Abstract
Multiple sclerosis is a chronic inflammatory disease of the central nervous system, associated with demyelination and neurodegeneration. The mechanisms of tissue injury are poorly understood, but recent data suggest that mitochondrial injury may play an important role in this process. Mitochondrial injury can be triggered by reactive oxygen and nitric oxide species, and we recently provided evidence for oxidative damage of oligodendrocytes and dystrophic axons in early stages of active multiple sclerosis lesions. In this study, we identified potential sources of reactive oxygen and nitrogen species through gene expression in carefully staged and dissected lesion areas and by immunohistochemical analysis of protein expression. Genome-wide microarrays confirmed mitochondrial injury in active multiple sclerosis lesions, which may serve as an important source of reactive oxygen species. In addition, we found differences in the gene expression levels of various nicotinamide adenine dinucleotide phosphate oxidase subunits between initial multiple sclerosis lesions and control white matter. These results were confirmed at the protein level by means of immunohistochemistry, showing upregulation of the subunits gp91phox, p22phox, p47phox, nicotinamide adenine dinucleotide phosphate oxidase 1 and nicotinamide adenine dinucleotide phosphate oxidase organizer 1 in activated microglia in classical active as well as slowly expanding lesions. The subunits gp91phox and p22phox were constitutively expressed in microglia and were upregulated in the initial lesion. In contrast, p47phox, nicotinamide adenine dinucleotide phosphate oxidase 1 and nicotinamide adenine dinucleotide phosphate oxidase organizer 1 expression were more restricted to the zone of initial damage or to lesions from patients with acute or early relapsing/remitting multiple sclerosis. Double labelling showed co-expression of the nicotinamide adenine dinucleotide phosphate oxidase subunits in activated microglia and infiltrated macrophages, suggesting the assembly of functional complexes. Our data suggest that the inflammation-associated oxidative burst in activated microglia and macrophages plays an important role in demyelination and free radical-mediated tissue injury in the pathogenesis of multiple sclerosis.
Keywords: multiple sclerosis, reactive oxygen species, oxidative injury, NADPH oxidase, demyelination, neurodegeneration
Introduction
Multiple sclerosis is a chronic inflammatory disease of the CNS leading to focal as well as diffuse demyelination and neurodegeneration in the CNS (Lassmann et al., 2007). Different mechanisms might contribute to tissue injury in multiple sclerosis, but one of the major driving forces was recently suggested to be mitochondrial damage and subsequent energy failure (Lu et al., 2000; Dutta et al., 2006; Mahad et al., 2008a; Trapp and Stys 2009; Witte et al., 2009, 2010). Mitochondrial injury in active multiple sclerosis lesions mainly affects complex IV and might explain characteristic pathological features of multiple sclerosis lesions, including demyelination and oligodendrocyte apoptosis (Veto et al., 2010), destruction of small diameter axons (Mahad et al., 2008b, 2009), neurodegeneration (Campbell et al., 2011), differentiation arrest of oligodendrocyte progenitor cells and remyelination failure (Ziabreva et al., 2010), as well as astrocyte dysfunction (Sharma et al., 2010). In vitro data and experimental multiple sclerosis animal models provide evidence that mitochondrial injury can be induced by reactive oxygen and nitrogen species (Bolanos et al., 1997; Higgins et al., 2010; Witte et al., 2010; Nikić et al., 2011). The mitochondrion itself is not only affected by reactive oxygen species-induced damage, but is also a potent source of reactive oxygen species production, as disturbed oxidative phosphorylation leads to increased reactive oxygen species generation (Murphy, 2009). Reactive oxygen and nitrogen species-induced damage to biological macromolecules, such as polyunsaturated fatty acids in membrane lipids, proteins and DNA/RNA have been described to occur in multiple sclerosis lesions (Cross et al., 1998; Liu et al., 2001; Diaz-Sanchez et al., 2006; van Horssen et al., 2008). In a recent study, we observed oxidation of DNA in oligodendrocytes, and oxidized lipids in myelin, oligodendrocytes and axons in association with active demyelination and neurodegeneration (Haider et al., 2011).
Active lesions in the relapsing–remitting as well as in the progressive course are always associated with inflammation (Frischer et al., 2009), and the extent of lipid and DNA oxidation correlated significantly with inflammation (Haider et al., 2011). Besides an unavoidable by-product of cellular respiration, reactive oxygen species are synthesized by dedicated enzyme systems, including myeloperoxidase (MPO), xanthine oxidase and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase in activated microglia and macrophages. MPO has been shown to be predominantly expressed by macrophages and activated microglia within and in close vicinity of multiple sclerosis plaques in white matter lesions (Marik et al., 2007; Gray et al., 2008a), as well as in a subtype of microglia surrounding cortical lesions (Gray et al., 2008b). Expression of NADPH oxidases, which convert molecular oxygen to superoxide, has so far not been analysed in multiple sclerosis lesions.
Hence, the aim of our current project was to identify possible sources for reactive oxygen species production in relation to demyelination and neurodegeneration in multiple sclerosis. In a first step, we studied global changes in the expression of genes involved in mitochondrial function and oxidative stress through genome-wide microarray analysis of gene expression in carefully dissected lesion areas of patients with fulminant acute multiple sclerosis. Molecules of the NADPH oxidase complexes were then analysed regarding protein expression by immunocytochemistry in a large set of multiple sclerosis lesions. Our study suggests that oxidative burst through reactive oxygen species production by NADPH oxidases is a major driving force for demyelination and neurodegeneration in multiple sclerosis lesions.
Materials and methods
Human autopsy tissues
This study was performed on autopsy brains of patients and control cases from paraffin blocks archived in the Centre of Brain Research, Medical University of Vienna, Austria and the Department of Neuropathology, University Medical Centre Amsterdam, The Netherlands. The multiple sclerosis samples from Vienna (total n = 30; female to male ratio 19:11; age range 34–84 years) contained seven cases of Marburg's type of acute multiple sclerosis, two of them with Balo type concentric lesions, eight cases of relapsing–remitting multiple sclerosis, seven cases of secondary progressive multiple sclerosis and seven cases of primary progressive multiple sclerosis. For one patient, who showed inactive lesions, the clinical course remained uncertain. As control, we included autopsy tissues from patients without neurological disease and without any CNS lesions (n = 18; female to male ratio 11:7; age range 30–97 years). Detailed clinical data on these patients have been published recently in our studies on inflammation and oxidative damage in multiple sclerosis (Frischer et al., 2009; Haider et al., 2011), and our current study has been performed on the cases and lesions, described in these studies. Furthermore, 7/8 cases of multiple sclerosis with aggressive acute or relapsing disease course, analysed in our present sample, were also included previously in our studies on mitochondrial injury (Mahad et al., 2008) and on initial multiple sclerosis lesions (Marik et al., 2007). The samples from Amsterdam (total n = 11; female to male ratio 8:3; age range 45–80 years) contained 10 cases of secondary progressive multiple sclerosis and 1 case of primary progressive multiple sclerosis. Detailed clinical data on these patients have been recently published (van Horssen et al., 2010).
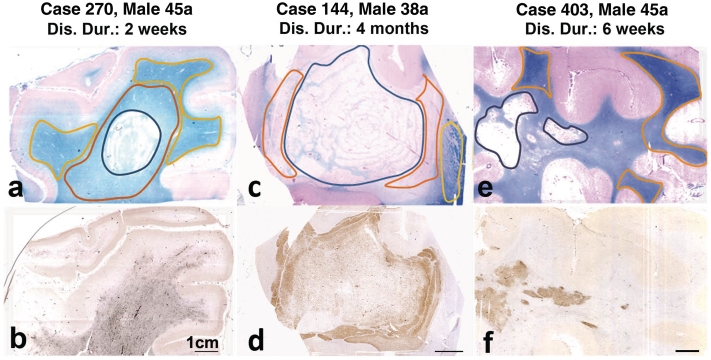
For microarray studies, three of the acute multiple sclerosis cases were selected on the basis of lesion size and activity as well as messenger RNA preservation, assessed by in situ hybridization for proteolipid protein messenger RNA (Fig. 1).
Figure 1.
Acute multiple sclerosis lesions used for gene expression analysis; the structure of the lesions is shown in sections stained with Luxol fast blue (myelin; a, c and e); the lower panel of figures shows the same lesions, stained for p22phox expression in activated macrophages and microglia. In the first patient (a and b), the active lesion (black outline) is surrounded by a broad area of microglia activation with p22phox expression and myelin pallor (red outline; initial lesion), which makes it difficult to see the lesion margin in the staining for macrophages and microglia. In the normal-appearing white matter (yellow outline), myelin density is normal, but there is still moderate microglia activation. In the second patient (c and d), the demyelinated lesion core (black outline) shows concentric rings of preserved myelin. This is surrounded by the initial lesion area with extensive immunoreactivity for p22phox (red outline). The normal-appearing white matter shows normal myelin density and low expression of macrophage antigens (yellow outline). In the third patient (e and f) a dense infiltrate of macrophages with p22phox expression is seen in the area of demyelination (active plaque). In the surrounding white matter, there is little expression of macrophage/microglia antigens. Areas of normal white matter used for gene expression analysis are shown by the yellow outline. Gene expression for proteins involved in oxidative damage and for mitochondrial proteins has been analysed separately in the indicated lesion areas. Dis. Dur = disease duration.
Whole-genome arrays
Whole-genome arrays were performed on material, micro-dissected from sections of formaldehyde-fixed paraffin-embedded archival tissue, cut and mounted onto glass slides. It was performed on material from three patients, who died with fulminant acute multiple sclerosis between 14 days and 4 months after disease onset (Fig. 1). All three patients showed a pattern of active demyelination, following pattern III (Luchinetti et al., 2000). From the sections, we dissected areas of initial lesions (Marik et al., 2007; Lassmann 2011), also defined as ‘pre-phagocytic’ lesions areas (Barnett and Prineas 2004; Henderson et al., 2009). These areas showed a moderate T cell infiltration, pronounced microglia activation, reduction of myelin staining intensity, selective loss of myelin-associated glycoprotein and oligodendrocyte apoptosis but no overt demyelination, and most pronounced presence of oxidized lipids and DNA (Marik et al., 2007; Henderson et al., 2009; Haider et al., 2011). In addition, we dissected areas of early demyelination, characterized by loss of myelin and infiltration with macrophages-containing myelin oligodendrocyte glycoprotein and proteolipid protein reactive myelin debris [early and late active lesion areas according to Brück et al. (1995)] and areas from the normal appearing white matter with moderate microglia activation only. For comparison, we obtained normal white matter from four control individuals without brain disease or neuropathologically detectable lesions.
After histological characterization, consecutively cut sections of 6–10 µm were mounted on glass slides in RNase-free conditions. With this archival formaldehyde-fixed paraffin-embedded tissue, several problems had to be overcome: the time interval between the initial sample acquisition and fixation was unclear, it was not known whether the tissue has been adequately cooled before fixation to prevent the action of RNA degrading enzymes, and the tissue has been fixed with formaldehyde, which induces the formation of methylol cross-links (von Ahlfen et al., 2007). This makes it essentially impossible to retrieve larger amounts of intact messenger RNA.
To overcome these problems, we performed in situ hybridization as described (Breitschopf et al., 1992), using a 1.4 kb RNA probe of proteolipid protein 1 (labelled with digoxigenin) to identify tissue blocks with good RNA preservation, and we only continued with tissues yielding a strong hybridization signal. From these tissues, unstained slides were used to scratch the different lesion areas (Fig. 1) as described previously (Nicolussi et al., 2009). The scratched material from each region of interest was collected into separate vials. We isolated total RNA from this material, using the High Pure FFPE RNA Micro Kit (Roche) as recommended by the manufacturer. We then transcribed the messenger RNA fragments contained in the total RNA pool to complementary DNA, using the Paradise® Reagent System (Arcturus) according to the instructions of the manufacturer. This system uses poly-T primers for the reverse transcription from total RNA to complementary DNA, thereby relying on the presence of the poly(A) tail on the messenger RNA fragments. The obtained complementary DNA was amplified by one round of in vitro transcription and reverse transcription, again using the Paradise Reagent® System as recommended. Then, we tested the quality of the amplified complementary DNA and its suitability for array analysis by polymerase chain reaction. For this purpose, we designed primers specific for the housekeeping gene β-actin (ACTB) in such a way that the binding site of the forward primer was located in a distance of 472 bases from the poly(A) tail of the corresponding messenger RNA. Only when the messenger RNA fragments obtained from the isolation process were sufficiently long, the forward primer was able to bind and a polymerase chain reaction product was detected (Supplementary Table 1). We only continued with isolates that fulfilled this quality requirement, and made a second round of in vitro transcription, again using the Paradise® Reagent System according to the instructions of the manufacturer.
The obtained purified antisense RNA was then sent to imaGenes (imaGenes GmbH www.imagenes-bio.de), where it was labelled with Cy3 and hybridized to Agilent-014850 Whole-Human Genome Microarrays 4 × 44 K G4112A, and where the microarray data images were scanned and analysed using the Agilent Feature Extraction Software (www.agilent.com/chem/fe). The resulting raw data were subjected to quantile normalization. We then evaluated the normalized microarray data based on log2 fold changes in gene expression between the samples of interest and the controls.
With the workflow described above, our RNA probes had a length of at least 480 bp. This was a useful size to identify many, but not all differentially expressed genes. For example, transcripts of p22phox (CYBA) could be detected: the CYBA-oligomere spotted on the Agilent array (A_23_P163506) binds in a distance of 407 bp from the poly(A) tail of the CYBA gene (NM_000101.2). Accordingly, we were able to obtain corresponding signals on the Agilent microarray. The situation was different when binding sites of oligomeres are located outside our RNA fragment size range. For example, the proteolipid protein 1 oligomere spotted on the Agilent array (A_23_P85201) binds at a distance of 1024 bp from the poly(A) tail of the proteolipid protein gene (NM_000533.3). Hence, such transcripts could not be detected. The data discussed in this publication have been deposited in NCBI's Gene Expression Omnibus and are accessible through GEO Series accession number GSE32915 (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE32915).
Neuropathological techniques
Expression of the NADPH oxidase 2 subunits p22phox, gp91phox, p47phox, NADPH oxidase 1 (NOX1) and NADPH oxidase organizer 1 (NOXO1) in different stages of multiple sclerosis lesions were studied with immunohistochemistry on paraffin sections according to established techniques (King et al., 1997; Bauer et al., 2007). A detailed list of primary antibodies, dilutions and corresponding pre-treatment of the sections are given in Table 1. Primary antibody binding was visualized with a biotin/avidin/peroxidase method or with alkaline phosphatase-coupled secondary antibodies (Haider et al., 2011). Diaminobenzidine and fast blue were used as substrates for visualization of peroxidase and alkaline phosphatase, respectively.
Table 1.
Antibodies used for immunocytochemistry
| Primary antibody | Antibody type | Target | Dilution | Pretreatment | Source |
|---|---|---|---|---|---|
| NOX1 | Rabbit (pAB) | NADPH-Oxidase subunit | 1:200 | EDTA pH 9 | Sigma-Aldrich, SAB 4200097 |
| gp91phox | Mouse (mAB) | NADPH-Oxidase subunit | 1:100 | Citrate pH6 | Verhoeven et al., 1989 |
| p22phox | Rabbit (pAB) | NADPH-Oxidase subunit | 1:100 | Citrate pH6 | Santa Cruz, sc-20781 |
| NOXO1 | Rabbit (pAB) | NADPH-Oxidase subunit | 1:200 | Citrate pH6 | Sigma-Aldrich, SAB 2900367 |
| p47phox | Goat (pAB) | NADPH-Oxidase subunit | 1:100 | Citrate pH6 | Abcam, ab 74095 and Lifespan Biosci, LS-B2365 |
| GFAP | Mouse (mAB) | NADPH-Oxidase subunit | 1:200 | Citrate pH6 | Thermo Scientific, USA; MS1376 |
| CD68 | Mouse (mAB) | Macrophages, microglia | 1:100 | EDTA pH 9 | Dako, M0814 |
| IBA-1 | Rabbit (pAB) | Microglia | 1:3000 | EDTA pH9 | WAKO Chemicals, 019-19741 |
| LN3 | Mouse (mAB) | MHCII | 1:50 | Citrate pH6 | Dako |
Double stainings
The cellular localization of the NADPH oxidase markers as well as the co-localization of the complex-forming subunits within the same cell type were examined with double staining with light microscopy (p22phox, p47phox and NOXO1) and/or fluorescent microscopy (p22phox, p47phox, gp91phox and NOX1).
All double labelling was performed using primary antibodies from different species.
The two different primary antibodies were applied together overnight. The secondary system was chosen in a way that couples one of the antibodies to a secondary antibody directly conjugated to peroxidase. The other primary antibody was first bound to a biotinylated secondary antibody, followed by coupling to avidin-linked alkaline phosphatase. Alkaline phosphatase was then first visualized by fast blue BB salt (blue reaction product) and peroxidase with amino ethyl carbazole (red reaction product; for details see Haider et al., 2011).
Double staining for fluorescent microscopy was done in a comparable way except fluorophore-coupled secondary antibodies were used (Cy2, DyLight488, Cy3 or Alexa 546 and 448). The signal of p22phox was enhanced by incubation with biotinylated secondary antibody followed by incubation with streptavidin coupled to the respective fluorophore. Fluorescent preparations were examined using a Leica SP2 confocal scan microscope.
Quantitative analysis
Expression levels of p22phox and gp91phox in different lesion areas and the normal appearing white matter were determined by densitometry, as described in detail (Haider et al., 2011). In short, different lesion areas and normal-appearing white matter were defined on sections stained with Luxol fast blue and for microglia activation (Iba-1 immunoreactivity). From each different multiple sclerosis or control case, 8–34 images (0.61 × 0.46 mm in size) were scanned and stored as JPEG files. The images were processed with Adobe Photoshop CS2 by setting a threshold level (output level = 128) and pixels above this level were deleted. Per cent areas, covered by the signal, were measured with ImageJ. Averages for individual densitometric values were calculated per lesion area per case and the averages compared between different multiple sclerosis lesion areas and control white matter.
Western blot analysis
To assess the protein expression of various key NADPH oxidase subunits, we selected three multiple sclerosis lesion blocks containing active demyelinated lesions and three white matter samples from non-neurological controls. First, two frozen sections were stained for proteolipid protein and MHC-II, to select the active demyelinating areas, which were subsequently outlined with a scalpel on the tissue block. After cutting 50-µm sections, outlined areas were collected (±40–60 mg) and tissue samples were homogenized by incubating the samples with M-PER® buffer (Thermo Scientific) with protease and phosphatase inhibitors (Roche diagnostics GmbH) on ice for 30 min and passing the samples 10 times through an 0.8 mm2 needle (Terumo). Protein concentrations were measured using BCA protein assay (Thermo Scientific) and equal amounts of protein were separated on 10% sodium dodecyl sulphate–polyacrylamide gel electrophoresis gels and transferred to PVDF membranes (Bio-Rad Laboratories). After blocking in Odyssey® blocking buffer (LI-COR Biosciences), membranes were incubated with either anti-gp91phox (1:200), p22phox (1:200) or Nox1 (1:1000) overnight in Odyssey® blocking buffer at 4°C. Primary antibodies were detected by incubation with appropriate IRDye® secondary antibodies (LI-COR Biosciences) for 1 h at room temperature in Odyssey® blocking buffer and quantified using the Odyssey® infrared imaging system (LI-COR Biosciences). Actin quantification was used to correct for total protein loading variation. GraphPad Prism software was used for statistical analyses and Student's t-test was used to compare differences among the control and multiple sclerosis samples with the control group as a reference point. Results were considered significant when P < 0.05.
Statistical analysis
Due to the uneven distribution of the histological data, statistical analysis was performed with non-parametric tests. Descriptive analysis included median value and range. Differences between two groups were assessed with Wilcoxon–Mann–Whitney U-test. Differences between more than two groups were assessed with Kruskal–Wallis test, followed by pair-wise Wilcoxon–Mann–Whitney U-tests. In case of multiple testing (comparison of more than two groups), significant values were corrected with Bonferroni procedure. Interdependence of variables was evaluated by Spearman non-parametric correlation test. The reported P-values are results of two-sided tests. A P < 0.05 is considered statistically significant. For all statistical analysis, mean values per patient for each lesion type and normal-appearing white matter were used.
Results
Pathological alterations in the brain of patients with multiple sclerosis are complex and differ between stages of the disease (relapsing versus progressive) or activity of the disease process (Frischer et al., 2009; Lassmann 2011). Active lesions consist of the classical acute or chronic active lesions, which are characterized by inflammation, blood–brain barrier injury and rapidly developing demyelination and tissue injury. They are most frequently seen in patients with acute or relapsing/remitting multiple sclerosis. In contrast, besides diffuse injury in the normal appearing white and grey matter, the brain of patients with progressive disease contain mainly inactive lesions or slowly expanding active lesions (Kutzelnigg et al., 2005). The latter are characterized by an inactive lesion centre surrounded by a margin with microglia activation, few macrophages with early myelin degradation products and some acute axonal injury (Frischer et al., 2009). In addition, both classical active lesions, and to a lesser extent, slowly expanding lesions are surrounded by a zone of microglia activation associated with initial stages of tissue injury (Lassmann, 2011), called the initial (Marik et al., 2007) or the ‘pre-phagocytic’ lesion stage (Barnett and Prineas, 2004). Since we recently provided evidence for oxidative tissue injury in active multiple sclerosis lesions (Haider et al., 2011), we focused here on the origin of reactive oxygen species and reactive nitrogen species in the early stages of multiple sclerosis lesion development. In a first step, we analysed mitochondrial genes and those that are involved in oxidative stress, to obtain a global view on their expression patterns in different stages of active lesions in three cases of fulminant acute multiple sclerosis in comparison to controls. In a second step, we concentrated on protein subunits of the NADPH complexes by immunocytochemistry in a large sample of different multiple sclerosis lesion types and disease stages.
Microarray studies
The raw data on gene expression in different types of multiple sclerosis lesions are deposited in the Gene Expression Omnibus data repository (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE32915). As potential sources for reactive oxygen species and the respective tissue reaction, we focused in our present analysis on mitochondrial genes and genes that are known to be involved in redox homoeostasis, such as oxidative burst, and in anti-oxidative defence. Highly up- or downregulated genes in comparison to controls were mainly seen in initial lesions and much less in established demyelinated lesions or normal-appearing white matter (Table 2).
Table 2.
Top-regulated mitochondrial genes and genes related to oxidative tissue injury in different stages of active multiple sclerosis lesions (average values per lesion category)
| Mitochondrial genes, normal-appearing white matter | Oxidative stress genes, normal-appearing white matter | Mitochondrial genes, initial lesion | Oxidative stress genes, initial lesion | Mitochondrial genes, early demyelinated lesions | Oxidative stress, early demyelinated lesion | |
|---|---|---|---|---|---|---|
| Downregulated | 7/19 | 0/1 | 48/55 | 7/12 | 11/21 | 0/5 |
| Upregulated | 2/4 | 0/1 | 18/22 | 5/9 | 1/9 | 0/2 |
From the global microarray data, we analysed how many genes encoding for mitochondrial proteins showed expression changes (up- or downregulated) of >3 log2-fold (bold) or >2 log2-fold.
Mitochondrial genes
Mitochondrial genes were highly enriched in the cohort of top-regulated genes (>3-fold; log2) in multiple sclerosis lesions and the most pronounced changes were seen in initial lesion areas. Downregulated expression was seen in 48 genes and upregulated expression in 18 genes (Table 2).
All mitochondrial DNA-encoded genes that were included in the arrays (ND1, ND2, ND3, ND5, ND6, COX1, CYTB) were downregulated in initial multiple sclerosis lesions (Table 3). A similar pattern was seen for nuclear-encoded genes of the respiratory chain, with marked downregulation of genes coding for complex I, and complex IV (Table 3). Regarding other mitochondria-related genes with expression changes of >3-fold (log2), again downregulation was seen in the majority (n = 31), whereas only 16 showed upregulated expression (Table 3). The latter included genes involved in mitochondrial protein synthesis (MRPL18, 14, 23; MRPS15, 22), adenine nucleotide translocation (SLC25A4), which are induced by oxidative stress and are also involved in oxidative stress defence (UCP3, GRPEL1, TXNRD2, ISCU, AASS, ACADL, DMGDH and ACADS).
Table 3.
Gene expression for molecules involved in mitochondrial function in initial (‘pre-phagocytic’) multiple sclerosis lesions: top-regulated genes (>3 log2-fold)
| Downregulated | Upregulated | |
|---|---|---|
| Respiratory chain genes | Complex I | Complex 1 |
| ND1, ND2, ND3, ND5, ND6, | NDUFB10 | |
| NDUFA3, NDUFA4, NDUFA8, NDUFB2. NDUFB8NDUFS5, | ||
| Complex III | ||
| CYTB, | ||
| UQCRQ | ||
| Complex IV | SURF1 | |
| COX1, | ||
| COX6A1, COX6B1, COX7A2 | ||
| Other genes | ACADVL, MRPS24, PTRH2, FXN, ACSM2B, SLC25A17, MRPL16, BCL2L1, PRDX3, ALDH18A1, FDXR, CPT1B, s75896, AW46717, APEX2, CLPP, CYP11A1, MRPL28, HTRA2, TUFM, FXC1, ENDOG, MRPS18B, ARG2, CASQ1, AF086790, NT5M, ALDH4A1, GFM2, s81524, MRPS25 | UCP3, GRPEL1, AASS, MRPL18, ACADL, LOC28521, MRPS15, MRPL23, SLC25A4, TXNRD2, ISCU, MRPS22, CS, DMGDH, MRPL14, ACADS |
This table shows those genes encoding for mitochondrial proteins, which were up- or downregulated (>3 log2-fold) in initial multiple sclerosis lesions compared with controls. Mitochondrial DNA-encoded genes are shown in bold; gene abbreviations and function can be found at www.ihop-net.org and www.sigmaaldrich.com/customer-services/services/basic-research.html.
Genes involved in radical production and response to oxidative stress
As seen for the above described mitochondrial genes, most pronounced changes in the expression of genes involved in the production of reactive oxygen and nitrogen species were seen in initial lesions, followed by demyelinated lesion areas and normal-appearing white matter (Table 2). The most pronounced changes were found for inducible (NOS2A) and endothelial (NOS3) nitric oxide synthases and for subunits of the NADPH oxidase complex 2 (CYBA, CYBB and NCF1; Table 4). In addition, we also found enhanced expression of the reactive oxygen species-generating enzymes MPO, eosinophil peroxidase (EPX) and lactoperoxidase (LPO), but not for xanthine oxidase (XDH). While the expression of genes involved in the production of reactive oxygen species were highly upregulated, expression of nitric oxide synthase genes was reduced compared with controls. In addition to genes involved in the production of reactive oxygen and nitrogen species, we found changes in the expression of genes involved in free radical detoxification, including glutathione peroxidases and peroxiredoxins (Table 4). These findings further support the concept of oxidative stress as a major pathogenic factor in initial multiple sclerosis lesions (Lassmann and van Horssen, 2011).
Table 4.
Expression of genes related to reactive oxygen species production and oxidative defence as well as genes induced by oxidative stress in initial multiple sclerosis lesions (>2 log2-fold changed expression values in at least one of three patients)
| Upregulated | Downregulated | |
|---|---|---|
| Reactive oxygen species production | CYBA, CYBB, NCF1 MPO, EPX, PTGS1, PXDN | NOS1, NOS2A, NOS3, NOX5, MIOX, RAC1, RAC3, |
| Reactive oxygen species detoxification | GPX4, PRDX1, 2, 4 | GPX3, GPX5, PRDX3 |
| Induced by reactive oxygen species | ALOX12, ATOX1, EPHX2, GPR156, MSRA, STK25, OSGIN, GLRX2, PRG3, SEPP1, SGK2, TXNRD2 | APOE, CYGB, PNKP, SCARA3, SFTPD, SIRT2, SRXN1 |
This table lists genes that are involved in production or detoxification of reactive oxygen species or are induced by oxidative stress and are up- or downregulated (>2 log2-fold) in initial multiple sclerosis lesions. Gene abbreviations and function can be found at www.ihop-net.org and www.sigmaaldrich.com/customer-services/services/basic-research.htlm.
Difference in gene expression between different cases in relation to lesion activity
All three cases included in this microarray analysis fulfilled the criteria of highly active acute multiple sclerosis and care was taken to select comparable lesion stages from the material by tissue microdissection. Despite these precautions, differences in gene expression were seen between the cases (Fig. 1). The most marked changes in gene expression were seen in Case 270, a patient with fulminant multiple sclerosis and disease duration of 2 weeks only. Intermediate changes were present in Case 144, who died within 4 months after disease onset and presented with a rapidly enlarging white matter lesion with concentric demyelination. Both of these cases showed, besides demyelinated lesions with massive macrophage infiltration, large areas of initial ‘pre-phagocytic’ lesions (Fig. 1). Only moderate changes in gene expression were seen in Case 403, who died with acute multiple sclerosis with a clinical duration of 1.5 months. The respective section analysed in our study contained large demyelinated plaques with densely packed macrophages with early myelin debris, but showed only a very small rim of initial ‘pre-phagocytic’ lesion area around the plaque. These data suggest a very tight regulation of the expression of molecules involved in oxidative stress, closely depending upon the state of activity of the lesion.
Immunocytochemistry for oxidative burst molecules in multiple sclerosis lesions
Expression of mitochondrial proteins in different types and stages of multiple sclerosis lesions has been extensively described (Lu et al., 2000; Dutta et al., 2006; Mahad 2008a, b, 2009; Witte et al., 2009, 2010) and these data were in part obtained from sections from the same blocks and patients as those used for microarray analysis in this study (Mahad et al., 2008). In addition, we and others have previously shown the expression of inducible nitric oxide synthase (iNOS) and MPO in active multiple sclerosis lesions (Cross et al., 1998; Liu et al., 2001; Marik et al., 2007; Gray et al., 2008a, b; Zeis et al., 2009). So far, however, data on the protein expression of molecules involved in oxidative burst in multiple sclerosis lesions are not available. We therefore analysed the expression of several components of the NADPH oxidase (Nox) complexes.
Nox2 complex
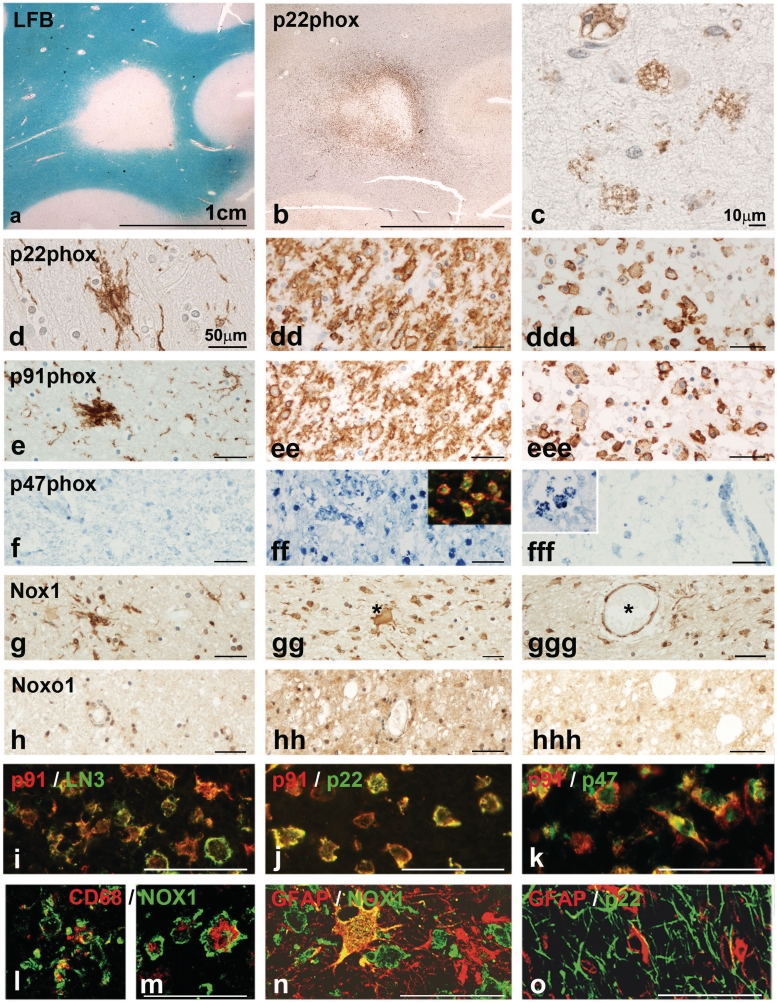
The Nox2 complex is composed of two transmembrane proteins: p22phox (reflected by CYBB in the gene expression arrays) and gp91phox (CYBA) and requires, for functional activation, the association with p47phox (NCF1) as a regulatory subunit, together with p67phox (NCF2) and p40phox (NCF4; Bedard and Krause, 2007). We have therefore analysed the expression of p22phox and gp91phox, as well as p47phox, as a representative of the regulatory elements in lesions and normal-appearing white matter of patients with multiple sclerosis (Figs 1 and 2) and age-matched controls (Supplementary Fig. 1A–E). The proteins p22phox and gp91phox showed very similar expression patterns in patients with multiple sclerosis and controls. In general, both proteins are expressed in microglia cells and macrophages (Table 5), revealing a staining pattern that is similar to that seen with the pan-microglia marker Iba-1. In the white matter of controls (Supplementary Fig. 1A and B) and in the normal-appearing white matter of patients with multiple sclerosis, a moderate density of p22phox- and gp91phox-positive microglia was seen, and these molecules were strongly expressed in microglia nodules when present in multiple sclerosis brains (Fig. 2d and e). At the lesion edge, in particular in areas of initial (‘pre-phagocytic’) lesions, the expression of p22 and gp91phox was intense, due to the marked increase in microglia density and increased expression of these molecules in individual cells (Fig. 2dd and ee). In the demyelinated regions, macrophages that had taken up myelin debris expressed p22phox and gp91phox, albeit to a lesser extent than the initial lesion area (Fig. 2c, ddd and eee). In contrast, the expression pattern of p47phox (NCF1) was more restricted. In control white matter, few perivascular macrophages were stained (Supplementary Fig. 1C). In multiple sclerosis lesions, we found p47phox expression in 5–20% of macrophages and activated microglia and this was predominantly localized in areas of initial tissue damage at the edge of actively demyelinating lesions [‘pre-phagocytic’ lesion areas; (Fig. 2f–fff)].
Figure 2.
(a–c) Active lesion in a patient with primary progressive multiple sclerosis. (a) Luxol fast blue (LFB) myelin staining shows a demyelinated lesion with defined borders. (b) In the adjacent section stained for p22phox intense expression is seen at the active lesion edge, spanning into the adjacent normal appearing white matter (initial lesion area). (c) In the inactive centre of the lesion p22phox is weakly expressed in some macrophages. [d–h(hh)] These images show the expression of oxidative burst associated molecules in the normal appearing white matter (left column), in the zone of initial tissue injury (centre column) and in the demyelinated zone (right column). Most pronounced expression of all proteins is seen in the initial lesion area (centre panel), while expression for p22phox and gp91phox is much weaker in lipid containing macrophages in the lesion centre (right panel). In the normal appearing white matter microglia nodules can be seen, which are intensely stained for p22phox, gp91phox and NOX1. P22phox, gp91phox and p47phox are only expressed in macrophages and microglia (see below), while Nox1 shows a broader expression also in astrocytes (asterisk in gg) and endothelial cells (asterisk labels the vessel with endothelial staining); the expression of Noxo1 is even broader compared with that of Nox1. P47phox staining is absent in the normal appearing white matter (g), while intense expression is seen in macrophages and small microglia like cells; the insert shows expression of p47phox (red) in macrophages stained with LN3 (green). In the lesion centre, only weak reactivity for p47phox is seen, mainly in perivascular macrophages (at higher magnification in the inset). (l–o) Confocal laser microscope images of double staining with different Nox markers and CNS cell-specific markers; the staining combinations are indicated on the figures. These data show co-localization of different Nox components within the same macrophages or microglia cells in the multiple sclerosis lesions. Nox 1 is also expressed in GFAP-positive astrocytes (n) in the absence of p22phox (o). Red and green staining depicts the individual antigens as indicated in the figure; yellow staining represents double staining.
Table 5.
Expression of NADPH subunits in microglia and astrocytes (confocal microscopy double staining)
| p22phox | gp91phox | p47phox | NOX1 | NOXO1 | |
|---|---|---|---|---|---|
| Microglia (Iba1 or CD68) | + | + | + | + | + |
| Astrocytes (GFAP) | − | − | − | + | + |
Immunofluorescence was performed by double staining with markers for NADPH oxidase subunits and for microglia (Iba1 and CD68) or astrocytes (GFAP). Stained sections were analysed by confocal laser microscopy as shown in Fig. 2. GFAP = glial fibrillary acidic protein.
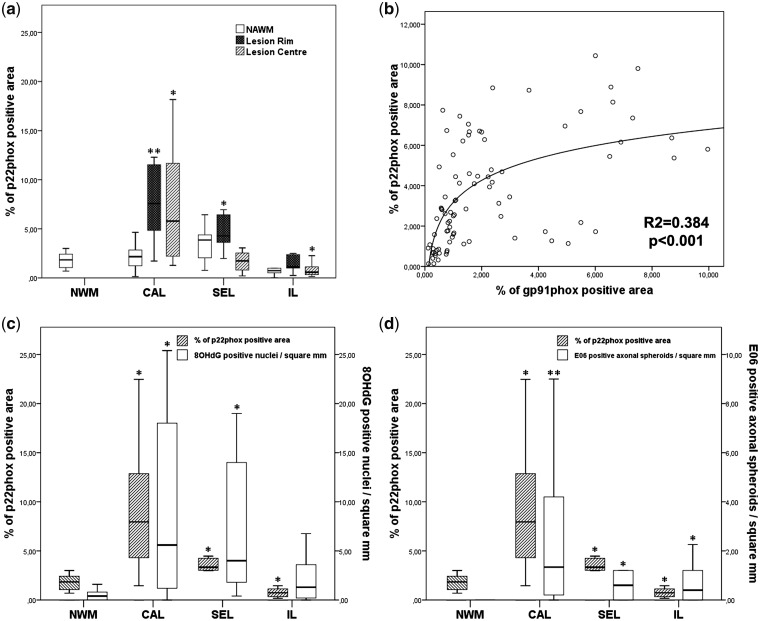
The quantitative analysis of p22phox expression by densitometry revealed additional insights (Fig. 3a). In classical active lesions from early multiple sclerosis, expression in the lesions was very high but in the surrounding normal-appearing white matter, it was similar to that seen in normal control white matter. In contrast, in slowly expanding lesions, p22phox expression in the active lesion parts was, as expected, lower than in classical active lesions and this reflects the lower/milder degree of active tissue injury. Finally, p22phox expression was low in the centre of inactive lesions, reflecting reduced microglia density in these areas compared with normal white matter (Lassmann, 2011). A similar expression pattern was seen with the gp91phox antibody, resulting in a significant correlation between p22phox and gp91phox expression (Fig. 3b).
Figure 3.
(a) Quantitative analysis of p22phox expression in different types of multiple sclerosis lesions. Compared with control white matter, there is a significantly higher expression (P < 0.01) in classical active (CAL) and slowly expanding multiple sclerosis lesions (SEL), and in the normal-appearing white matter (NWM) of slowly expanding lesions; in the lesions p22phox-positive microglia are mainly seen in the active lesion edge (initial lesions, IL) and less in the inactive lesion centre. Furthermore, we found a significant decrease of p22 expressing microglia in the centre of inactive lesions. (b) Correlation between p22 and gp91 expression in different multiple sclerosis cases and lesions. The same areas of normal-appearing white matter and lesions were scanned for p22phox and gp91phox expression and regression was analysed as described in the ‘Material and methods’ section. (c) Comparison between p22phox expression, determined by densitometry and the number of nuclei with oxidized DNA (8OHdG immunoreactivity) within multiple sclerosis lesions. (d) Comparison between p22phox expression and the number of dystrophic axons, immunoreactive for oxidized phospholipids (E06); p22phox and gp91phox immunoreactivity was determined by densitometry; nuclei with oxidized DNA and dystrophic axons, positive for E06 were counted manually (Haider et al., 2011). *P<0.05; **P<0.01.
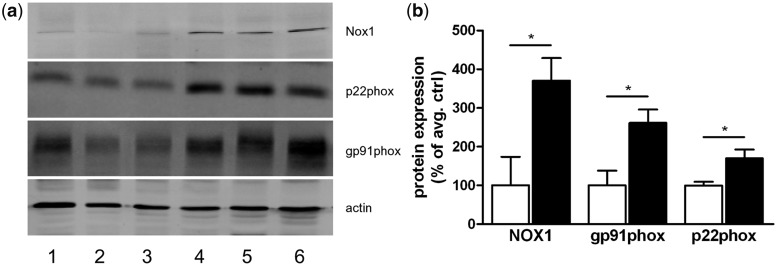
The significantly increased expression of proteins of the Nox2 and Nox1 complexes in multiple sclerosis lesions compared with control white matter was also confirmed by western blot analysis, performed in an independent set of samples (Amsterdam material; Fig. 4).
Figure 4.
(a) Western blot of three control samples (Lanes 1–3) and three multiple sclerosis samples (Lanes 4–6) demonstrating enhanced protein expression of NOX1, p22phox and gp91phox in active demyelinating multiple sclerosis lesions compared with white matter from non-neurological controls. (b) Quantitative densitometry of the blots reveals significantly increased expression levels for NOX1, gp91phox and p22phox in multiple sclerosis lesions compared with control white matter. *P.
The expression of p22phox, seen in different types of multiple sclerosis lesions, in general co-localized in the same areas with the presence of oxidized DNA and lipids (Fig. 3c and d), described in detail previously (Haider et al., 2011). In addition, we found a significant correlation between the extent of p22phox expression with the number of dystrophic axons immunoreactive for amyloid precursor protein (R = 0.47; P < 0.001) and oxidized phospholipids (R = 0.35; P < 0.006; Fig. 3d), with the number of CD3+ T cells (R = 0.55; P < 0.001) and the number of HLA-DR+ microglia cells and macrophages (R = 0.69; P < 0.001). The values for inflammatory cells, dystrophic axons and oxidized DNA and lipids have been determined in previous studies on the same material used in the present study (Frischer et al., 2009; Haider et al., 2011). We did not find significant differences of p22phox expression with regard to gender. There was, however, a significant decrease of p22phox expression with disease duration (R = 0.18; P < 0.021). Patients with acute or relapsing–remitting multiple sclerosis showed significantly more lesional p22phox expression than patients who died during the progressive stage of the disease (primary and secondary progressive multiple sclerosis; P < 0.012).
A more detailed analysis of p22phox expression in relation to inflammation showed the presence of T cells (mainly CD8+ cells) associated with intense p22phox expression in microglia and macrophages (Supplementary Fig. 1). This was seen even in microglia nodules in the normal-appearing white matter (Supplementary Fig. 1). In double staining at the level of individual cells, we did not find co-localization of p22phox with T cell markers (CD3, CD8); however, p22phox was expressed in a subset of CD20+ B-lymphocytes (Supplementary Fig. 1).
Nox1 complex
The Nox1 complex contains two transmembrane proteins (p22phox and Nox1), as well as cytoplasmic regulatory molecules (Noxo1 and Noxa1: Bedard and Krause 2007; Cheret et al., 2008). The expression patterns of Nox1 and Noxo1 were different from those of the Nox2 complex (Table 5). In 9 out of 16 controls, Nox1 was weakly expressed in some microglia, astrocytes and endothelial cells (Supplementary Fig. 1), while no staining was seen in the others. Weak Noxo1 expression was detected in microglia in controls (Supplementary Fig. 1e). In multiple sclerosis lesions, Nox1 and Noxo1 expression were mainly seen in and around active plaques of acute and relapsing multiple sclerosis (Fig. 2g and h). There, Nox1 was not only present in macrophages and microglia, but also in astrocytes and endothelial cells [Fig. 2g–g(gg)]. Expression was not restricted to initial (‘pre-phagocytic’) areas but more generally throughout the plaque area and the adjacent normal-appearing white matter, where it was also found in microglia nodules (Fig. 2g). In slowly expanding lesions, some Nox1 staining was found in microglia, macrophages, astrocytes and endothelial cells at the active lesion edge and in the adjacent normal-appearing white matter. Overall, Noxo1 immunoreactivity was weak and mainly present in initial lesions. Its expression was more diffuse, but accentuated in microglia and macrophages. Immunoreactivity in inactive lesions was similar to that seen in controls.
Co-localization of Nox subunits in multiple sclerosis lesions suggests functionally active oxidative burst
Superoxide generation through Nox molecules requires functionally assembled subunits. Therefore, we performed double labelling with confocal laser microscopy to test for co-localization of the respective molecules in the same cells (Fig. 2i–o). In macrophages and microglia, we identified co-localization of all the tested components of the Nox1 and Nox2 complexes. This was, however, not the case for Nox1 complex expression in astrocytes and endothelial cells. In these cells, no convincing expression of p22phox was seen (Fig. 2o).
Discussion
Our current results expand previous observations that suggest a prominent role of oxidative injury in the pathogenesis of demyelination and tissue injury in multiple sclerosis (Basagra et al., 1995; Vladimirova et al., 1998; Smith et al., 1999; Smith and Lassmann 2002; Bizzozero et al., 2005; van Horssen et al., 2011). In a recent study, we found that oxidized DNA and lipids are present in high amounts in active multiple sclerosis lesions, in particular at sites of initial tissue injury. Furthermore, the presence of oxidized epitopes was enriched in apoptotic oligodendrocytes and in acutely injured dystrophic axons (Haider et al., 2011). The prominent upregulation of gene expression of molecules induced by oxidative stress or involved in redox homoeostasis, as seen in our current study, provides additional support for the contribution of reactive oxygen species in the pathogenesis of early multiple sclerosis.
Reactive oxygen species production is accomplished by two principally different mechanisms: activation of free radical-producing enzymes, such as those involved in oxidative burst, and by mitochondrial dysfunction (Van Horssen et al., 2010; Smith 2011; Witte et al., 2011). Support for both mechanisms comes from our microarray study since we found marked changes in the expression of mitochondrial genes and, in particular, of those encoded by mitochondrial DNA. The present results are in line with previous biochemical, histochemical and immunocytochemical studies that showed an impairment of mitochondrial function in active multiple sclerosis lesions, which appears to be related to active degeneration of myelin, oligodendrocytes, axons and neurons (Mahad et al., 2008a). Mitochondrial dysfunction is transient, as seen in the comparison of initial lesion areas, with demyelinated lesion areas in our arrays. At later stages of lesion formation mitochondrial numbers and enzyme activity increase, apparently reflecting the increased metabolic demand of demyelinated axons in the lesions or a reaction to chronic mitochondrial insult (Mahad et al., 2009; Witte et al., 2009). Taken together, it is therefore likely that dysfunction of mitochondria contributes to reactive oxygen species production within multiple sclerosis lesions. It is, however, unlikely that this phenomenon is responsible for the initial stage of mitochondrial dysfunction in the lesions.
Our microarray data, in combination with the immunohistochemical results, identify activated macrophages and microglia as the major source of reactive oxygen species production in initial multiple sclerosis lesions. We demonstrate increased expression of the major components of the Nox2 and Nox1 complexes in active multiple sclerosis lesions, predominantly in areas of initial ‘pre-phagocytic’ tissue injury, as defined by Barnett and Prineas (2004), Marik et al. (2007) and Henderson et al. (2009). This is the area of active multiple sclerosis lesions, where myelin sheaths are still preserved, but distal oligodendrogliopathy, oligodendrocyte apoptosis and acute axonal injury take place in association with mild T cell infiltrates and microglia activation (Lucchinetti et al., 2000; Barnett and Prineas, 2004; Marik et al., 2007; Lassmann 2011). Furthermore, cells containing oxidized DNA and oxidized lipids are mainly concentrated at these sites (Haider et al., 2011) and the most pronounced damage to mitochondria in oligodendrocytes and axons is seen in this area (Mahad et al., 2008a). Experimental studies suggest that oxidative tissue damage under these conditions is most likely mediated by peroxynitrite. Nitrotyrosine expression, a footprint of peroxynitrite-induced injury, has been found at the edge of active multiple sclerosis lesions (Zeis et al., 2009) and is known to mediate oligodendrocyte injury in vitro and in autoimmune encephalomyelitis in vivo (Li et al., 2005, 2011; Nikic et al., 2011; Vana et al., 2011). Our present data strongly suggest that reactive oxygen species, which are also necessary for peroxynitrite formation, are mainly produced by activated microglia through classical Nox2-dependent oxidative burst. This view is supported by several observations. First, p22phox and gp91phox are more abundantly expressed in active multiple sclerosis lesions compared with other oxidases, such as MPO (see arrays and Marik et al., 2007; Gray et al., 2008a, b). Secondly, the co-expression of different components of the Nox2 complex in the same microglia cells indicates that these complexes are functionally active. Thirdly, it is interesting to note that p22phox and gp91phox expression are less intense in macrophages that have taken up myelin debris in comparison to microglia in the initial lesion zone. This observation supports the concept that myelin phagocytosis deactivates macrophages from a pro-inflammatory to an anti-inflammatory phenotype within multiple sclerosis lesions. Potential functional importance of Nox2 complexes in inflammatory demyelinating brain lesions is shown by the protective effect of gp91phox gene deletion in animals with autoimmune encephalomyelitis (Li et al., 2011). Furthermore, Nox2 attenuation, by either genetic knockdown or pharmacological compounds, is beneficial in animal models for neurodegeneration. Nox2 deficiency reduced oxidative stress and improved the outcome in a mouse model of Alzheimer's disease (Park et al., 2008), and neurodegeneration was markedly attenuated in an experimental animal model of Parkinson's disease compared with wild-type animals (Zhang et al., 2004).
In contrast, little is known about the role of the Nox1 complex in multiple sclerosis and experimental brain inflammation. In vitro, microglia toxicity is, in part, mediated through reactive oxygen species production by the Nox1 complex (Cheret et al., 2008). In addition, Nox1 expression was not restricted to macrophages and microglia, where p22phox is present for potential interaction. In astrocytes and endothelial cells, Nox1 was present in the absence of detectable p22phox. Whether astrocytic and endothelial Nox1 are also able to produce reactive oxygen species in the absence of p22phox or whether they serve other functions in these cells warrants future studies.
Despite the use of formaldehyde-fixed paraffin-embedded material, we found a good correspondence between the gene expression data obtained in microarrays and the respective immunohistochemical results. This was not only the case for the changes in relation to oxidative burst molecules, which were directly analysed here, but also for those related to mitochondrial function, where respective immunocytochemical analysis has been performed previously on the same material (Mahad et al., 2008a). It has been shown before that transcriptome analysis can be done on paraffin-embedded material (von Weizsäcker et al., 1991; Lewis et al., 2001; Waddell et al., 2010) and we show that this is even feasible on archival autopsy material from patients with multiple sclerosis. This is important since acute multiple sclerosis lesions are rare in pathological collections and so far not available in native frozen tissue blocks. There were, however, a number of exceptions and caveats. First, some messenger RNAs, such as iNOS, were downregulated in the arrays, despite increased protein expression seen in immunocytochemistry (Marik et al., 2007). In the normal white matter of the human brain, a low number of iNOS-positive microglia cells is present (Marik et al., 2007), which may reflect some basic activation of microglia in the human brain in comparison to animals, housed under specific pathogen-free conditions. This may explain the moderate basic level of iNOS messenger RNA expression in controls seen in our microarrays. Since iNOS messenger RNA expression after cytokine stimulation is transient due to its instability and to active regulation by the presence of nitric oxide (Park et al., 1997; Murphy, 2000), downregulation in the active multiple sclerosis lesions may not be unexpected. Secondly, one-third of the 33 000 genes analysed showed very low expression levels, which also showed no changes between cases or between patients with multiple sclerosis and controls. These values had to be excluded from the analysis due to insufficient RNA quantity. This is a technical limit of gene expression studies in archival, formaldehyde-fixed and paraffin-embedded tissue material, having a much lower sensitivity compared with those performed on native frozen tissue (Waddell et al., 2010) and where the gene sequences utilized for detection have to be located close to the poly(A) tail of the gene (see ‘Material and methods’ section). Finally, closer inspection of the data showed differences between the cases. As discussed above, these differences may best be explained by the complexity of lesion architecture and more subtle differences in the stage of the respective lesions (Lassmann, 2011). These data suggest that in a disease such as multiple sclerosis, transcriptome or proteome analysis should not be performed by simply comparing active with inactive lesions or control white matter, using standard criteria for lesion definition. What is needed for this type of research in the future is highly precise area selection and micro-dissection, similar but possibly even better than that carried out in our current study. However, our current study shows that additional important information can be obtained from such microarrays, when these precautions are considered.
One can argue that the brain samples selected for gene expression analysis in our study are not derived from typical multiple sclerosis cases. In fact, all three were from patients with acute multiple sclerosis and one showed prominent concentric demyelination, typical for Balo's disease. There are, however, two main arguments that support our view that the changes seen in these cases are representative of more classical multiple sclerosis lesions. First, the same changes, although in lower severity, were seen by immunocytochemistry in all other active multiple sclerosis lesions, including the slowly expanding lesions of progressive multiple sclerosis. Secondly, it has been shown previously that those tissue alterations characteristic for pattern III lesions, including elements of concentric sclerosis, are seen to a lesser extent in other classical active lesions from patients with relapsing or progressive multiple sclerosis (Barnett and Prineas, 2004; Marik et al., 2007).
In conclusion, our data provide further evidence for the importance of oxidative damage in the pathogenesis of demyelination and tissue injury in multiple sclerosis. We suggest that tissue damage is initiated by oxidative burst in activated microglia and macrophages, which is most likely induced by the inflammatory process. Oxidative damage leads to mitochondrial injury and disturbance of the mitochondrial respiratory chain, which not only results in energy deficiency but also in further propagation of reactive oxygen species production (Lassmann and van Horssen, 2011; Smith, 2011). Neuroprotective therapies, specifically focusing on the prevention of oxidative damage may, thus, become attractive in the future, and a current trial testing the effect of fumarates, which boost endogenous antioxidant enzymes in patients with multiple sclerosis, represents one possible example for this approach (Schreibelt et al., 2007; de Vries et al., 2008; Linker et al., 2011). However, experimental data have shown that reactive nitrogen species, as well as reactive oxygen species can, under certain circumstances also mediate beneficial, anti-inflammatory effects in autoimmune encephalomyelitis (Sahrbacher et al., 1998; Becanovic et al., 2006; Liu et al., 2006; Willenborg et al., 2007), possibly by repressing the T cell-mediated immune response. On this basis, stimulation of reactive oxygen species production has been suggested as a potential therapy for patients with multiple sclerosis (Becanovic et al., 2006). In light of our current observations such therapeutic trials should be met with caution.
Funding
This study was funded by the PhD programme Cell Communication in Health and Disease (CCHD, co-funded by the Austrian Science Fund and the Medical University Vienna), by the Austrian Science Fund (FWF, Projects P19854-B02 and P24245-B19 to H.L.), the Dutch MS Research Foundation (to J.L., J.D. and J.vH.) and the National Multiple Sclerosis Society (USA; Award Nr.: PP1443).
Supplementary material
Supplementary material is available at Brain online.
Acknowledgements
We thank Ulrike Köck, Angela Kury and Marianne Leisser for expert technical assistance.
Glossary
Abbreviations
- iNOS
inducible nitric oxide synthase
- NADPH
nicotinamide adenine dinucleotide phosphate
- NOX1
nicotinamide adenine dinucleotide phosphate oxidase 1
- NOXO1
nicotinamide adenine dinucleotide phosphate oxidase organizer 1
References
- Bagasra O, Michaels FH, Zheng YM, Bobroski LE, Spitsin SV, Fu ZF, et al. Activation of the inducible form of nitric oxide synthase in the brains of patients with multiple sclerosis. Proc Natl Acad Sci USA. 1995;92:12041–5. doi: 10.1073/pnas.92.26.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett MH, Prineas JW. Relapsing and remitting multiple sclerosis: pathology of the newly forming lesion. Ann Neurol. 2004;55:458–68. doi: 10.1002/ana.20016. [DOI] [PubMed] [Google Scholar]
- Bauer J, Elger CE, Hans VH, Schramm J, Urbach H, Lassmann H, et al. Astrocytes are a specific immunological target in Rasmussen's encephalitis. Ann Neurol. 2007;62:67–80. doi: 10.1002/ana.21148. [DOI] [PubMed] [Google Scholar]
- Becanovic K, Jagodic M, Sheng JR, Dahlman I, Aboul Enein F, Wallstrom E, et al. Advanced intercross line mapping of Eae5 reveals Ncf-1 and CLDN4 as candidate genes for experimental autoimmune encephalomyelitis. J Immunol. 2006;176:6055–64. doi: 10.4049/jimmunol.176.10.6055. [DOI] [PubMed] [Google Scholar]
- Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and Pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Bizzozero OA, Dejesus G, Callaha K, Pastuszyn A. Elevated protein carbonylation in the brain white matter and grey matter of patients with multiple sclerosis. J Neurosci Res. 2005;81:687–95. doi: 10.1002/jnr.20587. [DOI] [PubMed] [Google Scholar]
- Bolanos JP, Almeida A, Stewart V, Peuchen S, Land JM, Clark JB, et al. Nitric oxide mediated mitochondrial damage in the brain: mechanisms and implication for neurdegenerative diseases. J Neurochem. 1997;68:2227–40. doi: 10.1046/j.1471-4159.1997.68062227.x. [DOI] [PubMed] [Google Scholar]
- Breitschopf H, Suchanek G, Gould RM, Colman DR, Lassmann H. In situ hybridization with digoxigenin-labeled probes : sensitive and reliable detection method applied to myelinating rat brain. Acta Neuropathol. 1992;84:581–7. doi: 10.1007/BF00227734. [DOI] [PubMed] [Google Scholar]
- Brück W, Porada Ph, Poser S, Rieckmann P, Hanefeld F, Kretschmer HA, et al. Monocyte/macrophage differentiation in early multiple sclerosis. Ann Neurol. 1995;38:788–96. doi: 10.1002/ana.410380514. [DOI] [PubMed] [Google Scholar]
- Campbell GR, Ziabreva I, Reeve AK, Krishnan KJ, Reynolds R, Howell O, et al. Mitochondrial DNA deletions and neurodegeneration in multiple sclerosis. Ann Neurol. 2011;69:481–92. doi: 10.1002/ana.22109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheret C, Gervais A, Lelli A, Colin C, Amar L, Ravassard P, et al. Neurotoxic activation of microglia is promoted by a nox1 dependent NADPH oxidase. J Neurosci. 2008;28:12039–51. doi: 10.1523/JNEUROSCI.3568-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross AH, Manning PT, Keeling RM, Schmidt RE, Misko TP. Peroxynitrite formation within the central nervous system in active multiple sclerosis. J Neuroimmunol. 1998;88:45–56. doi: 10.1016/s0165-5728(98)00078-2. [DOI] [PubMed] [Google Scholar]
- de Vries HE, Witte ME, Hondius D, Rozemuller A, Drukarch B, Hoozemans J, et al. Nrf2-induced antioxidant protection: a promising target to counteract ROS-mediated damage in neurodegenerative disease. Free Radic Biol Med. 2008;45:1375–83. doi: 10.1016/j.freeradbiomed.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Diaz-Sanchez M, Williams K, DeLuca GC, Esiri MM. Protein co-expression with axonal injury in multiple sclerosis plaques. Acta Neuropathol. 2006;111:289–99. doi: 10.1007/s00401-006-0045-0. [DOI] [PubMed] [Google Scholar]
- Dutta R, McDonough J, Yin X, Peterson J, Chang A, Torres T, et al. Mitochondrial dysfunction as a cause of axonal degeneration in multiple sclerosis patients. Ann Neurol. 2006;59:478–89. doi: 10.1002/ana.20736. [DOI] [PubMed] [Google Scholar]
- Frischer JM, Bramow S, Dal Bianco A, Lucchinetti C, Rauschka H, Schmidbauer M, et al. The relation between inflammation and neurodegeneration in multiple sclerosis. Brain. 2009;132:1175–89. doi: 10.1093/brain/awp070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray E, Thomas TL, Betmouni S, Scolding N, Love S. Elevated myeloperoxidase activity in white matter in multiple sclerosis. Neurosci Lett. 2008a;442:195–8. doi: 10.1016/j.neulet.2008.08.035. [DOI] [PubMed] [Google Scholar]
- Gray E, Thomas TL, Betmouni S, Scolding N, Love S. Elevated activity of microglial expression of myeloperoxidase in demyelinated cerebral cortex in multiple sclerosis. Brain Pathol. 2008b;18:86–95. doi: 10.1111/j.1750-3639.2007.00110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider L, Fischer MT, Frischer JM, Bauer J, Höftberger R, Botond G, et al. Oxidative damage and neurodegeneration in multiple sclerosis lesions. Brain. 2011;134:1914–24. doi: 10.1093/brain/awr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson APD, Barnett MH, Parratt JDE, Prineas JW. Multiple sclerosis: distribution of inflammatory cells in newly forming lesions. Ann Neurol. 2009;66:739–53. doi: 10.1002/ana.21800. [DOI] [PubMed] [Google Scholar]
- Higgins GC, Beart PM, Shin YS, Chen MJ, Cheung NS, Nagley P. Oxidative stress: Emerging mitochondrial and cellular themes and variations in neuronal injury. J Alzheimer's disease. 2010;20:S453–73. doi: 10.3233/JAD-2010-100321. [DOI] [PubMed] [Google Scholar]
- King G, Payne S, Walker F, Murray GI. A highly sensitive detection method for immunohistochemistry using biotinylated tyramine. J Pathol. 1997;183:237–41. doi: 10.1002/(SICI)1096-9896(199710)183:2<237::AID-PATH893>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Kutzelnigg A, Lucchinetti CF, Stadelmann C, Bruck W, Rauschka H, Bergmann M, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain. 2005;128:2705–12. doi: 10.1093/brain/awh641. [DOI] [PubMed] [Google Scholar]
- Lassmann H. The architectures of active multiple sclerosis lesions. Neuropath Appl Neurobiol. 2011;37:698–710. doi: 10.1111/j.1365-2990.2011.01189.x. [DOI] [PubMed] [Google Scholar]
- Lassmann H, van Horssen J. The molecular basis of neurodegeneration in multiple sclerosis. FEBS Lett. 2011;585:3715–23. doi: 10.1016/j.febslet.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Lassmann H, Brück W, Lucchinetti C. The immunopathology of multiple sclerosis: an overview. Brain Pathol. 2007;17:210–8. doi: 10.1111/j.1750-3639.2007.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis F, Maughan NJ, Smith V, Hillan K, Quirke P. Unlocking the archive–gene expression in paraffin-embedded tissue. J Pathol. 2001;195:66–71. doi: 10.1002/1096-9896(200109)195:1<66::AID-PATH921>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Li J, Baud O, Vartanian T, Volpe JJ, Rosenberg PA. Peroxynitrite generated by inducible nitric oxide synthase and NADPH oxidase mediates microglial toxicity to oligodendrocytes. Proc Natl Acad Sci USA. 2005;102:9936–41. doi: 10.1073/pnas.0502552102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Vana AC, Ribeiro R, Zhang Y. Distinct role of nitric oxide and peroxinitrite in mediating oligodendrocyte toxicity in culture and in experimental autoimmune encephalomyelitis. Neuroscience. 2011;184:107–19. doi: 10.1016/j.neuroscience.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Linker RA, Lee DH, Ryan S, van Dam AM, Conrad R, Bista P, et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain. 2011;3:678–92. doi: 10.1093/brain/awq386. [DOI] [PubMed] [Google Scholar]
- Liu Y, Hao W, Letiembre M, Walter S, Kiúlanga M, Neumann H, et al. Suppression of microglial inflammatory activity by myelin phagocytosis: role of p47phox-mediated generation of reactive oxygen species. J Neurosci. 2006;26:12904–13. doi: 10.1523/JNEUROSCI.2531-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JSH, Zhao ML, Brosnan CF, Lee SC. Expression of inducible nitric oxide synthase and nitrotyrosine in multiple sclerosis lesions. Am J Pathol. 2001;158:2057–66. doi: 10.1016/S0002-9440(10)64677-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F, Selak M, O'Connor J, Croul S, Lorenzana C, Butunoi C, et al. Oxidative damage to mitochondrial DNA and activity of mitochondrial enzymes in chronic active lesions of multiple sclerosis. J Neurol Sci. 2000;177:95–103. doi: 10.1016/s0022-510x(00)00343-9. [DOI] [PubMed] [Google Scholar]
- Lucchinetti C, Brück W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47:707–17. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Mahad D, Ziabreva I, Lassmann H, Turnbull D. Mitochondrial defects in acute multiple sclerosis lesions. Brain. 2008a;131:1722–35. doi: 10.1093/brain/awn105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahad D, Lassmann H, Turnbull D. Mitochondria and disease progression in multiple sclerosis. Neuropathol Appl Neurobiol. 2008b;34:577–89. doi: 10.1111/j.1365-2990.2008.00987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahad D, Ziabreva I, Campbell G, Lax N, Hanson PS, Lassmann H, et al. Mitochondrial changes within axons in multiple sclerosis. Brain. 2009;132:1161–74. doi: 10.1093/brain/awp046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marik C, Felts P, Bauer J, Lassmann H, Smith KJ. Lesion genesis in a subset of patients with multiple sclerosis: a role for innate immunity? Brain. 2007;130:2800–15. doi: 10.1093/brain/awm236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S. Production of nitric oxide by glial cells: regulation and potential roles in the CNS. Glia. 2000;29:1–14. doi: 10.1002/(sici)1098-1136(20000101)29:1<1::aid-glia1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Nicolussi EM, Huck S, Lassmann H, Bradl M. The cholinergic anti-inflammatory system limits T cell infiltration into the neurodegenerative CNS, but cannot counteract complex CNS inflammation. Neurobiol Dis. 2009;35:24–31. doi: 10.1016/j.nbd.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Nikic I, Merkler D, Sorbara C, Brinkoetter M, Keutzfeldt M, Bareyre FM, et al. A reversible form of axon damage in experimental autoimmune encephalomyelitis and multiple sclerosis. Nat Med. 2011;17:495–9. doi: 10.1038/nm.2324. [DOI] [PubMed] [Google Scholar]
- Park SK, Lin HL, Murphy S. Nitric oxide regulates nitric oxide synthase-2 gene expression by inhibiting NF-kappa B binding to DNA. Biochem J. 1997;322:609–13. doi: 10.1042/bj3220609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park L, Zhou P, Pitstick R, Capone C, Anrather J, Norris EH, et al. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc Natl Acad Sci USA. 2008;105:1347–52. doi: 10.1073/pnas.0711568105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahrbacher UC, Lechner F, Eugster HP, Frei K, Lassmann H, Fontana A. Mice with an inactivation of the inducible nitric oxide sythase gene are susceptible to experimental autoimmune encephalomyelitis. Eur J Immunol. 1998;28:1332–8. doi: 10.1002/(SICI)1521-4141(199804)28:04<1332::AID-IMMU1332>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Sharma R, Fischer MT, Bauer J, Smith K, Misu T, Fujihara K, et al. Inflammation induced by innate immunity in the central nervous system leads to primary astrocyte dysfunction followed by demyelination. Acta Neuropathol. 2010;120:223–36. doi: 10.1007/s00401-010-0704-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KJ. Newly-lesioned tissue in MS – a role for oxidative damage? Brain. 2011;134:1877–81. doi: 10.1093/brain/awr144. [DOI] [PubMed] [Google Scholar]
- Smith KJ, Lassmann H. The role of nitric oxide in multiple sclerosis. Lancet Neurol. 2002;1:232–41. doi: 10.1016/s1474-4422(02)00102-3. [DOI] [PubMed] [Google Scholar]
- Smith KJ, Kapoor PA, Felts PA. Demyelination: the role of reactive oxygen and nitrogen species. Brain Pathol. 1999;9:69–92. doi: 10.1111/j.1750-3639.1999.tb00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreibelt G, van Horssen J, van Rossum S, Dijkstra CD, Drukarch B, de Vries HE. Therapeutic potential and biological role of endogenous antioxidant enzymes in multiple sclerosis pathology. Brain Res. Reviews. 2007;2:322–30. doi: 10.1016/j.brainresrev.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Trapp B, Stys P. Virtual hypoxia and chronic necrosis of demyelinated axons in multiple sclerosis. Lancet Neurol. 2009;8:280–91. doi: 10.1016/S1474-4422(09)70043-2. [DOI] [PubMed] [Google Scholar]
- Vana AC, Li S, Ribeiro R, Tchantchou F, Zhang Y. Arachidonyl trifluoromethyl ketone ameliorates experimental autoimmune encephalomyelitis via blocking peroxynitrite formation in mouse spinal cord white matter. Exp Neurol. 2011;231:45–55. doi: 10.1016/j.expneurol.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Van Horssen J, Witte ME, Schreibelt G, de Vries HE. Radical changes in multiple sclerosis pathogenesis. Biochem Biophys Acta. 2011;1812:141–50. doi: 10.1016/j.bbadis.2010.06.011. [DOI] [PubMed] [Google Scholar]
- van Horssen J, Drexhage J, Flor T, Gerritsen W, van der Valk P, de Vries HE. Nrf2 and DJ1 are consistently upregulated in inflammatory multiple sclerosis lesions. Free Radic Biol Med. 2010;8:1283–9. doi: 10.1016/j.freeradbiomed.2010.07.013. [DOI] [PubMed] [Google Scholar]
- Van Horssen J, Schreibelt G, Drexhage J, Hazes T, Dijkstra CD, van der Valk P, et al. Severe oxidative damage in multiple sclerosis lesions coincides with enhanced antioxidant enzyme expression. Free Radical Biol Med. 2008;45:1729–37. doi: 10.1016/j.freeradbiomed.2008.09.023. [DOI] [PubMed] [Google Scholar]
- Verhoeven AJ, Bolscher BG, Meerhof LJ, van Zwieten R, Keijer J, Weening RS, et al. Characterization of two monoclonal antibodies against cytochrome b558 of human neutrophils. Blood. 1989;6:1686–94. [PubMed] [Google Scholar]
- Veto S, Acs P, Bauer J, Lassmann H, Berente Z, Setalo G, et al. Inhibiting poly(ADP-ribose) polymerase: a potential therapy against oligodendrocyte death. Brain. 2010;133:822–34. doi: 10.1093/brain/awp337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vladimirova O, O'Connor J, Cahill A, Alder H, Butunoi C, Kalman B. Oxidative damage to DNA in plaques of MS brains. Mult Scler. 1998;4:413–8. doi: 10.1177/135245859800400503. [DOI] [PubMed] [Google Scholar]
- Von Ahlfen S, Missel A, Bendrat K, Schlumpberger M. Determinants of RNA quality from FFPE samples. PloS One. 2007;2:21261. doi: 10.1371/journal.pone.0001261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Weizsäcker F, Labeit S, Koch HK, Oehlert W, Gerok W, Blum HE. A simple and rapid method for the detection of RNA in formalin-fixed, paraffin-embedded tissues by PCR amplification. Biochem Biophys Res Commun. 1991;174:176–80. doi: 10.1016/0006-291x(91)90502-x. [DOI] [PubMed] [Google Scholar]
- Waddell N, Cocciardi S, Johnson J, Healey S, Marsh A, Riley J, et al. Gene expression profiling of formalin-fixed, paraffin-embedded familial breast tumours using the whole genome-DASL assay. J Pathol. 2010;221:452–61. doi: 10.1002/path.2728. [DOI] [PubMed] [Google Scholar]
- Willenborg DO, Staykova M, Fordham S, O'Brian N, Linares D. The contribution of nitric oxide and interferon gamma to the regulation of the neuro-inflammation in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2007;191:16–25. doi: 10.1016/j.jneuroim.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Witte ME, Bo L, Rodenburg R, Belien JA, Musters R, Hazes T, et al. Enhanced number and activity of mitochondria in multiple sclerosis lesions. J Pathol. 2009;2:193–204. doi: 10.1002/path.2582. [DOI] [PubMed] [Google Scholar]
- Witte ME, Geurts JJ, deVries HE, van der Valk P, van Horssen J. Mitochondrial dysfunction: a potential link between neuroinflammation and neurodegeneration. Mitochondrion. 2010;10:411–8. doi: 10.1016/j.mito.2010.05.014. [DOI] [PubMed] [Google Scholar]
- Zeis T, Propst A, Steck AJ, Stadelmann C, Brück W, Schaeren-Wiemers N. Molecular changes in white matter adjacent to an active demyelinating lesion in early multiple sclerosis. Brain Pathol. 2009;19:459–66. doi: 10.1111/j.1750-3639.2008.00231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziabreva I, Campbell G, Rist J, Zambonin J, Rorbach J, Wydro MM, et al. Injury and differentiation following inhibition of mitochondrial respiratory chain complex IV in rat oligodendrocytes. Glia. 2010;58:1827–37. doi: 10.1002/glia.21052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Wang T, Qin L, Gao HM, Wilson B, Ali SF, et al. Neuroprotective effect of dextromethorphan in the MPTP Parkinson's disease model: role of NADPH oxidase. FASEB J. 2004;18:589–91. doi: 10.1096/fj.03-0983fje. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.