Abstract
The chemokine receptor CXCR4, which normally regulates stromal stem cell interactions in the bone marrow, is highly expressed on a variety of malignant hematologic cells, including lymphoma and lymphocytic leukemias. A new treatment concept has arisen wherein CXCR4 may be an effective therapeutic target as an adjunct to treatment of hematologic neoplasms with chemo- and immunotherapy. In the present study, we developed pepducins, cell-penetrating lipopeptide antagonists of CXCR4, to interdict CXCL12-CXCR4 transmembrane signaling to intracellular G-proteins. We demonstrate that pepducins targeting the first (i1) or third (i3) intracellular loops of CXCR4 completely abrogate CXCL12-mediated cell migration of lymphocytic leukemias and lymphomas. Stromal-cell coculture protects lymphoma cells from apoptosis in response to treatment with the CD20-targeted Ab rituximab. However, combination treatment with CXCR4 pepducins and rituximab significantly increases the apoptotic effect of rituximab. Furthermore, treatment of mice bearing disseminated lymphoma xenografts with pepducins alone or in combination with rituximab significantly increased their survival. These data demonstrate that CXCL12-CXCR4 signaling can be effectively inhibited by cell-penetrating pepducins, which represents a potential new treatment strategy for lymphoid malignancies.
Introduction
Hematologic malignancies account for almost 10% of new cancer cases in the United States each year.1 The last decade has seen the introduction of rituximab, a humanized mAb directed against the CD20 Ag, as a treatment option for B-cell leukemia and lymphomas, and combination chemotherapy with rituximab is now standard treatment for aggressive non-Hodgkin lymphoma (NHL).2 However, because approximately 60% of patients with aggressive NHL are not cured, new biologic therapies and targets are urgently needed to further improve overall survival.
The chemokine G-protein–coupled receptor (GPCR) CXCR4 and its ligand, CXCL12 (also called stromal cell–derived factor-1α [SDF-1]), regulate a diverse array of cellular processes, including leukocyte trafficking, B-cell lymphopoiesis, and bone marrow myelopoiesis3; survival and proliferation of hematopoietic stem cells (HSCs)4; and homing of HSCs to the BM. Under normal physiologic conditions, HSCs and hematopoietic progenitor cells (HPCs) are predominantly present in the BM, where they give rise to the mature cells of the hematopoietic system that are released into the blood circulation.5 CXCL12 is constitutively secreted at high levels by BM stromal cells,6 and it is this chemokine gradient that retains HSCs and HPCs in the BM and regulates homing of CXCR4-expressing cells.7 The small-molecule antagonist plerixafor (AMD3100), which targets the CXCR4/CXCL12-SDF1 signaling axis, is an effective clinical tool with which to enhance mobilization of HSCs to the peripheral blood for subsequent autologous transplantation,8,9 and has recently been approved for use in combination with G-CSF as a stem cell–mobilizing agent in humans.
In recent years, CXCR4 has been implicated in the progression of several hematologic and nonhematologic malignancies. CXCR4 is expressed on a variety of human tumors and is a poor prognostic factor in cancers as diverse as breast carcinoma,6 melanoma,10 colorectal cancer,11 and acute myelogenous leukemia.12,13 CXCL12/CXCR4 signaling mediates metastasis to distal organs, including the BM14 and lymph nodes,15,16 where interaction with CXCL12-secreting stromal cells can mediate cell survival and resistance to chemotherapy.17,18 Recent studies have examined the potential of targeting CXCR4 as a therapeutic strategy in the treatment of hematologic malignancies19–21 and metastasis of solid tumors,22,23 and plerixafor is currently being evaluated for safety and efficacy in phase 1/2 clinical trials in patients with chronic lymphocytic leukemia (CLL) in combination with rituximab.24 A significant obstacle to curing hematologic malignancies is the occurrence of minimal residual disease. Stromal cells of the BM and secondary lymphoid organs support the survival and chemoresistance of CLL cells,25,26 and are thought to contribute to minimal residual disease and subsequent disease relapse. In this way, antagonism of CXCL12-SDF1/CXCR4 signaling with plerixafor disrupts interaction of myeloma cells with stromal cells of the BM, thereby increasing their sensitivity to the cytotoxic agent bortezomib.27 It has also been demonstrated that mAbs to CXCR4 mediate tumor cell extravasation and enhance survival of mice bearing human lymphoma xenografts.28
GPCRs such as CXCR4 are attractive therapeutic targets because of their involvement in a range of pathologic diseases. The majority of drugs targeting GPCRs interact with the receptor on the outside surface in competition with the natural ligand. However, the intracellular domains of GPCRs represent new drug targets because these regions mediate interaction of receptors with G proteins that activate subsequent downstream signaling pathways. In recent years, cell-penetrating lipidated peptides called pepducins have emerged as effective agonists or antagonists of their cognate GPCR.29–35 Pepducins are composed of a peptide sequence derived from the amino acid sequence of the intracellular domains of the target receptor, typically conjugated to a lipid moiety such as palmitate. The lipid moiety facilitates membrane translocation and tethering of the pepducin to the inner leaflet of the lipid bilayer, where the peptide sequence can selectively modulate GPCR activity.36 Pepducins have high bioavailability, efficacy, and long biologic half-lives when delivered systemically.37 Cell-penetrating pepducins have been designed to target several GPCRs and exhibit in vivo efficacy in several disease models, including inhibition of cancer cell metastasis,35,38 thrombosis,29,32 and sepsis.33,39
Our previous work demonstrated that CXCR4 pepducin antagonists were potent inhibitors of calcium mobilization and chemotaxis of human neutrophils in response to CXCL12 and could mobilize immature neutrophils to the peripheral blood of mice.33 In the present study, we examined the efficacy of CXCR4 pepducins based on the first (i1) and third (i3) intracellular loops in primary and established leukemia and lymphoma cells, and have demonstrated that the CXCR4 pepducins inhibit chemotaxis to gradients of CXCL12. Pepducins enhanced rituximab-mediated apoptotic cell death in primary CLL cells and in Raji and Ramos lymphoma cells. Moreover, CXCR4 pepducins, alone or in combination with rituximab, prolonged survival in a mouse model of disseminated lymphoma. These findings provide support for the use of pepducin antagonists of CXCR4 as a novel treatment strategy for lymphoid malignancies.
Methods
Reagents
N-palmitoylated peptides were synthesized as described previously40 with C-terminal amides by the Tufts Peptide Core Facility and purified by reverse-phase HPLC. The peptide sequence of PZ-218, MGYQKKLRSMTD, corresponds to the i1 loop of CXCR4; the peptide sequence of PZ-210, SKLSHSKGHQKRKALK, corresponds to the i3 loop of CXCR4. The control pepducins PZ-253 and PZ-254 are composed of the same peptide sequence as PZ-218 and PZ-210, but lack an N-terminal palmitate. PZ-217 (ATI-2339) is a negative control CXCR4 pepducin that was characterized previously by Tchernychev et al.41 Plerixafor was obtained from Sigma-Aldrich and rituximab was from Genentech.
Cell culture and clinical samples
All leukemia and lymphoma cell lines were obtained from the American Type Culture Collection (Manassas, VA). Cells were cultured in RPMI supplemented with 10% FBS. For primary leukemia samples, blood was obtained after informed consent in accordance with the Declaration of Helsinki following a protocol approved by the Institutional Review Board of Tufts Medical Center. Low-density mononuclear cells were isolated by density gradient separation on Ficoll-Paque PLUS, and cells were cryopreserved until required.
Measurement of cell-surface CXCR4 and CD20 expression
Cells were stained with PE-conjugated CXCR4 Ab (clone 12G5), FITC-conjugated CD20 Ab, or isotype controls (BD Pharmingen) for 20 minutes at room temperature. Samples were analyzed with a FACS Canto II flow cytometer (BD Biosciences).
Chemotaxis and migration assays
Chemotaxis of cells through a 5-μm cellulose nitrate filter to 30nM CXCL12 (Peprotech) was measured as described previously33 using a 48-well microchemotaxis chamber (Neuro Probe). Briefly, cells in migration medium (0.4% BSA/RPMI) in the upper wells of the chamber were treated with pepducins or plerixafor and allowed to migrate to CXCL12 in the bottom wells for 1 hour at 37°C. The migration depth of cells into the filter was quantified by light microscopy by measuring the distance from the surface of the filter to the leading front of the cells. Chemotaxis of vehicle-treated cells was set at 100% and was calculated as change in distance migrated toward CXCL12 compared with random migration (distance migrated by cells in the absence of chemoattractant). Migration assays were carried out essentially as described previously by Bluel et al using 5-μm pore polycarbonate Transwell migration chamber (Corning).42 Cells were treated with vehicle or pepducin, and 4 × 105 cells in 200 μL of 0.4% BSA/RPMI were seeded in the upper well of the migration chamber. CXCL12 at a final concentration of 3nM in 0.4% BSA/RPMI was added to the bottom wells. Cells that had migrated through the 5-μm polycarbonate membranes after 5 hours at 37°C were manually counted using a hemocytometer.
Measurement of intracellular calcium mobilization
Cells were resuspended, washed in KRB buffer,43 and loaded with 2.5μM Fura-2/AM (Molecular Probes) for 30 minutes at 37°C with 5% CO2 and gentle shaking. Fluorescence was measured in a Perkin-Elmer LS50B spectrofluorometer as described previously. The fluorescence emission was recorded at 510 nm with dual excitation at 340 and 380 nm at 25°C.
Western blotting
Cells were treated with inhibitors overnight in 0.4% BSA/RPM1 (10μM pepducin or plerixafor and 100 ng/mL of pertussis toxin). Stimulation with CXCL12 (6 ng/mL) was for 15 minutes at 37°C. Cells were lysed in NP-40 buffer (150mM NaCl; 1% NP-40; 50mM Tris, pH 8.0) supplemented with the proteinase inhibitors aprotinin and leupeptin (Sigma-Aldrich) and a phosphatase inhibitor cocktail (Roche) as described previously.44 Western blots were probed with phospho-ERK1/2 and total ERK1/2 Abs (Cell Signaling Technologies) overnight at 4°C.
Coculture experiments
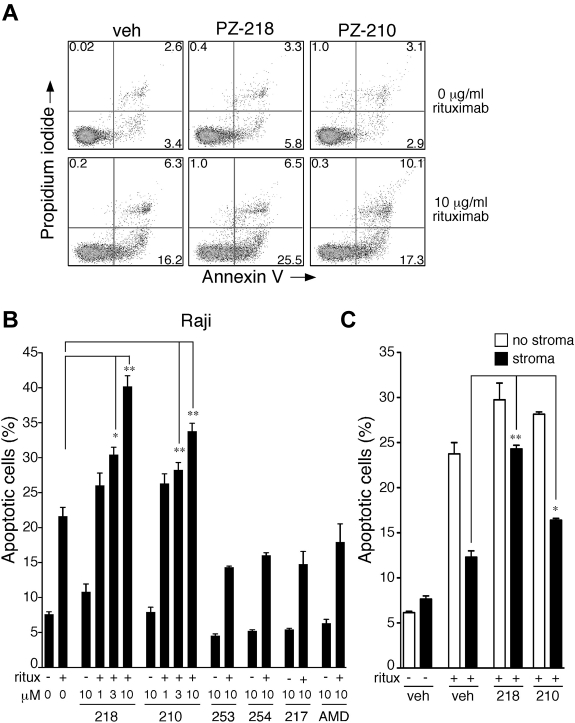
M210B4 stromal cells (American Type Culture Colleciton) were plated at a density of 4 × 104 cells per well in 12-well tissue culture plates and incubated overnight. The following day, Raji lymphoma cells were resuspended in M210B4 conditioned medium and treated with vehicle or rituximab (10 μg/mL) in the presence or absence of pepducins. These cells were then cocultured with M210B4 cells for 24 hours before assessment of apoptotic cells by annexin V/propidium iodide (PI) staining. Stromal cells were distinguished from Raji cells on the basis of their distinct forward scatter/side scatter properties by flow cytometry.
Annexin V/PI cell viability assay
Apoptotic/dead cells were quantified using the annexin V–FITC Apoptosis Detection Kit (BD Pharmingen) by dual staining with FITC-conjugated annexin V and PI. Briefly, Raji and Ramos cells (0.4 × 106/mL in 10% FBS/RPMI) were treated with pepducin and rituximab (10 μg/mL for Raji cells and 20 μg/mL for Ramos cells) for 24 hours at 37°C. Cells were then stained with annexin V–FITC and PI for 15 minutes at room temperature before analysis of fluorescence by flow cytometry. The percentage of apoptotic/dead cells was calculated as the sum of percentages of early apoptotic cells (annexin V+/PI−) and late apoptotic/dead cells (annexin V−/PI+ and annexin V+/PI+).
In vivo systemic lymphoma mouse model
Female 6-week-old NOD/SCID/Il2rg−/− (NSG) mice (The Jackson Laboratory) were injected intravenously into the lateral tail vein with 2 × 106 Raji lymphoma cells suspended in 200 μL of PBS. Raji cells had been maintained in culture for less than 2 weeks and were tested for surface expression of CD20 and CXCR4 by flow cytometry before injection into mice. After injection, mice were randomly divided into 8 groups for the following treatments: (1) vehicle, (2) rituximab, (3) PZ-218, (4) PZ-210, (5) plerixafor, (6) rituximab and PZ-218, (7) rituximab and PZ-210, and (8) rituximab and plerixafor. Equimolar concentrations of pepducins (3 mg/kg) and plerixafor (1 mg/kg) were administered subcutaneously each day starting on day 0 (the day of injection of Raji cells). Rituximab (10 mg/kg) was injected intraperitoneally twice weekly, with the first treatment administered on day 7. Mice were monitored daily for signs of disease progression and death, and were killed upon development of hind-limb paralysis.
Statistical analyses
Statistical analyses of chemotaxis and cell viability assays were performed using Prism Version 4.0 software (GraphPad). P values were calculated using the Student unpaired t test. For survival studies, Kaplan-Meier curves were generated for all treatment cohorts using the Prism Version 4.0 software. Statistical significance between treatment groups was analyzed using the log-rank test.
Results
Generation of lipid-conjugated peptides that antagonize CXCR4 signaling in lymphocytic leukemia and lymphoma cells
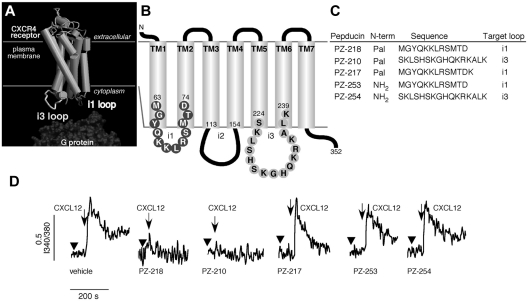
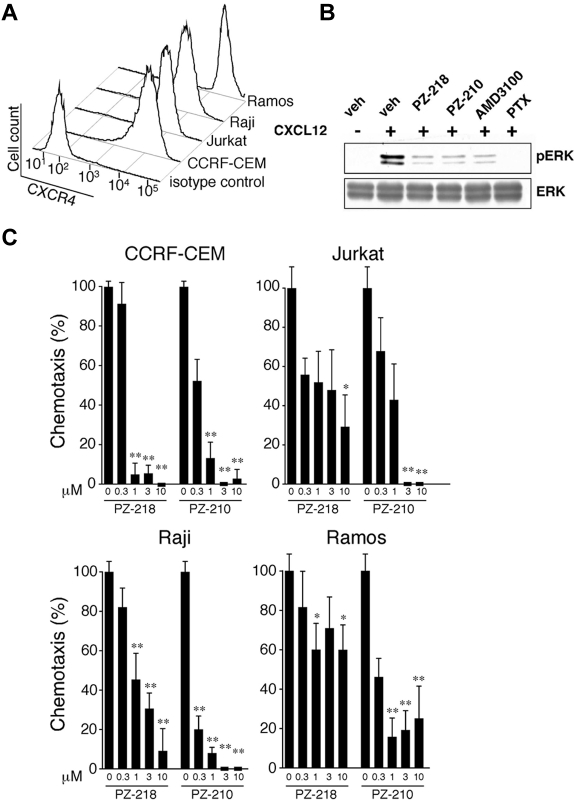
CXCR4 is a 7-transmembrane receptor that mediates intracellular signaling by interaction with cytosolic heterotrimeric G proteins, as illustrated in Figure 1A. Previous work by our group demonstrated that palmitoylated peptides, called pepducins, based on the sequences of the first or third intracellular loops of GPCRs, can modulate downstream signaling. In the present study, pepducins were designed to target the first (i1) and third (i3) intracellular loops of CXCR4 (Figure 1B-C), which are identical in human and mouse. These pepducins, designated PZ-218 and PZ-210, respectively, contain an N-terminal palmitate moiety that facilitates insertion into the cytosolic face of the plasma membrane. Peptides lacking an N-terminal palmitate moiety (PZ-253 and PZ-254) and an inactive analog (PZ-217) with an additional C-terminal positive charge (Figure 1C) were generated as controls to examine the specific activity of CXCR4-targeted pepducins. Our previous work demonstrated that PZ-218 and PZ-210 specifically inhibit CXCL12-mediated responses in human and mouse neutrophils.33 Both PZ-218 and PZ-210 could completely inhibit CXCL12-mediated Ca2+ signaling, whereas PZ-217, PZ-253, and PZ-254 negative control pepducins had no effect (Figure 1D). CXCR4 was highly expressed on the surface of several leukemia cell lines, including CEM and Jurkat cells, and on the lymphoma cell lines Raji and Ramos (Figure 2A). Consistent with previous studies,45 the mechanism of CXCR4-mediated activation of the MAPK cascade is Gi(βγ)–PI3K dependent, as shown by complete inhibition of CXCL12-mediated ERK activation by pertussis toxin treatment of Jurkat cells (Figure 2B). There was complete inhibition of CXCL12-induced ERK1/2 phosphorylation with the PZ-218 and PZ-210 pepducins and by plerixafor (AMD3100).
Figure 1.
Pepducins are lipopeptide antagonists of CXCR4 in human leukemia and lymphoma cell lines. (A) A model of the 3-dimensional structure of CXCR4 based on the structure of rhodopsin demonstrates the topological arrangement of the intracellular loops. G proteins are recruited to the intracellular loops of CXCR4 and mediate downstream signaling in response to ligand binding. (B) Schematic representation of CXCR4 with i1 and i3 loop amino acid sequences. (C) Sequences of the CXCR4 pepducins and control peptides. Pepducins with N-terminal palmitate (Pal) based on the sequence of the i1 (PZ-218 and PZ-217) and i3 (PZ-210) intracellular loops. Control peptides lacking an N-terminal palmitate moiety and corresponding to the i1 (PZ-253) and i3 (PZ-254) loop sequences were synthesized. (D) Effect of CXCR4 pepducins on human hematopoietic cell calcium flux. Cells were preincubated with vehicle or the indicated pepducins and stimulated with CXCL12.
Figure 2.
Pepducins targeting the i1 and i3 loop of CXCR4 inhibit CXCL12-mediated chemotaxis of leukemia and lymphoma cells. (A) CXCR4 surface expression on human leukemia (Jurkat and CEM) and lymphoma (Raji and Ramos) cell lines was analyzed by flow cytometry using PE-conjugated CXCR4 Ab. (B) Inhibition of CXCR4-ERK activation by i1- and i3-derived CXCR4 pepducins. Lysates from Jurkat cells untreated (veh) or treated as indicated before CXCL12 stimulation were immunoblotted with anti–phospho-ERK or total ERK. (C) Human leukemia and lymphoma cells were treated with 0.3-10μM PZ-218 or PZ-210 and chemotaxis to 30nM CXCL12 was determined by microscopically measuring the distance migrated by cells through a cellulose nitrate filter. Chemotaxis of vehicle-treated cells to CXCL12 was set at 100%, and random chemotaxis (chemotaxis of cells in the absence of CXCL12) was set at 0%. Data are presented as the means ± SEM of triplicate experiments with n = 3 for each experiment. *P < .05 and **P < .01 compared with vehicle-treated controls.
We also investigated the efficacy of the i1 and i3 loop pepducins as CXCR4 antagonists in human lymphocytic leukemia and lymphoma cell lines and primary cells derived from CLL patients using chemotaxis and Boyden chamber migration assays. Both PZ-218 and PZ-210 inhibited chemotaxis of CEM, Jurkat, Raji, and Ramos cells toward 30nM CXCL12 in a dose-dependent manner, with IC50 values of approximately 0.3-1μM (Figure 2C). In CEM, Jurkat, and Raji cell lines, PZ-218 and PZ-210 at high concentrations (≥ 3μM) had the additional ability to inhibit chemotaxis below baseline levels, suggesting suppression of chemokinesis. We also examined CXCL12-mediated chemotaxis of CEM and Ramos cells treated with the nonpalmitoylated peptides PZ-253 and PZ-254 and with the i1 loop pepducin, PZ-217, which has an additional C-terminal lysine. At concentrations of 10μM, neither the nonpalmitoylated peptides nor PZ-217 inhibited chemotaxis in these cell lines (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), indicating that both the palmitate moiety and the specific peptide composition are essential for antagonist activity of the CXCR4 pepducins.
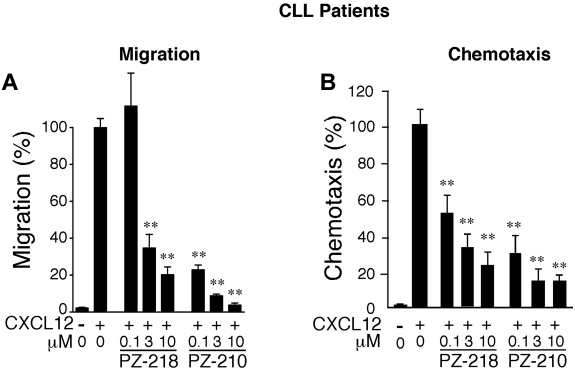
To confirm the efficacy of pepducins as CXCR4 antagonists, we tested their effect on CXCL12-mediated Boyden chamber migration and chemotaxis in leukemia cells isolated from 5 patients with CLL. All patient CLL B cells tested had high CXCR4 expression levels (data not shown). Both PZ-218 and PZ-210 significantly inhibited cell migration/chemotaxis to CXCL12 with comparable IC50 values in both types of migration assays (Figure 3A-B and supplemental Figure 2), indicating that pepducins are effective antagonists of CXCR4 activity in leukemic cells obtained from patients with CLL.
Figure 3.
Inhibition of CXCL12-mediated chemotaxis in primary human B-CLL cells. Primary leukemic cells isolated from patients with B-CLL were treated with PZ-218, PZ-210, or plerixafor (AMD, 0.1-10μM) and migration (A) and chemotaxis (B) toward CXCL12 was measured. Chemotaxis/migration of vehicle-treated cells to CXCL12 was set at 100%. All data are expressed as means ± SEM (n = 4) with assays done in triplicate or quadruplicate. **P < .01 compared with CXCL12-treated cells.
CXCR4 pepducins significantly enhance rituximab-mediated cytotoxicity of leukemic cells from patients with CLL
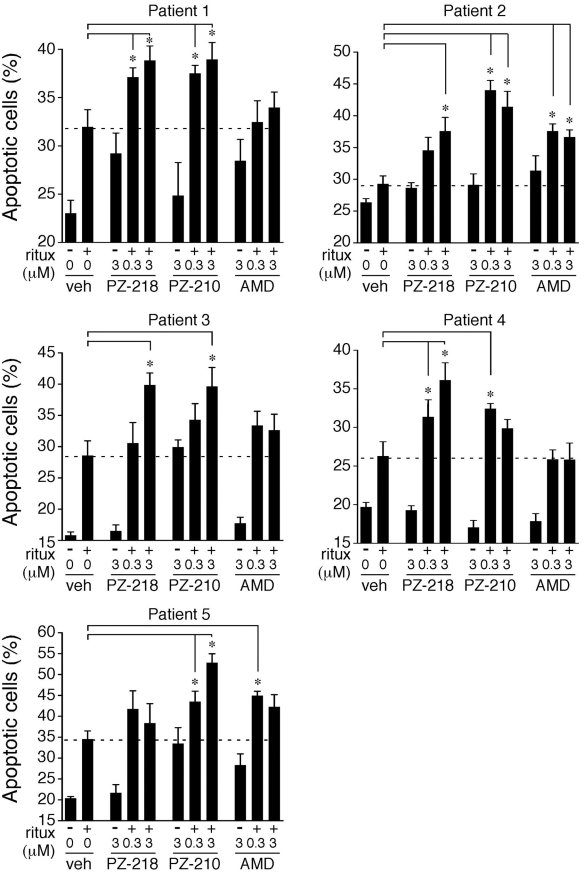
In view of recent reports examining the potential of CXCR4 antagonism as an adjunctive treatment strategy in hematologic malignancies,20,27,46 we next examined the cytotoxic activity of CXCR4 pepducins on leukemic cells obtained from patients with CLL in the presence or absence of rituximab. Rituximab, the humanized anti-CD20 Ab, is a component of standard therapy for previously treated and untreated B-CLL and NHL. We confirmed that cells from patients with CLL expressed CD20 on their cell surface (data not shown). As expected, treatment of CLL B cells with rituximab alone caused significant increases in the percentage of apoptotic cells for the majority of patients (Figure 4). The i3 loop pepducin PZ-210 also gave similar increases in apoptosis as rituximab alone in CLL B cells from patients 3 and 5. When used in combination with rituximab, PZ-218 and/or PZ-210 significantly increased apoptosis compared with rituximab alone in all patient samples and were more efficacious than plerixafor in the majority of patient CLL cells.
Figure 4.
CXCR4 pepducins enhance rituximab-mediated cytotoxicity in leukemic cells from patients with CLL. Leukemic cells isolated from 5 patients with CLL were treated overnight with vehicle (0.3% DMSO), PZ-218, PZ-210, or plerixafor (AMD) in the absence or presence of rituximab (20 μg/mL). Samples were stained with annexin V–FITC, and evaluated for apoptotic cells by flow cytometry. Data represent means ± SEM (n = 5). *P < .05 compared with rituximab-treated controls.
CXCR4 pepducins significantly enhance rituximab-mediated cytotoxicity of lymphoma cells
To further examine the synergistic effects of pepducins and rituximab, we performed cytotoxicity assays on CD20-expressing Raji and Ramos lymphoma cell lines.47 Treatment of Raji cells with 1-10μM PZ-218 or PZ-210 alone for 24 hours did not significantly increase the percentage of apoptotic cells (annexin V+/PI+/annexin V+PI+; Figure 5A-B). However, when Raji cells were treated with rituximab in combination with 3 and 10μM PZ-218 or PZ-210, the pepducins enhanced rituximab-mediated cytotoxicity in a dose-dependent manner, with 1.5- to 2-fold increases in the percentage of apoptotic cells compared with rituximab alone (Figure 5B). Treatment of Raji cells with 10μM concentrations of the negative control pepducins PZ-253, PZ-254, or PZ-217 did not increase cytotoxicity either alone or in combination with rituximab (Figure 5B). Plerixafor (AMD), the small-molecule inhibitor of CXCR4, had no effect on the survival of Raji lymphoma cells either alone or in combination with rituximab (Figure 5B). The cytotoxic effects of PZ-218 and PZ-210 in combination with rituximab were confirmed in the Ramos lymphoma cell line (supplemental Figure 3), demonstrating that pepducin antagonists of CXCR4 significantly and dose-dependently enhance rituximab-mediated killing of lymphoma cells in vitro.
Figure 5.
CXCR4 pepducins enhance rituximab-mediated cytotoxicity in lymphoma cells. (A) Raji cells were treated overnight with vehicle (0.3% DMSO), PZ-218 (10μM), or PZ-210 (10μM) in the absence or presence of rituximab (10 μg/mL). Samples were dual stained with annexin V–FITC and PI and evaluated for apoptotic/dead cells by flow cytometry. The x-axis depicts annexin V and y-axis depicts PI. Experiments were repeated 3 times with similar results. Representative dot plots are shown. (B) Raji cells were treated with the indicated concentrations of pepducins and plerixafor (AMD) for 24 hours in the presence (+) or absence (−) of rituximab (10 μg/mL). The percentage of apoptotic/dead cells was determined by annexin V/PI staining and flow cytometry. Data represent means of the sum of annexin V+/PI+/annexin V+PI+ cells ± 1 seconds (n = 3), with assays done in duplicate. *P < .05 and ** P < .01 compared with rituximab-treated samples. (C) CXCR4 pepducins inhibit stromal-mediated protective effect of rituximab and enhance rituximab-induced apoptosis. Raji cells were cocultured with or without M2-10B4 stromal cells and treated with 10μM concentrations of pepducins or vehicle control in the presence (+) or absence (−) of rituximab. Samples were dual stained with annexin V–FITC and PI and evaluated for apoptotic/dead cells by flow cytometry.
CXCR4 pepducins reverse the protective effect of stroma and enhance rituximab-induced apoptosis
Because survival of B-cell lymphomas is protected by stromal cells in the BM microenvironment,48,49 we investigated whether survival of Raji lymphoma cells could be enhanced by coculture with stromal M2 10B4 cells. Consistent with previous reports,50 coculture of Raji cells with M2 10B4 BM stromal cells increased their resistance to rituximab treatment (Figure 5C). To determine whether the protective effect of M2 10B4 cells could be reversed by blockade of the CXCR4 receptor, Raji cells were treated with vehicle or rituximab (10 μg/mL) in the presence or absence of CXCR4 pepducins. Lymphoma cells treated with PZ-218 and PZ-210 exhibited a highly significant enhancement in rituximab-induced apoptosis. The i1 loop pepducin PZ-218 was more effective than the i3 loop pepducin PZ-210 at suppressing stromal-mediated protection of the lymphoma cells in combination with rituximab.
CXCR4 pepducins enhance rituximab-mediated survival in a mouse model of lymphoma
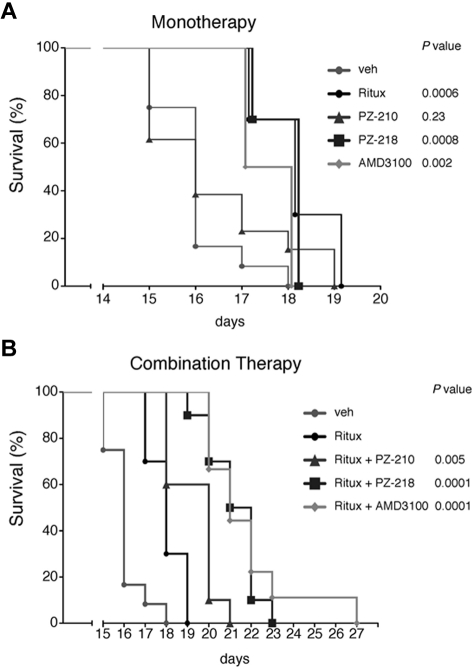
To assess the efficacy of i1 and i3 loop CXCR4 pepducins as potential treatments of lymphoma in vivo, we used a disseminated lymphoma xenograft model in which Raji cells were injected into the lateral tail vein of immunocompromised NOD/SCID mice. As expected, rituximab monotherapy prolonged survival of mice compared with the vehicle-treated cohort (P = .0006; Figure 6A). PZ-218 monotherapy also significantly increased survival compared with the vehicle-treated cohort (P = .0008), and there was no difference in survival between the rituximab and PZ-218 monotherapy groups. By comparison, the i3 loop pepducin PZ-210 gave no significant increase in survival as a monotherapy. Similar to PZ-218, plerixafor monotherapy yielded an increase in survival compared with vehicle (P = .002) in the lymphoma model.
Figure 6.
CXCR4 pepducins in combination with rituximab increase survival in a mouse model of disseminated lymphoma. (A) NOD/SCID/γ mice were injected IV with 2 × 106 Raji cells, then treated daily with subcutaneous injections of vehicle, PZ-210, PZ-218, plerixafor (AMD3100), or biweekly with rituximab, as described in “In vivo systemic lymphoma mouse model.” Survival is shown by Kaplan-Meier analysis (n = 10 mice per treatment group). (B) Mice were injected with Raji cells and then treated with a combination of rituximab and PZ-218, PZ-210, or plerixafor (AMD3100). Survival was monitored and plotted using Kaplan-Meier analysis (n = 10 mice per group, except for AMD3100-treated cohort, where n = 9). Statistical significance between groups was determined using the log-rank test.
Based on the observed synergistic effects in vitro of rituximab plus pepducins on lymphoma-cell cytotoxicity, we next determined whether PZ-218 and PZ-210 could further enhance the survival of rituximab-treated mice with disseminated lymphoma. Indeed, PZ-218 in combination with rituximab increased survival compared with rituximab alone (P = .0001; Figure 6B). Likewise, PZ-210 increased survival (P = .005), although the protective effect was less than that conferred by PZ-218. Combination therapy of rituximab and plerixafor also increased survival compared with rituximab alone (P = .0001). PZ-218 and plerixafor were equivalent in these survival studies. These data support the notion that CXCR4 is a promising clinical target for lymphoma treatment, and suggest that CXCR4 pepducins may be effective at increasing the salutary effects of rituximab.
Discussion
The involvement of GPCRs such as CXCR4 in a variety of diseases is well established, and agents targeting this class of receptor represent 30%-40% of all current pharmacotherapeutics. The majority of these therapeutics are orthosteric modulators of GPCR activity that compete with the natural ligand for receptor binding. In the present study, we used a novel approach to CXCR4 antagonism using cell-penetrating peptide antagonists targeted at specific intracellular loops of CXCR4. We demonstrated that pepducins targeting the i1 and i3 loops of CXCR4 completely abrogated CXCL12-mediated migration/chemotaxis of leukemia and lymphoma cells. Furthermore, activation of downstream CXCL12/CXCR4-Gi–mediated MAPK (ERK) signaling and calcium flux was completely blocked. Whereas pepducins alone exhibited little cytotoxic activity against leukemia and lymphoma cell lines, they enhanced rituximab-mediated cell death of primary CLL, Raji, and Ramos cells significantly in vitro. Most importantly, coculture of lymphoma cells with BM-derived stromal cells resulted in protection of cell survival in lymphoma cells. CXCR4 pepducin treatment was able to reverse stromal protection and synergized with rituximab to increase apoptotic cell death. Furthermore, pepducins in combination with rituximab significantly prolonged survival in a murine model of disseminated lymphoma.
Effective small-molecule antagonists of CXCR4 have been developed, and the bicyclam antagonist plerixafor (AMD3100) is used clinically in combination with G-CSF to enhance the mobilization of stem cells for subsequent transplantation. The therapeutic half-life of plerixafor is short, approximately 1.5 hours, which is permissive for stem cell mobilization but may be ineffective for other therapeutic indications targeting CXCR4 for which sustained inhibition of CXCR4 activity is desirable. It has been reported recently that plerixafor and TC14012, a peptidomimetic antagonist of CXCR4, are agonists of CXCR7,51,52 a GPCR that is activated by CXCL12 and I-TAC. CXCR7 signaling has been implicated in invasion, angiogenesis, and growth of human tumors.53 For this reason, chronic administration of agents that activate CXCR7 could potentially lead to undesirable tumor-promoting effects.
CXCR4 antagonists are predicted to enhance the efficacy of conventional cytoreductive treatment in CLL and other hematologic malignancies by abrogating stromal-mediated protection,48 and the safety and tolerability of plerixafor as an adjunctive therapy in CLL is currently under investigation in phase 1/2 clinical trials. Although rituximab is potentially a treatment option in combination with chemotherapy for B-cell malignancies, it has only modest activity in CLL and NHL as monotherapy and has been shown to have weak effects on some tumors.54 Therefore, combination treatment with orthogonal targets that enhance rituximab-mediated cytotoxicity is an attractive approach to improving therapeutic outcome. We propose that one mechanism by which pepducins enhance rituximab-mediated survival is by inhibition of the interaction of malignant cells with cytoprotective microenvironments. Indeed, in in vivo lymphoma model, both pepducins and plerixafor seemed equivalent, which implies that stromal protection may dominate the in vivo responses. Further supporting this hypothesis, mouse stem cell studies demonstrated that the CXCR4 pepducins, PZ-218 and PZ-210, enhance hematopoietic stem and progenitor cell mobilization from the BM.55 PZ-218 was a more effective stem cell–mobilizing agent than PZ-210, which is consistent with the enhanced efficacy of this pepducin in lymphoma survival studies. Consistent with these results are coculture stromal protection studies that identified PZ-218 as highly efficacious in reversing stromal protection to rituximab treatment. However, we also demonstrated that pepducin antagonists of CXCR4 enhanced the cytotoxic activity of rituximab in the absence of stromal cells, suggesting that inhibition of CXCR4 signaling may have pleiotropic effects on cells to influence survival. Moreover, PZ-218, when administered as a single agent to mice with disseminated lymphoma, enhanced survival compared with vehicle-treated mice, suggesting that pepducin antagonism of CXCR4 may itself be beneficial in the treatment of hematologic malignancies.
Rituximab mediates cytoreductive effects through Ab-dependent cell cytotoxicity (ADCC), complement-dependent cytotoxicity (CDC), and direct cytotoxic activity. Cell-viability assays demonstrated that pepducins did not augment rituximab-mediated CDC (data not shown), and we can further conclude that the cytotoxic effects of pepducins in combination with rituximab are not because of ADCC, because our in vitro assays did not include effector cells. CDC and ADCC rely on the Fc fragment of rituximab, which binds complement factor C1q and Fc receptors on macrophages and natural killer cells. Because the mouse strain used here (NSG) has severely depleted natural killer cell activity, this indicates that the increased survival mediated by CXCR4 pepducins is because of enhancement of the direct cytotoxic activity of rituximab.
In the present study, we have demonstrated that cell-penetrating CXCR4 pepducins provide effective antagonism of CXCL12-mediated signaling in leukemia and lymphoma cell lines. The synergistic effects of pepducins with rituximab in vitro and in vivo demonstrate that pepducins increase the susceptibility of lymphoma cells to rituximab-mediated cell death. Our findings therefore validate CXCR4 as a promising new target in lymphomas, and support further evaluation of pepducins as novel adjuvant therapies in the treatment of lymphoma and other hematological malignancies.
Supplementary Material
Acknowledgments
The authors thank Thanh Tran for help with mouse experiments, Monica Betancur for advice on growth conditions for cell lines, Valerie Relais for generously providing reagents, Güllü Görgün for assistance in FACS analysis, and Boris Tchernychev and Adam O'Shea for training in animal techniques.
This work was funded by National Institutes of Health grants (R01 HL-57905, R01 HL-64701, and R01 CA-122992 to A.K.; R01 CA104406 to L.C.; and R01 CA 090576 to R.A.V.E.) and by a sponsored research grant from Anchor Therapeutics (to A.K. and L.C.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: K.O., L.L., N.N., and N.C.K. performed the experiments; K.O., M.-Y.H., R.A.V.E., A.K., and L.C. designed the research; A.K.K and K.S. provided the clinical samples; K.O., A.K., and L.C. analyzed the results and produced the figures; and K.O., A.K., and L.C. wrote the manuscript.
Conflict-of-interest disclosure: A.K. and L.C. are founders and scientific advisory board members of Anchor Therapeutics, which licensed the pepducins used in this study. The remaining authors declare no competing financial interests.
Correspondence: Athan Kuliopulos or Lidija Covic, Molecular Oncology Research Institute, Division of Hematology/Oncology, Tufts Medical Center, Box 7510, 800 Washington St, Boston, MA 02111; e-mail: athan.kuliopulos@tufts.edu or lcovic@tuftsmedicalcenter.org.
References
- 1.Altekruse SFKC, Krapcho M, Neyman N, et al. SEER Cancer Statistics Review, 1975-2007. Bethesda, MD: National Cancer Institute; 2010. [Google Scholar]
- 2.Keating GM. Rituximab: a review of its use in chronic lymphocytic leukaemia, low-grade or follicular lymphoma and diffuse large B-cell lymphoma. Drugs. 2010;70(11):1445–1476. doi: 10.2165/11201110-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Nagasawa T, Hirota S, Tachibana K, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382(6592):635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 4.Morrison SJ, Uchida N, Weissman IL. The biology of hematopoietic stem cells. Annu Rev Cell Dev Biol. 1995;11:35–71. doi: 10.1146/annurev.cb.11.110195.000343. [DOI] [PubMed] [Google Scholar]
- 5.Nagasawa T, Kikutani H, Kishimoto T. Molecular cloning and structure of a pre-B-cell growth-stimulating factor. Proc Natl Acad Sci U S A. 1994;91(6):2305–2309. doi: 10.1073/pnas.91.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yasuoka H, Tsujimoto M, Yoshidome K, et al. Cytoplasmic CXCR4 expression in breast cancer: induction by nitric oxide and correlation with lymph node metastasis and poor prognosis. BMC Cancer. 2008;8:340. doi: 10.1186/1471-2407-8-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broxmeyer HE, Orschell CM, Clapp DW, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201(8):1307–1318. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liles WC, Broxmeyer HE, Rodger E, et al. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood. 2003;102(8):2728–2730. doi: 10.1182/blood-2003-02-0663. [DOI] [PubMed] [Google Scholar]
- 9.Flomenberg N, Devine SM, Dipersio JF, et al. The use of AMD3100 plus G-CSF for autologous hematopoietic progenitor cell mobilization is superior to G-CSF alone. Blood. 2005;106(5):1867–1874. doi: 10.1182/blood-2005-02-0468. [DOI] [PubMed] [Google Scholar]
- 10.Scala S, Ottaiano A, Ascierto PA, et al. Expression of CXCR4 predicts poor prognosis in patients with malignant melanoma. Clin Cancer Res. 2005;11(5):1835–1841. doi: 10.1158/1078-0432.CCR-04-1887. [DOI] [PubMed] [Google Scholar]
- 11.Kim J, Takeuchi H, Lam ST, et al. Chemokine receptor CXCR4 expression in colorectal cancer patients increases the risk for recurrence and for poor survival. J Clin Oncol. 2005;23(12):2744–2753. doi: 10.1200/JCO.2005.07.078. [DOI] [PubMed] [Google Scholar]
- 12.Spoo AC, Lubbert M, Wierda WG, Burger JA. CXCR4 is a prognostic marker in acute myelogenous leukemia. Blood. 2007;109:786–791. doi: 10.1182/blood-2006-05-024844. [DOI] [PubMed] [Google Scholar]
- 13.Konoplev S, Rassidakis GZ, Estey E, et al. Overexpression of CXCR4 predicts adverse overall and event-free survival in patients with unmutated FLT3 acute myeloid leukemia with normal karyotype. Cancer. 2007;109(6):1152–1156. doi: 10.1002/cncr.22510. [DOI] [PubMed] [Google Scholar]
- 14.Alsayed Y, Ngo H, Runnels J, et al. Mechanisms of regulation of CXCR4/SDF-1 (CXCL12)-dependent migration and homing in multiple myeloma. Blood. 2007;109(7):2708–2717. doi: 10.1182/blood-2006-07-035857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arai J, Yasukawa M, Yakushijin Y, Miyazaki T, Fujita S. Stromal cells in lymph nodes attract B-lymphoma cells via production of stromal cell-derived factor-1. Eur J Haematol. 2000;64(5):323–332. doi: 10.1034/j.1600-0609.2000.90147.x. [DOI] [PubMed] [Google Scholar]
- 16.Blades MC, Manzo A, Ingegnoli F, et al. Stromal cell-derived factor 1 (CXCL12) induces human cell migration into human lymph nodes transplanted into SCID mice. J Immunol. 2002;168(9):4308–4317. doi: 10.4049/jimmunol.168.9.4308. [DOI] [PubMed] [Google Scholar]
- 17.Panayiotidis P, Jones D, Ganeshaguru K, Foroni L, Hoffbrand AV. Human bone marrow stromal cells prevent apoptosis and support the survival of chronic lymphocytic leukaemia cells in vitro. Br J Haematol. 1996;92(1):97–103. doi: 10.1046/j.1365-2141.1996.00305.x. [DOI] [PubMed] [Google Scholar]
- 18.Konopleva M, Konoplev S, Hu W, Zaritskey AY, Afanasiev BV, Andreeff M. Stromal cells prevent apoptosis of AML cells by up-regulation of anti-apoptotic proteins. Leukemia. 2002;16(9):1713–1724. doi: 10.1038/sj.leu.2402608. [DOI] [PubMed] [Google Scholar]
- 19.Burger M, Hartmann T, Krome M, et al. Small peptide inhibitors of the CXCR4 chemokine receptor (CD184) antagonize the activation, migration, and antiapoptotic responses of CXCL12 in chronic lymphocytic leukemia B cells. Blood. 2005;106(5):1824–1830. doi: 10.1182/blood-2004-12-4918. [DOI] [PubMed] [Google Scholar]
- 20.Zeng Z, Shi YX, Samudio IJ, et al. Targeting the leukemia microenvironment by CXCR4 inhibition overcomes resistance to kinase inhibitors and chemotherapy in AML. Blood. 2009;113(24):6215–6224. doi: 10.1182/blood-2008-05-158311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeelenberg IS, Ruuls-Van Stalle L, Roos E. Retention of CXCR4 in the endoplasmic reticulum blocks dissemination of a T cell hybridoma. J Clin Invest. 2001;108(2):269–277. doi: 10.1172/JCI11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liang Z, Wu H, Reddy S, et al. Blockade of invasion and metastasis of breast cancer cells via targeting CXCR4 with an artificial microRNA. Biochem Biophys Res Commun. 2007;363(3):542–546. doi: 10.1016/j.bbrc.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 23.Liang Z, Yoon Y, Votaw J, Goodman MM, Williams L, Shim H. Silencing of CXCR4 blocks breast cancer metastasis. Cancer Res. 2005;65(3):967–971. [PMC free article] [PubMed] [Google Scholar]
- 24.Andritsos LA, Byrd J, Hewes B, Kipps T, Johns D, Burger J. Preliminary results from a phase I dose escalation study to determine the maxium tolerated dose of plerixafor in combination with rituximab in patients with relapsed chronic lymphocytic leukemia [abstract]. Haematologica. 2010;95(2):321. [Google Scholar]
- 25.Caligaris-Cappio F. Role of the microenvironment in chronic lymphocytic leukaemia. Br J Haematol. 2003;123(3):380–388. doi: 10.1046/j.1365-2141.2003.04679.x. [DOI] [PubMed] [Google Scholar]
- 26.Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. N Engl J Med. 2005;352(8):804–815. doi: 10.1056/NEJMra041720. [DOI] [PubMed] [Google Scholar]
- 27.Azab AK, Runnels JM, Pitsillides C, et al. CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood. 2009;113(18):4341–4351. doi: 10.1182/blood-2008-10-186668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bertolini F, Dell'Agnola C, Mancuso P, et al. CXCR4 neutralization, a novel therapeutic approach for non-Hodgkin's lymphoma. Cancer Res. 2002;62(11):3106–3112. [PubMed] [Google Scholar]
- 29.Covic L, Misra M, Badar J, Singh C, Kuliopulos A. Pepducin-based intervention of thrombin receptor signaling and systemic platelet activation. Nature Med. 2002;8:1161–1165. doi: 10.1038/nm760. [DOI] [PubMed] [Google Scholar]
- 30.Leger AJ, Covic L, Kuliopulos A. Protease-activated receptors in cardiovascular diseases. Circulation. 2006;114(10):1070–1077. doi: 10.1161/CIRCULATIONAHA.105.574830. [DOI] [PubMed] [Google Scholar]
- 31.Agarwal A, Tressel SL, Kaimal R, et al. Identification of a metalloprotease-chemokine signaling system in the ovarian cancer microenvironment: implications for antiangiogenic therapy. Cancer Res. 2010;70(14):5880–5890. doi: 10.1158/0008-5472.CAN-09-4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trivedi V, Boire A, Tchernychev B, et al. Platelet matrix metalloprotease-1 mediates thrombogenesis by activating PAR1 at a cryptic ligand site. Cell. 2009;137(2):332–343. doi: 10.1016/j.cell.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaneider NC, Agarwal A, Leger AJ, Kuliopulos A. Reversing systemic inflammatory response syndrome with chemokine receptor pepducins. Nat Med. 2005;11(6):661–665. doi: 10.1038/nm1245. [DOI] [PubMed] [Google Scholar]
- 34.Boire A, Covic L, Agarwal A, Jacques S, Sherifi S, Kuliopulos A. PAR1 is a matrix metalloprotease-1 receptor that promotes invasion and tumorigenesis of breast cancer cells. Cell. 2005;120(3):303–313. doi: 10.1016/j.cell.2004.12.018. [DOI] [PubMed] [Google Scholar]
- 35.Yang E, Boire A, Agarwal A, et al. Blockade of PAR1 signaling with cell-penetrating pepducins inhibits Akt survival pathways in breast cancer cells and suppresses tumor survival and metastasis. Cancer Res. 2009;69(15):6223–6231. doi: 10.1158/0008-5472.CAN-09-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wielders SJ, Bennaghmouch A, Reutelingsperger CP, Bevers EM, Lindhout T. Anticoagulant and antithrombotic properties of intracellular protease-activated receptor antagonists. J Thromb Haemost. 2007;5(3):571–576. doi: 10.1111/j.1538-7836.2007.02364.x. [DOI] [PubMed] [Google Scholar]
- 37.Tressel SL, Koukos G, Tchernychev B, Jacques SL, Covic L, Kuliopulos A. Pharmacology, Biodistribution, and Efficacy of GPCR-based Pepducins in Disease Models. Methods Mol Biol. 2011;683:259–275. doi: 10.1007/978-1-60761-919-2_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agarwal A, Covic L, Sevigny LM, et al. Targeting a metalloprotease-PAR1 signaling system with cell-penetrating pepducins inhibits angiogenesis, ascites, and progression of ovarian cancer. Mol Cancer Ther. 2008;7(9):2746–2757. doi: 10.1158/1535-7163.MCT-08-0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kaneider NC, Leger AJ, Agarwal A, et al. ‘Role reversal’ for the receptor PAR1 in sepsis-induced vascular damage. Nat Immunol. 2007;8(12):1303–1312. doi: 10.1038/ni1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Covic L, Gresser AL, Talavera J, Swift S, Kuliopulos A. Activation and inhibition of G protein-coupled receptors by cell-penetrating membrane-tethered peptides. Proc Natl Acad Sci U S A. 2002;99:643–648. doi: 10.1073/pnas.022460899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tchernychev B, Ren Y, Sachdev P, et al. Discovery of a CXCR4 agonist pepducin that mobilizes bone marrow hematopoietic cells. Proc Natl Acad Sci U S A. 2010;107(51):22255–22259. doi: 10.1073/pnas.1009633108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1). J Exp Med. 1996;184(3):1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Covic L, Gresser AL, Kuliopulos A. Biphasic kinetics of activation and signaling for PAR1 and PAR4 thrombin receptors in platelets. Biochemistry. 2000;39(18):5458–5467. doi: 10.1021/bi9927078. [DOI] [PubMed] [Google Scholar]
- 44.Cisowski J, O'Callaghan K, Kuliopulos A, et al. Targeting protease-activated receptor-1 with cell-penetrating pepducins in lung cancer. Am J Pathol. 2011;179(1):513–523. doi: 10.1016/j.ajpath.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roland J, Murphy BJ, Ahr B, et al. Role of the intracellular domains of CXCR4 in SDF-1-mediated signaling. Blood. 2003;101(2):399–406. doi: 10.1182/blood-2002-03-0978. [DOI] [PubMed] [Google Scholar]
- 46.Mandawat A, Fiskus W, Buckley KM, et al. Pan-histone deacetylase inhibitor panobinostat depletes CXCR4 levels and signaling and exerts synergistic antimyeloid activity in combination with CXCR4 antagonists. Blood. 2010;116(24):5306–5315. doi: 10.1182/blood-2010-05-284414. [DOI] [PubMed] [Google Scholar]
- 47.Bil J, Winiarska M, Nowis D, et al. Bortezomib modulates surface CD20 in B-cell malignancies and affects rituximab-mediated complement-dependent cytotoxicity. Blood. 2010;115(18):3745–3755. doi: 10.1182/blood-2009-09-244129. [DOI] [PubMed] [Google Scholar]
- 48.Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107:1761–1767. doi: 10.1182/blood-2005-08-3182. [DOI] [PubMed] [Google Scholar]
- 49.Kurtova AV, Balakrishnan K, Chen R, et al. Diverse marrow stromal cells protect CLL cells from spontaneous and drug-induced apoptosis: development of a reliable and reproducible system to assess stromal cell adhesion-mediated drug resistance. Blood. 2009;114(20):4441–4450. doi: 10.1182/blood-2009-07-233718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Buchner M, Brantner P, Stickel N, et al. The microenvironment differentially impairs passive and active immunotherapy in chronic lymphocytic leukaemia–CXCR4 antagonists as potential adjuvants for monoclonal antibodies. Br J Haematol. 2010;151(2):167–178. doi: 10.1111/j.1365-2141.2010.08316.x. [DOI] [PubMed] [Google Scholar]
- 51.Kalatskaya I, Berchiche YA, Gravel S, Limberg BJ, Rosenbaum JS, Heveker N. AMD3100 is a CXCR7 ligand with allosteric agonist properties. Mol Pharmacol. 2009;75(5):1240–1247. doi: 10.1124/mol.108.053389. [DOI] [PubMed] [Google Scholar]
- 52.Gravel S, Malouf C, Boulais PE, et al. The peptidomimetic CXCR4 antagonist TC14012 recruits beta-arrestin to CXCR7: roles of receptor domains. J Biol Chem. 2010;285(49):37939–37943. doi: 10.1074/jbc.C110.147470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Burns JM, Summers BC, Wang Y, et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203(9):2201–2213. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hainsworth JD, Litchy S, Barton JH, et al. Single-agent rituximab as first-line and maintenance treatment for patients with chronic lymphocytic leukemia or small lymphocytic lymphoma: a phase II trial of the Minnie Pearl Cancer Research Network. J Clin Oncol. 2003;21:1746–1751. doi: 10.1200/JCO.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 55.O'Callaghan KM, Hsieh M-Y, VanEtten RA, Covic L, Kuliopulos A. CXCR4 pepducins in stem cell mobilization [abstract]. Blood. 2009;114:2440a. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.