Abstract
Numerous kindreds with familial frontotemporal dementia and/or amyotrophic lateral sclerosis have been linked to chromosome 9, and an expansion of the GGGGCC hexanucleotide repeat in the non-coding region of chromosome 9 open reading frame 72 has recently been identified as the pathogenic mechanism. We describe the key characteristics in the probands and their affected relatives who have been evaluated at Mayo Clinic Rochester or Mayo Clinic Florida in whom the hexanucleotide repeat expansion were found. Forty-three probands and 10 of their affected relatives with DNA available (total 53 subjects) were shown to carry the hexanucleotide repeat expansion. Thirty-six (84%) of the 43 probands had a familial disorder, whereas seven (16%) appeared to be sporadic. Among examined subjects from the 43 families (n = 63), the age of onset ranged from 33 to 72 years (median 52 years) and survival ranged from 1 to 17 years, with the age of onset <40 years in six (10%) and >60 in 19 (30%). Clinical diagnoses among examined subjects included behavioural variant frontotemporal dementia with or without parkinsonism (n = 30), amyotrophic lateral sclerosis (n = 18), frontotemporal dementia/amyotrophic lateral sclerosis with or without parkinsonism (n = 12), and other various syndromes (n = 3). Parkinsonism was present in 35% of examined subjects, all of whom had behavioural variant frontotemporal dementia or frontotemporal dementia/amyotrophic lateral sclerosis as the dominant clinical phenotype. No subject with a diagnosis of primary progressive aphasia was identified with this mutation. Incomplete penetrance was suggested in two kindreds, and the youngest generation had significantly earlier age of onset (>10 years) compared with the next oldest generation in 11 kindreds. Neuropsychological testing showed a profile of slowed processing speed, complex attention/executive dysfunction, and impairment in rapid word retrieval. Neuroimaging studies showed bilateral frontal abnormalities most consistently, with more variable degrees of parietal with or without temporal changes; no case had strikingly focal or asymmetric findings. Neuropathological examination of 14 patients revealed a range of transactive response DNA binding protein molecular weight 43 pathology (10 type A and four type B), as well as ubiquitin-positive cerebellar granular neuron inclusions in all but one case. Motor neuron degeneration was detected in nine patients, including five patients without ante-mortem signs of motor neuron disease. While variability exists, most cases with this mutation have a characteristic spectrum of demographic, clinical, neuropsychological, neuroimaging and especially neuropathological findings.
Keywords: frontotemporal dementia, amyotrophic lateral sclerosis, motor neuron disease, TDP-43, neurogenetics, chromosome 9
Introduction
Familial frontotemporal dementia (FTD) with or without parkinsonism has been associated with mutations in genes encoding microtubule associated protein tau (MAPT), progranulin (PGRN), and less commonly valosin-containing protein, TAR DNA-binding protein (TDP) and fused in sarcoma (FUS) while familial amyotrophic lateral sclerosis (ALS) has been associated with mutations in genes encoding Cu/Zn superoxide dismutare-1, TDP and FUS (Rosen et al., 1993; Hutton et al., 1998; Watts et al., 2004; Baker et al., 2006; Cruts et al., 2006; Kabashi et al., 2008; Sreedharan et al., 2008; Kwiatkowski et al., 2009; Vance et al., 2009). The phenotype of familial FTD and/or ALS has been linked to chromosome 9 (Morita et al., 2006; Vance et al., 2006; Valdmanis et al., 2007; Luty et al., 2008; Le Ber et al., 2009; Gijselinck et al., 2010; Boxer et al., 2011; Pearson et al., 2011), and while the identification of the pathogenic genetic mutation has been challenging to identify, the cause of the disease in these families was recently discovered in our index family known as the Vancouver–San Francisco–Mayo Clinic Family 20 and many other families (Boxer et al., 2011; DeJesus-Hernandez et al., 2011; Renton et al., 2011). The mutation is an expansion of the GGGGCC hexanucleotide repeat in the non-coding intronic region of the chromosome 9 open reading frame 72 gene (C9ORF72) (DeJesus-Hernandez et al., 2011; Renton et al., 2011), and the disease is known as FTD and/or ALS linked to chromosome 9 (c9FTD/ALS) (DeJesus-Hernandez et al., 2011; Murray et al., 2011). The ante-mortem clinical features of affected individuals remain to be reported, but there are already reports of pathological features of c9FTD/ALS due to C9ORF72 mutations (Al-Sarraj et al., 2011; Murray et al., 2011). In this report, we describe demographic, clinical, neuropsychological, neuroimaging and neuropathological findings in a large series of individuals ascertained from the Mayo Clinics in Minnesota and Florida in whom this repeat expansion was found, either in them or their affected relatives.
Materials and methods
Subjects
The GGGGCC hexanucleotide repeat in C9ORF72 was screened for in all subjects with FTD or primary progressive aphasia in the Mayo Clinic Alzheimer Disease Research Centre database (n = 177 in Mayo Clinic Rochester, n = 197 in Mayo Clinic Florida), Mayo Clinic Alzheimer's Disease Patient Registry database (n = 1), and in subjects with ALS in the Mayo Clinic Florida Amyotrophic Lateral Sclerosis database (n = 229) (DeJesus-Hernandez et al., 2011). At least one affected individual from each family and all sporadic cases were enrolled in one of these research programmes, each of which is a Mayo Foundation Institutional Review Board-approved programme. All available clinical records, neuropsychological data, neuroimaging studies and pathological examinations on affected members of these kindreds were reviewed. Genetic analyses, clinical assessments, neuropsychological assessments, neuroimaging studies and autopsies were performed after subjects and/or appropriate proxies provided written consent.
Clinical cohorts
One proband was recruited and followed in the Alzheimer's Disease Patient Registry programme, which focused on subjects residing in Olmsted County, Minnesota, and once the FTD phenotype and familial nature of the disorder was appreciated, other relatives were recruited and followed longitudinally. The Mayo Clinic Rochester clinical cohort includes all cases with an FTD or primary progressive aphasia diagnosis regardless of family history, who were enrolled in the Mayo Clinic Alzheimer's Disease Research Centre or Alzheimer's Disease Patient Registry programmes from 1986 to 2011; since recruitment of familial FTD has been a focus of interest for many years, this cohort was expected to have a higher frequency of familial cases. The Mayo Clinic Florida Alzheimer's Disease Research Centre clinical cohort includes all cases with an FTD or primary progressive aphasia diagnosis regardless of family history who were enrolled from 1990 to 2011. The Mayo Clinic Florida ALS cohort includes all cases with ALS, regardless of family history, who were enrolled from December 2007 to August 2011.
The available information on each case with a diagnosis of FTD or primary progressive aphasia was reassessed to determine how each would be classified using the updated behavioural variant FTD (Rascovsky et al., 2011) and primary progressive aphasia (Gorno-Tempini et al., 2011) subtype criteria [almost all cases had been previously classified by the Neary et al. (1998) criteria]. Each case previously classified as FTD indeed met criteria for the behavioural variant FTD diagnosis, and DNA on all available cases were genotyped. Among the reclassified cases with primary progressive aphasia, only cases with the non-fluent/agrammatic and semantic subtypes were included in the DNA screen; since most with the logopenic subtype likely have underlying Alzheimer's disease, they were excluded from this screen. Subjects classified with the primary diagnoses of mild cognitive impairment, Alzheimer's disease, dementia with Lewy bodies, corticobasal syndrome, progressive supranuclear palsy and posterior cortical atrophy were also excluded from the DNA screen for purposes of this initial analysis focused on cases with FTD, primary progressive aphasia and/or ALS.
Subjects were classified as being familial (the presence of a positive family history) or sporadic based on specific sets of criteria. For subjects with a dementia syndrome as the major phenotype, a positive family history was defined as a first- or second-degree relative with FTD and/or ALS or a first-degree relative with memory problems, behavioural changes, parkinsonism or another suspected neurodegenerative disorder. A positive family history in the Mayo Clinic Florida ALS series was defined as a first- or second-degree relative with ALS (Byrne et al., 2011). Since the published criteria for determining familial ALS does not consider a family history of dementia or parkinsonism (Byrne et al., 2011), a looser definition of positive family history was also used, in which the presence of one or more first- or second-degree relatives with dementia, parkinsonism or ALS was also used in frequency calculations, with the strict definition of sporadic disease being the absence of any first or second-degree relatives with dementia, parkinsonism or ALS.
Clinical features and diagnoses
Age of onset for dementia was the age at which the subject first demonstrated behavioural/personality, cognitive ± parkinsonism as noted by themselves, their relatives or their clinicians. Age of onset for ALS was the age at which any symptom reflecting upper motor neuron and/or lower motor neuron dysfunction was noted by themselves, their relatives, or their clinicians. Survival (in years) was based on the number of years from onset of any neurological symptoms to death. All neurological clinical data (Members of the Department of Neurology, 1998) were reviewed. The presence or absence of the following clinical features were recorded: personality/behaviour changes (self explanatory), executive dysfunction (defined as impairment in sustained attention, multi-tasking, decision-making, problem solving, etc.), memory impairment (defined as forgetfulness for details of recent events or upcoming plans and poor delayed word list recall), aphasia (defined as difficulties with object or person naming, or receptive or expressive language functioning), parkinsonism (defined as some combination of masked facies, stooped posture, shuffling gait, rest tremor, bradykinesia, rigidity, postural instability), frontal release signs (defined as grasp, snout or palmomental reflex present), and pseudobulbar affect (defined as the tendency to cry or laugh inappropriately). Features of upper motor neuron and lower motor neuron dysfunction were considered present when specifically recorded in the clinical record, and were subclassified based on the level of involvement. The upper motor neuron features were characterized as follows: bulbar—pseudobulbar affect, snout or palmomental reflex, exaggerated jaw jerk, spastic dysarthria; cervical—hyper-reflexia and/or spasticity in the upper limbs, Hoffmann sign present; thoracic—absent superficial abdominal reflex; lumbar—hyper-reflexia and/or spasticity in the lower limbs, crossed adductor reflex, Babinski sign present. The lower motor neuron features were characterized as follows: bulbar—facial or tongue fasciculations, muscle wasting, and weakness, flaccid dysarthria; cervical—upper limb fasciculations, muscle wasting and weakness; thoracic—trunk or abdominal fasciculations, muscle wasting and weakness; lumbar—lower limb fasciculations, muscle wasting and weakness.
Clinical diagnoses were based on published criteria for FTD, progressive non-fluent aphasia, semantic dementia, ALS, etc. (Neary et al., 1998; Brooks et al., 2000), and as noted above, each case was reclassified using the updated behavioural variant FTD and primary progressive aphasia criteria (Gorno-Tempini et al., 2011; Rascovsky et al., 2011). Patients with ALS were classified as ‘clinically definite’, ‘probable’, ‘probable lab-supported’, ‘possible’ or ‘suspected’ ALS according to the El Escorial criteria, allowing for concomitant presence of signs of FTD in patients with FTD and ALS (Brooks et al., 2000). The site of onset—bulbar, upper and lower spinal level—was also noted based on available historical and clinical data for each case. Those who underwent electromyography were noted as such. The diagnosis of combined FTD/ALS was based on the presence of both FTD and ALS features. Those that had some combination of masked facies, stooped posture, shuffling gait, rest tremor, bradykinesia, rigidity, postural instability, etc. were characterized as having parkinsonism based on examination or informant report; the clinical diagnosis of Parkinson's disease was reserved for those who were formally examined and had the cardinal features based on published criteria (Hughes et al., 1992). Those that had a dementing illness characterized by an insidious onset and progressive course, but were either examined in the late stage of the illness or whose characterization was based on informant report alone, were labelled as having dementia. The designation of ‘clinically definite’ behavioural variant FTD, ALS, parkinsonism, or some combination of these, was reserved for only those subjects who were personally examined by one or more of the co-authors.
Since recent data suggest that psychosis and altered eating behaviour are associated with FTD/ALS and/or TAR DNA binding protein molecular weight 43 (TDP-43) pathology (Lillo et al., 2010; Burrell et al., 2011; Piguet et al., 2011), the presence or absence of these features was also sought by reviewing the available clinical records.
Laboratory analyses
Genomic DNA was extracted from peripheral blood samples using standard procedures. For each subject, genomic DNA was screened for the presence of the expanded hexanucleotide repeat in C9ORF72 using the repeat primed polymerase chain reaction method as previously described (DeJesus-Hernandez et al., 2011). In short, polymerase chain reaction was performed with 100 ng/µl of genomic DNA in the presence of 1 M betaine (Sigma), 5% dimethyl sulphoxide (Sigma), 5 mM of dCTP, dATP, dTTP (Promega) and 7-deaza-2-deoxy GTP (Roche) in substitution for dGTP. The cycling programme included an initial denaturation at 98°C for 10 min followed by 10 cycles of denaturation at 97°C for 35 s, annealing at 64°C for 2 min and extension at 68°C for 8 min, followed by 25 cycles of denaturation at 97°C for 35 s, annealing at 64°C for 2 min and extension at 68°C for 8 min plus an additional 20 s for each cycle.
Neuropsychological examinations
A battery of neuropsychological tests was available for 27 subjects. Four additional subjects were deemed too impaired to complete a full battery and their evaluation was limited to one or more cognitive screening exams, including the Mini-Mental State Examination (Folstein et al., 1975), Dementia Rating Scale-2 (Mattis, 1988) or ALS-Cognitive Behavioural Screen (Woolley et al., 2010). Some subjects who completed the neuropsychological battery also completed one or more screening exams.
The neuropsychological battery included tests of attention and executive functioning [Digit Span (n = 14) (Wechsler, 1981, 1997a), Trail Making Test (n = 20) (Reitan, 1958) and Stroop Colour-Word Test (n = 12) (Golden, 1978)]; language [Boston Naming Test (n = 25) (Kaplan et al., 1983), Controlled Oral Word Association Test (n = 21) (Benton et al., 1994), and semantic/category fluency for animals, fruits and vegetables (n = 21)]; learning and memory [delayed recall from Logical Memory (n = 22) (Wechsler, 1987, 1997b), Visual Reproduction (n = 19) (Wechsler, 1987, 1997b) and Auditory Verbal Learning Test (n = 20) (Rey, 1964) or California Verbal Learning Test-II (n = 4) (Delis et al., 2000)], visuospatial skills [Block Design (n = 13) (Wechsler, 1981, 1997a) and Judgement of Line Orientation (n = 11) (Benton et al., 1994)], and estimated premorbid functioning [Reading subtest from the Wide Range Achievement Test, 3rd edition (n = 17) (Wilkinson, 1993)]. Age-corrected normative data were obtained from the Mayo Older American Normative Studies (Ivnik et al., 1992, 1996, 1997; Lucas et al., 1998a, b) and published test manuals. To minimize variability due to normative reference sources, the youngest Mayo Older American Normative Studies age bracket was extended downward to include subjects aged 50–55 years. Normative data for adults aged <50 years were derived separately for the Trail Making Test (n = 2) (Bornstein, 1985), Boston Naming Test (n = 4) (Tombaugh and Hubley, 1997), and Judgement of Line Orientation (n = 1) (Benton, 1994). Cognitive impairment was defined as test scores 1.5 standard deviations below the respective normative group mean.
Neuroimaging examinations
Magnetic resonance imaging
MRIs were performed either at 1.5 or 3 tesla (GE Healthcare). At 1.5 tesla, a 3D spoiled gradient recalled acquisition in steady state (SPGR) and at 3 tesla a magnetization prepared rapid gradient echo acquisition (MPRAGE) were performed for the assessment of grey matter atrophy. A fluid attenuated inversion recovery (FLAIR) sequence was performed at both 1.5 and 3 tesla. To visualize the patterns of grey matter atrophy in individual subjects, we created Z-score maps or STAND-Maps (STructural Abnormality due to NeuroDegeneration-Maps), which indicate the atrophy in terms of Z-scores relative to a group of cognitively normal controls in 120 brain regions (Vemuri et al., 2011).
Single photon emission computed tomography
Brain single photon emission computed tomography (SPECT) using Tc-99 m-ECD (Neurolite) as the radiotracer was performed on select cases, as was brain PET using fluorodeoxyglucose (FDG) as the radiotracer, and the patterns of hypoperfusion (for SPECT) and hypometabolism (for PET) were noted.
In the SPECT acquisition protocol, 20 mCi of Tc-99 m-ECD (Neurolite®) were injected intravenously. Images were acquired on a dual-headed Elscint Helix gamma camera system (Elscint Inc.) with ultra-high-resolution fan beam collimators. Standard vendor acquisition and reconstruction parameters were used. These images were converted to Analyse format (Biomedical Imaging Resource, Mayo Foundation) for further processing including normalization, smoothing and segmentation, which was performed with SPM5. A two-sample t-test between subjects with C9ORF72 hexanucleotide repeat expansion and the control group was performed in SPM5 (Ashburner and Friston, 2005) with age and gender entered as nuisance covariates (uncorrected P < 0.05).
Given the largest difference between the groups in this analysis was in the cingulate cortex, we extracted the mean relative SPECT intensity within a cingulate region of interest for each SPECT scan. The cingulate region of interest is a combination of bilateral anterior and middle cingulate regions of the automated anatomic labelling atlas (Tzourio-Mazoyer et al., 2002).
An image of the salience network was derived from a high-dimensional group independent component analysis of resting state functional MRI data from 893 subjects from the Mayo Clinic Study of Ageing (Roberts et al., 2008). The topography of the salience network was compared with the topography of hypoperfusion on SPECT.
Positron emission tomography
For FDG-PET, subjects were injected with fluorodeoxyglucose (370–555 MBq range), in a dimly lit room with minimal auditory stimulation. Standard acquisition and vendor reconstruction parameters were used. FDG-PET scans were processed using CortexID software (GE Medical). The activity in each subject's PET data set was normalized to the pons and compared with an age-segmented normative database, yielding Z-score 3D-SSP images.
Neuropathological examination and diagnosis
Archival material was available for neuropathological examination for 14 cases. Haematoxylin and eosin stains were used to examine neuronal loss and gliosis in cortical, limbic, basal ganglia and brainstem structures. Luxol fast blue stains were available on medulla or spinal cord (n = 9), or both, supplemented by neurofilament or microglial immunohistochemistry in some cases, for assessment of corticospinal tract degeneration using methods previously described (Murray et al., 2011). In some cases, only haematoxylin and eosin material was available. Bielschowsky silver stains or thioflavin S fluorescent microscopy were used to assess Alzheimer type pathology and both a CERAD neuritic plaque score (Mirra et al., 1991) and a Braak neurofibrillary tangle stage (Braak and Braak, 1991) was assigned to each case. Midbrain and amygdala sections were examined for Lewy-related pathology with immunohistochemistry using a monoclonal antibody to α-synuclein (n = 10, LB509, 1:100, Zymed). Immunohistochemistry for phospho-TDP-43 was available on all but one case (a 1967 case from tissue archives) with a sensitive and specific antibody (ps409/410, 1:5000, Cosmobio Co.). Almost all cases also had immunohistochemistry for ubiquitin with a sensitive and specific monoclonal antibody (MAB1510, 1:60 000; Chemicon). Presence of cerebellar granular neuron inclusions was assessed in all 14 cases and scored based upon the density of inclusions as: 0 = absent, 1 = sparse, 2 = moderate and 3 = frequent.
Microscopic pathology methods for cortical degeneration, substantia nigra degeneration, lower motor neuron loss and evaluation of hippocampal sclerosis have been previously described (Murray et al., 2011). Lower motor neuron degeneration was assessed in hypoglossal nucleus and anterior horn cells of the spinal cord with respect to neuronal loss and gliosis, as well as presence of inclusions on haematoxylin and eosin stains (Lewy body-like hyaline inclusions and Bunina bodies) and on TDP-43 immunohistochemistry (skein-like inclusions).
Neuropathological diagnosis of frontotemporal lobar degeneration with TDP-43 positive inclusions (FTLD-TDP) was made if there was neuronal loss and gliosis affecting frontal or temporal lobe with ubiquitin-positive or TDP-43-positive neuronal inclusions in cortical and subcortical regions (Josephs et al., 2009). Cases were subtyped using the classification scheme of Mackenzie et al. (2006) as validated for subcortical brain regions (Josephs et al., 2009) and also assigned a recently recommended ‘harmonized’ type (Mackenzie et al., 2011). A neuropathological diagnosis of FTLD with motor neuron disease (MND) was made if there were findings of FTLD-TDP as well as evidence of motor neuron loss and neuronal inclusions with variable corticospinal tract degeneration (Josephs et al., 2006).
Results
Genetic/inheritance findings
In our largest and conclusively chromosome 9p-linked FTD/ALS kindred (Vancouver–San Francisco–Mayo Clinic Family 20) which was recently described (Boxer et al., 2011), the disease was associated with an expansion of the non-coding GGGGCC hexanucleotide repeat in C9ORF72 (DeJesus-Hernandez et al., 2011). All cases in our three cohorts were then screened for this expansion, with frequencies of expansion detected shown in Table 1. The frequency of expansion varied from 3.6% to 11.2% among all screened cases, and from 6.3% to 38.2% among cases with a positive family history. Note that the frequency data vary depending on how strictly the definition for positive family history is applied in the ALS cohort.
Table 1.
Frequency of the non-coding GGGGCC hexanucleotide repeat expansion in C9ORF72 among the three Mayo Clinic cohorts
| Characteristic/feature | Mayo Clinic Rochester FTD cohort | Mayo Clinic Florida FTD cohort | Mayo Clinic Florida ALS cohort |
|---|---|---|---|
| Total cases screened | 178 | 197 | 229 |
| Expansion detected | 20a (11.2%) | 7 (3.6%) | 16 (7.0%) |
| Family history of dementia, parkinsonism, or ALSb | 94 | 80 | 34 (39) |
| Expansion detected | 18 (19.1%) | 5 (6.3%) | 8 (23.5%) [13 (38.2%)] |
| Sporadic casesb | 84 | 117 | 195 [190] |
| Expansion detected | 2 (2.4%) | 2 (1.7%) | 8 (4.1%) [3 (1.6%)]c |
a Includes the Vancouver–San Francisco–Mayo Clinic Family 20 proband in the Mayo Clinic Rochester FTD cohort.
b Family history (see text for details). Of note, the eight cases from the Mayo Clinic Florida ALS cohort had no first- or second-degree relatives with ALS, which is the published criterion for considering ‘familial ALS’. Five of them had one or more first- or second-degree relatives with dementia or parkinsonism. Therefore, using strict criteria for sporadic disease being the absence of any first- or second-degree relatives with dementia, parkinsonism or ALS, the values in brackets reflect these strict criteria such that 2 + 2 + 3 = 7 (16%) out of 43 probands were sporadic.
c One of the three is adopted with no confirmed family history of dementia or ALS.
The frequency of the C9ORF72 expansion based on the clinical phenotype is shown in Table 2. The frequency of the expansion among all screened cases was highest in those with FTD/ALS (21.6%), and among cases with a positive family history, the frequency was highest in FTD/ALS (47.6%). Sporadic cases in all three phenotypes had the expansion detected, but these were uncommon (<5% in each group). Table 2 also shows the frequency of mutations in MAPT and PGRN in these three phenotypes; mutations in these genes were only identified in the behavioural variant FTD phenotype. Among cases with behavioural variant FTD with a positive family history, the frequencies of mutations in these two genes (12.7% for MAPT and 6.8% for PGRN) were slightly lower than the frequency of the C9ORF72 mutation (14.7%). Among the cases with familial ALS, the frequency of the C9ORF72 mutation (24%) was similar to the combination of three other genes (24%). These data therefore indicate that a sizable proportion of cases with familial FTD/ALS are now explained by the C9ORF72 mutation.
Table 2.
Frequency of the non-coding GGGGCC hexanucleotide repeat expansion in C9ORF72, and mutations in MAPT, PGRN and other genes among the syndromes of behavioural variant FTD, FTD/ALS and ALS
| Characteristic/feature | Behavioural variant FTD | FTD/ALS | ALS |
|---|---|---|---|
| Total cases screened, n | 210 | 51 | 195 |
| C9ORF72 expansion detected, n (%) | 19 (9.0) | 11 (21.6) | 13 (6.7) |
| MAPT mutation detected, n (%) | 16 (7.6) | 0 | 0 |
| PGRN mutation detected, n (%) | 10 (4.8) | 0 | 0 |
| Family history of dementia, parkinsonism or ALS | 102 | 21 | 25 |
| C9ORF72 expansion detected, n (%) | 15 (14.7) | 10 (47.6) | 6 (24.0) |
| MAPT mutation detected, n (%) | 13 (12.7) | 0 | 0 |
| PGRN mutation detected, n (%) | 7 (6.8) | 0 | 0 |
| Mutation in other gene detecteda, n (%) | 0 | 0 | 6 (24.0) |
| No mutation in C9ORF72, MAPT, or PGRN, or other genes detected, n (%) | 67 (65.7) | 11 (52.4) | 13 (52.0) |
| Sporadic casesb | 108 | 30 | 170 |
| C9ORF72 expansion detected, n (%) | 4 (3.7) | 1 (3.3) | 7 (4.1) [2 (1.2)]a |
| MAPT mutation detected, n (%) | 3 (2.8) | 0 | 0 |
| PGRN mutation detected, n (%) | 3 (2.8) | 0 | 0 |
a Mutations in other genes include TARDBP (n = 1), FUS (n = 1) and SOD1 (n = 4).
b See text for details on determining familial versus sporadic. Of note, while seven of the cases with ALS had no first- or second-degree relatives with ALS, which is the published criterion for considering ‘familial ALS’, five of them had one or more first- or second-degree relatives with dementia or parkinsonism. Therefore, using strict criteria for sporadic disease being the absence of any first- or second-degree relatives with dementia, parkinsonism or ALS, the values in brackets reflect these strict criteria such that 7 – 5 = 2 (1.2%) out of 170 cases were sporadic.
FUS = gene encoding fused in sarcoma; MAPT = gene encoding microtubule associated protein tau; PGRN = gene encoding progranulin; SOD1 = gene encoding superoxide dismutase-1; TARDBP = gene encoding TARD binding protein.
In addition to the proband in the Vancouver–San Francisco–Mayo Clinic Family 20 kindred, the repeat expansion was identified in 42 other probands with either sporadic (n = 7) or familial (n = 35) behavioural variant FTD, ALS or FTD/ALS, and in an additional 10 affected relatives (totalling 53 cases). Ten additional affected relatives were examined but did not have DNA available for analysis (total 63 examined cases). Clinical records were available for 40 additional relatives with dementia, parkinsonism or ALS, and data on the total of 103 cases were analysed for demographic analyses, realizing that some of these cases could have been phenocopies.
As shown in Table 3, the majority of kindreds (84%) were familial, demonstrating an autosomal dominant pattern of inheritance. Approximately 33% had dementia or behavioural variant FTD as the only phenotype expressed in the family, while 3% had only ALS expressed in the family. More than half (58%) had both behavioural variant FTD and ALS expressed in affected members of the kindred. Seven (16%) had no apparent family history of neurodegenerative disease. Two kindreds had one subject in each who died prior to the onset of any neurological symptoms yet must have been obligate carriers—one died at age 34 and the other at age 72. In 11 (26%) kindreds, the most recent generation of affected subjects had an age of onset >10 years younger than the previous generation (Supplementary Fig. 1).
Table 3.
Summary of inheritance observations among affected individuals in 43 kindreds with probands harbouring the non-coding GGGGCC hexanucleotide repeat expansion in C9ORF72
| Inheritance observation | n (%) |
|---|---|
| Kindreds with apparent autosomal dominant pattern of inheritance | 36 (84) |
| Only dementia or behavioural variant FTD present in same kindred | 12 (33) |
| Only ALS phenotype present in same kindred | 3 (8) |
| FTD and ALS phenotype present in same kindred | 21 (58) |
| Apparent sporadic cases with no known family history of neurodegenerative disease | 7 (16) |
| Apparent kindreds with incomplete penetrance | 2 (5) |
| Apparent younger age of onset (>10 years) from one generation to the next | 11 (26) |
Demographic and clinical findings
The demographic and clinical findings of pertinent members of the 43 kindreds are presented in Supplementary Table 1. A summary of the key demographic findings is presented in Table 4. Details regarding our index kindred have been previously reported (Boxer et al., 2011).
Table 4.
Summary of clinical phenotypes and demographic data among affected individuals in 43 kindreds with probands harbouring the non-coding GGGGCC hexanucleotide repeat expansion in the gene C9ORF72
| Dominant clinical phenotype or feature | Total | Male, n (%) | Age at onset Median (range) (in years) | Age at death Median (range) (in years) | Survival Median (range) (in years) | Sx<40, n (%) | Sx>60, n (%) | Sx>70, n (%) |
|---|---|---|---|---|---|---|---|---|
| Dementia, parkinsonism or ALS | 103 | 56 (54) | 56 (33–85) | 66 (34–90) | 5 (1–26) | 7 (7) | 47 (46) | 20 (19) |
| Clinically definite bvFTD and/or ALS ± parkinsonism | 63 | 33 (52) | 52 (33–72) | 59 (35–75) | 5 (1–17) | 6 (10) | 19 (30) | 3 (5) |
| Primary diagnosis of bvFTD ± parkinsonism | 30 | 19 (63) | 52 (33–69) | 61 (35–75) | 6 (1–17) | 2 (7) | 9 (30) | 0 |
| Primary diagnosis of ALS | 18 | 7 (39) | 53 (35–72) | 55 (37–73) | 4 (1–6) | 1 (4) | 4 (22) | 1 (4) |
| Primary diagnosis of FTD/ALS ± parkinsonism | 12 | 5 (42) | 53 (38–71) | 57 (39–75) | 3 (1–9) | 2 (14) | 3 (21) | 1 (7) |
| Primary diagnosis of PPAa | 0 | |||||||
| Presence of bvFTD features among subjects with a primary ALS diagnosisb (n = 18) | 3 (17) | |||||||
| Presence of ALS features among subjects with a primary bvFTD diagnosisb (n = 30) | 12 (40) | |||||||
| Presence of parkinsonismb (n = 63) | 22 (35) |
a Includes 76 cases with the non-fluent/agrammatic and 65 with the semantic subtypes of PPA.
b Only includes examined subjects. All subjects with parkinsonism had behavioural variant FTD or FTD/ALS, but not ALS, as the dominant clinical phenotype.
bvFTD = behavioural variant frontotemporal dementia; PPA = primary progressive aphasia.
Demographic features
All subjects were Caucasian. There was a very slight male predilection considering all cases and only clinically definite cases, and there was a trend towards subjects with behavioural variant FTD being more represented by males (63%) and those with ALS ± FTD being more represented by females (60%). Among the 63 examined subjects from the 43 families (33 male, 52%) with clinically definite behavioural variant FTD and/or ALS ± parkinsonism, age at onset ranged from 33 to 72 years (median 52 years). Age at death ranged from 35 to 75 years (median 60 years), and survival ranged from 1 to 17 years (median 5 years). Four of the clinically definite cases survived 10 years or more, all of whom had the behavioural variant FTD phenotype. Among the 63 with clinically definite disease, six (10%) had onset of symptoms prior to age 40, 19 (30%) had onset of symptoms after age 60, and three (5%) had onset of symptoms after age 70; the maximal age of onset was 72 years. Also, one man with an Alzheimer's disease-like phenotype who was not examined by any of the authors had a dementia onset of 74 years and death age of 84 years. He underwent neuropathological examination and FTLD-MND was identified. Therefore, the age of onset and duration of disease appear quite variable among those with c9FTD/ALS.
Clinical phenotypes
Similar data are shown in Table 4 for those with the dominant clinical phenotype (henceforth labelled as ‘primary diagnosis’) of behavioural variant FTD, ALS and combined FTD/ALS, and according to whether FTD or ALS was present. The median age of onset regardless of the major clinical phenotype was 52 years, and the survival tended to be shorter for those with ALS (median 3 years) compared with those without (median 6 years). Among the 12 subjects with combined FTD/ALS, the behavioural variant FTD syndrome preceded the ALS features in seven by a median of 2 years (range 1–4 years), the ALS features preceded the behavioural variant FTD syndrome in two by a median of 2.5 years (range 2–3 years), and the behavioural variant FTD and ALS evolved concurrently in three.
Absence of primary progressive aphasia phenotype
Although all subjects in the Mayo Clinic Rochester and Mayo Clinic Florida clinical cohorts with the progressive non-fluent aphasia (now termed non-fluent/agrammatic subtype of primary progressive aphasia, n = 76) and semantic dementia (now termed semantic subtype of primary progressive aphasia, n = 65) phenotypes were screened for the hexanucleotide repeat expansion, none with a primary progressive aphasia diagnosis (0 out of 141) were found to have the expansion (Tables 2 and 4).
Parkinsonism
Among the clinically examined subjects, 22 (35%) showed evidence of parkinsonism. In 14 (64%) of those with parkinsonism, the extrapyramidal features evolved within the first 2 years of the onset of the FTD ± ALS features. All who had parkinsonism had the dominant clinical phenotype of behavioural variant FTD (15 out of 30 or 50% of subjects with behavioural variant FTD) or FTD/ALS (5 out of 12 or 42% of subjects with FTD/ALS), but not ALS (0 out of 18). Parkinsonism was characterized as a relatively symmetric akinetic-rigid syndrome, and the few in whom tremor was present, it was prominent with posture and action but not at rest. None in whom levodopa was administered benefited from this treatment, and none were diagnosed with Parkinson's disease.
Amyotrophic lateral sclerosis features
Among the clinically examined subjects, 28 (44%) subjects had clinical findings allowing El Escorial classification of ALS, and 27 (43%) underwent electromyography. The frequency of ALS diagnoses using the El Escorial criteria designations considering all examined cases were as follows: definite (n = 10, 16%), probable (n = 8, 13%), possible (n = 4, 6%), probable laboratory-supported (n = 4, 6%), suspected (n = 1, 2%), and not applicable (n = 36, 57%). The site of onset was bulbar in seven, upper spinal cord in 13, and lower spinal cord in eight. A few rare phenotypes were present, with one subject having monomelic amyotrophy (one upper extremity affected), one had progressive muscular atrophy, and one had a combination of pseudobulbar affect, mixed flaccid/spastic dysarthria, profound spasticity in the limbs most consistent with a primary lateral sclerosis phenotype plus lower motor neuron findings in the bulbar musculature.
Evidence of upper motor neuron and/or lower motor neuron dysfunction was also found in many cases with dementia with or without parkinsonism, but did not involve multiple neuraxis levels to warrant a coexisting diagnosis of suspected, possible, probable or definite ALS by El Escorial criteria. Twelve (40%) of the 30 subjects with behavioural variant FTD or dementia had clinical evidence of upper motor neuron dysfunction, and one (3%) had evidence of lower motor neuron dysfunction.
Other clinical features
Also among all clinically examined subjects, the following clinical findings were identified: memory dysfunction (n = 23), aphasia (n = 20), frontal release signs (n = 8) and pseudobulbar affect (n = 4). The degree of memory dysfunction varied from mild to marked, and when present, this was often evident early in the disease course. Aphasia tended to be a late feature, with some becoming mute late in the course.
Detailed information regarding the presence/absence of neuropsychiatric features was available in 20 subjects (16 with behavioural variant FTD, three with FTD/ALS and one with ALS). The frequency of delusions was 45%, frequency of hallucinations was 50% and changes in appetite/eating behaviour was 100%. Two of the three cases with FTD/ALS had all three of these features.
Neuropsychological findings
Screening exams
Twelve subjects (mean age 58.1 ± 9.08 years) completed a Mini-Mental State Examination and obtained a mean score of 20.5 ± 6.75 (range 7–28). Seven of these 12 (58.3%) subjects obtained a Mini-Mental State Examination score <24/30. Twenty-three subjects (mean age 57.8 ± 7.66 years) completed a Dementia Rating Scale-2 and obtained a mean score of 108.5 ± 24.40 (range 56–139). Dementia Rating Scale-2 scores were in the impaired range for 18 of 23 (78.3%) subjects. Three additional subjects received the Amyotrophic Lateral Sclerosis-Cognitive Behavioural Screen: one individual obtained an impaired cognitive score of 13/20, another individual was unable to complete the test due to the severity of cognitive impairment, while the third individual obtained a cognitively normal score of 17/20.
Neuropsychological battery
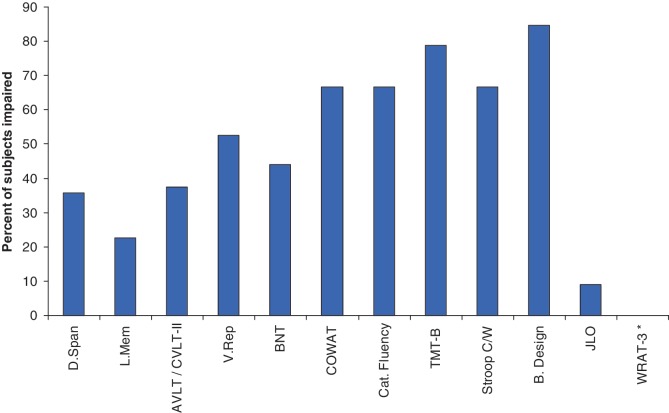
As shown in Fig. 1, subjects demonstrated predominant impairment across timed tasks, with a high percentage showing specific deficits on measures of complex attention/executive functioning and word fluency. There were no obvious differences in the neuropsychological profile of impairment between the behavioural variant FTD, FTD/ALS and ALS phenotypes. Fewer subjects were impaired on tests of basic attention, memory and visual naming. No subject was impaired on a test of single word reading, suggesting relative sparing of this ability and highlighting that test's utility as an index of estimated premorbid cognitive function in FTD/ALS. The discrepancy in impairment between two tests involving visuospatial functioning (Block Design and Judgement of Line Orientation) is worth noting. Block Design is a timed task that requires motor manipulation of blocks to match target designs and relies on processing speed, visuospatial skills and an executive problem-solving component. In contrast, Judgement of Line Orientation is a non-timed task that requires visual discrimination of angular relationships. Eleven of the 13 subjects who completed Block Design obtained an impaired score (84.6%), compared with 1 of 11 (9.1%) subjects who completed the Judgement of Line Orientation task. Of the five subjects who completed both tasks, all had lower scores on Block Design and their mean score was ∼1 standard deviation below their mean score on Judgement of Line Orientation. These results, although from a limited number of subjects, suggest that the high rate of impairment obtained on Block Design may be due to slowed processing speed and executive dysfunction, not visuospatial deficits.
Figure 1.
Per cent of subjects with impaired neuropsychological test scores. Cognitive impairment defined as test scores less than −1.5 SDs from respective normative sample. AVLT = Auditory Verbal Learning Test; B.Design = Block Design; BNT = Boston Naming Test; Cat. Fluency = Category Fluency; COWAT = Controlled Oral Word Association Test; CVLT-II = California Verbal Learning Test-II; D.Span = Digit Span; JLO = Judgement of Line Orientation; L. Mem = Logical Memory; Stroop C/W = Stroop colour-word interference trial; TMT-B = Trail Making Test part B; V. Rep = Visual Reproduction; WRAT-3 = Reading subtest from the Wide Range Achievement Test-3. Asterisk represents no subject impaired in WRAT-3 Reading test.
Neuroimaging findings
Magnetic resonance imaging
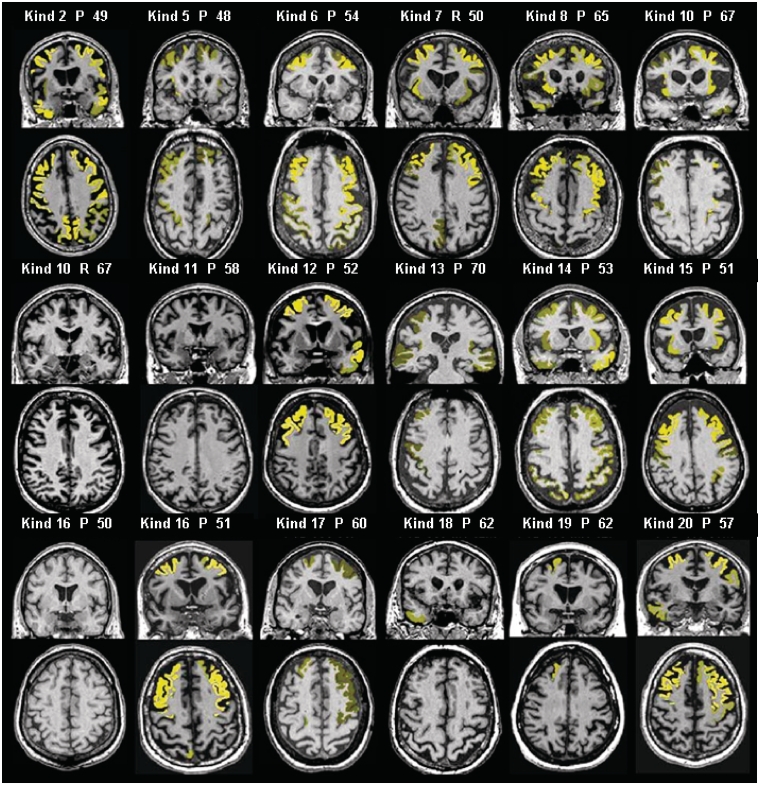
MRI was available for review in 18 patients (14 with behavioural variant FTD, three with FTD/ALS and one with ALS), and representative images highlighting the relatively consistent pattern of symmetric bilateral frontal ± temporal cortical atrophy are presented in Fig. 2. Significant atrophy was determined in individual subject MRI scans using a threshold of Z-score less than −2.5. Atrophy was identified in 15 patients, with frontotemporal predominance in 12. One patient with behavioural variant FTD, one with FTD/ALS and one with ALS had no detectable atrophy. Parietal cortical atrophy was present in five patients and insular atrophy was found in eight patients. Subcortical white matter signal changes were absent in all cases. Longitudinal MRI scans spanning ≥2 years were available in eight patients, which all showed progression of atrophy specifically in the frontal lobes and ventricular enlargement.
Figure 2.
Atrophy patterns in individual subjects. MRI scans of individual subjects overlaid with regions of severe atrophy in yellow using MRI STAND-maps (Z-score less than −2.5 relative to normals was used as a threshold). The brightness of the overlaid colour indicates the degree of atrophy. Numbers represent age of each subject in years. Kind = kindred; P = proband; R = relative.
SPECT and positron emission tomography
SPECT scans were available for analysis in four subjects (Fig. 3). The findings indicate statistically significant anterior and middle cingulate gyri hypoperfusion compared with controls (P = 0.037) (Supplementary Fig. 2). One subject (Kindred 16, Case IV.2) who had normal findings on his initial MRI (Fig. 1) also underwent both SPECT and PET imaging, and each showed anterior cingulate changes. However, the frontal cortical changes were more prominent on the PET when the subject was also 2 years older. Five subjects underwent FDG-PET. In four of five FDG-PET scans (Fig. 4), anterior cingulate Z-scores were significant (average right 2.2; average left 1.9) and showed consistent hypometabolism in these subjects. Posterior cingulate metabolism was normal in these subjects (average right 0.98; average left 0.98). Frontal cortical hypometabolism in these four subjects ranged from mild to severe. One of the five subjects had a parietal/precuneus pattern of hypometabolism with sparing of frontal cortical regions. These SPECT and PET findings have regional abnormalities that show localization similarities with the salience network (Supplementary Fig. 3).
Figure 3.
Individual subject relative SPECT intensity maps. SPECT maps shown for a 54-year-old healthy control subject (A), 51-year-old with behavioural variant FTD (B), 51-year-old with behavioural variant FTD (C), 56-year-old with FTDP/ALS (D), and 63-year-old with behavioural variant FTD (E). The relative SPECT intensity for each subject's normalized SPECT scans is overlaid on a template brain for anatomical reference. The healthy control subject is displayed at the top (blue box), and images for subjects B to E are arranged in increasing order of severity of anterior cingulate reduction in SPECT intensity. Colour bar encodes SPECT intensity relative to pontine region of interest.
Figure 4.
Fluorodeoxyglucose-PET Z-score 3D-SSP images. PET images shown for a 68-year-old with behavioural variant FTD (A), 69-year-old with behavioural variant FTD (B), 64-year-old with behavioural variant FTD (C), 53-year-old with behavioural variant FTD (D), and 57-year-old with behavioural variant FTD (E). The images from left to right are right lateral, left lateral, right medial, and left medial Z-score projection maps. The upper two rows show mild frontal cortical and cingulate hypometabolism while the lower two show moderate to severe dorsolateral frontal, anterior temporal cortical and cingulate hypometabolism. The middle subject images show a parietal/precuneus pattern of hypometabolism. Subject D in this figure is the same person as Subject B in Fig. 3.
Neuropathological findings
Neuropathological data were available for 14 affected individuals (summarized in Supplementary Table 2); data on two of these cases were reported previously (Murray et al., 2011). All had TDP-43 pathology associated with frontal and variable parietal or temporal cortical atrophy and microscopic evidence of neurodegeneration (Fig. 5 and Supplementary Fig. 4); nine had pathology consistent with MND (FTLD-MND). Alzheimer's type pathology was minimal and none met research criteria for Alzheimer's disease; two patients with moderate CERAD neuritic plaques and one subject with Braak neurofibrillary tangle stages III and IV were all 70 years of age or older. Substantia nigra degeneration was common and moderate-to-severe in seven of nine patients with FTLD-MND, but zero of five FTLD-TDP. None of the cases had Lewy bodies with α-synuclein immunohistochemistry. Of the seven cases with the most severe substantia nigra degeneration, three had parkinsonism that ranged from mild-to-severe. Hippocampal sclerosis was relatively uncommon and severe in only 4 of the 14 cases. Of the cases with FTLD-MND, seven showed both upper and lower motor neuron pathology consistent with ALS, the remaining two (Kindred 10, Case III.2; Kindred 12, Case IV.1) showed only involvement of lower motor neuron with no corticospinal tract degeneration and were consistent with progressive muscular atrophy. Two of the cases with FTLD-MND were considered to have preclinical MND since they had no documented clinical findings of MND. Bunina bodies were found in all cases with FTLD-MND, except one case who showed very subtle lower motor neuron neuronal loss and sparse TDP-43-positive pathology.
Figure 5.
Microscopic findings of c9FTD/ALS. Ubiquitin immunohistochemistry (A) compared with phospho-TDP-43 immunohistochemistry (B) in adjacent sections of dentate fascia of hippocampus (Kindred 14, Case 1.III.1). Note considerably more neuronal inclusions and neurites with ubiquitin. Ubiquitin-positive cerebellar inclusions in same case at lower magnification (C) and higher magnification (D). Frontal cortex phospho-TDP-43 immunohistochemistry: type A in Patient III.1 from Kindred 5 shows short neurites, neuronal inclusions and intranuclear inclusions (inset); and type B in Patient IV.1 from Kindred 12 shows mostly neuronal inclusions, including so-called ‘pre-inclusions’. Scale bar = 30 μm for A–C, E and F and 6 μm for D and E inset.
The cases were classified as Mackenzie type 1 (harmonized type A, n = 10), Mackenzie type 3 (harmonized type B, n = 4). Half of the cases with FTLD-MND were type A and half were type B. Two cases with type A FTLD-TDP had no evidence of motor neuron loss or corticospinal tract degeneration, but isolated motor neurons in spinal cord with either a Lewy body-like hyaline inclusion or a TDP-43 positive skein-like inclusion. If these cases were included in the group with MND the overwhelming majority of cases would be type A. Regardless, type A in our series is more common than type B in c9FTD/ALS, in contrast to the conclusions of the authors in the harmonization group (Mackenzie et al., 2011).
Cerebellar granular cell ubiquitin-immunoreactive neuronal inclusions were detected in all but three patients (all FTLD-MND, one type A and two type B). They tended to be more numerous in type A than type B and in FTLD-TDP rather than FTLD-MND. In almost all cases, ubiquitin tended to label more neuronal inclusions in the hippocampal dentate fascia and dystrophic neurites in the molecular layer of the dentate fascia than TDP-43 (Fig. 5), similar to findings recently reported by Al-Sarraj and colleagues (2011). Moreover, the frequency of ubiquitin-positive inclusions compared with TDP-43 could affect the interpretation of TDP-43 type.
A summary of the salient features and findings associated with the C9ORF72 mutation is shown in Table 5.
Table 5.
Salient features of c9FTD/ALS due to the GGGGCC hexapeptide repeat expansion in C9ORF72
| Demographic |
|
| Inheritance |
|
| Clinical |
|
| Neuropsychological |
|
| Neuroimaging |
|
| Neuropathology |
|
SPECT = single photon emission computed tomography.
Discussion
The core features of chromosome 9 FTD/ALS
There was a relatively characteristic spectrum of core findings among individuals with FTD and/or ALS associated with the hexanucleotide repeat expansion in C9ORF72, which we suggest should be termed c9FTD/ALS. The phenotype was typically behavioural variant FTD, ALS or a combination of both. Features of parkinsonism were found in nearly one half of patients with behavioural variant FTD or FTD-ALS but were not seen in patients with ALS in these cohorts. No patient had a primary dementia phenotype of primary progressive aphasia. The age of onset was in the 33–75 year range, and survival ranged widely, with most succumbing to the disease within 7 years from onset. Those with ALS by itself or in conjunction with behavioural variant FTD tended to have a shorter disease course. Most had one or more relatives with dementia and/or ALS. The neuroimaging findings of symmetric bilateral frontal ± parietal and/or temporal cortical changes were relatively consistent. All patients with autopsies and neuropathological examinations had TDP-43 pathology consistent with either type A or Type B, as well as frequent motor neuron pathology, frequent substantia nigra degeneration and ubiquitin-positive, TDP-43-negative cerebellar and hippocampal neuronal inclusions.
Demographic considerations
Among all subjects with dementia and/or ALS, and among only those who were examined by one of the co-authors, there was a slight male predominance (52–54%). Considering these three cohorts together, males with the primary diagnosis of behavioural variant FTD were over-represented (63%), and females with ALS ± FTD were slightly over-represented (18/30 or 60%), particularly in the ALS phenotype (61%), but whether these slight male–female differences reflect a sampling bias or have biological relevance in terms of sex factors impacting the topography of degeneration and hence the clinical phenotype will require further study.
Genetic/inheritance considerations
Among the three cohorts of subjects, the frequency of the mutation was highest in the Mayo Clinic Rochester FTD cohort (11.2% of all FTD ± ALS cases) and lowest in the Mayo Clinic Florida FTD cohort (3.6%), which likely reflects the strong focus of recruiting familial FTD kindreds in the Mayo Clinic Rochester centre and likely higher frequency of northern European ancestry in the Midwest (which is where most Mayo Clinic Rochester participants reside). Among those with a positive family history, the frequency of the expansion was highest in the Mayo Clinic Florida ALS cohort (at least 23%), but also reasonably high in the Mayo Clinic Rochester FTD cohort (19.1%). There are likely two factors that explain this. The newly identified C9ORF72 mutation explains a high proportion of familial ALS based on the available data (DeJesus-Hernandez et al., 2011; Renton et al., 2011). Furthermore, other data suggest that a common founder from Scandinavia might underlie the disease (Mok et al., 2012). More work is needed to determine if the mutation stems from a common founder, represents a ‘weak point’ in the genome which allows an increased frequency of expansions to randomly occur, or a combination of these and other factors.
As shown in Table 2, the frequency of the C9ORF72 mutation is highest in the FTD/ALS phenotype compared with the behavioural variant FTD and ALS phenotypes regardless of whether they are considered among all cases or familial cases only. Furthermore, no mutations in MAPT or PGRN were detected in the cases with sporadic or familial FTD/ALS and ALS. Also, at least in this Mayo Clinic series, the frequency of familial behavioural variant FTD being explained by the C9ORF72 mutation is higher than that explained by MAPT and PGRN despite the strong interest in recruiting familial FTD families over the past 20 years and contributions to the original identification of mutations in MAPT and PGRN causing familial FTD. These data therefore indicate that a sizable proportion of cases with familial FTD and/or ALS is explained by the C9ORF72 mutation. Importantly, however, an even greater proportion is not explained by mutations in these three or other known genes (>50% for each phenotype), indicating that other yet-to-be-identified genes are associated with FTD and/or ALS.
More than 80% of our probands were familial, of which more than half had both the behavioural variant FTD and ALS phenotypes represented in the kindreds, a third had only dementia or behavioural variant FTD, and the remaining minority had only ALS represented in the families. Therefore, the repeat expansion should be considered in any kindred with familial FTD and/or ALS. The other 16% of our probands did not meet predefined criteria for a positive family history and hence were considered sporadic.
The observation that the GGGGCC hexanucleotide repeat expansion in C9ORF72 may be associated with FTD, FTD/ALS and ALS in the same pedigree supports recommendations included in recently proposed criteria for the diagnosis of familial ALS (Byrne et al., 2011). In the Mayo Clinic Florida ALS cohort we classified eight patients who carried the C9ORF72 repeat mutation and had no known family history of ALS in a first- or second-degree relative, as sporadic ALS. However, available information suggests that five of these eight patients might be reasonably classified as having a familial disorder given a family history of dementia in a first-degree relative in four and in a second-degree relative in one patient. The latter classification is in keeping with criteria we applied in designating patients with FTD and FTD/ALS as familial or sporadic. This leaves a small number of patients with FTD or ALS in whom family history suggests sporadic disease, but evaluation of parental DNA will be important in future investigation to determine whether these pedigrees represent non-penetrance or spontaneous expansion of the GGGGCC hexanucleotide repeat from a non-pathogenic parental form. Adoption of stringent criteria for the diagnosis of familial ALS along the lines of those recently proposed by Byrne et al. (2011) seems especially important in giving increasing recognition that expanded phenotypes such as extrapyramidal disease may be associated with genes linked to FTD and ALS.
Whether the sporadic cases represent incomplete penetrance or de novo mutations is being investigated. Two subjects appeared to be obligate carriers but died while neurologically asymptomatic—one at age 34 from an accident, and another at age 72 from a myocardial infarction. The younger subject might have exhibited features had he survived decades more, and the 72-year old may have done likewise if he had survived a few more years. However, considering that 72 was the maximal age of onset of any clinically examined subject in this series, and the subject with the Alzheimer's disease phenotype whose onset was at the age of 74 and autopsy revealed FTLD-TDP-43 pathology (the repeat expansion has not been confirmed in this case but is presumed), this asymptomatic case who died at age 72 suggests that incomplete penetrance might occur even among aged individuals.
A curious observation is the apparent earlier age of onset (>10 years) in the youngest generation compared with the previous generation in 11 (26% of all probands) of our families with c9FTD/ALS (Supplementary Fig. 1). Considering that the mechanism of this genetic defect involves a hexanucleotide repeat expansion, and the concept of anticipation (i.e. an earlier age of onset in successive generations occurs with increasing repeat length) is well established in many of the nucleotide repeat disorders (Lindblad and Schalling, 1999), it is tempting to hypothesize that a similar mechanism may be at play in these families. However, this will be challenging to study. First, the repeat expansion is unstable and variable across cells, and variability in single subjects has already been seen (DeJesus-Hernandez et al., 2011). Furthermore, the current methodology for quantifying repeat length is imperfect, particularly when several hundreds of repeats are present. Investigating this issue further is clearly worthy to support or refute an anticipation mechanism associated with this hexanucleotide repeat. Repeat length may also impact the topography of neurodegeneration and hence the clinical phenotype expressed.
Clinical considerations
As noted above, the core phenotypes associated with this repeat expansion were behavioural variant FTD, ALS or combined FTD/ALS, with one-third of total cases and nearly half with behavioural variant FTD or FTD/ALS also having some degree of parkinsonism, which was typically an akinetic-rigid syndrome without rest tremor. When parkinsonism was present, it tended to evolve over the initial 2 years of the disease course. When both behavioural variant FTD and ALS occurred in the same individual, the features almost always evolved within 2 years. Even among subjects with the primary behavioural variant FTD syndrome, almost 40% in this series had some degree of upper and/or lower motor neuron dysfunction but not of sufficient breadth to warrant the FTD/ALS diagnosis. A comprehensive neurological examination in any patient with dementia will permit identification of these clinical findings. Almost 20% of the subjects with a primary diagnosis of ALS had some degree of behavioural variant FTD features but either did not fully meet criteria for the FTD/ALS diagnosis or could not be formally evaluated owing to motor impairment. Features of behavioural variant FTD may be subtle and not characteristic of fully developed dementia in ALS resulting in potential misattribution of signs of cognitive dysfunction to situational factors or depression. Weakness and fatigue may limit the practical assessment of FTD in subjects with ALS, and eliciting the historical and clinical features of behavioural variant FTD may not be routinely performed in clinics focused on standard elements of ALS patient care. Thus, the 20% frequency of behavioural variant FTD features in this cohort of subjects with ALS may be an underestimate. Yet the overlapping features of behavioural variant FTD and ALS in many subjects with the repeat expansion in C9ORF72 underscores several key points: (i) this genetic alteration can impact cognition, behaviour and motor functioning via degeneration in cerebral and/or spinal cord neurons; (ii) clinicians will often identify ALS features in subjects with behavioural variant FTD and behavioural variant FTD features in subjects with ALS if detailed questioning, clinical examinations and electromyograms are performed (Lomen-Hoerth et al., 2002, 2004); and (iii) assessments over time permit the identification of more widespread findings as the disease process evolves.
Neuropsychiatric morbidity was frequent in the subjects in whom this information was collected. The caregivers of subjects endorsed the presence of changes in appetite and eating behaviour in all who were queried, and almost half endorsed the presence of delusions or hallucinations. These findings are consistent with other recent publications on the neuropsychiatric features in FTD/ALS and/or those with TDP-43 positive pathology (Lillo et al., 2010; Burrell et al., 2011; Piguet et al., 2011).
Among the clinically examined subjects, most with ALS features fulfilled El Escorial criteria for definite, probable or possible ALS, and the site of onset was approximately equal across the bulbar, upper and lower spinal cord segments. The rare variants included atypical primary lateral sclerosis (no DNA available), monomelic ALS (mutation identified), and progressive muscular atrophy (mutation identified). Although most had ALS-like findings, neuropathological studies also showed patients with motor neuron degeneration consistent with progressive muscular atrophy. Hence, this genetic alteration should be considered in any ALS variant, particularly with a positive family history of FTD and/or ALS.
Importantly, none of our cases with FTD/ALS with early and profound aphasia, nor any of our non-fluent/agrammatic and semantic subtypes of primary progressive aphasia, were found to have a hexanucleotide repeat expansion. This observation not only has diagnostic relevance, but also suggests the repeat expansion on C9ORF72 affects cerebral networks in a more bilateral, patchy or diffuse manner as opposed to a focal/asymmetric manner which is what the primary progressive aphasia syndromes reflect. If this repeat expansion is also found to be rare or non-existent in other focal/asymmetric cortical degeneration syndromes such as the corticobasal syndrome, associative agnosia/prosopagnosia, posterior cortical atrophy, etc., this concept of clinical features reflecting more symmetric, patchy or relatively diffuse cerebral neurodegeneration will need to be explained based on the mechanism of how the repeat expansion causes cerebral dysfunction.
Neuropsychological considerations
Consistent with the phenotypic presentation of FTD/ALS, the predominant cognitive profile in subjects with the C9ORF72 hexanucleotide repeat expansion is associated with slowed processing speed, complex attention/executive dysfunction and impairment in rapid word retrieval. This pattern of dysfunction implicates frontal lobe structures and subcortical pathways involving dorsolateral prefrontal cortex and anterior cingulate. In contrast, naming, episodic memory, word reading and gross visuoperceptual/spatial skills are relatively spared, suggesting infrequent or minimal involvement of temporal and parietal lobe structures.
Approximately one-third of our subjects did not demonstrate impairment on cognitive screening measures. This finding may reflect the known poor sensitivity of screening measures to executive dysfunction or the presence of cases with behavioural variant FTD that have yet to develop cognitive deficits. It also bears noting that neuropsychological tests in common practice may not capture the entire spectrum of cognitive impairment possible in behavioural variant FTD or FTD/ALS (Torralva et al., 2009). Thus, the need for broad-based and more sensitive measures of cognitive assessment will become paramount when evaluating subjects in the presymptomatic and early symptomatic stages of the disease.
Neuroimaging considerations
The syndrome of behavioural variant FTD is typically associated with mild to marked frontal and/or temporal lobe abnormalities, particularly in the non-dominant cerebral hemisphere (usually right), on structural and functional neuroimaging studies (Miller et al., 1991; Rosen et al., 2002; Foster, 2003; Whitwell et al., 2004, 2005, 2009; Whitwell and Jack, 2007; Rohrer, 2011). On MRI, the degree of cerebral cortical atrophy in patients with C9ORF72 mutation tended to be rather mild, and was most apparent in the dorsolateral prefrontal and insular cortex. The temporal lobes were normal to only mildly affected. Some degree of parietal cortical atrophy was evident in some cases. And, the pattern of atrophy was never in a strikingly focal or asymmetric manner in any of our cases. Signal changes on T2-weighted and fluid attenuation inversion recovery images in the cortex or subcortical white matter were also minimal or non-existent.
The findings on SPECT and PET were similar to those on MRI, with the dorsolateral prefrontal cortex, and anterior ± medial cingulate cortex involved. This topography of atrophy, hypoperfusion and hypometabolism correlates well with other features such as the prominent apathy and inertia as part of the behavioural variant FTD phenotype. The neuroimaging findings are also correlated with poor performance on neuropsychological measures that assess psychomotor speed, word fluency and sustained attention. Similar to the MRI findings in some subjects, the degree of hypoperfusion on SPECT or hypometabolism on PET was quite mild in some despite the presence of an obvious behavioural variant FTD syndrome. Also, a few cases had atrophy on MRI or hypometabolism on PET that was apparent in the biparietal/precuneus regions.
Therefore, abnormalities on neuroimaging studies that maximally involve the dorsolateral prefrontal cortex and anteromedial cingulate cortex were the most consistent findings. Parietal and/or temporal cortex abnormalities were present in some. Findings were mild in many, particularly early in the course, and relatively symmetric cerebral cortical involvement was the rule.
Neuropathological considerations
All cases had TDP-43 pathology, and more than half also had evidence of motor neuron degeneration and substantia nigra degeneration. Overt clinical evidence of MND or parkinsonism was not always associated with motor neuron or substantia nigra degeneration, respectively. Cortical neuronal loss, gliosis and spongiosis was most prominent in frontal lobe, with less in parietal and temporal lobes. Hippocampal sclerosis, which is common in FTLD-TDP (Josephs and Dickson, 2007) was detected in less than half the cases with c9FTD/ALS, possibly owing to the greater frequency of FTLD-MND in this series, where it has been previously shown that hippocampal sclerosis is less frequent (Josephs and Dickson, 2007). In an unselected autopsy series of c9FTD/ALS, MND was less common, while hippocampal sclerosis was more common than in this series (Murray et al., 2011). The paucity of prominent amnestic syndromes in the present series may be accounted for partly by the low frequency of hippocampal sclerosis.
The TDP-43 pathology was variable, with the majority being type A. Type A (Mackenzie type 1) is characterized by many cortical neuronal cytoplasmic inclusions and dystrophic neurites often with intranuclear inclusions and widespread involvement of subcortical areas, while type B (Mackenzie type 3) have many neuronal cytoplasmic inclusions, but few dystrophic neurites and more limited subcortical pathology (Josephs et al., 2009). These findings contrast to what was expected from previously suggested pathological correlates of c9FTD/ALS (Boxer et al., 2011; Mackenzie et al., 2011), which was suggested to be associated with type B (Mackenzie type 3) TDP-43 pathology. The explanation for the heterogeneity in TDP-43 pathology remains to be determined. It is of note that none of the cases had type C (Mackenzie type 2) pathology, which is associated with prominent cortical neuritic pathology with few cortical neuronal inclusions and clinically by the semantic subtype of primary progressive aphasia, was also not a clinical syndrome found in any patient in this series. The present findings contrast with detection of at least a few cases with type C in an unselected autopsy series of cases with the C9ORF72 mutation (Murray et al., 2011).
It has been suggested that ubiquitin-positive (P62-positive) inclusions in cerebellar granule neurons are characteristic of c9FTD/ALS (Al-Sarraj et al., 2011; Boxer et al., 2011; Murray et al., 2011), and we were able to confirm this except for three cases in which such inclusions could not be detected. It has also been suggested that in the hippocampus, cases with c9FTD/ALS have neuronal inclusions that are disproportionately positive for ubiquitin compared with TDP-43 (Al-Sarraj et al., 2011). We were able to confirm this finding. These are promising pathological features that might one day prove to lead to a specific and sensitive biomarker for c9FTD/ALS when their protein composition is eventually determined.
Comparison of ante-mortem and post-mortem features and findings in subjects with mutations in C9ORF72, MAPT and PGRN
Comparing and contrasting the clinical, neuropsychological, neuroimaging and neuropathological data between C9ORF72, MAPT and PGRN features will provide insights for clinicians to better target genetic testing (particularly for those with the behavioural variant FTD phenotype), and also enlighten our understanding of neurodegeneration associated with different mutations. A summary of the known features associated with these three mutations is shown in Table 6. Future analyses will likely include comparisons such as those shown in Supplementary Figs 5 and 6.
Table 6.
Key features of c9FTD/ALS due to the GGGGCC hexanucleotide repeat expansion in C9ORF72 compared with FTLD due to mutations in the genes encoding MAPT and PGRN
| Feature | C9ORF72 | MAPT | PGRN |
|---|---|---|---|
| Demographic | |||
| Sex | Male = Female | Male = Female | Male = Female |
| Age at onset, median (range) (years) | 52 (33–72) | 45 (25–65) | 59 (35–85) |
| Survival, median (range) (years) | 5 (1–17) | 7 (2–25) | 6 (3–15) |
| Inheritance | |||
| Inheritance pattern | Autosomal dominant | Autosomal dominant | Autosomal dominant |
| Frequency of sporadic cases | ++ | + | + |
| Penetrance | Probably high | Almost 100% | 90% by age 70 |
| Anticipation suggested | ++ | 0 | + |
| Clinical | |||
| Behavioural variant FTD phenotype | ++++ | ++++ | +++ |
| ALS phenotype | ++++ | + | + |
| FTD/ALS phenotype | +++ | + | + |
| Parkinsonism | ++ | +++ | ++ |
| PPA/corticobasal syndrome phenotypea | 0 | + | ++ |
| Alzheimer-like phenotypea | + | + | + |
| Neuropsychological | |||
| Impaired psychomotor speed | ++++ | ++++ | ++++ |
| Impaired complex attention/executive functioning | ++++ | ++++ | ++++ |
| Impaired word retrieval | ++++ | +++ | +++ |
| Impaired episodic memory | ++ | ++ | ++ |
| Impaired gross visuospatial functioning | + | + | + |
| Impaired confrontation naming | + | +++ | ++ |
| Impaired word reading | 0 | + | + |
| MRI | |||
| Dorsolateral frontal/insular atrophy | +++ | ++ | ++ |
| Parietal cortex atrophy | ++ | + | ++ |
| Temporal cortex atrophy | + | +++ | ++ |
| Markedly focal or asymmetric atrophy | 0 | + | +++ |
| White matter signal changes | 0 | + | ++ |
| SPECT/PET | |||
| Dorsomedial frontal/cingulate abnormal | +++ | ++ | ++ |
| Parietal cortex abnormal | ++ | + | ++ |
| Temporal cortex abnormal | + | +++ | ++ |
| Markedly focal or asymmetric findings | 0 | + | +++ |
| Subcortical structures abnormal | 0 | + | + |
| Neuropathology | |||
| Key proteinopathy | TDP-43 | tau | TDP-43 |
| TDP-43 type pathology | A and B | Not applicable | A only |
0 = not present;+ =infrequent;++ occurred with some frequency; +++ moderately frequent; ++++ very frequent.
a Since only probands with the behavioural variant FTD, non-fluent/agrammatic and semantic subtypes of primary progressive aphasia (PPA), and ALS were screened for the GGGGCC expansion, the frequency of primary progressive aphasia, corticobasal syndrome, autosomal dominant, and other phenotypes with this mutation may be higher than suggested here. Yet among those probands screened, and their affected relatives, the frequencies of these syndromes were low.
Implications for research and future clinical practice
These findings suggest that genetic testing for the hexanucleotide repeat expansion in C9ORF72 should be considered in anyone with the FTD and/or ALS phenotype and a positive family history of dementia, parkinsonism or ALS. The presence of multiple affected relatives, absence of primary progressive aphasia in any affected relatives, symmetric and relatively mild frontal ± parietal and/or temporal atrophy on MRI, and FTLD-TDP pathology in any relative should particularly raise suspicion of c9FTD/ALS. Clinical genetic testing will surely be developed in the near future. Considering that the frequency of the hexanucleotide repeat expansion in C9ORF72 already exceeds the frequency of mutations in the other known genes associated with familial FTD and/or ALS, it is highly likely that a significant proportion of individuals with familial FTD and/or ALS without a mutation in the other known genes will be found to harbour the hexanucleotide repeat expansion in C9ORF72.
Funding
The ‘Mayo Alzheimer's Disease Research Centre’ (P50 AG016574); the ‘Mayo Alzheimer's Disease Patient Registry’ (UO1 AG006786); ‘Identifying Mechanisms of Dementia: Role for MRI in the Era of Molecular Imaging’ (RO1 AG011378); the ALS Association (to R.R.); the ALS Therapy Alliance (to R.R.); the MCF ALS Centre donor funds (to K.B.B.); the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer's Disease Research Program of the Mayo Foundation, and the NIH/NINDS Morris K. Udall Centre of Excellence for Parkinson's Disease Research at Mayo Clinic Florida (P50 NS072187 and P50 NS072187-01S2); NIH (grants R01 NS065782 and R01 AG026251, to R.R.).
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
We thank Drs Ian Mackenzie, Adam Boxer and Bruce Miller for collaborating on the VSM-20 kindred, and many colleagues for referring subjects to our centre. We also extend our appreciation to the late Dr Emre Kokmen who personally examined many of the affected relatives in Kindred 4, Drs Glenn E. Smith PhD and Robert J. Ivnik PhD for their expertise on neuropsychological aspects of these subjects, and to the staff of the ADRC, ADPR and ALS Centre for participating in the evaluation and care of the patients and families. The assistance of Pamela Desaro, Amelia Johnston and Thomas Kryston is acknowledged for the collection of ALS DNA samples and clinical data in the MCF ALS Centre. We particularly thank the patients and their families for participating in ageing and neurodegenerative disease research.
Glossary
Abbreviations
- ALS
amyotrophic lateral sclerosis
- FTD
frontotemporal dementia
- FTLD
frontotemporal lobar degeneration
- MND
motor neuron disease
- SPECT
single photon emission computed tomography
- TDP-43
Transactive response DNA binding protein molecular weight 43
References
- Al-Sarraj S, King A, Troakes C, Smith B, Maekawa S, Bodi I, et al. p62 positive, TDP-43 negative, neuronal cytoplasmic and intranuclear inclusions in the cerebellum and hippocampus define the pathology of C9orf72-linked FTLD and MND/ALS. Acta Neuropathol. 2011;122:691–702. doi: 10.1007/s00401-011-0911-2. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston K. Unified segmentation. Neuroimage. 2005;26:839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Baker M, Mackenzie I, Pickering-Brown S, Gass J, Rademakers R, Lindholm C, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–9. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- Benton A, Sivan A, Hamsher K, Varney N, Spreen O. Contributions to neuropsychological assessment: A clinical manual. second edn. New York: Oxford University Press; 1994. [Google Scholar]
- Bornstein R. Normative data on selected neuropsychological measures from a nonclinical sample. J Clin Psychol. 1985;42:651–9. [Google Scholar]
- Boxer AL, Mackenzie IR, Boeve BF, Baker M, Seeley WW, Crook R, et al. Clinical, neuroimaging and neuropathological features of a new chromosome 9p-linked FTD-ALS family. J Neurol Neurosurg Psychiatry. 2011;82:196–203. doi: 10.1136/jnnp.2009.204081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Brooks B, Miller R, Swash M, Munsat T. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–9. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- Burrell J, Kiernan M, Vucic S, Hodges J. Motor neuron dysfunction in frontotemporal dementia. Brain. 2011;134:2582–94. doi: 10.1093/brain/awr195. [DOI] [PubMed] [Google Scholar]
- Byrne S, Bede P, Elamin M, Kenna K, Lynch C, McLaughlin R, et al. Proposed criteria for familial amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2011;12:157–9. doi: 10.3109/17482968.2010.545420. [DOI] [PubMed] [Google Scholar]
- Cruts M, Gijselinck I, van der Zee J, Engelborghs S, Wils H, Pirici D, et al. Null mutations in progranulin cause ubiquitin positive frontotemporal dementia linked to chromosome 17q21. Nature. 2006;442:920–4. doi: 10.1038/nature05017. [DOI] [PubMed] [Google Scholar]
- DeJesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–56. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis D, Kramer J, Kaplan E, Ober B. California verbal learning test. 2nd edn. San Antonio, TX: Psychological Corporation; 2000. [Google Scholar]
- Folstein M, Folstein S, McHugh P. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Foster NL. Validating FDG-PET as a biomarker for frontotemporal dementia. Exp Neurol. 2003;184(Suppl 1):S2–8. doi: 10.1016/s0014-4886(03)00360-1. [DOI] [PubMed] [Google Scholar]
- Gijselinck I, Engelborghs S, Maes G, Cuijt I, Peeters K, Mattheijssens M, et al. Identification of 2 Loci at chromosomes 9 and 14 in a multiplex family with frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Arch Neurol. 2010;67:606–16. doi: 10.1001/archneurol.2010.82. [DOI] [PubMed] [Google Scholar]
- Golden C. The STROOP color and word test (Manual) Chicago: Stoelting Company; 1978. [Google Scholar]
- Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–14. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes A, Ben-Shlomo Y, Daniel S, Lees A. What features improve the accuracy of clinical diagnosis in Parkinson's disease? A clinicopathologic study. Neurology. 1992;42:1142–6. doi: 10.1212/wnl.42.6.1142. [DOI] [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, et al. Association of missense and 5'-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393:702–5. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- Ivnik R, Malec J, Smith G, Tangalos E, Petersen R, Kokmen E, et al. Mayo's Older American Normative Studies: WAIS-R, WMS-R, and AVLT norms for ages 56–97. The Clin Neuropsychol. 1992;6(Suppl):1–104. [Google Scholar]
- Ivnik R, Malec J, Smith G, Tangalos E, Petersen R. Neuropsychological tests' norms above age 55: COWAT, BNT, MAE Token, WRAT-R reading, AMNART, STROOP, TMT and JLO. Clin Neuropsychol. 1996;10:262–78. [Google Scholar]
- Ivnik RJ, Smith GE, Lucas JA, Tangalos EG, Kokmen E, Petersen RC. Free and cued selective reminding test: MOANS norms. J Clin Exper Neuropsychol. 1997;19:676–91. doi: 10.1080/01688639708403753. [DOI] [PubMed] [Google Scholar]
- Josephs K, Stroh A, Dugger B, Dickson D. Evaluation of subcortical pathology and clinical correlations in FTLD-U subtypes. Acta Neuropathol. 2009;118:349–58. doi: 10.1007/s00401-009-0547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Parisi JE, Knopman DS, Boeve BF, Petersen RC, Dickson DW. Clinically undetected motor neuron disease in pathologically proven frontotemporal lobar degeneration with motor neuron disease. Arch Neurol. 2006;63:506–12. doi: 10.1001/archneur.63.4.506. [DOI] [PubMed] [Google Scholar]
- Josephs KA, Dickson DW. Hippocampal sclerosis in tau-negative frontotemporal lobar degeneration. Neurobiol Aging. 2007;28:1718–22. doi: 10.1016/j.neurobiolaging.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Kabashi E, Valdmanis PN, Dion P, Spiegelman D, McConkey BJ, Vande Velde C, et al. TARDBP mutations in individuals with sporadic and familial amyotrophic lateral sclerosis. Nat Genet. 2008;40:572–4. doi: 10.1038/ng.132. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. The Boston naming test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- Kwiatkowski TJ, Jr, Bosco DA, Leclerc AL, Tamrazian E, Vanderburg CR, Russ C, et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323:1205–8. doi: 10.1126/science.1166066. [DOI] [PubMed] [Google Scholar]
- Le Ber I, Camuzat A, Berger E, Hannequin D, Laquerriere A, Golfier V, et al. Chromosome 9p-linked families with frontotemporal dementia associated with motor neuron disease. Neurology. 2009;72:1669–76. doi: 10.1212/WNL.0b013e3181a55f1c. [DOI] [PubMed] [Google Scholar]
- Lillo P, Garcin B, Hornberger M, Bak T, Hodges J. Neurobehavioral features in frontotemporal dementia with amyotrophic lateral sclerosis. Arch Neurol. 2010;67:826–30. doi: 10.1001/archneurol.2010.146. [DOI] [PubMed] [Google Scholar]
- Lindblad K, Schalling M. Expanded repeat sequences and disease. Semin Neurol. 1999;19:289–99. doi: 10.1055/s-2008-1040845. [DOI] [PubMed] [Google Scholar]
- Lomen-Hoerth C, Anderson T, Miller B. The overlap of amyotrophic lateral sclerosis and frontotemporal dementia. Neurology. 2002;59:1077–9. doi: 10.1212/wnl.59.7.1077. [DOI] [PubMed] [Google Scholar]
- Lomen-Hoerth C. Characterization of amyotrophic lateral sclerosis and frontotemporal dementia. Dement Geriatr Cogn Disord. 2004;17:337–41. doi: 10.1159/000077167. [DOI] [PubMed] [Google Scholar]
- Lucas JA, Ivnik RJ, Smith GE, Bohac DL, Tangalos EG, Graff-Radford NR, et al. Mayo's older Americans normative studies: category fluency norms. J Clin Exp Neuropsychol. 1998a;20:194–200. doi: 10.1076/jcen.20.2.194.1173. [DOI] [PubMed] [Google Scholar]
- Lucas JA, Ivnik RJ, Smith GE, Bohac DL, Tangalos EG, Kokmen E, et al. Normative data for the Mattis Dementia Rating Scale. J Clin Exp Neuropsychol. 1998b;20:536–47. doi: 10.1076/jcen.20.4.536.1469. [DOI] [PubMed] [Google Scholar]
- Luty AA, Kwok JB, Thompson EM, Blumbergs P, Brooks WS, Loy CT, et al. Pedigree with frontotemporal lobar degeneration–motor neuron disease and Tar DNA binding protein-43 positive neuropathology: genetic linkage to chromosome 9. BMC Neurol. 2008;8:32. doi: 10.1186/1471-2377-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie I, Neumann M, Baborie A, Sampathu D, Du Plessis D, Jaros E, et al. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol. 2011;122:111–3. doi: 10.1007/s00401-011-0845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie IR, Baborie A, Pickering-Brown S, Du Plessis D, Jaros E, Perry RH, et al. Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: classification and relation to clinical phenotype. Acta Neuropathol. 2006;112:539–49. doi: 10.1007/s00401-006-0138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattis S. Dementia Rating Scale: Professional Manual. Odessa: Psychological Assessment Resources; 1988. [Google Scholar]
- Members of the Department of Neurology MC. Clinical Examinations in Neurology. 6th edn. St. Louis: Mosby - Year Book, Inc.; 1998. [Google Scholar]
- Miller BL, Cummings JL, Villanueva-Meyer J, Boone K, Mehringer CM, Lesser IM, et al. Frontal lobe degeneration: clinical, neuropsychological, and SPECT characteristics. Neurology. 1991;41:1374–82. doi: 10.1212/wnl.41.9.1374. [DOI] [PubMed] [Google Scholar]
- Mirra S, Heyman A, McKeel D, Sumi S, Crain B, Brownlee L, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer's disease. Neurology. 1991;41:479–86. doi: 10.1212/wnl.41.4.479. [DOI] [PubMed] [Google Scholar]
- Mok K, Traynor BJ, Schymick J, Tienari PJ, Laaksovirta H, Peuralinna T, et al. The chromosome 9 ALS and FTD locus is probably derived from a single founder. Neurobiol Aging. 2012;33(209):e3–e8. doi: 10.1016/j.neurobiolaging.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita M, Al-Chalabi A, Andersen PM, Hosler B, Sapp P, Englund E, et al. A locus on chromosome 9p confers susceptibility to ALS and frontotemporal dementia. Neurology. 2006;66:839–44. doi: 10.1212/01.wnl.0000200048.53766.b4. [DOI] [PubMed] [Google Scholar]
- Murray ME, Dejesus-Hernandez M, Rutherford NJ, Baker M, Duara R, Graff-Radford NR, et al. Clinical and neuropathologic heterogeneity of c9FTD/ALS associated with hexanucleotide repeat expansion in C9ORF72. Acta Neuropathol. 2011;122:673–90. doi: 10.1007/s00401-011-0907-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neary D, Snowden J, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–54. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- Pearson JP, Williams NM, Majounie E, Waite A, Stott J, Newsway V, et al. Familial frontotemporal dementia with amyotrophic lateral sclerosis and a shared haplotype on chromosome 9p. J Neurol. 2011;258:647–55. doi: 10.1007/s00415-010-5815-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet O, Petersen A, Yin Ka Lam B, Gabery S, Murphy K, Hodges JR, et al. Eating and hypothalamus changes in behavioral-variant frontotemporal dementia. Ann Neurol. 2011;69:312–9. doi: 10.1002/ana.22244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134:2456–77. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitan R. Validity of the Trail-Making Test as an indication of organic brain damage. Percept Mot Skills. 1958;8:271–6. [Google Scholar]
- Renton AE, Majounie E, Waite A, Simon-Sanchez J, Rollinson S, Gibbs JR, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–68. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey A. L'Examen Clinique en Psychologie. Paris, France: Presses Universitaires de France; 1964. [Google Scholar]
- Roberts R, Geda Y, Knopman D, Cha R, Pankratz V, Boeve B, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30:58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer JD. Behavioural variant frontotemporal dementia-defining genetic and pathological subtypes. J Mol Neurosci. 2011;45:583–8. doi: 10.1007/s12031-011-9542-2. [DOI] [PubMed] [Google Scholar]
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Rosen HJ, Gorno-Tempini ML, Goldman WP, Perry RJ, Schuff N, Weiner M, et al. Patterns of brain atrophy in frontotemporal dementia and semantic dementia. Neurology. 2002;58:198–208. doi: 10.1212/wnl.58.2.198. [DOI] [PubMed] [Google Scholar]
- Sreedharan J, Blair IP, Tripathi VB, Hu X, Vance C, Rogelj B, et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319:1668–72. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh T, Hubley A. Normative data for the 60-item Boston Naming Test (BNT) for cognitively intact adults aged 25 to 88 years. J Clin Exp Neuropsychol. 1997;19:922–32. doi: 10.1080/01688639708403773. [DOI] [PubMed] [Google Scholar]
- Torralva T, Roca M, Gleichgerrcht E, Bekinschtein T, Manes F. A neuropsychological battery to detect specific executive and social cognitive impairments in early frontotemporal dementia. Brain. 2009;132:1299–309. doi: 10.1093/brain/awp041. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Valdmanis P, Dupre N, Bouchard J, Camu W, Salachas F, Meininger V, et al. Three families with amyotrophic lateral sclerosis and frontotemporal dementia with evidence of linkage to chromosome 9p. Arch Neurol. 2007;64:240–5. doi: 10.1001/archneur.64.2.240. [DOI] [PubMed] [Google Scholar]
- Vance C, Al-Chalabi A, Ruddy D, Smith BN, Hu X, Sreedharan J, et al. Familial amyotrophic lateral sclerosis with frontotemporal dementia is linked to a locus on chromosome 9p13.2–21.3. Brain. 2006;129:868–76. doi: 10.1093/brain/awl030. [DOI] [PubMed] [Google Scholar]
- Vance C, Rogelj B, Hortobagyi T, De Vos KJ, Nishimura AL, Sreedharan J, et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323:1208–11. doi: 10.1126/science.1165942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemuri P, Simon G, Kantarci K, Whitwell JL, Senjem ML, Przybelski SA, et al. Antemortem differential diagnosis of dementia pathology using structural MRI: Differential-STAND. NeuroImage. 2011;55:522–31. doi: 10.1016/j.neuroimage.2010.12.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts G, Wymer J, Kovach M, Mehta S, Mumm S, Darvish D, et al. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet. 2004;36:377–81. doi: 10.1038/ng1332. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Revised (Manual) San Antonio: The Psychological Corporation; 1981. [Google Scholar]
- Wechsler D. Wechsler Memory Scale-Revised (Manual) New York: Psychological Corporation; 1987. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3rd edn. San Antonio, TX: The Psychological Corporation; 1997a. [Google Scholar]
- Wechsler D. Wechsler Memory Scale. 3rd edn. San Antonio, TX: The Psychological Corporation; 1997b. [Google Scholar]
- Whitwell JL, Warren JD, Josephs KA, Godbolt AK, Revesz T, Fox NC, et al. Voxel-based morphometry in tau-positive and tau-negative frontotemporal lobar degenerations. Neurodegener Dis. 2004;1:225–30. doi: 10.1159/000080990. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Josephs KA, Rossor MN, Stevens JM, Revesz T, Holton JL, et al. Magnetic resonance imaging signatures of tissue pathology in frontotemporal dementia. Arch Neurol. 2005;62:1402–8. doi: 10.1001/archneur.62.9.1402. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Jack CR., Jr Neuroimaging in dementia. Neurol Clin. 2007;25:843–57. doi: 10.1016/j.ncl.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Whitwell JL, Przybelski SA, Weigand SD, Ivnik R, Prashanthi V, Gunter JL, et al. Distinct anatomical subtypes of the behavioural variant of frontotemporal dementia: A Cluster Analysis Study. Brain. 2009;132:2932–46. doi: 10.1093/brain/awp232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson G. The Wide Range Achievement Test: Manual. Wilmington, DE: Wide Range; 1993. [Google Scholar]
- Woolley S, York M, Moore D, Strutt A, Murphy J, Schulz P, et al. Detecting frontotemporal dysfunction in ALS: utility of the ALS Cognitive Behavioral Screen (ALS-CBS) Amyotroph Lateral Scler. 2010;11:303–11. doi: 10.3109/17482961003727954. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.