Abstract
Adverse effects of benzodiazepines limit their clinical use; these effects might be reduced without altering therapeutic effects by administering other positive GABAA modulators (i.e., neuroactive steroids) with benzodiazepines. One concern with this strategy involves reversing these combined effects in case of overdose. The current study examined whether flumazenil can attenuate the combined effects of two benzodiazepines, midazolam and flunitrazepam, and the combined effects of midazolam and the neuroactive steroid pregnanolone, in four monkeys discriminating midazolam. Each positive modulator produced ≥80% midazolam-lever responding. Interactions between midazolam and either flunitrazepam or pregnanolone were additive. Flumazenil antagonized the benzodiazepines when they were administered alone or in combination. Schild analyses yielded slopes that did not deviate from unity, regardless of whether benzodiazepines were administered alone or together; the pA2 value for flumazenil was 7.58. In contrast, flumazenil enhanced the effects of pregnanolone with 0.32 mg/kg flumazenil shifting the pregnanolone dose-effect curve 2-fold leftward. Flumazenil attenuated the combined effects of midazolam and pregnanolone, although antagonism was not dose-dependent. Thus, the interaction between two benzodiazepines was similar to that of a benzodiazepine and a neuroactive steroid; however, flumazenil more efficiently attenuated a combination of two benzodiazepines compared with a combination of a benzodiazepine and a neuroactive steroid. Although the magnitude of antagonism of a benzodiazepine combined with a neuroactive steroid was reduced, these results support continued exploration of the use of combinations of positive modulators to enhance therapeutic effects while reducing adverse effects.
Introduction
Benzodiazepines have been widely used to treat anxiety, insomnia, convulsions, and ethanol withdrawal. Although they are safe and effective, long-term use has revealed adverse effects, particularly tolerance and dependence. One strategy that might retain the therapeutic effects of benzodiazepines while reducing adverse effects is to develop other positive modulators of GABAA receptors, such as neuroactive steroids. Although benzodiazepines and neuroactive steroids act at distinct sites on GABAA receptors, they both facilitate the actions of GABA, thereby increasing Cl− flux and producing similar behavioral effects. Like benzodiazepines, neuroactive steroids have anxiolytic (Wieland et al., 1997), sedative (Lancel, 1999; Vanover et al., 1999), and anticonvulsant effects (Kokate et al., 1994; Gasior et al., 2000; Reddy and Rogawski, 2001) and can reverse ethanol withdrawal (Finn et al., 2000). Despite these similarities, the effects of neuroactive steroids and benzodiazepines are not identical, with differences emerging during long-term treatment; for example, tolerance and dependence are less likely to develop during long-term treatment with neuroactive steroids than with benzodiazepines (Kokate et al., 1998; Reddy and Rogawski, 2000; Eppolito and Gerak, 2010). Although the ramifications of long-term therapeutic use of neuroactive steroids are not known, lack of tolerance could provide a clinical advantage for neuroactive steroids over benzodiazepines. In contrast, there are other factors that might make the therapeutic use of benzodiazepines more appealing than that of neuroactive steroids, such as the availability of a pharmacological antagonist (e.g., flumazenil) that can reverse the effects of benzodiazepines in the event of overdose; no such antagonist is available to reverse the effects of neuroactive steroids. Thus, clinical benefits are different among positive GABAA modulators, and if benzodiazepines and neuroactive steroids could be combined in one therapeutic drug, that drug might retain the clinical effectiveness of benzodiazepines with fewer adverse effects and could be at least partially attenuated by flumazenil.
One way to combine the benefits of benzodiazepines and neuroactive steroids is to administer them concurrently. Drug combinations have been used successfully to treat other conditions. For example, when drugs (e.g., opioids and nonsteroidal anti-inflammatory drugs) are given together to relieve pain, smaller doses of each drug are needed to produce the desired effect; adverse effects are reduced by using smaller doses of drugs that act through different mechanisms. A similar approach might be used with positive modulators acting at different sites on GABAA receptors to retain positive aspects of each drug while reducing less desirable features.
Studies in monkeys suggest that combinations of benzodiazepines and neuroactive steroids might provide clinical advantages by retaining therapeutic effects while reducing adverse effects. For example, combinations of the benzodiazepine triazolam and the neuroactive steroid pregnanolone produced supra-additive effects in a conflict procedure, which provides a measure of anxiolytic actions (Fischer and Rowlett, 2011). When discriminative stimulus effects or rates of lever pressing were measured, the interaction was additive (McMahon and France, 2005; Fischer and Rowlett, 2011), and in monkeys self-administering a combination of triazolam and pregnanolone, the interaction was either additive or infra-additive, depending on the ratio of doses used (Fischer and Rowlett, 2011). Thus, the combined effects of positive GABAA modulators can vary, enhancing some effects more than others.
Although benzodiazepines are generally safe, severe respiratory depression can occur when benzodiazepines are given with other drugs (e.g., ethanol), and toxicity can be reduced with flumazenil, which reverses the benzodiazepine component of the mixture. Flumazenil might also be expected to attenuate the combined effects of a benzodiazepine and a neuroactive steroid. Flumazenil antagonizes benzodiazepines in monkeys discriminating midazolam, shifting dose-effect curves rightward (Lelas et al., 1999, 2000; McMahon et al., 2002); however, it enhances the effects of neuroactive steroids, shifting dose-effect curves leftward (McMahon and France, 2005; Gerak and France, 2011), which reflects the positive efficacy of flumazenil (Dantzer and Pério, 1982). Thus, flumazenil could enhance or attenuate the combined effects of benzodiazepines and neuroactive steroids, depending on the proportion of each drug in the mixture.
This study examined the ability of flumazenil to alter the effects of pregnanolone, midazolam, and another benzodiazepine, flunitrazepam, given alone or together. Drug combinations were administered using a fixed-ratio design such that the proportion remained constant on the basis of the potency of each drug to produce midazolam-lever responding. According to receptor theory, flumazenil should attenuate the effects of all benzodiazepines, regardless of whether they are administered alone or together. These studies tested that hypothesis by comparing the combined effects of two benzodiazepines with the combined effects of a benzodiazepine and a neuroactive steroid and determining the extent to which flumazenil attenuates each drug combination.
Materials and Methods
Subjects.
Five adult female rhesus monkeys were housed individually on a 14-h light and 10-h dark cycle. Their weights (4.0–9.0 kg) did not markedly change throughout the experiments and were maintained with primate chow (High Protein Monkey Diet; Harlan Teklad, Madison, WI), fresh fruit, and peanuts provided in the home cage. Monkeys had free access to water while in their home cages. They were trained to discriminate midazolam at least 1 year before the start of these studies (Gerak and France, 2011). Three monkeys (subjects SA, LI, and NI) contributed to all data presented. A fourth monkey (subject RO) participated in studies in which 1) midazolam was given alone and with flumazenil, 2) flunitrazepam was given alone, and 3) a mixture of midazolam and flunitrazepam was administered with and without flumazenil. That monkey was replaced by a fifth monkey (subject HE), that participated in studies in which 1) midazolam was studied alone, 2) pregnanolone was given alone and with flumazenil, and 3) a mixture of midazolam and pregnanolone was administered with and without flumazenil. Monkeys used in these studies were maintained in accordance with the Institutional Animal Care and Use Committee, The University of Texas Health Science Center at San Antonio (San Antonio, TX) and with the 1996 Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, 1996).
Apparatus.
During experimental sessions, monkeys were seated in chairs (Primate Products, Miami, FL) with their feet placed in shoes mounted on the front of the chair. Shoes contained brass electrodes to which a brief (250 ms, 3 mA) electric shock could be delivered. During sessions, chairs were placed in ventilated, sound-attenuating chambers that were equipped with two stimulus lights and two response levers. A commercially available interface (Med Associates, Inc., St. Albans, VT) connected experimental chambers to computers that controlled experiments and recorded data using MED-PC IV software (MED Associates, Inc.). White noise was present in each chamber.
Procedure.
At the beginning of these studies, three monkeys discriminated 0.178 mg/kg midazolam and two other monkeys discriminated 0.32 mg/kg midazolam from vehicle while responding under a fixed-ratio 10 schedule of stimulus-shock termination (Gerak and France, 2011). For the two monkeys that began the experiment discriminating the larger dose of midazolam (subjects RO and LI), the training dose was decreased to 0.178 mg/kg during these studies because the larger dose decreased the rate of lever pressing; dose-effect curves for midazolam and flunitrazepam (subject RO) and for midazolam and pregnanolone (subject LI) determined before and after the change in training dose were similar. Daily experimental sessions were divided into 15-min cycles with up to eight cycles in each session. Cycles began with 10-min timeout periods during which chambers were dark and responding had no programmed consequence. Response periods occurred during the last 5 min of each cycle and were signaled by illumination of red stimulus lights. Under the schedule of stimulus-shock termination, monkeys could extinguish stimulus lights and postpone the shock schedule for 30 s by responding 10 consecutive times (fixed-ratio 10) on the lever designated correct by an injection administered during the 1st min of the cycle. The lever designated correct after administration of midazolam was counterbalanced across monkeys. Incorrect responses reset the response requirement on the correct lever. If monkeys did not satisfy the response requirement within 15 s of illumination of red lights, a brief electric shock was delivered every 15 s until the response requirement was satisfied or the cycle ended, which occurred either when four shocks were delivered or 5 min elapsed since the beginning of the response period.
Injections were given during the 1st min of each cycle. For some training sessions, the training dose of midazolam was administered at the beginning of one cycle; during that cycle as well as during one subsequent cycle, which began with a sham injection, responding on the drug-appropriate lever extinguished lights and postponed the shock schedule. The session ended after the sham cycle. The cycle during which midazolam was administered was preceded by zero to six cycles during which saline or sham injections were administered. For other training sessions, monkeys received saline or sham injections before all cycles, with the number of cycles varying between two and eight. Stimulus control was considered adequate for testing when the following criteria were satisfied: ≥80% responding on the injection-appropriate lever during each cycle and fewer than 10 responses on the incorrect lever before completion of the first fixed ratio on the correct lever. Before their first test session, monkeys satisfied these criteria for five consecutive or six of seven training sessions; thereafter, test sessions were conducted when these criteria were satisfied for two consecutive training sessions. When monkeys did not satisfy the testing criteria, training continued until the criteria were satisfied during two consecutive sessions.
Test sessions were identical to training sessions except that 10 consecutive responses on either lever postponed shock and various doses of test compounds were administered during the 1st min of each cycle. Dose-effect curves for midazolam, flunitrazepam, and pregnanolone were generated by administering vehicle on the first cycle and test compound during subsequent cycles with the cumulative dose increasing by 1/4 log unit per cycle. Dosing continued until monkeys responded ≥80% on the midazolam lever. From these dose-effect curves, the dose needed to produce 50% responding on the drug lever (ED50) was estimated for individual monkeys using linear regression. The discriminative stimulus effects of combinations of positive modulators were studied by administering increasing doses during each cycle while maintaining a constant proportion of the two drugs; the proportion was based on the group mean ED50 values (McMahon and France, 2005). During the 1st min of the 1st cycle, two separate injections of vehicle were administered; one injection was saline and the second injection was the vehicle appropriate for either flunitrazepam or pregnanolone, depending on which drug was studied in combination with midazolam during subsequent cycles. Thereafter, two drugs were given in separate injections on each cycle. When midazolam was studied in combination with flunitrazepam, doses that were one-eighth of the group mean ED50 value for each drug were administered during the second cycle; when midazolam was studied in combination with pregnanolone, doses that were one-fourth of the group mean ED50 value for each drug were administered during the second cycle. The dose of each drug doubled on each subsequent cycle up to the dose combination that produced ≥80% responding on the midazolam lever. To examine whether flumazenil alters the discriminative stimulus effects of positive modulators administered alone or in combination, a single dose of flumazenil (0.01–1 mg/kg) was given on the first cycle followed by cumulative doses of the test compound(s) up to doses that produced ≥80% responding on the midazolam lever. When one positive modulator was administered after flumazenil, doses increased in 1/4 log unit increments, whereas when two positive modulators were administered concurrently, doses of both drugs doubled on each subsequent cycle. Dose-effect curves for a single positive modulator or a combination of two positive modulators were determined twice in the absence of flumazenil: once before and once after they were studied in the presence of three doses of flumazenil.
Drugs.
Midazolam hydrochloride (Bedford Laboratories, Bedford, OH) was purchased as a commercially prepared solution and diluted with sterile water. Flunitrazepam (Sigma-Aldrich, St. Louis, MO) was dissolved in a vehicle containing 50% Emulphor and 50% ethanol. Pregnanolone (5β-pregnan-3α-ol-20-one; Bujno Synthesis, Warsaw, Poland) was dissolved in 45% (w/v) hydroxypropyl-γ-cyclodextrin. Flumazenil was dissolved in a vehicle comprising 40% propylene glycol, 50% sterile water, and 10% ethanol. Doses are expressed in terms of the forms listed above in milligrams per kilogram body weight. Drugs were administered subcutaneously in the back.
Data Analyses.
Control response rates were determined when monkeys received only saline or sham injections and satisfied the testing criteria during each cycle of the session. A mean response rate for each training session was calculated by averaging response rates across cycles; control response rates represent the average of the means for five training sessions. Discrimination data are expressed as the average percentage of total responses emitted on the midazolam lever (± 1 S.E.M.) and plotted as a function of dose. Once an individual monkey responded at least 80% on the midazolam lever during drug test sessions, cumulative dosing stopped and a percentage of 100 was used in the analyses for larger doses. Effects of positive modulators administered in the absence of flumazenil were doubly determined and averaged for individual monkeys before the mean of the group was obtained.
ED50 values were estimated by fitting straight lines to individual dose-effect curves for each monkey; each line was estimated by linear regression using one dose that produced not more than 25% responding on the drug lever, one dose that produced at least 75% responding on the drug lever, and all doses in between. Potency ratios were calculated for each animal by dividing ED50 values obtained after administration of flumazenil by ED50 values obtained after administration of vehicle. A significant change in the potency of a positive modulator or combination of positive modulators was detected when the 95% confidence intervals (CIs) of the potency ratios averaged among monkeys did not include 1. When flumazenil was studied with either one or two benzodiazepines, dose ratios were also used to construct Schild plots. Using the method of Arunlakshana and Schild (1959), log (dose ratio − 1) was determined for individual monkeys, averaged, and plotted as a function of flumazenil dose [−log (moles per kilogram)]. Straight lines were fitted to each Schild plot using GraphPad Prism (version 5.01 for Windows; GraphPad Software Inc., San Diego, CA) and the following equation: log (dose ratio − 1) = −log(molar dose of flumazenil) × slope + intercept. Schild plots generated using midazolam and flunitrazepam either alone or in combination were compared using an F-ratio test (Kenakin, 1993; Koek et al., 2000). The slope of the Schild plot was constrained to unity in the simpler model and allowed to vary in the more complex model. If the calculated F value was not significant, then the data were best fit by the simpler model (i.e., slope constrained to −1). When slopes of Schild plots were constrained to unity, apparent pA2 values were compared using an F-ratio test. When slopes were not constrained, apparent pA2 values (95% CI) were determined using linear regression of Schild plots. Significance was set at P < 0.05.
Results
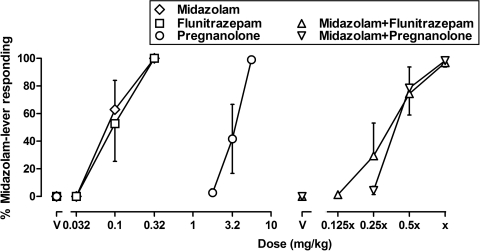
Among the four monkeys that received combinations of midazolam and flunitrazepam (subjects RO, SA, LI, and NI), the group mean response rate (mean ± 1 S.E.M.) obtained in the absence of drug was 2.53 ± 0.32 responses/s, and among the four monkeys that received combinations of midazolam and pregnanolone (subjects HE, SA, LI, and NI), the group mean response rate was 2.62 ± 0.24 responses/s. Mean response rates were lower after administration of the training dose of midazolam: 1.47 ± 0.15 responses/s in the first group and 1.44 ± 0.51 responses/s in the second. When monkeys received saline, they responded on the saline lever and response rates were similar to control rates (data not shown). Midazolam, flunitrazepam, and pregnanolone dose-dependently increased responding on the midazolam lever (Fig. 1, left). When midazolam and flunitrazepam were administered alone (subjects RO, SA, LI, and NI), ED50 values (mean ± 1 S.E.M.) were 0.097 ± 0.028 and 0.12 ± 0.03 mg/kg, respectively; when midazolam and pregnanolone were administered alone (subjects HE, SA, LI, and NI), ED50 values were 0.051 ± 0.011 and 3.45 ± 0.49 mg/kg, respectively. To study the combined effects of drugs, multiples of these ED50 values were administered. The smallest dose combination of midazolam and flunitrazepam (0.013 and 0.015 mg/kg, respectively, which were one eighth of their ED50 values) produced 1.4% responding on the midazolam lever; as the dose doubled on each subsequent cycle, responding on the midazolam lever increased and was ≥80% when the ED50 values were administered concurrently (Fig. 1, right, ▵). The dose combination that was estimated to produce 50% midazolam-lever responding was 0.042 ± 0.010 mg/kg midazolam together with 0.051 ± 0.012 mg/kg flunitrazepam. Likewise, the smallest dose combination of midazolam and pregnanolone studied (0.013 and 0.86 mg/kg, respectively) was one fourth of their ED50 values and produced 4.4% responding on the midazolam lever; as the dose doubled on each subsequent cycle, responding on the midazolam lever increased, with concurrent administration of the ED50 values resulting in ≥80% drug-lever responding (Fig. 1, right, ▿). The dose combination that was estimated to produce 50% midazolam-lever responding was 0.023 ± 0.003 mg/kg midazolam together with 1.54 ± 0.23 mg/kg pregnanolone. The effects of positive GABAA modulators, administered alone or in combination, on rate of lever pressing were similar to those obtained with the training dose of midazolam; doses producing ≥80% drug-lever responding decreased response rates to <60% of control rates (data not shown).
Fig. 1.
Discriminative stimulus effects of midazolam, flunitrazepam, and pregnanolone administered alone (left) and midazolam administered in combination with flunitrazepam or pregnanolone (right). Each dose-effect curve represents data from four monkeys; dose-effect curves shown in this figure for midazolam, flunitrazepam, and a combination of midazolam and flunitrazepam were determined in subjects RO, SA, LI, and NI, and dose-effect curves for pregnanolone and a combination of midazolam and pregnanolone were determined in subjects HE, SA, LI, and NI. Abscissa, left: dose in milligrams per kilogram body weight; right: dose expressed as multiples of ED50 values, which are denoted by ×. In monkeys that received combinations of midazolam and flunitrazepam, the ED50 value for midazolam when it was administered alone was 0.097 mg/kg and the ED50 for flunitrazepam alone was 0.12 mg/kg. In monkeys that received combinations of midazolam and pregnanolone, the ED50 value for midazolam when it was studied alone was 0.051 mg/kg and the ED50 for pregnanolone alone was 3.45 mg/kg. Data above V represent the effects of vehicle. Ordinates, mean (±1 S.E.M.) percentage of total responding that occurred on the midazolam lever.
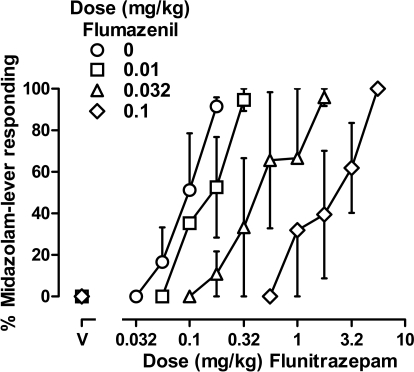
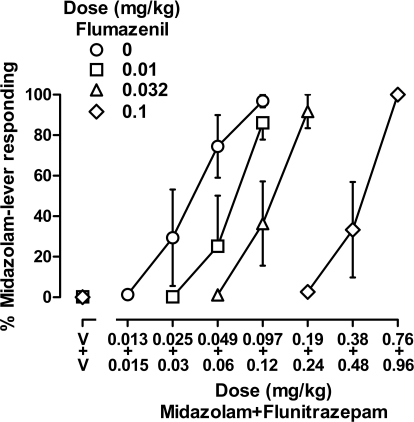
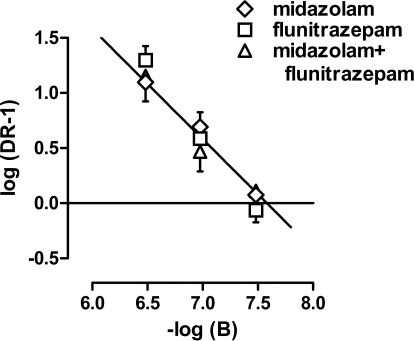
Flumazenil dose-dependently antagonized the discriminative stimulus effects of midazolam and flunitrazepam, whether the benzodiazepines were administered alone or together. Doses of 0.01, 0.032, and 0.1 mg/kg flumazenil shifted the midazolam dose-effect curve 2.3-, 6.5-, and 16.5-fold to the right, respectively (data not shown) and shifted the flunitrazepam dose-effect curve 1.9-, 6.4-, and 22.8-fold, respectively (Fig. 2). When midazolam and flunitrazepam were given concurrently, flumazenil also produced orderly, rightward shifts in the dose-effect curve (2.3-, 4.8-, and 18.5-fold, respectively) (Fig. 3). Schild plots were constructed from data obtained when flumazenil was studied with midazolam alone, flunitrazepam alone, or a combination of midazolam and flunitrazepam (Fig. 4); straight lines fitted to the data indicated that slopes (F2,27 = 0.806, n.s.) and intercepts (F2,29 = 0.082, n.s.) were not significantly different among the three Schild plots. Moreover, the common slope was not different from unity (F1,29 = 1.134, n.s.). Thus, the simplest model that could be used to fit the data obtained when flumazenil was studied with midazolam, flunitrazepam, or a combination of midazolam and flunitrazepam was one with a common slope and a common intercept. With the common slope constrained to unity, the apparent pA2 value was 7.58. When the three Schild plots were analyzed independently, the unconstrained slope of each line was not significantly different from unity, yielding apparent pA2 values that were also not significantly different (Table 1).
Fig. 2.
Discriminative stimulus effects of flunitrazepam administered alone (○) or after administration of various doses of flumazenil in three monkeys (subjects SA, LI, and NI). Abscissa, dose in milligrams per kilogram body weight; data above V represent the effects of vehicle. Ordinate, mean (±1 S.E.M.) percentage of total responding that occurred on the midazolam lever.
Fig. 3.
Discriminative stimulus effects of a combination of midazolam and flunitrazepam after administration of vehicle (○) or various doses of flumazenil in four monkeys (subjects RO, SA, LI, and NI). Abscissa, dose in milligrams per kilogram body weight with the top label on the abscissa indicating the dose of midazolam and the bottom label indicating the dose of flunitrazepam; data above V represent the effects of vehicle. Doses of midazolam and flunitrazepam were multiples of ED50 values obtained when that drug was studied alone; those values were 0.097 mg/kg for midazolam and 0.12 mg/kg for flunitrazepam. Ordinate, mean (±1 S.E.M.) percentage of total responding that occurred on the midazolam lever.
Fig. 4.
Schild plots for flumazenil studied with midazolam, flunitrazepam, or a combination of the two benzodiazepines. Raw data are shown in Fig. 2 (flumazenil with flunitrazepam) and Fig. 3 (flumazenil with the combination of midazolam and flunitrazepam) or are not shown (flumazenil with midazolam). Abscissa, negative log of the molar dose of flumazenil. Ordinate, log(dose ratio − 1). DR, dose ratio.
TABLE 1.
Slopes of Schild plots and pA2 values (95% CI) for flumazenil determined with constrained and unconstrained slopes
| Slope (Unconstrained) | pA2 (Unconstrained) | pA2 (Constrained) | |
|---|---|---|---|
| Midazolam | −1.02 (−1.43 to −0.61) | 7.59 (7.36–8.05) | 7.60 (7.44–7.76) |
| Flunitrazepam | −1.36 (−1.88 to −0.84) | 7.43 (7.24–7.77) | 7.59 (7.36–7.82) |
| Midazolam + flunitrazepam | −1.04 (−1.48 to −0.60) | 7.54 (7.31–7.99) | 7.56 (7.39–7.73) |
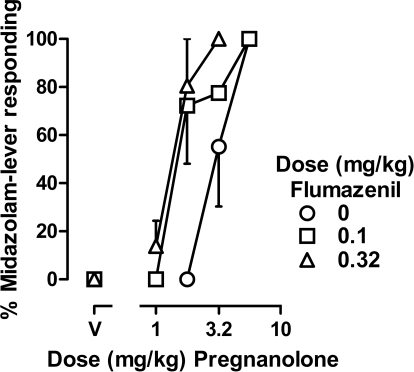
Although flumazenil antagonized the discriminative stimulus effects of benzodiazepines, it enhanced the discriminative stimulus effects of pregnanolone, shifting the pregnanolone dose-effect curve to the left (Fig. 5). Larger doses of flumazenil were needed to shift the pregnanolone dose-effect curve leftward, compared with doses that shifted the benzodiazepine dose-effect curves rightward (Figs. 2 and 3). Mean potency ratios (95% CI) obtained after administration of 0.032, 0.1, and 0.32 mg/kg flumazenil were 0.85 (0.42–1.29), 0.66 (0.07–1.24), and 0.48 (0.27–0.69); the largest dose of flumazenil produced a significant (2-fold) increase in the potency of pregnanolone.
Fig. 5.
Discriminative stimulus effects of pregnanolone administered alone (○) or after administration of various doses of flumazenil in four monkeys (subjects HE, SA, LI, and NI). Abscissa, dose in milligrams per kilogram body weight; data above V represent the effects of vehicle. Ordinate, mean (±1 S.E.M.) percentage of total responding that occurred on the midazolam lever.
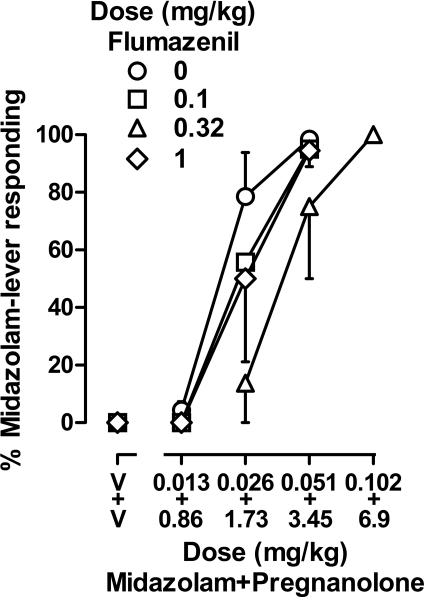
A dose of 0.32 mg/kg flumazenil, which significantly enhanced the effects of pregnanolone when it was administered alone, attenuated the combined effects of midazolam and pregnanolone (Fig. 6). Mean dose ratios (95% CI) obtained after administration of 0.1, 0.32, and 1 mg/kg flumazenil were 1.28 (0.51–2.05), 1.97 (1.44–2.49), and 1.31 (0.59–2.02). Although a dose of 0.32 mg/kg flumazenil significantly decreased the potency of the combination of midazolam and pregnanolone, this effect was not dose-dependent.
Fig. 6.
Discriminative stimulus effects of a combination of midazolam and pregnanolone after administration of vehicle (○) or various doses of flumazenil in four monkeys (subjects HE, SA, LI, and NI). Abscissa, dose in milligrams per kilogram body weight with the top label on the abscissa indicating the dose of midazolam and the bottom label indicating the dose of pregnanolone; data above V represent the effects of vehicle. Doses of midazolam and pregnanolone were multiples of ED50 values obtained when that drug was studied alone; those values were 0.051 mg/kg for midazolam and 3.45 mg/kg for pregnanolone. Ordinate, mean (±1 S.E.M.) percentage of total responding that occurred on the midazolam lever.
Discussion
Benzodiazepines act at the benzodiazepine binding site on GABAA receptors, where they have high positive efficacy. When two benzodiazepines are given together, their discriminative stimulus effects are additive in monkeys (current study; McMahon and France, 2005). A different type of interaction occurs when a benzodiazepine is given with flumazenil, which acts at the benzodiazepine binding site with low positive efficacy. Flumazenil antagonizes the discriminative stimulus effects of benzodiazepines, producing orderly, rightward shifts in dose-effect curves. Schild analyses of these antagonism data yield slopes that are not different from unity and are consistent with a simple, competitive, and reversible interaction. The pA2 values, which indicate the potency of flumazenil to antagonize benzodiazepines, has been determined for flumazenil with several different benzodiazepines in monkeys discriminating midazolam; pA2 values range from 7.41 to 7.69 (Lelas et al., 1999, 2000; McMahon et al., 2002), suggesting that the same population of receptors mediates the discriminative stimulus effects of all benzodiazepines studied to date. When flumazenil is examined with midazolam and with flunitrazepam, results are similar to previous data with slopes not different from unity and pA2 values for flumazenil within the range of values obtained previously (pA2 = 7.59 with flunitrazepam; pA2 = 7.60 with midazolam). Thus, the potency of flumazenil in antagonizing various benzodiazepines is remarkably consistent across a wide range of conditions (Lelas et al., 1999, 2000; McMahon et al., 2002).
Given the similarities in the potency of flumazenil to antagonize benzodiazepines across many conditions, it might be expected that its potency would be the same when two benzodiazepines are given together. Consistent with that hypothesis, flumazenil antagonizes the discriminative stimulus effects of a combination of midazolam and flunitrazepam, yielding pA2 values that are not different from those obtained when flumazenil is studied with a single benzodiazepine. These results further demonstrate the similarity in the potency of flumazenil across a range of drugs and conditions.
There are multiple modulatory sites on GABAA receptors, and positive modulators acting at several of these sites can produce midazolam-lever responding in monkeys. Moreover, concurrent administration of high-efficacy positive modulators acting at different sites results in additive effects between drugs (current study; McMahon and France, 2005). Thus, when high-efficacy drugs are administered together, their combined effects are qualitatively the same, regardless of which modulatory site mediates those effects. One way that actions at different sites can be detected is to study high-efficacy drugs with a low-efficacy drug that acts at one of the two sites (e.g., flumazenil). Under these conditions, flumazenil is expected to attenuate the effects of drugs acting at the same (e.g., benzodiazepine) site and should not alter the effects of drugs acting at different sites. Flumazenil enhances the discriminative stimulus effects of pregnanolone, shifting its dose-effect curve leftward and confirming that flumazenil has some positive efficacy (Dantzer and Pério, 1982).
Given that interactions between flumazenil and positive modulators vary according to the type of positive modulator being studied, antagonism by flumazenil should be less for a combination of midazolam and pregnanolone, compared with antagonism of midazolam alone or of a combination of midazolam and flunitrazepam. For example, 0.1 mg/kg flumazenil shifts a benzodiazepine dose-effect curve 17- to 23-fold rightward and does not alter the pregnanolone dose-effect curve. This dose of flumazenil might be expected to attenuate the combination of midazolam and pregnanolone; however, it does not change their combined discriminative stimulus effects (Fig. 6). Although flumazenil is less potent in antagonizing a combination of midazolam and pregnanolone, compared with its potency in antagonizing benzodiazepines, it can attenuate the combined effects of midazolam and pregnanolone with 0.32 mg/kg producing a small, rightward shift. Moreover, flumazenil fully antagonizes the effects of midazolam in the mixture, as evidenced by similar ED50 values for pregnanolone when it is administered alone (3.45 ± 0.49 mg/kg), compared with the ED50 value for pregnanolone when it is given in a mixture with midazolam and that mixture is maximally shifted by flumazenil (3.08 ± 0.71 mg/kg). Thus, even under conditions in which flumazenil enhances the effects of one component of the mixture, a combination of a benzodiazepine and a neuroactive steroid can be attenuated by flumazenil.
One possible strategy for retaining the therapeutic effects of benzodiazepines while reducing their adverse effects is to administer benzodiazepines and neuroactive steroids together. This strategy would allow smaller doses of each drug to be used, which might decrease adverse effects, such as the development of tolerance. The consequences of repeated daily treatment are different for benzodiazepines compared with those for neuroactive steroids. During long-term benzodiazepine treatment, tolerance develops readily to many effects of benzodiazepines, including decreases in lever pressing (McMahon and France, 2002b) and anticonvulsant effects (Gonsalves and Gallager, 1987; Löscher et al., 1996), although cross-tolerance does not develop to decreases in lever pressing produced by neuroactive steroids (Gerak, 2009). During long-term treatment with neuroactive steroids, tolerance can develop to some [e.g., decreases in locomotor activity (Marshall et al.,1997) or impairment in Morris water maze (Türkmen et al., 2006)] but not all [e.g., anticonvulsant effects (Kokate et al., 1998; Reddy and Rogawski, 2000) or decreases in lever pressing (McMahon and France, 2002a)] effects of neuroactive steroids. Thus, giving smaller doses of benzodiazepines and neuroactive steroids together compared with doses of either drug that would be needed when administered alone might decrease the likelihood of tolerance and cross-tolerance developing. In addition, the proportion of each drug in the mixture might be titrated to optimize the therapeutic effects while minimizing the possibility that tolerance develops.
Unlike the development of tolerance and dependence, some undesired effects are common among positive modulators, regardless of the binding site that mediates their effects, and would be expected to occur when they are administered in combination. For example, positive modulators decrease ventilation and they can produce severe respiratory depression when administered together (e.g., benzodiazepines and ethanol). Although these effects will only occur when large doses are administered concurrently, it is important to know whether the effects could be reversed in case of overdose. In the current study, flumazenil antagonizes the effects of a benzodiazepine given alone and with another benzodiazepine. Although flumazenil enhances the effects of pregnanolone administered alone, it does not enhance the effects of a combination of midazolam and pregnanolone and modestly attenuates their combined effects at a dose of 0.32 mg/kg. It is not clear whether a combination of benzodiazepines and neuroactive steroids would produce life-threatening adverse effects; however, flumazenil might be useful in reducing toxicity caused by the combination. Moreover, because one component of the mixture, the neuroactive steroid, will be resistant to antagonism by flumazenil, the proportion of each component in the mixture could be adjusted to obtain the most ideal combination of a benzodiazepine and a neuroactive steroid; this proportion would decrease the likelihood that tolerance and dependence develop to the mixture while retaining the ability to attenuate the effects in case of overdose.
The generality of these findings to other dependent variables needs to be determined empirically; however, drug discrimination seems to be predictive of effects across a wide range of conditions. For example, many, if not all, effects of benzodiazepines are mediated by benzodiazepine sites on GABAA receptors, including their discriminative stimulus, therapeutic, and adverse effects. Although absolute potency of drugs acting at benzodiazepine sites can vary across procedures, relative potencies do not change (Lelas et al., 1999; Rowlett et al., 2006). Drug discrimination has been used extensively to examine interactions between GABAA modulators that vary in efficacy and site of action on GABAA receptors (Lelas et al., 2000; McMahon and France, 2005; Gerak and France, 2011; current study), providing an empirical and theoretical framework against which results could be compared and demonstrating that drug discrimination studies are highly reliable and predictable. Finally, under the conditions used in the current study, additive interactions have been observed between positive modulators acting at different sites, and flumazenil has been shown to enhance the effects of pregnanolone, providing a baseline against which the relative enhancement or attenuation of the combined effects of positive modulators can be compared. Consequently, there appears to be little evidence to suggest that results would be different with other dependent variables and a number of reasons to believe that the discriminative stimulus effects of positive GABAA modulators will be predictive of other effects.
In summary, the ability of flumazenil to antagonize benzodiazepines administered concurrently could not be distinguished from its ability to antagonize a single benzodiazepine. Moreover, despite qualitative differences in the interaction between flumazenil and either benzodiazepines or neuroactive steroids, flumazenil attenuated the discriminative stimulus effects of a combination of midazolam and pregnanolone, although the magnitude of antagonism was less for the combination, compared with antagonism of midazolam alone. Although different interactions with flumazenil might have been observed had different proportions of midazolam and pregnanolone been studied (Fischer and Rowlett, 2011), the data obtained to date support the view that combinations of benzodiazepines and neuroactive steroids could be useful in retaining the therapeutic effects of benzodiazepines and reducing their individual adverse effects while at least partially retaining the ability to reverse accidental overdose.
Acknowledgments
We thank B. Harrington, A. Hernandez, M. Hernandez, D. Logan, B. Taylor, and J. Wallis for their excellent technical assistance.
This work was supported by the National Institutes of Health National Institute on Drug Abuse [Grant DA009157 (to L.R.G.), Senior Scientist Award K05-DA17918 (to C.P.F.)].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
- CI
- confidence interval
- n.s.
- not significant.
Authorship Contributions
Participated in research design: Gerak and France.
Performed data analysis: Gerak.
Wrote or contributed to the writing of the manuscript: Gerak and France.
References
- Arunlakshana O, Schild HO. (1959) Some quantitative uses of drug antagonists. Br J Pharmacol 14:48–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Pério A. (1982) Behavioural evidence for partial agonist properties of RO 15-1788, a benzodiazepine receptor antagonist. Eur J Pharmacol 81:655–658 [DOI] [PubMed] [Google Scholar]
- Eppolito AK, Gerak LR. (2010) Tolerance to the rate-increasing and not rate-decreasing effects of pregnanolone in rats. Behav Pharmacol 21:736–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn DA, Gallaher EJ, Crabbe JC. (2000) Differential change in neuroactive steroid sensitivity during ethanol withdrawal. J Pharmacol Exp Ther 292:394–405 [PubMed] [Google Scholar]
- Fischer BD, Rowlett JK. (2011) Anticonflict and reinforcing effects of triazolam + pregnanolone combinations in rhesus monkeys. J Pharmacol Exp Ther 337:805–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasior M, Ungard JT, Beekman M, Carter RB, Witkin JM. (2000) Acute and chronic effects of the synthetic neuroactive steroid, ganaxolone, against the convulsive and lethal effects of pentylenetetrazol in seizure-kindled mice: comparison with diazepam and valproate. Neuropharmacology 39:1184–1196 [DOI] [PubMed] [Google Scholar]
- Gerak LR. (2009) Selective changes in sensitivity to benzodiazepines, and not other positive GABAA modulators, in rats receiving flunitrazepam chronically. Psychopharmacology 204:667–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerak LR, France CP. (2011) Chronic benzodiazepine treatment does not alter interactions between positive GABAA modulators and flumazenil or pentylenetetrazole in monkeys. Behav Pharmacol 22:49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsalves SF, Gallager DW. (1987) Time course for development of anticonvulsant tolerance and GABAergic subsensitivity after chronic diazepam. Brain Res 405:94–99 [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals, 7th ed, Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, Washington DC [Google Scholar]
- Kenakin T. (1993) Pharmacologic Analysis of Drug-Receptor Interaction, 2nd ed, Raven Press, New York [Google Scholar]
- Koek W, Assié MB, Zernig G, France CP. (2000) In vivo estimates of efficacy at 5-HT1A receptors: effects of EEDQ on the ability of agonists to produce lower-lip retraction in rats. Psychopharmacology 149:377–387 [DOI] [PubMed] [Google Scholar]
- Kokate TG, Svensson BE, Rogawski MA. (1994) Anticonvulsant activity of neurosteroids: correlation with γ-aminobutyric acid-evoked chloride current potentiation. J Pharmacol Exp Ther 270:1223–1229 [PubMed] [Google Scholar]
- Kokate TG, Yamaguchi S, Pannell LK, Rajamani U, Carroll DM, Grossman AB, Rogawski MA. (1998) Lack of anticonvulsant tolerance to the neuroactive steroid pregnanolone in mice. J Pharmacol Exp Ther 287:553–558 [PubMed] [Google Scholar]
- Lancel M. (1999) Role of GABAA receptors in the regulation of sleep: initial sleep responses to peripherally administered modulators and agonists. Sleep 22:33–42 [DOI] [PubMed] [Google Scholar]
- Lelas S, Gerak LR, France CP. (1999) Discriminative-stimulus effects of triazolam and midazolam in rhesus monkeys. Behav Pharmacol 10:39–50 [DOI] [PubMed] [Google Scholar]
- Lelas S, Gerak LR, France CP. (2000) Antagonism of the discriminative stimulus effects of positive γ-aminobutyric acidA modulators in rhesus monkeys discriminating midazolam. J Pharmacol Exp Ther 294:902–908 [PubMed] [Google Scholar]
- Löscher W, Rundfeldt C, Hönack D, Ebert U. (1996) Long-term studies on anticonvulsant tolerance and withdrawal characteristics of benzodiazepine receptor ligands in different seizure models in mice. I. Comparison of diazepam, clonazepam, clobazam and abecarnil. J Pharmacol Exp Ther 279:561–572 [PubMed] [Google Scholar]
- Marshall FH, Stratton SC, Mullings J, Ford E, Worton SP, Oakley NR, Hagan RM. (1997) Development of tolerance in mice to the sedative effects of the neuroactive steroid minaxolone following chronic exposure. Pharmacol Biochem Behav 58:1–8 [DOI] [PubMed] [Google Scholar]
- McMahon LR, France CP. (2002a) Acute and chronic effects of the neuroactive steroid pregnanolone on schedule-controlled responding in rhesus monkeys. Behav Pharmacol 13:545–555 [DOI] [PubMed] [Google Scholar]
- McMahon LR, France CP. (2002b) Daily treatment with diazepam differentially modifies sensitivity to the effects of γ-aminobutyric acidA modulators on schedule-controlled responding in rhesus monkeys. J Pharmacol Exp Ther 300:1017–1025 [DOI] [PubMed] [Google Scholar]
- McMahon LR, France CP. (2005) Combined discriminative stimulus effects of midazolam with other positive GABAA modulators and GABAA receptor agonists in rhesus monkeys. Psychopharmacology 178:400–409 [DOI] [PubMed] [Google Scholar]
- McMahon LR, Gerak LR, Carter L, Ma C, Cook JM, France CP. (2002) Discriminative stimulus effects of benzodiazepine (BZ)1 receptor-selective ligands in rhesus monkeys. J Pharmacol Exp Ther 300:505–512 [DOI] [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. (2000) Chronic treatment with the neuroactive steroid ganaxolone in the rat induces anticonvulsant tolerance to diazepam but not to itself. J Pharmacol Exp Ther 295:1241–1248 [PubMed] [Google Scholar]
- Reddy DS, Rogawski MA. (2001) Enhanced anticonvulsant activity of neuroactive steroids in a rat model of catamenial epilepsy. Epilepsia 42:337–344 [DOI] [PubMed] [Google Scholar]
- Rowlett JK, Lelas S, Tornatzky W, Licata SC. (2006) Anti-conflict effects of benzodiazepines in rhesus monkeys: relationship with therapeutic doses in humans and role of GABAA receptors. Psychopharmacology 184:201–211 [DOI] [PubMed] [Google Scholar]
- Türkmen S, Löfgren M, Birzniece V, Bäckström T, Johansson IM. (2006) Tolerance development to Morris water maze test impairments induced by acute allopregnanolone. Neuroscience 139:651–659 [DOI] [PubMed] [Google Scholar]
- Vanover KE, Suruki M, Robledo S, Huber M, Wieland S, Lan NC, Gee KW, Wood PL, Carter RB. (1999) Positive allosteric modulators of the GABAA receptor: differential interaction of benzodiazepines and neuroactive steroids with ethanol. Psychopharmacology 141:77–82 [DOI] [PubMed] [Google Scholar]
- Wieland S, Belluzzi J, Hawkinson JE, Hogenkamp D, Upasani R, Stein L, Wood PL, Gee KW, Lan NC. (1997) Anxiolytic and anticonvulsant activity of a synthetic neuroactive steroid Co 3–0593. Psychopharmacology 134:46–54 [DOI] [PubMed] [Google Scholar]