Abstract
The identification of a hexanucleotide repeat expansion in the C9ORF72 gene as the cause of chromosome 9-linked frontotemporal dementia and motor neuron disease offers the opportunity for greater understanding of the relationship between these disorders and other clinical forms of frontotemporal lobar degeneration. In this study, we screened a cohort of 398 patients with frontotemporal dementia, progressive non-fluent aphasia, semantic dementia or mixture of these syndromes for mutations in the C9ORF72 gene. Motor neuron disease was present in 55 patients (14%). We identified 32 patients with C9ORF72 mutations, representing 8% of the cohort. The patients’ clinical phenotype at presentation varied: nine patients had frontotemporal dementia with motor neuron disease, 19 had frontotemporal dementia alone, one had mixed semantic dementia with frontal features and three had progressive non-fluent aphasia. There was, as expected, a significant association between C9ORF72 mutations and presence of motor neuron disease. Nevertheless, 46 patients, including 22 familial, had motor neuron disease but no mutation in C9ORF72. Thirty-eight per cent of the patients with C9ORF72 mutations presented with psychosis, with a further 28% exhibiting paranoid, deluded or irrational thinking, whereas <4% of non-mutation bearers presented similarly. The presence of psychosis dramatically increased the odds that patients carried the mutation. Mutation bearers showed a low incidence of motor stereotypies, and relatively high incidence of complex repetitive behaviours, largely linked to patients’ delusions. They also showed a lower incidence of acquired sweet food preference than patients without C9ORF72 mutations. Post-mortem pathology in five patients revealed transactive response DNA-binding protein 43 pathology, type A in one patient and type B in three. However, one patient had corticobasal degeneration pathology. The findings indicate that C9ORF72 mutations cause some but not all cases of frontotemporal dementia with motor neuron disease. Other mutations remain to be discovered. C9ORF72 mutations are associated with variable clinical presentations and pathology. Nevertheless, the findings highlight a powerful association between C9ORF72 mutations and psychosis and suggest that the behavioural characteristics of patients with C9ORF72 mutations are qualitatively distinct. Mutations in the C9ORF72 gene may be a major cause not only of frontotemporal dementia with motor neuron disease but also of late onset psychosis.
Keywords: frontotemporal lobar degeneration, clinical characteristics, motor neuron disease, psychosis, neuropathology
Introduction
The association between frontotemporal dementia (FTD) and motor neuron disease (MND) is well established. Patients presenting with FTD may develop MND (Neary et al., 1990; Lomen-Hoerth et al., 2002; Seelaar et al., 2007) and patients with MND may develop FTD (Phukan et al., 2007; Strong, 2008; Strong et al., 2009). Pathologically, FTD and MND are linked by the presence of ubiquitin immunoreactive neuronal changes, and transactive response DNA-binding protein 43 (TDP-43) as the major pathological protein (Neumann et al., 2006). Genetically, chromosome 9 linkage and genome-wide association studies have revealed families in whom some members have FTD and others MND (Morita et al., 2006; Le Ber et al., 2009; Vance et al., 2009; Van Es et al., 2009; Laaksovirta et al., 2010; Shatunov et al., 2010; Pearson et al., 2011). The identification of the hexanucleotide GGGGCC repeat expansion in C9ORF72 in such families (DeJesus-Hernandez et al., 2011; Renton et al., 2011) consolidates the relationship.
The genetic discovery raises a number of questions. Does the C9ORF72 mutation account for all patients with familial FTD and MND (FTD–MND)? In patients with the C9ORF72 mutation who present with FTD is MND an inevitable finding as the disease progresses? MND has previously been associated not only with the behavioural variant of FTD, but also with other prototypical syndromes of frontotemporal lobar degeneration (FTLD), progressive non-fluent aphasia (Caselli et al., 1993; Doran et al., 1995; Bak et al., 2001; Catani et al., 2004) and semantic dementia (de Souza et al., 2009; Kim et al., 2009; Ostberg and Bogdanovic, 2011). Can C9ORF72 mutations be found in these syndromes too? Finally and crucially, are there clinical characteristics, aside from MND, that discriminate patients with FTD with C9ORF72 mutations from those without?
These questions are germane to the broader issue of the status of FTD–MND within FTLD. The pathology underpinning FTLD is heterogeneous (Cairns et al., 2007; Mackenzie et al., 2009, 2010). TDP-43 pathology accounts for only around half of cases, most others showing a tau-positive pathology, and a small minority fused-in-sarcoma (FUS) pathology. Moreover, TDP-43 pathology is itself not uniform. Distinct subtypes have been identified, reflecting the relative preponderance of TDP-43 immunoreactive pathological changes within neurons and/or neurites (Mackenzie et al., 2006, 2009, 2010; Sampathu et al., 2006). FTD–MND is typically associated with subtype B (Josephs et al., 2011; Rohrer et al., 2011; Snowden et al., 2011a), as defined by the most recent classification (Mackenzie et al., 2011), characterized by a predominance of cytoplasmic inclusions. The specific and distinctive pathology associated with FTD–MND lends credence to the view that FTD–MND represents a disorder that is aetiologically distinct from other forms of FTLD. In this regard, it would be important to determine whether, in a cohort of patients with clinical syndromes of FTLD, there are clinical characteristics that set apart those patients who have C9ORF72 mutations from those who do not.
The present study documents clinical and demographical characteristics of patients presenting to a specialist dementia clinic, in whom mutations in C9ORF72 have been identified, and compares them with characteristics of a larger cohort of patients with syndromes of FTLD who do not have the mutation. We predicted that a principle discriminating feature would be the presence of MND in the patient or family member. However, we sought also to determine whether patients’ behavioural/cognitive disorder conformed to a distinct phenotype. A proportion of patients have come to autopsy and we ascertained the pathological characteristics of those patients.
Patients and methods
Patients
The study cohort comprised 398 consecutive patients, investigated in the Cerebral Function Unit, Greater Manchester Neuroscience Centre, who had a diagnostic classification of FTD, progressive non-fluent aphasia or semantic dementia (Neary et al., 1998) or mixed syndrome, and for whom genomic DNA was available. Clinical diagnostic investigations of patients, at the time of referral, included a clinical history using a structured proforma, which documents systematically alterations in behaviour and cognition, a full neurological examination and neuropsychological evaluation using the Manchester Neuropsychological Profile, which incorporates both published and locally developed tests (Thompson et al., 2005; Snowden et al., 2007a, 2011a; Stopford et al., 2008). Naming was assessed by the Graded Naming Test (McKenna and Warrington, 1983), and the less demanding Manchester Picture Naming Test, which consists of 10 animals, 10 fruits/vegetables, 10 articles of clothing and 10 objects. The presence of semantic impairment was determined on the basis of semantic errors in naming (e.g. ‘dog’ for rabbit), and impaired performance on a four-choice word–picture matching test, which uses the same 40 stimulus items as the Manchester Picture Naming Test, and within-category distracters. Perceptual and spatial skills were assessed using the Visual Object and Space Perception Battery (Warrington and James, 1991), and face recognition using a 10-item screening test of famous faces. Gestural praxis assessment incorporated both orofacial and limb actions, and included repetitive and alternating speech sounds for assessment of speech apraxia. Limb praxis was assessed independently in the right and left upper limbs using eight transitive and intransitive gestures. Memory was assessed by story (immediate and delayed), object (immediate and delayed) and face (delayed) recall and recognition tasks. Memory performance was interpreted as being in keeping with a ‘frontal’ type of memory loss on the basis of (i) disproportionate impairment on open-ended recall compared with recognition; and (ii) no abnormal forgetting over a delay, together with qualitative features of confabulation, perseveration, verbatim reporting of story elements without theme abstraction. Generation, set shifting and sequencing skills were assessed, by verbal fluency (animals and the letters F, A and S), Weigls blocks and picture sequencing tasks, respectively. Concreteness was determined by self-referential responses on sentence comprehension tasks and literal interpretations of proverbs. Motor perseveration was evaluated by Luria motor tasks.
Structural and/or functional neuroimaging were available for the majority of patients. Electrophysiological investigations were carried out in patients with suspected MND. Patients had been followed up for sufficient time to confirm the progressive nature of patients’ symptoms. Patients were excluded from the study if their symptoms and signs did not progress, if there were strong risk factors for vascular disease, or the presentation was of a form of progressive aphasia deemed likely to be associated with Alzheimer's disease rather than FTLD pathology.
Of the 398 patients, 211 (53%) had a cognitive/behavioural classification of FTD, 66 (17%) progressive non-fluent aphasia, 53 (13%) semantic dementia and the remaining 68 (17%) mixed syndromes. MND was documented in 55 of these patients, representing 14% of the total cohort.
At present, 78 of the patients have come to autopsy; 30 had FTLD-tau pathology [nine Pick-type, 10 with microtubule associated protein tau (MAPT) mutations and 11 with corticobasal degeneneration], 45 had FTLD-TDP pathology (20 type A, 18 type B and seven type C) histology, two had FTLD-FUS [with atypical FTLD-U (ubiquitin-positive inclusions)], and one FTLD-ni (no inclusions; see Mackenzie et al., 2009, 2010, 2011 for definitions). Clinical and pathological descriptions for most of these patients have been reported previously (Snowden et al., 2007b; 2011a; Pickering-Brown et al., 2008; Baborie et al., 2011). Brains of 21 patients with MND, 20 with Alzheimer's disease and 16 elderly healthy control subjects, donated to the Manchester Brain Bank, served as histopathological reference groups. All brains had been obtained with full ethical permission using informed consent and consultee declaration procedures.
Genetic, clinical and pathological procedures
Blood samples from patients were obtained with informed written consent as part of a molecular genetic study of neurodegenerative disorders approved by the local Research Ethics Committee. In order to screen for mutations in the C9ORF72 gene, the repeat primed polymerase chain reaction was performed using the method described by Renton et al. (2011). The presence of the mutation was inferred on the basis of repeat lengths in excess of 30. It is acknowledged that present analytical methods (employing repeat primed polymerase chain reaction) do not ascertain the exact length of the expansion, which can appear in many instances to exceed several hundred repeats.
For the 398 patients included in the study, demographic, neurological, behavioural and cognitive findings were recorded. Recorded data were based on information available to and documented by the consultant neurologist at the time of the patient's initial referral, and information elicited on initial diagnostic neuropsychological assessment. The rationale was to ensure maximum uniformity of information available for each patient. However, where signs of MND were absent at referral we noted whether such signs emerged during the course of follow-up.
Where post-mortem brain tissue was available, brains were fixed routinely for at least 3 months in 10% buffered formaldehyde and following fixation, 14 standard blocks were taken to include frontal and temporal cortex (with hippocampus), cerebellar cortex, medulla oblongata (at level of hypoglossus nucleus) and spinal cord (if available). Paraffin sections were cut at a thickness of 6 µm and stained with antibodies for ubiquitin (rabbit polyclonal antibody Z0458, Dako Cytomation, 1:750–3000), TDP-43 (rabbit polyclonal antibody, 10782-2-AP, Proteintech, 1:1000–12 500), FUS protein (rabbit polyclonal antibody HPA-008784, Sigma, 1:50–200 and rabbit polyclonal antibody 11570-1-AP, Proteintech, 1:100) and tau (mouse monoclonal antibody AT8, Innogenetics, 1:750) from which their histological subtype according to Mackenzie et al. (2009, 2010, 2011) was ascribed. A subset of sections was immunostained for C9ORF72 using two rabbit polyclonal antibodies (Insight Biotechnology and HPA023873, Sigma-Aldrich) at dilutions of 1:50–200. Sections were initially immunostained at 1:200 dilution, but restained at 1:50 if a weak or negative staining reaction was obtained at 1:200 dilution. Sections of cerebellar cortex were additionally stained using p62-lck ligand (rabbit polyclonal antibody, B D Biosciences, 1:100). All subsequent steps employed a standard VECTASTAIN® ABC Elite kit (Vector) with diamonbenzidene as chromagen. Antigen retrieval by microwaving in 0.1 M citrate buffer, pH 6.0 was performed routinely, except for amyloid β-protein immunostaining when pretreatment in 100% formic acid was preferred.
Results
Thirty-two patients were identified with C9ORF72 mutations, representing 8% of the total cohort. None of these patients had mutations in MAPT or progranulin (PGRN) genes.
Demographics and clinical classification
The 32 patients comprised 18 males and 14 females, giving a male:female ratio of 1.3:1 (Table 1). This sex distribution did not differ significantly from that of the 366 patients with FTLD in the cohort without C9ORF72 mutations (202 males, 164 females, χ2 = 0.01, P = 0.91).
Table 1.
Clinical overview of patients with C9ORF72 mutations
| ID | Sex | Age at onset (years) | Years onset to referral death | Family history |
Family members affected | Clinical classification at presentation | Dominant presenting problem | ||
|---|---|---|---|---|---|---|---|---|---|
| FTD | MND | ||||||||
| 1 | F | 54 | <1 | ? | − | + | Two brothers | FTD/MND | Speech production |
| 2 | F | 54 | 1 | 1 | + | − | Sister | FTD/MND | Speech production |
| 3 | F | 72 | 1 | 2 | − | − | – | FTD/MND | Speech production |
| 4 | M | 58 | <1 | ? | ? | ? | ? | FTD/MND | Speech production |
| 5 | M | 57 | <1 | ? | + | − | Father | FTD/MND | Psychosis |
| 6 | F | 68 | 4 | 5 | + | + | Brother FTD, sister MND | FTD/MND | Psychosis |
| 7 | F | 70 | 1 | ? | + | + | Two sisters FTD-MND; mother, son and daughter MND | FTD/MND | Behaviour |
| 8 | M | 58 | 1 | 2 | − | − | FTD/MND | Behaviour | |
| 9 | M | 57 | 1 | 2 | + | − | Mother, uncle | FTD/MND | Behaviour |
| 10 | M | 49 | 6 | 9 | + | − | Four sibs | FTD | Psychosis |
| 11 | M | 55 | 1 | ? | + | + | Mother (FTD-MND) | FTD | Psychosis |
| 12 | M | 64 | 1 | ? | + | − | Father, brother, three aunts | FTD | Psychosis |
| 13 | M | 65 | 2 | ? | + | − | Father | FTD | Psychosis |
| 14 | F | 53 | <1 | ? | − | − | – | FTD | Psychosis |
| 15 | M | 58 | 3 | ? | − | − | – | FTD | Behaviour |
| 16 | M | 62 | 10 | ? | + | − | Brother-dementia, father- parkinsonian | FTD | Behaviour |
| 17 | M | 68 | 5 | ? | − | − | – | FTD | Behaviour |
| 18 | M | 39 | 1 | ? | + | − | Mothera, grandfather | FTD | Behaviour |
| 19 | M | 70 | 2 | ? | − | − | – | FTD | Behaviour |
| 20 | F | 52 | 10 | ? | + | − | Mother, sister | FTD | Behaviour/psychosis |
| 21 | M | 55 | 4 | ? | + | − | Father, uncle | FTD | Behaviour |
| 22 | F | 62 | ? | + | + | Mother, uncle, grandmother (dementia), brother (MND) | FTD | Behaviour/psychosis | |
| 23 | M | 46 | 2 | ? | + | − | Mother | FTD | Behaviour/psychosis |
| 24 | M | 59 | 4 | 11 | −b | − | – | FTD | Behaviour/psychosis |
| 25 | M | 55 | 2 | ? | − | − | – | FTD | Behaviour |
| 26 | F | 72 | 1 | ? | − | − | – | FTD | Behaviour |
| 27 | M | 56 | 4 | ? | + | − | Mother, grandparent | FTD | Psychosis |
| 28 | F | 60 | 1 | 6 | + | − | Father, sonc | FTD | Behaviour |
| 29 | F | 47 | 3 | ? | + | − | Mother | SD/FTD | Language/behaviour |
| 30 | F | 52 | 1 | 4 | − | − | – | PNFA | Expressive language |
| 31 | F | 62 | 2 | 4 | + | − | Mother | PNFA | Expressive language |
| 32 | F | 58 | 4 | ? | − | − | – | PNFA | Expressive language |
a Mother = Patient 28.
b Father and five siblings had genetically confirmed Huntington's disease, for which screening was negative in patient.
c Son = Patient 18.
PNFA = progressive non-fluent aphasia; SD/FTD = semantic dementia with frontal features; + = present; − = absent; ? = not known.
The mean age at onset of illness in patients with C9ORF72 mutations was 58.3 years [standard deviation (SD) 7.7], ranging from 46 to 72 years. Onset age did not differ significantly from that of patients with no mutation (mean 60.3 years; SD 8.8, t = 1.2, P = 0.22). The mean duration of symptoms at the time of referral was 2.7 years (SD 2.4) in patients with C9ORF72 mutations and 3.1 years (SD 2.3) in those without mutations. These differences are not significant (t = 0.80, P = 0.43).
Information about survival time after onset was unavailable for many patients with C9ORF72 mutations. However, the available information indicated variability, ranging from 1 to at least 11 years.
A positive family history of early onset dementia or MND in at least one first-degree relative was present in 20 of 31 patients with C9ORF72 mutations (65%). For one additional patient, no family history information was available. In 15 of the 31 patients, there was a family history of FTD, in one MND and in four both FTD and MND. In 12 patients (39%), more than one family member was affected. The presence of a positive family history was higher in patients with the C9ORF72 mutation than those without (χ2 = 6.24, P = 0.01). A positive family history more than doubled the likelihood of having the C9ORF72 mutation [odds ratio (OR) 2.6, 95% confidence interval (CI) 1.2–5.6].
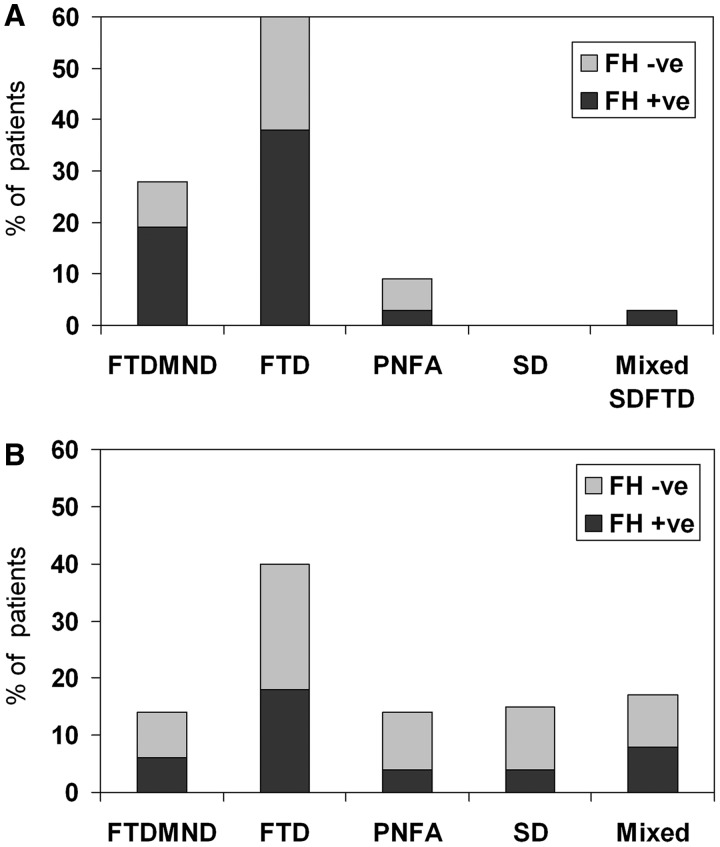
Clinical phenotype varied across the 32 patients with C9ORF72 mutations (Table 1). Nine patients (28%) were classified clinically as having FTD–MND, 19 (59%) FTD, one (3%) semantic dementia with frontal behavioural features and three (9%) progressive non-fluent aphasia. Figure 1 shows that the behavioural variant of FTD accounted for a relatively high proportion of patients with C9ORF72 mutations (Fig. 1A) compared with those without the mutation (Fig. 1B). The presence of FTD increased significantly the odds of having the mutation (OR 4.1, 95% CI 1.6–10.3). Figure 1 also illustrates the higher relative incidence of a positive family history in patients with the mutation.
Figure 1.
Relative frequency of clinical phenotypes and familial cases in patients with the C9ORF72 mutation (A) and those with no mutation (B). FH = family history; FTD = behavioural variant FTD; FTDMND = FTD with motor neuron disease. PNFA = progressive non-fluent aphasia; SD = semantic dementia.
Four patients with FTD–MND presented initially with symptoms of difficulty speaking associated with bulbar signs, and behavioural changes were noted subsequently, whereas in five patients, behavioural/psychiatric changes preceded the onset of MND. Strikingly, 12 patients presented with psychosis or a mixture of behavioural and psychotic symptoms. These symptoms are described in more detail below.
Neurological signs and neuroimaging findings
The onset of illness involved bulbar neurons in all but one of the nine patients with MND (Table 2). In two of these patients, neurological signs were confined to the cranial nerves consistent with a classification of progressive bulbar palsy. Other patients showed limb involvement, with upper and lower motor neuron signs consistent with a classification of amyotrophic lateral sclerosis. Electrophysiological investigations revealed widespread denervation in 5 out of 9 patients, normal electromyography in Patients 2 and 6, and was unavailable in Patients 5 and 9. The 23 patients (72%) who showed no signs of MND at referral did not develop signs of MND during their period of follow-up, which ranged from 1 to 8 years.
Table 2.
Neurological signs and imaging findings in patients with C9ORF72 mutations
| ID | Neurological signs |
Imaging findings |
||||
|---|---|---|---|---|---|---|
| MND/type | Onset | Parkinsonism | Grasp reflexes | MR/CT atrophy | SPECT hypoperfusion | |
| 1 | Yes/PBP | Bulbar | No | ? | n/a | n/a |
| 2 | Yes/ALS | Bulbar | Yes | Left | Normal | Temporal L = R |
| 3 | Yes/ALS | Bulbar | No | Bilateral | n/a | n/a |
| 4 | Yes/ALS | Bulbar | No | ? | n/a | n/a |
| 5 | Yes/ALS | Limb | Yes | Bilateral | General | Frontal = temporal L = R |
| 6 | Yes/PBP | Bulbar | No | No | Frontal > temporal R > L | n/a |
| 7 | Yes/ALS | Bulbar + limb | Yes | ? | n/a | n/a |
| 8 | Yes/ALS | Bulbar + limb | No | ? | n/a | n/a |
| 9 | Yes/ALS | Bulbar + limb | No | Bilateral | Frontal > temporal L > R | n/a |
| 10 | No | – | No | Bilateral | General | Frontal L = R |
| 11 | No | – | No | No | Normal | Frontal = temporal L = R |
| 12 | No | – | Yes | No | Temporal > frontal | n/a |
| 13 | No | – | No | No | Temporal > frontal L > R | Temporal L > R |
| 14 | No | – | No | No | Frontal = temporal L = R | n/a |
| 15 | No | – | No | Bilateral | n/a | Frontal = temporal L = R |
| 16 | No | – | No | Bilateral | n/a | n/a |
| 17 | No | – | No | No | n/a | Frontal = temporal L > R |
| 18 | No | – | No | No | Temporal L = R | n/a |
| 19 | No | – | No | Bilateral | Normal | Frontal = temporal |
| 20 | No | – | No | Bilateral | Frontal = temporal | n/a |
| 21 | No | – | No | No | Temporal L | n/a |
| 22 | No | – | No | Bilateral | Temporal > frontal | Temporal > frontal L > R |
| 23 | No | – | Yes | No | Normal | n/a |
| 24 | No | – | Yes | Bilateral | Normal | Frontal = temporal L > R |
| 25 | No | – | No | No | Frontal L = R | n/a |
| 26 | No | – | No | No | Normal | n/a |
| 27 | No | – | No | No | Normal | Frontal = temporal |
| 28 | No | – | No | Bilateral | Frontal L = R | Frontal = temporal L = R |
| 29 | No | – | No | No | Frontal = temporal L > R | Frontal = temporal L > R |
| 30 | No | – | Yes | No | n/a | Frontal > temporal L > R |
| 31 | No | – | No | No | Temporal L > R | Normal |
| 32 | No | – | Yes | Bilateral | Frontal = temporal L > R | Frontotemporal > parietal L |
ALS = amyotrophic lateral sclerosis; CT = computed tomography; L = left; MR = magnetic resonance; n/a = not available; PBP = progressive bulbar palsy; R = right; SPECT = single photon emission computed tomography.
Neurological signs aside from MND consisted of mild rigidity of the limbs in eight patients and positive grasp reflexes in 13 (Table 2). In 11 patients (34%), the neurological examination was entirely normal.
Comparison of patients with and without C9ORF72 mutations showed a higher prevalence of MND in patients with the mutation (χ2 = 6.0, P = 0.01). Odds ratio analysis showed that the presence of MND more than doubled the likelihood of the patient having the C9ORF72 mutation (OR 2.7, 95% CI 1.2–6.2). Nevertheless, the C9ORF72 mutation did not account for all cases of FTD–MND in the study cohort. Patients without the C9ORF72 mutation included 41 with FTD–MND, two with progressive non-fluent aphasia and MND and three with semantic dementia and MND. These 46 patients represent 12% of non-mutation carriers in the study cohort. In almost half of these 46 cases (47%) the disorder was familial.
Imaging findings (Table 2) were in keeping with FTD. In the majority of cases, there was frontotemporal atrophy or hypoperfusion. There was variability in terms of the relative preponderance of involvement of frontal and temporal lobes, and left–right sided asymmetries. There was no evident difference in this regard in patients presenting with or without psychosis. Predictably, however, in all four language-disordered patients left-sided damage was predominant.
Psychotic symptoms
A prominent feature of most patients was their highly abnormal behaviour, which had a distinctly psychiatric character (Table 3). Twelve patients (38%) presented with florid psychotic symptoms, resulting in initial psychiatric diagnoses of delusional psychosis, somatoform psychosis or paranoid schizophrenia. Patient 5 presented to the Accident and Emergency department of his local hospital complaining of pieces of plastic emanating from his head. He had been noted in preceding months to being increasingly suspicious and to pluck repetitively at his skull. He carried a pocket-knife with which he threatened to cut out the offending plastic. Patient 6 described visions of the devil and had developed behavioural strategies for keeping him at bay. She reported pain and spent all day in bed, saying that bed-rest would allow her ‘nerves to heal’. Despite her symptoms she showed no distress and her affect was of ‘belle indifference’. Patient 10 developed persecutory delusions. He threw lit fireworks though his neighbour's letterbox, and carried a knife and pistol for self-defence. He telephoned the police at the same time each night demanding protection. Patient 11 acquired a mono-delusion of weakness of the gluteal muscles, necessitating that he maintain his finger in his anus to prevent incontinence. Patient 12 believed that he was under surveillance and reported hearing the voice of God. Patient 13 exhibited delusional parasitosis believing that he was infested by mites, which crawled under his skin and into his extremities. He reported that the mites congregated in his ear lobe, and that he could reduce their number by pinching his ear lobe at regular 10-min intervals. Patient 14 contacted the police complaining of men hiding in her garden, including a man dressed in a gorilla outfit. Thereafter she reported seeing disembodied faces, which she believed to be spirits. Patient 16 barricaded himself into his home in the belief that his son was trying to kill him. Patient 17 believed that he was being contacted by letter or phone by dead friends and hatched plans to meet them. Patient 22 became suspicious about relatives and believed characters on the television screen to be communicating with her. Patient 23 threatened to shoot his wife in the belief that she was trying to harm him. Patient 27 developed a paranoid psychosis, believing that people around him and on the television screen were talking about him and calling him names. Three other patients expressed paranoid ideation as part of their behavioural disorder, although this was not the most prominent presenting symptom.
Table 3.
Behavioural changes in patients with C9ORF72 mutations
| Patients |
|||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Behavioural domains | Symptoms | 01 | 02 | 03 | 04 | 05 | 06 | 07 | 08 | 09 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 |
| Psychotic symptoms | Delusions/paranoia | − | − | − | − | + | + | − | − | − | + | + | + | + | + | − | + | + | − | − | + | − | + | + | + | + | − | + | − | − | − | − | − |
| Somatoform delusions | − | − | − | − | + | + | − | − | − | − | + | − | + | + | − | − | − | − | + | − | − | − | − | − | − | + | + | − | − | − | − | − | |
| Hallucinations | − | − | − | − | − | + | − | − | − | − | + | + | − | + | − | − | − | − | − | − | − | − | − | − | + | + | − | − | − | − | − | ||
| Bizarre/irrational behaviour | − | − | − | − | + | + | + | + | + | + | + | + | + | + | + | + | + | − | + | − | + | + | + | + | − | + | + | − | − | − | − | − | |
| Disinhibition | Socially inappropriate | − | + | + | + | + | − | − | + | + | − | + | + | − | + | + | + | − | − | − | + | − | + | − | + | + | + | − | − | − | − | − | − |
| Loss of manners | + | + | + | + | + | − | − | + | + | + | + | + | − | + | + | + | − | − | + | + | + | + | − | + | + | + | − | + | − | − | − | − | |
| Impulsive (overspends) | − | − | + | − | − | − | + | − | − | − | + | + | − | + | + | + | + | − | + | − | − | − | − | − | − | + | − | + | − | − | − | − | |
| Apathy | Apathy | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | − | − | − | − |
| Inertia | + | + | − | + | + | + | + | + | + | + | − | + | − | + | + | + | + | + | + | + | + | + | − | + | + | + | − | + | − | − | − | − | |
| Loss sympathy and empathy | Neglects feelings | + | + | + | + | + | + | + | + | + | + | + | − | + | + | + | + | + | − | + | + | + | + | + | + | + | + | − | + | + | − | − | − |
| Less interest/warmth | + | + | + | − | + | − | − | + | + | + | + | − | + | + | + | + | + | − | + | + | + | − | − | + | + | − | + | + | − | − | − | − | |
| Repetitive behaviours, stereotypies and obsessionality | Simple mannerisms | − | − | − | − | + | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | + | − | − | − | − | − | + | + | − | − | − | − |
| Complex routines/rituals | + | − | − | − | − | + | + | + | + | + | + | + | + | + | + | + | − | − | + | − | + | + | + | − | − | − | + | + | + | − | − | − | |
| Repetitive speech | + | − | + | − | + | − | + | + | − | + | + | + | + | + | + | − | − | − | + | − | + | + | + | + | − | + | + | − | + | − | − | − | |
| Clock watches | − | − | − | − | − | − | + | − | − | − | − | + | − | − | − | + | − | − | + | − | + | − | − | − | − | − | + | − | + | − | − | − | |
| Excess cleaning | − | − | − | − | − | − | + | − | − | − | − | + | − | − | − | − | + | − | + | − | + | − | − | − | − | − | − | − | + | − | − | − | |
| Dietary change | Sweet food preference | − | − | + | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | + | − | − | + | + | − | − | − |
| Food fads | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| Binge eating/cramming | + | − | − | − | − | + | + | − | + | + | − | − | + | − | + | + | − | − | + | − | + | − | − | − | + | − | − | − | − | − | − | − | |
| Mouths inedible objects | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| Loss of insight | Denial of illness | + | − | + | + | + | − | + | + | + | + | + | + | + | + | + | + | + | − | + | + | + | + | + | + | + | + | − | + | + | − | − | − |
| Unconcern | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | − | + | + | + | + | + | + | + | + | − | + | + | − | − | − | |
+ = present at initial referral; − = absent at initial referral.
Several patients had symptoms of a somatic character for which no medical cause could be found (Table 3). As noted above, Patient 5 complained of plastic emanating from him and Patient 6 reported pain. Patient 14 also complained of leg pain. She drank excessive quantities of water as a remedy for her symptoms, leading to hyponatraemia. Patient 19 complained of excessive heat. He threw open doors and windows, refused to allow any heating in the home and dressed in summer attire in mid-winter. He took to pouring cold water over tepid food to ‘cool it down’. Patient 11, as noted above, complained of gluteal muscle weakness and was preoccupied by bowel function. Patient 22 was similarly preoccupied by bowel function.
Several patients exhibited behaviour that was described by relatives as irrational, ‘confused’ or frankly bizarre. For example, Patient 15 combed his hair repetitively and vigorously leading to bleeding of the scalp. He wore multiple watches on his arm and donned clothes on the wrong part of his body (e.g. trousers on his head; underpants on his arms). He used objects inappropriately (e.g. spoon to clean teeth; toilet brush to brush hair). There was no evidence on cognitive examination of impaired object recognition to account for the object misuse. Patient 9 cleaned his teeth with the toilet brush using the water from the toilet bowl. Again, this could not be explained in cognitive terms. Patient 19 searched for personal documents in the garden in the middle of the night, despite having no immediate need for them or logical reason to believe that they would be located outside the home. Patient 22 took to wrapping faeces in paper and cooking it in the oven.
There was no previous history of psychiatric disorder in any of the patients. Delusional symptoms consistently failed to respond to anti-psychotic medication.
Psychosis was rare in patients without the C9ORF72 mutation, being present in <4% of patients. Moreover, the psychosis was described either as part of a prodromal depressive illness (psychotic depression) or in the context of previous history of psychiatric illness such as bipolar disorder. No patient exhibited the intense mono-delusional psychoses, such as delusional parasitosis, found in people with C9ORF72 gene mutations. In the case of the 46 patients with FTD–MND with no C9ORF72 mutation, only one (2%) exhibited psychiatric symptoms: she had a long-standing history of bipolar affective disorder.
The difference in frequency of psychosis in patients with and without the C9ORF72 mutation is highly significant (χ2 = 47.6, P < 0.0001). Odds ratio analysis showed that the presence of psychosis, as a presenting feature of FTD, dramatically increased the likelihood of having the C9ORF72 mutation (OR 15.4, 95% CI 5.9–40.0).
Behavioural characteristics of frontotemporal dementia
Table 3 shows that, in addition to the psychiatric symptoms described above, the majority of patients with C9ORF72 mutations exhibited a range of behavioural changes commonly associated with FTD (Neary et al., 1998; Rascovsky et al., 2011). Patient 18 showed few abnormalities at the time of initial presentation, but subsequently developed frank features of FTD. A prevailing feature across the patient group was increased apathy and a reduction in motivation and initiative, being reported in 88% of cases. Overtly, socially disinhibited behaviour occurred although this was less ubiquitous, being reported in 66%. Moreover, the ‘disinhibition’ was sometimes context-bound rather than generalized, centring around patients’ delusional belief. For example, Patient 11 took to baring his buttocks in order to demonstrate to people the weakness of his gluteal muscles. Some patients, despite their profound delusional state, were noted at interview to be polite and deferential.
A high proportion of patients with C9ORF72 mutation (75%) exhibited repetitive/stereotypic behaviour. This commonly took the form of complex behavioural routines (59%) and sometimes had an obsessive quality. Several patients became obsessed by cleanliness, which they took to extremes. For example, Patient 19 constantly wiped surfaces and washed pots, and carried with him a cloth to wipe his shoes before getting into his car, and plastic bags to wipe his hands. He washed his hands, filed his nails and combed his hair repetitively. Patient 21 cleaned the house obsessively and followed his dog around cleaning the surfaces that it had walked on. Simple motor mannerisms such as hand rubbing, humming or tapping were rare (16%). In some cases, where it occurred, the motor stereotypy constituted part of the patient's delusional belief. Patient 5 plucked repetitively at his scalp to remove the ‘plastic bits’ embedded in his skin; Patient 13 tugged at his ear lobe at 10-min intervals to counteract the invasion of mites. Repetitiveness in speech, present in 59% of cases, commonly constituted fixation on a particular theme, often related to patients’ delusional belief.
Alterations in dietary habits were reported in 14 patients with the C9ORF72 mutation. This typically constituted excessive or indiscriminate eating with tendency to cram food (Table 3). Food fads/restricted diet, reported in two patients, appeared to be one manifestation of the patients’ repetitive routine-bound behaviour (e.g. cornflakes every day for breakfast, meat pie for dinner). An altered preference for sweet foods occurred relatively infrequently. A comparison of patients with and without C9ORF72 mutations showed that the overall incidence of dietary change in mutation bearers (44%) was significantly lower than that (70%) in non-mutation bearers (χ2 = 8.4, P = 0.004). Closer examination of the nature of dietary change indicated that the frequency of overeating/food cramming behaviour did not differ in patients with and without the C9ORF72 mutation (χ2 = 0.50, P = 0.48). The presence of food fads was only marginally less common in mutation bearers (χ2 = 4.3, P = 0.04). The source of the overall group difference lay in an acquired preference for sweet foods, which was present in 19% of mutation bearers but 48% of patients with no mutation (χ2 = 9.5, P = 0.002). Odds ratio analysis indicated that an acquired preference for sweet foods was associated with a 4-fold reduction in the likelihood of having the C9ORF72 mutation (OR 0.25, 95% CI 0.10–0.63).
Insight was impaired in 78% of patients with C9ORF72 mutations. The three patients with progressive non-fluent aphasia showed insight and appropriate concern.
Cognitive changes
The salient feature on cognitive examination was the presence of profound deficits in executive functions in the majority of patients (Table 4). One exception to this rule was preserved performance on executive tests in Patient 11, contrasting with his striking delusional disorder. Patient 31, who presented with progressive non-fluent aphasia, also performed normally on executive tasks.
Table 4.
Cognitive changes in patients with C9ORF72 mutations
| Patients |
|||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cognitive domains | Nature of deficits | 01 | 02 | 03 | 04 | 05 | 06 | 07 | 08 | 09 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 |
| Language | ↓ Propositional speech | + | ? | + | ? | + | − | + | + | + | + | − | ? | + | − | + | + | + | − | + | + | − | + | + | − | + | − | + | + | − | + | + | + |
| Naming disorder | + | − | − | + | + | − | + | − | + | + | + | + | + | − | + | + | + | − | + | ? | − | + | + | − | − | + | − | + | + | + | + | + | |
| Phonological errors | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | + | |
| Semantic loss/errors | − | − | + | − | + | − | + | − | + | − | − | − | + | − | + | − | − | − | − | − | − | + | + | − | − | − | − | − | + | − | − | − | |
| Speech apraxia | − | − | − | ? | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| Perceptuo-spatial | Face/object agnosia | − | − | − | + | + | ? | − | − | − | − | + | + | + | − | − | − | − | − | − | − | − | − | + | − | − | − | − | − | + | − | − | − |
| Spatial impairment | − | − | − | − | − | ? | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| Praxis | Gestural apraxia | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| Memory | ‘Frontal'memory disorder | + | ? | + | − | + | − | + | + | + | + | + | + | + | + | − | + | − | + | ? | − | + | + | + | + | + | + | + | ? | + | − | − | |
| Executive | Generation impairment | + | + | + | + | + | + | + | + | + | + | + | + | + | − | + | + | + | − | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| Set switching impairment | + | − | + | + | + | − | + | + | + | ? | − | + | + | + | + | + | + | − | + | + | − | + | + | + | + | + | + | + | − | + | − | + | |
| Sequencing impairment | + | − | + | + | + | + | + | + | + | ? | − | + | + | + | + | + | + | − | + | + | + | + | + | + | + | + | + | + | ? | + | − | + | |
| Concrete responses | + | − | + | − | + | + | + | + | + | ? | − | − | + | + | + | + | + | − | + | + | − | + | + | + | − | + | + | + | + | + | − | + | |
| Verbal/motor perseveration | + | − | + | + | + | − | + | + | + | + | − | + | + | + | + | + | + | − | + | + | − | + | + | + | + | + | + | − | − | + | − | − | |
| Economy/impersistence | + | − | + | − | + | + | + | + | + | + | − | − | + | + | + | + | + | − | + | + | + | + | + | − | + | − | + | + | − | − | − | − | |
+ = deficit present at initial assessment; − = deficit absent at initial assessment; ? = information unavailable or difficult to interpret; ↓ = reduced in quantity and range of grammatical structure.
Most patients with FTD also exhibited changes in language (Table 4). This typically took the form of reduced generation of propositional speech. Patients did not initiate conversation and they responded to questions in short phrases or monosyllables, with little variation in syntactic structure. Echolalic tendencies and perseveration were observed. The language profile was in keeping predominantly with a dynamic aphasia. However, problems in word finding were also present and some patients made frank semantic errors, suggesting a semantic contribution to their language disorder. Deficits in object and face recognition were detected in some patients, providing further evidence of the presence of semantic impairment. None of the patients with FTD exhibited phonological errors or speech apraxia. Spatial skills and gestural limb praxis were also well preserved. Performance on memory tests was poor in the majority of patients, although this typically has a ‘frontal’ quality: inefficient, assimilation of information, particularly on open-ended recall tasks, but no abnormal loss over a delay.
Four patients were phenotypically different from the rest. Patient 29 exhibited a profile of semantic dementia, albeit in the context of some ‘frontal’ behaviour. She was garrulous, but had profound impairment of naming and word comprehension, as well as impaired face and object recognition. Patient 30 exhibited a contrasting picture of non-fluent, agrammatic speech, with phonological errors. Comprehension of nominal terms was preserved but understanding of syntax was impaired. Patients 31 and 32 exhibited a severe disorder of access to the phonological word form, but they made neither phonological nor semantic errors in naming. They exhibited no behavioural changes and Patient 31 showed no impairment on standard executive tests, aside from a verbal fluency task that makes linguistic demands.
Pathological findings
Gross pathology
Five of the 78 autopsied patients had C9ORF72 mutations (Patients 6 and 9 with FTD–MND and Patients 10, 20 and 24 with FTD). All patients apart from Patient 9 had exhibited psychosis at presentation. Brain weight was grossly reduced in Patient 10, moderately reduced in Patients 9 and 24, but normal in Patient 6. Brain weight was not recorded in Patient 20. All five patients displayed bilateral frontal and temporal lobe atrophy. The hippocampus was normal in all patients, but the substantia nigra was severely depigmented in Patients 9 and 20. There were no obvious differences in topographical distribution of anatomy in the patients.
Histopathology
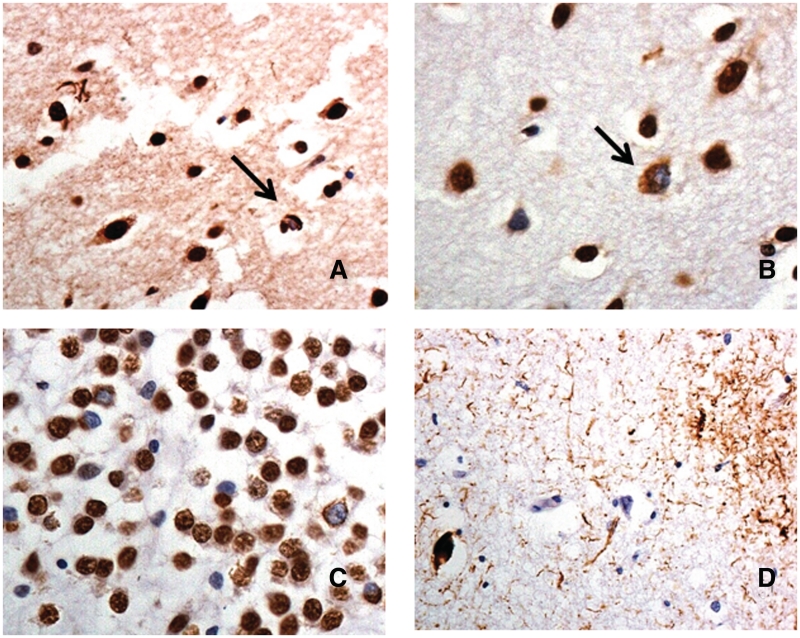
Four of the patients (Patients 6, 9, 10 and 20) showed ubiquitinated TDP-43-positive histopathological changes within the frontal and temporal cortex, and hippocampus. In Patient 10, with clinical FTD, these presented in the cerebral cortex as small, rounded or crescent-shaped neuronal cytoplasmic inclusions within pyramidal cells of layer II together with a few short, comma shaped dystrophic neurites (Fig. 2A); neuronal intranuclear inclusions were occasionally seen. In the hippocampus, a moderate number of more granular ubiquitinated TDP-43 positive inclusions were seen within the dentate gyrus granule cells. This pattern of changes was considered to best conform to FTLD-TDP type A histological change (Mackenzie et al., 2011). In Patients 6, 9 and 20 a few small, rounded or crescent-shaped neuronal cytoplasmic inclusions were present within pyramidal cells of layer II, with other cells showing a more granular ubiquitin TDP-43 pattern of staining (Fig. 2B). Dystrophic neurites were rare and neuronal intranuclear inclusions absent. In the hippocampus, a moderate number of granular ubiquitin TDP-43-positive inclusions were seen within the dentate gyrus granule cells (Fig. 2C). This pattern of changes best conformed to FTLD-TDP type B (Mackenzie et al., 2011). Occasional ubiquitin TDP-43 positive histopathological changes were observed within motor neurons of hypoglossus nucleus in Patient 9. The fifth patient with a C9ORF72 mutation (Patient 24) had a tauopathy consistent with corticobasal degeneration (Fig. 2D), and did not show any TDP-43 pathological changes in the hippocampus or cerebral cortex, though physiological nuclear staining was observed, as in other cases.
Figure 2.
Immunohistochemical staining for TDP-43 and tau proteins in patients bearing the hexanucleotide repeat expansion in C9ORF72. Patient 10 shows occasional TDP-43 immunoreactive neuronal cytoplasmic inclusions (arrow) and dystrophic neurites in the outer layers of the temporal cortex consistent with FTLD-TDP type A histology (A). Patient 6 shows occasional, often granular, TDP-43 immunoreactive neuronal cytoplasmic inclusions (arrow) in the outer layers of the temporal cortex (B), and many granular inclusions in dentate gyrus granule cells (C), consistent with FTLD-TDP type B histology. Patient 24 shows a tauopathy with astrocytic plaque and neurofibrillary tangle-like structures, consistent with corticobasal degeneration (D). Immunoperoxidase—haematoxylin, magnification ×40.
C9ORF72 immunostaining
Immunostaining for C9ORF72 failed to detect any of the ubiquitin/TDP-43 positive histopathological changes within the frontal and temporal cortex and hippocampus in the four patients with TDP-43 pathology; nor did it detect the tau pathology in Patient 24 or, the pathological inclusions of tau, TDP-43 or FUS in the 73 pathological cases without C9ORF7 mutations. Antigen retrieval by microwaving sections in citrate buffer was performed routinely, but omission of this did not appear to adversely affect immunostaining outcomes.
Nonetheless, inspection of C9ORF72 immunostained sections of temporal cortex and hippocampus in the study cohort of 78 patients, and in MND, Alzheimer's disease and healthy control reference groups revealed certain notable histological features. Such histological changes were, however, present irrespective of diagnostic group or mutation status and quantitatively similar across groups, so are considered to represent normal patterns of physiological staining of cells and pathways containing or utilizing C9ORF72 protein.
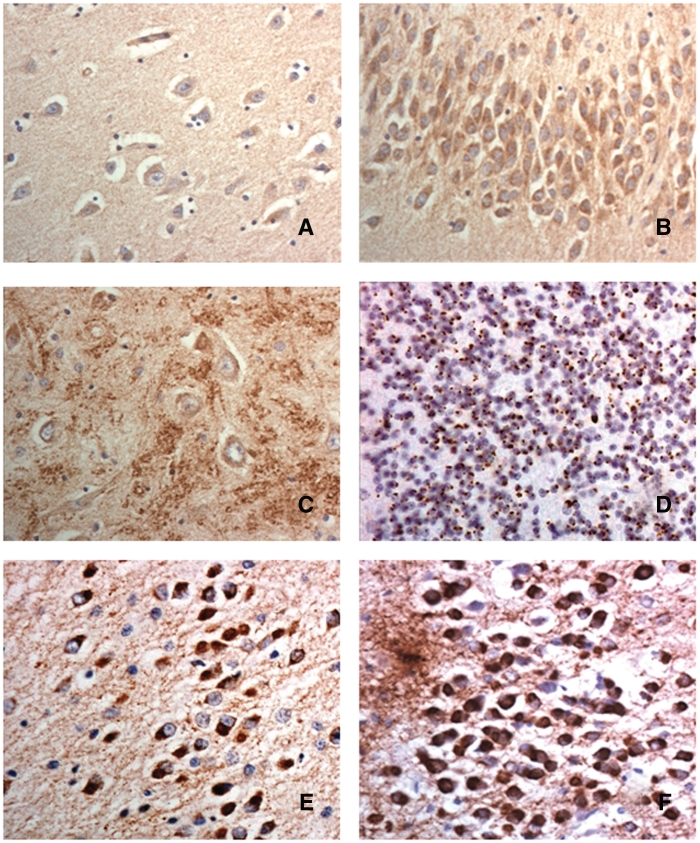
In healthy controls, pyramidal cells of the frontal and temporal cortex generally displayed a weak immunoreactivity containing sparse numbers of small C9ORF72 immunoreactive granules within the cytoplasm, but no nuclear staining was observed (Fig. 3A). However, dentate gyrus granule cells contained a greater, but variable, number of small C9ORF72 immunoreactive granules within the cytoplasm, but again no nuclear staining was observed (Fig. 3B). More strikingly, within areas CA4 there were arborizations of large C9ORF72 immunoreactive granules, resembling synaptic boutons, around the pyramidal cells of this region (Fig. 3D). These usually extended into CA2 and CA3 regions, but were not seen in CA1 and subiculum. Such synaptic arborizations were not seen in frontal or temporal cortical regions. Nonetheless, there was very little, or sometimes no C9ORF72 synaptic staining in some cases with FTLD of all disease types, even when antibody dilutions of 1:50 were employed. Cases with FTLD-TDP with mutations in C9ORF72 appeared to be no more likely to show less C9ORF72 synaptic staining than those without mutation. This same kind of synaptic staining was also variably preserved in reference cases of Alzheimer's disease and MND.
Figure 3.
Immunostaining for C9ORF72 protein in FTLD (A–C). There is slight granular staining of the cytoplasm in pyramidal neurons in the cerebral cortex (A) though dentate gyrus granule cells are strongly immunoreactive containing much granular material within the cytoplasm (B). There are strongly immunostained clusters resembling synaptic terminals around pyramidal cells of CA4 region of hippocampus (C). The cerebellum show many small p62-immunoreactive cytoplasmic inclusions within granule cells (D). In FTLD-tau, Pick bodies within the dentate gyrus granule cells are strongly immunoreactive to antibodies both to C9ORF72 (E) and tau (F). Immunoperoxidase—haematoxylin, magnification ×40.
Interestingly, in all eight cases of FTLD-tau with Pick bodies investigated, there was variable, but usually strong, C9ORF72 immunostaining of Pick bodies within dentate gyrus granule cells (Fig. 3E); but Pick bodies in hippocampal or cerebral cortical pyramidal cells were not immunostained, nor were the tau-positive inclusions in FTLD-tau due to (exons 10, +13 or +16) MAPT mutation. Double immunolabelling for C9ORF72 and tau proteins was not performed, though it was noted that in all instances the number of neurons containing C9ORF72 immunoreactive structures (Fig. 3E) was similar to that containing (AT8-positive) tau Pick bodies (Fig. 3F).
Medulla and spinal cord
C9ORF72 immunopositive neurons were seen within the motor divisions of the hypoglossus and accessory cranial nerve nuclei, and within neurons of the inferior and superior olivary nuclei. The cytoplasm of immunopositive neurons was diffusely stained, but no neuronal cytoplasmic inclusions were detected in any cell type. The nucleus was unstained. Anterior horn cells of the spinal cord were similarly (to hypoglossus) immunostained. There did not appear to be any quantitative differences in the level of immunostaining in any cell type between C9ORF72 mutation bearers and non-bearers and control patients.
Cerebellum
The four patients with C9ORF72 mutation with cortical TDP-43 pathology also displayed ubiquitin/p62-positive, TDP-43-negative neuronal cytoplasmic inclusions within cerebellar granule cells, of the kind described by Boxer et al. (2011). Granule cell neuronal cytoplasmic inclusions appeared as small rounded or oat-shaped bodies, though occasionally larger, more rounded and solid neuronal cytoplasmic inclusions were observed (Fig. 3D). A few, thin and short neuritic profiles were seen, and neuronal intranuclear inclusions were occasionally observed. In Patients 10 and 20, granule cell neuronal cytoplasmic inclusions were abundant, whereas in Patients 6 and 9 they were relatively few in number. Similar, more granular, neuronal cytoplasmic inclusions were present in basket cells, but none were seen within Golgi neurons, or within Bergmann glia. Occasional Purkinje cells and neurons in the dentate nucleus contained small, spicular or granular p62-immunoreactive structures that were not immunoreactive with ubiquitin, TDP-43 or FUS antibodies. In most cases, occasional astrocytes within the granule cell layer were seen with neuronal intranuclear inclusions. No ubiquitin/p62-positive, TDP-43-negative neuronal cytoplasmic inclusions were present, however, in the granule cells of the cerebellum, or any other cell type, in Patient 24.
The presence of p62-immunoreactive, TDP-43 negative neuronal cytoplasmic inclusions within cerebellar granule cells was not exclusive to patients with C9ORF72 mutations. They were present too in varying degrees in three patients with no mutation. Occasional Purkinje cells and neurons in the dentate nucleus showed p62-immunoreactive structures similar to those in C9ORF72 mutation bearers.
In general, the cerebellum appeared histologically normal on haematoxylin and eosin staining in all patients, irrespective of the presence of C9ORF72 mutation, with no undue loss of granule or Purkinje cells, or reactive astroglial changes. Alpha-internexin immunostaining showed occasional axonal torpedoes on Purkinje cells, but these were not obviously more frequent in cases with granule cell neuronal cytoplasmic inclusions compared with cases without.
Discussion
The study examined characteristics of 32 patients with mutations in the C9ORF72 gene, identified from a large cohort of 398 patients with clinical syndromes of FTLD. Demographic characteristics of gender, age at onset of symptoms and duration of illness at referral did not distinguish patients with C9ORF72 gene mutations from those without, indicating that the mutation bearers were representative of the larger group with respect to those demographic variables. Not surprisingly, however, a positive family history of dementia and/or MND was significantly more common in patients with C9ORF72 mutations.
There was, as anticipated, a significant association with MND: the presence of MND more than doubled the likelihood of a patient having the C9ORF72 mutation. Nevertheless, there was no absolute concordance. First, there were patients with the C9ORF72 mutation who had no signs of MND (to our knowledge at any time during the disease course), nor family history of MND. This strongly suggests that MND is not an inevitable manifestation of mutations in the C9ORF72 gene. Secondly, and crucially, there were patients in the study cohort with familial FTD–MND, who did not have the C9ORF72 mutation. This important finding indicates that hexanucleotide repeat expansion in C9ORF72 is not the cause of all cases of FTD–MND. Another genetic mutation or mutations must be responsible.
A striking and unanticipated finding was the strong association of C9ORF72 gene mutations with psychotic symptoms: delusions, hallucinations, paranoid ideation and disordered thinking. More than a third of patients presented with florid psychosis and were initially classified by their psychiatrist using conventional psychiatric diagnostic labels: delusional psychosis, mono-delusional psychosis, somatoform psychosis, paranoid schizophrenia. Other patients exhibited paranoid and delusional thinking as part of their behavioural disorder. In two-thirds of patients behaviour was bizarre and illogical. None of these patients had a history of psychiatric illness. The high prevalence of psychotic symptomatology is important because of its rarity in FTD in general (Bathgate et al., 2001; Mendez et al., 2008a, b). In their study of 86 patients with FTD Mendez et al. (2008b) reported delusions in only 2.3% and hallucinations in 0%, figures significantly lower than for Alzheimer's disease. Although psychotic symptoms have been described in association with MAPT mutations (Sumi et al., 1992; Poorkaj et al., 1998), this appears to be unusual. In line with previous findings, in the present cohort, <4% of patients without C9ORF72 mutations presented with psychosis or exhibited psychotic symptoms at the time of their referral. The presence of psychosis increased the odds of having the C9ORF72 gene mutation by 15-fold. It is noteworthy, moreover, that where psychosis was recorded in patients without the C9ORF72 mutation this was in the context either of a prodromal depressive illness or else a long-standing psychiatric disorder, and bore little resemblance to the florid, yet often circumscribed, delusional disorder found in people with the C9ORF72 mutation.
Interestingly, and in contrast to the paucity of psychosis in FTD in general, there have been several case reports of patients with FTD–MND or a family history of MND who present with psychotic symptoms (Nitrini and Rosenberg, 1998; Larner, 2008; Lillo et al., 2011; Loy et al., 2011). Moreover, in a series of 43 patients with behavioural FTD and 18 with FTD–MND (Lillo et al., 2011), delusions were found to be significantly more common in the FTD–MND group. The prediction would be that those patients had mutations in the C9ORF72 gene. This prediction remains to be substantiated. Psychosis has previously been reported in individual patients with chromosome 9-linked FTD–MND (Pearson et al., 2011) but there has thus far, to our knowledge, been no systematic study of its incidence. It is, however, pertinent that only one of the patients with FTD–MND without C9ORF72 mutations in the present series exhibited psychotic symptoms, this being in the context of a longstanding bipolar affective disorder. This finding is important because it suggests that it is the C9ORF72 mutation that is crucial to the development of psychotic symptoms and not FTD–MND per se.
Aside from patients with FTD–MND, the only other subgroup of FTD patients that has been associated with a relatively high prevalence of psychotic symptoms are rare cases with FUS pathology (Seelaar et al., 2010; Urwin et al., 2010; Snowden et al., 2011b). Importantly, FUS pathology and mutations in the FUS gene have been associated with MND. Psychosis has not typically been reported in association with mutations in either the MAPT (Hutton et al., 1998) or PGRN (Baker et al., 2006) genes, which are not linked to MND.
Is psychosis the only feature that distinguishes the behaviour of patients who have C9ORF72 gene mutations from those who do not? Patients exhibited a range of behaviours associated with FTD: apathy, disinhibition, loss of sympathy and empathy, repetitive behaviours, dietary change and loss of insight. At first glance, therefore, they appear indistinguishable from other patients with FTD. Nevertheless, close examination suggests some distinctive features. First, abnormal behaviours are, to some extent, influenced by patients’ delusional beliefs: for example, repeated telephone calls to the police by a patient who believes himself to be persecuted. Secondly, repetitive behaviours are typically complex. Simple, low-level motor mannerisms such as hand rubbing, tapping, humming, or grunting, which are found in many patients with FTD (Bathgate et al., 2001; Mateen and Josephs, 2009) were rare. This is particularly noteworthy in view of the patients’ general apathy: simple motor stereotypies have been reported most frequently in apathetic patients with FTD (Snowden et al., 2001). Moreover, repeated cleaning and hand washing observed in some patients has characteristics of obsessive–compulsive disorder, a feature that is rare in FTD in general (Bathgate et al., 2001). In the dietary domain, an altered preference for sweet foods was notable for its rarity. Gluttony or increased appetite, a feature that has been linked particularly to TDP-43 pathology (Piguet et al., 2011), did not differ in frequency in patients with and without the C9ORF72 mutation, suggesting that it is the sweet food preference in particular, rather than dietary change in general that is a relevant discriminator. There are therefore behavioural pointers that, taken together, might predict the presence of C9ORF72 mutations: psychotic symptoms, complex repetitive behaviours linked to a mono-delusion or with an obsessional–compulsive quality, absence of sweet food preference. The additional presence of MND in the patient or family member would greatly strengthen the prediction.
Notwithstanding these clinically distinct characteristics, absolute accuracy of prediction is likely to prove difficult by virtue of the phenotypic variability in patients with C9ORF72 mutations. Not only do some patients have MND and others not, the cognitive/behavioural phenotype also varies. As found in the Finnish cohort reported by Renton et al. (2011), patients may present with progressive non-fluent aphasia or semantic dementia, as well as with behavioural FTD. Patient 30 with progressive non-fluent aphasia, showed no psychotic symptoms, none of the behavioural features of FTD, and no signs of MND. There would, therefore be no a priori basis on which to predict the presence of a C9ORF72 gene mutation. In general, however, the prevailing cognitive profile of patients with C9ORF72 mutations was similar, characterized by executive impairment, combined with a dynamic form of aphasia, with additional semantic deficits present in some patients. No patient showed evidence of speech or limb apraxia.
The limitation of any retrospective study is the possibility that symptoms were present but not recorded. The likelihood is reduced, in this study, by the fact that the clinical history uses a structured proforma, which addresses individual behavioural and cognitive symptoms systematically. Moreover, the history was taken for the most part by the same consultant neurologist (D.N.) or neurologist trained by him, providing uniformity of data collection. Similarly, the neuropsychological examination was uniform in all patients and carried out or supervised by the same neuropsychologist (J.S.). Salient features of patients’ clinical presentation, such as psychosis, were recorded on a database prior to the results of genetic screening becoming available, so the recording procedure was carried out in blind fashion. Most importantly, any potential biases arising from ‘missing data’ cannot explain statistical differences in people with and without mutations in the C9ORF72 gene, since these patients were drawn from a single cohort and examined by the same people in the same way.
Findings from the present study not only highlight features likely to be of predictive value in identifying patients with mutations in the C9ORF72 gene but also inform the debate about the nature of the relationship between FTD and MND. Some authors have referred to a ‘continuum’ between FTD and MND (Talbot et al., 1995; Murphy et al., 2007a, b), implying that they are different manifestations of the same disease process. Others propose that FTD–MND should be regarded as an aetiological entity distinct from FTD (Bak et al., 2001; Mitsuyama et al., 2009; Bak, 2011). The present findings point to truth in both views. Mutations in the C9ORF72 gene are associated with a spectrum—or continuum—of clinical manifestations, with isolated MND at one end, isolated FTD or progressive aphasia at the other and a combination of behavioural/cognitive and motor neuron symptomatology in the middle. Yet, the form of FTD associated with C9ORF72 mutations appears qualitatively distinct from other forms of FTD, suggesting that it represents a distinct clinical phenotypic variant linked to a distinct aetiology.
Neuropathological investigation carried out in five patients with C9ORF72 mutations revealed some unexpected findings. The prediction had been that patients would consistently show FTLD-TDP pathology and this would likely conform to type B (Mackenzie et al., 2011). Three patients did indeed exhibit type B pathology, in keeping with prediction. However, in Patient 10 the pathology was more akin to type A, suggesting that the underlying pathological characteristics are not entirely uniform. A recent study (Murray et al., 2011) of 15 cases of FTLD with C9ORF72 mutations (eight with FTLD + MND and seven with FTLD) showed similar heterogeneity, with cases of FTLD-MND being more likely to have type B histology and those with FTLD alone tending to be type A, though there was no absolute concordance.
More surprisingly, in the present study, in one mutation bearer (Patient 24) TDP-43 based histology was absent, and a corticobasal degeneration pathology (not associated with parkinsonism or apraxia) was seen. It is not possible, at present, to know whether the chromosome 9 hexanucleotide expansion in this patient was silent, or whether it in some way promoted the corticobasal degeneration pathology. The lack of TDP-43 staining in this case was unlikely to be due to post-mortem interval or agonal state since normal physiological nuclear staining was present and TDP-43 pathology was adequately immunostained in other patients who died in hypoxic states with longer post-mortem delays. Interestingly, Patient 24 had a strong family history of Huntington's disease. The patient did not himself carry the huntingtin mutation. Nevertheless, since Huntington's disease is a trinucleotide repeat expansion disorder it raises the question whether there is an increased susceptibility within the family to have an altered replication of repeat expansions. In any event, the absence of TDP-43 pathology inevitably raises questions regarding the specificity of the C9ORF72 mutation for FTLD.
Pathogenic repeat expansions can cause disease through haploinsufficiency, in which expression or splicing of the gene in question is disrupted. The expansion can also generate abnormal amounts of toxic RNA species, which interfere with normal cellular activity by sequestering normal RNA and proteins involved in transcription regulation (Wojciechowska and Krzyzosiak, 2011). The large size of the expansion and its non-coding localization within C9ORF72 gene may favour the latter (Renton et al., 2011). Presently, however, the function of C9ORF72 protein is unknown, though in normal individuals at least three alternatively spliced transcripts are expressed (DeJesus-Hernandez et al., 2011). The absence of C9ORF72 protein within the TDP-43 pathological lesions that characterize cases with the mutation suggests that the C9ORF72 expansion does not induce a ‘toxic gain-of-function’ by the protein per se through some process of aggregation and inclusion body formation akin to TDP-43 and FUS proteins in cases of MND bearing mutations in transactive response DNA-binding protein (TARDBP) and FUS genes (Kabashi et al., 2008; Kwiatkowski et al., 2009; Vance et al., 2009). The presence of C9ORF72 RNA intranuclear inclusions in mutation carriers suggest that gain of RNA toxicity is a likely mechanism in these cases (DeJesus-Hernandez et al., 2011).
However, a putative loss-of-function effect (irrespective of the actual mechanism whereby this might be induced) might be anticipated to lead to loss of protein within brain tissue. Nonetheless, present immunostaining with antibodies against C9ORF72 did not detect any obvious loss of immunoreactivity or change in pattern of staining in mutation bearers compared with cases with FTLD associated with MAPT or GRN mutations, or with FTLD-tau, FTLD-TDP, FTLD-FUS, MND or Alzheimer pathology. However, given that careful characterization of the present commercial antibodies available to us for this study has not been performed, (we were unable to obtain recombinant C9ORF72 protein ourselves in order to pre-adsorb the antibody prior to immunostaining), the specificity of the histological findings reported here cannot be guaranteed. Nonetheless, the observations that many of those particular cells and neuroanatomical pathways that are affected by TDP-43 pathological changes in FTD–MND (i.e. hippocampus, inferior olives, motor neurons) are those that appear normally rich in C9ORF72 protein would argue that the findings presented are credible and suggest a functional dependency between C9ORF72 and TDP-43, though the nature of this remains to be elucidated. Present immunohistochemical staining may represent patterns of normal physiological activity, and this would be consistent with the variable preservation or accessibility of the antigen following death even with antigen retrieval, post-mortem delay or differential fixation effects.
The finding of C9ORF72 protein within Pick bodies of the granule cells of the dentate gyrus is interesting. However, this observation might simply reflect the passive sequestration and recruitment of C9ORF72 protein into these tau containing lesions, given that such cells normally appear to be unusually rich in C9ORF72 protein, and that Pick bodies within the relatively C9ORF72 impoverished pyramidal cells of the hippocampal pyramidal cell layer, and the cerebral cortex do not apparently display such immunoreactivity. Nonetheless, a specific interaction between C9ORF72 protein and tau is possible because the TDP-43 positive inclusions in cells of the dentate gyrus in cases with FTLD-TDP apparently did not contain C9ORF72 protein as might be expected if the argument that C9ORF72 protein is being merely sequestered into Pick bodies is correct.
The pathological findings of p62-positive, TDP-43 negative neuronal cytoplasmic inclusions within granule cells of the cerebellum in the four C9ORF72 mutation bearers with TDP-43 pathology are broadly consistent with previous reports (Pikkarainen et al., 2008; King et al., 2011; Murray et al., 2011). These particular cases would support the suggestion by Boxer et al. (2011) that the presence of ubiquitin/p62-positive, TDP-43 negative neuronal cytoplasmic inclusions within the cerebellar granule cells might potentially be used as a pathological surrogate to indicate linkage to chromosome 9p, and the presence of hexanucleotide repeat expansion in C9ORF72, even in the absence of formal linkage studies or genetic analysis. However, three other cases of FTLD showed similar ubiquitin/p62-positive, TDP-43 negative neuronal cytoplasmic inclusions within cerebellar granule cells, in the absence of hexanucleotide repeat expansion in C9ORF72. Moreover, no such inclusions were present in the cerebellum in the fifth patient with C9ORF72 mutation with corticobasal degeneration histology. Hence, although it is likely that most patients/families with FTLD and/or MND possessing repeat expansions in C9ORF72 will display a ubiquitin/p62-positive, TDP-43 negative cerebellar pathology, this does not seem to be a universal finding, and the presence of this cerebellar pathology per se does not appear to be predictive of the presence of this particular mutation, or even linkage to chromosome 9.
The variability in clinical presentation of patients with C9ORF72 mutations is intriguing. Currently, it is unclear why some patients show signs of MND and others not, why some patients present with psychosis and others not, and why patients occasionally present with a highly circumscribed language disorder with neither behavioural nor psychotic features. The imaging and pathology data available in this study do not resolve these issues. The imaging findings reveal no obvious differences in terms of relative preponderance of frontal or temporal atrophy and hemispheric asymmetries in patients with and without psychosis. Predictably, patients with progressive aphasia all showed predominant left-sided atrophy, but asymmetries were present in other patients as well. No obvious pathological difference was apparent between the four patients with psychosis and the one patient without. There is a need for more sophisticated prospective neuroimaging studies of patients screened for the C9ORF72 mutation and pathological studies in larger cohorts of patients to address directly the question of the anatomical correlates of phenotypic variation. It is, of course, possible that phenotypic differences reflect differences in relative time course of symptom development rather than absolute differences. Patients who present with MND are likely to have an attenuated disease course, so it is plausible that they too have a susceptibility to the development of psychosis, but that this does not have time to become manifest. These issues require systematic investigation.
In conclusion, mutations in the C9ORF72 gene are responsible for some but not all cases of FTD–MND. The clinical presentation of patients who have the mutation is variable, the earliest symptoms being physical, behavioural or linguistic. The pathological characteristics are also variable. There are, nevertheless, clinical features that set these patients apart from other patients with FTD. The powerful association between C9ORF72 mutations and psychosis suggests that mutations in the C9ORF72 gene may have a crucial role not only in FTD–MND but also in the development of late onset psychosis.
Funding
S.P.-B. and D.M.A.M. receive Medical Research Council and Wellcome Trust support through the Neurodegeneration Programme. Q.H. is supported through 111 Project funding to Prof. Jinzhou Tian from People's Republic of China. The Manchester brain donation scheme and the work of the Manchester Brain Bank is supported by Alzheimer's Research UK and the Alzheimer's Society through the Brains for Dementia Research Initiative.
Acknowledgements
We thank neurological and psychiatric colleagues from the North West Region of the UK for referring patients, and DeNDRoN for assistance in acquisition of blood samples.
Glossary
Abbreviations
- FTD
frontotemporal dementia
- FTLD
frontotemporal lobar degeneration
- FUS
fused-in-sarcoma
- MND
motor neuron disease
- TDP-43
transactive response DNA-binding protein 43
References
- Baborie A, Griffiths TD, Jaros E, McKeith IG, Burn DJ, Richardson A, et al. Pathological correlates of frontotemporal lobar degeneration in the elderly. Acta Neuropathol. 2011;121:365–71. doi: 10.1007/s00401-010-0765-z. [DOI] [PubMed] [Google Scholar]
- Bak TH. Motor neuron disease and frontotemporal dementia: one, two or three diseases? Ann Indian Acad Neurol. 2011;13(Suppl. 2):S81–8. doi: 10.4103/0972-2327.74250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak TH, O'Donovan DG, Xuereb JH, Boniface S, Hodges JR. Selective impairment of verb processing associated with pathological changes in Brodmann areas 44 and 45 in the motor neurone disease-dementia-aphasia syndrome. Brain. 2001;124:103–20. doi: 10.1093/brain/124.1.103. [DOI] [PubMed] [Google Scholar]
- Baker M, Mackenzie IRA, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, et al. Mutations in Progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–9. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- Bathgate D, Snowden JS, Varma A, Blackshaw A, Neary D. Behaviour in frontotemporal dementia: a comparison with Alzheimer's disease and vascular dementia. Acta Neurol Scand. 2001;103:367–78. doi: 10.1034/j.1600-0404.2001.2000236.x. [DOI] [PubMed] [Google Scholar]
- Boxer AL, Mackenzie IR, Boeve BF, Baker M, Seeley WW, Crook R, et al. Clinical, neuroimaging and neuropathological features of a new chromosome 9p-linked FTD-ALS family. J Neurol Neurosurg Psychiatry. 2011;82:196–203. doi: 10.1136/jnnp.2009.204081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns NJ, Bigio EH, Mackenzie IRA, Neumann M, Lee VMY, Hatanpaa KJ, et al. Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol. 2007;114:2–22. doi: 10.1007/s00401-007-0237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caselli RJ, Windebank AJ, Petersen RC, Komori T, Parisi J, Okazaki H, et al. Rapidly progressive aphasic dementia and motor neuron disease. Ann Neurol. 1993;33:200–7. doi: 10.1002/ana.410330210. [DOI] [PubMed] [Google Scholar]
- Catani M, Piccirilli M, Geloso MC, Cherubini A, Finali G, Pelliccioli G, et al. Rapidly progressive aphasic dementia with motor neuron disease: a distinctive clinical entity. Dement Geriatr Cogn Disord. 2004;17:21–8. doi: 10.1159/000074139. [DOI] [PubMed] [Google Scholar]
- DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, et al. Expanded GGGGCC Hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–56. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Souza LC, Sarazin M, Samri D, Kas A, Lehericy S, Levy R, Dubois B. Démence sémantique associée à une sclérose latérale amyotrophique. [Semantic dementia associated with amyotrophic lateral sclerosis] Rev Neurol (Paris) 2009;165:278–81. doi: 10.1016/j.neurol.2008.06.008. [DOI] [PubMed] [Google Scholar]
- Doran M, Xuereb J, Hodges JR. Rapidly progressive aphasia with bulbar motor neurone disease: a clinical and neuropsychological study. Behav Neurol. 1995;8:169–80. [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, et al. Association of missense and 5'-splice mutations in tau with the inherited FTDP-17. Nature. 1998;393:702–5. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Hodges JR, Snowden JS, Mackenzie IR, Neumann M, Mann DM, Dickson DW. Neuropathological background of phenotypical variability in frontotemporal dementia. Acta Neuropathol. 2011;122:137–53. doi: 10.1007/s00401-011-0839-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabashi E, Valdmanis PN, Dion P, Spiegelman D, McConkey BJ, Vande Welde C, et al. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nature Genet. 2008;40:572–4. doi: 10.1038/ng.132. [DOI] [PubMed] [Google Scholar]
- Kim SH, Seo SW, Go SM, Suh MK, Chin J, Jeong JH, Na DL. Semantic dementia combined with motor neuron disease. J Clin Neurosci. 2009;16:1683–5. doi: 10.1016/j.jocn.2009.05.005. [DOI] [PubMed] [Google Scholar]
- King A, Maekawa S, Bodi I, Troakes C, Al-Sarraj S. Ubiquitinated, p62 immunopositive cerebellar cortical neuronal inclusions are evident across the spectrum of TDP-43 proteinopathies but are only rarely additionally immunopositive for phosphorylation-dependent TDP-43. Neuropathology. 2011;31:239–49. doi: 10.1111/j.1440-1789.2010.01171.x. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski TJ, Bosco DA, LeClerc AL, Tamrazian E, Vanderburg CR, Russ C, et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323:1205–8. doi: 10.1126/science.1166066. [DOI] [PubMed] [Google Scholar]
- Laaksovirta H, Peuralinna T, Schymick JC, Scholz SW, Lai S-L, Myllykangas L, et al. Chromosome 9p21 in amyotrophic lateral sclerosis in Finland: a genome-wide association study. Lancet Neurol. 2010;9:978–85. doi: 10.1016/S1474-4422(10)70184-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larner AJ. Delusion of pregnancy in frontotemporal lobar degeneration with motor neurone disease (FTLD/MND) Behav Neurol. 2008;19:199–200. doi: 10.1155/2008/149086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Ber I, Camuzat A, Berger E, Hannequin D, Laquerriere A, Golfier V, et al. Chromosome-linked 9p-linked families with frontotemporal dementia associated with motor neuron disease. Neurology. 2009;72:1669–76. doi: 10.1212/WNL.0b013e3181a55f1c. [DOI] [PubMed] [Google Scholar]
- Lillo P, Garcin B, Hornberger M, Bak TH, Hodges JR. Neurobehavioural features in frontotemporal dementia with amyotrophic lateral sclerosis. Arch Neurol. 2011;67:826–30. doi: 10.1001/archneurol.2010.146. [DOI] [PubMed] [Google Scholar]
- Lomen-Hoerth C, Anderson T, Miller B. The overlap of amyotrophic lateral sclerosis and frontotemporal dementia. Neurology. 2002;59:1077–9. doi: 10.1212/wnl.59.7.1077. [DOI] [PubMed] [Google Scholar]
- Loy CT, Krill JJ, Troller JN, Kiernan MC, Kwok JB, Vucic S, et al. The case of a 48 year-old woman with bizarre and complex delusions. Nat Rev Neurol. 2011;6:175–9. doi: 10.1038/nrneurol.2010.3. [DOI] [PubMed] [Google Scholar]
- Mackenzie IRA, Baborie A, Pickering-Brown S, Du Plessis D, Jaros E, Perry RH, et al. Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: classification and relation to clinical phenotype. Acta Neuropathol. 2006;112:539–49. doi: 10.1007/s00401-006-0138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IRA, Neumann M, Baborie A, Sampathu DM, Du Plessis D, Jaros E, et al. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol. 2011;122:111–3. doi: 10.1007/s00401-011-0845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IRA, Neumann M, Bigio E, Cairns NJ, Alafuzoff I, Kril J, et al. Nomenclature for neuropathologic subtypes of frontotemporal lobar degeneration: consensus recommendations. Acta Neuropathol. 2009;117:15–8. doi: 10.1007/s00401-008-0460-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IR, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, et al. Nomenclature for neuropathologic subtypes of frontotemporal lobar degeneration: consensus recommendations: an update. Acta Neuropathol. 2010;119:1–4. doi: 10.1007/s00401-009-0612-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateen FJ, Josephs KA. The clinical spectrum of stereotypies in frontotemporal lobar degenerate on. Mov Disord. 2009;24:1237–40. doi: 10.1002/mds.22555. [DOI] [PubMed] [Google Scholar]
- McKenna P, Warrington EK. Graded Naming Test. Windsor, UK: NFER-Nelson; 1983. [Google Scholar]
- Mendez MF, Lauterbach EC, Sampson SM. An evidence-based review of the psychopathology of frontotemporal dementia: a report of the ANPA Committee on Research. J Neuropsychiatry Clin Neurosci. 2008a;20:130–49. doi: 10.1176/jnp.2008.20.2.130. [DOI] [PubMed] [Google Scholar]
- Mendez MF, Shapira JS, Woods RJ, Licht EA, Saul RE. Psychotic symptoms in frontotemporal dementia: prevalence and review. Dement Geriatr Cogn Disord. 2008b;25:206–11. doi: 10.1159/000113418. [DOI] [PubMed] [Google Scholar]
- Mitsuyama Y, Inoue T. Clinical entity of frontotemporal dementia with motor neuron disease. Neuropathology. 2009;29:649–54. doi: 10.1111/j.1440-1789.2009.01059.x. [DOI] [PubMed] [Google Scholar]
- Morita M, Al-Chalabi A, Andersen PM, Hosler B, Sapp P, Englund E, et al. A locus on chromosome 9p confers susceptibility to ALS and frontotemporal dementia. Neurology. 2006;66:839–844. doi: 10.1212/01.wnl.0000200048.53766.b4. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Henry RG, Langmore S, Kramer JH, Miller BL, Lomen-Hoerth C. Continuum of frontal lobe impairment in amyotrophic lateral sclerosis. Arch Neurol. 2007b;64:530–4. doi: 10.1001/archneur.64.4.530. [DOI] [PubMed] [Google Scholar]
- Murphy J, Henry R, Lomen-Hoerth C. Establishing subtypes of the continuum of frontal lobe impairment in amyotrophic lateral sclerosis. Archives of Neurology. 2007a;64:330–4. doi: 10.1001/archneur.64.4.530. [DOI] [PubMed] [Google Scholar]
- Murray ME, DeJesus-Hernandez M, Rutherford NJ, Baker M, Duara R, Graff-Radford N, et al. Clinical and neuropathological heterogeneity of c9FTD/ALS associated with hexanucleotide repeat expansion in C9ORF72. Acta Neuropathol. 2011;122:673–90. doi: 10.1007/s00401-011-0907-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration. A consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–54. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Neary D, Snowden JS, Mann DMA, Northen B, Goulding PJ, Macdermott N. Frontal lobe dementia and motor neuron disease. J Neurol Neurosurg Psychiatry. 1990;53:23–32. doi: 10.1136/jnnp.53.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–3. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- Nitrini R, Rosenberg S. Psychotic symptoms in dementia associated with motor neuron disease: a pathophysiological hypothesis. J Neuropsychiatry Clin Neurosci. 1998;10:456–8. doi: 10.1176/jnp.10.4.456. [DOI] [PubMed] [Google Scholar]
- Ostberg P, Bogdanovic N. Semantic dementia with lower motor neuron disease showing FTLD-TDP type 3 pathology (sensu Mackenzie) Neuropathology. 2011;31:271–9. doi: 10.1111/j.1440-1789.2010.01154.x. [DOI] [PubMed] [Google Scholar]
- Pearson JP, Williams NM, Majounie E, Waite A, Stott J, Newsway V, et al. Familial frontotemporal dementia with amyotrophic lateral sclerosis and a shared haplotype on chromosome 9p. J Neurol. 2011;258:647–55. doi: 10.1007/s00415-010-5815-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phukan J, Pender NP, Hardiman O. Cognitive impairment in amyotrophic lateral sclerosis. Lancet Neurol. 2007;6:994–1003. doi: 10.1016/S1474-4422(07)70265-X. [DOI] [PubMed] [Google Scholar]
- Pickering-Brown SM, Rollinson S, Du Plessis D, Morrison KE, Varma A, Richardson AMT, et al. Frequency and clinical characteristics of progranulin mutation carriers in the Manchester Frontotemporal Lobar Degeneration cohort: Comparison to patients with MAPT and no known mutations. Brain. 2008;131:721–31. doi: 10.1093/brain/awm331. [DOI] [PubMed] [Google Scholar]
- Piguet O, Petersen A, Yin Ka Lam B, Gabery S, Murphy K, Hodges JR, et al. Eating and hypothalamic changes in behavioural-variant frontotemporal dementia. Ann Neurol. 2011;69:312–9. doi: 10.1002/ana.22244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikkarainen M, Hartikainen P, Alafuzoff I. Neuropathologic features of frontotemporal lobar degeneration with ubiquitin-positive inclusions visualized with ubiquitin-binding protein p62 immunohistochemistry. J Neuropathol Exp Neurol. 2008;67:280–98. doi: 10.1097/NEN.0b013e31816a1da2. [DOI] [PubMed] [Google Scholar]
- Poorkaj P, Bird TD, Wijsman E, Nemens E, Garruto RM, Anderson L, et al. Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann Neurol. 1998;43:815–25. doi: 10.1002/ana.410430617. [DOI] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criterial for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–77. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renton AE, Majounie E, Waite A, Simon-Sanchez J, Rollinson S, Gibbs JR, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–68. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD, Lashley T, Schott JM, Warren JE, Mead S, Isaacs AM, et al. Clinical and neuranatomical signatures of tissue pathology in frontotemporal lobar degeneration. Brain. 2011;134:2565–81. doi: 10.1093/brain/awr198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampathu DM, Neumann M, Kwong LK, Chou TT, Micsenyi M, Truax A, et al. Pathological heterogeneity of frontotemporal lobar degeneration with ubiquitin-positive inclusions delineated by ubiquitin immunohistochemistry and novel monoclonal antibodies. Am J Pathol. 2006;169:1343–52. doi: 10.2353/ajpath.2006.060438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelaar H, Klijnsma KY, de Koning I, van der Lugt A, Zheng Chiu W, Azmani A, et al. Frequency of ubiquitin and FUS-positive, TDP-43-negative frontotemporal lobar degeneration. J Neurol. 2010;257:747–53. doi: 10.1007/s00415-009-5404-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelaar H, Schlhaas HJ, Azmani A, Kusters B, Rosso S, Majoor-Krakauer D, et al. TDP-43 pathology in familial frontotemporal dementia and motor neuron disease without Progranulin mutations. Brain. 2007;130:1375–85. doi: 10.1093/brain/awm024. [DOI] [PubMed] [Google Scholar]
- Shatunov A, Mok K, Newhouse S, Weale ME, Smith B, Vance C, et al. Chromosome 9p21 in sporadic amyotrophic lateral sclerosis in the UK and seven other countries: a genome-wide association study. Lancet Neurol. 2010;9:986–94. doi: 10.1016/S1474-4422(10)70197-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden JS, Bathgate B, Varma A, Blackshaw A, Gibbons ZC, Neary D. Distinct behavioural profiles in frontotemporal dementia and semantic dementia. J Neurol Neurosurg Psychiatry. 2001;70:323–32. doi: 10.1136/jnnp.70.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden JS, Hu Q, Rollinson S, Halliwell N, Robinson A, Davidson YS, et al. The most common type of FTLD-FUS (aFTLD-U) is associated with a distinct clinical form of frontotemporal dementia but is not related to mutations in the FUS gene. Acta Neuropathol. 2011b;122:99–110. doi: 10.1007/s00401-011-0816-0. [DOI] [PubMed] [Google Scholar]
- Snowden JS, Neary D, Mann D. Frontotemporal lobar degeneration: clinical and pathological relationships. Acta Neuropathol. 2007b;114:31–8. doi: 10.1007/s00401-007-0236-3. [DOI] [PubMed] [Google Scholar]
- Snowden JS, Stopford CL, Julien CL, Thompson JC, Davidson Y, Gibbons L, et al. Cognitive phenotypes in Alzheimer's disease and genetic risk. Cortex. 2007a;43:835–45. doi: 10.1016/s0010-9452(08)70683-x. [DOI] [PubMed] [Google Scholar]
- Snowden JS, Thompson JC, Stopford CL, Richardson AMT, Gerhard A, Neary D, et al. The clinical diagnosis of early-onset dementias: diagnostic accuracy and clinicopathological relationships. Brain. 2011a;134:2478–92. doi: 10.1093/brain/awr189. [DOI] [PubMed] [Google Scholar]
- Stopford CL, Snowden JS, Thompson JC, Neary D. Variability in cognitive presentation of Alzheimer's disease. Cortex. 2008;44:185–95. doi: 10.1016/j.cortex.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Strong MJ. The syndromes of frontotemporal dysfunction in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2008;9:323–38. doi: 10.1080/17482960802372371. [DOI] [PubMed] [Google Scholar]
- Strong MJ, Grace GM, Freedman M, Lomen-Hoerth C, Woolley S, Goldstein LH, et al. Consensus criteria for the diagnosis of frontotemporal cognitive and behavioural syndromes in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2009;10:131–46. doi: 10.1080/17482960802654364. [DOI] [PubMed] [Google Scholar]
- Sumi SM, Bird TD, Nochlin D, Raskind MA. Familial presenile dementia with psychosis associated with cortical neurofibrillary tangles and degeneration of the amygdala. Neurology. 1992;42:120–7. doi: 10.1212/wnl.42.1.120. [DOI] [PubMed] [Google Scholar]
- Talbot PR, Goulding PJ, Lloyd JJ, Snowden JS, Neary D, Testa HJ. Inter-relationship between ‘classic’ motor neurone disease and frontotemporal dementia: a neuropsychological and single photon emission tomographic study. J Neurol Neurosurg Psychiatry. 1995;58:541–7. doi: 10.1136/jnnp.58.5.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JC, Stopford C, Snowden JS, Neary D. Qualitative neuropsychological performance characteristics in frontotemporal dementia and Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2005;76:920–7. doi: 10.1136/jnnp.2003.033779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urwin H, Josephs KA, Rohrer JD, Mackenzie IR, Neumann M, Authier A, et al. FUS pathology defines the majority of tau- and TDP-43 negative frontotemporal lobar degeneration. Acta Neuropathol. 2010;120:33–41. doi: 10.1007/s00401-010-0698-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance C, Roegelj B, Hortobagyi T, De Vos KJ, Nishimura AL, Sreedharan J, et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323:1208–11. doi: 10.1126/science.1165942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Es MA, Veldink JH, Saris CG, Blauw HM, van Vught PW, Birve A, et al. Genome-wide association study identifies 19p13.3 (UNC13A) and 9p21.2 as susceptibility loci for sporadic amyotrophic lateral sclerosis. Nat Genet. 2009;41:1083–7. doi: 10.1038/ng.442. [DOI] [PubMed] [Google Scholar]
- Warrington EK, James M. Visual Object and Space Perception Battery (VOSP) Oxford: Pearson Assessment; 1991. [Google Scholar]
- Wojciechowska M, Krzyzosiak WJ. Cellular toxicity of expanded RNA repeats: focus on RNA foci. Hum Mol Genet. 2011;20:3811–21. doi: 10.1093/hmg/ddr299. [DOI] [PMC free article] [PubMed] [Google Scholar]