Abstract
Antipsychotics are an important class of drugs for the management of schizophrenia and other psychotic disorders. They act by blocking dopamine receptors; however, because these receptors are present throughout the brain, prolonged antipsychotic use also leads to serious side effects. These include tardive dyskinesia, repetitive abnormal involuntary movements of the face and limbs for which there is little treatment. In this study, we investigated whether nicotine administration could reduce tardive dyskinesia because nicotine attenuates other drug-induced abnormal movements. We used a well established model of tardive dyskinesia in which rats injected with the commonly used antipsychotic haloperidol develop vacuous chewing movements (VCMs) that resemble human orofacial dyskinesias. Rats were first administered nicotine (minipump; 2 mg/kg per day). Two weeks later, they were given haloperidol (1 mg/kg s.c.) once daily. Nicotine treatment reduced haloperidol-induced VCMs by ∼20% after 5 weeks, with a significant ∼60% decline after 13 weeks. There was no worsening of haloperidol-induced catalepsy. To understand the molecular basis for this improvement, we measured the striatal dopamine transporter and nicotinic acetylcholine receptors (nAChRs). Both haloperidol and nicotine treatment decreased the transporter and α6β2* nAChRs (the asterisk indicates the possible presence of other nicotinic subunits in the receptor complex) when given alone, with no further decline with combined drug treatment. By contrast, nicotine alone increased, while haloperidol reduced α4β2* nAChRs in both vehicle and haloperidol-treated rats. These data suggest that molecular mechanisms other than those directly linked to the transporter and nAChRs underlie the nicotine-mediated improvement in haloperidol-induced VCMs in rats. The present results are the first to suggest that nicotine may be useful for improving the tardive dyskinesia associated with antipsychotic use.
Introduction
Tardive dyskinesia is a serious side effect of long-term antipsychotic treatment for schizophrenia and other psychotic disorders. Antipsychotics are drugs with dopamine (DA) antagonist properties that form the mainstay of treatment for schizophrenia. They exert their beneficial effect by dampening excess dopaminergic activity, which is currently of unknown pathophysiologic origin (Turrone et al., 2003; Seeman, 2010; Aia et al., 2011; Gershanik and Gómez Arévalo, 2011; Tarsy et al., 2011). Antipsychotics are effective in alleviating psychosis. However, their long-term use also leads to the development of abnormal involuntary movements or tardive dyskinesia, which can be very disruptive and eventually debilitating. Dyskinesia is quite common, with an incidence of up to 24% with continued treatment (Correll et al., 2004; Aia et al., 2011; Gershanik and Gómez Arévalo, 2011; Tarsy et al., 2011). Because schizophrenia has a prevalence rate of 0.5%, tardive dyskinesia afflicts a considerable proportion of the population (Saha et al., 2005).
The development of tardive dyskinesia with antipsychotic use prompted a search for novel drugs with fewer extrapyramidal side effects. The second-generation antipsychotics are associated with a reduced risk of tardive dyskinesia; however, they still develop particularly in older adults, probably because the mechanisms that improve psychoses overlap with those that result in tardive dyskinesia (Correll et al., 2004; Tarsy et al., 2011). In addition, second-generation drugs are associated with side effects, including weight gain and severe disturbances in lipid and glucose regulation (Tandon et al., 2008; Volavka and Citrome, 2009). Thus, there is a critical need for better treatment options for psychotic disorders or at least to reduce the side effects that develop with their use.
Our recent studies have shown that nicotine attenuates the dyskinesias or abnormal involuntary movements that develop with long term l-DOPA treatment for Parkinson's disease. Nicotine decreased l-DOPA-induced dyskinesias in parkinsonian nonhuman primates when given either before the onset of dyskinesias or when they were established (Quik et al., 2007a). Similar results were obtained in parkinsonian rat and mouse models of l-DOPA-induced dyskinesias, attesting to the robustness of the effect of nicotine across species (Bordia et al., 2008, 2010; Huang et al., 2011a,b). Nicotine reduced dyskinesias when given via several modes of administration, including drinking water, minipumps, or injection (Bordia et al., 2008, 2010; Huang et al., 2011a,b). Several of these treatment paradigms readily lend themselves to use in patients, for instance, as an oral formulation or a slow release mode (nicotine patch). It is noteworthy that nicotine did not worsen the anti-parkinsonian effect of l-DOPA in any species.
Antipsychotic administration resembles l-DOPA treatment in that both lead to abnormal involuntary movements, possibly due to an enhanced dopaminergic tone (Barroso-Chinea and Bezard, 2010; Cenci and Konradi, 2010; Gershanik and Gómez Arévalo, 2011). l-DOPA-induced dyskinesias arise because of the increased conversion of l-DOPA to DA. Antipsychotics may enhance dopaminergic activity because of a compensatory antagonist-induced DA receptor up-regulation. On the basis of this comparison, we hypothesized that nicotine might also reduce antipsychotic-induced tardive dyskinesia.
To investigate this possibility, we tested the effect of nicotine in a rat model of tardive dyskinesia, which involved injection of the commonly used antipsychotic haloperidol. This model shares many characteristics typical of tardive dyskinesia in humans (Turrone et al., 2002). The present results show that long-term nicotine treatment reduces haloperidol-induced abnormal involuntary movements or vacuous chewing movements (VCMs) in rats. Molecular studies, including measurement of the DA transporter (DAT), α4β2* and α6β2* nAChRs, were done as an approach to understand the mechanisms associated with the changes in behavior. Overall, these data suggest that nicotine or nAChR drugs may be useful for the treatment of antipsychotic-induced tardive dyskinesia in humans.
Materials and Methods
Animals.
Adult male Sprague-Dawley rats weighing ∼250 g were purchased from Charles River Laboratories, Inc. (Wilmington, MA). They were housed two per cage in a temperature- and humidity-controlled environment under a 12-h light/dark cycle with free access to food and water. All procedures conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996) and were approved by the Institutional Animal Care and Use Committee.
Drug Treatments.
One set of 36 rats was used for all behavioral and biochemical experiments. After habituation, the rats were randomly divided into two groups. Both groups were surgically implanted with osmotic minipumps (Alzet model 2006, delivery rate, 0.15 μl/h; Durect Corporation, Cupertino, CA) filled with either vehicle (water) or nicotine (2 mg/kg per day, free base; Sigma-Aldrich, St. Louis, MO) as described previously (Bordia et al., 2008). Plasma cotinine levels were similar to those in moderate smokers (Matta et al., 2007), suggesting that the nicotine dose is one that would be tolerated in humans. Two weeks after the start of nicotine treatment, a subset of rats from both vehicle and nicotine-treated groups was injected with haloperidol (1 mg/kg s.c.; Sigma-Aldrich) or vehicle (water) once daily (5 days per week) throughout the entire study. This was followed by a 5-week nicotine withdrawal period, which was initiated by surgical removal of the nicotine-containing minipumps. The rats were then given various doses of nicotine (0.1–0.3 mg/kg free base s.c.) or saline once only or once daily for 4 days. They were then implanted with new minipumps containing nicotine (2 mg/kg per day) or vehicle, after which they were euthanized.
Haloperidol-Induced VCMs.
Rats were evaluated for haloperidol-induced VCMs, as described previously (Rogoza et al., 2004; Schleimer et al., 2005). These abnormal movements, which resemble human orofacial dyskinesias, are characterized by purposeless mouth openings in a vertical plane, with or without tongue protrusion and jaw tremors that are presented as high-frequency fasciculations of the mouth or jaw (Turrone et al., 2003). The rats were individually placed in a circular Plexiglas chambers for 15 min to allow them to adapt to the novel environment. Mirrors were strategically placed around the chamber to evaluate VCMs that arose when the animal faced away from the observer. After habituation, rats received injections of haloperidol, and the VCMs were counted for 5 min, with average scores of ≥8 VCMs per 5 min considered positive for abnormal movements (Turrone et al., 2003). VCMs were assessed every 2nd week until week 13, after which they were rated on a weekly basis.
Haloperidol-Induced Catalepsy.
The bar test was used to quantify haloperidol-induced catalepsy. This test measures the ability of the animal to respond to drug-induced immobility (Kuschinsky and Hornykiewicz, 1972). Thirty minutes before treatment, rats were rated for baseline catalepsy by placing their front paws over a 10-cm high horizontal bar. Cataleptic measurements were defined as the time taken to remove the paw from the horizontal bar within a 1-min period. After baseline measurements, the rats were injected with haloperidol. Ten minutes after injection, catalepsy was assessed again.
Autoradiography.
Rats were killed by decapitation 90 min after vehicle or haloperidol injection (Samaha et al., 2008). The brains were immediately frozen in isopentane on dry ice and stored at −80°C. When required, 8-μm-thick coronal sections were cut from the frozen half of the brain using a cryostat (Leica Microsystem, Deerfield, IL) at −18°C. The cut sections were thaw mounted onto Superfrost Plus slides (Thermo Fisher Scientific, Waltham, MA), air-dried, and stored at −80°C for autoradiography. After each experiment, slides were air-dried and exposed to Kodak MR film (PerkinElmer Life and Analytical Sciences, Waltham, MA) along with [3H] standards (GE Healthcare, Chalfont St. Giles, Buckinghamshire, UK).
[125I]RTI-121 Binding.
DAT levels were measured using [125I]3β-(4-iodophenyl)tropane-2β-carboxylic acid isopropyl ester(RTI-121) (specific activity, 2200 Ci/mmol; PerkinElmer Life and Analytical Sciences) autoradiography as described previously (Bordia et al., 2008). Sections were preincubated twice for 15 min in buffer (50 mM Tris-HCl, pH 7.4, 120 mM NaCl, and 5 mM KCl) and then incubated for 2 h with 50 pM [125I]RTI-121 in the same buffer also containing 0.025% bovine serum albumin and 1 μM fluoxetine. Sections were washed 4 × 15 min in ice-cold buffer and 1 × 10 s in ice-cold deionized H2O. Nonspecific binding was determined in the presence of the DA uptake inhibitor nomifensine (100 μM).
[125I]α-Conotoxin MII Binding.
α6β2* nAChR levels were measured using [125I]α-conotoxin MII(α-CtxMII) (specific activity, 2200 Ci/mmol), as described previously (Quik et al., 2003). Sections were preincubated for 15 min in binding buffer (20 mM HEPES buffer, 144 mM NaCl, 1.5 mM KCl, 2.0 mM CaCl2, and 1.0 mM MgSO4) plus 1.0 mM phenylmethylsulfonyl fluoride. This was followed by a 1-h incubation in binding buffer plus 0.5% bovine serum albumin, also containing 5 mM EDTA; 5 mM EGTA; 10 μg/ml each of aprotinin, leupeptin, and pepstatin A; and 0.5 nM [125I]α-CtxMII. The assay was terminated by washing the slides for 10 min in ice-cold binding buffer at room temperature, 2 × 10 min in 0.1× buffer at 0°C, and two final 5-s washes in ice-cold deionized water. Nicotine (100 μM) was used to determine nonspecific binding.
[125I]Epibatidine Binding.
α4β2* nAChRs levels were measured using [125I]epibatidine (specific activity, 2200 Ci/mmol; PerkinElmer Life and Analytical Sciences) (Quik et al., 2003). Thawed sections were preincubated at room temperature for 15 min in buffer containing 50 mM Tris, pH 7.5, 120 mM NaCl, 5 mM KCl, 2.5 mM CaCl2, and 1.0 mM MgCl2. This was followed by a 40-min incubation with 0.015 nM [125I]epibatidine without or with 100 nM α-CtxMII to define α4β2* nAChRs. Sections were washed 2 × 5 min in ice-cold buffer, 1 × 10 s in ice-cold deionized H2O. Nicotine (100 μM) was used to determine nonspecific binding.
Data Analysis.
For quantification of the optical densities from autoradiographic images, we used the ImageQuant system (GE Healthcare). Specific binding (total binding minus nonspecific binding) was converted to femtomoles per milligram of tissue using standard curves generated from radioactive [3H] standards that were simultaneously exposed to the films. The standards were calibrated for [125I] autoradiography, as described previously (Artymyshyn et al., 1990). The optical density readings for the samples fell within the linear range of the film. The receptor levels for any one animal were obtained from at least two independent experiments using two to three consecutive sections per experiment.
Statistical Analysis.
All statistics were conducted using GraphPad Prism (GraphPad Software, Inc., La Jolla, CA). Comparisons were performed using two-way analysis of variance (ANOVA), followed by a Bonferroni post hoc test. A level of 0.05 was considered significant. Values are expressed as the mean ± S.E.M. of the indicated number of rats and represent data pooled from up to two separate experiments.
Results
Long-Term Nicotine Treatment Reduces Haloperidol-Induced VCMs.
Rats were implanted with minipumps containing vehicle or nicotine (2 mg/kg per day). Such a nicotine regimen results in cotinine levels of ∼300 ng/ml (Bordia et al., 2008), which is similar to those in moderate smokers (Matta et al., 2007). Two weeks later, they were injected subcutaneously once daily with 1 mg/kg haloperidol or vehicle, as outlined in Fig. 1. They were rated for VCMs biweekly until week 13 and then every week thereafter by a blinded rater. Administration of haloperidol resulted in the development of significant VCMs by 7 weeks of treatment, which plateaued at ∼13 weeks, consistent with previous studies (Turrone et al., 2002). Long-term nicotine dosing significantly (P < 0.01) decreased VCMs after 13 weeks of haloperidol treatment, with a trend for a decline starting at 5 weeks (Fig. 2). Two-way ANOVA yielded a significant main effect of both nicotine and haloperidol treatment (P < 0.001), as well as a significant interaction (P < 0.01). The extended time course suggests that the effect of nicotine on haloperidol-induced VCMs involves long-term molecular changes.
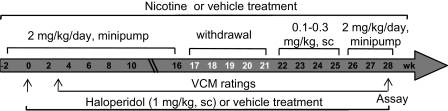
Fig. 1.
Experimental design depicting the time line and dosing of nicotine and haloperidol treatments. Rats were surgically implanted with minipumps containing vehicle or nicotine (2 mg/kg per day). Two weeks later, a subset of rats from the vehicle and nicotine-treated groups was administered vehicle or haloperidol (1 mg/kg s.c.) once daily until the end of the study with the nicotine continued. After 3 weeks of haloperidol treatment, rats were rated bi-weekly and every week thereafter for haloperidol-induced VCMs throughout the entire course of the study.
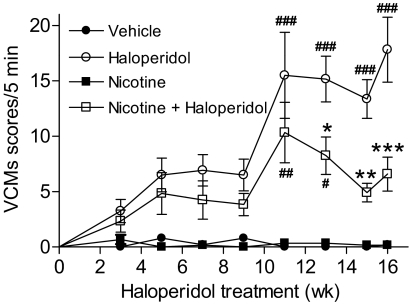
Fig. 2.
Time course of the nicotine-mediated reduction in haloperidol-induced VCMs. Rats were implanted with minipumps containing vehicle or nicotine (2 mg/kg per day). Two weeks later, a subset of rats receiving vehicle or nicotine was injected subcutaneously once daily with haloperidol (1 mg/kg). They were then evaluated for the development of VCMs at the indicated time points as described under Materials and Methods. Nicotine treatment significantly reduced the severity of haloperidol-induced VCMs. Values are the mean ± S.E.M. of six rats in the vehicle and nicotine-treated and 12 rats in the haloperidol and nicotine + haloperidol-treated groups. Significance of difference from the vehicle-treated control, #, P < 0.05, ##, P < 0.01, ###, P < 0.001; from the haloperidol-treated group, *, P < 0.05, **, P < 0.01, ***, P < 0.001 using two-way ANOVA followed by a Bonferroni's post hoc test.
Nicotine Reduces Haloperidol-Induced Abnormal Involuntary Movements in Rats during the Light and Dark Phase of the Light/Dark Cycle.
Because rats are nocturnal, we also evaluated haloperidol-induced VCMs in the dark phase of the light/dark cycle to determine whether these movements were altered by diurnal rhythms (Table 1). Haloperidol treatment resulted in comparable levels of VCMs in the dark and light phase. Moreover, nicotine treatment reduced VCMs (P < 0.001) to a similar extent in either phase, with a significant main effect of nicotine (P < 0.01) and haloperidol treatments (P < 0.001), as assessed by ANOVA. Thus, the magnitude of haloperidol-induced VCMs and the effect of nicotine seem to be independent of circadian rhythms.
TABLE 1.
Similar reduction in haloperidol-induced VCMs with chronic nicotine treatment (2 mg/kg per day) in the light or dark phase of the light-dark cycle
Rats were rated for haloperidol-induced VCMs in the light or dark phase of the daily cycle as described under Materials and Methods. Values are the mean ± S.E.M. of six rats in the vehicle and nicotine-treated and 12 rats in the haloperidol and nicotine + haloperidol-treated groups.
| Lighting Condition | Treatment | Number of VCMs per 5 Min |
|
|---|---|---|---|
| Control | Haloperidol | ||
| Light | Vehicle | 0.4 ± 0.2 | 17.8 ± 2.9### |
| Nicotine | 0.3 ± 0.2 | 6.6 ± 1.5*** | |
| Dark | Vehicle | 0.2 ± 0.2 | 13.4 ± 1.7### |
| Nicotine | 0.1 ± 0.1 | 4.9 ± 0.8#*** | |
Significance of difference from vehicle-treated control, # P < 0.05, ### P < 0.001; from vehicle-treated group, *** P < 0.001 using two-way ANOVA followed by a Bonferroni's post hoc test.
Nicotine Removal Worsens Haloperidol-Induced VCMs, Whereas Its Re-exposure Reduces VCMs.
The results in Fig. 2 show that nicotine pretreatment reduces the development of haloperidol-induced VCMs. An important question that arises is the reversibility of the nicotine-mediated decline in haloperidol-induced VCMs. To address this, the nicotine-containing minipumps were removed, and VCMs were rated weekly thereafter, with continued treatment of haloperidol. The results in Fig. 3 show that there was a gradual increase in VCMs with nicotine withdrawal, reaching levels similar to that of the vehicle-treated group 5 weeks after nicotine removal.
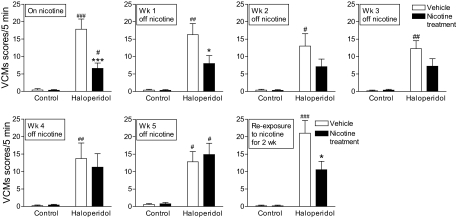
Fig. 3.
Effect of nicotine removal and re-exposure on haloperidol-induced VCMs. Nicotine (2 mg/kg per day) containing minipumps were removed, with haloperidol treatment continued. The rats were then rated for VCMs every week for 5 weeks. Removal of nicotine resulted in a gradual increase in haloperidol-induced VCMs overtime, reaching levels similar to that of the vehicle treated group by 5 weeks (left to right). It is noteworthy that two weeks of nicotine (2 mg/kg per day) re-exposure led to a significant reduction in haloperidol-induced VCMs. Values are the mean ± S.E.M. of six rats in the vehicle and nicotine-treated and 12 rats in the haloperidol and nicotine + haloperidol-treated groups. Significance of difference from vehicle-treated control, #, P < 0.05, ##, P < 0.01, ###, P < 0.001; from own vehicle-treated group, *, P < 0.05, ***, P < 0.001 using two-way ANOVA followed by a Bonferroni's post hoc test.
We next tested whether nicotine re-administration would decrease haloperidol-induced VCMs in the rats previously exposed to nicotine. We first evaluated the efficacy of a single acute dose of either 0.1 or 0.3 mg/kg s.c. nicotine injected 10 min before haloperidol (Table 2). However, no decline was observed in haloperidol-induced VCMs with either dose given short-term. We also tested the effect of a slightly longer nicotine treatment regimen (once-daily injections for 4 days), with a similar lack of effect. The rats were then reimplanted with nicotine-containing minipumps (2 mg/kg per day) (Fig. 3). A significant decrease in haloperidol-induced VCMs (P < 0.05) was observed after 2 weeks of long-term treatment compared with the vehicle-treated group. These data suggest that continued nicotine dosing is required to maintain its beneficial effects against VCMs.
TABLE 2.
Effect of acute and subchronic nicotine treatment on haloperidol-induced VCMs
Rats received injections of haloperidol 10 min after nicotine treatment and were rated for haloperidol-induced VCMs on weeks 22 through 25 (see Fig. 1). Nicotine treatment had no effect on haloperidol-induced VCMs when administered on an acute (single dose) or subchronic (once daily for 4 days) basis. Values are the mean ± S.E.M. of six rats in the vehicle and nicotine-treated and 12 rats in the haloperidol and nicotine + haloperidol-treated groups.
| Length of Treatment | Treatment | Dose | Number of VCMs per 5 Min |
|
|---|---|---|---|---|
| Control | Haloperidol | |||
| mg/kg | ||||
| Single dose | Vehicle | 0.4 ± 0.3 | 20.4 ± 5.1# | |
| Nicotine | 0.1 | 0 ± 0 | 19.1 ± 4.6# | |
| Vehicle | 0.4 ± 0.2 | 15.8 ± 4.7# | ||
| Nicotine | 0.3 | 0 ± 0 | 13.1 ± 3.4# | |
| Once daily for 4 days | Vehicle | 0 ± 0 | 17.9 ± 5.7# | |
| Nicotine | 0.1 | 0.2 ± 0.2 | 16.8 ± 4.5# | |
| Vehicle | 0.8 ± 0.4 | 15.4 ± 3.9# | ||
| Nicotine | 0.2 | 0.2 ± 3.8 | 17.4 ± 3.6# | |
Significance of difference from own control, # P < 0.05 using two-way ANOVA followed by a Bonferroni's post hoc test.
Long-Term Nicotine Treatment Does Not Reduce Haloperidol-Induced Catalepsy.
In addition to VCMs, haloperidol treatment also induces catalepsy. To test whether nicotine modified this behavior, we used the bar test. This test measures the time required for the rat to remove its forelimbs from an elevated horizontal bar 10 min after haloperidol compared with vehicle treatment (Kuschinsky and Hornykiewicz, 1972). Haloperidol-induced catalepsy (descent latency in seconds) was similar with nicotine and vehicle treatments. Thus, nicotine selectively affects VCMs (Table 3).
TABLE 3.
Nicotine treatment had no effect on haloperidol-induced catalepsy
Rats were assessed for haloperidol-induced catalepsy using the bar test, which measures the time taken (latency) for the rat to remove its forelimbs from an elevated horizontal bar. Nicotine or vehicle was injected 10 min before haloperidol treatment. Cataleptic effects of haloperidol were measured for 60 s, 10 min after haloperidol injection, immediately after rating of the VCMs. Values are the mean ± S.E.M. of six rats in the vehicle and nicotine-treated and 12 rats in the haloperidol and nicotine + haloperidol-treated groups.
| Length of Treatment | Treatment | Dose | Descent Latency |
|
|---|---|---|---|---|
| Control | Haloperidol | |||
| mg/kg per day | s | |||
| One injection | Vehicle | 0.40 ± 0.24 | 17.1 ± 3.5### | |
| Nicotine | 0.1 | 0.66 ± 0.21 | 11.0 ± 2.5## | |
| Once daily for 4 days | Vehicle | 0.20 ± 0.20 | 19.1 ± 3.4### | |
| Nicotine | 0.1 | 0.51 ± 0.22 | 18.2 ± 4.6### | |
| Chronic (minipump) | Vehicle | 0.42 ± 0.23 | 22.7 ± 3.5### | |
| Nicotine | 2 | 0.83 ± 0.30 | 25.1 ± 2.5### | |
Significance of difference from own control, ## P < 0.01, ### P < 0.001 using two-way ANOVA followed by a Bonferroni's post hoc test.
Long-Term Nicotine and Haloperidol Treatments Decrease the Striatal DA Transporter.
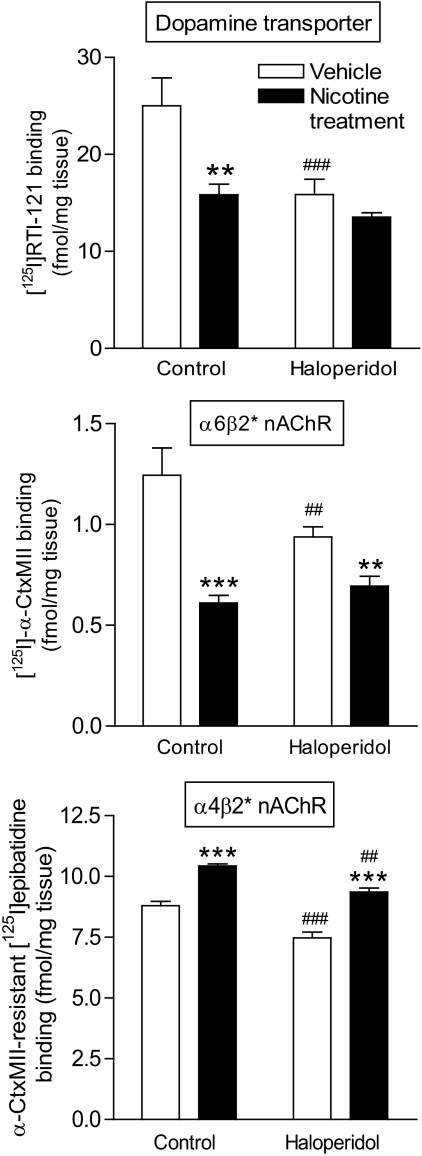
Previous studies measuring tyrosine hydroxylase immunoreactivity had suggested that haloperidol induces VCMs by down-regulating nigrostriatal DA neurons (Marchese et al., 2002; Zhang et al., 2007; Reynolds et al., 2011). To investigate whether haloperidol modulated dopaminergic measures in our studies, we measured the DAT, a DA nerve terminal marker (Quik et al., 2006). Haloperidol treatment reduced (P < 0.001) striatal DAT levels (Fig. 4, top), consistent with previous studies measuring tyrosine hydroxylase immunoreactivity (Marchese et al., 2002; Zhang et al., 2007; Reynolds et al., 2011). The long-term nicotine treatment also decreased DAT (P < 0.01). ANOVA showed a significant interaction (P < 0.05) between nicotine and haloperidol treatments, with results indicating that the combined effect of nicotine and haloperidol was not additive (Fig. 4, top). These data suggest that the nicotine- and haloperidol-induced declines in DAT share a common mechanism.
Fig. 4.
Long-term nicotine and/or haloperidol treatment alters the striatal dopamine transporter, as well as α6β2* and α4β2* nAChR levels. Rats were chronically administered nicotine (2 mg/kg per day) and/or haloperidol (1 mg/kg) as described under Materials and Methods. After treatment, they were killed 90 min after the last injection of haloperidol. Top panel shows striatal dopamine transporter levels measured using [125I]RTI-121. Long-term nicotine and haloperidol treatments resulted in a significant reduction in transporter levels, with no further reduction with the combined treatment. The middle panel depicts striatal α6β2* nAChR levels assessed using [125I]α-CtxMII. Both nicotine and haloperidol treatments resulted in significant reductions in α6β2* nAChR levels, with no further decrease with the combined treatment. Striatal α4β2* nAChR levels were determined by measuring binding of [125I]epibatidine in the presence of α-CtxMII. Nicotine treatment increased α4β2* nAChRs in the absence or presence of haloperidol, whereas long-term treatment of haloperidol alone led to a significant decrease in binding. Values are the mean ± S.E.M. of six rats in the vehicle and nicotine-treated and 12 rats in the haloperidol and nicotine + haloperidol-treated groups. Significance of difference from vehicle-treated control, ##, P < 0.01, ###, P < 0.001; from own vehicle-treated group, **, P < 0.01, ***, P < 0.001 using two-way ANOVA followed by a Bonferroni test.
Nicotine and/or Haloperidol Treatments Modulate Striatal α6β2* and α4β2* nAChR Levels.
Given that nicotine generally exerts its CNS effects by acting at nAChRs, we next investigated the impact of long-term nicotine treatment on α6β2* and α4β2* nAChRs, the primary subtypes in striatum. The effect on binding of α6β2* nAChRs, measured using [125I]α-CtxMII, is shown in Fig. 4 (middle). Nicotine reduced α6β2* nAChRs (P < 0.001), consistent with studies showing that it down-regulates this subtype (Quik et al., 2006). Haloperidol treatment also decreased α6β2* nAChRs (P < 0.01). Two-way ANOVA yielded a significant interaction (P < 0.01) between nicotine and haloperidol treatments. These data suggest that the nicotine- and haloperidol-induced declines in α6β2* nAChRs occur via a similar mechanism, analogous to our observations for DAT.
We also measured α4β2* receptors, the other major nAChR subtype in striatum using [125I]epibatidine binding in the presence of α-CtxMII (Fig. 4, bottom). Long-term haloperidol treatment alone led to a small but significant decrease (P < 0.001) in receptor levels in the striatum. This decline was region-specific with no decrease in the cortex (vehicle, 5.8 ± 0.23: haloperidol, 5.3 ± 0.07 fmol/mg). Long-term nicotine treatment alone increased (P < 0.001) α4β2* nAChRs in both striatum and in cortex (nicotine, 7.2 ± 0.13: nicotine plus haloperidol; 7.6 ± 0.06 fmol/mg), in agreement with previous findings (Quik et al., 2011). In addition, nicotine administration increased α4β2* nAChRs in striatum of haloperidol-treated rats (P < 0.001). Two-way ANOVA yielded a significant (P < 0.01) main effect of both nicotine and haloperidol treatments, with no significant interaction. Thus, haloperidol treatment decreased in α4β2* receptors in both vehicle- and nicotine-treated rats.
Discussion
The present results are the first to show that nicotine administration reduces the severity of haloperidol-induced VCMs in a rat model of tardive dyskinesia. Nicotine was equally effective in reducing VCMs in either phase of the light/dark cycle, attesting to the robustness of this effect. Time-course studies suggest that the effect of nicotine on haloperidol-induced VCMs involves long-term molecular changes. These data support the idea that nicotine or nAChR drugs may be useful for reducing antipsychotic-induced tardive dyskinesia.
For our studies, we used an established animal model of tardive dyskinesias in which rats are administered the commonly used antipsychotic drug haloperidol for several months (Turrone et al., 2002). Rats treated long-term with antipsychotics develop VCMs that share many characteristics typical of tardive dyskinesia, including similarities in appearance, developmental time course, and response to DA drugs (Waddington, 1990). Although rats are considered one of the best animal models, there are limitations as VCMs have been reported to normalize after haloperidol removal in some studies (Marchese et al., 2002; Zhang et al., 2007), whereas antipsychotic-induced tardive dyskinesia in humans may be irreversible (Gershanik and Gómez Arévalo, 2011).
Experiments were first done to determine whether the nicotine-mediated reduction in haloperidol-induced VCMs developed on a short-term basis or over the long-term. Nicotine did not attenuate haloperidol-induced VCMs when administered as a single acute dose. Once-daily nicotine injection for 4 days also failed to improve VCMs. Instead, 2 weeks of long-term nicotine administration was necessary for a significant reduction in VCMs, whereas 4 to 5 weeks of nicotine removal was required before its beneficial effects were lost. These combined data suggest that nicotine-mediated effects on haloperidol-induced VCMs involve long-term molecular and/or cellular mechanisms.
A chronic morphological change associated with the development of tardive dyskinesia is drug-induced declines in tyrosine hydroxylase-positive nigrostriatal dopaminergic neurons (Marchese et al., 2002; Andreassen et al., 2003; Zhang et al., 2007; Reynolds et al., 2011). Haloperidol treatment causes a shrinkage or loss of tyrosine hydroxylase immunoreactive neurons in the substantia nigra and a reduction in tyrosine hydroxylase immunoreactivity in rat striatum, which correlates with the development of VCMs (Levinson et al., 1998; Mazurek et al., 1998; Marchese et al., 2002; Zhang et al., 2007). These changes selectively occur in the nigrostriatal pathway, with no declines in the mesolimbic DA system (Reynolds et al., 2011). The reduced tyrosine hydroxylase immunoreactivity in the striatum and the presence of VCMs were both normalized after ∼1 month of haloperidol removal, suggesting a causal link (Marchese et al., 2002; Zhang et al., 2007). It is noteworthy that a more recent study showed that the haloperidol-induced decline in tyrosine hydroxylase-immunoreactive cells in the nigra was blocked by administration of a DAT blocker (Reynolds et al., 2011). These data could suggest that haloperidol-induced morphological changes are the result of an increase in DA turnover, which leads to elevated intracellular DA, subsequent inhibition of complex I, and enhanced oxidative stress.
In contrast to these detrimental effects of haloperidol, extensive in vitro and in vivo studies show that nicotine protects against nigrostriatal dopaminergic damage. Long-term nicotine treatment improves neurotoxin-induced dopaminergic degeneration in rodents and nonhuman primates (Quik et al., 2007b; Picciotto and Zoli, 2008). Nicotine is thought to exert this effect by acting at CNS nAChRs. These are pentameric ion channels composed of different α-subunits (α2–α10) or of α- and β-subunits (β2–β4) (Albuquerque et al., 2009; Gotti et al., 2009; Millar and Gotti, 2009; Changeux, 2010; Quik and Wonnacott, 2011). The principle subtypes in the peripheral nervous system are the α3β4* and α7 nAChRs, whereas the α4β2*, α6β2*, and α7 nAChRs are the primary ones in the nigrostriatal pathway. The asterisk indicates the possible presence of other subunits in the receptor complex. The α6β2* nAChR subtypes have a restricted CNS distribution, including dopaminergic pathways, whereas the α4β2* subtypes are widely distributed in the brain and also present on dopaminergic nerve terminals (Quik and Wonnacott, 2011; Quik et al., 2011; Threlfell and Cragg, 2011).
The present results showing a decline in striatal DAT with haloperidol treatment complement previous work that demonstrate a loss in tyrosine hydroxylase immunoreactivity in the nigrostriatal pathway (Marchese et al., 2002; Zhang et al., 2007; Reynolds et al., 2011). This loss in tyrosine hydroxylase immunoreactivity has been attributed to haloperidol-induced down-regulation. Long-term nicotine treatment via minipump also decreased DAT. Because nicotine is generally thought to protect against nigrostriatal damage (Quik et al., 2007b), this decline may reflect transporter down-regulation. Such an interpretation is consistent with other studies showing that drugs, such as amphetamine and methylphenidate, also down-regulate DAT (Madras et al., 2005). It is noteworthy that the effect of combined nicotine and haloperidol treatments was similar to that with either drug alone. These data suggest that nicotine and haloperidol may reduce DAT via an analogous mechanism of action, possibly down-regulation.
The drug-induced changes in α6β2* nAChRs were very similar to those in DAT. This analogous regulation may relate to the fact that both DAT and α6β2* nAChRs share a similar localization on dopaminergic nerve terminals in the striatum. These data contrast with the effects of nicotine and haloperidol on α4β2* receptors, the other major nAChR subtype in striatum. These differential changes in α4β2* compared with α6β2* nAChRs may relate to their differential cellular localization, with only a small proportion (20%) of α4β2* receptors present on DA terminals and the greater majority (80%) on other striatal elements (Quik et al., 2003). Long-term haloperidol treatment alone significantly decreased striatal α4β2* receptor binding in both vehicle- and haloperidol-treated rats. Furthermore, long-term nicotine treatment alone increased α4β2* nAChRs in the vehicle-treated group, consistent with previous findings (Quik et al., 2011), and also elevated α4β2* nAChRs in striata of haloperidol-treated rats.
How these molecular changes in DAT, α6β2* nAChRs, and/or α4β2* nAChRs relate to nicotine's beneficial action against antipsychotic-induced VCMs is an important question. The current studies did not identify unique changes in α4β2* nAChRs with nicotine and haloperidol treatment that might explain the beneficial effect of nicotine to reduce VCMs. Moreover, the observations that DAT and α6β2* nAChR levels were similar with nicotine or haloperidol treatment alone and with combined nicotine and haloperidol treatment suggest that these measures are not directly associated with the nicotine-mediated decline in haloperidol-induced VCMs. On the other hand, the DAT and nAChR studies were done at a subsaturating concentration of the radioligand. Studies at higher radioligand concentrations may yield information on the potential changes in maximal responsiveness under the different treatment conditions. Overall, the current data indicate that changes other than those directly at the DAT and nAChR level underlie the nicotine-mediated improvement in haloperidol-induced VCMs in rats. This may include adaptive changes in intracellular signaling mechanisms associated with nAChRs and/or in other neurotransmitter systems linked to the nicotinic cholinergic and dopaminergic systems.
Clinical trials have been done to investigate the link between smoking and tardive dyskinesias, with conflicting results (Yassa et al., 1987; Menza et al., 1991; Nilsson et al., 1997; Diehl et al., 2009; Zhang et al., 2011). In studies in which smoking correlated with the severity of tardive dyskinesia, data interpretation was complicated by findings that tardive dyskinesia was also associated with significantly higher exposure to antipsychotics, frequencies of psychiatric morbidity, and/or alcohol dependence (Yassa et al., 1987; Nilsson et al., 1997). In another study, patients who smoked received significantly higher doses of antipsychotics but did not have more frequent or more severe tardive dyskinesia (Menza et al., 1991). Altogether, these data indicate that further work is required to understand the relationship between smoking and tardive dyskinesia.
In summary, our data show that nicotine treatment decreases haloperidol-induced VCMs in an established rat model of tardive dyskinesia. The demonstration that nicotine removal leads to a return of VCMs, whereas nicotine re-exposure reduced haloperidol-induced VCMs, suggests a causal relationship. These data have clinical applications for the treatment of tardive dyskinesias associated with long-term antipsychotic treatment using nicotine. It is noteworthy that multiple nicotine formulations are currently available in humans for other indications primarily smoking cessation, including the nicotine patch, gum, lozenge, nasal spray, and nasal inhalant (Matta et al., 2007). Any one of these has the potential to treat tardive dyskinesia. A clinical trial in subjects with tardive dyskinesias would be necessary for development of the optimal therapy. Alternatively, the development of subtype-selective nAChR drugs may better target the receptors directly linked to the development of tardive dyskinesias, with optimal beneficial results and a minimum of side effects.
Acknowledgments
We thank Matthew Chin for excellent technical assistance.
This work was supported by the National Institutes of Health National Institute of Neurological Disorders and Stroke [Grants NS47162, NS59910]; and the National Institutes of Health National Institute of Mental Health [Grant MH53631].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- DA
- dopamine
- DAT
- DA transporter
- VCMs
- vacuous chewing movements
- RTI-121
- 3β-(4-iodophenyl)tropane-2β-carboxylic acid isopropyl ester
- α-CtxMII
- α-conotoxin MII
- ANOVA
- analysis of variance
- nAChRs
- nicotinic acetylcholine receptors (the asterisk indicates the possible presence of other nicotinic subunits in the receptor complex)
- CNS
- central nervous system.
Authorship Contributions
Participated in research design: Bordia and Quik.
Conducted experiments: Bordia.
Contributed new reagents or analytic tools: McIntosh.
Performed data analysis: Bordia and Quik.
Wrote or contributed to the writing of the manuscript: Bordia, McIntosh, and Quik.
References
- Aia PG, Revuelta GJ, Cloud LJ, Factor SA. (2011) Tardive dyskinesia. Curr Treat Options Neurol 13:231–241 [DOI] [PubMed] [Google Scholar]
- Albuquerque EX, Pereira EF, Alkondon M, Rogers SW. (2009) Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev 89:73–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreassen OA, Ferrante RJ, Aamo TO, Beal MF, Jørgensen HA. (2003) Oral dyskinesias and histopathological alterations in substantia nigra after long-term haloperidol treatment of old rats. Neuroscience 122:717–725 [DOI] [PubMed] [Google Scholar]
- Artymyshyn R, Smith A, Wolfe BB. (1990) The use of 3H standards in 125I autoradiography. J Neurosci Methods 32:185–192 [DOI] [PubMed] [Google Scholar]
- Barroso-Chinea P, Bezard E. (2010) Basal ganglia circuits underlying the pathophysiology of levodopa-induced dyskinesia. Front Neuroanat 14:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordia T, Campos C, Huang L, Quik M. (2008) Continuous and intermittent nicotine treatment reduces l-3,4-dihydroxyphenylalanine (l-DOPA)-induced dyskinesias in a rat model of Parkinson's disease. J Pharmacol Exp Ther 327:239–247 [DOI] [PubMed] [Google Scholar]
- Bordia T, Campos C, McIntosh JM, Quik M. (2010) Nicotinic receptor-mediated reduction in l-DOPA-induced dyskinesias may occur via desensitization. J Pharmacol Exp Ther 333:929–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cenci MA, Konradi C. (2010) Maladaptive striatal plasticity in l-DOPA-induced dyskinesia. Prog Brain Res 183:209–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changeux JP. (2010) Nicotine addiction and nicotinic receptors: lessons from genetically modified mice. Nat Rev Neurosci 11:389–401 [DOI] [PubMed] [Google Scholar]
- Correll CU, Leucht S, Kane JM. (2004) Lower risk for tardive dyskinesia associated with second-generation antipsychotics: a systematic review of 1-year studies. Am J Psychiatry 161:414–425 [DOI] [PubMed] [Google Scholar]
- Diehl A, Reinhard I, Schmitt A, Mann K, Gattaz WF. (2009) Does the degree of smoking effect the severity of tardive dyskinesia? A longitudinal clinical trial. Eur Psychiatry 24:33–40 [DOI] [PubMed] [Google Scholar]
- Gershanik OS, Gómez Arévalo GJ. (2011) Typical and atypical neuroleptics. Handb Clin Neurol 100:579–599 [DOI] [PubMed] [Google Scholar]
- Gotti C, Clementi F, Fornari A, Gaimarri A, Guiducci S, Manfredi I, Moretti M, Pedrazzi P, Pucci L, Zoli M. (2009) Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem Pharmacol 78:703–711 [DOI] [PubMed] [Google Scholar]
- Huang LZ, Grady SR, Quik M. (2011a) Nicotine reduces l-DOPA-induced dyskinesias by acting at β2* nicotinic receptors. J Pharmacol Exp Ther 338:932–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang LZ, Campos C, Ly J, Ivy Carroll F, Quik M. (2011b) Nicotinic receptor agonists decrease l-DOPA-induced dyskinesias most effectively in moderately lesioned parkinsonian rats. Neuropharmacology 60:861–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals 7th ed Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington DC [Google Scholar]
- Kuschinsky K, Hornykiewicz O. (1972) Morphine catalepsy in the rat: relation to striatal dopamine metabolism. Eur J Pharmacol 19:119–122 [DOI] [PubMed] [Google Scholar]
- Levinson AJ, Garside S, Rosebush PI, Mazurek MF. (1998) Haloperidol induces persistent down-regulation of tyrosine hydroxylase immunoreactivity in substantia nigra but not ventral tegmental area in the rat. Neuroscience 84:201–211 [DOI] [PubMed] [Google Scholar]
- Madras BK, Miller GM, Fischman AJ. (2005) The dopamine transporter and attention-deficit/hyperactivity disorder. Biol Psychiatry 57:1397–1409 [DOI] [PubMed] [Google Scholar]
- Marchese G, Casu MA, Bartholini F, Ruiu S, Saba P, Gessa GL, Pani L. (2002) Sub-chronic treatment with classical but not atypical antipsychotics produces morphological changes in rat nigro-striatal dopaminergic neurons directly related to “early onset” vacuous chewing. Eur J Neurosci 15:1187–1196 [DOI] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, et al. (2007) Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl) 190:269–319 [DOI] [PubMed] [Google Scholar]
- Mazurek MF, Savedia SM, Bobba RS, Garside S, Rosebush PI. (1998) Persistent loss of tyrosine hydroxylase immunoreactivity in the substantia nigra after neuroleptic withdrawal. J Neurol Neurosurg Psychiatry 64:799–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menza MA, Grossman N, Van Horn M, Cody R, Forman N. (1991) Smoking and movement disorders in psychiatric patients. Biol Psychiatry 30:109–115 [DOI] [PubMed] [Google Scholar]
- Millar NS, Gotti C. (2009) Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology 56:237–246 [DOI] [PubMed] [Google Scholar]
- Nilsson A, Waller L, Rosengren A, Adlerberth A, Wilhelmsen L. (1997) Cigarette smoking is associated with abnormal involuntary movements in the general male population–a study of men born in 1933. Biol Psychiatry 41:717–723 [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M. (2008) Neuroprotection via nAChRs: the role of nAChRs in neurodegenerative disorders such as Alzheimer's and Parkinson's disease. Front Biosci 13:492–504 [DOI] [PubMed] [Google Scholar]
- Quik M, Cox H, Parameswaran N, O'Leary K, Langston JW, Di Monte D. (2007a) Nicotine reduces levodopa-induced dyskinesias in lesioned monkeys. Ann Neurol 62:588–596 [DOI] [PubMed] [Google Scholar]
- Quik M, O'Neill M, Perez XA. (2007b) Nicotine neuroprotection against nigrostriatal damage: importance of the animal model. Trends Pharmacol Sci 28:229–235 [DOI] [PubMed] [Google Scholar]
- Quik M, Parameswaran N, McCallum SE, Bordia T, Bao S, McCormack A, Kim A, Tyndale RF, Langston JW, Di Monte DA. (2006) Chronic oral nicotine treatment protects against striatal degeneration in MPTP-treated primates. J Neurochem 98:1866–1875 [DOI] [PubMed] [Google Scholar]
- Quik M, Perez XA, Grady SR. (2011) Role of α6 nicotinic receptors in CNS dopaminergic function: relevance to addiction and neurological disorders. Biochem Pharmacol 82:873–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Sum JD, Whiteaker P, McCallum SE, Marks MJ, Musachio J, McIntosh JM, Collins AC, Grady SR. (2003) Differential declines in striatal nicotinic receptor subtype function after nigrostriatal damage in mice. Mol Pharmacol 63:1169–1179 [DOI] [PubMed] [Google Scholar]
- Quik M, Wonnacott S. (2011) α6β2* and α4β2* Nicotinic acetylcholine receptors as drug targets for Parkinson's disease. Pharmacol Rev 63:938–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds KB, MacGillivray L, Zettler M, Rosebush PI, Mazurek MF. (2011) Role of the dopamine transporter in mediating the neuroleptic-induced reduction of tyrosine hydroxylase-immunoreactive midbrain neurons. Brain Res 1394:24–32 [DOI] [PubMed] [Google Scholar]
- Rogoza RM, Fairfax DF, Henry P, N-Marandi S, Khan RF, Gupta SK, Mishra RK. (2004) Electron spin resonance spectroscopy reveals alpha-phenyl-N-tert-butylnitrone spin-traps free radicals in rat striatum and prevents haloperidol-induced vacuous chewing movements in the rat model of human tardive dyskinesia. Synapse 54:156–163 [DOI] [PubMed] [Google Scholar]
- Saha S, Chant D, Welham J, McGrath J. (2005) A systematic review of the prevalence of schizophrenia. PLoS Med 2:e141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaha AN, Reckless GE, Seeman P, Diwan M, Nobrega JN, Kapur S. (2008) Less is more: antipsychotic drug effects are greater with transient rather than continuous delivery. Biol Psychiatry 64:145–152 [DOI] [PubMed] [Google Scholar]
- Schleimer SB, Johnston GA, Henderson JM. (2005) Novel oral drug administration in an animal model of neuroleptic therapy. J Neurosci Methods 146:159–164 [DOI] [PubMed] [Google Scholar]
- Seeman P. (2010) Dopamine D2 receptors as treatment targets in schizophrenia. Clin Schizophr Relat Psychoses 4:56–73 [DOI] [PubMed] [Google Scholar]
- Tandon R, Belmaker RH, Gattaz WF, Lopez-Ibor JJ, Jr, Okasha A, Singh B, Stein DJ, Olie JP, Fleischhacker WW, Moeller HJ, et al. (2008) World Psychiatric Association Pharmacopsychiatry Section statement on comparative effectiveness of antipsychotics in the treatment of schizophrenia. Schizophr Res 100:20–38 [DOI] [PubMed] [Google Scholar]
- Tarsy D, Lungu C, Baldessarini RJ. (2011) Epidemiology of tardive dyskinesia before and during the era of modern antipsychotic drugs. Handb Clin Neurol 100:601–616 [DOI] [PubMed] [Google Scholar]
- Threlfell S, Cragg SJ. (2011) Dopamine signaling in dorsal versus ventral striatum: the dynamic role of cholinergic interneurons. Front Syst Neurosci 5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turrone P, Remington G, Kapur S, Nobrega JN. (2003) The relationship between dopamine D2 receptor occupancy and the vacuous chewing movement syndrome in rats. Psychopharmacology (Berl) 165:166–171 [DOI] [PubMed] [Google Scholar]
- Turrone P, Remington G, Nobrega JN. (2002) The vacuous chewing movement (VCM) model of tardive dyskinesia revisited: is there a relationship to dopamine D(2) receptor occupancy? Neurosci Biobehav Rev 26:361–380 [DOI] [PubMed] [Google Scholar]
- Volavka J, Citrome L. (2009) Oral antipsychotics for the treatment of schizophrenia: heterogeneity in efficacy and tolerability should drive decision-making. Expert Opin Pharmacother 10:1917–1928 [DOI] [PubMed] [Google Scholar]
- Waddington JL. (1990) Spontaneous orofacial movements induced in rodents by very long-term neuroleptic drug administration: phenomenology, pathophysiology and putative relationship to tardive dyskinesia. Psychopharmacology (Berl) 101:431–447 [DOI] [PubMed] [Google Scholar]
- Yassa R, Lal S, Korpassy A, Ally J. (1987) Nicotine exposure and tardive dyskinesia. Biol Psychiatry 22:67–72 [DOI] [PubMed] [Google Scholar]
- Zhang XY, Yu YQ, Sun S, Zhang X, Li W, Xiu MH, Chen da C, Yang FD, Zhu F, Kosten TA, et al. (2011) Smoking and tardive dyskinesia in male patients with chronic schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 35:1765–1769 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Xu H, He J, Yan B, Jiang W, Li X, Li XM. (2007) Quetiapine reverses altered locomotor activity and tyrosine hydroxylase immunoreactivity in rat caudate putamen following long-term haloperidol treatment. Neurosci Lett 420:66–71 [DOI] [PubMed] [Google Scholar]