Abstract
Amyotrophic lateral sclerosis is a fatal neurodegenerative disease characterized by progressive motoneuron loss. Redistribution of transactive response deoxyribonucleic acid-binding protein 43 from the nucleus to the cytoplasm and the presence of cystatin C-positive Bunina bodies are considered pathological hallmarks of amyotrophic lateral sclerosis, but their significance has not been fully elucidated. Since all reported rodent transgenic models using wild-type transactive response deoxyribonucleic acid-binding protein 43 failed to recapitulate these features, we expected a species difference and aimed to make a non-human primate model of amyotrophic lateral sclerosis. We overexpressed wild-type human transactive response deoxyribonucleic acid-binding protein 43 in spinal cords of cynomolgus monkeys and rats by injecting adeno-associated virus vector into the cervical cord, and examined the phenotype using behavioural, electrophysiological, neuropathological and biochemical analyses. These monkeys developed progressive motor weakness and muscle atrophy with fasciculation in distal hand muscles first. They also showed regional cytoplasmic transactive response deoxyribonucleic acid-binding protein 43 mislocalization with loss of nuclear transactive response deoxyribonucleic acid-binding protein 43 staining in the lateral nuclear group of spinal cord innervating distal hand muscles and cystatin C-positive cytoplasmic aggregates, reminiscent of the spinal cord pathology of patients with amyotrophic lateral sclerosis. Transactive response deoxyribonucleic acid-binding protein 43 mislocalization was an early or presymptomatic event and was later associated with neuron loss. These findings suggest that the transactive response deoxyribonucleic acid-binding protein 43 mislocalization leads to α-motoneuron degeneration. Furthermore, truncation of transactive response deoxyribonucleic acid-binding protein 43 was not a prerequisite for motoneuronal degeneration, and phosphorylation of transactive response deoxyribonucleic acid-binding protein 43 occurred after degeneration had begun. In contrast, similarly prepared rat models expressed transactive response deoxyribonucleic acid-binding protein 43 only in the nucleus of motoneurons. There is thus a species difference in transactive response deoxyribonucleic acid-binding protein 43 pathology, and our monkey model recapitulates amyotrophic lateral sclerosis pathology to a greater extent than rodent models, providing a valuable tool for studying the pathogenesis of sporadic amyotrophic lateral sclerosis.
Keywords: TDP-43, Bunina bodies, cystatin C, cynomolgus monkeys, amyotrophic lateral sclerosis
Introduction
Amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig's disease, is an incurable progressive neurodegenerative disease characterized by muscle weakness and atrophy resulting from the combined loss of upper and lower motoneurons. Most cases of ALS are sporadic, and only 10% of ALS cases are of a familial form. Protein aggregates are one histopathological characteristic of ALS. A breakthrough in understanding ALS pathogenesis was the discovery of the 43-kDa transactive response DNA-binding protein (TDP-43), which was recently identified as the major component of the protein aggregates and of the insoluble fraction in the brains of patients with sporadic ALS and frontotemporal lobar degeneration (FTLD) (Arai et al., 2006; Neumann et al., 2006). TDP-43 is now expected to play an essential role in the pathogenesis of sporadic ALS, possibly equivalent to that of tau and beta amyloid in Alzheimer's disease or α-synuclein in Parkinson's disease.
Human TDP-43 is a highly conserved and ubiquitously expressed 414 amino acid nuclear protein that binds to both DNA and RNA (Ou et al., 1995; Buratti et al., 2001). In normal settings, TDP-43 is a primarily nuclear protein that functions in transcription regulation, alternative splicing and RNA stabilization (Buratti et al., 2008), as well as in microRNA metabolism (Buratti et al., 2010). Pathological TDP-43 can be abnormally truncated, phosphorylated and ubiquitinated, and most TDP-43 is mislocalized from the nucleus to the cytoplasm or neurites (Arai et al., 2006; Neumann et al., 2006). Of note, almost all neurons with cytoplasmic TDP-43 accumulations show a dramatic depletion of normal nuclear TDP-43. Thus, both gain and loss of functions are potential disease mechanisms, either due to the loss of normal nuclear TDP-43 expression, or cytoplasmic mislocalization (Arai et al., 2006; Neumann et al., 2006; Cairns et al., 2007). Therefore, cytoplasmic TDP-43 mislocalization with loss of its nuclear staining is a key feature found in the majority of patients’ brains and spinal cords (Arai et al., 2006; Neumann et al., 2006).
TDP-43 strictly regulates its messenger RNA levels by directly binding to an intron in the 3′-untranslated region of its own transcript and enhancing its splicing (Ayala et al., 2010; Polymenidou et al., 2011), however, the expression level of TDP-43 can be upregulated ∼1.5-fold (Mishra et al., 2007; Gitcho et al., 2009) in FTLD/ALS. Moreover, mutations in the TDP-43 gene are associated with familial ALS (Kabashi et al., 2008; Yokoseki et al., 2008), in which TDP-43 is also frequently mislocalized within motoneurons of the spinal cord. These reports support the hypothesis that mislocalization of this protein plays a central role in the disease pathogenesis. The rodent, Drosophila, Ceanorhabditis elegans and zebrafish models with overexpressed mutant as well as wild-type TDP-43 show severe motor symptoms and wild-type TDP-43 localizes exclusively or primarily to nuclei (Ash et al., 2010; Hanson et al., 2010; Kabashi et al., 2010; Li et al., 2010; Shan et al., 2010; Voigt et al., 2010; Wils et al., 2010; Xu et al., 2010; Swarup et al., 2011), although mutant TDP-43 is more likely to accumulate in the cytoplasm (Swarup et al., 2011). The results of the rodent models suggested that overexpressed nuclear wild-type TDP-43 is toxic, but provide little insight for the significance of mislocalized wild-type TDP-43. Even in mouse models that overexpress wild-type TDP-43 with mutated nuclear localization signals, total human and mouse nuclear TDP-43 was not reduced (Igaz et al., 2011) when compared with that in littermate wild-type controls. Together, these reported mouse models might have a different TDP-43 pathology from that found in patients with ALS. Expecting that a primate model of ALS might more closely reflect the TDP-43 pathology in human patients with ALS, we overexpressed human wild-type TDP-43 in the spinal motoneurons of a non-human primate, the cynomolgus monkey, using an adeno-associated virus (AAV) 1 vector.
Materials and methods
Human subjects
Neurologists clinically diagnosed ALS with the aid of electrophysiological examinations. The clinical diagnosis of definite ALS was based on El Escorial (Brooks et al., 2000) and electrodiagnostic (De Carvalho et al., 2008) criteria and confirmed by neuropathological examination in accordance with published guidelines (Piao et al., 2003).
The patient study protocol was approved by the institutional clinical study committee at Tokyo Medical and Dental University (No. 799). Consent forms for autopsy were obtained from legal representatives of all patients in accordance with the guidelines of the institutional review boards.
Animals
Ten male adult cynomolgus monkeys (Macaca fascicularis; 3–7 years old, 3.28–5.10 kg) were bred and treated at Tsukuba Primate Research Centre. The number of monkeys and concentrations of viral stocks were as follows: one monkey was injected with high-dose Flag-TDP-43 AAV1 [1 × 1013 viral genomes (vg)/ml]; six monkeys were injected with low-dose Flag-TDP-43 AAV1 (3 × 1012 vg/ml); three monkeys were injected with low-dose mock AAV1 (3 × 1012 vg/ml) as a negative control. Three monkeys injected with low-dose TDP-43 AAV1 were pathologically examined in the early stage, 3–5 days after the onset of motor symptoms and the other three monkeys were examined in the late stage, 4–7 weeks after injection.
Eleven adult male Fisher rats (10 weeks old, Sankyo-lab) were used. The number of rats and concentrations of viral stocks were as follows: eight rats were injected with low-dose Flag-TDP-43 AAV1 (3 × 1012 vg/ml) and three rats were injected with low-dose control AAV1 (3 × 1012 vg/ml) as a negative control. Three rats injected with TDP-43 AAV1 were pathologically examined in the early stage, 1–2 weeks after injection, and the others were examined in the late stage, 4–9 weeks after injection.
All animal experiments were conducted according to the U.S. National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the Guidelines for the Animal Care and Management of the Tsukuba Primate Research Center and Tokyo Medical and Dental University.
Constructs
Human wild-type TDP-43 was purchased from Invitrogen. The TDP-43 and Flag-TDP-43 fragments were generated by polymerase chain reaction using the following primer pairs: 5′-CCGCTCGAGGCCACCATGGATTAC AAGGATGACGACGATAAGTCTGAATATATTCGGGTAACCGG-3′ and 5′-CCGCTCGAGCTACATTCCCCAG CCAGAAGACTTA-3′ for TDP-43, and 5′-CCGCTCGAGGCCACCATGGATTACAAGGATGACGACGAT AAGTCTGAATATATTCGGGTAACCGG-3′ and 5′-CCGCTCGAGCTACATTCCCCAGCCAGAAGACTTA-3′ for Flag-TDP-43, which contained XhoI digestion sites at the 3′- and 5′-ends. The Flag-TDP-43 complementary DNA was subcloned into an expression cassette flanked with AAV2 inverted terminal repeats (Stratagene). The cytomegalovirus (CMV) promoter was used to drive expression.
Adeno-associated virus preparations
Human embryonic kidney (HEK) 293 cells at ∼70% confluence were transfected with the AAV1 packaging plasmid pRep2/Cap9 (gift from Dr James M. Wilson, University of Pennsylvania) and adenovirus helper plasmid (Stratagene) at a ratio of 1:1:1. At 6 h after transfection, the culture medium was replaced with fresh medium, and the cells were incubated for 48 h. The cells were then harvested from the culture dishes and pelleted by centrifugation, resuspended in phosphate-buffered saline and subjected to three rounds of freeze–thawing. Cell debris was then pelleted by centrifugation at 1200g for 15 min. AAV vectors were purified using ammonium sulphate precipitation and iodixanol (Axis-Shield) continuous gradient centrifugation.
Size-exclusion chromatography was performed using an AKTA Explorer 100 HPLC system (GE Healthcare) equipped with a 2-ml sample loop. A Superdex 200 10/300 GL column (GE Healthcare) was equilibrated with MHA buffer (3.3 mM MES, 3.3 mM HEPES, 3.3 mM NaOAc, 50 mM NaCl, pH 6.5). The vector-containing fractions were loaded onto the column at a flow rate of 0.5 ml/min, and the eluate was collected as 0.5 ml fractions over the duration of one column volume (23 ml). AAV peak fractions were identified by 280/260 nm absorbance and real-time quantitative polymerase chain reaction using vector-specific primers. The purified AAVs were then concentrated further by using Amico Ultra-4 tubes (Ultracel-30k, Millipore) to a final concentration of 1 × 1013 genome copies/ml, as determined by real-time quantitative polymerase chain reaction.
The genome copy number was calculated by TaqMan® PCR (Applied Biosystems). The vectors were treated with Benzonase® and digested with proteinase K (Wako Pure Chemical Industries) for 1 h and purified by phenol–chloroform extraction. The TaqMan® primers and probe were designed as follows: forward primer: 5′-CAGGCTGGTCCAACTCCTA-3′, reverse primer: 5′-GCAGTGGTTCACGCCTGTAA-3′, and probe: 5′-TACCCACCTTGGCCTC-3′. The designed TaqMan® PCR fragment was located in the human growth hormone polyadenylation site in the vector.
Successful viral assembly of control AAV and transgene expression were confirmed by immunoblot analysis using HEK 293 cells infected with AAV (Supplementary Fig. 1), as described below.
HEK-293 cells were cultured in Dulbecco's modified Eagle's medium containing 10% foetal bovine serum with 1% penicillin/streptomycin. The cells in 12-well plates were infected by Flag-TDP-43 AAV1 (5 × 1010 vg/ml). At 48 h after infection, cells were harvested by gentle scraping in lysis buffer [20 mM Tris–HCl, 150 mM NaCl, 1% NP-40, 0.1% deoxicolate, 1% sodium dodecyl sulphate, 1 mM EDTA, 1 mM EGTA, 10 mM β-glycerophosphate, 5 mM NaF and Complete protease inhibitor cocktail (Roche Diagnostics)]. Equal amounts of total cellular protein were mixed with 5× Laemmli sample buffer, denatured at 95°C for 5 min, and separated with 10% sodium dodecyl sulphate polyacrylamide gel electrophoresis. The proteins were transferred to PVDF membranes. After blocking with 3% gelatin (Wako Pure Chemical Industry) in Tris-buffered saline or 5% skimmed milk (Wako Pure Chemical Industry) in Tris-buffered saline-Triton X-100, the membranes were incubated overnight with the following primary antibodies: anti-M2 (1:2000); anti-pan-TDP-43 (1:2000); and anti-VP1, VP2 and VP3 of AAV (1:1000). After incubation with an appropriate horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology), labelling was detected with the ECL Plus™ Chemiluminescent Detection System (GE Healthcare) or SuperSignal (Thermo Scientific).
Stereotaxic injection of adeno-associated viral vectors
All surgical operations were performed under general anaesthesia. Ketamine hydrochloride (Ketalar, Sankyo) was intramuscularly administered at a dose of 5 mg/kg as a pre-anaesthetic agent, and general anaesthesia was maintained with isoflurane (Forane, Abbott) and oxygen after tracheal intubation. The monkeys were positioned in a stereotaxic frame. After a bilateral laminectomy and opening the dura in the midline at C5–6, AAV vectors were stereotaxically injected into the side ipsilateral to the dominant hand. The injection site was determined and depth of needle insertion was calculated from the preoperatively taken MRI of cervical spinal cord. AAV stock (5 µl) was injected through a 31-gauge needle connected to a 10-µl Hamilton microsyringe in 2 min. The needle remained in place for 10 min and was removed slowly. The dura and skin were sutured and monkeys returned to their individual cages. The monkeys received 0.5 mg/kg butorphanol tartrate (Stadol, Bristol-Myers Squibb) intramuscularly for 3 days to alleviate any postoperative pain.
The rats were anaesthetized with an intraperitoneally administered cocktail of 1.5 ml chloral hydrate (70 mg/ml, Wako Pure Chemical Industry) and 0.1 ml ketamine hydrochloride (70 mg/ml) at a dose of 6 ml/kg. A left side hemi-laminectomy was performed from C4 to C6. On the left side of the C6 segment, 1.5 µl of viral stock was manually injected through sharpened microcapillary glass (PN-30 puller, Narishige) connected via silicone to a 10-µl Hamilton microsyringe at a rate of 0.5 µl/min. The sharpened microcapillary glass remained in place for 3 min and was removed slowly. The skin was sutured, the rats placed on a heating pad until they began to recover from surgery, and then returned to their individual cages.
For detection of the AAV genome in the spinal cord, total DNA was extracted from spinal cord with homogenized buffer containing 0.5% sodium dodecyl sulphate, 10 mM Tris–HCl pH 8.0, and 10 mM EDTA pH 8.0, and polymerase chain reactions were carried out with the following primer pair: 5′-CGCTGTTTTGACCTCCATAGAA-3′ and 5′-AGGCGGTACTTACGTCACTCTTG-3′ for the cytomegalovirus β-globin intron.
Behavioural analysis
For monkeys, to evaluate weakness of the forelimb muscles on the AAV-injected side (the dominant-hand side), we performed the ‘apple test’. The front fence of the cage was altered to have two holes and trays on the right and left side. A piece of apple was placed in line from back (monkey side) to front (observer side) on the left or right tray at 3, 6, 9 and 12 cm from the front fence during a session. The monkeys were trained to reach a small piece of apple on the trays through the hole. Four sessions were performed alternately for each side. We analysed how frequently a monkey used his dominant hand to pick up apples before and every week after the operations. We also carefully observed behaviour of the monkeys in daily life every day and recorded video monitoring for 30 min per week.
For rats, to evaluate weakness of the forelimb muscles, we measured grip strength using a special device (Muromachi Kiki) as previously described (Anderson, 2005).
Electrophysiological assessment
The nerve conduction study and needle EMG studies were performed under anaesthesia with a combination of 7 mg/kg ketamine hydrochloride and 1.2 mg/kg xylazine administrated intramuscularly. Nerve conduction studies were performed in the bilateral median nerves using conventional procedures and an EMG Machine (MEB-2300, Nihon-koden). The recording surface electrode was placed over the belly of the thenar eminence with a reference electrode at the metacarpophalangeal joint of the thumb, and compound muscle action potentials were elicited after the stimulation of the median nerve at the wrist. The peak-to-peak amplitudes were measured for all compound muscle action potentials. Nerve conduction studies were examined before AAV injection and every 1 or 2 weeks after. The needle EMG study was performed in the first dorsal interosseous, flexor carpi ulnaris and biceps brachii muscles using a conventional concentric needle electrode used in human studies (TECA Elite, CareFusion), 4–6 weeks after AAV injection.
Neuropathological examinations
Animals were deeply anaesthetized first with intramuscularly administered 7 mg/kg ketamine hydrochloride and then with intravenously administered 25 mg/kg pentobarbital. After confirming the absence of a blink reflex, the spinal cord and skeletal muscles were removed. For neuropathological examination, human and animal spinal cord samples were immersion fixed in 10% neutral buffered formalin, processed conventionally, embedded in paraffin, cut into 4-µm-thick sections and stained with haematoxylin and eosin. For immunostaining, sections were deparaffinized, pretreated in 0.5% periodic acid, autoclaved for 5 min at 121°C and then incubated free-floating overnight at 4°C with the following primary antibodies: M2 (1:500), anti-pan-TDP-43 (1:1000), anti-pS409/410-TDP-43 (1:500), anti-ubiquitin (1:500), anti-p62 (1:500), anti-cystatin-C (1:1000), anti-GFAP (1:500), anti-GLUT-5 (1:500) and SMI31 (1:200). Following brief washes, the sections were sequentially incubated with polymer immunocomplex (Dako)
Immunoreactive elements were visualized by treating sections with 3,3′ diaminobenzidine tetroxide (DAB-4HCl, Dojin Kagaku) with or without nickel ammonium chloride. The sections were then counterstained with haematoxylin. For double-immunostaining, the deparaffinized sections were stained with Sudan Black B to avoid autofluorescence. The free-floating sections were incubated overnight at 4°C in solutions containing the primary antibodies. The sections were then incubated with AlexaFluor 488- or 555-conjugated secondary antibodies (1:500, Invitrogen), and DAPI nuclear stain (1:500, Santa Cruz Biotechnology) for 1 h. All sections were examined using a confocal microscope (NIKON or Carl Zeiss).
Animal anterior root samples were fixed with a mixture of 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) at 4°C overnight, and then further fixed in 1% osmium tetroxide in 0.1 M phosphate buffer (pH 7.4) for 1 h. The well-fixed tissues were dehydrated in graded ethanol and embedded in Epon 812 (Poly/Bed® 812, Polyscience). The fixed roots were transversely cut into 1-µm-thick sections and stained with toluidine blue.
For counting of anterior horn neurons in the spinal cord, monkey eighth cervical segments were serially cut at 4-µm thickness, and every fifth section was stained with haematoxylin and eosin. The previous and next serial sections (that is, every fourth and sixth sections) were immunostained with anti-pan-TDP-43 or anti-Flag antibodies. The number and minimum diameters of neurons with nuclei in the lateral or medial nuclear groups in 15 sections were evaluated using the Image J software program from the U.S. National Institutes of Health.
Antibody information
We used the following primary antibodies for immunostaining and immunoblot analyses: mouse monoclonal anti-Flag (M2, Sigma); rabbit polyclonal anti-pan-TDP-43 (10782-1-AP, ProteinTech Group); rabbit polyclonal anti-C-terminal TDP-43 (12892-1-AP, ProteinTech Group); rabbit polyclonal anti-phosphorylated S409/410 TDP-43 (Cosmo Bio); rabbit polyclonal anti-ubiquitin (Dako); rabbit polyclonal anti-cystatin-C (Dako); mouse monoclonal anti-phosphorylated neurofilament (SMI31, Sternberger Monoclonals); rabbit polyclonal anti-glial fibrillary acidic protein (GFAP) (Dako); rabbit polyclonal anti-glucose transporter 5 (GLUT-5, IBL); rabbit polyclonal and mouse monoclonal anti-peripeherin (AB1530 and AB1527, Chemicon); mouse monoclonal anti-neurofilament light (N5139, Sigma) antibody; anti-p62 (GP62-C, Progen); anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH, Biodesign), and anti-AAV capsid proteins VP1, VP2 and VP3 (Progen Biotechnik).
Sequential biochemical fractionation, dephosphorylation and immunoblot analysis
Frozen frontal cortex or spinal cord (50–250 mg) was homogenized in 10 volumes of buffer A (10 mM Tris–HCl pH 7.5, containing 1 mM EGTA, 10% sucrose and 0.8 M NaCl). After the addition of Triton X-100 at a final concentration of 1%, the homogenate was incubated for 30 min at 37°C and spun at 100 000g for 20 min at 20°C. The pellet was homogenized in 20 volumes of buffer A containing 1% sarkosyl, incubated for 30 min at 37°C and spun at 100 000 g for 20 min at 20°C. The sarkosyl-insoluble pellet was homogenized in four volumes of buffer A containing 1% CHAPS [3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulphonate] and spun at 100 000g for 20 min. The pellet was sonicated in 0.5 volume of 8 mol/l urea buffer, cleared by centrifugation at 100 000g for 20 min at 20°C, and used for immunoblotting. The samples before (−) and after (+) treatment with lambda protein phosphatase (1600 U/ml, New England Biolabs) were subjected to 10% sodium dodecyl sulphate polyacrylamide gel electrophoresis. Proteins in the gel were then transferred onto a polyvinylidene difluoride (PVDF) membrane (Millipore). After blocking with 3% gelatin (Wako Pure Chemical Industries) in Tris-buffered saline (50 mM Tris–HCl pH 7.5, 150 mM NaCl), the membranes were incubated overnight with the following primary antibodies: anti-M2 (1:2000); anti-C-TDP-43 (1:1200); anti-phosphorylated S409/410 TDP-43 (1:2000); anti-p62 (1:3000) and anti-GAPDH (1:2000). After incubation with an appropriate horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology), labelling was detected by a 3,3′diaminobendizene reaction intensified with nickel chloride (Metal-Enhanced DAB Substrate Kit, Thermo Scientific), the ECL Plus™ Chemiluminescent Detection System (GE Healthcare), or SuperSignal (Thermo Scientific).
Quantitative real-time polymerase chain reaction assay
Total RNA was extracted from whole-brain homogenates with Isogen (Nippon Gene). DNase-treated RNA (2.5 µg) was reverse-transcribed with SuperScript® III and random hexamers (Life Technologies). Complementary DNA was amplified by the quantitative TaqMan® system by using the Light Cycler 480 Real-Time PCR Instrument (Roche Diagnostics). The primers and probes specific for Flag-tagged human TDP-43, rat TDP-43 (NM_001011979.2) and monkey TDP-43 (XM_001102660.2) were designed. Relative Flag-human TDP-43 messenger RNA levels were calculated in comparison to endogenous rat or cynomolgus messenger RNA levels. A list of all primers and probes used in this study is provided in Supplementary Table 1.
Statistical analysis
The data obtained from independent experiments are presented as means ± SEM.
Statistical analysis of spinal neuron counts and axonal density among TDP-43-expressed monkey group in early, late stage and control monkey group by one-way ANOVA with Bonferroni's post hoc test. P < 0.05 was considered significant.
Results
Overexpressing wild-type TDP-43 in the monkey cervical cord leads to progressive motor weakness and muscle atrophy with fasciculation
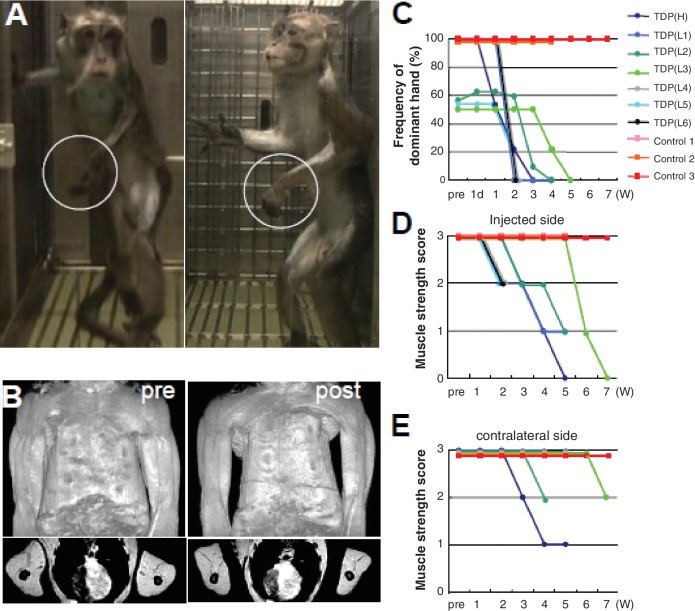
AAV expressing Flag-tagged TDP-43 (Supplementary Fig. 2) was directly injected into the sixth cervical segment on the dominant-hand side of seven monkeys. All monkeys developed progressive motor weakness and muscle atrophy with marked fasciculation in the forelimb on the injected side (Fig. 1A and B, Supplementary Video 1). Two to 3 weeks after the injection, the TDP-43-expressing monkeys first showed some difficulty in taking time to pick up pieces of apple using the dominant hand on the injected side in the apple test, and changed to use the non-dominant hand (Fig. 1C). On observation by video monitoring, they could reach and grasp the ceiling fence at the onset of clumsiness of picking apples, but 1–3 weeks later could not flex the elbow joint and raise the forelimb, indicating the motor weakness had spread to the proximal muscles (Fig. 1D). In the late stage, 2–5 weeks after the onset, the dominant hand muscles became completely paralysed (Fig. 1A), and muscle weakness and atrophy had spread to the contralateral, un-injected side limb muscles (Fig. 1B and E). One of TDP-43-expressing monkeys showed respiratory failure at the end stage. The control monkeys did not show obvious motor symptoms, indicating surgical procedure or virus toxicity was minimal (Fig. 1C–E and Supplementary Video 2).
Figure 1.
Overexpressing wild-type TDP-43 in the monkey cervical cord leads to progressive motor weakness and muscle atrophy. (A) Motor paresis of the forelimb on the injected side (encircled) in TDP-43 AAV-injected monkeys. (B) Constructed (top) and axial (bottom) MRI images of upper arm muscles before (pre) and 4 weeks after (post) injection, indicating a marked muscle atrophy of upper and forearms as well as hand muscles, predominantly on the injected side (left side). (C) Apple test. Frequency with which the dominant hand was used to pick up apples. (D and E) Behavioural analysis assessing muscle strength of forearms on the injected side (D) and contralateral side (E). A score of 3 indicates that the monkeys can hang on the ceiling fence; score of 2, they can grasp but not hang on the ceiling fence; score of 1, they can raise the forelimb but not reach ceiling fence; score of 0, they cannot raise the forelimb. Measurements that appear to end before the end of the experiment are from the three monkeys that were pathologically examined in the early stage.
Electrophysiological findings
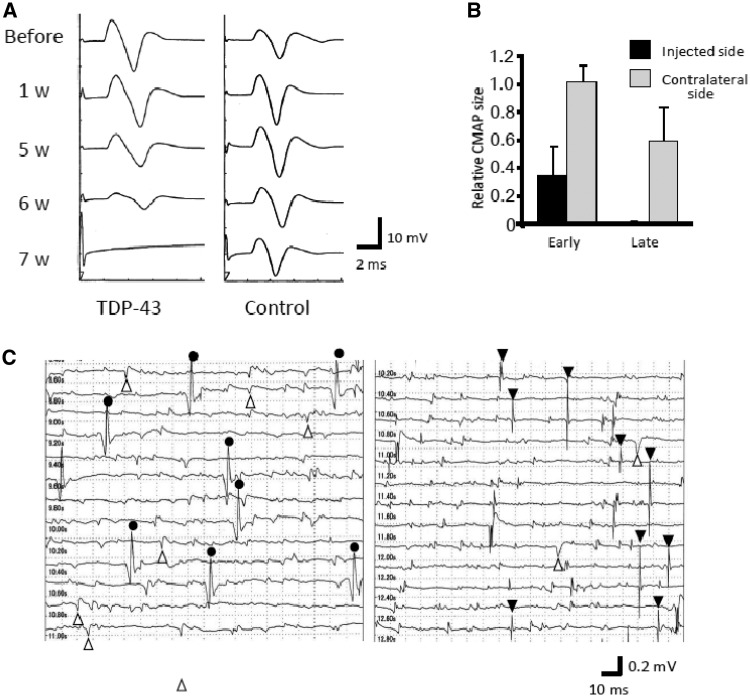
Electrophysiologically, in all TDP-43-expressing monkeys, the compound muscle action potential of the thenar muscle evoked by stimulation of the median nerve at the wrist progressively decreased in size with preservation of conduction velocity, and the muscle became unexcitable in the late stage (Fig. 2A). In contrast, three monkeys injected with control AAV did not show a marked change compound muscle action potential size (Fig. 2A). In TDP-43-expressing monkeys, the compound muscle action potential size on the contralateral side was not changed in the early stage (3–5 days after the onset of motor symptoms), but showed milder reduction of compound muscle action potential size in the late stage (Fig. 2B). Needle EMG revealed robust fasciculation potentials, and denervating potentials of positive sharp waves and fibrillation potentials in the late stage (Fig. 2C).
Figure 2.
Electrophysiological findings for motor symptoms of the monkeys. (A) Compound muscle action potentials (CMAPs) in the thenar muscle after stimulation of the median nerve at the wrist. They gradually decreased in size, and became inexcitable in the late stage. There was no change of compound muscle action potential size in the control monkey. (B) Ratio of the compound muscle action potential size 3–5 days after the onset (early stage) or 2–5 weeks after the onset (late stage) to the size before injection. In the early stage, there was no reduction in compound muscle action potential size on the contralateral side, but moderately attenuated in the late stage. (C) Needle EMG findings of the forearm muscle. Circles indicate fasciculation potentials; open triangles, positive sharp waves; filled triangles, fibrillation potentials.
Cytoplasmic mislocalization with loss of endogenous monkey TDP-43, dystrophic neurites and cystatin C-positive granules in the cytoplasm
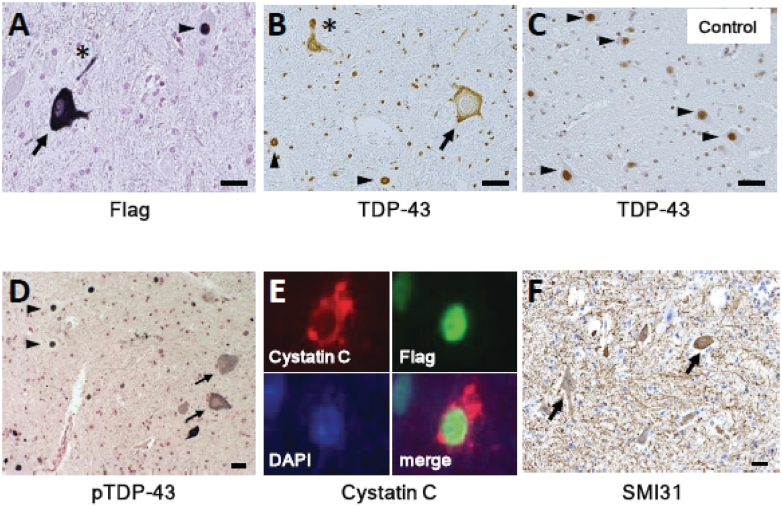
Neuropathologically, an anti-Flag antibody widely detected exogenous TDP-43 in neurons from the second cervical to the second thoracic segments on both sides, and was observed in almost all neurons on the injected side from the fourth to eighth cervical segments. Generally, Flag immunoreactivity was not detected in the glial cells. There was no inflammatory reaction except for in the area around the injection site. In the late stage, exogenous TDP-43 was observed in either the nucleus or cytoplasm (Fig. 3A). Pan-TDP-43 staining revealed that most motoneurons with cytoplasmic TDP-43 lost endogenous monkey TDP-43 staining (Fig. 3B), which normally localized in the nucleus (Fig. 3C). Mislocalized TDP-43 was diffusely distributed in the cytoplasm and, in some neurons, granularly aggregated (Fig. 3B). Proximal and distal dystrophic neurites were occasionally observed (Fig. 3A and B). Phosphorylation of TDP-43 in the nucleus or cytoplasm was not clear in the early stage, but became obvious in the late stage (Fig. 3D). Anti-ubiquitin and anti-p62 antibodies did not show a clear abnormal signal. Astrogliosis and microgliosis were observed (Supplementary Fig. 3). A small fraction of the motoneurons expressing TDP-43 in the nucleus characteristically displayed coarse cystatin C-positive granules in the cytoplasm (Fig. 3E) in the neurons with exogenously expressed TDP-43 in the nucleus. There were no neurons with co-localized cystatin C-positive granules and TDP-43 aggregates in the cytoplasm. We also observed aberrant accumulation of phosphorylated neurofilaments (Fig. 3F) and peripherin (Supplementary Fig. 4) in the cytoplasm of spinal motoneurons, a common pathological feature in patients with ALS (Munoz et al., 1988; Corbo and Hays, 1992). Immunostaining with an anti-Flag antibody identified its nuclear or cytoplasmic immunoreactivity in some Betz cells in the precentral gyrus restricted to the forelimb area contralateral to the injection. This Flag immunoreactivity was not observed in other cortical areas (including hippocampus and frontal lobe, which are preferentially affected in patients with FTLD), thalamus, basal ganglia and white matter, or in glial cells (Supplementary Fig. 5).
Figure 3.
Neuropathological findings of monkey spinal cords of TDP-43–overexpressed monkeys at the late stage (A, D–F), and control with mock AAV (C). (A–D) TDP-43-overexpressed spinal cord immunostaining using antibodies to Flag (A), pan-TDP-43 (B) and pS409/410 TDP-43 (D) demonstrated mislocalization in cytoplasm (arrows), and dystrophic neurites (asterisks) as well as normal localization in nuclei (arrowheads), whereas normal spinal cord showed only nuclear localization of TDP-43. (E) Co-labelling of a motoneuron expressing TDP-43 in the nucleus with antibodies to cystatin C (red) and Flag (green). The nucleus is labelled with DAPI. (F) Immunostaining using SMI31 revealed the aberrant presence of phosphorylated neurofilament in the neuronal cytoplasm (arrows). Scale bars: 20 µm. Immunostainings of spinal cord with control mock AAV using the antibodies to Flag, pS409/410 TDP-43, cystatin C and SMI31 are shown in Supplementary Fig. 7.
Characteristic regional mislocalization of TDP-43 in motoneurons of the anterior horn similar to amyotrophic lateral sclerosis spinal cords
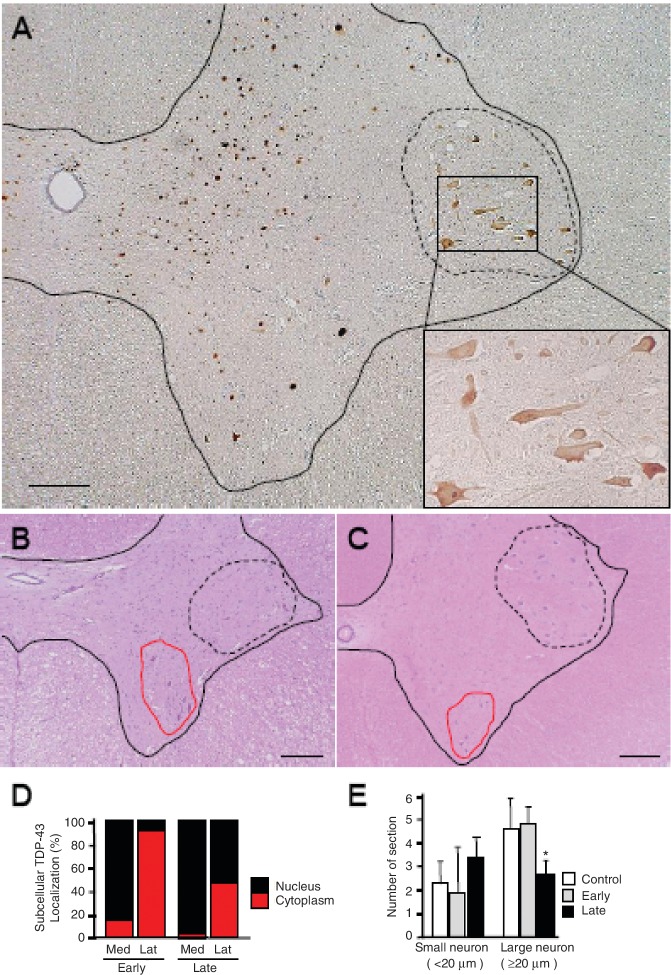
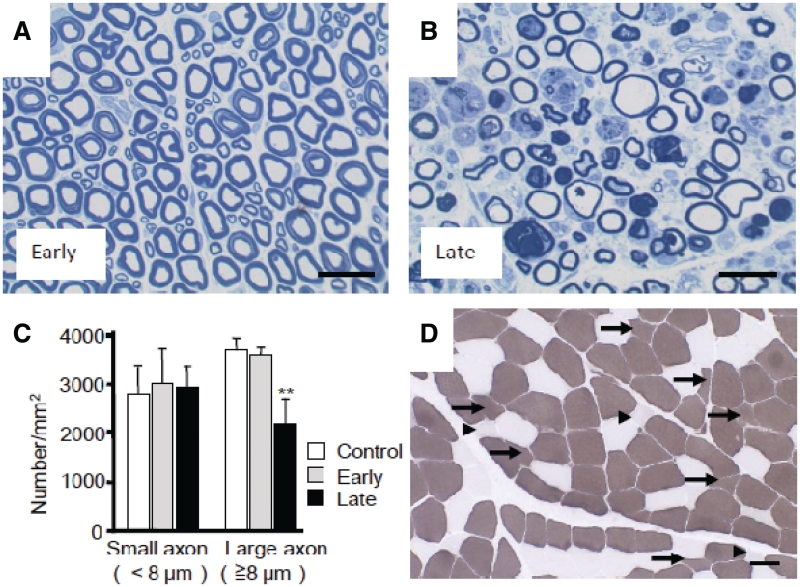
In the early stage, 3–5 days after the onset of hand clumsiness, TDP-43 mislocalization of diffuse staining pattern was observed in most motoneurons in the lateral nuclear group of the anterior horn. In contrast, almost all neurons in other areas of the spinal cord including the posterior horn showed flag signal of exogenously expressed TDP-43 only in the nucleus (Fig. 4A and D). Importantly, the contralateral lateral nuclear group also exhibited TDP-43 mislocalization on the side of forelimb that did not yet show obvious motor symptoms (Supplementary Fig. 6). Signals of exogenous Flag-TDP-43 were detected by real-time polymerase chain reaction on the contralateral half of the spinal cord (Supplementary Fig. 7). However, this distribution indicates that this regional selectivity is not due to differences in the concentration of the injected AAV, but rather is due to properties of the affected neurons. In the late stage, 2–5 weeks after onset, the percentage of motoneurons with TDP-43 mislocalization decreased ∼47% in the lateral nuclear group, and was <2% in the ventromedial nuclear group (Figs 3B and 4D). The number of large motoneurons (≥20 µm) in the early stage in this lateral nuclear group did not change, but in the late stage, was reduced by ∼42% (Fig. 4B, C and E). In contrast, the reduction in the number of large neurons in the ventromedial nuclear group was not significant (control, 1.78 ± 0.20 versus TDP-43, 1.68 ± 0.18/section, P = 0.80). Astrogliosis was also more prominent in the lateral area than in the ventromedial area of the anterior horn (data not shown). Motoneuronal degeneration of the lateral nuclear group was also confirmed by studying the anterior roots of the eighth cervical segment, which showed frequent myelin ovoids and loss of large myelinated axons (≥8 µm) in the late stage, although they were almost normal in the early stage (Fig. 5A–C). This axonal loss in the anterior roots is consistent with pathological change of the thenar muscle, showing numerous small angulated atrophic fibres (Fig. 5D).
Figure 4.
Regional mislocalization of TDP-43 and cell death in monkey spinal cords. (A) Sections from the injected side of the eighth cervical segment of the cord taken at the early stage and immunostained with an anti-Flag antibody. Most neurons in the lateral nuclear group (area encircled by broken line) showed cytoplasmic mislocalization of TDP-43 (inset), but almost all neurons in other areas expressed exogenous TDP-43 in the nucleus. Scale bars: 200 µm. (B and C) The eighth cervical level of cord from monkeys injected with TDP-43–expressing (B) and control (C) AAV, taken at the late stage and stained with haematoxylin and eosin. The number of large motoneurons decreased in the lateral nuclear group (areas encircled by broken line), but not in ventromedial nuclear group (areas encircled by red solid line). Scale bars: 200 µm. (D) Percentage of neurons with nuclear (black) or cytoplasmic (red) localization of exogenous TDP-43 in the lateral nuclear groups on the injected side. Neurodegeneration affects the lateral nuclear group more than the ventromedial nuclear group. (E) Cell count of neurons in the lateral nuclear group on haematoxylin and eosin staining. Mean ± SEM. n = 3, *P < 0.05. Lat = lateral nuclear group; Med = ventromedial nuclear group.
Figure 5.
Pathological finding of monkey anterior root and skeletal muscle. Toluidine blue staining of the eighth cervical anterior roots on the injected side in the early (A) and late (B) stages, and their myelinated axon densities (C). Mean ± SEM, n = 3, **P < 0.01. (D) Transverse section of the biceps brachii muscle from a TDP-43–expressing monkey 4 weeks after injection, stained with ATPase (pH 10.6). Small angulated atrophic changes of type I (arrowheads) and type II (arrows) fibres, with predominant involvement of type II fibres, can be seen. Scale bar: 50 µm.
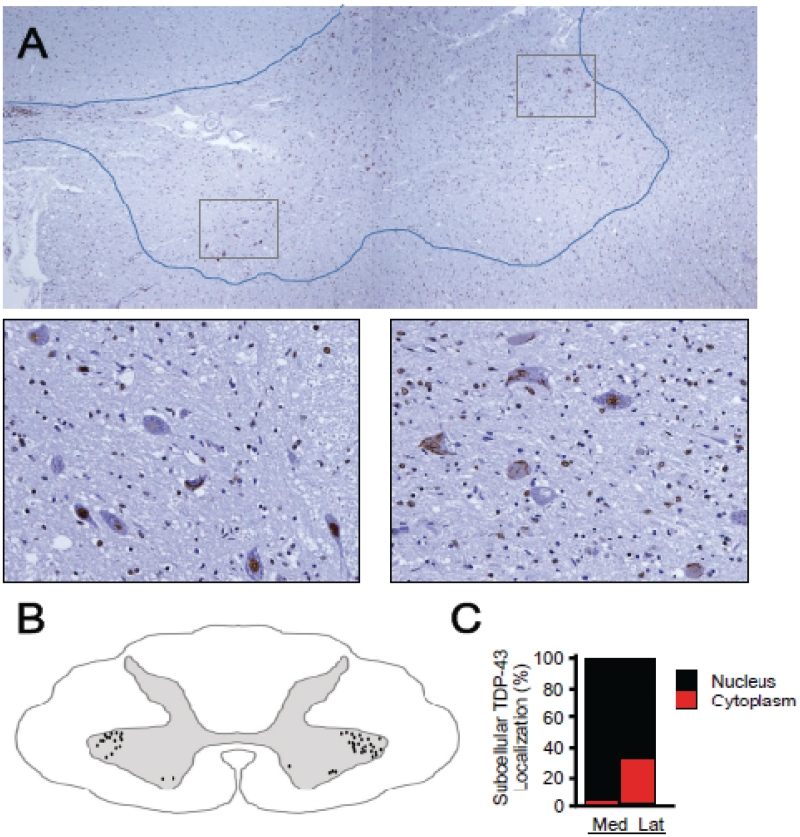
We furthermore examined whether such a regional change of TDP-43 mislocalization occurs in spinal cord of nine patients with ALS with upper limb weakness and hand muscle atrophy. TDP-43 mislocalization was observed much more in the lateral nuclear group than in the ventromedial nuclear group of the cord at the eighth cervical segment (Fig. 6A–C).
Figure 6.
Pan-TDP-43 staining of spinal cords of patients with ALS. (A) Autopsied eighth cervical segment of spinal cord immunostained with pan-TDP-43 antibody. Scale bars: 200 µm in A and 50 µm in window insets. (B) Schematic illustration of the distribution of neurons with TDP-43 mislocalization, made by summing data from five sections (at 20-µm intervals). (C) Percentage of neurons with nuclear or cytoplasmic localization of exogenous TDP-43 in the lateral and medial nuclear groups. More frequent TDP-43 mislocalization in the lateral nuclear group in spinal cords of patients with ALS than in the medial nuclear group. Mean ± SEM, n = 10, P < 0.01. Scale bars: 50 µm. Lat = lateral nuclear group; Med = ventromedial nuclear group.
Interspecies differences in TDP-43 pathology in rodents and primates
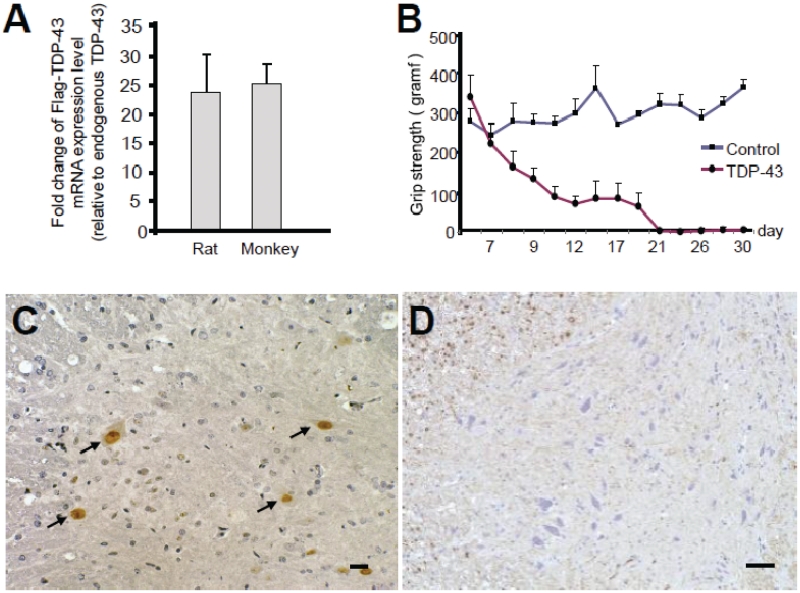
To investigate interspecies differences in TDP-43 pathology, we injected the identical TDP-43-expressing AAV at the same concentration into rat cervical cords. Expression level of Flag-TDP-43 messenger RNA around the injection site in rat spinal cord was >20-fold higher than that of endogenous TDP-43 level, and this fold change was similar to that in monkey spinal cord (Fig. 7A). Rats injected with TDP-43 AAV showed progressive motor weakness (Fig. 7B), measured by grip strength. Importantly, exogenous TDP-43 was observed only in the nuclei of motoneurons in both early (14 days after injection of AAV) and late (4 weeks after injection of AAV) stages (Fig. 7C). Since mislocalization of TDP-43 in the monkey spinal cords was more prominent in the early stage (14 days), we also examined the pathology of rat spinal cords at a very early stage (7 days); however, the weak Flag immunoreactivity was still limited to the nucleus of motoneurons (data not shown). Furthermore, this rat model failed to exhibit cystatin C-positive aggregates, dystrophic neurites, or aberrant accumulation of phosphorylated neurofilaments in the cytoplasm of spinal motoneurons (Fig. 7D). These neuropathological findings indicate that this rat model was less similar to human ALS than our monkey model in TDP-43 localization and other characteristic features of ALS.
Figure 7.
Effect of TDP-43-expressing AAV in rat spinal cords. (A) Ratio of exogenously expressed Flag-TDP-43 messenger RNA level to endogenous rat or cynomolgus TDP-43 messenger RNA level evaluated by quantitative real-time polymerase chain reaction. Mean ± SEM, rat, n = 4; cynomolgus, n = 3, P = 0.74. (B) Time course of grip strength. Mean ± SEM. (C) Nuclear staining of exogenous TDP-43 in cervical cord sections of AAV-injected rats by immunostaining with an anti-Flag antibody (arrows). (D) Immunostaining of cervical cord sections of TDP-43–expressing rat, 4 weeks after injection, with SMI31 did not show aberrant phosphorylated neurofilament in the neuronal cytoplasm. Scale bars: 20 µm.
Detection of the 25-kDa C-terminal fragment and phosphorylated TDP-43 in the early stage
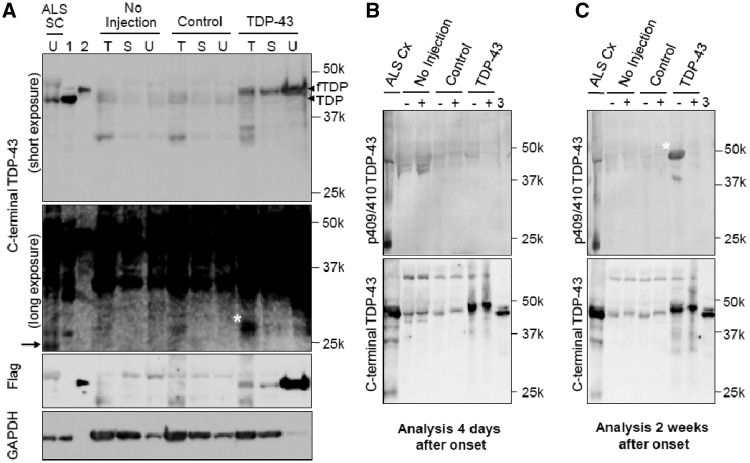
Biochemically, immunoblot analysis of monkey spinal cord demonstrated that the exogenous Flag-tagged TDP-43 became much more insoluble than endogenous TDP-43 (Fig. 8A). The phosphorylation of TDP-43 was unclear in the early stage (Fig. 8B) but clearly detected later (Fig. 8C). Neither a C-terminal nor a phosphospecific TDP-43 antibody detected the 25-kDa C-terminal fragment (Fig. 8A–C). These suggest that neither phosphorylation of TDP-43 or its 25-kDa C-terminal fragment in spinal cord is necessary to initiate motoneuronal dysfunction and degeneration in our monkeys.
Figure 8.
Biochemical analysis of monkey spinal cords. (A) Immunoblot of cervical spinal cord lysates from TDP-43–expressing monkeys (4 weeks after injection) and patients with ALS using antibodies recognizing the C-terminus of TDP-43 and Flag. SC = spinal cord of patient with ALS; 1 = TDP-43–expressing HEK 293 T cell lysate; 2 = Flag-TDP-43–expressing HEK 293T cell lysate; T = 1% Triton X-100-soluble; S = 1% sarkosyl-soluble; U = 8 mol/l urea-soluble fraction; fTDP = Flag-TDP-43. A longer exposure (second panel from top) revealed the 25-kDa C-terminal fragment in the spinal cord of a patient with ALS (arrow). The ∼30-kDa band noted in the Triton-soluble fraction from the spinal cord of a TDP-43–expressing monkey (asterisk) was different from the 25-kDa C-terminal fragment (arrow). (B and C) Immunoblot of 8 mol/l urea-soluble fraction from the monkey spinal cord harvested 4 days (B) and 2 weeks (C) after onset of symptoms, using antibodies to pS409/410 TDP-43 (top) and C-TDP-43 (bottom) before (−) and after (+) treatment with lambda protein phosphatase (λPPase). 3 = Mixture of Flag-TDP-43- and TDP-43-expressing HEK 293T cell lysates. The phosphorylated TDP-43 was detected only in the late stage (asterisk).
Discussion
Frequent mislocalization of TDP-43 in the cytoplasm and loss of its nuclear staining are major pathological hallmarks in the histological diagnosis of ALS and FTLD (Geser et al., 2010). The classification of TDP-43 proteinopathy is based on a combination of neuronal cytoplasmic inclusions and dystrophic neurites (Mackenzie et al., 2011). The morphological features in our monkeys are close to type B TDP-43 proteinopathy, which is usually observed in the brains of patients with ALS. The only difference between the pathology of our monkeys and type B TDP-43 proteinopathy is that mislocalized cytoplasmic TDP-43 was usually diffuse and neuronal cytoplasmic inclusions were less frequent in our monkeys. Since this monkey is an acute model for TDP-43 pathology, it possibly takes more time for diffusely mislocalized TDP-43 to be aggregated. Moreover, in the spinal cords of patients with ALS, diffuse cytoplasmic TDP-43 staining is more common, and neuronal cytoplasmic inclusions are less frequent than in the brain and may even be absent (Giordana et al., 2010). Thus, our monkey model shows the key features of TDP-43 proteinopathy as seen in the ALS spinal cord.
Interestingly, despite the diffuse expression of exogenous TDP-43 in the spinal cord, TDP-43 mislocalization and neuron loss predominantly occurred in the lateral nuclear group in Rexed lamina IX, in which large neurons are mostly α-motoneurons (Carpenter et al., 1983). The sensory neurons and interneurons in laminae III–VIII rarely showed TDP-43 mislocalization, and large motoneurons in the ventromedial nuclear group, most of which are also α-motoneurons, showed much less TDP-43 mislocalization and neuron loss. Within lamina IX, the lateral nuclear group innervates the distal, fast-contracting muscles of the extremities, and the ventromedial nuclear group innervates the posture-related, continuously contracting muscles attached to the axial skeleton (Carpenter et al., 1983). This regional vulnerability among α-motoneurons is consistent with the distal hand or foot muscles being the first involved in 73% of patients with non-bulbar ALS (Harverkamp et al., 1995; Körner et al., 2011) and might be related to axon length, which affects axonal transport (Bilsland et al., 2010), or to the preferential susceptibility of fast-fatigue rather than slow motoneurons (Dengler et al., 1990; Pun et al., 2006). Furthermore, in nine patients with ALS, more TDP-43 mislocalization was observed in the lateral nuclear group than in the ventromedial nuclear group of the eighth cervical cord segments. Taking these results together, we think that the tropism of TDP-43 mislocalization was similar to that of ALS pathology. However, expression levels of exogenous wild-type TDP-43 in our monkey and rat models were very high (∼20-fold higher than that of endogenous TDP-43), which was partly due to lack of 3′-untranslated region in our TDP-43 expression construct. This is probably because TDP-43 controls its own expression through a negative feedback loop by binding to 3′-untranslated region sequences in its own messenger RNA (Ayala et al., 2010; Polymenidou et al., 2010). The unphysiologically high level of TDP-43 expression in our animal models should be taken into consideration when interpreting our findings.
Since Flag TDP-43 messenger RNA was detected in the spinal cord contralateral to the injected side by real-time polymerase chain reaction analysis, the AAV virus was shown to spread contralaterally through the spinal cord causing motor paresis and reduction of compound muscle action potential size in the opposite forelimb. However, it is still possible that there was concomitant cell-to-cell or trans-synaptic propagation of Flag TDP-43 protein in the spinal cord. Moreover, it is interesting that the Flag-TDP-43 signal was selectively extended into Betz cells in the forelimb area of precentral gyrus contralateral to the injection side, which can be explained by a retrograde progression from α-motoneuron in the cervical cord. More sophisticated experimental paradigms are necessary to distinguish whether it is the AAV vector itself, transcribed messenger RNA or Flag-TDP protein that is the molecule responsible for this progression, which is a prime objective for our future study.
Bunina bodies are small, cystatin C-positive, eosinophilic cytoplasmic inclusions and are generally considered a specific hallmark of sporadic ALS (Okamoto et al., 1993; Mitsumoto et al., 1998). Importantly, Bunina bodies are absent in familial ALS that is due to the SOD1 mutation (Tan et al., 2007) or FUS/TLS mutation (Tateishi et al., 2010), but they have been detected in familial ALS with the TDP-43 mutation (Yokoseki et al., 2008) as well as in sporadic ALS. These imply an association between Bunina bodies and TDP-43 pathology in sporadic ALS. From this point of view, the generation of cystatin C-positive cytoplasmic aggregates in our monkeys might strengthen their pathological value as a model of sporadic ALS.
Biochemically, TDP-43 proteinopathy is characterized by decreased solubility, phosphorylation and the generation of 25-kDa C-terminal fragment (Arai et al., 2006; Neumann et al., 2006; Hasegawa et al., 2008). In TDP-43-overexpressing monkeys, the exogenous TDP-43 became much more insoluble than endogenous TDP-43 of control monkeys, indicating that expression of large amounts of exogenous wild-type TDP-43 can render it insoluble. Unexpectedly, the solubility of endogenous monkey TDP-43 did not become insoluble in TDP-43-overexpressing monkeys. The expectation would be that exogenous insoluble TDP-43 would recruit endogenous monkey TDP-43 and alter its solubility. In this biochemical aspect of TDP-43 solubility, our monkey model differs from patients with ALS.
The pathological role of phosphorylated TDP-43 is still unclear; TDP-43 phosphorylation in culture cells enhances its oligomerization (Hasegawa et al., 2008), but experiments with a phosphorylation-resistant mutant TDP-43 indicated that phosphorylation is not required for inclusion formation or cellular toxicity (Zhang et al., 2009). In our monkeys, phosphorylation of TDP-43 was a late event but not observed at 4 days after symptom onset. This finding suggests that TDP-43 phosphorylation is not necessary to initiate motor symptoms and is a late event in motoneuron degeneration.
In the spinal cords of our monkeys, neither a C-terminal nor a phosphospecific TDP-43 antibody detected the 25-kDa C-terminal fragment that is found in patients with ALS. Overexpressed 25-kDa C-terminal fragment in cultured cells is reported to be toxic (Igaz et al., 2009; Zhang et al., 2009), and the accumulation of 25-kDa C-terminal fragment in transgenic mouse brain correlates with disease progression (Xu et al., 2010). Interestingly, unlike the FTLD/ALS brain, the 25-kDa C-terminal fragment is often absent in the ALS spinal cord (Neumann et al., 2009). The absence of 25-kDa C-terminal fragment in ALS spinal cord does not necessarily preclude a primary role for this form; rather it can be pathologically crucial if its absence is due to the accelerated degeneration of motoneurons with 25-kDa C-terminal fragment. It is difficult to deny that small amounts of C-terminal truncated species are actually present, because mislocalization of TDP-43 was focal in the spinal cord of our monkeys. However, the failure to detect 25-kDa C-terminal fragment in our monkey spinal cord at the early stage may have an implication that full-length TDP-43 is sufficient to be toxic, because α-motor axonal excitability was impaired but their cell bodies were preserved at autopsy.
The results of studies on the relationship between TDP-43 mislocalization and neuron loss remain controversial. The overexpression of wild-type TDP-43 in the nuclei in a transgenic rodent model was sufficient to be toxic to spinal motoneurons (Li et al., 2010; Shan et al., 2010; Wils et al., 2010; Xu et al., 2010), which is consistent with our observations in the rat model. In this context, it can be interpreted that the cytoplasmic mislocalization of wild-type TDP-43 is an epiphenomenon and not a necessary condition for the disease. However, in our monkey model, TDP-43 mislocalization was detected in almost all of the large motoneurons of the lateral nuclear group at the early or even the presymptomatic stage, and these motoneurons later showed neuron loss. In contrast, overexpressed exogenous TDP-43 in the large motoneurons of the ventromedial nuclear group was restricted to the nucleus, but did not produce neuron loss. Mice with over-expression of human TDP-43 engineered to localize in the cytoplasm showed progressive neuronal loss and downregulation of endogenous nuclear mouse TDP-43 expression (Igaz et al., 2011). These suggest that TDP-43 mislocalization is an upstream event in the cascade of motoneuronal degeneration. This finding is consistent with the observation that the highest percentage of neurons with TDP-43 mislocalization was found in the early stage of ALS in patients (Giordana et al., 2010).
In conclusion, our monkey model is superior to rodent models in recapitulating the TDP-43 pathology and in the presence of Bunina body-like inclusion and is expected to be a powerful tool for investigating developing effective therapies as well as the disease pathogenesis of sporadic ALS.
Funding
Comprehensive Research on Disability Health and Welfare (Grant Nos 20301501 and 23161501 to T.Y. and H.M.); Research on Neurodegenerative Diseases/ALS from Ministry of Health, Labor and Welfare, Japan; Grant-in-Aid for Scientific Research (A) (Grant No. 22240039 to T.Y.) and Grant-in-Aid for Research Activity Start-up (Grant No. 22890051 to T.T.) and Strategic Research Program for Brain Science, Field E from Ministry of Education, Culture, Sports and Technology, Japan.
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
The authors are grateful to Drs Masato Hasegawa, Makoto Urushitani and Hiroshi Tsukagoshi for discussion and technical advices on Western blotting and pathological analysis; Dr Takashi Shimada, and Ms Fumiko Sunaga for AAV preparation; Dr Satoshi Ikeda, Dr Masumi Ichikawa, Dr Kinya Ishikawa, Miss Tomoko Ueno, Miss Minako Suzuki, Ms Michiko Imanishi, and Ms Hiromi Kondo for technical support on pathological analysis; Dr Kazuo Kusano for surgical support; Ms Yuki Yamamoto, Dr Miho Akaza for their help.
Glossary
Abbreviations
- AAV
adeno-associated virus
- ALS
amyotrophic lateral sclerosis
- FTLD
frontotemporal lobar degeneration
- HEK
human embryonic kidney
- TDP-43
transactive response DNA-binding protein 43
References
- Anderson KD, Gunawan A, Steward O. Spinal pathways involved in the control of forelimb motor function in rats. Exp Neurol. 2005;194:161–74. doi: 10.1016/j.expneurol.2007.05.024. [DOI] [PubMed] [Google Scholar]
- Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–11. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- Ash PE, Zhang YJ, Roberts CM, Saldi T, Hutter H, Buratti E, et al. Neurotoxic effects of TDP-43 overexpression in C. elegans. Hum Mol Genet. 2010;19:3206–18. doi: 10.1093/hmg/ddq230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayala YM, De Conti L, Avendaño-Vázquez SE, Dhir A, Romano M, D'Ambrogio A, et al. TDP-43 regulates its mRNA levels through a negative feedback loop. EMBO J. 2011;30:277–88. doi: 10.1038/emboj.2010.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilsland LG, Sahai E, Kelly G, Golding M, Greensmith L, Schiavo G. Deficits in axonal transport precede ALS symptoms in vivo. Proc Natl Acad Sci USA. 2010;107:20523–8. doi: 10.1073/pnas.1006869107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–9. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- Buratti E, Baralle FE. Characterization and functional implications of the RNA binding properties of nuclear factor TDP-43, a novel splicing regulator of CFTR exon 9. J Biol Chem. 2001;276:36337–43. doi: 10.1074/jbc.M104236200. [DOI] [PubMed] [Google Scholar]
- Buratti E, Baralle FE. Multiple roles of TDP-43 in gene expression, splicing regulation, and human disease. Front Biosci. 2008;13:867–8. doi: 10.2741/2727. [DOI] [PubMed] [Google Scholar]
- Buratti E, De Conti L, Stuani C, Romano M, Baralle M, Baralle F. Nuclear factor TDP-43 can affect selected microRNA levels. FEBS J. 2010;277:2268–81. doi: 10.1111/j.1742-4658.2010.07643.x. [DOI] [PubMed] [Google Scholar]
- Cairns NJ, Neumann M, Bigio EH, Holm IE, Troost D, Hatanpaa KJ, et al. TDP-43 in familial and sporadic frontotemporal lobar degeneration with ubiquitin inclusions. Am J Pathol. 2007;171:227–40. doi: 10.2353/ajpath.2007.070182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter MB, Sutin J. Human neuroanatomy. 8th edn. Baltimore: Williams & Wilkins; 1983. [Google Scholar]
- Corbo M, Hays AP. Peripherin and neurofilament protein coexist in spinal spheroids of motor neuron disease. J Neuropathol Exp Neurol. 1992;51:531–7. doi: 10.1097/00005072-199209000-00008. [DOI] [PubMed] [Google Scholar]
- De Carvalho M, Dengler R, Eisen A, England JD, Kaji R, Kimura J, et al. Electrodiagnostic criteria for diagnosis of ALS. Clin Neurophysiol. 2008;119:497–503. doi: 10.1016/j.clinph.2007.09.143. [DOI] [PubMed] [Google Scholar]
- Dengler R, Konstanzer A, Küther G, Hesse S. Amyotrophic lateral sclerosis: Macro–EMG and twitch forces of single motor units. Muscle Nerve. 1990;13:545–50. doi: 10.1002/mus.880130612. [DOI] [PubMed] [Google Scholar]
- Geser F, Lee VM, Trojanowski JQ. Amyotrophic lateral sclerosis and frontotemporal lobar degeneration: A spectrum of TDP-43 proteinopathies. Neuropathology. 2010;30:103–12. doi: 10.1111/j.1440-1789.2009.01091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordana MT, Piccinini M, Grifoni S, De Marco G, Vercellino M, Magistrello M, et al. TDP-43 redistribution is an early event in sporadic amyotrophic lateral sclerosis. Brain Pathol. 2010;20:351–60. doi: 10.1111/j.1750-3639.2009.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitcho MA, Bigio EH, Mishra M, Johnson N, Weintraub S, Mesulam M, et al. TARDBP 3′-UTR variant in autopsy-confirmed frontotemporal lobar degeneration with TDP-43 proteinopathy. Acta Neuropathol. 2009;11:633–45. doi: 10.1007/s00401-009-0571-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson KA, Kim SH, Wassarman DA, Tibbetts RS. Ubiquilin modifies TDP-43 toxicity in a Drosophila model of amyotrophic lateral sclerosis (ALS) J Biol Chem. 2010;285:11068–72. doi: 10.1074/jbc.C109.078527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harverkamp LJ, Appel V, Appel SH. Natural history of amyotrophic lateral sclerosis in a data base population. Validation of a scoring system and a model for survival prediction. Brain. 1995;118:707–19. doi: 10.1093/brain/118.3.707. [DOI] [PubMed] [Google Scholar]
- Hasegawa M, Arai T, Nonaka T, Kametani F, Yoshida M, Hashizume Y, et al. Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Ann Neurol. 2008;64:60–70. doi: 10.1002/ana.21425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igaz LM, Kwong LK, Chen-Plotkin A, Winton MJ, Unger TL, Xu Y, et al. Expression of TDP-43 C-terminal fragments in vitro recapitulates pathological features of TDP-43 proteinopathies. J Biol Chem. 2009;284:8516–24. doi: 10.1074/jbc.M809462200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igaz LM, Kwong LK, Lee EB, Chen-Plotkin A, Swanson E, Unger T, et al. Dysregulation of the ALS-associated gene TDP-43 leads to neuronal death and degeneration in mice. J Clin Invest. 2011;121:726–38. doi: 10.1172/JCI44867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabashi E, Lin L, Tradewell ML, Dion PA, Bercier V, Bourgouin P, et al. Gain and loss of function of ALS-related mutations of TARDBP (TDP-43) cause motor deficits in vivo. Hum Mol Genet. 2010;19:671–83. doi: 10.1093/hmg/ddp534. [DOI] [PubMed] [Google Scholar]
- Kabashi E, Valdmanis PN, Dion P, Spiegelman D, McConkey BJ, Vande Velde C, et al. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat Genet. 2008;40:572–4. doi: 10.1038/ng.132. [DOI] [PubMed] [Google Scholar]
- Körner S, Kollewe K, Fahlbusch M, Zapf A, Dengler R, Krampfl K, et al. Onset and spreading patterns of upper and lower motor neuron symptoms in amyotrophic lateral sclerosis. Muscle Nerve. 2011;43:636–42. doi: 10.1002/mus.21936. [DOI] [PubMed] [Google Scholar]
- Li Y, Ray P, Rao EJ, Shi C, Guo W, Chen X, et al. A Drosophila model for TDP-43 proteinopathy. Proc Natl Acad Sci USA. 2010;107:3169–74. doi: 10.1073/pnas.0913602107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IRA, Neumann M, Baborie A, Sampathu DM, Plessis DD, Jaros E, et al. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol. 2011;122:111–3. doi: 10.1007/s00401-011-0845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra M, Paunesku T, Woloschak GE, Siddique T, Zhu LJ, Lin S, et al. Gene expression analysis of frontotemporal lobar degeneration of the motor neuron disease type with ubiquitinated inclusions. Acta Neuropathol. 2007;114:81–94. doi: 10.1007/s00401-007-0240-7. [DOI] [PubMed] [Google Scholar]
- Mitsumoto H, Chad DA, Pioro EP. Amyotrophic lateral sclerosis. Philadelphia: F.A. Davis; 1998. [Google Scholar]
- Munoz DG, Greene C, Perl DP, Selkoe DJ. Accumulation of phosphorylated neurofilaments in anterior horn motoneurons of amyotrophic lateral sclerosis patients. J Neuropathol Exp Neurol. 1988;47:9–18. doi: 10.1097/00005072-198801000-00002. [DOI] [PubMed] [Google Scholar]
- Neumann M, Kwong LK, Lee EB, Kremmer E, Flatley A, Xu Y, et al. Phosphorylation of S409/410 of TDP-43 is a consistent feature in all sporadic and familial forms of TDP-43 proteinopathies. Acta Neuropathol. 2009;117:137–49. doi: 10.1007/s00401-008-0477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–3. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Hirai S, Amari M, Watanabe M, Sakurai A. Bunina bodies in amyotrophic lateral sclerosis immunostained with rabbit anti-cystatin C serum. Neurosci Lett. 1993;162:125–8. doi: 10.1016/0304-3940(93)90576-7. [DOI] [PubMed] [Google Scholar]
- Ou SH, Wu F, Harrich D, Garcia-Martinez LF, Gaynor RB. Cloning and characterization of a novel cellular protein, TDP-43, that binds to human immunodeficiency virus type 1 TAR DNA sequence motifs. J Virol. 1995;69:3584–96. doi: 10.1128/jvi.69.6.3584-3596.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao YS, Wakabayashi K, Kakita A, Yamada M, Hayashi S, Morita T, et al. Neuropathology with clinical correlations of sporadic amyotrophic lateral sclerosis:102 autopsy cases examined between 1962 and 2000. Brain Pathol. 2003;13:10–22. doi: 10.1111/j.1750-3639.2003.tb00002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymenidou M, Lagier-Tourenne C, Hutt KR, Huelga SC, Moran J, Liang TY, et al. Long pre-mRNA deletion and RNA missplicing contribute to neuronal vulnerability from loss of TDP-43. Nat Neurosci. 2011;14:459–68. doi: 10.1038/nn.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pun S, Santos AF, Saxena S, Xu L, Caroni P. Selective vulnerability and pruning of phasic motoneuron axons in motoneuron disease alleviated by CNTF. Nat Neurosci. 2006;9:408–19. doi: 10.1038/nn1653. [DOI] [PubMed] [Google Scholar]
- Shan X, Chiang PM, Price DL, Wong PC. Altered distributions of gemini of coiled bodies and mitochondria in motor neurons of TDP-43 transgenic mice. Proc Natl Acad Sci USA. 2010;107:16325–30. doi: 10.1073/pnas.1003459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup V, Phaneuf D, Bareil C, Robertson J, Rouleau GA, Kriz J, et al. Pathological hallmarks of amyotrophic lateral sclerosis/frontotemporal lobar degeneration in transgenic mice produced with TDP-43 genomic fragments. Brain. 2011;134:2610–26. doi: 10.1093/brain/awr159. [DOI] [PubMed] [Google Scholar]
- Tan CF, Eguchi H, Tagawa A, Onodera O, Iwasaki T, Tsujino A, et al. TDP-43 immunoreactivity in neuronal inclusions in familial amyotrophic lateral sclerosis with or without SOD1 gene mutation. Acta Neuropathol. 2007;113:535–42. doi: 10.1007/s00401-007-0206-9. [DOI] [PubMed] [Google Scholar]
- Tateishi T, Hokonohara T, Yamasaki R, Miura S, Kikuchi H, Iwaki A, et al. Multiple system degeneration with basophilic inclusions in Japanese ALS patients with FUS mutation. Acta Neuropathol. 2010;119:355–64. doi: 10.1007/s00401-009-0621-1. [DOI] [PubMed] [Google Scholar]
- Voigt A, Herholz D, Fiesel FC, Kaur K, Müller D, Karsten P, et al. TDP-43-mediated neuron loss in vivo requires RNA-binding activity. PLoS ONE. 2010;5:e12247. doi: 10.1371/journal.pone.0012247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wils H, Kleinberger G, Janssens J, Pereson S, Joris G, Cuijt I, et al. TDP-43 transgenic mice develop spastic paralysis and neuronal inclusions characteristic of ALS and frontotemporal lobar degeneration. Proc Natl Acad Sci USA. 2010;107:3858–63. doi: 10.1073/pnas.0912417107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YF, Gendron TF, Zhang YJ, Lin WL, D'Alton S, Sheng H, et al. Wild-type human TDP-43 expression causes TDP-43 phosphorylation, mitochondrial aggregation, motor deficits, and early mortality in transgenic mice. J Neurosci. 2010;30:10851–9. doi: 10.1523/JNEUROSCI.1630-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoseki A, Shiga A, Tan CF, Tagawa A, Kaneko H, Koyama A, et al. TDP-43 mutation in familial amyotrophic lateral sclerosis. Ann Neurol. 2008;63:538–42. doi: 10.1002/ana.21392. [DOI] [PubMed] [Google Scholar]
- Zhang YJ, Xu YF, Cook C, Gendron TF, Roettges P, Link CD, et al. Aberrant cleavage of TDP-43 enhances aggregation and cellular toxicity. Proc Natl Acad Sci USA. 2009;106:7607–12. doi: 10.1073/pnas.0900688106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.