Abstract
Failure to develop normal language comprehension is an early warning sign of autism, but the neural mechanisms underlying this signature deficit are unknown. This is because of an almost complete absence of functional studies of the autistic brain during early development. Using functional magnetic resonance imaging, we previously observed a trend for abnormally lateralized temporal responses to language (i.e. greater activation on the right, rather than the expected left) in a small sample (n = 12) of sleeping 2–3 year olds with autism in contrast to typically developing children, a finding also reported in autistic adults and adolescents. It was unclear, however, if findings of atypical laterality would be observed in a larger sample, and at even earlier ages in autism, such as around the first birthday. Answers to these questions would provide the foundation for understanding how neurofunctional defects of autism unfold, and provide a foundation for studies using patterns of brain activation as a functional early biomarker of autism. To begin to examine these issues, a prospective, cross-sectional design was used in which brain activity was measured in a large sample of toddlers (n = 80) during the presentation of a bedtime story during natural sleep. Forty toddlers with autism spectrum disorder and 40 typically developing toddlers ranging in age between 12–48 months participated. Any toddler with autism who participated in the imaging experiment prior to final diagnosis was tracked and diagnoses confirmed at a later age. Results indicated that at-risk toddlers later diagnosed as autistic display deficient left hemisphere response to speech sounds and have abnormally right-lateralized temporal cortex response to language; this defect worsens with age, becoming most severe in autistic 3- and 4-year-olds. Typically developing children show opposite developmental trends with a tendency towards greater temporal cortex response with increasing age and maintenance of left-lateralized activation with age. We have now demonstrated lateralized abnormalities of temporal cortex processing of language in autism across two separate samples, including a large sample of young infants who later are diagnosed with autism, suggesting that this pattern may reflect a fundamental early neural developmental pathology in autism.
Keywords: autism, language, functional magnetic resonance imaging, sleep, temporal cortex
Introduction
Early subclinical behavioural signs of autism appear in the first years of life (Palomo et al., 2006; Zwaigenbaum et al., 2009; Pierce et al., 2011) but the underlying brain dysfunction remains a mystery. This is because of a virtual absence of functional imaging studies of the autistic brain during this crucial developmental period.
One of the earliest appearing signs of autism is a failure to develop sophisticated expressive and receptive language. While most typical toddlers begin to say words around their first birthday (Fenson et al., 1994), some toddlers who will eventually develop autism miss or are slow to achieve this important milestone. In fact, delay in language is reported as the strongest and earliest red flag by most experts (De Giacomo and Fombonne, 1998; Wetherby et al., 2004). Later in development, children with autism often, but not always, display unusual patterns of language that may include the presence of neologisms [i.e. made up words (Volden and Lord, 1991)], speech that is highly repetitive, ritualized or echolalic (McEvoy et al., 1988) or characterized by high rates of pronoun reversals (Rapin and Dunn, 2003). Early studies on the topic found that comprehension of speech was more severely compromised in children with autism than those with global developmental delay (Bartak et al., 1975). Studies examining samples of individuals from the 1980s reported that ∼50% of the children with autism spectrum disorder have little to no functional language (Sigman and McGovern, 2005), although more recent studies suggest better language outcomes with at least 75% of the individuals acquiring some functional language (Luyster et al., 2008). Non-verbal social communication engagement, including the use of gestures such as pointing and waving, are reduced in autism (Loveland et al., 1988). Not surprisingly, the pragmatic use of language (e.g. conversational speech) is severely impaired in affected children (Loveland et al., 1988; Philofsky et al., 2007). Overall, brain systems that support language development must be abnormal in autism. Moreover, given the early emergence of language capacity in humans, with typical infants demonstrating the ability to discriminate phonemes within the first weeks of life (Eimas et al., 1971; Werker and Lalonde, 1988), recognize their own name at 4 months (Mandel et al., 1995) and discriminate familiar from unfamiliar words by 7 months (Tincoff and Jusczyk, 1999), this neural abnormality must begin very early in development in babies that will eventually show autism.
Integrity of temporal and frontal cortices is essential for normal language development, but in autistic infants, toddlers and young children these structures display pathological overgrowth (Carper et al., 2002; Carper and Courchesne, 2005; Schumann et al., 2010; Hazlett et al., 2011). For example, Carper and Courchesne (2002) showed that frontal and temporal cortices were ∼13% greater in volume in children with autism spectrum disorder relative to typically developing children, whereas other brain regions, such as occipital cortex, were not significantly enlarged. When brain systems directly engaged in language comprehension were challenged in studies of older autistic children, adolescents and adults, abnormal functional lateralization in frontal and temporal cortices has consistently been observed, including increased right hemisphere (Boddaert et al., 2004; Wang et al., 2006; Kleinhans et al., 2008; Mason et al., 2008; Tesink et al., 2009; Knaus et al., 2010) and decreased left hemisphere (Just et al., 2004; Gendry Meresse et al., 2005; Harris et al., 2006; Gaffrey et al., 2007; Groen et al., 2009) responsiveness. When differences in brain response between hemispheres are measured directly, individuals with autism generally display less functional lateralization (Muller et al., 1999; Kleinhans et al., 2008; Minagawa-Kawai et al., 2009; Anderson et al., 2010), or a tendency towards abnormal right hemisphere lateralization (Dawson et al., 1989; Bruneau et al., 1999; Flagg et al., 2005).
Whether or not such abnormal response and rightward functional lateralization occurs at the beginning stages of autism has never been tested. This is largely due to substantial practical and technical difficulties in measuring brain function in infants and toddlers with disabilities using functional MRI, a technique that traditionally requires subjects to remain still for extended periods of time. Fortunately, several recent studies have demonstrated that it is possible to obtain functional MRI data in response to language sounds from infants during natural sleep, thus eliminating the requirement for infants and toddlers to hold still (for review, see Pierce, 2011). Moreover, the ‘sleep functional MRI’ method has demonstrated responsiveness of superior temporal gyrus to auditory stimuli in non-autistic infants as young as 1 week to 3 months old (Anderson et al., 2001; Dehaene-Lambertz et al., 2002, 2006). More recently, our laboratory examined brain response to language stimuli during natural sleep in two age groups of typically developing children (mean ages of 21 and 39 months; Redcay et al., 2008). Both age groups showed superior temporal gyrus response to language stimuli, and the younger group's response included additional frontal, occipital and cerebellar sites of activation, demonstrating that expected cortical responses during language comprehension can be measured in very young typically developing children using functional MRI during natural sleep.
Sleep functional MRI provides many scientific as well as safety advantages for examining brain function in very young autistic and typically developing subjects including the ability to test subjects from all functioning levels. Despite its strengths, only one study has examined differences between very young children with autism and typically developing children using this technology. In response to a bedtime story during natural sleep, Redcay and Courchesne (2008) found reduced functional activity in temporal cortices in a small sample (n = 12) of 2–3 year olds children with autism compared with both chronological and mental age-matched groups and a non-statistically significant, but interesting trend towards abnormal rightward laterality of brain response to language stimuli in the autism group.
There are several natural questions that follow: can abnormal functional activation in response to language be detected even at the first signs of autism? Is abnormally right lateralized brain response present at that at-risk time-point or does it emerge gradually with age after full symptom onset? What is the developmental trajectory of normal left lateralization for language in the first several years of life in autism when the explosion in language abilities normally takes place in typical infants? Given the relatively late age of diagnosis, which remains around the age of 4 years nationally (Network, 2009), it has historically been challenging to identify and study toddlers with autism spectrum disorder prospectively before the onset of full-blown clinical symptoms. However, new advances in the early detection of autism, such as the 1-Year Well-Baby Check-Up Approach, have made it possible to study autism prospectively from the first year of life in the general population (Pierce et al., 2011).
Here, we report results of the first functional MRI study of autism in the earliest years of life that addresses these foundational questions. We analysed functional MRI activation to language stimuli, a baby’s bedtime story, measured during natural sleep from the largest sample collected to date of infants and toddlers at-risk for autism spectrum disorder whose diagnosis was confirmed at older ages, as well as from 3- to 4-year-old children with autism spectrum disorder. Functional MRI activation patterns were compared with those in typically developing infants, toddlers and very young children. We predicted abnormalities of functional response and lateralization at the earliest ages in autism spectrum disorder, with right instead of left hemisphere specialization, and a failure to develop normal left lateralization patterns across the years from infancy to young childhood.
Subjects and methods
Participants
As part of an ongoing study (www.autism-center.ucsd.edu) toddlers ranging in ages between 12 and 48 months were brought to a scanning facility on one or more nights. Toddlers at-risk for an autism spectrum disorder were obtained from one of two sources: general community referral (e.g. website or outside agency) and a population-based screening method called the 1-Year Well-Baby Check-Up Approach (Pierce et al., 2011). Using this method, toddlers at-risk for an autism spectrum disorder as young as 12 months were identified in paediatric offices with a broadband screening instrument, the Communication and Symbolic Behaviour Scales-Developmental Profile Infant Toddler Checklist (Wetherby et al., 2002), and were recruited and tracked every 6 months until their third birthday. This method thus allowed for the prospective study of autism beginning at 12 months. Typically developing controls were obtained from community referrals.
A total of 111 toddlers attempted participation in the scanning session and a total of 80 infants were included in final analyses (40 autism spectrum disorder and 40 typically developing). Infants and toddlers were included in the final sample if both a structural MRI scan and a functional MRI scan examining speech processing were acquired before the child awoke. A total of 12 participants with autism spectrum disorder and 10 typically developing participants woke during the functional scan before two-thirds of the time-points had been collected, so their data were not included in analyses. One typically developing participant was included who woke after completing more than two-thirds of the scan; all other participants slept through the entire scan. Data from an additional four participants were excluded for other imaging-related reasons [given an over-the-counter medication by the family (n = 1), given earplugs to aid in staying asleep (n = 1), primary language in the home was German (n = 1) and found to have a temporal lobe cyst (n = 1)].
A total of 21 participants were younger than 30 months (an age at which autism spectrum disorder diagnoses can be reliably made) at the time of scanning and were judged to be at-risk for autism spectrum disorder at that time. Five of these children were later judged to no longer warrant a spectrum diagnosis and were not included in the analyses [revised diagnoses were: typical (n = 2), language delayed (n = 1), developmental delay/borderline IQ (n = 1) and autistic features of insufficient severity to warrant a diagnosis (n = 1)]. Of the remaining 16 children judged at-risk prior to 30 months of age, all but one (who moved out of the area) have participated in at least one follow-up clinical evaluation 6 months to 1 year later [at a mean (SD) age of 31.9 (5.2) months], and all of these have had their provisional diagnoses confirmed by a psychologist (one currently has autism diagnostic features with diagnosis pending a second follow-up). Total Autism Diagnostic Observation Schedule-Toddler Module (ADOS-T) scores [social and communication plus repetitive scores; mean (SD)] at follow-up were 17.6 (5.0).
All toddlers participated in a series of psychometric tests across multiple 2-h sessions that included the Autism Diagnostic Observation Schedule-Toddler Module newly validated for use with infants as young as 12 months (Luyster et al., 2009), the Mullen Scales of Early Learning (Mullen, 1995) and the McArthur-Bates Communicative Development Inventories (Fenson et al., 1993). Toddlers participated in additional behavioural (e.g. examinations of play behaviour) and biological tests as part of a larger study. All standardized assessments were administered by three highly experienced doctoral-level psychologists with over 15 years combined experience in the field of autism.
Demographic and clinical summary data for the groups at the time of scanning are presented in Table 1. As expected, the typically developing group had significantly higher receptive language and visual T-scores and significantly lower Autism Diagnostic Observation Schedule scores than the autism spectrum disorder group. In addition, the typically developing group was significantly younger than the autism spectrum disorder group (P = 0.005); however, the difference in mean age was only 6 months. This study was approved by the University of California San Diego Institutional Review Board. Parents provided written informed consent according to the Declaration of Helsinki and were paid for their participation.
Table 1.
Participant characteristics
| Measure | Autism spectrum disorder |
Typically developing |
Group comparison |
|||
|---|---|---|---|---|---|---|
| n | Mean (SD), range or number (%) | n | Mean (SD), range or number (%) | Student's t (or χ2) | P-value | |
| Age (months) | 40 | 32.0 (12.5–47.6) | 40 | 25.6 (12.3–45.3) | −2.9 | 0.005 |
| Sex (no. of males) (%) | 40 | 29 (73) | 40 | 25 (63) | 0.91 | 0.34 |
| Receptive language age (months) | 37 | 20.1 (9.8) | 36 | 23.2 (8.9) | 1.39 | 0.17 |
| Receptive language T-score | 37 | 30.5 (11.9) | 36 | 51.2 (7.2) | 8.93 | 6.2 × 10−14 |
| Receptive visual age (months) | 37 | 27.1 (10.7) | 36 | 26.1 (11.7) | −0.39 | 0.70 |
| Receptive visual T-score | 37 | 40.9 (15.8) | 36 | 58.4 (9.3) | 5.78 | 1.6 × 10−7 |
| ADOS social and communication score | 40 | 14.2 (3.7) | 40 | 2.0 (1.7) | −18.8 | 1.1 × 10−30 |
| ADOS repetitive and restrictive | 40 | 3.5 (1.6) | 40 | 0.2 (0.4) | −13.3 | 9.4 × 10−22 |
| Number of words produced | 39 | 126.6 (153.0) | 39 | 206.6 (241.6) | 1.75 | 0.09 |
| Minutes asleep before task | 40 | 53.6 (22.3) | 40 | 51.4 (23.8) | −0.41 | 0.68 |
ADOS = Autism Diagnostic Observation Schedule; SD = standard deviation.
Stimuli and task design
The speech task was identical to that used in our previous pilot study (Redcay et al., 2008) and consisted of three types of stimuli, presented in 20-s blocks: complex forward speech, simple forward speech and backward speech. All speech conditions were created using the same female speaker. During the complex forward speech condition, toddlers were exposed to segments of a children's story that was written at a comprehension level of over 48 months. During the simple forward speech condition, toddlers were exposed to excerpts from a children's story written at a comprehension level between 12 and 36 months. Finally, during the backward speech condition, toddlers were exposed to the simple story segments played backwards. There were also 20-s rest blocks (no presented stimuli) between each stimulus type. Each stimulus type was repeated three times in a pseudorandom order. The total task length was 6 min 25 s. The stimuli were presented using commercially available music presentation software with maximum volume set both for the software and the computer's speakers. Stimulus presentation was through pneumatic headphones (Confon, Inc.) set to a volume attenuation of −40 dB for all participants.
Scanning procedure
Infants and toddlers were imaged in a 1.5 Tesla General Electric MRI scanner during natural sleep; no sedation was used. Parents were encouraged to forgo any usual naps by the child and engage the child in rigorous physical activity during the day. Families arrived at the scanning facility 1 h after the child's typical bedtime and most children had been asleep in the car for ∼15 min prior to arrival. If not already asleep or if awakened by placement on the scanner bed, the child was allowed to fall asleep in the waiting or scanning room and was placed on the scanner bed after ∼15 min of sleep. The time that the child fell asleep was recorded for every participant. After placement of the headphones, padding of the head for comfort and motion reduction and covering the child with a weighted blanket for warmth and motion reduction, the scan was started (∼15 min later). A research assistant was present in the room next to the child during the entire scan and stopped the scan if the child woke up or made a large movement.
The order of scans varied somewhat between individuals, but all participants first received a high-resolution, T1-weighted anatomical scan (repetition time = 6.5, flip angle = 12°, bandwidth = 31.25, field of view = 24 cm, in-plane resolution = 1 × 1 mm, slice thickness = 1.2 mm, 170 slices, scan length = 7 min 24 s) for localization of functional signals and warping into standard atlas space. On average, the speech task was presented 18.3 ± 13.3 min after the onset of scanning. Other functional and anatomical scans were acquired before and after the speech task; data from these will not be presented in this article. If the child remained asleep for the entire procedure, the total scan time was 1 h 15 min.
Blood oxygenation level-dependent signal was measured across the whole brain with echoplanar imaging during the language paradigm. Scan parameters were: echo time = 30 ms, repetition time = 2500 ms, flip angle = 90°, bandwidth = 70 kHz, field of view = 25.6 cm, in-plane resolution = 4 × 4 mm, slice thickness = 4 mm, 31 slices.
Data analyses
Analysis of Functional NeuroImages software was used to detect and correct for head motion. Head motion in the analysed participants, who slept through the entire scan (or, for one participant, more than two-thirds of the scan), was minimal: no participant was excluded for having visually apparent residual motion following motion correction on more than one-third of time-points. In addition, there were no group differences in the amount of motion as determined by t-tests comparing the average across the scan of the squared movement parameters in each in-plane direction and each axis of rotation (all P > 0.19). Analysis of Functional NeuroImages software was also used to carry out regression analyses in which we compared blood oxygenation level-dependent response between speech blocks and periods of no stimulation while controlling for linear trends and residual motion. Specifically, we examined the contrast between blood oxygenation level-dependent response during periods of both complex and simple forward speech and backward speech to the response during periods of non-stimulation (AllSpeech versus Rest). Individual subject activation maps were then spatially blurred with an 8-mm full-width at half-maximum Gaussian kernel and normalized to standard atlas space (Talairach and Tournoux, 1988) by rigid-body transformation based on manual placement of markers at the anterior and posterior commissures and at the extremes of the brain in each plane. We have found in previous investigations (Redcay et al., 2008) that group averaging following Talairach transformation is at least as accurate for toddlers with and without autism spectrum disorder as it is for adults. Group maps of significant language-related response were then created for the typical and autism spectrum disorder groups individually and for the direct comparison between them. For all whole-brain analyses, we retained clusters with an individual-voxel P < 0.01 (t ≥ 2.86 for within group; t ≥ 2.42 for a one-tailed between-group comparison) and a volume of at least 32 voxels (2048 µl). This threshold and cluster size combination protects a whole-brain rate of false-positive clusters less than P = 0.05 as determined by Monte–Carlo simulation. Whole-brain analyses were repeated with age or sleep latency as a covariate in the model using the Analysis of Functional NeuroImages program 3dttest ++. To examine the generality of the voxel-wise findings based on AllSpeech to each different speech condition, we separately compared the mean response to Simple Forward, Complex Forward and Backward speech between groups in the observed region of group difference. Furthermore, follow-up analyses of the relationship of age to the amplitude and extent of activation within the identified region of differential speech response were conducted. We tested for a group × age interaction and also calculated the within-group age correlations. Correlation analyses were also conducted for the clinical variables within the region of differential response to speech.
We were also interested in examining the laterality of response to speech stimuli within the superior temporal gyrus. We confined our analysis to voxels falling within the boundaries of the superior temporal gyrus as defined by the Talairach Daemon (Lancaster et al., 2000). For the region of interest analysis, we divided bilateral superior temporal gyrus into three sections [anterior (left: 279 voxels, right: 277 voxels), middle (left: 157 voxels, right: 156 voxels) and posterior (left: 219 voxels, right: 218 voxels)] with equal anterior–posterior extents. For each speech condition, we used a repeated-measures general linear model to test for main effects of group (a between-subjects factor), hemisphere (a within-subjects factor), region (a within-subjects factor) and two- and three-way interactions between them on the number of voxels that showed a positive response (t > 2.0). Follow-up analyses within each group examined the effects of hemisphere and region and their interaction. To examine how age was related to laterality of response, we added age to the model as a between-subject covariate for any condition that had shown hemisphere effects.
To further examine the localization of possible group laterality differences in the superior temporal gyrus and to replicate our previously published findings, we conducted a voxel-wise analysis in which the magnitude of response to forward speech in each voxel of the right superior temporal gyrus was subtracted from the value in the corresponding voxel in the left hemisphere to create a laterality map. These maps were then compared between the autism spectrum disorder and typically developing groups with a two-sample t-test. Clusters were considered to be significant if they were >32 voxels in volume and each voxel t-value was >1.66 (Pone-tailed < 0.05). This combination protected a superior temporal gyrus-wide probability of false positives of <0.05. Age and clinical correlations with mean activity in clusters of significant group effects were then examined.
Results
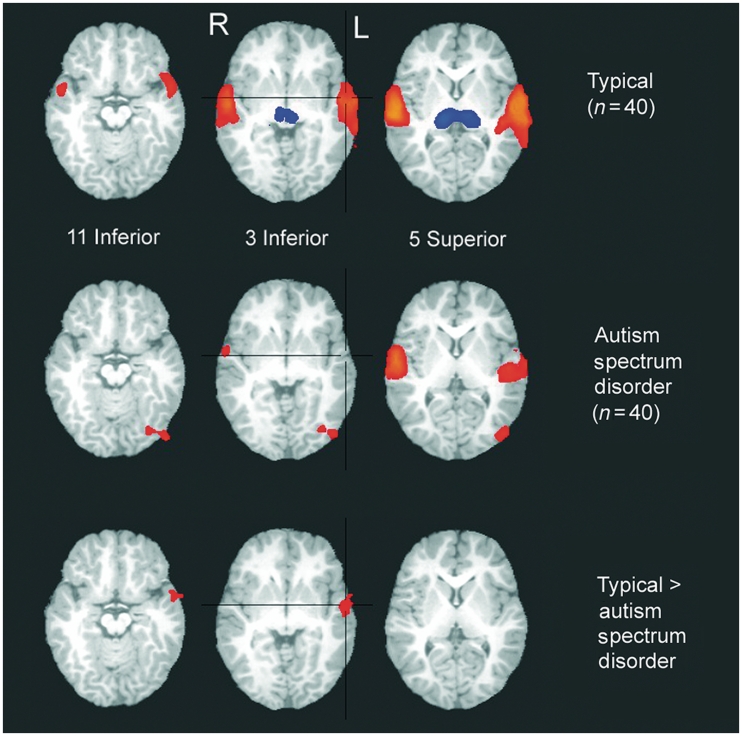
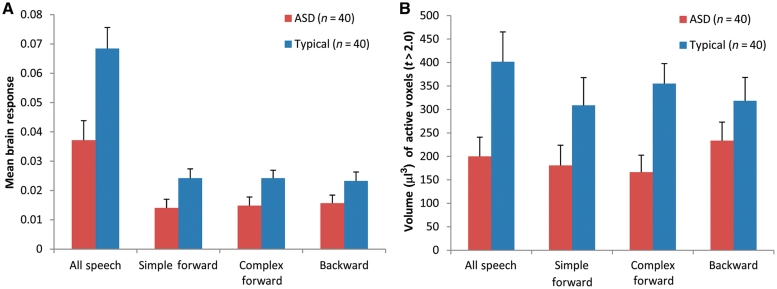
Among typically developing infants and toddlers, listening to storybook passages resulted in strong bilateral superior temporal gyrus activation, positive response in midline precuneus and deactivation (i.e. less response during speech than rest) in bilateral thalamus and midline cerebellum (Fig. 1 and Table 2). Infants and toddlers in the autism spectrum disorder group showed less extensive, but still significant, response in clusters within bilateral superior temporal gyrus and activation of a region within the right inferior occipital gyrus (Fig. 1 and Table 2). Upon direct comparison, a region of left superior temporal gyrus (in Brodmann's area 22) was significantly less responsive to speech stimuli in the group with autism spectrum disorder than the typical group (Fig.1 and Table 2), a difference that remained significant after controlling for age (Supplementary Fig. 1). Our initial analysis found no regions in which the autism spectrum disorder group had significantly greater responsiveness than the typically developing group, although the age-corrected analysis revealed that middle occipital gyrus response was greater in the group with autism spectrum disorder than the typically developing group (Supplementary Fig. 1). Within the region of group difference in the superior temporal gyrus, there was a significantly larger mean brain response in the typical than autism spectrum disorder group when examining simple and complex story passages separately [simple: t(78) = 2.12, Pone-tailed = 0.01; complex: t(78) = 3.05, Pone-tailed = 0.001] and when examining response to the backward passages [backward: t(78) = 2.06, Pone-tailed = 0.02; Fig. 2]. The extent of activation in this region, as indicated by the number of voxels with t > 2.0 (individual voxel P < 0.05), also was significantly different for the forward speech conditions [simple: t(78) = 1.75, Pone-tailed = 0.04; complex: t(78) = 1.83, Pone-tailed = 0.03] but only a trend for the backward speech condition [t(78) = 1.3, Pone-tailed = 0.09]. When we examined age-related changes in responsiveness of this region of differential activation, we found no significant age × group interactions for the amplitude [F (1,78) = 3.1, P = 0.08] or extent [F (1,78) = 1.1, P = 0.30]. However, brain response was significantly negatively correlated with age in the autism spectrum disorder group for both amplitude [r(38) = −0.32, P = 0.04] and extent [r(38) = −0.35, P = 0.03], suggesting that the deficient response in autism spectrum disorder does not normalize with increasing age. Neither the extent nor amplitude of response in this region of the superior temporal gyrus was significantly related to clinical severity in the autism spectrum disorder group (P's > 0.15) or to our measures of expressive and receptive language ability in either group (all P's > 0.09).
Figure 1.
Clusters of significant response to all speech stimuli in the typically developing (n = 40) and autism spectrum disorder (n = 40) groups, and clusters of significant difference in brain response between groups. Colours indicate relative strength and direction of the effect size (η2) [for individual group maps, positive values (warm colours) indicate greater brain response to speech compared with non-stimulation; and negative values (cool colours) indicate lower brain response to speech compared with non-stimulation in individual group maps; for group comparison map, positive values (warm colours) indicate greater brain response in the typically developing compared with autism spectrum disorder group]. R = right; L = left.
Table 2.
Significant clusters of brain response to speech in typically developing and autism spectrum disorder infants and toddlers and the difference between them
| Cluster | Location | Voxel count | Centre of mass coordinates | Peak coordinates | All speech mean effect size (η2) | Simple forward speech mean effect size (η2) | Complex forward speech mean effect size (η2) | Backward speech mean effect size (η2) |
|---|---|---|---|---|---|---|---|---|
| Typically developing | ||||||||
| 1 | Left superior temporal gyrus | 393 | 56L, 15P, 4S | 54L, 13P, 8S | 0.30 | 0.20 | 0.24 | 0.18 |
| 2 | Right superior temporal gyrus | 285 | 57R, 13P, 6S | 58R, 17P, 4S | 0.32 | 0.24 | 0.21 | 0.20 |
| 3 | Bilateral thalamus | 148 | 0R, 21P, 5S | 14R, 21P, 8S | −0.23a | −0.05 | −0.12 | −0.18 |
| 4 | Midline cerebellum | 110 | 3L, 58P, 31I | 6L, 61P, 28I | −0.22 | −0.08 | −0.08 | −0.17 |
| 5 | Midline precuneus | 58 | 1L, 82P, 41S | 6L, 61P, 40S | 0.22 | 0.14 | 0.15 | 0.11 |
| Autism spectrum disorder | ||||||||
| 1 | Right superior temporal gyrus | 181 | 56R, 14P, 10S | 58R, 5P, 8S | 0.29 | 0.22 | 0.17 | 0.17 |
| 2 | Left superior temporal gyrus | 131 | 54L, 18P, 9S | 66L, 17P, 8S | 0.23 | 0.17 | 0.12 | 0.18 |
| 3 | Left inferior occipital gyrus | 52 | 40L, 75P, 2I | 46L, 81P, 12I | 0.19 | 0.16 | 0.08 | 0.10 |
| Typically developing > autism spectrum disorder | ||||||||
| 1 | Left superior temporal gyrus | 40 | 55L, 1A, 6I | 54L, 5P, 4I | 0.09 | 0.03 | 0.11 | 0.04 |
a η2 values are signed to indicate direction of the effect. Negative values indicate negative response (i.e. less response during speech than during rest).
A = anterior; I = inferior; L = left, P = posterior; R = right, S = superior.
Figure 2.
Graphs of amplitude (mean fit coefficient) (A) and extent (volume in µl of active voxels with t > 2.0) (B) of brain response within the cluster of significant group difference in brain response to all speech in the left hemisphere superior temporal gyrus. Values are shown for all speech (used to identify the significant cluster) and separately for the component conditions. Mean values for the autism spectrum disorder (ASD) group are shown in red and those for the typically developing group are shown in blue. Error bars indicate the standard error of the mean.
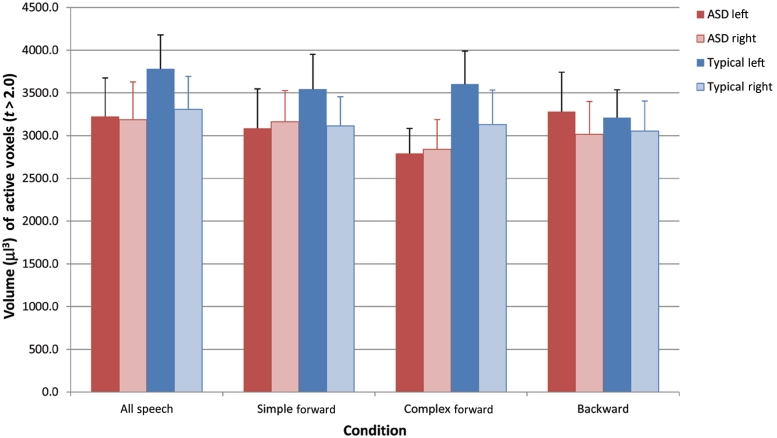
Next, we tested the degree to which responses were lateralized in each group and examined potential laterality differences between autism spectrum disorder and typical groups within three anatomically defined portions of the superior temporal gyrus in each hemisphere. There were no main effects of group, hemisphere or region for any of the conditions, and none of the interactions with region was significant. Thus, we summed across the three superior temporal gyrus regions to examine laterality effects. Figure 3 presents the means of the two groups for extent of activation (volume of voxels in microlitres with t > 2.0) for all speech and for each individual condition in the entire superior temporal gyrus. There was a trend towards an interaction between group and hemisphere for response to complex [F(1,78) = 2.5, P = 0.06] speech, but differential laterality effects were not significant for all speech [F(1,78) = 1.44, P = 0.15], simple forward speech [F(1,78) = 1.65, P = 0.10] or backward speech [F(1,78) = 0.08, P = 0.77]. In each condition, there was no evidence of laterality of total superior temporal gyrus response within the autism spectrum disorder group (all P's for within-group hemisphere effect >0.4). In contrast, there was evidence for leftward laterality in the typically developing group in all but the backward condition [within-group hemisphere effects: all speech: F(1,39) = 5.3, P = 0.03; simple forward: F(1,39) = 3.9, P = 0.05; complex forward: F(1,39) = 3.8, P = 0.06; backward: F(1,39) = 0.54, P = 0.5].
Figure 3.
Graph of extent (volume in µl of active voxels with t > 2.0) of brain response within each hemisphere of the entire superior temporal gyrus anatomical region of interest. Values are shown for all speech and separately for the component conditions. Mean values for the autism spectrum disorder (ASD) group are shown in red for left hemisphere and pink for right hemisphere, and those for the typically developing group are shown in blue for the left hemisphere and light blue for the right hemisphere. Error bars indicate the standard error of the mean.
To examine the relationship of age to lateralized brain response patterns, we examined the effects of age, hemisphere and group and their interactions on volume of activation summed across the three superior temporal gyrus subregions (given the lack of regional effects) for response to all speech and simple and complex forward speech (the contrasts that showed hemispheric effects). There were no significant main effects of age or group × age × hemisphere interactions. For both hemispheres, there were group × age interactions for all speech [left: F(1,76) = 3.16, P = 0.04; right: F(1,76) = 3.56, P = 0.03] and simple forward speech [left: F(1,76) = 4.09, P = 0.02; right: F(1,76) = 3.35, P = 0.03], but not for complex speech [left: F(1,76) = 0.05, P = 0.82; right: F(1,76) = 0.47, P = 0.50]. Table 3 shows the correlations between age and extent of response in each hemisphere of the entire superior temporal gyrus during all speech and simple and complex forward speech. It can be seen that the group × age interactions are driven by opposite developmental trends in the two groups—small positive relationships with age in the typically developing group and negative relationships with age in the autism spectrum disorder group. Extent of response to all speech and simple and complex forward speech in left and right superior temporal gyrus was not significantly related to clinical severity in the autism spectrum disorder group (all P's > 0.25) or to our measures of expressive and receptive language ability in either group (all P's > 0.14).
Table 3.
Relationship of age and extent of hemispheric brain response in all speech and simple and complex forward speech conditions in the entire superior temporal gyrus region of interest
| Measure of extent of response | Group |
|||
|---|---|---|---|---|
| Autism spectrum disorder (n = 40) | Typically developing (n = 40) | |||
| Response to all speech | r | P | r | P |
| Left | −0.20 | 0.22 | 0.20 | 0.20 |
| Right | −0.26 | 0.11 | 0.17 | 0.31 |
| Response to simple forward speech | ||||
| Left | −0.32 | 0.04 | 0.12 | 0.47 |
| Right | −0.28 | 0.08 | 0.13 | 0.41 |
| Response to complex forward speech | ||||
| Left | −0.05 | 0.77 | 0.01 | 0.95 |
| Right | −0.13 | 0.44 | 0.04 | 0.81 |
The bold values indicate the one significant correlation (and its P-value).
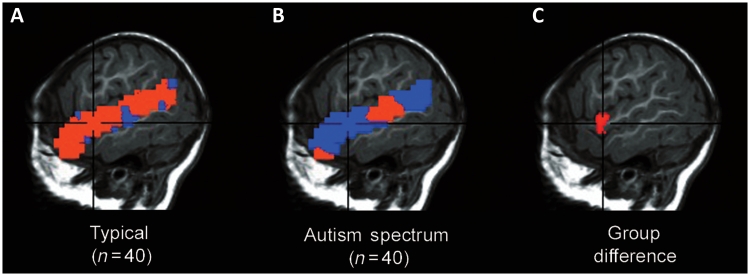
To explore the precise localization of group laterality differences and to replicate previously published trends (Redcay and Courchesne, 2008), we undertook a voxel-based analysis in which we directly subtracted response levels between homologous left and right hemisphere voxels during the forward speech condition (Fig. 4). The typical group showed significantly greater left-lateralized magnitude of response compared with the autism spectrum disorder group within a region of anterior superior temporal gyrus [36 voxels (each with t = 1.66, Pone-tailed = 0.05), with a peak at 50L, 5P, 4S (Brodmann's area 22)]. Similar to the anatomical region of interest-based analyses, there was a significant age × group interaction for left hemisphere extent of response to forward speech [F(1,76) = 2.98, P = 0.04] in this region of differential laterality. Specifically, left hemisphere responsiveness in this region was not related to age in the typical group [r(38) = −0.01, P = 0.96], but was negatively related to age in the autism spectrum disorder group [r(38) = −0.45, P = 0.003]. In the right hemisphere, the direction of the interaction was similar but not significant [F(1,76) = 1.1, P = 0.15]. We did not find any relationship of the measured clinical variables with speech response in these regions (all P's > 0.08).
Figure 4.
(A and B) Voxel-based within-group laterality score maps during the forward speech condition (expressed as signed η2 effect size values; warm colours = left greater than right; cool colours = right greater than left) for typically developing and autism spectrum disorder groups in the superior temporal gyrus (shown at 51 left/right). (C) Group difference map showing the cluster of significant difference in laterality between groups, with more left-lateralized values in the typically developing compared with autism spectrum disorder group [36 voxels (each with t = 1.66, Pone-tailed = 0.05), superior temporal gyrus-wide P < 0.05, with a peak at 50 left, 5 posterior and 4 superior].
Discussion
In response to a simple bedtime story, sleep functional MRI revealed that infants and toddlers with autism display abnormally reduced left temporal cortex activity and that this difference from normal grows more pronounced with age. Furthermore, whereas typical toddlers show the expected pattern of left side dominance in response to stories, toddlers with autism exhibit reversed or absent laterality patterns. Specifically, toddlers with autism display stronger activation on the right relative to the left in the anterior portion of the superior temporal gyrus, the brain region most strongly responsive to language sounds in typically developing toddlers. This abnormal lateralization is consistent with studies of older autistic children and adults (Dawson et al., 1989; Bruneau et al., 1999; Muller et al., 1999; Flagg et al., 2005; Kleinhans et al., 2008; Minagawa-Kawai et al., 2009; Anderson et al., 2010). Thus, one of the earliest appearing signature deficits in autism, failure to develop normal language comprehension, may be due to very early defects in the superior temporal gyrus and these defects may last across the lifetime.
We hypothesize that a failure of left hemisphere specialization during language comprehension in early life may not only delay basic language acquisition in infants and toddlers with autism, but may also impair the development of social language behaviours, such as analysis of prosodic information, which is typically mediated by right hemisphere neural processes (Friederici and Alter, 2004). In our study, the right hemisphere, relative to the left, was disproportionately involved in processing speech sounds in toddlers with autism spectrum disorder. One possibility is that the right superior temporal gyrus is compensating for ineffectual processing by the left superior temporal gyrus, thus crowding out the development of more social language abilities that would normally require activity of that hemisphere. Developmental neuroscientists have long known that language and social development are inextricably linked. Consider the elegant study by Kuhl and colleagues (2003) that revealed that American babies only learned Mandarin phonemes within the context of natural play interactions with a Chinese Mandarin speaker. Babies performed at chance levels when exposed to the identical linguistic information delivered via television or audio tape alone.
Why is the left superior temporal gyrus hypoactive in autism to begin with and why does it fail to show a steady increase in activity across development (in fact, showing less activity with age)? One possibility may relate to a wider problem of a failure of the superior temporal gyrus to develop effective functional connections during early development. Successful function of brain systems that support sophisticated language depend on the initial coordinated activity of widespread networks. For example, between 6 and 12 months, Imada and colleagues (2006) showed that infants recruit both traditional auditory areas in superior temporal gyrus as well motor areas in the frontal lobes in response to auditory signals. Slightly later in development, Redcay and colleagues (2008) showed that in response to bedtime stories, typical 2- and 3-year olds recruited multiple brain areas in frontal, occipital, cerebellar as well as temporal cortices. In the current study, the typically developing group engaged parietal, subcortical and cerebellar regions in addition to the strong response within the superior temporal gyrus. In autism, functional connectivity studies generally report highly aberrant patterns of functional connectivity usually in the form of underconnectivity (Just et al., 2004; Pierce and Eyler, 2011), although sometimes overconnectivity (Muller et al., 2011). These studies, however, have almost exclusively been conducted with older children, adolescents and adults with the disorder. Connectivity studies during the toddler years in autism are almost non-existent, with the single exception of a study examining inter-hemispheric correlation patterns between brain areas key to language development, such as the superior temporal gyrus and inferior frontal gyrus, in toddlers with autism (Dinstein et al., 2011). Results from that study showed reduced correlations between right and left superior temporal gyrus and inferior frontal gyrus in 2-year olds with autism relative to typically developing as well as language delay contrast groups. The current study demonstrated negative correlations of superior temporal gyrus activations with age in the autism spectrum disorder group, which may suggest that early connectivity problems lead to further failure of functional specialization during early development. One key to answering how frontal and temporal systems connect with each other and mature in autism would be to perform diffusion tensor imaging as well on subjects and examine the cross relationship between diffusion tensor imaging and functional activation results, which will be a future line of inquiry. The relatively anterior location of the region of abnormal brain response and laterality within the superior temporal gyrus among the autism spectrum disorder group may suggest particular connectivity issues in the ventral pathway from Broca's area to superior temporal gyrus via the extreme capsule fibre system (Brauer et al., 2010). This pathway has been shown to be involved in sentence processing, along with more immature dorsal connections to posterior superior temporal gyrus via the arcuate fasciculus, in typically developing 7-year-olds (Brauer et al., 2010). In adults, the ventral pathway to anterior superior temporal gyrus seems to subserve processing of local syntax whereas the dorsal pathway to posterior superior temporal gyrus is more involved in hierarchical syntactic processing. At the young ages we tested, this functional specialization has not yet developed (Brauer et al., 2010), but the disruption of response and lateralization of anterior regions in autism spectrum disorder early in life may lead to later syntactic problems and aberrant functional organization of the superior temporal gyrus.
Our study also adds to a body of literature on typical brain function documenting left lateralization in response to language very early in development (Dehaene-Lambertz et al., 2002, 2006; Dehaene-Lambertz and Gliga, 2004). The early emergence of left lateralization in response to language has led to the idea that left lateralization in response to language has a very strong genetic component (Dehaene-Lambertz et al., 2006). Interestingly, as compared with the right, the left side of the brain has enlarged white matter underlying Heschl's gyrus (Penhune et al., 1996), increased area measures (Lyttelton et al., 2009), larger pyramidal neurons (Hutsler, 2003), increased contact by afferent fibres (Seldon, 1981), increased width of cortical columns (Seldon, 1981), longer sylvian fissure (Geschwind and Levitsky, 1968) and thicker myelinated fibres (Anderson et al., 1999). Most of what is known regarding left–right structural asymmetries, however, has been gleaned from studies using adults or older children and it is unclear whether these anatomic asymmetries are the cause of language abilities in our species or only the consequence of heavy exposure to the particular acoustic properties of speech (Dehaene-Lambertz et al., 2006; Price, 2010). However, some regions of temporal cortex, such as the left planum temporale, appear to be enlarged even in foetuses and infants (Witelson and Pallie, 1973; Chi et al., 1977). Development of functional lateralization of speech processing has also been examined using functional near-infrared spectroscopy in awake typically developing infants and toddlers. Our sleep functional MRI findings are consistent with near-infrared spectroscopy results showing that activation of temporal cortex is reliably observed in response to hearing language stimuli (Rossi et al., 2011) and showing evidence of early emergence of leftward lateralization, at least for natural speech (Pena et al., 2003; Kotilahti et al., 2010). Rightward lateralization of functional near-infrared spectroscopy response to prosodic versus flattened stimuli (Homae et al., 2007) has also been shown at 10 months of age, but our stimuli were not designed to isolate prosody from other types of speech processing.
Interestingly, there were no statistically significant relationships found between the volume or strength of activation in the left superior temporal gyrus and language ability or symptom severity in toddlers with autism spectrum disorder. This could be due to some instability of language test scores for the youngest toddlers in our study, which were in some cases as young as 12 months in age. Clearer relationships may emerge based on language and symptom profiles at slightly older ages (e.g. 3 years old) as we have found in other studies from our laboratory (Redcay and Courchesne, 2008; Dinstein et al., 2011). The relationship between early functional brain profiles ascertained between 12 and 18 months and outcome at 3–4 years in age will be examined in follow-up studies.
Language development most certainly involves a complex interplay between environmentally driven and genetic factors (Quartz and Sejnowski, 1997; Kuhl, 2010) and disentangling contributions of each has puzzled scientists for centuries. Considering the influence of environment, studies have shown that exposure to language in the first year of life impacts the brain's neural circuitry (Kuhl, 2010 for review). For example, while babies are born with a universal ability to detect phoneme contrasts from all the world's languages (Eimas et al., 1971), by the time they reach 6–10 months in age, they become increasingly more skilled in discriminating phonemes from their native language environment (i.e. what they hear most frequently) and lose the ability to discriminate sounds from other cultures (Kuhl et al., 2006). This critical period, which peaks around 9-months in age, is within a time window before parents become clearly aware of their child's autism diagnosis, which typically is confirmed sometime around the child's fourth birthday in the USA (Prevention, 2009). As such, the early language environment between birth and the first birthday for babies destined to develop autism, in contrast to those destined for a typical trajectory, are likely similar. Yet, by the first birthday, our study demonstrates that the brain region highly engaged in language processing, the superior temporal gyrus, is under-responsive in toddlers with autism spectrum disorder and shows reversed laterality. Why is this the case? Although adverse environmental influences that may be occurring selectively in this group during this time period cannot be ruled out, our results of very early superior temporal gyrus abnormality in autism, at face value, suggest a strong genetic component. While the genetics of language development are far from clear, studies have indeed demonstrated that genes related to language development such as contactin-associated protein-like 2 (CNTNAP2), are dysregulated in autism (Alarcon et al., 2008; Scott-Van Zeeland et al., 2010). Ongoing studies in our laboratory aim to elucidate the pathways from genes to aberrant functional lateralization in autism spectrum disorder.
Some potential limitations to this study should be noted. First, although this is the largest functional MRI study of very young children with autism spectrum disorder to date, it is still possible that we failed to detect small differences between groups or complicated developmental interactions. In addition, the cross-sectional nature of the presented imaging data limits our ability to make inferences about within-person trajectories of change, although our longitudinal measurement of clinical status is a strength of the design. In the present analysis, we were not able to test the specificity of our findings to those who eventually develop autism compared with those who go on to experience other developmental delays. In our ongoing study, however, we are collecting imaging data from relevant comparison groups and are also conducting follow-up functional scans for future longitudinal analyses. Finally, although our functional MRI results from sleeping babies are similar to those seen in awake typical children using other methods, we have limited information about the sleep stage of the participants in the study. Sleep latency was not different between the groups and did not impact the findings of group differences, however.
In sum, the cortical functional lateralization abnormality observed in this study appears to be early and invariant and may reflect a core, fundamental neurodevelopmental pathology in autism. As such, these aberrant response patterns may serve as the platform for the first neurofunctional biomarker of autism. Given the very early emergence of these functional deficits, our data highlight the importance of very early detection. In this way, affected infants stand the best chance to correct and refine functional defects before they become a permanent hindrance to actualizing their full cognitive and emotional potential.
Funding
National Institutes of Health (R01-MH036840 to E.C., P50-MH081755 to E.C., R01-MH080134 to K.P.).
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
We would like to thank the Autism Center of Excellence brain imaging team: S. Solso, C. Ahrens-Barbeau, K. Campbell, R. Stoner, J. Young, and R. Znamirowski, and the clinical and administrative team: C. Carter, S. Spendlove, M. Weinfeld, J. Desmond, R. Hazin, N. Gallagher, and L. Lopez. We also wish to thank E. Redcay for task development and seminal work with this paradigm. We would especially like to thank the families of the children who participated in the study.
Glossary
Abbreviation
- ADOS
autism diagnostic observation schedule
- ASD
austism spectrum disorder
References
- Alarcon M, Abrahams BS, Stone JL, Duvall JA, Perederiy JV, Bomar JM, et al. Linkage, association, and gene-expression analyses identify CNTNAP2 as an autism-susceptibility gene. Am J Hum Genet. 2008;82:150–9. doi: 10.1016/j.ajhg.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JS, Lange N, Froehlich A, DuBray MB, Druzgal TJ, Froimowitz MP, et al. Decreased left posterior insular activity during auditory language in autism. AJNR Am J Neuroradiol. 2010;31:131–9. doi: 10.3174/ajnr.A1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson AW, Marois R, Colson ER, Peterson BS, Duncan CC, Ehrenkranz RA, et al. Neonatal auditory activation detected by functional magnetic resonance imaging. Magn Reson Imaging. 2001;19:1–5. doi: 10.1016/s0730-725x(00)00231-9. [DOI] [PubMed] [Google Scholar]
- Anderson B, Southern BD, Powers RE. Anatomic asymmetries of the posterior superior temporal lobes: a postmortem study. Neuropsychiatry Neuropsychol Behav Neurol. 1999;12:247–54. [PubMed] [Google Scholar]
- Bartak L, Rutter M, Cox A. A comparative study of infantile autism and specific development receptive language disorder. I. The children. Br J Psychiatry. 1975;126:127–45. doi: 10.1192/bjp.126.2.127. [DOI] [PubMed] [Google Scholar]
- Boddaert N, Chabane N, Belin P, Bourgeois M, Royer V, Barthelemy C, et al. Perception of complex sounds in autism: abnormal auditory cortical processing in children. Am J Psychiatry. 2004;161:2117–20. doi: 10.1176/appi.ajp.161.11.2117. [DOI] [PubMed] [Google Scholar]
- Brauer J, Anwander A, Friederici AD. Neuroanatomical prerequisites for language functions in the maturing brain. Cereb Cortex. 2010;21:459–66. doi: 10.1093/cercor/bhq108. [DOI] [PubMed] [Google Scholar]
- Bruneau N, Roux S, Adrien JL, Barthelemy C. Auditory associative cortex dysfunction in children with autism: evidence from late auditory evoked potentials (N1 wave-T complex) Clin Neurophysiol. 1999;110:1927–34. doi: 10.1016/s1388-2457(99)00149-2. [DOI] [PubMed] [Google Scholar]
- Carper RA, Courchesne E. Localized enlargement of the frontal cortex in early autism. Biol Psychiatry. 2005;57:126–33. doi: 10.1016/j.biopsych.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Carper RA, Moses P, Tigue ZD, Courchesne E. Cerebral lobes in autism: early hyperplasia and abnormal age effects. Neuroimage. 2002;16:1038–51. doi: 10.1006/nimg.2002.1099. [DOI] [PubMed] [Google Scholar]
- Chi JG, Dooling EC, Gilles FH. Left-right asymmetries of the temporal speech areas of the human fetus. Archives of Neurology. 1977;34:346–8. doi: 10.1001/archneur.1977.00500180040008. [DOI] [PubMed] [Google Scholar]
- Dawson G, Finley C, Phillips S, Lewy A. A comparison of hemispheric asymmetries in speech-related brain potentials of autistic and dysphasic children. Brain Lang. 1989;37:26–41. doi: 10.1016/0093-934x(89)90099-0. [DOI] [PubMed] [Google Scholar]
- De Giacomo A, Fombonne E. Parental recognition of developmental abnormalities in autism. Eur Child Adolesc Psychiatry. 1998;7:131–6. doi: 10.1007/s007870050058. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Dehaene S, Hertz-Pannier L. Functional neuroimaging of speech perception in infants. Science. 2002;298:2013–5. doi: 10.1126/science.1077066. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Gliga T. Common neural basis for phoneme processing in infants and adults. J Cogn Neurosci. 2004;16:1375–87. doi: 10.1162/0898929042304714. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Hertz-Pannier L, Dubois J. Nature and nurture in language acquisition: anatomical and functional brain-imaging studies in infants. Trends Neurosci. 2006;29:367–73. doi: 10.1016/j.tins.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Hertz-Pannier L, Dubois J, Meriaux S, Roche A, Sigman M, et al. Functional organization of perisylvian activation during presentation of sentences in preverbal infants. Proc Natl Acad Sci USA. 2006;103:14240–5. doi: 10.1073/pnas.0606302103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinstein I, Pierce K, Eyler L, Solso S, Malach R, Behrmann M, et al. Disrupted neural synchronization in toddlers with autism. Neuron. 2011;70:1218–25. doi: 10.1016/j.neuron.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimas PD, Siqueland ER, Jusczyk P, Vigorito J. Speech perception in infants. Science. 1971;171:303–6. doi: 10.1126/science.171.3968.303. [DOI] [PubMed] [Google Scholar]
- Fenson L, Dale PS, Reznick JS, Bates E, Thal DJ, Pethick SJ. Variability in early communicative development. Monogr Soc Res Child Dev. 1994;59:1–173. discussion 4–85. [PubMed] [Google Scholar]
- Fenson L, Dale PS, Reznick JS, Thal D, Bates E, Hartung JP, et al. The MacArthur communicative development inventories: user's guide and technical manual. Baltimore. Paul H. Brokes Publishing Co. 1993 [Google Scholar]
- Flagg EJ, Cardy JE, Roberts W, Roberts TP. Language lateralization development in children with autism: insights from the late field magnetoencephalogram. Neurosci Lett. 2005;386:82–7. doi: 10.1016/j.neulet.2005.05.037. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Alter K. Lateralization of auditory language functions: a dynamic dual pathway model. Brain Lang. 2004;89:267–76. doi: 10.1016/S0093-934X(03)00351-1. [DOI] [PubMed] [Google Scholar]
- Gaffrey MS, Kleinhans NM, Haist F, Akshoomoff N, Campbell A, Courchesne E, et al. Atypical [corrected] participation of visual cortex during word processing in autism: an fMRI study of semantic decision. Neuropsychologia. 2007;45:1672–84. doi: 10.1016/j.neuropsychologia.2007.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendry Meresse I, Zilbovicius M, Boddaert N, Robel L, Philippe A, Sfaello I, et al. Autism severity and temporal lobe functional abnormalities. Ann Neurol. 2005;58:466–9. doi: 10.1002/ana.20597. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Levitsky W. Human brain: left-right asymmetries in temporal speech region. Science. 1968;161:186–7. doi: 10.1126/science.161.3837.186. [DOI] [PubMed] [Google Scholar]
- Groen WB, van Orsouw L, Huurne N, Swinkels S, van der Gaag RJ, Buitelaar JK, et al. Intact spectral but abnormal temporal processing of auditory stimuli in autism. J Autism Dev Disord. 2009;39:742–50. doi: 10.1007/s10803-008-0682-3. [DOI] [PubMed] [Google Scholar]
- Harris GJ, Chabris CF, Clark J, Urban T, Aharon I, Steele S, et al. Brain activation during semantic processing in autism spectrum disorders via functional magnetic resonance imaging. Brain Cogn. 2006;61:54–68. doi: 10.1016/j.bandc.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Hazlett HC, Poe MD, Gerig G, Styner M, Chappell C, Smith RG, et al. Early brain overgrowth in autism associated with an increase in cortical surface area before age 2 years. Arch Gen Psychiatry. 2011;68:467–76. doi: 10.1001/archgenpsychiatry.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homae F, Watanabe H, Nakano T, Taga G. Prosodic processing in the developing brain. Neurosci Res. 2007;59:29–39. doi: 10.1016/j.neures.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Hutsler JJ. The specialized structure of human language cortex: pyramidal cell size asymmetries within auditory and language-associated regions of the temporal lobes. Brain Lang. 2003;86:226–42. doi: 10.1016/s0093-934x(02)00531-x. [DOI] [PubMed] [Google Scholar]
- Imada T, Zhang Y, Cheour M, Taulu S, Ahonen A, Kuhl PK. Infant speech perception activates Broca's area: a developmental magnetoencephalography study. Neuroreport. 2006;17:957–62. doi: 10.1097/01.wnr.0000223387.51704.89. [DOI] [PubMed] [Google Scholar]
- Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004;127:1811–21. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- Kleinhans NM, Muller RA, Cohen DN, Courchesne E. Atypical functional lateralization of language in autism spectrum disorders. Brain Res. 2008;1221:115–25. doi: 10.1016/j.brainres.2008.04.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus TA, Silver AM, Kennedy M, Lindgren KA, Dominick KC, Siegel J, et al. Language laterality in autism spectrum disorder and typical controls: a functional, volumetric, and diffusion tensor MRI study. Brain Lang. 2010;112:113–20. doi: 10.1016/j.bandl.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotilahti K, Nissila I, Nasi T, Lipiainen L, Noponen T, Merilainen P, et al. Hemodynamic responses to speech and music in newborn infants. Hum Brain Mapp. 2010;31:595–603. doi: 10.1002/hbm.20890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl PK. Brain mechanisms in early language acquisition. Neuron. 2010;67:713–27. doi: 10.1016/j.neuron.2010.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl PK, Tsao FM, Liu HM. Foreign-language experience in infancy: effects of short-term exposure and social interaction on phonetic learning. Proc Natl Acad Sci USA. 2003;100:9096–101. doi: 10.1073/pnas.1532872100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl PK, Stevens E, Hayashi A, Deguchi T, Kiritani S, Iverson P. Infants show a facilitation effect for native language phonetic perception between 6 and 12 months. Dev Sci. 2006;9:F13–21. doi: 10.1111/j.1467-7687.2006.00468.x. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–31. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveland KA, Landry SH, Hughes SO, Hall SK, McEvoy RE. Speech acts and the pragmatic deficits of autism. J Speech Hear Res. 1988;31:593–604. doi: 10.1044/jshr.3104.593. [DOI] [PubMed] [Google Scholar]
- Luyster R, Gotham K, Guthrie W, Coffing M, Petrak R, Pierce K, et al. The autism diagnostic observation schedule-toddler module: a new module of a standardized diagnostic measure for autism spectrum disorders. J Autism Dev Disord. 2009;39:1305–20. doi: 10.1007/s10803-009-0746-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyster RJ, Kadlec MB, Carter A, Tager-Flusberg H. Language assessment and development in toddlers with autism spectrum disorders. J Autism Dev Disord. 2008;38:1426–38. doi: 10.1007/s10803-007-0510-1. [DOI] [PubMed] [Google Scholar]
- Lyttelton OC, Karama S, Ad-Dab'bagh Y, Zatorre RJ, Carbonell F, Worsley K, et al. Positional and surface area asymmetry of the human cerebral cortex. Neuroimage. 2009;46:895–903. doi: 10.1016/j.neuroimage.2009.03.063. [DOI] [PubMed] [Google Scholar]
- Mandel DR, Jusczyk PW, Pisoni D. Infants' recognition of the sound patterns of their own names. Psychological Science. 1995;6:314–7. doi: 10.1111/j.1467-9280.1995.tb00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason RA, Williams DL, Kana RK, Minshew N, Just MA. Theory of mind disruption and recruitment of the right hemisphere during narrative comprehension in autism. Neuropsychologia. 2008;46:269–80. doi: 10.1016/j.neuropsychologia.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEvoy RE, Loveland KA, Landry SH. The functions of immediate echolalia in autistic children: a developmental perspective. J Autism Dev Disord. 1988;18:657–68. doi: 10.1007/BF02211883. [DOI] [PubMed] [Google Scholar]
- Minagawa-Kawai Y, Naoi N, Kikuchi N, Yamamoto J, Nakamura K, Kojima S. Cerebral laterality for phonemic and prosodic cue decoding in children with autism. Neuroreport. 2009;20:1219–24. doi: 10.1097/WNR.0b013e32832fa65f. [DOI] [PubMed] [Google Scholar]
- Mullen EM. Circle Pine, MN: American Guidance Service, Inc; 1995. Mullen scales of early learning. [Google Scholar]
- Muller RA, Behen ME, Rothermel RD, Chugani DC, Muzik O, Mangner TJ, et al. Brain mapping of language and auditory perception in high-functioning autistic adults: a PET study. J Autism Dev Disord. 1999;29:19–31. doi: 10.1023/a:1025914515203. [DOI] [PubMed] [Google Scholar]
- Muller RA, Shih P, Keehn B, Deyoe JR, Leyden KM, Shukla DK. Underconnected, but How? A Survey of functional connectivity MRI studies in autism spectrum disorders. Cereb Cortex. 2011 doi: 10.1093/cercor/bhq296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Network AaDDM. Prevalence of autism spectrum disorders - autism and developmental disabilities monitoring network, United States, 2006. MMWR Surveill Summ. 2009;58:1–20. [PubMed] [Google Scholar]
- Palomo R, Belinchon M, Ozonoff S. Autism and family home movies: a comprehensive review. J Dev Behav Pediatr. 2006;27:S59–68. doi: 10.1097/00004703-200604002-00003. [DOI] [PubMed] [Google Scholar]
- Pena M, Maki A, Kovacic D, Dehaene-Lambertz G, Koizumi H, Bouquet F, et al. Sounds and silence: an optical topography study of language recognition at birth. Proc Natl Acad Sci USA. 2003;100:11702–5. doi: 10.1073/pnas.1934290100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penhune VB, Zatorre RJ, MacDonald JD, Evans AC. Interhemispheric anatomical differences in human primary auditory cortex: probabilistic mapping and volume measurement from magnetic resonance scans. Cereb Cortex. 1996;6:661–72. doi: 10.1093/cercor/6.5.661. [DOI] [PubMed] [Google Scholar]
- Philofsky A, Fidler DJ, Hepburn S. Pragmatic language profiles of school-age children with autism spectrum disorders and Williams syndrome. Am J Speech Lang Pathol. 2007;16:368–80. doi: 10.1044/1058-0360(2007/040). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce K. Early functional brain development in autism and the promise of sleep fMRI. Brain Res. 2011;1380:162–74. doi: 10.1016/j.brainres.2010.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce K, Carter C, Weinfeld M, Desmond J, Hazin R, Bjork R, et al. Detecting, studying, and treating autism early: the one-year well-baby check-up approach. J Pediatr. 2011;159:458–65. doi: 10.1016/j.jpeds.2011.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce K, Eyler LT. Structural and functional brain development in autism: the impact of early brain overgrowth and considerations for treatment. In: Fein DA, editor. The neuropsychology of autism. Oxford: Oxford University Press; 2011. pp. 407–50. [Google Scholar]
- Prevention CfDCa. Prevalence of autism spectrum disorders - Autism and Developmental Disabilities Monitoring Network, United States, 2006. MMWR Surveill Summ. 2009;58:1–20. [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: a review of 100 fMRI studies published in 2009. Ann N Y Acad Sci. 2010;1191:62–88. doi: 10.1111/j.1749-6632.2010.05444.x. [DOI] [PubMed] [Google Scholar]
- Quartz SR, Sejnowski TJ. The neural basis of cognitive development: a constructivist manifesto. Behav Brain Sci. 1997;20:537–56. doi: 10.1017/s0140525x97001581. discussion 56–96. [DOI] [PubMed] [Google Scholar]
- Rapin I, Dunn M. Update on the language disorders of individuals on the autistic spectrum. Brain Dev. 2003;25:166–72. doi: 10.1016/s0387-7604(02)00191-2. [DOI] [PubMed] [Google Scholar]
- Redcay E, Courchesne E. Deviant functional magnetic resonance imaging patterns of brain activity to speech in 2-3-year-old children with autism spectrum disorder. Biol Psychiatry. 2008;64:589–98. doi: 10.1016/j.biopsych.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redcay E, Haist F, Courchesne E. Functional neuroimaging of speech perception during a pivotal period in language acquisition. Dev Sci. 2008;11:237–52. doi: 10.1111/j.1467-7687.2008.00674.x. [DOI] [PubMed] [Google Scholar]
- Rossi S, Telkemeyer S, Wartenburger I, Obrig H. Shedding light on words and sentences: near-infrared spectroscopy in language research. Brain Lang. 2011 doi: 10.1016/j.bandl.2011.03.008. Available online at: http://dx.doi.org/10.1016/j.bandl.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Schumann CM, Bloss CS, Barnes CC, Wideman GM, Carper RA, Akshoomoff N, et al. Longitudinal magnetic resonance imaging study of cortical development through early childhood in autism. J Neurosci. 2010;30:4419–27. doi: 10.1523/JNEUROSCI.5714-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Van Zeeland AA, Abrahams BS, Alvarez-Retuerto AI, Sonnenblick LI, Rudie JD, Ghahremani D, et al. Altered functional connectivity in frontal lobe circuits is associated with variation in the autism risk gene CNTNAP2. Sci Transl Med. 2010;2:56ra80. doi: 10.1126/scitranslmed.3001344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seldon HL. Structure of human auditory cortex. I. Cytoarchitectonics and dendritic distributions. Brain Res. 1981;229:277–94. doi: 10.1016/0006-8993(81)90994-x. [DOI] [PubMed] [Google Scholar]
- Seldon HL. Structure of human auditory cortex. II. Axon distributions and morphological correlates of speech perception. Brain Res. 1981;229:295–310. doi: 10.1016/0006-8993(81)90995-1. [DOI] [PubMed] [Google Scholar]
- Sigman M, McGovern CW. Improvement in cognitive and language skills from preschool to adolescence in autism. J Autism Dev Disord. 2005;35:15–23. doi: 10.1007/s10803-004-1027-5. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. New York: Thieme Medical Publishers; 1988. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system - an approach to cerebral imaging. [Google Scholar]
- Tesink CM, Buitelaar JK, Petersson KM, van der Gaag RJ, Kan CC, Tendolkar I, et al. Neural correlates of pragmatic language comprehension in autism spectrum disorders. Brain. 2009;132:1941–52. doi: 10.1093/brain/awp103. [DOI] [PubMed] [Google Scholar]
- Tincoff R, Jusczyk PW. Some beginnings of word comprehension in 6-month-olds. Psychol Sci. 1999;10:172–5. [Google Scholar]
- Volden J, Lord C. Neologisms and idiosyncratic language in autistic speakers. J Autism Dev Disord. 1991;21:109–30. doi: 10.1007/BF02284755. [DOI] [PubMed] [Google Scholar]
- Wang AT, Lee SS, Sigman M, Dapretto M. Neural basis of irony comprehension in children with autism: the role of prosody and context. Brain. 2006;129:932–43. doi: 10.1093/brain/awl032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werker JF, Lalonde C. Cross-langauge speech perception: initial capabilities and developmental change. Dev Psychol. 1988;24:672–83. [Google Scholar]
- Wetherby AM, Allen L, Cleary J, Kublin K, Goldstein H. Validity and reliability of the communication and symbolic behavior scales developmental profile with very young children. J Speech Lang Hear Res. 2002;45:1202–18. doi: 10.1044/1092-4388(2002/097). [DOI] [PubMed] [Google Scholar]
- Wetherby AM, Woods J, Allen L, Cleary J, Dickinson H, Lord C. Early indicators of autism spectrum disorders in the second year of life. J Autism Dev Disord. 2004;34:473–93. doi: 10.1007/s10803-004-2544-y. [DOI] [PubMed] [Google Scholar]
- Witelson SF, Pallie W. Left hemisphere specialization for language in the newborn. Neuroanatomical evidence of asymmetry. Brain. 1973;96:641–6. doi: 10.1093/brain/96.3.641. [DOI] [PubMed] [Google Scholar]
- Zwaigenbaum L, Bryson S, Lord C, Rogers S, Carter A, Carver L, et al. Clinical assessment and management of toddlers with suspected autism spectrum disorder: insights from studies of high-risk infants. Pediatrics. 2009;123:1383–91. doi: 10.1542/peds.2008-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.