Abstract
Dystonia is a neurological disorder characterized by involuntary muscle contractions that cause twisting movements and abnormal postures. Functional imaging consistently reveals cerebellar overactivity in dystonic patients regardless of the type or etiology of the disorder. To explore mechanisms that might explain the basis for the cerebellar overactivity in dystonia, normal mice were challenged with intracerebellar application of a variety of agents that induce hyperexcitability. A nonspecific increase in cerebellar excitability, such as that produced by picrotoxin, was not associated with dystonia. Instead, glutamate receptor activation, specifically AMPA receptor activation, was necessary to evoke dystonia. AMPA receptor agonists induced dystonia, and AMPA receptor antagonists reduced the dystonia induced by glutamate receptor agonists. AMPA receptor antagonists also ameliorated the dystonia exhibited by the dystonic mouse mutant tottering, suggesting that AMPA receptors may play a role in some other genetic models of dystonia. Furthermore, AMPA receptor desensitization mediated the expression of dystonia. Preventing AMPA receptor desensitization with cyclothiazide or the nondesensitizing agonist kainic acid exacerbated the dystonic response. These results suggest the novel hypothesis that the cerebellar overactivity observed in neuroimaging studies of patients with dystonia may be an indirect reflection of abnormal glutamate signaling. In addition, these results imply that reducing AMPA receptor activation by blocking AMPA receptors and promoting AMPA receptor desensitization or negative allosteric modulators may prove to be beneficial for treating dystonia.
Introduction
Dystonia, the third most common movement disorder after tremor and Parkinson's disease, is characterized by involuntary muscle contractions that cause debilitating twisting movements and postures (Jankovic and Fahn, 1998). Dystonia is a heterogeneous disorder classified by the distribution of affected muscle groups. Focal or segmental forms of the disorder involve a small number of muscles, such as those of the hand in writer's cramp. Generalized dystonia is characterized by involvement of muscles throughout the body. Because the biological basis of dystonia is not well understood, therapies are largely unsatisfactory, palliative, and/or highly invasive. Small molecule drugs, including anticholinergics, neuroleptics, or benzodiazepines, are ineffective in most patients (Schwarz and Bressman, 2009; Kartha, 2010). Botulinum toxin treatment effectively reduces the abnormal muscle contractions. It requires injection directly into the affected muscles and is therefore practical only for the treatment of dystonias that affect a small number of muscles (Lim and Seet, 2010). Deep brain stimulation of the globus pallidus is another option but is obviously highly invasive (Krauss et al., 2004; Toda et al., 2004; Vidailhet et al., 2005). Development of treatments has been difficult because, unlike other movement disorders such as Parkinson's disease or Huntington disease where cell death provides clues to the pathogenesis, dystonia is not overtly degenerative. Because dystonia results from dysfunction, rather than degeneration, localizing mechanisms of dysfunction is challenging.
Functional imaging in patients is one approach used to localize dysfunction in dystonia. These studies often reveal abnormalities in basal ganglia, cortex, and brainstem. Neuroimaging studies also frequently reveal involvement of the cerebellum in many forms of dystonia (Neychev et al., 2011). Indeed, diffusion tensor imaging in dystonia patients implicates defects in cerebellothalamic connectivity as a proximal defect in the disorder (Argyelan et al., 2009). Both positron emission tomographic and functional magnetic resonance imaging studies implicate the cerebellum in different forms of generalized and segmental dystonias including DYT1 generalized dystonia (Eidelberg et al., 1998), hemidystonia (Ceballos-Baumann et al., 1995), and exercise-induced paroxysmal dystonia (Kluge et al., 1998). Likewise, cerebellar abnormalities are observed in functional imaging studies of focal dystonias, such as writer's cramp (Odergren et al., 1998; Preibisch et al., 2001), cervical dystonia (Galardi et al., 1996), and blepharospasm (Hutchinson et al., 2000). More specifically, increases in cerebellar perfusion or metabolism are consistently observed across functional imaging studies in dystonic patients, regardless of form or etiology.
Although it is difficult to determine mechanism from functional imaging in humans, animal models can be used to test hypotheses suggested by the results of studies performed in humans. Similar to the imaging studies in patients, abnormal cerebellar function is observed in genetic mouse and rat models of dystonia (LeDoux and Lorden, 2002; Chen et al., 2009; Zhang et al., 2011). Indeed, an increase in metabolic activity in the cerebellum is observed in mouse models of DYT1 dystonia (Uluğ et al., 2011; Zhao et al., 2011), similar to imaging studies in DYT1 patients. Furthermore, in normal mice, generalized dystonia is induced by delivery of the excitatory nonselective glutamate receptor agonist kainic acid to the midline cerebellum (Pizoli et al., 2002). However, cerebellar microinjection of the nonselective glutamate receptor antagonist 2,3-dihydroxy-6-nitro-7-sulfamoylbenzo(f)quinoxaline (NBQX) has no behavioral effects, suggesting that nonspecifically disrupting glutamatergic signaling is not sufficient to produce dystonia. Rather, the excitatory properties of kainate are likely necessary. The induction of dystonia via an excitatory agent applied to the cerebellum is in line with the human functional imaging studies that associate cerebellar hyperactivity with dystonia. Therefore, we explored the relationship between abnormal cerebellar excitation and dystonia in normal mice to determine whether a nonspecific increase in excitability was associated with dystonia or whether specific pathways were involved.
Materials and Methods
Animals.
Normal C57BL/6J mice, tottering mice (B6.D2-Cacna1atg/J), and Purkinje cell degeneration mice (B6.Br-Agtpbp1pcd/J) were obtained from The Jackson Laboratory (Bar Harbor, ME) and bred at Emory University. The pcd mutation causes the death of ∼99% of Purkinje cells by 2 months of age (Wassef et al., 1987). Male and female mice (8–12 weeks of age) were used in all experiments. All procedures conformed to the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996) and were approved by the Emory University Animal Care and Use Committee.
Microinjections.
Cerebellar microinjections were performed as described previously (Pizoli et al., 2002). The short-acting inhalant anesthetic isoflurane (IsoSol; Vedco, Inc., St. Joseph, MO) was used because rapid recovery after surgery was required to observe the behavioral effects of the microinjections. To limit the amount of time mice remained under anesthesia, all injections were made freehand using a standard needle length for reproducible injections. A midline incision was made over the skull, and a small hole was drilled over the cerebellum (approximate coordinates: −4.0 mm anteroposterior to the lamba, 0 mm lateral). A Hamilton syringe with a needle cut to 2 mm was used to deliver 0.5 μl of compound over 10 s. The wound was reapproximated and sealed with a drop of wound glue (3M Vetbond; 3M, St. Paul, MN). Mice recovered within 10 min. Microinjection sites, which were midline at the primary fissure, were histologically verified. We have previously demonstrated that interanimal injection sites are consistent and reproducible (Pizoli et al., 2002).
Cannula Implantation and Drug Delivery in Tottering Mice.
For NBQX experiments, cannulae were implanted in tottering mice for repeated injection so that each tottering mouse could be treated with both vehicle and NBQX to serve as their own controls. Kainate-treated tottering mice were treated as described above for normal mice. Tottering mice were anesthetized with 2,2,2-tribromoethanol. A hole was drilled at the same position as above. A guide cannula (Plastic One, Roanoke, VA) was implanted 1 mm below the skull surface and affixed with dental cement. Three to 5 days later, tottering mice were injected with 15 mg/kg caffeine (i.p.), which consistently induces generalized dystonia, and 0.5 μl of NBQX or saline delivered into the cerebellum. For each mouse, the order of drug and vehicle was pseudorandom.
Dystonia Assessments.
Animals were placed in an empty cage immediately after drug delivery, and behaviors were assessed for 1 h. To quantify dystonia, mice were rated using timed behavioral sampling. Observers were blinded to treatment. Each mouse was rated for 1 min every 10 min for a 1-h sampling period using a well established disability rating scale (Jinnah et al., 2000), where 0 = normal motor behavior; 1 = slightly slowed or abnormal movements; 2 = fleeting abnormal postures; 3 = moderate impairment, limited ambulation even when disturbed, frequent abnormal postures; and 4 = severe impairment, sustained abnormal postures. The six scores obtained during the 1-h scoring period were added to obtain a total score for the entire scoring period for each mouse. Therefore, the highest cumulative score for the 1-h scoring period was 24. Cumulative scores lower than 10, which would be achieved by ratings of only 1 or 2 in each scoring interval, suggest abnormal motor behavior such as motor slowing or an unsteady gait like that exhibited by the tottering mouse mutant at baseline, but not overt dystonia. If a mouse was quiescent at the start of a rating period, the animal was gently prodded to encourage movement because dystonia is movement-induced. Because veratridine delayed recovery from anesthesia, the scoring session was extended to 2 h to capture all relevant behaviors. For some experiments, each 1-min scoring period was divided into four bins of 15 s, so mice received four scores over the course of a minute. Mice were encouraged to move every 15 s during the 1-min scoring period.
Drugs.
Kainic acid [(2S,3S,4S)-carboxy-4-(1-methylethenyl)-3-pyrrolidineacetic acid], (2S,4R)-4-methylglutamic acid (SYM 2081), (S)-AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid), (R)-AMPA, 4-(8-methyl-9H-1,3-dioxolo[4,5-h][2,3]benzodiazepin-5-yl)-benzenaminedihydrochloride (GYKI 52466 dihydrochloride), NBQX disodium salt [3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo(f)quinoxaline-7-sulfonamide], (5S,10R)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine (MK 801 maleate), (1S,3R)-1-aminocyclopentane-1,3-dicarboxylic acid (ACPD), (RS)-α-methyl-4-carboxyphenylglycine (MCPG disodium salt), (αS)-α-amino-3-([4-carboxyphenyl]methyl)-3,4-dihydro-5-iodo-2,4-dioxo-1(2H)-pyrimidinepropanoic acid (UBP 301), 4-aminopyridine (4-AP), cyclothiazide [6-chloro-3,4-dihydro-3-(5-norbornen-2-yl)-2H-1,2,4-benzothiazidiazine-7-sulfonamide-1,1-dioxide], and picrotoxin were obtained from Tocris Bioscience (Ellisville, MO) and dissolved in 0.9% saline or phosphate-buffered saline. Veratridine (4α,9-epoxy-3β-veratroyloxy-5β-cevan-4β,12,14,16β,17, 20-hexaol) was purchased from Sigma-Aldrich (St. Louis, MO) and dissolved in 0.9% saline. 1,4-Dihydro-2,6-dimethyl-5-nitro-4-(2-(trifluoromethyl)phenyl)-3-pyridinecarboxylic acid (Bay K 8644) was purchased from Sigma-Aldrich and dissolved in 0.4% dimethyl sulfoxide in phosphate-buffered saline with sonication.
Data Analysis.
An arithmetic sum of the disability scores was used to calculate a total score for the entire 1-h session. These data approximate a continuous variable when total scores from one animal are added; thus, data were analyzed using parametric tests, in this case, one-way analyses of variance (ANOVAs). Variance was determined for each mouse by calculating the variance of the four scores from each of the 1-min scoring intervals. Then mean variance for each subject was obtained using the six values obtained in the 1-h scoring session. StatView software version 5.0.1 (SAS Institute, Inc., Cary, NC) was used for statistical analyses. All data are expressed as means ± S.E.M.
Results
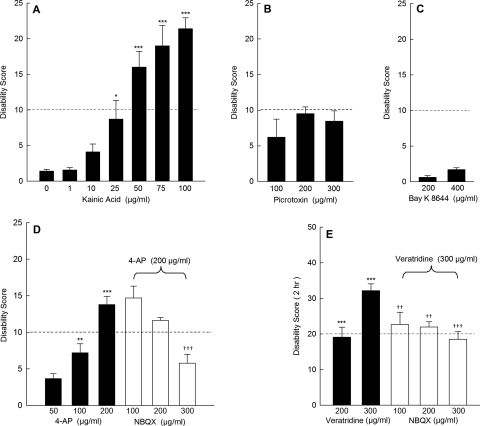
To determine whether cerebellar excitation is associated with dystonia, regardless of the specific action of the stimulus, mice were challenged with a variety of excitatory compounds via direct cerebellar microinjection. As reported previously, intracerebellar administration of the nonselective glutamate receptor agonist kainic acid, which acts at both AMPA and kainate receptors, dose-dependently induced generalized dystonia [Fig. 1A; for photographs of the behavior, see Pizoli et al. (2002)]. We have previously demonstrated that the action of microinjected kainic acid is restricted to the cerebellum and that lesioning cerebellar Purkinje cells abolishes kainate-induced dystonia, suggesting that the cerebellar cortex is necessary for the induction of dystonia (Pizoli et al., 2002). In contrast to kainic acid, cerebellar microinjection of the GABAA receptor antagonist picrotoxin did not provoke dystonia even at concentrations that have previously been shown to increase Purkinje cell firing rates in vivo (Thomsen et al., 2004; Marshall and Lang, 2009). However, abnormal motor coordination and slowing were observed accounting for the increase in disability scores above baseline (Fig. 1B), suggesting that picrotoxin exerted an effect on the central nervous system.
Fig. 1.
Effects of excitatory agents delivered to the cerebellum on dystonia. A, the mixed AMPA/kainate receptor agonist kainic acid dose-dependently induced dystonia (one-way ANOVA; F6,27 = 19.4, p < 0.0001, n = 4–5/dose). B, the GABAA antagonist picrotoxin did not induce dystonia but caused abnormal coordination that resulted in an increase in disability scores (one-way ANOVA; F3,16 = 5.8, p < 0.01, n = 4–6/dose). C, the l-type calcium channel activator Bay K 8644 did not induce dystonia (n = 4–5/dose). D, the potassium channel blocker 4-AP dose-dependently induced dystonia (one-way ANOVA; F3,24 = 30.0, p < 0.0001, n = 5–12/dose), which was dose-dependently reduced by the nonselective glutamate receptor antagonist NBQX (one-way ANOVA; F3,27 = 10.3, p < 0.0001, n = 6–12/dose). E, the sodium channel activator veratridine dose-dependently induced dystonia (one-way ANOVA; F2,16 = 52.9, p < 0.00001, n = 5–8/dose), which was reduced by the nonselective glutamate receptor antagonist NBQX (one-way ANOVA; F3,23 = 6.5, p < 0.01, n = 6–8/dose). For ease of presentation, a vehicle control bar is included in only one panel. Disability scores lower than 10 (dashed lines) suggest abnormal motor behavior, such as an unsteady gait, but not overt dystonia. Values represent the mean of the cumulative disability score in 1 h (or 2 h for veratridine) ± S.E.M. and **, p < 0.01 and ***, p < 0.001 compared with vehicle; ††, p < 0.01 and †††, p < 0.001 compared with 200 μg/ml 4-AP alone or compared with 300 μg/ml veratridine alone.
Cerebellar microinjection of the l-type calcium channel activator Bay K 8644 did not induce dystonia at doses up to 400 μg/ml (∼1 mM; Fig. 1C). This result was surprising because peripheral administration of Bay K 8644 induces dystonia in mice (Jinnah et al., 2000). To verify that abnormal cerebellar excitation is not involved in the dystonia provoked by peripheral administration of Bay K 8644, mice lacking cerebellar Purkinje cells (pcd mice) were challenged with peripheral administration of vehicle or Bay K 8644. Normal control mice given intraperitoneal injections of vehicle did not exhibit abnormal motor behavior (average cumulative disability score = 0; n = 4). The pcd mice treated with a peripheral injection of vehicle exhibited mild motor disability consistent with their mildly ataxic gait (average cumulative disability score = 8 ± 1.5; n = 3). After peripheral administration of 4 mg/kg Bay K 8644, which reliably induces generalized dystonia in normal mice, both normal control mice (n = 4) and pcd mice (n = 4) had similar dystonic responses (average cumulative disability score for control = 16 ± 0.5 and for pcd = 20 ± 1). These results imply that the dystonia induced by peripherally administered Bay K 8644 is not dependent on signaling from the cerebellar cortex, consistent with the idea that dystonia may arise from many different brain regions (Neychev et al., 2011). These results are also consistent with the lack of response to intracerebellar delivery of Bay K 8644.
In contrast to picrotoxin and Bay K 8644, intracerebellar administration of either the sodium channel activator veratridine or the Kv potassium channel blocker 4-AP induced dystonia (Fig. 1, D and E). At high concentrations (>100 μM), such as the highest doses used here, 4-AP potentiates cerebellar activity by enhancing glutamate-evoked Purkinje cell excitatory postsynaptic currents in vitro (Alviña and Khodakhah, 2010). Therefore, to determine whether the dystonia produced by 4-AP was actually contingent on glutamatergic transmission rather than the excitatory effects of blocking potassium channels per se, the nonselective glutamate antagonist NBQX was coadministered with 4-AP. NBQX dose-dependently blocked the dystonia evoked by 4-AP (Fig. 1D). In light of these results, NBQX was coadministered with veratridine to determine whether glutamatergic transmission was also integral to the dystonic response to veratridine. Similar to 4-AP, the dystonia induced by veratridine was dose-dependently reduced by NBQX (Fig. 1E). Taken together, these results suggest that dystonia was not induced by nonspecific hyperexcitability but was dependent on glutamatergic signaling.
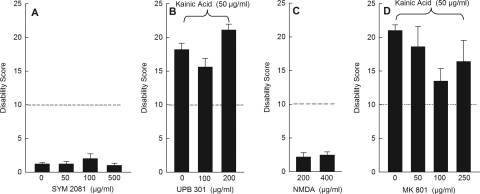
To identify the glutamate receptor subtypes that mediate dystonia, receptor-selective agonists were tested for their ability to induce dystonia. Conversely, receptor-selective antagonists were tested for their ability to reduce the dystonic response to the nonselective glutamate receptor agonist kainic acid. All antagonists were first tested alone without coadministration of kainic acid to determine effects on motor behavior and disability scores. Antagonists alone had no behavioral effect. To test the involvement of kainate receptors, the kainate receptor agonist SYM 2081, which has 3000-fold selectivity for kainate receptors over AMPA receptors, was microinjected into the cerebellum. Unlike kainic acid, SYM 2081 did not induce dystonia over a wide range of doses (Fig. 2A). Furthermore, the kainate receptor antagonist UBP 301 did not block dystonia when coadministered with kainic acid (Fig. 2B). To test the involvement of NMDA receptors, the agonist NMDA was microinjected into the cerebellum but did not cause dystonia (Fig. 2C). In addition, administration of the NMDA receptor antagonist MK 801 with kainic acid did not affect kainic acid-induced dystonia (Fig. 2D). These results suggest that kainate receptors and NMDA receptors play little role, if any, in the dystonic response.
Fig. 2.
Effects of kainate receptor and NMDA receptor agents on dystonia. A, the selective kainate receptor agonist SYM 2081 did not induce dystonia (n = 5/dose). B, the selective kainate receptor antagonist UBP 301 did not reduce kainic acid-induced dystonia (n = 8–10/dose). C, the selective agonist NMDA did not alter kainic acid-induced dystonia (n = 5–6/dose). D, the selective NMDA receptor antagonist MK 801 did not block or reduce kainic acid-induced dystonia (n = 4–5/dose). For ease of presentation, a vehicle control bar is included in only one panel. Disability scores lower than 10 (dashed lines) suggest abnormal motor behavior, such as an unsteady gait, but not overt dystonia. Values represent the mean of the cumulative disability score in 1 h ± S.E.M.
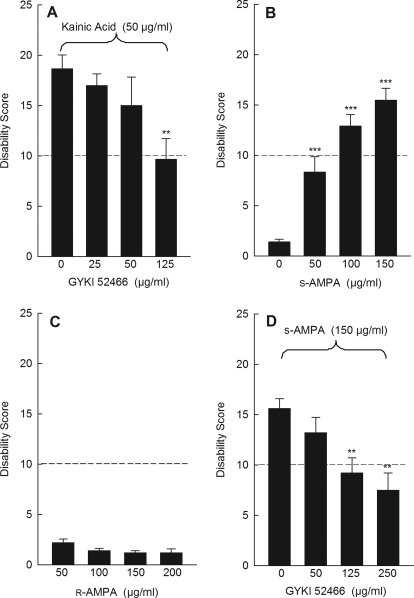
Kainic acid is not particularly specific for its cognate receptor. Indeed, kainic acid acts at AMPA receptors to induce large sustained currents (Kiskin et al., 1986; Patneau and Mayer, 1991). To determine whether AMPA receptors mediate dystonia, mice were challenged with kainic acid while blocking AMPA receptors with the noncompetitive AMPA receptor antagonist GYKI 52466, which has 40-fold selectivity for AMPA receptors over kainate receptors. Although intracerebellar administration of GYKI 52466 by itself had no effect on motor behavior (data not shown), GYKI 52466 dose-dependently inhibited kainic acid-induced dystonia (Fig. 3A). To determine whether AMPA receptor activation induces dystonia, (S)-AMPA, an AMPA receptor-selective agonist, was used. (S)-AMPA dose-dependently induced dystonia (Fig. 3B). In contrast, cerebellar microinjection of the inactive enantiomer (R)-AMPA had no effect, demonstrating the receptor specificity of the effect (Fig. 3C). Consistent with an AMPA receptor effect, the AMPA receptor antagonist GYKI 52466 dose-dependently reduced (S)-AMPA-induced dystonia (Fig. 3D). Curiously, although AMPA has higher affinity than kainic acid for AMPA receptors and the molarity of AMPA exceeded that of kainic acid at the highest doses tested, dystonia induced by (S)-AMPA was consistently less severe than that induced by kainic acid. Because kainate is an agonist at both AMPA and kainate receptors, it was possible that coactivation of AMPA and kainate receptors potentiated the dystonia. However, dystonia induced by coadministration of AMPA plus the kainate receptor agonist SYM 2081 did not differ from dystonia induced by AMPA alone (data not shown).
Fig. 3.
AMPA receptors mediate dystonia. A, the selective AMPA receptor antagonist GYKI 52466 dose-dependently blocked kainic acid -induced dystonia (one-way ANOVA; F3,20 = 4.0, p < 0.05, n = 6/dose). B, the selective AMPA receptor agonist (S)-AMPA dose-dependently induced dystonia (one-way ANOVA; F3,18 = 27.4, p < 0.0001, n = 5–7/dose). C, (R)-AMPA, the inactive enantiomer of (S)-AMPA, did not induce dystonia (one-way ANOVA; F4,20 = 1.9, p > 0.1, n = 5/dose). D, the selective AMPA receptor antagonist GYKI 52466 dose-dependently blocked (S)-AMPA-induced dystonia (one-way ANOVA; F3,20 = 5.7, p < 0.01, n = 5–7/dose). For ease of presentation, a vehicle control bar is included in only one panel. Disability scores lower than 10 (dashed lines) suggest abnormal motor behavior, such as an unsteady gait, but not overt dystonia. Values represent the mean of the cumulative disability score in 1 h ± S.E.M.; **, p < 0.01 and ***, p < 0.001 compared with vehicle.
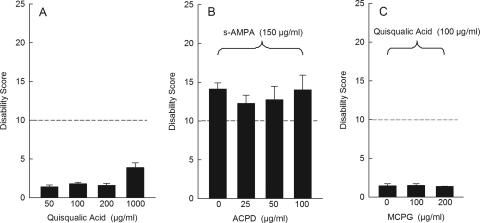
To further explore the role of AMPA receptor activation in dystonia, we also tested quisqualic acid, an AMPA receptor-selective agonist that has a high affinity for AMPA receptors (Giberti et al., 1991). We were surprised to find that intracerebellar administration of quisqualate did not induce dystonia at any dose, even at doses as high as 1 mg/ml (Fig. 4A). The unique features of quisqualate may explain the response. Although quisqualic acid is a potent AMPA receptor agonist, it is also an agonist at type 1 and type 2 metabotropic glutamate receptors (mGluR). Conjunctive activation of AMPA receptors and mGluR1 receptors, as occurs with quisqualic acid, causes long-term depression in parallel fiber-Purkinje cell synapses (Linden et al., 1991). The induction of long-term depression might explain the lack of dystonia with quisqualic acid challenge. To test this hypothesis, AMPA was coadministered with the nonselective mGluR agonist ACPD to mimic the simultaneous activation caused by quisqualic acid. ACPD did not attenuate AMPA-induced dystonia (Fig. 4B). Similar results were obtained in response to coadministration of AMPA plus 3,5-dihydroxyphenylglyine, a selective mGluR1 agonist (data not shown). Conversely, quisqualic acid was coadministered with the mGluR antagonist (RS)-α-methyl-4-carboxyphenylglycine (MCPG) to block conjunctive activation. MCPG did not potentiate the effects of quisqualate (Fig. 4C), suggesting that coactivation of AMPA and mGluR does not explain the failure of quisqualate to induce dystonia.
Fig. 4.
Effects of the mixed AMPA/mGluR agonist quisqualic acid on dystonia. A, intracerebellar administration of quisqualic acid did not induce dystonia (one-way ANOVA; F3,20 = 3.9, p > 0.1, n = 5). B, coadministration of the nonselective mGluR agonist ACPD failed to inhibit (S)-AMPA-induced dystonia (one-way ANOVA: F2,12 = 0.1, p > 0.1, n = 5/dose). C, coadministration of the nonselective mGluR antagonist MCPG did not facilitate quisqualic acid-induced dystonia (one-way ANOVA: F3,25 = 0.5, p > 0.1, n = 5/dose). For ease of presentation, a vehicle control bar is included in only one panel. Disability scores lower than 10 (dashed lines) suggest abnormal motor behavior, such as an unsteady gait, but not overt dystonia. Values represent the mean of the cumulative disability score in 1 h ± S.E.M.
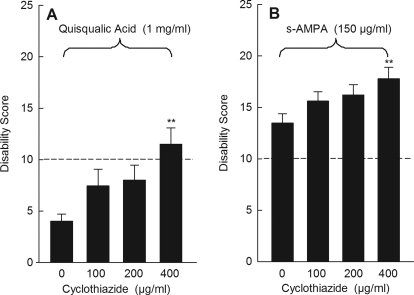
Another unique feature of quisqualic acid is that it causes rapid AMPA receptor desensitization (Huettner, 1990; Patneau and Mayer, 1991), whereas kainic acid elicits nondesensitizing or slowly desensitizing responses (Kiskin et al., 1986; Patneau and Mayer, 1991). This important difference may explain the strong induction of dystonia with kainate whereby prolonged receptor activation may be necessary to elicit dystonia. To determine whether receptor desensitization accounts for the lack of response to quisqualate, we blocked desensitization with cyclothiazide, an allosteric inhibitor of AMPA receptor desensitization (Yamada and Tang, 1993). Cyclothiazide alone had no behavioral effect (data not shown). When coadministered with quisqualic acid, cyclothiazide dose-dependently increased the dystonic response (Fig. 5A). To determine whether receptor desensitization also mediates the response to AMPA, which is also a desensitizing agonist, we again blocked desensitization with cyclothiazide. When coadministered with AMPA, cyclothiazide dose-dependently increased the dystonic response (Fig. 5B).
Fig. 5.
Effect of cyclothiazide on quisqualic acid- and AMPA-induced dystonia. A, cyclothiazide dose-dependently promoted quisqualic acid-induced dystonia (one-way ANOVA: F3,25 = 3.9, p < 0.05, n = 6–8/dose). B, cyclothiazide dose-dependently potentiated (S)-AMPA-induced dystonia (one-way ANOVA: F3,22 = 3.4, p < 0.05, n = 6–7/dose). Disability scores lower than 10 (dashed lines) suggest abnormal motor behavior, such as an unsteady gait, but not overt dystonia. Values represent the mean of the cumulative disability score in 1 h ± S.E.M.; **, p < 0.01 compared with vehicle.
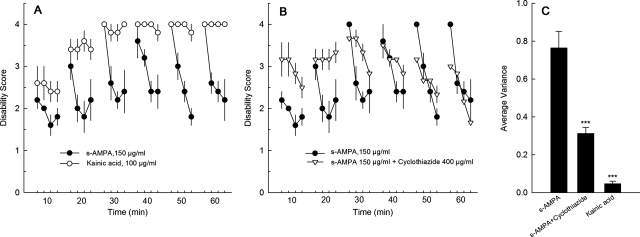
Desensitization could also explain why the dystonia provoked by kainate was more robust than that provoked by AMPA. Whereas kainate is nondesensitizing, AMPA causes desensitization of glutamate receptors. However, the desensitizing response to AMPA is less vigorous than that of quisqualate. Therefore, to better define the characteristics of the dystonic response to kainate and AMPA, mice were rated every 15 s within the 1-min scoring period. Mice were encouraged to move every 15 s during the 1-min scoring period with gentle prodding because dystonia is movement-induced. The response to kainate was relatively constant across the entire minute, with consistently high scores that indicate sustained abnormal postures (Fig. 6A) and low variance in the scores across the 1-min scoring period (Fig. 6C). In contrast, the dystonia induced by (S)-AMPA fluctuated over the course of the 1-min scoring period (Fig. 6A). Mice treated with AMPA exhibited dystonia that was comparable in severity to kainate-induced dystonia at the beginning of the 1-min scoring period. However, the severity of the dystonia gradually subsided during the scoring period, ending with mild disability. The fluctuation in the severity of the AMPA-induced dystonia across the 1-min scoring interval is reflected by a variance that is significantly greater than the variance observed with kainate-induced dystonia (Fig. 6C) and suggests an effect of desensitization. To test this hypothesis, AMPA was coadministered with cyclothiazide to block desensitization. Cyclothiazide potentiated (S)-AMPA-induced dystonia, increasing the overall severity of the dystonia (Fig. 6, B and C). In addition, the behavioral pattern produced by coadministration of cyclothiazide and AMPA was more sustained than that produced by AMPA alone, as reflected by a significant reduction in the variance (Fig. 6C).
Fig. 6.
Temporal effects of kainic acid- and AMPA-induced dystonia. Each individual mouse was observed for 1 min every 10 min for 1 h; each 1-min observation period was divided into four intervals of 15 s, so mice received four scores in each 1-min observation period. Each 15-s bin is plotted. Mice were then left undisturbed for 9 min until the next scoring period. A, kainic acid- and AMPA-induced dystonia plotted over the course of 1 h (n = 5/drug). B, effects of cyclothiazide on AMPA-induced dystonia plotted over the course of 1 h (n = 5–6/drug); data for AMPA alone is repeated from (A). Values represent the mean disability score ± S.E.M. C, average variance ± S.E.M. of the disability scores presented in A and B for kainate, AMPA, and AMPA + cyclothiazide (***, p < 0.001 compared with AMPA alone; Student's t test, n = 5–6/drug).
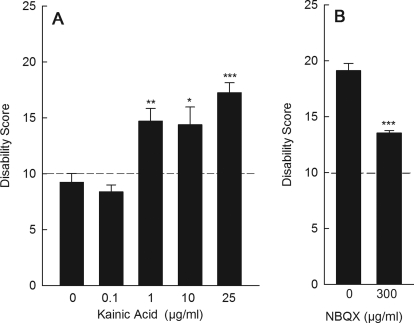
To determine whether glutamate receptor signaling also may be relevant in other models of dystonia, tottering mice were challenged with kainic acid. Tottering mice exhibit episodes of generalized dystonia superimposed on a mildly ataxic baseline that are caused by a mutation in the Cacna1a gene, which encodes the P/Q-type calcium channel. In both humans and mice, some mutations in CACNA1A are associated with dystonia (Raike et al., 2005). As in normal mice, cerebellar microinjection of kainic acid in tottering mice dose-dependently induced dystonia. However, tottering mice were exquisitely sensitive to kainic acid, exhibiting dystonia in response to as little as 1 μg/ml kainic acid (Fig. 7A), a dose that does not induce dystonia in normal mice (Fig. 1A). Furthermore, the severity of the dystonia in tottering mice was significantly reduced by intracerebellar delivery of the glutamate receptor antagonist NBQX (Fig. 7B).
Fig. 7.
Effect of kainic acid and NBQX on tottering mouse dystonia. A, intracerebellar administration of the mixed AMPA/kainate receptor agonist kainic acid dose-dependently induced dystonia in tottering mice (one-way ANOVA; F4,45 = 17.0, p < 0.0001, n = 8–13/dose). B, intracerebellar administration of the nonselective glutamate receptor antagonist NBQX reduced the severity of the dystonia in tottering mice (paired t test, n = 6). Disability scores lower than 10 (dashed lines) suggest abnormal motor behavior, such as an unsteady gait, but not overt dystonia. Values represent the mean of the cumulative disability score in 1 h ± S.E.M.; *, p < 0.05, **, p < 0.01, and ***, p < 0.001 compared with vehicle.
Discussion
Here, we demonstrated that a nonspecific increase in cerebellar excitability was not associated with dystonia but glutamate receptor activation was. The effect was specific for AMPA receptors. Whereas kainate and NMDA receptor-selective compounds did not affect dystonia, AMPA receptor agonists induced dystonia, and AMPA receptor antagonists ameliorated the dystonia induced by glutamate receptor agonists. AMPA receptor agonists and antagonists similarly affected the dystonia exhibited by the mouse mutant tottering, a genetic model of generalized dystonia, suggesting that AMPA receptors may play a role in different types of dystonia. Furthermore, AMPA receptor desensitization mediated the expression of dystonia whereby blocking desensitization exacerbated the dystonic response.
The specificity for AMPA receptors is not surprising, because AMPA receptors dominate glutamate responses in Purkinje cells (Häusser and Roth, 1997), the only efferents of the cerebellar cortex. Although NMDA receptors are abundant in cerebellar granule cells (Watanabe et al., 1994) and are also expressed in mature Purkinje cells, where they mediate long-term depression (Piochon et al., 2010), this local synaptic function may be too subtle to influence the expression of dystonia. Kainate receptors are predominantly expressed presynaptically on granule cells, where they mediate glutamate release onto Purkinje cells (Delaney and Jahr, 2002). Neither a kainate receptor agonist alone nor a kainate receptor agonist combined with an AMPA receptor agonist influenced the expression of dystonia, suggesting that the presynaptic effects of kainate receptors are not adequate to influence the dystonic response.
Kainate and AMPA but not quisqualic acid induced dystonia. This was surprising because quisqualic acid has a higher affinity for AMPA receptors than kainate or AMPA (Giberti et al., 1991). In addition, quisqualate has greater efficacy, as determined by the agonist-induced maximal transient peak current, Ca2+ current, or the EC50 of transient peak current (Kiskin et al., 1986; Patneau and Mayer, 1991; Wyllie and Cull-Candy, 1994). Receptor desensitization, rather than acute activation properties such as affinity or efficacy, may explain the difference in action. When receptor activation is determined as steady-state current or the ratio between steady-state current and peak current, rank order is kainic acid>AMPA>quisqualic acid (Huettner, 1990; Patneau and Mayer, 1991; Wyllie and Cull-Candy, 1994; Wilding and Huettner, 1997), demonstrating that quisqualic acid causes rapid desensitization relative to kainate and AMPA. Cyclothiazide allosterically binds to AMPA receptors, increasing agonist-induced steady-state current and preventing AMPA desensitization (Yamada and Tang, 1993). Here, cyclothiazide potentiated AMPA-induced dystonia and induced dystonia in combination with quisqualic acid, suggesting that AMPA receptor desensitization contributes to the expression of dystonia.
AMPA induced a cyclical dystonic response. Severe dystonia occurred at the start of the scoring period when the mouse was encouraged to move and then subsided over the course of a minute, despite additional nudges. After mice were left undisturbed for 10 min, the severity of the AMPA-induced dystonia rebounded, and the pattern recurred. This phasic response was significantly diminished by cyclothiazide, suggesting a cycle of AMPA receptor desensitization and resensitization. However, it is difficult to reconcile the rebound to a more severe dystonic state at the beginning of the scoring period with the continuous presence of the desensitizing agonist AMPA. Although AMPA receptor activation does mediate receptor desensitization, other factors also contribute. In hippocampal neurons, activity-dependent increases in intracellular calcium reduce AMPA receptor mobility, resulting in a reduction in functional receptors available for activation by a subsequent stimulus (Heine et al., 2008). This decrement in response can persist for more than 5 min (Heine et al., 2008). It is known that the initiation of movement, as when the mice are encouraged to move at the beginning of a scoring session, induces glutamate release via climbing fibers (Kim et al., 1987), which may facilitate the immobilization of AMPA receptors through mass activation of glutamate receptors and hence calcium mobilization. After 10 undisturbed minutes, which fits well with the long-lasting time course reported for hippocampal neurons, receptors recover and the cycle starts again.
A generic increase in excitation was not associated with the induction of dystonia. Others have demonstrated that delivery of 0.5 mM picrotoxin to the cerebellar surface of anesthetized rats increases Purkinje cell firing rates by 2- to 3-fold (Thomsen et al., 2004; Marshall and Lang, 2009). However, in the experiments presented here with a similar method of delivery, this concentration of picrotoxin did not produce dystonia. Although the excitatory agents 4-AP and veratridine induced dystonia, the effect was abrogated when glutamatergic transmission was blocked, suggesting that increasing overall excitability is not adequate to induce dystonia without accompanying glutamatergic transmission. The quality of the neuronal firing patterns may explain the dissociation between cerebellar excitation and glutamatergic signaling in the induction of dystonia. For example, picrotoxin increases Purkinje cell firing rates of both simple spikes and complex spikes (Marshall and Lang, 2009). In contrast, a reduction in complex spike frequency and irregular bursts of simple spikes is observed in single unit recordings of Purkinje cells from the dystonic (dt) rat, a model of generalized dystonia (LeDoux and Lorden, 2002), but overall firing rates are not changed, suggesting that abnormal patterns rather than absolute excitability is important. Although AMPA receptors appear to be central to this effect, it is worth noting that veratridine and 4-AP also induced dystonia by indirectly perturbing AMPA receptor signaling. Thus, there are several different routes to producing these abnormal signals.
Functional neuroimaging in humans consistently reveals cerebellar overactivity in dystonia. Functional imaging is based on the premise that there is a tight correlation between neuronal activity and blood flow or metabolism. This association is based on studies of forebrain regions. However, in cerebellum, neuronal activity and blood flow are not so tightly linked. In fact, even a 300% increase in Purkinje cell firing rate is not sufficient to elicit a change in blood flow (Thomsen et al., 2004). Indeed, cerebellar blood flow is not responsive to large changes in local field potentials in the cerebellum (Caesar et al., 2003). Instead, stimulation of parallel fibers or climbing fibers, which elicits glutamate release, increases cerebellar blood flow even when spike firing is inhibited (Mathiesen et al., 1998; Offenhauser et al., 2005). Conversely, AMPA receptor antagonists attenuate changes in both blood flow and tissue oxygen partial pressure (Mathiesen et al., 1998; Offenhauser et al., 2005). This glutamate-induced increase in cerebellar blood flow is attributed, at least in part, to an AMPA receptor-mediated induction of nitric oxide, which leads to vasodilation and therefore increased blood flow (Yang and Iadecola, 1996). Similar to the data presented here, this effect is apparently specific to AMPA receptors because NMDA receptor antagonists have no effect on glutamate-induced increases in blood flow.
Animal models are useful for generating mechanistic explanations for phenomena observed in patient studies. Such hypotheses can then be tested in humans to promote our understanding of the disease process. Although an increase in cerebellar metabolic activity is consistently observed in patients with dystonia, the mechanisms underlying the increase are unknown. Furthermore, it is not clear whether the overactivity observed in the cerebellum of dystonic patients contributes to or is a consequence of the dystonia, although a recent diffusion tensor imaging study suggests that abnormalities in cerebellothalamic connectivity are a primary defect (Argyelan et al., 2009). The work presented here in animals coupled with the results from imaging and blood flow studies suggests the novel hypothesis that the increase in cerebellar signal observed in neuroimaging studies of patients with dystonia may be an indirect reflection of abnormal AMPA receptor activation. Furthermore, this abnormal glutamatergic signaling may play a causal role. The obvious correlate of this hypothesis is that reducing AMPA receptor signaling by directly blocking AMPA receptors, by promoting AMPA receptor desensitization or by negative allosteric modulation of AMPA receptors, may prove useful in the treatment of dystonia.
This work was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grants R01-NS33592, R01-NS040470].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- NBQX
- 2,3-dihydroxy-6-nitro-7-sulfamoylbenzo(f)quinoxaline
- AMPA
- α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- ACPD
- (1S,3R)-1-aminocyclopentane-1,3-dicarboxylic acid
- ANOVA
- analysis of variance
- NMDA
- N-methyl-d-aspartate
- mGluR
- metabotropic glutamate receptor
- MCPG
- (RS)-α-methyl-4-carboxyphenylglycine
- SYM 2081
- (2S,4R)-4-methylglutamic acid
- GYKI 52466 dihydrochloride
- 4-(8-methyl-9H-1,3-dioxolo[4,5-h][2,3]benzodiazepin-5-yl)-benzenaminedihydrochloride
- MK 801 maleate
- (5S,10R)-(+)-5-methyl-10,11-dihydro-5H-dibenzo[a,d]cyclohepten-5,10-imine
- UBP 301
- (αS)-α-amino-3-([4-carboxyphenyl]methyl)-3,4-dihydro-5-iodo-2,4-dioxo-1(2H)-pyrimidinepropanoic acid
- 4-AP
- 4-aminopyridine
- Bay K 8644
- 1,4-dihydro-2,6-dimethyl-5-nitro-4-(2-(trifluoromethyl)phenyl)-3-pyridinecarboxylic acid.
Authorship Contributions
Participated in research design: Fan, Hughes, Jinnah, and Hess.
Conducted experiments: Fan and Hughes.
Performed data analysis: Fan and Hess.
Wrote or contributed to the writing of the manuscript: Fan, Jinnah, and Hess.
References
- Alviña K, Khodakhah K. (2010) The therapeutic mode of action of 4-aminopyridine in cerebellar ataxia. J Neurosci 30:7258–7268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argyelan M, Carbon M, Niethammer M, Ulug AM, Voss HU, Bressman SB, Dhawan V, Eidelberg D. (2009) Cerebellothalamocortical connectivity regulates penetrance in dystonia. J Neurosci 29:9740–9747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caesar K, Thomsen K, Lauritzen M. (2003) Dissociation of spikes, synaptic activity, and activity-dependent increments in rat cerebellar blood flow by tonic synaptic inhibition. Proc Natl Acad Sci USA 100:16000–16005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos-Baumann AO, Passingham RE, Marsden CD, Brooks DJ. (1995) Motor reorganization in acquired hemidystonia. Ann Neurol 37:746–757 [DOI] [PubMed] [Google Scholar]
- Chen G, Popa LS, Wang X, Gao W, Barnes J, Hendrix CM, Hess EJ, Ebner TJ. (2009) Low-frequency oscillations in the cerebellar cortex of the tottering mouse. J Neurophysiol 101:234–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney AJ, Jahr CE. (2002) Kainate receptors differentially regulate release at two parallel fiber synapses. Neuron 36:475–482 [DOI] [PubMed] [Google Scholar]
- Eidelberg D, Moeller JR, Antonini A, Kazumata K, Nakamura T, Dhawan V, Spetsieris P, deLeon D, Bressman SB, Fahn S. (1998) Functional brain networks in DYT1 dystonia. Ann Neurol 44:303–312 [DOI] [PubMed] [Google Scholar]
- Galardi G, Perani D, Grassi F, Bressi S, Amadio S, Antoni M, Comi GC, Canal N, Fazio F. (1996) Basal ganglia and thalamo-cortical hypermetabolism in patients with spasmodic torticollis. Acta Neurol Scand 94:172–176 [DOI] [PubMed] [Google Scholar]
- Giberti A, Ratti E, Gaviraghi G, van Amsterdam FT. (1991) Binding of DL-[3H]alpha-amino-3-hydroxy-5-methyl-isoxazole-4-propionic acid (AMPA) to rat cortex membranes reveals two sites or affinity states. J Recept Res 11:727–741 [DOI] [PubMed] [Google Scholar]
- Häusser M, Roth A. (1997) Dendritic and somatic glutamate receptor channels in rat cerebellar Purkinje cells. J Physiol 501 (Pt 1):77–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine M, Groc L, Frischknecht R, Béïque JC, Lounis B, Rumbaugh G, Huganir RL, Cognet L, Choquet D. (2008) Surface mobility of postsynaptic AMPARs tunes synaptic transmission. Science 320:201–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettner JE. (1990) Glutamate receptor channels in rat DRG neurons: activation by kainate and quisqualate and blockade of desensitization by Con A. Neuron 5:255–266 [DOI] [PubMed] [Google Scholar]
- Hutchinson M, Nakamura T, Moeller JR, Antonini A, Belakhlef A, Dhawan V, Eidelberg D. (2000) The metabolic topography of essential blepharospasm: a focal dystonia with general implications. Neurology 55:673–677 [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington DC [Google Scholar]
- Jankovic J, Fahn S. (1998) Dystonic disorders, in Parkinson's Disease and Movement Disorders (Jankovic J, Tolosa E. eds) pp 513–551, Williams & Wilkins, Baltimore [Google Scholar]
- Jinnah HA, Sepkuty JP, Ho T, Yitta S, Drew T, Rothstein JD, Hess EJ. (2000) Calcium channel agonists and dystonia in the mouse. Mov Disord 15:542–551 [DOI] [PubMed] [Google Scholar]
- Kartha N. (2010) Therapeutic challenges in dystonia. Neurol Clin 28:927–940 [DOI] [PubMed] [Google Scholar]
- Kim JH, Wang JJ, Ebner TJ. (1987) Climbing fiber afferent modulation during treadmill locomotion in the cat. J Neurophysiol 57:787–802 [DOI] [PubMed] [Google Scholar]
- Kiskin NI, Krishtal OA, Tsyndrenko AYa. (1986) Excitatory amino acid receptors in hippocampal neurons: kainate fails to desensitize them. Neurosci Lett 63:225–230 [DOI] [PubMed] [Google Scholar]
- Kluge A, Kettner B, Zschenderlein R, Sandrock D, Munz DL, Hesse S, Meierkord H. (1998) Changes in perfusion pattern using ECD-SPECT indicate frontal lobe and cerebellar involvement in exercise-induced paroxysmal dystonia. Mov Disord 13:125–134 [DOI] [PubMed] [Google Scholar]
- Krauss JK, Yianni J, Loher TJ, Aziz TZ. (2004) Deep brain stimulation for dystonia. J Clin Neurophysiol 21:18–30 [DOI] [PubMed] [Google Scholar]
- LeDoux MS, Lorden JF. (2002) Abnormal spontaneous and harmaline-stimulated Purkinje cell activity in the awake genetically dystonic rat. Exp Brain Res 145:457–467 [DOI] [PubMed] [Google Scholar]
- Lim EC, Seet RC. (2010) Use of botulinum toxin in the neurology clinic. Nat Rev Neurol 6:624–636 [DOI] [PubMed] [Google Scholar]
- Linden DJ, Dickinson MH, Smeyne M, Connor JA. (1991) A long-term depression of AMPA currents in cultured cerebellar Purkinje neurons. Neuron 7:81–89 [DOI] [PubMed] [Google Scholar]
- Marshall SP, Lang EJ. (2009) Local changes in the excitability of the cerebellar cortex produce spatially restricted changes in complex spike synchrony. J Neurosci 29:14352–14362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiesen C, Caesar K, Akgören N, Lauritzen M. (1998) Modification of activity-dependent increases of cerebral blood flow by excitatory synaptic activity and spikes in rat cerebellar cortex. J Physiol 512 (Pt 2):555–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neychev VK, Gross RE, Lehéricy S, Hess EJ, Jinnah HA. (2011) The functional neuroanatomy of dystonia. Neurobiol Dis 42:185–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odergren T, Stone-Elander S, Ingvar M. (1998) Cerebral and cerebellar activation in correlation to the action-induced dystonia in writer's cramp. Mov Disord 13:497–508 [DOI] [PubMed] [Google Scholar]
- Offenhauser N, Thomsen K, Caesar K, Lauritzen M. (2005) Activity-induced tissue oxygenation changes in rat cerebellar cortex: interplay of postsynaptic activation and blood flow. J Physiol 565:279–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patneau DK, Mayer ML. (1991) Kinetic analysis of interactions between kainate and AMPA: evidence for activation of a single receptor in mouse hippocampal neurons. Neuron 6:785–798 [DOI] [PubMed] [Google Scholar]
- Piochon C, Levenes C, Ohtsuki G, Hansel C. (2010) Purkinje cell NMDA receptors assume a key role in synaptic gain control in the mature cerebellum. J Neurosci 30:15330–15335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizoli CE, Jinnah HA, Billingsley ML, Hess EJ. (2002) Abnormal cerebellar signaling induces dystonia in mice. J Neurosci 22:7825–7833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preibisch C, Berg D, Hofmann E, Solymosi L, Naumann M. (2001) Cerebral activation patterns in patients with writer's cramp: a functional magnetic resonance imaging study. J Neurol 248:10–17 [DOI] [PubMed] [Google Scholar]
- Raike RS, Jinnah HA, Hess EJ. (2005) Animal models of generalized dystonia. NeuroRx 2:504–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz CS, Bressman SB. (2009) Genetics and treatment of dystonia. Neurol Clin 27:697–718, vi [DOI] [PubMed] [Google Scholar]
- Thomsen K, Offenhauser N, Lauritzen M. (2004) Principal neuron spiking: neither necessary nor sufficient for cerebral blood flow in rat cerebellum. J Physiol 560:181–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda H, Hamani C, Lozano A. (2004) Deep brain stimulation in the treatment of dyskinesia and dystonia. Neurosurg Focus 17:E2. [DOI] [PubMed] [Google Scholar]
- Uluğ AM, Vo A, Argyelan M, Tanabe L, Schiffer WK, Dewey S, Dauer WT, Eidelberg D. (2011) Cerebellothalamocortical pathway abnormalities in torsinA DYT1 knock-in mice. Proc Natl Acad Sci USA 108:6638–6643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidailhet M, Vercueil L, Houeto JL, Krystkowiak P, Benabid AL, Cornu P, Lagrange C, Tézenas du Montcel S, Dormont D, Grand S, et al. (2005) Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. N Engl J Med 352:459–467 [DOI] [PubMed] [Google Scholar]
- Wassef M, Sotelo C, Cholley B, Brehier A, Thomasset M. (1987) Cerebellar mutations affecting the postnatal survival of Purkinje cells in the mouse disclose a longitudinal pattern of differentially sensitive cells. Dev Biol 124:379–389 [DOI] [PubMed] [Google Scholar]
- Watanabe M, Mishina M, Inoue Y. (1994) Distinct spatiotemporal expressions of five NMDA receptor channel subunit mRNAs in the cerebellum. J Comp Neurol 343:513–519 [DOI] [PubMed] [Google Scholar]
- Wilding TJ, Huettner JE. (1997) Activation and desensitization of hippocampal kainate receptors. J Neurosci 17:2713–2721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyllie DJ, Cull-Candy SG. (1994) A comparison of non-NMDA receptor channels in type-2 astrocytes and granule cells from rat cerebellum. J Physiol 475:95–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada KA, Tang CM. (1993) Benzothiadiazides inhibit rapid glutamate receptor desensitization and enhance glutamatergic synaptic currents. J Neurosci 13:3904–3915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Iadecola C. (1996) Glutamate microinjections in cerebellar cortex reproduce cerebrovascular effects of parallel fiber stimulation. Am J Physiol 271:R1568–R1575 [DOI] [PubMed] [Google Scholar]
- Zhang L, Yokoi F, Jin YH, DeAndrade MP, Hashimoto K, Standaert DG, Li Y. (2011) Altered dendritic morphology of Purkinje cells in Dyt1 ΔGAG knock-in and purkinje cell-specific Dyt1 conditional knockout mice. PLoS One 6:e18357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Sharma N, LeDoux MS. (2011) The DYT1 carrier state increases energy demand in the olivocerebellar network. Neuroscience 177:183–194 [DOI] [PMC free article] [PubMed] [Google Scholar]