Abstract
Antagonists of the muscarinic acetylcholine receptors (mAChRs) were among the first treatments for Parkinson's disease. However, the clinical utility of mAChR antagonists is limited by adverse effects associated with the blockade of multiple mAChR subtypes. Understanding the roles of specific mAChR subtypes in regulating basal ganglia and motor function could lead to the development of novel agents that have antiparkinsonian activity with fewer adverse effects. Using the novel, highly selective M1 antagonist N-[3-oxo-3-[4-(4-pyridinyl)-1-piperazinyl]propyl]-2,1,3-benzothiadiazole-4-sulfonamide (VU0255035) and the M1 positive allosteric modulator benzylquinolone carboxylic acid, we investigated the roles of M1 receptors in cholinergic excitation and regulation of synaptic transmission in striatal medium spiny neurons (MSNs) and neurons in the subthalamic nucleus (STN) and substantia nigra pars reticulata (SNr). Electrophysiological studies demonstrate that M1 activation has excitatory effects on MSNs but plays little or no role in mAChR-mediated increases in firing frequency or the regulation of synaptic transmission in STN and SNr neurons. On the basis of this profile, M1-selective antagonists may have weak antiparkinsonian activity but would not have the full efficacy observed in nonselective mAChR antagonists. Consistent with this, the M1-selective antagonist VU0255035 partially reversed reserpine-induced akinesia and decreased haloperidol-induced catalepsy in rats but did not have the full efficacy observed with the nonselective mAChR antagonist scopolamine. These results suggest that the M1 receptor participates in the overall regulation of basal ganglia function and antiparkinsonian effects of mAChR antagonists but that other mAChR subtype(s) also play important roles at multiple levels of the basal ganglia motor circuit.
Introduction
Parkinson's disease (PD) is a common movement disorder with primary motor symptoms, including resting tremor, rigidity, and bradykinesia (Davie, 2008). The pathophysiological hallmark of this disorder is a loss of dopamine neurons in the substantia nigra pars compacta. Traditional therapies are based on dopamine replacement strategies and include levodopa (l-DOPA) and dopamine receptor agonists (Chen and Swope, 2007; Davie, 2008). These drugs are initially effective in virtually all patients with PD but eventually fail in most patients due to the emergence of motor complications, such as motor fluctuations and dyskinesias. Thus, there is a need to develop an understanding of the roles of other neurotransmitter systems in regulating the function of the basal ganglia motor circuit in the hope of developing novel strategies for the treatment of PD.
Cholinergic systems provide one of the most important neuromodulators of basal ganglia function (Lester et al., 2010). It is noteworthy that muscarinic acetylcholine receptor (mAChR) antagonists were among the first available treatments for PD and still are used for the treatment of this disorder (Katzenschlager et al., 2003; Chen and Swope, 2007). Unfortunately, the clinical utility of mAChR antagonists is limited by central and peripheral adverse effects, some of which are mediated likely by the blockade of mAChR subtypes that are not involved in the regulation of basal ganglia motor function. In addition, it is not entirely clear which regions of the basal ganglia motor circuit are involved in the antiparkinsonian actions of muscarinic antagonists. The primary effects of acetylcholine (ACh) in regulating the basal ganglia and motor function often are thought to be mediated by its actions in the striatum, where ACh is released from tonically active cholinergic interneurons that project to neighboring neurons within the striatum, including medium spiny neurons (MSNs) (Graybiel, 1990; Pisani et al., 2007). However, ACh also plays important roles in regulating other structures in the basal ganglia. For instance, the subthalamic nucleus (STN) and the output nuclei of the basal ganglia, substantia nigra pars reticulata (SNr) and internal globus pallidus, receive cholinergic innervation from the pedunculopontine tegmental nucleus (PPN) (Lavoie and Parent, 1994a,b; Bevan and Bolam, 1995). Electrophysiological studies reveal an increase in burst firing in STN and SNr neurons in parkinsonian animals and patients with PD (DeLong and Wichmann, 2007). If the PPN cholinergic projection contributes to increases in the activity of STN and/or SNr neurons, then this could exacerbate parkinsonian motor disability and thus also could provide an important site of action for mAChR antagonists. Indeed, it has been shown that the microinjection of mAChR antagonists into the STN has antiparkinsonian effects in a rodent model of PD (Hernández-López et al., 1996). Therefore, it is important to develop a detailed understanding of the physiological roles of individual mAChR subtypes that mediate the cholinergic regulation of basal ganglia function, which could provide the basis for the development of improved anticholinergic therapies for PD.
The mAChRs are class A G protein-coupled receptors and include five subtypes, termed M1 to M5. Of the five mAChR subtypes, M1 receptors are the most abundant mAChR subtypes expressed in the brain, including the striatum, and proposed to play important roles in a variety of brain functions, including motor control as well as attention, memory, and sleep-wake cycle regulation (Felder et al., 2000). Unfortunately, it has been difficult to develop a detailed understanding of the physiological roles of each mAChR subtype because of a lack of pharmacological tools that are highly selective for individual subtypes. We developed and characterized a panel of novel compounds that are highly selective for M1 and M4 mAChR subtypes, including the selective M1 antagonist N-[3-oxo-3-[4-(4-pyridinyl)-1-piperazinyl]propyl]-2,1,3-benzothiadiazole-4-sulfonamide (VU0255035) and M1 positive allosteric modulator (PAM) benzylquinolone carboxylic acid (BQCA) (Sheffler et al., 2009; Shirey et al., 2009). In the present studies, we took advantage of these selective M1 ligands to determine the roles of M1 in the modulation of membrane excitability of striatal MSNs and neurons in the STN and SNr. In addition, we assessed the involvement of M1 in cholinergic modulation of synaptic transmission in the STN and SNr with the goal of focusing on cholinergic modulation in the indirect pathway, particularly the GABAergic transmission in STN neurons and glutamatergic transmission in SNr neurons. The body of literature suggests that modulation of transmission through the indirect pathway can have antiparkinsonian activity (for a review, see Johnson et al., 2009) and therefore could contribute to the antiparkinsonian effect of mAChR antagonists. Finally, we tested the hypothesis that selective M1 antagonists have antiparkinsonian activity in rodent models of PD.
Materials and Methods
Animals.
All of the animals used in the present studies were group housed with food and water available ad libitum. Animals were kept under a 12 h light/dark cycle with lights on from 6:00 AM to 6:00 PM and were tested during the light phase unless stated otherwise. All of the experimental procedures were approved by the Vanderbilt University Animal Care and Use committee and followed the guidelines set forth by the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996).
In Vitro Electrophysiological Studies.
Coronal brain slices (290–300 μm) containing the striatum were obtained from C57BL/6Hsd mice (postnatal days 21–27; Harlan, Indianapolis, IN). Sagittal brain slices (290–300 μm) containing the STN and SNr were obtained from Sprague-Dawley rats (postnatal days 16–22; Charles River Laboratories, Inc., Wilmington, MA). Animals were anesthetized with isoflurane, and brains were removed rapidly from skulls and submerged in ice-cold modified artificial cerebrospinal fluid (ACSF) oxygenated with 95% O2/5% CO2. The modified ACSF was composed of the following: 220 mM glucose, 2.5 mM KCl, 8 mM MgSO4, 0.5 mM CaCl2, 1.25 mM NaH2PO4, 26 mM NaHCO3, and 10 mM d-glucose. Brain slices containing the striatum or STN and SNr were cut using a Vibratome 3000 (Vibratome, St. Louis, MO). Slices were incubated in oxygenated ACSF at 32°C for 30 min and then maintained at room temperature (20–22°C) afterward until being transferred to a recording chamber. The recording chamber was perfused continuously with oxygenated ACSF containing: 126 mM NaCl, 2.5 mM KCl, 2.0 mM CaCl2, 1.3 mM MgSO4, 1.25 mM NaH2PO4, 26 mM NaHCO3, and 10 mM d-glucose.
Whole-cell or cell-attached recordings were made from visually identified striatal MSNs, STN neurons, or SNr neurons under an Olympus BX50WI upright microscope (Olympus, Tokyo, Japan). A low-power objective (4×) was used to identify the brain region, and a 40× water-immersion objective coupled with Hoffman optics was used to visualize the individual neurons of interest. Whole-cell current- or voltage-clamp signals were amplified using an Axon Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA). Patch pipettes were prepared from borosilicate glass (Sutter Instrument Company, Novato, CA) using a Narishige puller (model PP-830; Narishige, Greenvale, NY). The electrode resistance was 3 to 5 MΩ when filled with the following intracellular solution: 120 mM K-MeSO4, 1 mM MgCl2, 0.1 mM CaCl2, 10 mM HEPES, 1 mM EGTA, 12 mM phosphocreatine, 0.4 mM GTP, and 2 mM ATP. The pH of the pipette solution was adjusted to 7.3 with 1 M KOH, and osmolarity was adjusted to 290 to 295. Striatal MSNs, subthalamic neurons, and SNr GABAergic projection neurons were identified based on previously established electrophysiological characteristics (Richards et al., 1997; Beurrier et al., 1999; Wilson, 2004). The change in the excitability of MSNs was assessed in current-clamp mode by monitoring the change in the membrane potential and change in the number of spike discharges in response to near rheobase depolarization current injection. The changes in the excitability of STN neurons and SNr neurons were determined by monitoring the changes in the frequency of spontaneous firing. Inhibitory postsynaptic currents (IPSCs) in STN neurons or excitatory postsynaptic current (EPSCs) in SNr neurons were evoked every 10 s using a concentric bipolar tungsten electrode (Frederick Haer Company, Bowdoinham, ME) placed in the internal capsule rostral to the STN or SNr. IPSCs were recorded at a holding potential of −55 mV in the presence of the ionotropic glutamate receptor antagonists 2-amino-5-phosphonopentanoic acid (50 μM; Tocris Bioscience, Ellisville, MO) and 2,3-dihydroxy-6,7-dinitroquinoxaline (20 μM; Tocris Bioscience). EPSCs were recorded at a holding potential of −60 mV in the presence of the GABAA receptor antagonist (−)-bicuculline methobromide (20 μM; Tocris Bioscience). To determine the role of M1 in MSNs, STN neurons, and SNr neurons, we used the selective M1 antagonist VU0255035 and the M1 PAM BQCA. Use of these tools required us to establish the concentration response curve for carbachol (CCh) for each response so that we could use the appropriate CCh concentrations for each experiment. Thus, to assess the effects of these selective M1 ligands on CCh-induced responses, we first determined the dose-response relationship of CCh responses in MSNs, STN neurons, and SNr neurons. This allowed us to use concentrations of CCh that provide EC20 and EC80 responses. We then used an approximate EC80 concentration of CCh for the studies of the effect of the M1 antagonist VU0255035 and an EC20 CCh concentration to measure potentiation by BQCA. All of the drugs were bath-applied. The electrical signal was low-pass-filtered at 3 kHz, digitized at 20 kHz, and acquired using a Clampex9.2/Digidata1332 system (Molecular Devices). ClampFit (Molecular Devices), Origin (Origin Lab Corp., Northampton, MA), and MiniAnalysis (Synaptosoft, Decatur, GA) were used for data analysis.
Behavioral Studies.
Male Sprague-Dawley rats (250–300 g, approximately 9–10 weeks old) were used in behavioral studies. For reserpine-induced akinesia, rats were given injections of reserpine (3 mg/kg s.c., dissolved in 1% acetic acid) and returned to their home cages for 2 h, followed by random assignment to treatment groups and the administration of a dose of either VU0255035 (0.3, 1, or 3 mg/kg i.p.), scopolamine (0.1, 0.3 or 1 mg/kg s.c.), or vehicle. Animals then were placed in a locomotor activity chamber for 30 min. Thirty minutes after the administration of the test compound or vehicle (2.5 h after reserpine injection), motor activity was recorded for an additional 30 min in the locomotor activity chamber equipped with 16 × 16 infrared beams (Hamilton-Kinder, Poway, CA).
For haloperidol-induced catalepsy, rats were given injections of haloperidol (0.75 mg/kg i.p., dissolved in 0.2% lactic acid) and monitored for catalepsy 2 h later. Two hours after haloperidol injection, rats were assigned randomly to treatment groups and then given a single administration of either VU0255035 (0.3, 1, or 3 mg/kg i.p.), scopolamine (0.3, 1, or 3 mg/kg s.c.), or vehicle. Thirty minutes later (2.5 h after haloperidol injection), catalepsy was assessed using a horizontal bar placed 6 cm from the testing surface. The forepaws of each rat were placed gently on the bar, with the body positioned at an angle of ∼45° to the testing surface. The latency for the rat to remove one or both forepaws from the bar was measured manually using a stopwatch with a cut-off time of 60 s. Any rat that remained on the bar between 45 and 60 s was considered to be cataleptic.
Statistics.
Grouped data were presented as mean ± S.E.M. The data from electrophysiological studies were compared using the t test, and the data from behavioral studies were compared using Dunnett's test. p < 0.05 was considered to be statistically significant.
Results
Activation of M1 mAChR Has Excitatory Effects in Striatal MSNs.
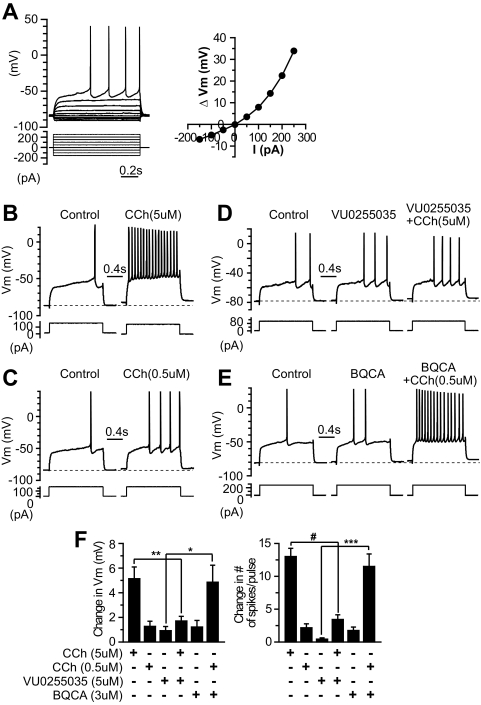
Striatal MSNs were identified based on their electrophysiological characteristics described previously, including a hyperpolarized resting membrane potential, inward rectification, and delayed action potential discharges in response to near rheobase current injection (Fig. 1A). Using novel compounds that are highly selective for the M1 subtype, we studied the involvement of M1 mAChRs in the modulation of membrane excitability of MSNs by monitoring the changes in membrane potential and the number of spike discharges in response to a near threshold depolarization current pulse in the current-clamp condition. The amplitude of the depolarization current pulse was adjusted such that only one to three spikes were elicited before any pharmacological manipulation. Bath application of the muscarinic agonist CCh increased the excitability of MSNs in a concentration-dependent manner. As illustrated in Fig. 1, B, C, and F, 0.5 and 5 μM depolarized the membrane potential (ΔVm) by 1.27 ± 0.42 mV (n = 6) and 5.13 ± 0.95 mV (n = 9), respectively, and increased the number of spike discharges in response to the depolarization current pulse (change in the number of spikes per pulse) by 2.17 ± 0.60 (n = 6) and 13.0 ± 1.3 (n = 9), respectively. The mAChR antagonist VU0255035, which has been shown to be highly selective for M1 relative to the M2 to M5 subtypes (Sheffler et al., 2009), blocked the CCh-induced excitation of MSNs. As shown in Fig. 1, D and F, in the presence of 5 μM VU0255035, 5 μM CCh only slightly depolarized the membrane potential (ΔVm = 1.70 ± 0.38 mV, n = 7, compared with ΔVm = 5.13 ± 0.95 mV with the application of 5 μM CCh alone, n = 9, p < 0.01) and marginally increased the number of spikes per pulse (3.43 ± 0.72, n = 7, compared with 13.0 ± 1.3 with 5 μM CCh alone, n = 9, p < 0.0001). Furthermore, the novel selective M1 PAM BQCA (Shirey et al., 2009) potentiated the submaximal concentration of CCh-induced excitation in MSNs. As shown in Fig. 1, E and F, in the presence of 3 μM BQCA, CCh (0.5 μM) caused a robust depolarization of membrane potential (ΔVm = 4.86 ± 1.38 mV, n = 8, compared with 1.27 ± 0.42 mV with 0.5 μM CCh alone, n = 6, p < 0.05) and a marked increase in the number of spikes per pulse (11.5 ± 1.90, n = 8, compared with 2.17 ± 0.60 with 0.5 μM CCh alone, n = 6, p < 0.005). The results suggest that the muscarinic excitation of MSNs is mediated by the M1 mAChR subtype. It is worth noting that BQCA alone also caused slight excitation in MSNs (Fig. 1, E and F); the number of spikes per pulse increased by 1.75 ± 0.53 (p < 0.05, n = 8), although ΔVm, 1.21 ± 0.54 mV, did not reach a statistically significant level (p = 0.06, n = 8), which suggested that BQCA might potentiate endogenous ambient ACh action on M1 receptors in MSNs.
Fig. 1.
VU0255035 blocks and BQCA potentiates CCh-induced excitation in MSNs. A, electrophysiological properties of striatal MSNs. Membrane potential responses to a series of hyperpolarization and depolarization current steps in a typical striatal MSN recorded in current-clamp conditions (left) and voltage-current relationship of this MSN showing the inward rectification (right). B and C, membrane potential responses to a depolarization current step in the control and after the application of different concentrations of CCh (B, 5 μM; C, 0.5 μM) in MSNs, showing that CCh excites MSNs in a dose-dependent manner. D and E, membrane potential responses to a depolarization current step in the control, during the application of 5 μM VU0255035 and coapplication of VU0255035 with 5 μM CCh in a MSN (D), and in the control, during the application of 3 μM BQCA and coapplication of BQCA with 0.5 μM CCh in another MSN (E). F, bar graphs summarize the changes in membrane potential (left) and number of spikes in response to the depolarization current step (right) after the application of different ligands. *, p < 0.05; **, p < 0.01; ***, p < 0.005; #, p < 0.0001.
M1 mAChRs Are Not Involved in Cholinergic Excitation in STN and SNr Neurons.
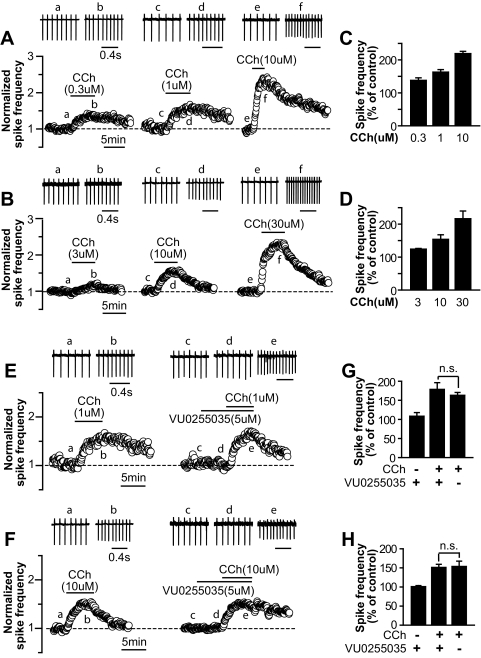
Both glutamatergic STN neurons and GABAergic SNr neurons displayed spontaneous rhythmic firing. We used cell-attached recordings to access the effect of CCh on the firing frequencies of STN and SNr neurons and determined the effect of the M1 antagonist VU0255035 on CCh-induced responses. As shown in Fig. 2, A–D, bath application of CCh increased the spontaneous firing of STN and SNr neurons in a concentration-dependent manner. CCh at concentrations of 0.3, 1, and 10 μM increased the firing rate of STN neurons to 137.8 ± 7.8% (n = 7), 163.0 ± 7.6% (n = 8), and 219.0 ± 7.0% (n = 7), respectively (Fig. 2, A and C). For SNr neurons, CCh at concentrations of 3, 10, and 30 μM increased the firing rate to 124.1 ± 2.1% (n = 3), 153.7 ± 14.0% (n = 5), and 216.3 ± 23.5% (n = 4), respectively (Fig. 2, B and D). The selective M1 antagonist VU0255035 failed to block the excitation induced by a submaximal concentration of CCh in STN and SNr neurons (Fig. 2, E–H). In the presence of 5 μM VU0255035, 1 μM CCh increased the firing rate to 178.6 ± 17.6% of the control value in STN neurons (n = 6), compared with 163.0 ± 7.6% in the absence of VU0255035 (Fig. 2, E and G, n = 8, p > 0.1). For SNr neurons, 10 μM CCh increased the firing rate to 151.1 ± 8.2% of the control value when 5 μM VU0255035 was coapplied (n = 5), compared with 153.7 ± 9.8% in the absence of VU0255035 (Fig. 2, F and H, n = 5, p > 0.5). These data suggest that M1 activation does not play a major role in cholinergic modulation of neuronal excitability in these two nuclei. We noted that higher concentrations of CCh were needed in SNr neurons than in STN neurons and MSNs to induce increased firing rates. This probably reflects a combination of different mAChR subtypes that mediate CCh responses in SNr neurons, STN neurons, and MSNs and/or differences in receptor reserve in these neuronal populations.
Fig. 2.
VU0255035 does not block CCh-induced excitation in STN and SNr neurons. A and B, sample traces (top) and time courses of normalized spike frequencies (bottom) of cell-attached recordings from typical experiments, showing that CCh induces a concentration-dependent increase in the spike frequency of STN (A) and SNr neurons (B). C and D, bar graphs summarize the grouped data of CCh-induced increases in spike frequencies of STN (C) and SNr neurons (D). E and F, sample traces (top) and time courses of normalized spike frequencies (bottom) of cell-attached recordings from representative cells, showing the effect of 1 or 10 μM CCh on the spontaneous firing of STN (E) or SNr neurons (F), respectively, in the absence (left) and presence of 5 μM VU0255035 (right). G and H, summary of grouped data shows that VU0255035 (5 μM) does not block CCh-induced increases in the spike frequencies of STN (G) and SNr neurons (H). Lowercase letters in A, B, E, and F indicate the time points where the sample traces are taken. Grouped data in C, D, G, and H are normalized to the corresponding control values.
M1 mAChRs Are Involved Partially in Cholinergic Inhibition of GABAergic Synaptic Transmission in STN Neurons but Not Involved in Cholinergic Inhibition of Excitatory Transmission in SNr Neurons.
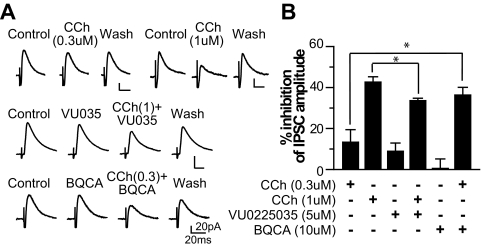
Whole-cell voltage-clamp recordings were used to determine the involvement of the M1 mAChR subtype in cholinergic modulation of GABAergic transmission in STN neurons and glutamatergic transmission in SNr neurons. Using CCh, the M1 antagonist VU0255035, and the M1 PAM BQCA, we found that mAChR activation inhibits IPSCs in STN neurons and this cholinergic inhibition is mediated in part by M1 receptors. As illustrated in Fig. 3, application of 0.3, 1, and 10 μM CCh reduced IPSC amplitude by 13.3 ± 6.1% (n = 5), 42.6 ± 2.6% (n = 6), and 53.9 ± 3.5% (n = 7), respectively. The M1 antagonist VU0255035 partially blocked CCh-induced inhibition of IPSCs (Fig. 3). In the presence of 5 μM VU0255035, CCh (1 μM) reduced IPSC amplitude by 35.2 ± 1.8% (n = 7), compared with 42.6 ± 2.6% with 1 μM CCh alone (n = 6, p < 0.05). To confirm M1 involvement, we determined whether the effect of CCh could be potentiated by M1 PAM BQCA. As shown in Fig. 3, in the presence of 10 μM BQCA, 0.3 μM CCh inhibited IPSC amplitude by 36.3 ± 3.8% (n = 6), which is significantly greater than the effect of 0.3 μM CCh alone (13.3 ± 6.1%, n = 5, p < 0.05). Taken together, the results suggest that M1 plays a role in cholinergic inhibition of GABAergic synaptic transmission in STN. However, on the basis of the failure of saturating concentrations of VU0255035 to fully block the response to a relatively low concentration of CCh, it is likely that other mAChR subtypes also are involved.
Fig. 3.
Cholinergic depression of inhibitory synaptic transmission in STN neurons is mediated in part by M1 mAChRs. A, averaged IPSC traces obtained from typical experiments where the effects of the following ligands on IPSC amplitudes in STN neurons were examined: 0.3 μM CCh, 1 μM CCh, 5 μM VU0225035, 5 μM VU0225035 with 1 μM CCh, 10 μM BQCA, and 10 μM BQCA with 0.3 μM CCh. B, bar graph summarizing the partial blockade effect of VU0225035 and the potentiation effect of BQCA on the CCh-induced depression of IPSCs in STN neurons. *, p < 0.05.
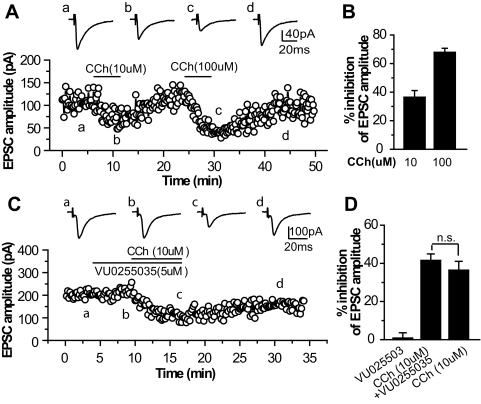
Muscarinic receptor activation also inhibited excitatory synaptic transmission in SNr neurons. CCh at 10 and 100 μM reduced EPSC amplitude by 36.4 ± 4.7% (n = 6) and 67.9 ± 2.8% (n = 4), respectively (Fig. 4). To determine whether M1 mAChRs are involved in CCh-induced reduction of EPSCs, we assessed the effect of the M1 antagonist VU0355025 on CCh action. Application of 5 μM VU0355025 failed to block 10 μM CCh-induced inhibition of EPSCs (41.5 ± 3.4%, n = 5, compared with 36.4 ± 4.7% in the absence of VU0355025, n = 6, p > 0.1). This result suggests that M1 mAChRs are not involved in mAChR-mediated inhibition of excitatory transmission in the SNr.
Fig. 4.
Cholinergic depression of excitatory synaptic transmission in SNr neurons is not mediated by M1 mAChRs. A and C, averaged EPSC traces (top) and time courses (bottom) of the EPSC amplitudes obtained from typical experiments, showing the effects of 10 and 100 μM CCh (A) and 5 μM VU0255035 with 10 μM CCh (C) on EPSC amplitudes. B and D, bar graphs summarizing the grouped data showing that VU0255035 does not block CCh-induced inhibition of EPSCs in SNr neurons.
Effect of VU0355025 on Rodent Models of PD.
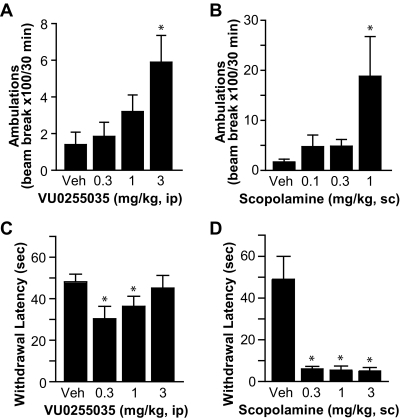
To determine whether the M1-selective antagonist VU0255035 has any antiparkinsonian efficacy, we tested VU0255035 in two preclinical models of PD that are used commonly to test drugs for their ability to reverse parkinsonian motor deficits, reserpine-induced akinesia and haloperidol-induced catalepsy in rats, in comparison with the effects of the nonselective mAChR antagonist scopolamine. We found that VU0225035 partially reversed akinesia induced by reserpine (3 mg/kg s.c.), as demonstrated by a dose-dependent increase in locomotor activity, with 3 mg/kg being the most effective (Fig. 5A, n = 8 per group, *, p < 0.05, compared with vehicle group, Dunnett's test). In addition, VU0255035 reduced haloperidol (0.75 mg/kg i.p.)-induced catalepsy, as indicated by a decrease in the latency to withdrawal of the forepaws when placed on a horizontal grid (Fig. 5C). The cutoff for termination of the experiment was 60 s. As shown in Fig. 5C, the withdrawal latency was reduced significantly for 0.3 and 1 mg/kg dosing groups, compared with the vehicle group (n = 8 per group, *, p < 0.05, Dunnett's test). In comparison, we tested the nonselective mAChR antagonist scopolamine in these two animal models and found that scopolamine had more robust effects in reversing reserpine (3 mg/kg s.c.)-induced akinesia and haloperidol (0.75 mg/kg i.p.)-induced catalepsy (Fig. 5, B and D). These results suggest that additional mAChR subtype(s) other than M1 also participated in antiparkinsonian actions of muscarinic antagonists.
Fig. 5.
Antiparkinsonian effects of VU0255035 in rodent models of PD, in comparison with the effect of scopolamine. A and C, effects of VU0255035 at different concentrations on reserpine (3 mg/kg s.c.)-induced akinesia (A) and haloperidol (0.75 mg/kg i.p.)-induced catalepsy (C) in rats (n = 8 per group). *, p < 0.05, compared with the vehicle group. B and D, effects of scopolamine at different concentrations on reserpine-induced (B) and haloperidol-induced motor deficits in rats (D) (n = 8 per group). *, p < 0.05, compared with the vehicle group.
Discussion
Hypercholinergic tone in the basal ganglia has long been proposed to be associated with motor deficits of PD. Most previous studies have focused on cholinergic modulation of striatal function as the primary site of action of cholinergic modulation of motor function (Barbeau, 1962; Pisani et al., 2007). However, previous animal and human studies provide strong evidence that changes in the activity in the indirect pathway of the basal ganglia motor circuit can contribute to motor dysfunction in patients with PD. For instance, STN neurons are hyperactive in patients with PD and display an increased incidence of burst activity (DeLong and Wichmann, 2007). The glutamatergic STN neurons send a primary excitatory projection to GABAergic neurons in the SNr, and this contributes to the overexcitation of SNr GABAergic neurons in patients with PD (DeLong and Wichmann, 2007). It is noteworthy that inhibition of the activity of either STN or SNr neurons using surgical lesions or deep brain stimulation can reduce parkinsonian motor disability (Bergman et al., 1990; Starr et al., 1998; DeLong and Wichmann, 2007).
The actions of mAChR activation at each level of the basal ganglia assessed in the present studies could contribute to parkinsonian motor impairments. Likewise, the antiparkinsonian efficacy of mAChR antagonists may involve actions at the level of the striatum as well as the STN and SNr. Muscarinic agonists increase the activity of STN neurons (Feger et al., 1979; Flores et al., 1996), and the microinjection of mAChR antagonists in the STN alleviates motor deficits in an animal model of PD (Hernández-López et al., 1996). Likewise, mAChRs are present on SNr neurons (Cross and Waddington, 1980), which receive cholinergic innervations from the PPN (Lavoie and Parent, 1994a). The activity of SNr neurons increases after the electrophoresis of ACh in vivo (Collingridge and Davies, 1981), and the microinjection of muscarinic agonists in the SNr induces parkinsonian-like motor deficits that are blocked by the muscarinic antagonist scopolamine (De Montis et al., 1979; Turski et al., 1984). These previous studies are consistent with the present findings that CCh has excitatory effects and reduces inhibitory transmission in STN and SNr neurons and suggest that, in addition to the striatum, the STN and SNr also could be targets for the actions of anticholinergic drugs in reducing motor symptoms of PD.
The present studies used highly selective M1 ligands, the M1 antagonist VU0255035 and the M1 PAM BQCA. The selectivity of these two compounds has been evaluated rigorously in our previous studies (Sheffler et al., 2009; Shirey et al., 2009). For instance, BQCA has been shown to lack an effect on responses to activation in cell lines expressing M2 to M5, lack an effect in cellular backgrounds lacking M1 expression and in broad profiling for activity at other G protein-coupled receptors and other central nervous system targets, and lack effect in M1 knockout (KO) mice (Shirey et al., 2009). For VU0255035, a similar series of studies have been performed to rigorously evaluate activity at all of the mAChR subtypes and multiple other potential targets. We found that VU0255035 has 75-fold or greater selectivity in antagonizing CCh-induced responses in the cells expressing M1 relative to that in those expressing M2, M3, M4, or M5 (Sheffler et al., 2009). We also found that CCh has no effect on M1-mediated responses in M1 KO mice that are blocked by VU0255035, including the evaluation of responses to VU0255035 in hippocampal and cortical neurons and present studies of muscarinic excitation of MSNs that has been shown to be absent in M1 KO mice (Shen et al., 2005; Sheffler et al., 2009; Shirey et al., 2009).
The present finding that the M1 mAChR subtype is responsible for excitatory effects of mAChR activation on striatal MSNs but not neurons in the STN and SNr suggests that the inhibition of multiple mAChR subtypes, including M1, is likely important for the overall effects of mAChR antagonists on basal ganglia function and motor activity. More importantly, although M1 is not involved in direct excitatory effects on STN and SNr neurons, our studies suggest that M1 does participate in cholinergic depression of IPSCs in STN neurons (Shen and Johnson, 2000). The primary source of GABAergic inhibitory inputs to STN neurons are from the external segment of the globus pallidus (van der Kooy et al., 1981). In parkinsonism, GABAergic pallidosubthalamic transmission is reduced (DeLong and Wichmann, 2007), which would contribute, at least in part, to the hyperactivity of STN neurons. Thus, M1 activation and disinhibition of the STN could increase further the activity of STN neurons and exacerbate the parkinsonian motor deficits.
It is noteworthy that some of the muscarinic antagonists used in the treatment of PD have been purported to have an “M1-like” preferential pharmacological profile, leading some investigators to postulate that selective M1 blockade would be an effective strategy for the treatment of PD (Tien and Wallace, 1985; Giachetti et al., 1986). However, these clinically available M1-preferring drugs are not sufficiently selective to ascribe their effects to M1, and our findings that M1 is involved in some but not all of the actions of mAChR activation in basal ganglia suggests that blockade may not have the same efficacy as can be achieved with nonselective mAChR antagonists. Consistent with this, we found that the selective M1 antagonist VU0255035 has a modest effect in reducing parkinsonian motor disability in rodent models but does not achieve the efficacy that can be seen with the nonselective mAChR antagonist scopolamine. More importantly, the dose of VU0255035 used in these studies was shown previously to achieve high brain levels and maximal inhibition of M1 activation in the central nervous system (Sheffler et al., 2009). These results raise a need to develop novel highly selective ligands for the mAChR subtype(s) to allow the development of a complete understanding of the roles of specific subtypes in regulating the different aspects of basal ganglia function.
Although these studies do not suggest that M1 antagonists could provide sufficient efficacy to be used as a stand-alone therapy for PD, it is possible that the modest efficacy achieved could be useful to augment other therapeutic approaches in patients with PD. In addition, it is possible that M1 antagonists could prove useful in some other basal ganglia disorders that have been shown to be effectively treated by nonselective mAChR antagonists, such as dystonia. It will be important to explore this possibility in future studies.
One concern associated with the use of M1 antagonists as therapeutic agents is possible adverse effects on cognitive functions, because nonselective mAChR antagonists have long been known to cause severe side effects, such as impairment of learning and memory (Drachman and Leavitt, 1974). However, we reported that VU0255035 does not have any effects on the acquisition of contextual fear conditioning in rats, a model of hippocampus-dependent learning, at doses that block pilocarpine-induced seizures (Sheffler et al., 2009). In contrast, the nonselective mAChR antagonist scopolamine causes a dose-dependent disruption in the acquisition of this conditioning response (Sheffler et al., 2009). These results are consistent with previous reports using M1 mAChR KO mice, which demonstrate that mice lacking M1 mAChRs show no impairments in certain forms of hippocampus-dependent learning (Miyakawa et al., 2001; Anagnostaras et al., 2003), suggesting that M1 receptors play subtle roles in learning and memory. Taken together, these results suggest that selective M1 antagonists could be used as potential therapeutic agents in the treatment of brain disorders, such as PD and other movement disorders, with limited side effects on cognitive functions.
In previous studies, the mechanisms underlying the overall excitatory effects of mAChR activation on MSNs have been evaluated in detail. For instance, mAChR activation inhibits various K+ currents, including KCNQ channel currents in MSNs, and this effect was lost in M1 KO mice (Shen et al., 2005). Another type of K+ channels that regulate MSN excitability and also are modulated by mAChR signaling is inwardly rectifying potassium (Kir2) channels (Galarraga et al., 1999; Shen et al., 2007). Unlike KCNQ channels, which are inhibited uniformly by mAChR activation in MSNs that reside in both direct and indirect pathways (Shen et al., 2005), Kir2 currents are inhibited differentially by muscarinic activation in MSNs depending on their projection targets; specifically, it preferentially reduces Kir2 channel currents in striatopallidal MSNs but has little effect on striatonigral MSNs (Shen et al., 2005). In the present studies, we did not note large variation or bimodal distribution of CCh-induced responses and BQCA potentiation of CCh responses across MSNs. This suggests that the VU0255035- and BQCA-sensitive, CCh-induced excitation in MSNs is likely to be primarily mediated by the inhibition of KCNQ channels. However, future studies will be needed to determine whether M1 is responsible for the modulation of other specific ion channels in these cells, and it is likely that the effects of M1 receptor activation on KCNQ channels along with Kir2 channels and possibly other ion channels contribute to cholinergic modulation of overall excitability of MSNs.
This work was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grants 1R01-NS065867, 5P50-NS071669] (to Z.X. and P.J.C., respectively); the National Institutes of Health National Institute of Mental Health [Grant 1U54-MH084659] (to C.W.L.); and the Dystonia Medical Research Foundation (to Z.X.).
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- PD
- Parkinson's disease
- ACh
- acetylcholine
- ACSF
- artificial cerebrospinal fluid
- BQCA
- benzylquinolone carboxylic acid
- CCh
- carbachol
- EPSC
- excitatory postsynaptic current
- IPSC
- inhibitory postsynaptic current
- Kir
- inwardly rectifying potassium channel
- KO
- knockout
- PPN
- pedunculopontine tegmental nucleus
- mAChR
- muscarinic acetylcholine receptor
- MSN
- medium spiny neuron
- PAM
- positive allosteric modulator
- SNr
- substantia nigra pars reticulata
- STN
- subthalamic nucleus
- VU0255035
- N-[3-oxo-3-[4-(4-pyridinyl)-1-piperazinyl]propyl]-2,1,3-benzothiadiazole-4-sulfonamide.
Authorship Contributions
Participated in research design: Xiang, Thompson, Jones, and Conn.
Conducted experiments: Xiang and Thompson.
Contributed new reagents or analytic tools: Lindsley and Conn.
Performed data analysis: Xiang and Thompson.
Wrote or contributed to the writing of the manuscript: Xiang, Thompson, Jones, and Conn.
References
- Anagnostaras SG, Murphy GG, Hamilton SE, Mitchell SL, Rahnama NP, Nathanson NM, Silva AJ. (2003) Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nat Neurosci 6:51–58 [DOI] [PubMed] [Google Scholar]
- Barbeau A. (1962) The pathogenesis of Parkinson's disease: a new hypothesis. Can Med Assoc J 87:802–807 [PMC free article] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, DeLong MR. (1990) Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science 249:1436–1438 [DOI] [PubMed] [Google Scholar]
- Beurrier C, Congar P, Bioulac B, Hammond C. (1999) Subthalamic nucleus neurons switch from single-spike activity to burst-firing mode. J Neurosci 19:599–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan MD, Bolam JP. (1995) Cholinergic, GABAergic, and glutamate-enriched inputs from the mesopontine tegmentum to the subthalamic nucleus in the rat. J Neurosci 15:7105–7120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Swope DM. (2007) Pharmacotherapy for Parkinson's disease. Pharmacotherapy 27:161S–173S [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Davies J. (1981) The influence of striatal stimulation and putative neurotransmitters on identified neurones in the rat substantia nigra. Brain Res 212:345–359 [DOI] [PubMed] [Google Scholar]
- Cross AJ, Waddington JL. (1980) [3H] Quinuclidinyl benzylate and [3H] GABA receptor binding in rat substantia nigra after 6-hydroxy-dopamine lesions. Neurosci Lett 17:271–275 [DOI] [PubMed] [Google Scholar]
- Davie CA. (2008) A review of Parkinson's disease. Br Med Bull 86:109–127 [DOI] [PubMed] [Google Scholar]
- De Montis GM, Olianas MC, Serra G, Tagliamonte A, Scheel-Krüger J. (1979) Evidence that a nigral gabaergic–cholinergic balance controls posture. Eur J Pharmacol 53:181–190 [DOI] [PubMed] [Google Scholar]
- DeLong MR, Wichmann T. (2007) Circuits and circuit disorders of the basal ganglia. Arch Neurol 64:20–24 [DOI] [PubMed] [Google Scholar]
- Drachman DA, Leavitt J. (1974) Human memory and the cholinergic system. A relationship to aging? Arch Neurol 30:113–121 [DOI] [PubMed] [Google Scholar]
- Feger J, Hammond C, Rouzaire-Dubois B. (1979) Pharmacological properties of acetylcholine-induced excitation of subthalamic nucleus neurones. Br J Pharmacol 65:511–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder CC, Bymaster FP, Ward J, DeLapp N. (2000) Therapeutic opportunities for muscarinic receptors in the central nervous system. J Med Chem 43:4333–4353 [DOI] [PubMed] [Google Scholar]
- Flores G, Hernandez S, Rosales MG, Sierra A, Martines-Fong D, Flores-Hernandez J, Aceves J. (1996) M3 muscarinic receptors mediate cholinergic excitation of the spontaneous activity of subthalamic neurons in the rat. Neurosci Lett 203:203–206 [DOI] [PubMed] [Google Scholar]
- Galarraga E, Hernández-López S, Reyes A, Miranda I, Bermudez-Rattoni F, Vilchis C, Bargas J. (1999) Cholinergic modulation of neostriatal output: a functional antagonism between different types of muscarinic receptors. J Neurosci 19:3629–3638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giachetti A, Giraldo E, Ladinsky H, Montagna E. (1986) Binding and functional profiles of the selective M1 muscarinic receptor antagonists trihexyphenidyl and dicyclomine. Br J Pharmacol 89:83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel AM. (1990) Neurotransmitters and neuromodulators in the basal ganglia. Trends Neurosci 13:244–254 [DOI] [PubMed] [Google Scholar]
- Hernández-López S, Flores G, Rosales MG, Sierra A, Martínez-Fong D, Aceves J. (1996) Muscarinic antagonists microinjected into the subthalamic nucleus decrease muscular rigidity in reserpinized rats. Neurosci Lett 213:157–160 [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals, 7th ed, Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington, DC [Google Scholar]
- Johnson KA, Conn PJ, Niswender CM. (2009) Glutamate receptors as therapeutic targets for Parkinson's disease. CNS Neurol Disord Drug Targets 8:475–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzenschlager R, Sampaio C, Costa J, Lees A. (2003) Anticholinergics for symptomatic management of Parkinson's disease. Cochrane Database Syst Rev CD003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie B, Parent A. (1994a) Pedunculopontine nucleus in the squirrel monkey: cholinergic and glutamatergic projections to the substantia nigra. J Comp Neurol 344:232–241 [DOI] [PubMed] [Google Scholar]
- Lavoie B, Parent A. (1994b) Pedunculopontine nucleus in the squirrel monkey: projections to the basal ganglia as revealed by anterograde tract-tracing methods. J Comp Neurol 344:210–231 [DOI] [PubMed] [Google Scholar]
- Lester DB, Rogers TD, Blaha CD. (2010) Acetylcholine-dopamine interactions in the pathophysiology and treatment of CNS disorders. CNS Neurosci Ther 16:137–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa T, Yamada M, Duttaroy A, Wess J. (2001) Hyperactivity and intact hippocampus-dependent learning in mice lacking the M1 muscarinic acetylcholine receptor. J Neurosci 21:5239–5250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani A, Bernardi G, Ding J, Surmeier DJ. (2007) Re-emergence of striatal cholinergic interneurons in movement disorders. Trends Neurosci 30:545–553 [DOI] [PubMed] [Google Scholar]
- Richards CD, Shiroyama T, Kitai ST. (1997) Electrophysiological and immunocytochemical characterization of GABA and dopamine neurons in the substantia nigra of the rat. Neuroscience 80:545–557 [DOI] [PubMed] [Google Scholar]
- Sheffler DJ, Williams R, Bridges TM, Xiang Z, Kane AS, Byun NE, Jadhav S, Mock MM, Zheng F, Lewis LM, et al. (2009) A novel selective muscarinic acetylcholine receptor subtype 1 antagonist reduces seizures without impairing hippocampus-dependent learning. Mol Pharmacol 76:356–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen KZ, Johnson SW. (2000) Presynaptic dopamine D2 and muscarine M3 receptors inhibit excitatory and inhibitory transmission to rat subthalamic neurones in vitro. J Physiol 525:331–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Hamilton SE, Nathanson NM, Surmeier DJ. (2005) Cholinergic suppression of KCNQ channel currents enhances excitability of striatal medium spiny neurons. J Neurosci 25:7449–7458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Tian X, Day M, Ulrich S, Tkatch T, Nathanson NM, Surmeier DJ. (2007) Cholinergic modulation of Kir2 channels selectively elevates dendritic excitability in striatopallidal neurons. Nat Neurosci 10:1458–1466 [DOI] [PubMed] [Google Scholar]
- Shirey JK, Brady AE, Jones PJ, Davis AA, Bridges TM, Kennedy JP, Jadhav SB, Menon UN, Xiang Z, Watson ML, et al. (2009) A selective allosteric potentiator of the M1 muscarinic acetylcholine receptor increases activity of medial prefrontal cortical neurons and restores impairments in reversal learning. J Neurosci 29:14271–14286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr PA, Vitek JL, Bakay RA. (1998) Deep brain stimulation for movement disorders. Neurosurg Clin N Am 9:381–402 [PubMed] [Google Scholar]
- Tien XY, Wallace LJ. (1985) Trihexyphenidyl–further evidence for muscarinic receptor subclassification. Biochem Pharmacol 34:588–590 [DOI] [PubMed] [Google Scholar]
- Turski L, Havemann U, Kuschinsky K. (1984) Role of muscarinic cholinergic mechanisms in the substantia nigra pars reticulata in mediating muscular rigidity in rats. Naunyn Schmiedebergs Arch Pharmacol 327:14–17 [DOI] [PubMed] [Google Scholar]
- van der Kooy D, Hattori T, Shannak K, Hornykiewicz O. (1981) The pallido-subthalamic projection in rat: anatomical and biochemical studies. Brain Res 204:253–268 [DOI] [PubMed] [Google Scholar]
- Wilson CJ. (2004) Basal ganglia, in The Synaptic Organization of the Brain (Shepherd GM. ed) pp 361–413, Oxford University Press, New York [Google Scholar]