Abstract
The demographics, immunologic parameters, medical complications, and mortality statistics from 473 subjects with common variable immune deficiency followed over 4 decades in New York were analyzed. Median immunoglobulin levels were IgG, 246 mg/dL; IgA, 8 mg/dL; and IgM, 21 mg/dL; 22.6% had an IgG less than 100 mg/dL. Males were diagnosed earlier (median age, 30 years) than females (median age, 33.5 years; P = .004). Ninety-four percent of patients had a history of infections; 68% also had noninfectious complications: hematologic or organ-specific autoimmunity, 28.6%; chronic lung disease, 28.5%; bronchiectasis, 11.2%; gastrointestinal inflammatory disease, 15.4%; malabsorption, 5.9%; granulomatous disease, 9.7%; liver diseases and hepatitis, 9.1%; lymphoma, 8.2%; or other cancers, 7.0%. Females had higher baseline serum IgM (P = .009) and were more likely to develop lymphoma (P = .04); 19.6% of patients died, a significantly shorter survival than age- and sex-matched population controls (P < .0001). Reduced survival was associated with age at diagnosis, lower baseline IgG, higher IgM, and fewer peripheral B cells. The risk of death was 11 times higher for patients with noninfectious complications (hazard ratio = 10.95; P < .0001). Mortality was associated with lymphoma, any form of hepatitis, functional or structural lung impairment, and gastrointestinal disease with or without malabsorption, but not with bronchiectasis, autoimmunity, other cancers, granulomatous disease, or previous splenectomy.
Introduction
Common variable immune deficiency (CVID) is a primary immune deficiency characterized by reduced serum levels of immunoglobulin (Ig)G, IgA, and/or IgM with reduced or absent specific antibody production.1–4 The diagnosis is typically made between the ages of 20 and 40 years, but ∼ 20% are less than 20 years of age.5 Potentially because of the symptom onset in young adult life and the heterogenous nature of the disease, a delay in diagnosis of 6 to 7 years is common.5–7 Because of the relative prevalence, 1:25 000 to 1:50 000, and numbers of medical encounters, CVID is a clinically important immune defect.4,5,7 The majority of subjects have normal numbers of peripheral blood B cells, but there are depleted numbers of circulating isotype switched memory B cells (IgD−IgM−CD27+), defective somatic hypermutation, and impaired formation of plasma cells in bone marrow and other tissues.8–10
Although there have been many investigations into the nature of this immune defect since it was first recognized in 1953,11 the fundamental genetic or other causes of CVID remain unclear for the majority of patients. In a few rare cases, CVID has been linked to autosomal recessive genetic mutations, including inducible costimulatory,12 CD19,13,14 B cell–activating factor receptor,15 CD20,16 and CD81.17 Both heterozygous and homozygous mutations in the gene for the B-cell receptor transmembrane activator and calcium-modulating cyclophilin ligand interactor (TACI) are found in 8% to 10% of subjects.18–20 Although these are significantly associated with both immune deficiency and autoimmunity in CVID,21,22 some of the same mutations also may be found in healthy controls and clinically “normal” relatives, suggesting that these are important disease-associated polymorphisms.23 Recent genome-wide studies have demonstrated the unique genetic characteristics of this immune defect, showing both novel chromosomal associations and highly significant copy number loss and gain.24
The standard of care in CVID is replacement Ig given at frequent intervals for life,25 and this replacement reduces the number of bacterial infections26 and probably enhances survival.7 However, immune globulin does not seem to protect against or treat the largely noninfectious complications such as functional and structural lung disease, autoimmunity, granulomatous disease, liver diseases and hepatitis, gastrointestinal inflammatory disease, or the development of cancer or lymphoma that are found in varying percentages of patients in different series.6,7,27–32 These conditions are important, because in aggregate, the autoimmune and inflammatory conditions over time, lead to increased morbidity and mortality.6,7,27 However, it is not clear whether all complications are equally deleterious or whether some of these are relatively benign with no increased risk to survival over long periods. We also lack biomarkers, preferably available at the time of diagnosis, to distinguish those subjects who are more likely to have selected clinical complications and poorer outcomes. Here, we examine the outcomes of a cohort of 473 CVID subjects followed by 1 group over 4 decades, and we present the demographic and immunologic markers associated with these complications and poorer survival.
Methods
CVID subjects
Subjects with CVID were seen in the Immune Deficiency Clinic at Mount Sinai Medical Center from 1986 through the present. Part of the cohort was previously seen at Memorial Sloan-Kettering Cancer Center (1974-1986). The diagnosis of CVID was made by standard criteria, including reduced serum IgG, IgA and/or IgM, by at least 2 SDs below the mean for age, with poor or absent antibody production to both protein and carbohydrate vaccines and exclusion of other causes of hypogammaglobulinemia.1,2,4,5,7 Subjects under age 4 years without continued follow-up and subjects with lymphoid cancer diagnosed within 2 years after the diagnosis of CVID were excluded. Immunologic parameters included enumeration of T and B cells, CD4, and CD8 T cells and IgM−IgD−CD27+ isotype switched memory B cells as a proportion of total B cells. Lymphocyte proliferative responses to mitogens, including phytohemagglutinin, concanavalin A, and pokeweed, were performed by standard methods. All subjects received Ig replacement therapy after diagnosis. Clinical information was obtained during patient visits; for subjects no longer receiving care at Mount Sinai, follow-up information was obtained from patients, their physicians, chart and medical records review, or a combination. All studies were undertaken with the consent of the Mount Sinai Medical Center Institutional Review Board.
Infectious and inflammatory complications
Both infectious and noninfectious illnesses were assessed from the clinical history and examination of medical records. Confirmation of malignancy, lymphoma, and granulomatous disease were based on pathology reports of tissues examined either at Mount Sinai or an outside facility. The diagnosis of chronic lung disease was based on radiologically evident or biopsy-proven structural lung disease, impaired lung function, or both. Structural lung disease included pulmonary infiltrates, nodules, fibrocystic parenchymal changes, granulomatous or lymphocytic infiltrates (confirmed on lung biopsy), bronchiectasis, or a combination. Impaired lung function was defined as a loss of vital capacity, restrictive or obstructive lung disease, reduced diffusion capacity of the lungs for carbon monoxide, reduced oxygen saturation with or without need for chronic oxygen therapy, or a combination. Infectious and noninfectious gastrointestinal disease was diagnosed by clinical history, stool and blood studies, and endoscopy with mucosal biopsy as required. Chronic diarrhea was defined as frequent loose or liquid stools for 3 or more months with no infectious cause identified. Malabsorption was defined as intestinal dysfunction causing substantial and chronic weight loss, documented vitamin or nutritional deficiencies, or a comnbination. Liver diseases and hepatitis were diagnosed by liver function tests above the upper limit of normal, sonographic studies, computerized tomography scans, specific PCR studies, and liver biopsy as needed.
Statistical methods
Baseline laboratory results before initiating immune globulin replacement are used for serum immune globulins; other test results were those done on the first clinic visit when possible. The percentage of patients meeting a specific immunologic criterion (eg, switched memory B cells, < 0.55%)33 represents a percentage of the total number of observed values for that immunologic parameter. Immunologic differences between males and females were assessed using Mann-Whitney Wilcoxon tests. Complication rates in males versus females were assessed using Fisher exact tests. Statistical significance was defined as P < .05. Associations between age at diagnosis, age at death, and other immunologic factors were assessed with Spearman correlation coefficients using Prism 4 software (GraphPad). For mortality analysis, the time since diagnosis was determined using the age at diagnosis of CVID if known; otherwise, the age at initial evaluation was used for this diagnosis. The endpoint used was the time of last known follow-up or the date of death. Probabilities of survival after diagnosis of CVID were estimated from Kaplan-Meier life tables and compared with the expected survival of males and females in the general population based on US mortality rates. The median year of diagnosis in our cohort was 1994; thus, our population was compared with the 1994 US population life tables for each sex.34 Patients for whom the date of death or the date of last follow-up could not be accurately determined were excluded from the mortality analysis. The Cox proportional hazards model was used for the analysis of factors that might be associated with increased risk of death. For this analysis, the time between the age at diagnosis and the age at either death, or at last known follow-up, was used as the “time” variable. These analyses were performed using SAS/STAT Version 9.2 of the SAS system for Windows software.35
Results
Demographics and immunologic parameters
The cohort included 473 patients (208 males and 265 females) confirmed as having CVID at Memorial Sloan-Kettering Cancer Center (1974-1986) or Mount Sinai Medical Center (1986-2010). The median age at characteristic symptom onset (major infection or other characteristic condition) was 24 years for males and 27 years for females (not significantly different), but males were diagnosed with CVID earlier, at a median age of 30 years, than females at a median age of 33.5 years (P = .004). Twenty-eight percent were under age 21 years at diagnosis. Median immunoglobulin levels were IgG, 246 mg/dL; IgA, 8 mg/dL; and IgM, 21 mg/dL; 22.6% had an IgG less than 100 mg/dL. For the group as a whole, baseline serum IgG levels were closely correlated with both baseline serum IgA (r = 0.37, P < .0001) and serum IgM (r = 0.41, P < .0001). Serum IgA was less than 7 mg/dL in 31.7%, and serum IgM was less than 25 mg/dL in 53.7%. Ten percent of subjects had 1% or fewer peripheral B cells, and 35% had 0.55% or fewer isotype switched memory B cells, compatible with the class 1 grouping described previously.9,33 As reported previously,33 females with CVID had both significantly higher serum IgM levels (P = .009) and greater numbers of isotype-switched memory B cells (P = .03) compared with CVID males. Peripheral CD4+ T cells were less than normal controls (480 cu/mm) in 29% and less than 200 cu/mm in 3.6%. Lymphocyte proliferative defects (defined as below the lower limit of normal) to 1 or more of the mitogens were found in 50%, and 6% of patients had lymphocyte proliferation less than 10% of normal (Table 1).
Table 1.
Immunologic parameters by sex
| Normal range* | Males (n = 208), median (range) | Females, (n = 265) median (range) | Mann-Whitney test | |
|---|---|---|---|---|
| Immunoglobulins, mg/dL | ||||
| IgG | 700-1 600 | 244 (6-630) | 246 (0-594) | 0.66 |
| IgA | 70-400 | 8 (0-221) | 8 (0-495) | 0.38 |
| IgM | 40-230 | 19 (0-247) | 24 (0-400) | 0.009† |
| Lymphocytes (%) | ||||
| T cells (n = 338) | 55-89 | 74 (0-94) | 75 (0-97) | 0.40 |
| B cells (n = 396) | 5-15 | 9 (0-38) | 10 (0-53) | 0.16 |
| Isotype switched memory B cells (n = 208) | 6.5-29.2 | 0.88 (0-21) | 1.3 (0-23) | 0.03† |
| Absolute CD4 count (n = 140) | 480-1 700 CU/MM | 582 (96-1 661) | 698 (130-2 383) | 0.13 |
| CD4/CD8 ratio (n = 262) | 1-3 | 1.5 (0-8) | 1.6 (0-9) | 0.31 |
| Lymphocyte proliferative response (cpm) | ||||
| Phytohemagglutinin (n = 160) | 16 000-29 000 | 16 848 (183-40 165) | 16 383 (370-39 870) | 0.49 |
| Concanavalin A (n = 191) | 10 000-24 000 | 10 077 (66-34 600) | 9 540 (56-34 300) | 0.53 |
| Pokeweed mitogen (n = 196) | 5 000-13 000 | 2 665 (32-19 800) | 4 284 (28-18 734) | 0.36 |
Normal range listed is for adult patients.
Statistically significant (P < .05).
Infectious conditions
Although 6% of the group reported no infections, 94% of patients had a history of at least 1 significant infection, in most cases, of the sinopulmonary tract. Of these, 187 patients (40%) had had at least 1 episode of pneumonia. When known, the predominant organisms included Streptococcus species and Hemophilus influenza. Other unusual infections were noted in 15.4%, including shingles because of herpes zoster (in 12), giardia (in 11), Pneumocystis jiroveci (in 6), mycoplasma and salmonella (in 4 each), candidiasis, mycobacterial disease, and papilloma viral infections (in 3 each), Molluscum contagiosusm, measles, progressive multifocal leukoencephalopathy, cytomegalovirus, and Clostridium difficile (in 2 each), and unknown anaerobic organism (probably Clostridium perfringins), campylobacter, cryptococcus, histoplasmosis, listeriosis, nocardia, varicella, and HIV (in 1 each). Of the 6 patients with P jiroveci, 3 were being treated with steroids, 1 was being treated with steroids and 6-mercaptopurine for gastrointestinal inflammatory disease, and 2 were not receiving any immunosuppressive therapy. Of note, 1 of the patients being treated with steroids had T-cell lymphopenia and died of Pneumocystis infection.
Inflammatory and autoimmune complications
Although 32% of the group had infections as their only manifestation of immune deficiency, 68% of the group had 1 or more inflammatory or autoimmune manifestation (Table 2). Chronic lung disease leading to radiographic changes, with or without functional impairment, developed in 28.5%, equally in males and females. Bronchiectasis, included in the total number of subjects with chronic lung disease but also assessed separately, was documented in 11.2%. Progressive lung disease led to the need for chronic oxygen therapy in 29 patients (6.1%) and lung transplantation in 3 of these. Hematologic or organ-specific autoimmune disease was diagnosed in 28.6% of subjects, equally in males and females. As in previous studies, immune thrombocytopenia (ITP) and autoimmune hemolytic anemia (AIHA) were the most common of these diseases. Seven patients were found to have anti-IgA antibodies. Other autoimmune conditions included anticardiolipin antibody, antiphospholipid syndrome, diabetes mellitus, inflammatory bowel disease, pernicious anemia, rheumatoid arthritis, juvenile rheumatoid arthritis, uveitis, multiple sclerosis, neutropenia, primary biliary cirrhosis, systemic lupus erythematosis, autoimmune thyroid disease, vasculitis, psoriasis, and vitiligo.
Table 2.
Selected complications
| Associated condition | No. | % of cohort (n = 473) |
|---|---|---|
| Infections only (no complications) | 151 | 31.9 |
| Chronic lung disease (functional/structural) | 135 | 28.5 |
| Bronchiectasis | 53 | 11.2 |
| Autoimmunity | 134 | 28.6 |
| ITP | 67 | 14.2 |
| AIHA | 33 | 7 |
| Evans syndrome | 20 | 4.2 |
| Rheumatoid arthritis | 15 | 3.2 |
| Anti-IgA antibody | 7 | 1.5 |
| Alopecia | 5 | 1.1 |
| Neutropenia, pernicious anemia, anticardiolipin antibody, antiphospholipid syndrome, diabetes mellitus, juvenile rheumatoid arthritis, uveitis, multiple sclerosis, systemic lupus erythematosis, autoimmune thyroid disease, lichen planus, vasculitis, vitiligo, psoriasis | < 5 | < 1 |
| Gastrointestinal disease | 73 | 15.4 |
| Malabsorption | 28 | 5.9 |
| Inflammatory bowel disease (Crohn disease, ulcerative colitis, ulcerative proctitis) | 20 | 4.2 |
| Chronic diarrhea | 9 | 1.9 |
| Idiopathic mucosal inflammation | 6 | 1.3 |
| Nodular lymphoid hyperplasia | 5 | 1.1 |
| Gastrointestinal bleeding, irritable bowel syndrome, partial gastrectomy, diverticulitis, esophagitis | 1 | < 1 |
| Liver disease/hepatitis | 43 | 9.1 |
| Hepatitis C | 9 | 1.9 |
| Liver granuloma | 8 | 1.7 |
| Idiopathic liver disease | 8 | 1.7 |
| Non-A, non-B hepatitis* | 6 | 1.3 |
| Chronic hepatitis of unknown origin | 5 | 1.1 |
| Primary biliary cirrhosis | 3 | < 1 |
| Nodular regenerative hyperplasia | 2 | < 1 |
| Hepatitis B, cirrhosis of unknown etiology | 1 | < 1 |
Diagnosed before availability of the hepatitis C PCR.
Noninfectious gastrointestinal disease was documented in 15.4% of patients, including inflammatory bowel disease (Crohn disease, ulcerative colitis, ulcerative proctitis), chronic diarrhea of unknown etiology, gastrointestinal bleeding (1 case because of ITP), diverticulitis, irritable bowel syndrome, and esophagitis. Malabsorption developed in 28 subjects (6%). Nodular lymphoid hyperplasia was known in 5 patients, but this is likely to be an underestimate. A history of hepatic disease was found in 9.1% of the CVID group; 16 of these had infectious hepatitis, and the rest had noninfectious causes, including granulomatous disease, known in 8 patients, or otherwise poorly understood cryptogenic cirrhosis or hepatitis.
Granulomatous disease
Granulomatous disease diagnosed by tissue biopsy was found in 46 subjects (9.7%). Depending on the tissues biopsied, 20 had granulomas in the lung, 6 in lymph nodes, 4 in liver, 3 in skin, and 2 in the spleen. One patient in each of the following categories had granuloma found in the bone marrow, brain, neck, and at a surgical site postoperatively. Seven of these patients had granuloma found in biopsy specimens from multiple locations (eg, liver, lungs, and spleen; Table 3).
Table 3.
Granulomatous disease by location
| Tissue location | No. (n = 46) |
|---|---|
| Lung | 20 |
| Multiple locations (ie, liver, lung, and spleen) | 7 |
| Lymph node | 6 |
| Liver | 4 |
| Skin | 3 |
| Spleen | 2 |
| Bone marrow | 1 |
| Brain | 1 |
| Neck tissue | 1 |
| Operative site | 1 |
Lymphoma and other malignancies
Thirty-nine patients (8.2%) had a lymphoid malignancy, all B cell in type (Table 4). As noted in previous reports,6,36 lymphoma was more common in females (28) than males (11; P = .04). Of these, non-Hodgkin B-cell lymphomas were the most common, with some of these being further classified into specific B-cell phenotypes, including mucosa-associated lymphoid tissue (MALT) lymphoma, marginal zone lymphoma, and T cell–rich B cell EBV-associated lymphoma. One patient was initially diagnosed with MALT lymphoma; however the pathology was reviewed at the National Cancer Institute and the diagnosis was reclassified as monoclonal B lymphocytosis.37 Cancers of other sorts developed in 33 patients (7%), 22 females and 11 males (P = .28; Table 5). Two patients developed 2 distinct primary malignancies; 1 with colon and prostate cancer and 1 with prostate and skin cancer.
Table 4.
Lymphoma and selected outcomes
| Lymphoma type | No. (n = 39) | Outcome |
|---|---|---|
| Non-Hodgkin lymphoma, B-cell type, not further classified | 23 | 11 died of lymphoma, 12 alive |
| Diffuse large B-cell lymphoma | 3 | 2 died of lymphoma, 1 also had severe lung disease, 1 alive |
| Hodgkin disease | 4 | 3 developed B-cell lymphoma years after treatment for Hodgkin disease, 2 of these died of lymphoma, 2 alive |
| MALT | 5 | 3 no treatment, 2 chemotherapy, 5 alive |
| Marginal zone lymphoma/monoclonal B lymphocytosis | 1 | No treatment given, 1 alive |
| Monoclonal B lymphocytosis | 1 | No treatment given, 1 alive |
| Diffuse poorly differentiated lymphoma with IgM-κ macroglobulinemia | 1 | 1 died of lymphoma |
| T cell–rich B cell EBV + lymphoma | 1 | 1 died of lymphoma |
Table 5.
Other cancers and selected outcomes
| Malignancy type | No. (n = 33) | Outcome |
|---|---|---|
| Breast cancer | 9 | 1 died of breast cancer, 2 died of other causes, 6 alive |
| Gastric cancer | 3 | 2 died of gastric cancer, 1 alive |
| Melanoma | 3 | 1 died of other causes, 2 alive |
| Malignancy of unknown primary | 3 | 3 alive |
| Colon cancer | 2 | 1 died of colon cancer, 1 died of other causes |
| Lung cancer | 2 | 2 died of lung cancer |
| Oral cancer | 2 | 1 died of oral cancer, 1 alive |
| Skin cancer | 2 | 2 alive |
| Hepatic carcinoid tumor | 1 | 1 died of carcinoid tumor |
| Colon, prostate cancer | 1 | 1 alive |
| Prostate, skin cancer | 1 | 1 alive |
| Thyroid cancer | 1 | 1 alive |
| Vaginal cancer | 1 | 1 alive |
| Ovarian cancer | 1 | 1 died of ovarian cancer |
| Esophageal cancer | 1 | 1 died of esophageal cancer |
Splenectomy
Thirty-nine patients (19 males and 20 females), 8.2% of the cohort, are known to have undergone a splenectomy. The majority of these were for either uncontrolled cytopenias (ITP or AIHA) or hypersplenism. In only 1 case was splenectomy performed after the introduction of rituximab, which has greatly reduced the need for splenectomy for either ITP or AIHA in subsequent subjects. However, 2 patients required rituximab after splenectomy, which also successfully led to remission of cytopenias. Only 1 subject, not yet on immunoglobulin replacement, had severe postoperative bacterial sepsis, but other complications developed in a few cases on Ig replacement, including fistulas to other organs or the exterior skin in 2, and unexplained portal hypertension and secondary liver failure in 2 (Table 6).
Table 6.
Splenectomy reasons and selected outcomes
| Reason | No. (n = 39) | Outcome |
|---|---|---|
| ITP | 12 | 11 cases resolved ITP, 1 case complicated by postoperative sepsis but this patient was not yet on immunoglobulin therapy |
| Hypersplenism | 9 | 6 cases resolved with no sequelae, 2 cases resolved hypersplenism but later developed liver failure with portal hypertension of unclear etiology, 1 case later developed bone marrow failure |
| AIHA | 6 | 4 cases resolved AIHA, 2 cases complicated by fistulae, 1 from the pancreas to the back, 1 to the stomach and abdominal wall |
| Hodgkin disease staging | 2 | 2 patients received chemotherapy for Hodgkin disease, both later died of lymphoma |
| Enlarged spleen and hypersplenism | 2 | 1 case resolved, 1 case improved |
| Unknown reason | 2 | 1 patient eventually developed lymphoma |
| Lymphoma | 1 | Patient found to have lymphoma |
| ITP/AIHA | 1 | Patient needed rituxumab for recurrence |
| Enlarged spleen | 1 | Splenomegaly with no other pathology |
| Abscess of spleen | 1 | Patient with splenic abscess with extension to psoas muscle |
| Enlarged spleen, presumed lymphoma | 1 | Patient with granulomata on pathology after splenectomy, no lymphoma found |
| Presumed lymphoma (medical error) | 1 | Spleen lost; pathology unknown, but patient given chemotherapy |
Survival
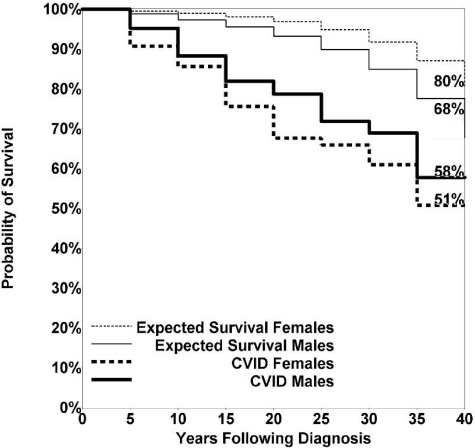
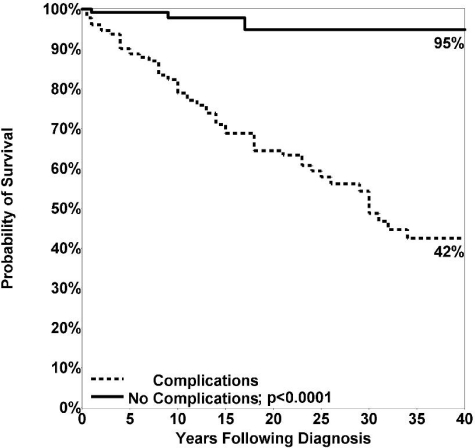
Of the 411 subjects with known follow-up (87% of the cohort), 93 patients (19.6%) had died. The median age at death was 44 years for females (range, 10-90 years) and 42 years for males (range, 9-79 years), not significantly different. The predominant causes of death included respiratory failure from chronic lung disease, lymphoid or other malignancy, or overwhelming infections (Table 7). To further examine the mortality of this cohort, we compared those with known long-term follow-up, 226 females (48%) and 185 males (39%), to age-matched population controls. The survival of both male (P = .0001) and female (P < .0001) CVID subjects was significantly reduced compared with 1994 US reference information34 (Figure 1). The risk of death in this interval was nearly 11 times higher for CVID patients with 1 or more of the noninfectious complications than for subjects who had infections only (hazard ratio [HR] = 10.96; P < .0001; Table 8). Kaplan-Meier analysis confirmed this observation, with a long-term survival of 95% for patients without versus 42% for those with noninfectious complications (Figure 2). These data agree with a previous study on CVID subjects from the European Society for Immunodeficiencies (ESID) registry.7 However, we found here that not all complications were associated with reduced survival. Patients with gastrointestinal disease (HR = 2.78; P = .0004); liver diseases and hepatitis (HR = 2.48; P = .0003); lymphoma (HR = 2.44; P = .001); chronic lung disease, including radiologic or functional lung disease, or both as described here (HR = 2.06; P = .001); or malabsorption (HR = 2.06; P = .022) had reduced survival in this interval, compared with CVID patients without these particular complications. In contrast, patients with any of the autoimmune conditions, cancers other than lymphoma, history of splenectomy, presence of granulomatous disease, or the development of bronchiectasis alone did not have significantly reduced survival over the 4 decades of study. Kaplan-Meier survival curves also affirmed these observations, showing significantly reduced survival for patients with gastrointestinal disease (P = .005), malabsorption (P = .0196), chronic lung disease (P = .0002), liver diseases and hepatitis (P < .0001), and lymphoma (P < .0001) but not for subjects with a history of autoimmunity (P = .1), cancer other than lymphomas (P = .21), previous splenectomy (P = .08), granuloma (P = .78), or the radiologic observation of bronchiectasis with lack of other findings (P = .54).
Table 7.
Causes of death
| Cause of death | No. (n = 93) | Comments |
|---|---|---|
| Lung failure | 34 | 14 of these from respiratory failure, 3 after lung transplant |
| Lymphoma | 17 | 2 cases with coexistent lung disease and 1 with coexistent liver disease contributing to death |
| Cancers | 10 | 2 lung, 2 stomach, 1 ovarian, 1 esophageal, 1 carcinoid, 1 oral, 1 breast, 1 colon |
| Liver disease | 8 | 1 after liver transplant, 4 infectious hepatitis |
| Other infections | 5 | Pneumocystis pneumonia, measles, nocardia brain abscess, multiple anaerobes, meningitis |
| Heart disease | 4 | 1 with coexisting lung disease |
| Aplastic anemia | 4 | 1 with coexisting neurodegenerative disease |
| Unknown cause of death | 4 | |
| Progressive multifocal leukoencephalopathy | 2 | |
| Vasculitis | 1 | |
| Neurodegenerative disease | 1 | |
| Autonomic amyloidosis | 1 | |
| Accidental death | 1 | |
| Suicide | 1 |
Figure 1.
Kaplan-Meier overall survival curves. CVID females and CVID males versus expected survival of females and males from the 1994 US population life tables. Both CVID females (P < .0001) and CVID males (P = .0001) had significantly shorter survival than population controls.
Table 8.
Cox proportional hazards modeling of complications
| HR | 95% CI | P | |
|---|---|---|---|
| Any complication | 10.96 | (3.46, 34.69) | < .0001* |
| Gastrointestinal disease | 2.78 | (1.44, 3.59) | .0004* |
| Liver disease/hepatitis | 2.48 | (1.51, 4.09) | .0003* |
| Lymphoma | 2.44 | (1.43, 4.16) | .0010* |
| Chronic lung disease (structural and functional) | 2.06 | (1.34, 3.16) | .0010* |
| Malabsorption | 2.06 | (1.11, 3.81) | .0218* |
| Splenectomy | 1.69 | (0.91, 3.12) | .0957 |
| Cancer | 1.51 | (0.795, 2.87) | .2084 |
| Autoimmunity | 1.36 | (0.87, 2.12) | .1735 |
| Granuloma | 1.27 | (0.65, 2.48) | .4939 |
| Bronchiectasis | 0.76 | (0.39, 1.48) | .4235 |
Adjusted for age at diagnosis.
P value significant at the .05 level.
Figure 2.
Kaplan-Meier curve for patients with and without noninfectious complications. Patients with noninfectious complications were significantly more likely to die than those with infections only (P < .0001).
Laboratory markers, complications, and survival
To identify demographic parameters associated with mortality, the Cox proportional hazards model, adjusted for age at diagnosis, was used. For each year of increase in age at diagnosis, the risk of death increased by 2.7% overall (HR = 1.027; P < .0001). The age at death was significantly correlated with both earlier age at symptom onset (r = 0.8, P < .0001) and age at diagnosis (r = 0.88, P < .0001). Examining immunologic parameters associated with mortality by Cox proportional hazards modeling, a lower baseline serum IgG level (HR = 0.998; P = .0079), fewer peripheral blood B cells (HR = 0.933; P = .0004), or an increased serum IgM level at diagnosis (HR = 1.005; P = .0021) were all associated with an increased mortality in this interval (Table 9).
Table 9.
Cox proportional hazards modeling of immunologic variables
| HR | 95% CI | P | |
|---|---|---|---|
| Age at diagnosis alone | 1.027 | (1.01, 1.04) | < .0001* |
| IgG | 0.998 | (0.996, 0.999) | .0079* |
| IgA | 0.995 | (0.99, 1.01) | .1577 |
| IgM | 1.005 | (1.00, 1.01) | .0021* |
| T cells | 0.994 | (0.98, 1.01) | .3437 |
| B cells | 0.933 | (0.898, 0.969) | .0004* |
Adjusted for age at diagnosis.
P value significant at the .05 level.
Finally, we examined the relationship between initial immunologic parameters—serum IgG, IgA, IgM, and percentage of B cells and isotype-switched memory B cells—and the odds of developing any of the noninfectious inflammatory and autoimmune complications. Higher levels of baseline serum IgG or IgA and higher percentages of peripheral blood B cells or isotype-switched memory B cells at the time of diagnosis were found significantly protective against noninfectious complications in general. Lower levels of isotype-switched memory B cells increased the risk of autoimmunity, as noted previously.33,38 Lower percentages of peripheral B cells increased the risk of nonlymphomatous cancers and higher serum IgM levels increased the risk of lymphoma. Higher levels of baseline serum IgM and lower levels of B cells increased the risk of liver diseases and hepatitis. Lower levels of serum IgG, IgA, and IgM at baseline; fewer B cells; or fewer isotype-switched memory B cells increased the risk for chronic lung disease (Table 10).
Table 10.
Odds ratios for specific complications given immunologic parameters
| Immunologic parameter | Odds ratio | 95% CI | P | |
|---|---|---|---|---|
| Any complication | IgG (lower) | 0.998 | 0.997, 1.000 | .0146* |
| B cells (lower) | 0.965 | 0.941, 0.989 | .0049* | |
| IgA (lower) | 0.993 | 0.987, 0.998 | .0097* | |
| Isotype switched memory B cells (lower) | 0.914 | 0.860, 0.971 | .0037* | |
| Autoimmunity | Isotype switched memory B cells (lower) | 0.924 | 0.854, 1.000 | .0487* |
| Cancer | B cells (lower) | 0.923 | 0.862, 0.989 | .0220* |
| Lymphoma | IgM (higher) | 1.007 | 1.001, 1.013 | .0139* |
| Liver disease/hepatitis | IgM (higher) | 1.006 | 1.001, 1.012 | .0219* |
| B cells (lower) | 0.939 | 0.890, 0.991 | .0228* | |
| Chronic lung disease (structural and functional) | IgG (lower) | 0.998 | 0.997, 1.000 | .0201* |
| IgM (lower) | 0.993 | 0.987, 0.998 | .0139* | |
| B cells (lower) | 0.951 | 0.922, 0.981 | .0015* | |
| IgA (lower) | 0.990 | 0.982, 0.998 | .0207* | |
| Isotype switched memory B cells (lower) | 0.910 | 0.830, 0.998 | .0451* |
P value significant at the .05 level.
Discussion
We report the demographics, immunologic parameters, clinical characteristics, associated conditions, and mortality statistics for 473 patients diagnosed with CVID seen in New York over 4 decades. In contrast to previous large studies on CVID that are aggregates of subjects followed at a number of medical centers, the current report is based at 1 medical center with more uniform case management. It should be noted that the population seen at this referral center may represent more severe and complicated cases of CVID.
The overall mortality in this cohort over this period was 19.6%, reduced from the 24% noted in our previous study a decade ago, and significantly improved from the data collected by Healy et al39 in 1971 before the introduction of intravenous immunoglobulin replacement (29% mortality). Both males and female subjects had significantly decreased survival at every time point, compared with sex- and age-matched controls. This is greater than that found for the ESID CVID cohort, with a 15% mortality over a similarly extended period,7 and greater than Quinti et al at 6%,31 for a significantly younger cohort followed for 11 years. The enhanced survival in CVID over time is probably because of the introduction of Ig replacement, and rising Ig trough levels over time that have greatly reduced the number of bacterial infections and improved the quality of life of subjects with immune defects in general.6,7,25,26,30,31,40 We also can speculate that the introduction of better and longer acting antibiotics have improved treatment regimens and extended life expectancy in CVID patients. Although female CVID patients tended to be older at diagnosis, they did not demonstrate a significantly enhanced survival over males. The more striking observation was that the 68% of subjects who had developed any of the noninfectious complications had markedly decreased survival compared with the 32% subjects with infections only (HR = 10.96; P < .0001). In fact, 89 of the 93 subjects who died had 1 or more of the noninfectious complications, and only the 4 others had infections only.
Because the noninfectious complications include an aggregate of inflammatory and autoimmune conditions, we then analyzed these separately to identify those more likely to cause mortality. Somewhat surprisingly, we found that only chronic lung disease, lymphoma, liver diseases and hepatitis, and gastrointestinal inflammatory disease with or without documented malabsorption were associated with worse survival. We did not find that the presence of bronchiectasis alone on chest tomography, history of autoimmunity, splenectomy, or cancers other than lymphoma significantly increased the risk of death in this 4-decade interval. As found in our previous study,6 lung disease, found in 28.5% of the cohort overall, was the main cause of death. In contrast to a previous study,30 we did not find that granuloma found in a variety of tissues, and in the lung in 26 cases, was associated with shorter survival. Bates et al had reported that patients with granulomatous lung disease along with lymphocytic interstitial pneumonia, follicular bronchiolitis, and pulmonary lymphoid hyperplasia, taken together, had shorter survival.30 Potentially, granulomatous disease in the lungs (found in > 50% of our 46 subjects with granulomata), as opposed to other organs, could lead to greater tissue destruction and ultimately respiratory failure. It should be noted that granulomatous disease is likely to be underdiagnosed in our cohort, because many patients did not undergo tissue biopsy.
Lymphoma, found in 39 cases (8.2%), was the second most common cause of death in our cohort. For unclear reasons, this is a higher overall incidence than other studies, including the ESID cohort (3%),7 a CVID group in Italy (1.8%),31 the United Kingdom (3.8%),27 and a combined Danish-Swedish cohort (2%).41 Although a significant cause of mortality, 22 of these subjects are alive, 4 of whom (3 with MALT and 1 with marginal zone lymphoma) are stable and had not received treatment up to 8 years after diagnosis of lymphoma. Although the presence of nonlymphoid cancers was not significantly associated with mortality, these were the third most common cause of death in this group. In contrast to older published data that suggested a higher incidence,27,42 we noted only 3 cases of gastric cancer, potentially related to the reduced incidence of this cancer overall, or greater use of antibiotics in these subjects that could eliminate colonizing Helicobacter pylori.43
By examining immunologic parameters using Cox regression analysis adjusted for age at diagnosis, we detected that lower baseline serum levels of IgG and higher levels of IgM were associated with poorer survival. Lower levels of IgG or IgA significantly increased the odds ratio for the development of any complication. In contrast, higher baseline serum IgM levels were associated with increased risk of lymphoma or any form of hepatitis. In this regard, as found previously,6,33 females with CVID had higher levels of serum IgM, and greater numbers of isotype switched memory B cells, and possibly not coincidentally, a higher incidence of B-cell lymphoma.6,36,41,44 Fewer peripheral B cells continued to be associated with reduced survival in CVID.6 Although the phenotype of circulating B cells was available for only about half of this patient population, we found that lower numbers of isotype switched memory B cells were associated with both autoimmunity and chronic lung disease, in accordance with previous studies.9,33,45–49
Although the genetic causes of CVID are likely to be multiple and most remain to be elucidated, these data show that the overall survival of patients has improved over time. More than 50% of the surviving subjects in this cohort are either students, working, retired but healthy, or otherwise pursuing normal activities of daily living, which suggests that the majority of patients carry out normal lives on replacement Ig therapy. However, selected, but not all, complications are still associated with increased morbidity and mortality. Baseline biomarkers as outlined here may provide clues to these clinical outcomes. Additional biomarkers are necessary to improve both prognostic and therapeutic information, including possibly the need for much higher doses of Ig, earlier use of biologic therapies, and if clear phenotypes emerge, recommendation for stem cell transplant in selected subjects. Further large cohort studies, particularly international efforts that include data from subjects with diverse genetic backgrounds, may provide improved biomarkers and a better understanding of the genetics of this syndrome.
Acknowledgments
The authors thank the following clinical staff of the Mt Sinai Immunodeficiency Clinic: Cynthia Medina, Denise Rosario, Sandy Leon, Monica Reiter-Wong, Stanley Vano, Aurora Barriga, and Maria Rodriguez. They also thank everyone in the Cunningham-Rundles laboratory: Lin Radigan, Li Zhang, and Thomas Marron.
This work was supported by National Institutes of Health grants AI-101093, AI-467320, and AI-48693; National Institute of Allergy and Infectious Diseases contract 03-22; National Institutes of Health National Center for Research Resources grant UL1RR029887; and the David S. Gottesman Immunology Chair.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: E.S.R. and C.C.-R. contributed data collection and analysis and wrote the manuscript; and E.L.M. and J.H.G. contributed statistical analysis and figure preparation.
Conflict-of-interest disclosure: C.C.-R. reports research grants from Baxter Heathcare and Octapharma and Medical Advisory Boards of Baxter Healthcare and Grifols. The remaining authors declare no competing financial interests.
Correspondence: Charlotte Cunningham-Rundles, Departments of Medicine and Pediatrics, The Immunology Institute, Rm 11-20, Mount Sinai School of Medicine, 1425 Madison Ave, New York, NY 10029; e-mail: charlotte.cunningham-rundles@mssm.edu.
References
- 1.Conley ME, Notarangelo LD, Etzioni A. Diagnostic criteria for primary immunodeficiencies. Representing PAGID (Pan-American Group for Immunodeficiency) and ESID (European Society for Immunodeficiencies). Clin Immunol. 1999;93(3):190–197. doi: 10.1006/clim.1999.4799. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham-Rundles C. Common variable immunodeficiency. Curr Allergy Asthma Rep. 2001;1(5):421–429. doi: 10.1007/s11882-001-0027-1. [DOI] [PubMed] [Google Scholar]
- 3.Chapel H, Cunningham-Rundles C. Update in understanding common variable immunodeficiency disorders (CVIDs) and the management of patients with these conditions. Br J Haematol. 2009;145(6):709–727. doi: 10.1111/j.1365-2141.2009.07669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Notarangelo LD, Fischer A, Geha RS, et al. Primary immunodeficiencies: 2009 update. J Allergy Clin Immunol. 2009;124(6):1161–1178. doi: 10.1016/j.jaci.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cunningham-Rundles C. How I treat common variable immune deficiency. Blood. 2010;116(1):7–15. doi: 10.1182/blood-2010-01-254417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cunningham-Rundles C, Bodian C. Common variable immunodeficiency: clinical and immunological features of 248 patients. Clin Immunol. 1999;92(1):34–48. doi: 10.1006/clim.1999.4725. [DOI] [PubMed] [Google Scholar]
- 7.Chapel H, Lucas M, Lee M, et al. Common variable immunodeficiency disorders: division into distinct clinical phenotypes. Blood. 2008;112(2):277–286. doi: 10.1182/blood-2007-11-124545. [DOI] [PubMed] [Google Scholar]
- 8.Agematsu K, Hokibara S, Nagumo H, Shinozaki K, Yamada S, Komiyama A. Plasma cell generation from B-lymphocytes via CD27/CD70 interaction. Leuk Lymphoma. 1999;35(3-4):219–225. doi: 10.3109/10428199909145724. [DOI] [PubMed] [Google Scholar]
- 9.Warnatz K, Denz A, Drager R, et al. Severe deficiency of switched memory B cells (CD27(+)IgM(-)IgD(-)) in subgroups of patients with common variable immunodeficiency: a new approach to classify a heterogeneous disease. Blood. 2002;99(5):1544–1551. doi: 10.1182/blood.v99.5.1544. [DOI] [PubMed] [Google Scholar]
- 10.Ochtrop ML, Goldacker S, May AM, et al. T and B lymphocyte abnormalities in bone marrow biopsies of common variable immunodeficiency. Blood. 2011;118(2):309–318. doi: 10.1182/blood-2010-11-321695. [DOI] [PubMed] [Google Scholar]
- 11.Janeway CA, Apt L, Gitlin D. Agammaglobulinemia. Trans Assoc Am Physicians. 1953;66:200–202. [PubMed] [Google Scholar]
- 12.Grimbacher B, Hutloff A, Schlesier M, et al. Homozygous loss of ICOS is associated with adult-onset common variable immunodeficiency. Nat Immunol. 2003;4(3):261–268. doi: 10.1038/ni902. [DOI] [PubMed] [Google Scholar]
- 13.van Zelm MC, Reisli I, van der Burg M, et al. An antibody-deficiency syndrome due to mutations in the CD19 gene. N Engl J Med. 2006;354(18):1901–1912. doi: 10.1056/NEJMoa051568. [DOI] [PubMed] [Google Scholar]
- 14.Kanegane H, Agematsu K, Futatani T, et al. Novel mutations in a Japanese patient with CD19 deficiency. Genes Immun. 2007;8(8):663–670. doi: 10.1038/sj.gene.6364431. [DOI] [PubMed] [Google Scholar]
- 15.Warnatz K, Salzer U, Rizzi M, et al. B-cell activating factor receptor deficiency is associated with an adult-onset antibody deficiency syndrome in humans. Proc Natl Acad Sci U S A. 2009;106(33):13945–13950. doi: 10.1073/pnas.0903543106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuijpers TW, Bende RJ, Baars PA, et al. CD20 deficiency in humans results in impaired T cell-independent antibody responses. J Clin Invest. 2010;120(1):214–222. doi: 10.1172/JCI40231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Zelm MC, Smet J, Adams B, et al. CD81 gene defect in humans disrupts CD19 complex formation and leads to antibody deficiency. J Clin Invest. 2010;120(4):1265–1274. doi: 10.1172/JCI39748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salzer U, Chapel HM, Webster AD, et al. Mutations in TNFRSF13B encoding TACI are associated with common variable immunodeficiency in humans. Nat Genet. 2005;37(8):820–828. doi: 10.1038/ng1600. [DOI] [PubMed] [Google Scholar]
- 19.Castigli E, Wilson SA, Garibyan L, et al. TACI is mutant in common variable immunodeficiency and IgA deficiency. Nat Genet. 2005;37(8):829–834. doi: 10.1038/ng1601. [DOI] [PubMed] [Google Scholar]
- 20.Pan-Hammarström Q, Salzer U, Du L, et al. Reexamining the role of TACI coding variants in common variable immunodeficiency and selective IgA deficiency. Nat Genet. 2007;39(4):429–430. doi: 10.1038/ng0407-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L, Radigan L, Salzer U, et al. Transmembrane activator and calcium-modulating cyclophilin ligand interactor mutations in common variable immunodeficiency: clinical and immunologic outcomes in heterozygotes. J Allergy Clin Immunol. 2007;120(5):1178–1185. doi: 10.1016/j.jaci.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salzer U, Bacchelli C, Buckridge S, et al. Relevance of biallelic versus monoallelic TNFRSF13B mutations in distinguishing disease-causing from risk-increasing TNFRSF13B variants in antibody deficiency syndromes. Blood. 2009;113(9):1967–1976. doi: 10.1182/blood-2008-02-141937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Conley ME, Dobbs AK, Farmer DM, et al. Primary B cell immunodeficiencies: comparisons and contrasts. Annu Rev Immunol. 2009;27:199–227. doi: 10.1146/annurev.immunol.021908.132649. [DOI] [PubMed] [Google Scholar]
- 24.Orange JS, Glessner JT, Resnick E, et al. Genome-wide association identifies diverse causes of common variable immunodeficiency. J Allergy Clin Immunol. 2011;127(6):1360–1367. doi: 10.1016/j.jaci.2011.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orange JS, Hossny EM, Weiler CR, et al. Use of intravenous immunoglobulin in human disease: a review of evidence by members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol. 2006;117(4 suppl):S525–553. doi: 10.1016/j.jaci.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 26.Lucas M, Lee M, Lortan J, Lopez-Granados E, Misbah S, Chapel H. Infection outcomes in patients with common variable immunodeficiency disorders: relationship to immunoglobulin therapy over 22 years. J Allergy Clin Immunol. 2010;125(6):1354–1360. doi: 10.1016/j.jaci.2010.02.040. [DOI] [PubMed] [Google Scholar]
- 27.Hermaszewski RA, Webster AD. Primary hypogammaglobulinaemia: a survey of clinical manifestations and complications. Q J Med. 1993;86(1):31–42. [PubMed] [Google Scholar]
- 28.Kainulainen L, Nikoskelainen J, Ruuskanen O. Diagnostic findings in 95 Finnish patients with common variable immunodeficiency. J Clin Immunol. 2001;21(2):145–149. doi: 10.1023/a:1011012023616. [DOI] [PubMed] [Google Scholar]
- 29.Van der Hilst JC, Smits BW, van der Meer JW. Hypogammaglobulinaemia: cumulative experience in 49 patients in a tertiary care institution. Neth J Med. 2002;60(3):140–147. [PubMed] [Google Scholar]
- 30.Bates CA, Ellison MC, Lynch DA, Cool CD, Brown KK, Routes JM. Granulomatous-lymphocytic lung disease shortens survival in common variable immunodeficiency. J Allergy Clin Immunol. 2004;114(2):415–421. doi: 10.1016/j.jaci.2004.05.057. [DOI] [PubMed] [Google Scholar]
- 31.Quinti I, Soresina A, Spadaro G, et al. Long-term follow-up and outcome of a large cohort of patients with common variable immunodeficiency. J Clin Immunol. 2007;27(3):308–316. doi: 10.1007/s10875-007-9075-1. [DOI] [PubMed] [Google Scholar]
- 32.Mouillot G, Carmagnat M, Gerard L, et al. B-cell and T-cell phenotypes in CVID patients correlate with the clinical phenotype of the disease. J Clin Immunol. 2010;30(5):746–755. doi: 10.1007/s10875-010-9424-3. [DOI] [PubMed] [Google Scholar]
- 33.Sánchez-Ramón S, Radigan L, Yu JE, Bard S, Cunningham-Rundles C. Memory B cells in common variable immunodeficiency: clinical associations and sex differences. Clin Immunol. 2008;128(3):314–321. doi: 10.1016/j.clim.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Center for Health Statistics. Hyattsville, MD: National Center for Health Statistics; 1998. Vital statistics of the United States, 1994, preprint of vol II, mortality, part A sec 6 life tables. [Google Scholar]
- 35.SAS Institute. SAS System for Windows Version 9.2 computer program. Cary, NC: SAS Institute; [Google Scholar]
- 36.Cunningham-Rundles C, Lieberman P, Hellman G, Chaganti RS. Non-Hodgkin lymphoma in common variable immunodeficiency. Am J Hematol. 1991;37(2):69–74. doi: 10.1002/ajh.2830370202. [DOI] [PubMed] [Google Scholar]
- 37.Lanasa MC, Allgood SD, Slager SL, et al. Immunophenotypic and gene expression analysis of monoclonal B-cell lymphocytosis shows biologic characteristics associated with good prognosis CLL. Leukemia. 2011;25(9):1459–1466. doi: 10.1038/leu.2011.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wehr C, Kivioja T, Schmitt C, et al. The EUROclass trial: defining subgroups in common variable immunodeficiency. Blood. 2008;111(1):77–85. doi: 10.1182/blood-2007-06-091744. [DOI] [PubMed] [Google Scholar]
- 39.Healy MJ. Hypogammaglobulinaemia in the United Kingdom. XII. Statistical analyses: prevalence, mortality and effects of treatment. Spec Rep Ser Med Res Counc. 1971;310:115–123. [PubMed] [Google Scholar]
- 40.Busse PJ, Razvi S, Cunningham-Rundles C. Efficacy of intravenous immunoglobulin in the prevention of pneumonia in patients with common variable immunodeficiency. J Allergy Clin Immunol. 2002;109(6):1001–1004. doi: 10.1067/mai.2002.124999. [DOI] [PubMed] [Google Scholar]
- 41.Mellemkjaer L, Hammarstrom L, Andersen V, et al. Cancer risk among patients with IgA deficiency or common variable immunodeficiency and their relatives: a combined Danish and Swedish study. Clin Exp Immunol. 2002;130(3):495–500. doi: 10.1046/j.1365-2249.2002.02004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kinlen LJ, Webster AD, Bird AG, et al. Prospective study of cancer in patients with hypogammaglobulinaemia. Lancet. 1985;1(8423):263–266. doi: 10.1016/s0140-6736(85)91037-2. [DOI] [PubMed] [Google Scholar]
- 43.Dhalla F, da Silva SP, Lucas M, Travis S, Chapel H. Review of gastric cancer risk factors in patients with common variable immunodeficiency disorders, resulting in a proposal for a surveillance programme. Clin Exp Immunol. 2011;165(1):1–7. doi: 10.1111/j.1365-2249.2011.04384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cunningham-Rundles C, Siegal FP, Cunningham-Rundles S, Lieberman P. Incidence of cancer in 98 patients with common varied immunodeficiency. J Clin Immunol. 1987;7(4):294–299. doi: 10.1007/BF00915550. [DOI] [PubMed] [Google Scholar]
- 45.Piqueras B, Lavenu-Bombled C, Galicier L, et al. Common variable immunodeficiency patient classification based on impaired B cell memory differentiation correlates with clinical aspects. J Clin Immunol. 2003;23(5):385–400. doi: 10.1023/a:1025373601374. [DOI] [PubMed] [Google Scholar]
- 46.Wehr C, Eibel H, Masilamani M, et al. A new CD21low B cell population in the peripheral blood of patients with SLE. Clin Immunol. 2004;113(2):161–171. doi: 10.1016/j.clim.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 47.Ko J, Radigan L, Cunningham-Rundles C. Immune competence and switched memory B cells in common variable immunodeficiency. Clin Immunol. 2005;116(1):37–41. doi: 10.1016/j.clim.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 48.Alachkar H, Taubenheim N, Haeney MR, Durandy A, Arkwright PD. Memory switched B cell percentage and not serum immunoglobulin concentration is associated with clinical complications in children and adults with specific antibody deficiency and common variable immunodeficiency. Clin Immunol. 2006;120(3):310–318. doi: 10.1016/j.clim.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 49.Isnardi I, Ng YS, Menard L, et al. Complement receptor 2/CD21-negative human naive B cells mostly contain autoreactive unresponsive clones. Blood. 2010;115(24):5026–5036. doi: 10.1182/blood-2009-09-243071. [DOI] [PMC free article] [PubMed] [Google Scholar]