A expression vector (30 ng/well) by using a Fugene 6 Transfection Kit following the manufacturer's instruction (Roche Applied Science, Indianapolis, IN). Twenty four hours after transfection, cells were treated for another 24 h with solvent (0.1% DMSO) or test compounds at concentrations of RIF (10 μM), CITCO (1 μM), and BMS-665351 (1 and 5 μM). Subsequently, cell lysates were assayed for firefly activities normalized against the activities of cotransfected renilla luciferase by using a Dual-Luciferase Kit (Promega). Data were represented as mean ± S.D. of three individual transfections. To analyze PXR- and CAR-mediated induction of CYP3A4 in hepatoma cells, HepG2 cells seeded in 12-well plates were transfected with hCAR or hPXR expression vectors, and 24 h after transfection cells were treated for another 24 h with RIF (10 μM), CITCO (1 μM), PK11195 (10 μM), BMS-665351 (1 and 5 μM), or cotreated for 24 h with PK11195 plus CITCO or BMS-665351, respectively. Total RNA was isolated and subjected to real-time RT-PCR analysis as described above. In separate experiments, HPHs seeded in 24-well BioCoat plates were transfected with CYP3A4-PXRE/XREM or CYP2B6-2.2kb construct in the presence of pGL-Tk vector by using Effectene reagent (QIAGEN) as described previously (Faucette et al., 2007). Transfected HPHs were treated with DMSO (0.1% v/v), RIF (10 μM), CITCO (1 μM), PB (1 mM), or BMS-665351 (1 and 5 μM) for 24 h. Cell lysates were subjected to dual-luciferase analysis as described above.
Translocation of Ad/EYFP-hCAR in Human Primary Hepatocytes.
The adenovirus expressing enhanced yellow fluorescent protein tagged hCAR (Ad/EYFP-hCAR), which infects HPHs with high efficiency, was generated as described previously (Li et al., 2009). Hepatocyte cultures in 24-well BioCoat plates were cultured in nonserum Williams' E medium containing Ad/EYFP-hCAR at a multiplicity of infection of 50 for 12 h before treatment with the vehicle control (0.1% DMSO), PB (1 mM), and BMS-665351 (1 and 5 μM). After 8 h of incubation, treated cells were subjected to confocal microscopy analysis with a Nikon (Tokyo, Japan) C1-LU3 instrument based on an inverted Nikon Eclipse TE2000 microscope. The subcellular localization of hCAR was visualized and quantitatively characterized as nuclear, cytosolic, and mixed (nuclear + cytosolic) expression by counting 100 Ad/EYFP-hCAR-expressing hepatocytes from each group.
Western Blot Analyses.
The whole-cell homogenate proteins (20 μg) from treated HPHs were resolved on SDS-polyacrylamide gels (12%) and electrophoretically transferred onto Immobilon-P polyvinylidene difluoride membranes. Subsequently, membranes were incubated with specific antibodies against CYP3A4 (Millipore Corporation) diluted 1:5000. β-Actin was used as an internal control. Blots were washed and incubated with horseradish peroxidase goat anti-rabbit IgG antibody diluted 1:4000 and developed by using enhanced chemiluminescence Western blotting detection reagent (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK).
Statistical Analysis.
All data represent at least three independent experiments and are expressed as the mean ± S.D. Statistical comparisons were made by using one-way analysis of variance followed by post hoc Dunnett's test and Students' t test where appropriate. Statistical significance was set at p < 0.05 and < 0.01.
Results
BMS-665351 Induces CYP3A4 Expression in Human Primary Hepatocytes.
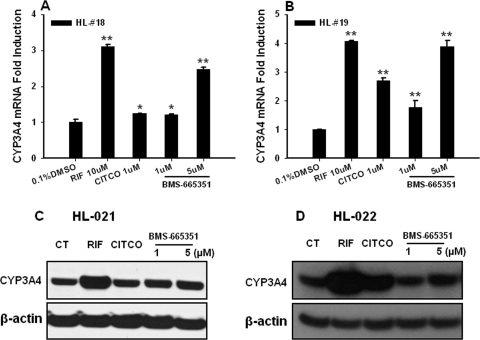
Given that CYP3A4 represents the most abundant drug-metabolizing enzyme in the human liver and small intestine and is involved in the metabolism of more than 60% of marketed drugs, we first evaluated the effect of BMS-665351 on CYP3A4 expression in HPHs. HPHs prepared from four different donors were treated with RIF (10 μM), CITCO (1 μM), or BMS-665351 (1 and 5 μM) as detailed under Materials and Methods. As expected, RIF, as a prototypical PXR activator, exhibited potent induction of CYP3A4 expression (Fig. 2), whereas CITCO (a prototypical CAR agonist) showed a relatively moderate effect on CYP3A4 induction. It is noteworthy that BMS-665351 displayed significant induction of CYP3A4 mRNA at both 1 and 5 μM concentrations (Fig. 2), and the maximal induction of CYP3A4 at 5 μM reached approximately 90% of RIF-mediated induction.
Fig. 2.
BMS-665351-mediated induction of CYP3A4 expression in human primary hepatocytes. Human primary hepatocyte cultures from donors were treated for 24 h with RIF (10 μM), CITCO (1 μM), or BMS-665351 (1 and 5 μM). A and B, total RNA was collected, reverse-transcribed, and subjected to real-time RT-PCR for detecting the CYP3A4 [donor HL-18 (A) and donor HL-19 (B)] expression levels as outlined under Materials and Methods. RT-PCR data are expressed as mean ± S.D. (n = 3). *, P < 0.05; **, P < 0.01. C and D, in separate experiments, HPHs from donors HL-21 (C) and HL-22 (D) were treated with the same batch of chemicals as indicated in A and B for 72 h. After harvesting, whole-cell homogenates (20 μg/each) were subjected to CYP3A4 immunoblotting analysis as described under Materials and Methods.
Effects of BMS-665351 on the Activation of PXR and CAR in HepG2 Cells and HPHs.
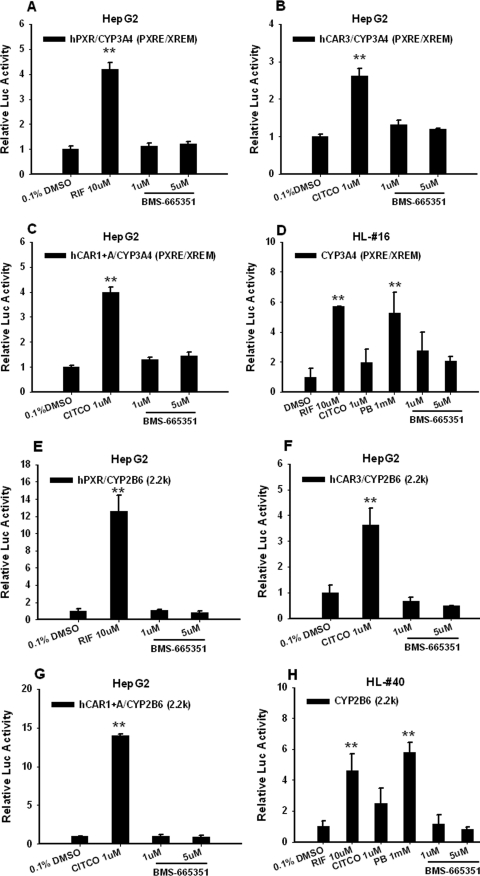
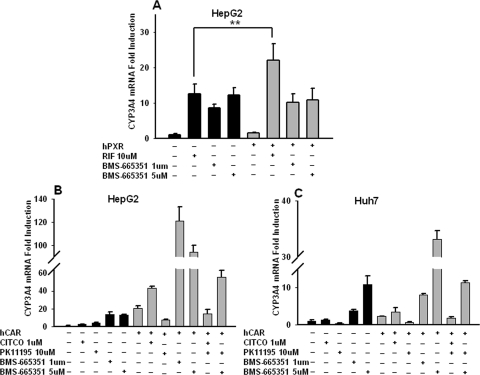
PXR and CAR are known transcription factors that mediate inductive expression of the CYP3A4 gene; therefore, we investigated the ability of BMS-665351 to transactivate these two nuclear receptors in cell-based reporter experiments. In contrast to the majority of CYP3A4 inducers, BMS-665351 at 1 and 5 μM concentrations failed to activate hPXR in HepG2 cells cotransfected with either CYP3A4 or CYP2B6 reporter vector, respectively (Fig. 3, A and E). Using the chemically responsive hCAR constructs (hCAR3 and hCAR1+A) as reported previously (Auerbach et al., 2005; Chen et al., 2010), cell-based reporter assays showed that BMS-665351 was unable to transactivate hCAR in HepG2 cells (Fig. 3, B, C, F, and G) either. In separate experiments, CYP3A4 or CYP2B6 reporter vectors were transfected in HPHs without the simultaneous transfection of exogenous PXR or CAR. As indicated in Fig. 3, D and H, endogenous PXR and CAR maintained in HPHs were sufficient to activate CYP3A4 and CYP2B6 reporters by RIF and PB, whereas BMS-665351 only marginally increased CYP3A4 but not CYP2B6 luciferase activity. Together, these data indicate BMS-665351 is not a typical activator of either PXR or CAR.
Fig. 3.
Effects of BMS-665351 on PXR and CAR activation in transfected HepG2 cells and HPHs. A, B, C, E, F, and G, HepG2 cells were transfected with CYP3A4 (PXRE/XREM) reporter construct in the presence of hPXR (A), hCAR3 (B), or hCAR1+A (C) expression vectors or in the combination of CYP2B6-2.2kb reporter construct with hPXR (E), hCAR3 (F), or hCAR1+A (G) expression vectors. Transfected cells were then treated with BMS-665351 (1 and 5 μM) for 24 h. RIF (10 μM) and CITCO (1 μM) were used as positive controls for hPXR and hCAR, respectively. D and H, in separate experiments, human primary hepatocytes from donors HL-16 and HL-40 in 24-well Biocoat plates were transfected with CYP3A4 (PXRE/XREM) (D) or CYP2B6-2.2kb (H) reporter vector, followed by treatment of RIF (10 μM), CITCO (1 μM), PB (1 mM), and BMS-665351 (1 and 5 μM) for 24 h. Luciferase activities were determined and expressed relative to vehicle control. Data represent the mean ± S.D. (n = 3). **, P < 0.01.
Nuclear Translocation of hCAR in Human Primary Hepatocytes.
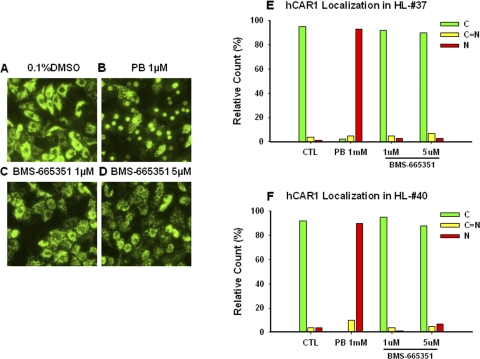
Another important feature of CAR is its nuclear accumulation in primary hepatocytes or in vivo upon chemically stimulated activation (Kawamoto et al., 1999). We further investigated whether BMS-665351 could translocate human CAR to the nucleus in HPHs as the initial step of hCAR activation. Ad-EYFP-hCAR has been generated, which demonstrates high infection efficiency in HPHs and displays the unique characteristics of hCAR cellular distribution and activation in HPHs (Li et al., 2009). As described under Materials and Methods, Ad-EYFP-hCAR-infected HPHs (donors HL-37 and HL-40) were treated with vehicle control (0.1% DMSO), positive control PB (1 mM), or BMS-665351 at 1 and 5 μM for 8 h. As expected, Ad-EYFP-hCAR was localized primarily in the cytoplasm (95 and 92%) of infected HPHs without activation (0.1% DMSO) and efficiently accumulated in the nucleus (93 and 90%) by the prototypical CAR activator PB (1 mM). It is noteworthy that BMS-665351 at 1 and 5 μM concentrations exhibited essentially no effect on hCAR nuclear accumulation in donors HL-37 and HL-40 (92 and 95% and 90 and 88% located in cytoplasm, respectively) (Fig. 4).
Fig. 4.
Nuclear translocation of hCAR in human primary hepatocytes. Human primary hepatocytes from donors HL-37 and HL-40 were infected with Ad/EYFP-hCAR as outlined under Materials and Methods. Twelve hours after infection, HPHs were treated with vehicle control (0.1% DMSO), PB (1 mM), or BMS-665351 (1 and 5 μM) for 8 h. Subsequently, cellular localizations of infected hCAR were visualized under a confocal microscope. A to D, representative Ad-EYFP-hCAR localizations from vehicle control, PB-treated, and BMS-665351-treated hepatocytes (donor HL-37). E and F, 100 Ad/EYFP-hCAR expressing HPHs from each treatment group were calculated with a percentage of cytosol, nucleus, and mixed (cytosol + nucleus) localizations for donors HL-37 (E) and HL-40 (F).
BMS-665351 Enhances CAR- but Not PXR-Mediated Induction of CYP3A4 Expression in Hepatoma Cells.
To investigate the association of BMS-665351-mediated induction of CYP3A4 with the expression levels of PXR and CAR, real-time PCR analysis was carried out in HepG2 and/or Huh7 cells transfected with PXR or CAR expression vectors as outlined under Materials and Methods. Consistent with the results from cell-based reporter assays, the induction of CYP3A4 mRNA by BMS-665351 was not further increased in HepG2 cells overexpressing hPXR, whereas the selective hPXR activator RIF-mediated induction of CYP3A4 was significantly augmented in hPXR-transfected HepG2 cells (Fig. 5A). Nonetheless, in contrast to the fact that BMS-665351 failed to activate hCAR in cell-based reporter assays and nuclear translocation experiments, BMS-665351 markedly increased CYP3A4 expression in hCAR-transfected HepG2 and Huh7 cells (Fig. 5, B and C). Moreover, the induction of CYP3A4 by BMS-665351 in these hCAR-overexpressing cells was repressed significantly in the presence of PK11195, a selective hCAR deactivator (Li et al., 2008). Together, these results suggest that the induction of CYP3A4 by BMS-665351 is CAR- but not PXR-dependent, although BMS-665351 itself does not activate either of these receptors.
Fig. 5.
Influence of hPXR and hCAR expression on BMS-665351-mediated induction of CYP3A4 expression in human hepatoma cells. A, HepG2 cells were transfected with empty vector or hPXR expression vector, then treated with RIF (10 μM) or BMS-665351 (1 and 5 μM) 24 h after transfection. **, P < 0.01. B and C, in separate experiments, HepG2 (B) and Huh7 (C) cells were transfected with hCAR expression vector (pCR3-hCAR) or empty vector followed by the treatment of CITCO (1 μM), PK11195 (10 μM), BMS-665351 (1 and 5 μM), or cotreatment with PK11195 as indicated. All transfected cells were treated for another 24 h before harvesting of the total RNA. Real-time PCR was used to detect the mRNA expression of CYP3A4. Data are expressed as mean ± S.D. (n = 3).
BMS-665351 Induces the Expression of CAR, Not PXR, in HPHs and Hepatoma Cells.
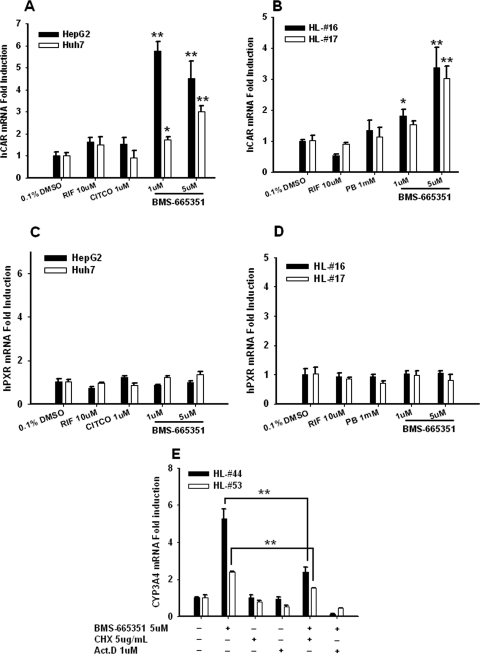
Previous evidence suggests that hepatic expression of PXR and CAR is positively correlated with that of their target P450 enzymes in humans (Chang et al., 2003). Here, we evaluated the effect of BMS-665351 on the expressions of PXR and CAR in HPHs and hepatoma cells (HepG2 and Huh7). Intriguingly, our results indicated that BMS-665351 at concentrations that enhanced CYP3A4 expression significantly induced the expression of hCAR in HepG2 and Huh7 cells, as well as HPHs (Fig. 6, A and B). On the other hand, the expression of PXR was not altered after BMS-665351 treatment at the same concentrations in these cells (Fig. 6, C and D). In agreement with previous reports, the prototypical PXR and CAR activators, such as RIF, CITCO, and PB, exhibited no induction of either CAR or PXR expression.
Fig. 6.
BMS-665351 induces the expression of hCAR, not hPXR, and de novo protein synthesis is required for BMS-665351-induced CYP3A4 expression. Cultured HepG2, Huh7, and human primary hepatocytes from donors HL-16 and HL-17 were treated with RIF (10 μM), CITCO (1 μM), PB (1 mM), or BMS-665351 (1 and 5 μM) for 24 h. A to D, total RNA was collected, reverse-transcribed, and subjected to quantitative PCR for detecting hCAR (A and B) and hPXR (C and D) expression levels. E, in separate experiments, HPHs from donors HL-44 and HL-53 were exposed to CHX (5 μg/ml), Act.D (1 μM), or vehicle 1 h before BMS-665351 treatment (5 μM, 24 h) for quantitative PCR detection of CYP3A4 mRNA expression. All quantitative PCR data are expressed as mean ± S.D. (n = 3). *, P < 0.05; **, P < 0.01.
Effects of Cycloheximide and Actinomycin D on BMS-665351-Mediated Induction of CYP3A4 mRNA.
Without exhibiting the prototypical features of CAR activation, BMS-665351 significantly induced CAR expression at concentrations that increased CYP3A4 expression. To further explore whether protein synthesis is required for BMS-665351-mediated induction of CYP3A4, HPHs were treated with BMS-665351 in the presence or absence of CHX (5 μg/ml), a typical inhibitor of protein synthesis, or Act.D (1 μM), a typical inhibitor of mRNA synthesis. As demonstrated in Fig. 6E, induction of CYP3A4 mRNA was significantly repressed in the presence of CHX, indicating that de novo protein synthesis is necessary for the optimal induction of CYP3A4 by BMS-665351. Moreover, inhibition of mRNA synthesis by Act.D totally abolished this induction.
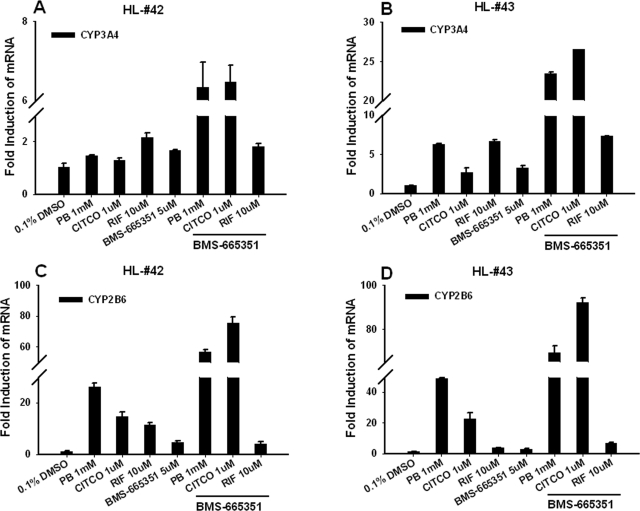
In a separate experiment, a synergistic induction of CYP3A4 and CYP2B6 was observed in HPHs cotreated with BMS-665351 and the prototypical hCAR activators CITCO and PB (Fig. 7). However, BMS-665351 did not enhance RIF (the selective hPXR activator)- mediated induction of either CYP3A4 or CYP2B6 (Fig. 7). These results indicate that BMS-665351-mediated induction of CAR expression may contribute atypically to its CAR-related induction of CYP3A4.
Fig. 7.
BMS-665351 enhances the induction of CYP3A4 by prototypical hCAR, not hPXR, activators. HPHs from donors HL-42 and HL-43 were treated with vehicle control (0.1% DMSO), RIF (10 μM), CITCO (1 μM), PB (1 mM), BMS-665351 (5 μM), or cotreated with BMS-665351 and each of these prototypical activators as indicated. Twenty four hours after treatment, total RNA was isolated from treated cells and subjected to real-time PCR for measuring the induction of CYP3A4 (A and B) and CYP2B6 (C and D). RT-PCR data are expressed as mean ± S.D. (n = 3).
Discussion
Early prediction of potentially serious DDIs throughout drug discovery and development has become a common exercise in the pharmaceutical industry. Because of the well known importance of CYP3A4 in the biotransformation of many marketed and future drugs, pharmacokinetic-associated DDIs resulting from induction or inhibition of this isozyme are of great clinical significance. To date, it is well established that the induction of CYP3A4 occurs predominantly at the transcriptional level through the transactivation of the nuclear receptor PXR and to a lesser extent of CAR (Blumberg et al., 1998; Goodwin et al., 2002; Wang and LeCluyse, 2003). PXR contains a large and flexible ligand-binding pocket and is more promiscuous than CAR in its interactions with drugs; nevertheless, these receptors share a subset of common xenobiotic activators and target genes, including CYP3A4. Given the simplistic nature of ligand-based activation assays and the importance of its target genes, PXR activation or elimination of PXR activation has been used to evaluate or avoid potential CYP3A4 induction at the earliest stages of drug development. Such a strategy has been used in structurally modifying the IGF-1R inhibitor (R)-4-(3-(3-chlorophenyl)-3-hydroxypropyl)-3-(4-methyl-6-morpholino-1H-benzo[d]imidazol-2-yl)pyridin-2(1H)-one (BMS-536924), with the aim to produce compounds such as BMS-665351, which retain in vivo activity toward IGF-1R inhibition while not transactivating PXR (Fig. 1). In the current study, our data unexpectedly revealed that BMS-665351 exhibited significant CYP3A4 induction in HPHs and HepG2 cells, although it did not activate either PXR or CAR (Figs. 2–4). In addition, BMS-665351 failed to activate glucocorticoid receptor (GR) and vitamin D receptor (VDR), two nuclear receptors that may play indirect or moderate roles in CYP3A4 induction (data not shown). Instead, this compound displayed selective induction of CAR but not PXR expression; and BMS-665351-mediated induction of CYP3A4 was positively associated with the expression level of CAR rather than PXR. Moreover, BMS-665351 exhibited synergistic induction of CYP3A4 and CYP2B6 in HPHs in the presence of prototypical CAR but not PXR activators. To the best of our knowledge, this report is the first to demonstrate a PXR-independent, nonclassical CAR-related induction of CYP3A4 by a benzimidazole-based IGF-1R inhibitor.
Chemical-induced expression of hepatic CYP3A4 is strongly associated with the transactivation of hPXR. To date, the majority of identified CYP3A4 inducers are confirmed hPXR activators (e.g., RIF), coactivators of human PXR and CAR (e.g., PB), or hPXR activators but CAR deactivators (e.g., clotrimazole) (Sueyoshi et al., 1999; Moore et al., 2000). On the other hand, a number of compounds, such as CITCO, the anti-HIV reagent efavirenz, and the antiepileptic phenytoin, have been shown to induce the expression of CYP3A4 preferentially through the activation of CAR instead of PXR (Maglich et al., 2003; Faucette et al., 2007). Therefore, it was reasonable to speculate that BMS-665351-mediated induction of CYP3A4 expression most likely occurs via the transactivation of hCAR not PXR. Nevertheless, unlike the ligand-dependent activation of PXR, CAR can be activated through both direct ligand binding (e.g., CITCO) and ligand-independent indirect mechanisms (e.g., PB). Moreover, CAR spontaneously accumulates in the nucleus of immortalized cell lines and exhibits constitutive activity in the absence of chemical activators (Baes et al., 1994), which further increases the complexity associated with in vitro evaluation of CAR activation. Alternatively, in human primary hepatocyte cultures, CAR is compartmented primarily in the cytoplasm without activation and translocates to the nucleus upon exposure to chemical activators, which reflects the initial step of CAR activation (Kawamoto et al., 1999). The hCAR splicing variant (hCAR3) and the hCAR3-based chimerical construct (hCAR1+A) have been established as chemical-responsive surrogates of CAR with chemical specificities closely correlated with the reference hCAR (Auerbach et al., 2005; Chen et al., 2010). In the current study, we evaluated hCAR activation by BMS-665351 using these newly established approaches. Unexpectedly, our results showed that BMS-665351 can neither transactivate hCAR3 or hCAR1+A in cell-based reporter assays nor translocate hCAR to the nucleus in HPHs at concentrations that significantly induce CYP3A4 expression. These observations, together with the fact that BMS-665351 is not an activator of hPXR, raise the possibility that BMS-665351 may induce CYP3A4 through other nuclear receptors such as GR and/or VDR.
The paradoxical roles of GR in the induction of CYP3A was somewhat clarified with the establishment of the predominant role of PXR in CYP3A-inductive expression (Pascussi et al., 2003). Several lines of evidence indicate that, instead of regulating CYP3A genes directly, GR may function as the regulator of PXR and CAR that indirectly influences the expression of CYP3A. In HPHs, submicromolar concentrations of dexamethasone induce the expression of both PXR and CAR and enhance RIF- and PB-mediated induction of CYP3A4 (Pascussi et al., 2000). In separate studies, VDR was recognized as an additional mediator of intestine and liver induction of CYP3A4 (Thummel et al., 2001; Drocourt et al., 2002), where the active form of vitamin D, 1α,25-(OH)2-D3, induces CYP3A4 expression through the enhanced interaction between VDR and the promoter regions of the CYP3A4 gene (Drocourt et al., 2002). In exploring these possibilities, our cell-based reporter assays demonstrated that BMS-665351 is not an activator of either GR or VDR at concentrations that induce CYP3A4, leaving the mechanisms of BMS-665351-mediated induction of CYP3A4 unknown.
Although our results showed that BMS-665351 fails to activate either CAR or PXR, we have observed that BMS-665351-mediated induction of CYP3A4 was significantly enhanced when hCAR but not hPXR was overexpressed in transfected hepatoma cells. Further evidence revealed that BMS-665351 is a selective inducer of CAR in human liver cells (HepG2, Huh7, and HPHs). Given that BMS-665351 is not an activator of GR and only induces the expression of CAR but not PXR, BMS-665351-mediated induction of hCAR may represent a GR-independent mechanism. It is noteworthy that previous reports indicated that hepatic CYP3A4 mRNA levels were correlated significantly with the expression levels of CAR and PXR (Chang et al., 2003; Pascussi et al., 2003). In addition, our results revealed that induction of the CYP3A4 gene by BMS-665351 is sensitive to CHX, suggesting de novo protein synthesis is required for the inductive response. Nevertheless, inhibition of protein synthesis by CHX does not completely abolish the induced CYP3A4 expression (Fig. 6E), indicating BMS-665351-increased CAR expression alone cannot explain the optimal induction of CYP3A4 expression. To this end, it is postulated that BMS-665351-mediated induction of the CYP3A4 gene might be associated with the increased hCAR expression that facilitates interaction of CAR with some yet unidentified endogenous activators. Moreover, we showed that cotreatment of BMS-665351 with CAR, but not PXR, activators synergistically enhanced the inductive expression of CYP3A4 and CYP2B6, further supporting the CAR-dependent effect of BMS-665351 in the induction of hepatic cytochromes P450.
In summary, we have identified a benzimidazole-based IGF-1R inhibitor, BMS-665351, that induces the expression of CYP3A4 through a PXR-independent, nonclassical CAR-related mechanism. The results demonstrated that BMS-665351 significantly increased the expression of CYP3A4 mRNA but failed to transactivate either PXR or CAR. Instead it selectively induced the expression of CAR in all liver cells tested. The correlation between CAR expression and BMS-665351-mediated CYP3A4 induction further implies the requirement of CAR in BMS-665351 inductivity. Although the precise molecular details involved in BMS-665351 induction of CYP3A4 are still elusive, our data clearly indicate that BMS-665351 represents a class of compounds that exert their cytochrome P450 induction through a unique, atypical CAR-dependent pathway. Given that multiple anticancer drugs are often coadministered as a combined regimen, significant induction of CYP3A4 expression by BMS-665351 would raise concerns for clinically unwanted DDIs.
Acknowledgments
We thank Drs. Masahiko Negishi (National Institute of Environmental Health Sciences, National Institute of Health, Research Triangle Park, NC), Steven Kliewer (University of Texas Southwestern Medical Center, Dallas, TX), Curtis Omiecinski (Pennsylvania State University, University Park, PA), and Bryan Goodwin (GlaxoSmith-Kline, Research Triangle Park, NC) for providing PXR, CAR expression, and CYP3A4 reporter constructs; Jennifer Fuhrman (University of Maryland Medical Center, Baltimore, MD) for providing the human liver samples used in this study; and Mark Wittman, Joan Carboni, and Sean Kim (Bristol-Myers Squibb) for their continual advice.
This research was supported by a research agreement with Bristol-Meyer Squibb; and funding from the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases [Grant DK061652] (to H.W.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- IGF-1R
- insulin-like growth factor-1 receptor
- Act.D
- actinomycin D
- CAR
- constitutive androstane receptor
- hCAR
- human CAR
- Ad/EYFP-hCAR
- adenovirus-expressing enhanced yellow florescent protein tagged hCAR
- CHX
- cycloheximide
- DDI
- drug-drug interaction
- DMSO
- dimethyl sulfoxide
- GAPDH
- glyceraldehyde-3-phosphate dehydrogenase
- GR
- glucocorticoid receptor
- HPH
- human primary hepatocyte
- CITCO
- 6-(4-chlorophenyl)imidazo[2,1-b][1,3]thiazole-5-carbaldehyde-O-(3,4-dichlorobenzyl) oxime
- PB
- phenobarbital
- PXR
- pregnane X receptor
- hPXR
- human PXR
- PXRE
- proximal everted repeat-6 element
- RIF
- rifampicin
- PK11195
- 1-(2-chlorophenyl-N-methylpropyl)-3-isoquinoline-carboxamide
- RT-PCR
- reverse transcription-polymerase chain reaction
- VDR
- vitamin D receptor
- XREM
- xenobiotic-responsive enhancer module
- BMS-665351
- 4-(1-(2-(4-((2-(4-chloro-1H-pyrazol-1-yl)ethyl)amino)-2-oxo-1,2-dihydropyridin-3-yl)-4-methyl-1H-benzo[d]imidazol-6-yl)piperidin-4-yl)piperazine-1-carboxylate
- BMS-536924
- (R)-4-(3-(3-chlorophenyl)-3-hydroxypropyl)-3-(4-methyl-6-morpholino-1H-benzo[d]imidazol-2-yl)pyridin-2(1H)-one.
Authorship Contributions
Participated in research design: Li, Sinz, and Wang.
Conducted experiments: Li, Sinz, and Wang.
Contributed new reagents or analytic tools: Sinz and Zimmermann.
Wrote or contributed to the writing of the manuscript: Li, Sinz, and Wang.
References
- Auerbach SS, Stoner MA, Su S, Omiecinski CJ. (2005) Retinoid X receptor-α-dependent transactivation by a naturally occurring structural variant of human constitutive androstane receptor (NR1I3). Mol Pharmacol 68:1239–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baes M, Gulick T, Choi HS, Martinoli MG, Simha D, Moore DD. (1994) A new orphan member of the nuclear hormone receptor superfamily that interacts with a subset of retinoic acid response elements. Mol Cell Biol 14:1544–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baserga R, Peruzzi F, Reiss K. (2003) The IGF-1 receptor in cancer biology. Int J Cancer 107:873–877 [DOI] [PubMed] [Google Scholar]
- Blumberg B, Sabbagh W, Jr, Juguilon H, Bolado J, Jr, van Meter CM, Ong ES, Evans RM. (1998) SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev 12:3195–3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TK, Bandiera SM, Chen J. (2003) Constitutive androstane receptor and pregnane X receptor gene expression in human liver: interindividual variability and correlation with CYP2B6 mRNA levels. Drug Metab Dispos 31:7–10 [DOI] [PubMed] [Google Scholar]
- Chen T, Tompkins LM, Li L, Li H, Kim G, Zheng Y, Wang H. (2010) A single amino acid controls the functional switch of human constitutive androstane receptor (CAR) 1 to the xenobiotic-sensitive splicing variant CAR3. J Pharmacol Exp Ther 332:106–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drocourt L, Ourlin JC, Pascussi JM, Maurel P, Vilarem MJ. (2002) Expression of CYP3A4, CYP2B6, and CYP2C9 is regulated by the vitamin D receptor pathway in primary human hepatocytes. J Biol Chem 277:25125–25132 [DOI] [PubMed] [Google Scholar]
- Faucette SR, Zhang TC, Moore R, Sueyoshi T, Omiecinski CJ, LeCluyse EL, Negishi M, Wang H. (2007) Relative activation of human pregnane X receptor versus constitutive androstane receptor defines distinct classes of CYP2B6 and CYP3A4 inducers. J Pharmacol Exp Ther 320:72–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin B, Hodgson E, D'Costa DJ, Robertson GR, Liddle C. (2002) Transcriptional regulation of the human CYP3A4 gene by the constitutive androstane receptor. Mol Pharmacol 62:359–365 [DOI] [PubMed] [Google Scholar]
- Goodwin B, Hodgson E, Liddle C. (1999) The orphan human pregnane X receptor mediates the transcriptional activation of CYP3A4 by rifampicin through a distal enhancer module. Mol Pharmacol 56:1329–1339 [DOI] [PubMed] [Google Scholar]
- Guengerich FP. (1999) Cytochrome P-450 3A4: regulation and role in drug metabolism. Annu Rev Pharmacol Toxicol 39:1–17 [DOI] [PubMed] [Google Scholar]
- Kawamoto T, Sueyoshi T, Zelko I, Moore R, Washburn K, Negishi M. (1999) Phenobarbital-responsive nuclear translocation of the receptor CAR in induction of the CYP2B gene. Mol Cell Biol 19:6318–6322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Dinchuk JE, Anthony MN, Orcutt T, Zoeckler ME, Sauer MB, Mosure KW, Vuppugalla R, Grace JE, Jr, Simmermacher J, et al. (2010) Evaluation of cynomolgus monkey pregnane X receptor, primary hepatocyte, and in vivo pharmacokinetic changes in predicting human CYP3A4 induction. Drug Metab Dispos 38:16–24 [DOI] [PubMed] [Google Scholar]
- Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, McKee DD, Oliver BB, Willson TM, Zetterström RH, et al. (1998) An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell 92:73–82 [DOI] [PubMed] [Google Scholar]
- LeCluyse EL, Alexandre E, Hamilton GA, Viollon-Abadie C, Coon DJ, Jolley S, Richert L. (2005) Isolation and culture of primary human hepatocytes. Methods Mol Biol 290:207–229 [DOI] [PubMed] [Google Scholar]
- Lehmann JM, McKee DD, Watson MA, Willson TM, Moore JT, Kliewer SA. (1998) The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J Clin Invest 102:1016–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AP, Kaminski DL, Rasmussen A. (1995) Substrates of human hepatic cytochrome P450 3A4. Toxicology 104:1–8 [DOI] [PubMed] [Google Scholar]
- Li H, Chen T, Cottrell J, Wang H. (2009) Nuclear translocation of adenoviral-enhanced yellow fluorescent protein-tagged-human constitutive androstane receptor (hCAR): a novel tool for screening hCAR activators in human primary hepatocytes. Drug Metab Dispos 37:1098–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Chen T, Stanton JD, Sueyoshi T, Negishi M, Wang H. (2008) The peripheral benzodiazepine receptor ligand 1-(2-chlorophenyl-methylpropyl)-3-isoquinoline-carboxamide is a novel antagonist of human constitutive androstane receptor. Mol Pharmacol 74:443–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglich JM, Parks DJ, Moore LB, Collins JL, Goodwin B, Billin AN, Stoltz CA, Kliewer SA, Lambert MH, Willson TM, et al. (2003) Identification of a novel human constitutive androstane receptor (CAR) agonist and its use in the identification of CAR target genes. J Biol Chem 278:17277–17283 [DOI] [PubMed] [Google Scholar]
- Moore LB, Parks DJ, Jones SA, Bledsoe RK, Consler TG, Stimmel JB, Goodwin B, Liddle C, Blanchard SG, Willson TM, et al. (2000) Orphan nuclear receptors constitutive androstane receptor and pregnane X receptor share xenobiotic and steroid ligands. J Biol Chem 275:15122–15127 [DOI] [PubMed] [Google Scholar]
- Neudauer CL, McCarthy JB. (2003) Insulin-like growth factor I-stimulated melanoma cell migration requires phosphoinositide 3-kinase but not extracellular-regulated kinase activation. Exp Cell Res 286:128–137 [DOI] [PubMed] [Google Scholar]
- Pascussi JM, Drocourt L, Fabre JM, Maurel P, Vilarem MJ. (2000) Dexamethasone induces pregnane X receptor and retinoid X receptor-α expression in human hepatocytes: synergistic increase of CYP3A4 induction by pregnane X receptor activators. Mol Pharmacol 58:361–372 [DOI] [PubMed] [Google Scholar]
- Pascussi JM, Gerbal-Chaloin S, Drocourt L, Maurel P, Vilarem MJ. (2003) The expression of CYP2B6, CYP2C9 and CYP3A4 genes: a tangle of networks of nuclear and steroid receptors. Biochim Biophys Acta 1619:243–253 [DOI] [PubMed] [Google Scholar]
- Qatanani M, Moore DD. (2005) CAR, the continuously advancing receptor, in drug metabolism and disease. Curr Drug Metab 6:329–339 [DOI] [PubMed] [Google Scholar]
- Ryan PD, Goss PE. (2008) The emerging role of the insulin-like growth factor pathway as a therapeutic target in cancer. Oncologist 13:16–24 [DOI] [PubMed] [Google Scholar]
- Samani AA, Yakar S, LeRoith D, Brodt P. (2007) The role of the IGF system in cancer growth and metastasis: overview and recent insights. Endocr Rev 28:20–47 [DOI] [PubMed] [Google Scholar]
- Sarfstein R, Maor S, Reizner N, Abramovitch S, Werner H. (2006) Transcriptional regulation of the insulin-like growth factor-I receptor gene in breast cancer. Mol Cell Endocrinol 252:241–246 [DOI] [PubMed] [Google Scholar]
- Staudinger JL, Goodwin B, Jones SA, Hawkins-Brown D, MacKenzie KI, LaTour A, Liu Y, Klaassen CD, Brown KK, Reinhard J, et al. (2001) The nuclear receptor PXR is a lithocholic acid sensor that protects against liver toxicity. Proc Natl Acad Sci U S A 98:3369–3374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueyoshi T, Kawamoto T, Zelko I, Honkakoski P, Negishi M. (1999) The repressed nuclear receptor CAR responds to phenobarbital in activating the human CYP2B6 gene. J Biol Chem 274:6043–6046 [DOI] [PubMed] [Google Scholar]
- Tarn C, Rink L, Merkel E, Flieder D, Pathak H, Koumbi D, Testa JR, Eisenberg B, von Mehren M, Godwin AK. (2008) Insulin-like growth factor 1 receptor is a potential therapeutic target for gastrointestinal stromal tumors. Proc Natl Acad Sci U S A 105:8387–8392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thummel KE, Brimer C, Yasuda K, Thottassery J, Senn T, Lin Y, Ishizuka H, Kharasch E, Schuetz J, Schuetz E. (2001) Transcriptional control of intestinal cytochrome P-4503A by 1α,25-dihydroxy vitamin D3. Mol Pharmacol 60:1399–1406 [DOI] [PubMed] [Google Scholar]
- Velaparthi U, Wittman M, Liu P, Carboni JM, Lee FY, Attar R, Balimane P, Clarke W, Sinz MW, Hurlburt W, et al. (2008) Discovery and evaluation of 4-(2-(4-chloro-1H-pyrazol-1-yl)ethylamino)-3-(6-(1-(3-fluoropropyl)piperid in-4-yl)-4-methyl-1H-benzo[d]imidazol-2-yl)pyridin-2(1H)-one (BMS-695735), an orally efficacious inhibitor of insulin-like growth factor-1 receptor kinase with broad spectrum in vivo antitumor activity. J Med Chem 51:5897–5900 [DOI] [PubMed] [Google Scholar]
- Wang D, Li L, Fuhrman J, Ferguson S, Wang H. (2011) The role of constitutive androstane receptor in oxazaphosphorine-mediated induction of drug-metabolizing enzymes in human hepatocytes. Pharm Res 28:2034–2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Faucette S, Sueyoshi T, Moore R, Ferguson S, Negishi M, LeCluyse EL. (2003) A novel distal enhancer module regulated by pregnane X receptor/constitutive androstane receptor is essential for the maximal induction of CYP2B6 gene expression. J Biol Chem 278:14146–14152 [DOI] [PubMed] [Google Scholar]
- Wang H, LeCluyse EL. (2003) Role of orphan nuclear receptors in the regulation of drug-metabolising enzymes. Clin Pharmacokinet 42:1331–1357 [DOI] [PubMed] [Google Scholar]
- Wang H, Negishi M. (2003) Transcriptional regulation of cytochrome p450 2B genes by nuclear receptors. Curr Drug Metab 4:515–525 [DOI] [PubMed] [Google Scholar]
- Wittman M, Carboni J, Attar R, Balasubramanian B, Balimane P, Brassil P, Beaulieu F, Chang C, Clarke W, Dell J, et al. (2005) Discovery of a (1H-benzoimidazol-2-yl)-1H-pyridin-2-one (BMS-536924) inhibitor of insulin-like growth factor I receptor kinase with in vivo antitumor activity. J Med Chem 48:5639–5643 [DOI] [PubMed] [Google Scholar]
- Xie W, Barwick JL, Downes M, Blumberg B, Simon CM, Nelson MC, Neuschwander-Tetri BA, Brunt EM, Guzelian PS, Evans RM. (2000) Humanized xenobiotic response in mice expressing nuclear receptor SXR. Nature 406:435–439 [DOI] [PubMed] [Google Scholar]
- Zimmermann K, Wittman MD, Saulnier MG, Velaparthi U, Sang X, Frennesson DB, Struzynski C, Seitz SP, He L, Carboni JM, et al. (2010) SAR of PXR transactivation in benzimidazole-based IGF-1R kinase inhibitors. Bioorg Med Chem Lett 20:1744–1748 [DOI] [PubMed] [Google Scholar]