Abstract
A close homologue to protein disulfide isomerase (PDI) called ERp57 forms disulfide bonds in glycoproteins in the endoplasmic reticulum and is expressed on the platelet surface. We generated 2 rabbit Abs to ERp57. One Ab strongly inhibited ERp57 in a functional assay and strongly inhibited platelet aggregation. There was minimal cross-reactivity of this Ab with PDI by Western blot or in the functional assay. This Ab substantially inhibited activation of the αIIbβ3 fibrinogen receptor and P-selectin expression. Furthermore, adding ERp57 to platelets potentiated aggregation. In contrast, adding a catalytically inactive ERp57 inhibited platelet aggregation. When infused into mice the inactive ERp57 prolonged the tail bleeding times. We generated 2 IgG2a mAbs that reacted with ERp57 by immunoblot. One of these Abs inhibited both ERp57 activity and platelet aggregation. The other Ab did not inhibit ERp57 activity or platelet aggregation. The inhibitory Ab inhibited activation of αIIbβ3 and P-selectin expression, prolonged tail bleeding times, and inhibited FeCl3-induced thrombosis in mice. Finally, we found that a commonly used mAb to PDI also inhibited ERp57 activity. We conclude that a glycoprotein-specific member of the PDI family, ERp57, is required for platelet aggregation, hemostasis, and thrombosis.
Introduction
The prototypic disulfide isomerase (PDI) was discovered approximately 50 years ago as an enzyme that forms disulfide bonds in nascent proteins in the endoplasmic reticulum (ER).1,2 Extracellular PDI is now known to mediate platelet aggregation3,4 and thrombus formation in vivo.5,6 ERp5 is another member of the PDI family that has a role in platelet aggregation.7 ERp57 is a member of the PDI family of enzymes8 that is also found in platelets.9,10 On platelet activation, ERp57 is secreted by platelets and recruited to the platelet surface.9,10 ER substrates of ERp57 are mostly heavily glycosylated disulfide-bonded proteins that include integrin subunits (α2, α3, α6, β1, β5).11 The role of ERp57 in platelet function is unknown.
ERp57 interacts cotranslationally with the ER lectins calnexin and calreticulin in the folding of glycoproteins8 and has been studied for its role in the assembly of the MHC (MHC class 1 molecules).12 While it was thought that ERp57 requires the chaperones calnexin and calreticulin to gain access to its substrates, not all ERp57-dependent disulfide bond formation in the ER requires calnexin and calreticulin.13
ERp57 is of similar size and domain structure to PDI with 33% identity in its amino acid sequences.14 ERp57 has 505 amino acids while PDI has 508 with the catalytic a and a′ domains sharing 50% amino acid identity.14 Like PDI it contains 2 CGHC active-site sequences and catalyzes the reversible oxidation of thiols to disulfides and the isomerization of disulfide bonds. The substrate binding b and b′ domains of PDI and ERp57 domains share only approximately 20% amino acid identity and the small C-terminal region of ERp57 contains multiple lysine residues whereas in PDI, the region is highly acidic.14
In the current studies, we raised both rabbit and mAbs to ERp57 to study the role of this enzyme in platelet function and hemostasis. Because of the high homology between ERp57 and PDI, we examined the ability of these Abs to inhibit PDI. Using Abs that preferentially inhibited ERp57, we document a role for ERp57 in platelet aggregation, hemostasis, and thrombosis.
Methods
Materials
Glutathione (GSH), glutathione disulfide (GSSG), DTT, Sephadex G-25, normal rabbit IgG, and a monoclonal mouse IgG2a (raised against pristane) were purchased from Sigma-Aldrich. The IgG2a mouse monoclonal anti-PDI Ab RL90 was purchased from Affinity BioReagents. The thrombin receptor agonist peptide SFLLRN was synthesized as previously described15 and was also synthesized by Enzo Life Sciences.
Ab generation
The cDNA for ERp57 was provided by Dr Peppi Koivunen (University of Oulu, Oulu, Finland). The cDNA was subcloned into a pTriEX-4 Neo vector (Novagen) containing an N-terminal histidine tag. ERp57 was expressed in Escherichia coli strain BL21 (DE3)pLysS (Promega) and purified on a Ni Sepharose High-Performance column (GE Healthcare). The cDNAs for PDI and ERp5 were obtained from Dr Bao-bing Zhu (University of Kansas Medical Center, Kansas City, KS) and Dr M. Kikuchi (Ritsumeikan University, Kyoto, Japan), respectively. PDI and ERp5 were prepared using identical methods as for ERp57. Polyclonal Abs to ERp57 were raised in 2 New Zealand white rabbits by the GenScript Company, and affinity purified against an ERp57 column and a protein A column.
Abs were raised to human ERp57 in mice by the GenScript Company. Hybridomas expressing anti-ERp57 mAbs were identified by Western blot and the mAbs were purified using a protein A column. The Abs were screened for inhibition of platelet aggregation, and an IgG2a Ab (3G1; herein called Mab1) was found that inhibited platelet aggregation. Another IgG2a Ab (1B12; herein called Mab2) reacted with ERp57 by Western blot but did not inhibit platelet aggregation. This Ab was used as a control and compared with the isotype-specific IgG2a control Ab.
Preparation of the PDI substrate, Di-E-GSSG
Dieosin glutathione disulfide (Di-E-GSSG) prepared by the method of Raturi and Mutus16 was kindly provided by Dr Mutus (University of Windsor, Windsor, ON). To make this PDI substrate, 2 eosin moieties are attached to 2 free N termini of GSSG.16 PDI reductase activity cleaves the SS bond of Di-E-GSSG resulting in the release of 2 eosin-GSH (EGSH) molecules and an increase in fluorescence.
Preparation of EGSH and an EGSH standard curve
To convert the fluorescent intensity of a sample to the amount of eosin-GSH (EGSH) generated, we used a standard curve of the fluorescent intensities of different EGSH concentrations. EGSH was prepared as described16 by treating Di-E-GSSG with DTT and separating the mixture with a Sephadex G-25 column. The eluted sample did not show any increase in the fluorescence after the addition of DTT (10mM), confirming that all the Di-E-GSSG has been converted to EGSH. The aliquots were pooled together and quantified using molar absorption coefficient e = 88 000M−1 cm−1. The increase in fluorescence was monitored as a function of [EGSH] (e = 88 000M−1 cm−1). The standard plot was generated with excitation at 525 nm and emission at 545 nm and used to quantify the reduction of [Di-E-GSSG] to [EGSH].
Assay of enzyme-dependent disulfide reduction
ERp57, PDI, and ERp5 disulfide reductase activities against Di-E-GSSG were monitored in PDI assay buffer (0.1M potassium phosphate buffer, 2mM EDTA, pH 7.0) by adding ERp57, PDI, or ERp5 (20nM) to Di-E-GSSG (150nM) in the presence of 5μM DTT. The increase in fluorescence was monitored at 545 nm with excitation at 525 nm.16 The degree of inhibition was relative to the total amount of EGSH formed over the time period of the assay or by the initial velocity of the reaction (nM EGSH formed per minute).
Western blotting
Western blotting was performed using the Odyssey Western blot kit according to the manufacturer's instructions and developed using the Odyssey Infrared Imaging System (Li-Cor Biosciences).
Preparation of catalytically inactive ERp57
A catalytically inactive mutant ERp57, with the 4-cysteine residues of both active sites mutated to serine residues (C57S, C60S, C406S, and C409S) was prepared using the QuikChange Site-Directed Mutagenesis Kit (Stratagene). The mutant ERp57 is also designated as ERp57 (oo-oo; representing the Cys/SH change to Ser/OH in the 2 CGHC active sites). DNA sequencing confirmed the correct base substitutions.
Platelet isolation and platelet aggregation
All studies on human platelets were performed after approval by the Temple University Institution Review Board. Platelets were prepared as described previously.17 Briefly, 8.6 mL of blood was drawn into 1.4 mL of acid-citrate dextrose solution, and the platelet-rich plasma, obtained after centrifugation of whole blood, was gel-filtered on a Sepharose 2B column equilibrated in Tyrode-albumin solution at pH 7.35. Calcium (1mM) was then added to the platelets. Platelet aggregation was as described using the Chronolog lumiaggregometer.17 The percentage of aggregation was calculated from the amplitude of the tracings and normalized to the response of the IgG control within an individual experiment.
Flow cytometric studies of platelets
Flow cytometric analysis was performed as described.3 Briefly, citrated platelet-rich plasma was diluted into an equal volume of PBS and incubated for 10 minutes with the rabbit anti-ERp57 IgG (30 μg/mL) or normal rabbit IgG and then with the SFLLRN peptide for 5 minutes without stirring at room temperature. In some samples, the anti-ERp57 Ab was added after the SFLLRN peptide had been incubated with the platelets. In other studies, the inhibitory monoclonal anti-ERp57 Ab (30 μg/mL) was used. A total of 10 μL of FITC-conjugated PAC-1 (BD Biosciences) was then incubated with 10 μL of platelets for 30 minutes in the dark. In some experiments, convulxin (Enzo Life Sciences) was used as the agonist and PE-conjugated anti-P-selectin Ab (eBioscience) was incubated with the platelets. To study surface expression of ERp57 on platelets, 20 μg/mL rabbit anti-ERp57 Ab or normal rabbit IgG was incubated with nonstimulated platelets for 10 minutes. FITC-labeled goat anti–rabbit Ab was then added for 10 minutes. The cells were fixed with 2% paraformaldehyde. Ten thousand platelets per sample were analyzed by flow cytometry. The data were combined to obtain the mean with the fluorescent intensity of Ab binding to nonactivated platelets subtracted out.
Tail bleeding times
Bleeding times were determined as previously described5 with some modifications. Mice were obtained from Charles River Laboratories and experiments were performed in accordance with institutional guidelines using IP pentobarbital for anesthesia. Male mice (C57BL/6, 6-8 weeks old) were anesthetized and recombinant mutant ERp57 (oo-oo; 100 μg/mouse) in a volume of 100 μL of PBS, PBS only, or mAbs (200 μg/mouse) were injected through the iliac vein. After 5 minutes, the mouse tail was transected 5 mm from the tip with a razor blade. Because of the relatively long tail bleeding times with this method, the procedure was revised to transect the mouse tail 3 mm from the tip. The bleeding tail was immersed in a 12-mL test tube containing PBS, which was warmed in a water bath at 37°C. Bleeding times were determined when the bleeding stopped for > 10 seconds.5 In initial experiments, bleeding times exceeding 30 minutes, and in a second set of experiments 15 minutes, were noted as 30 or 15 minutes, respectively.
Studies using FeCl3-induced thrombosis of the carotid artery
Male mice weighing 20 to 25 g (C57BL/6 age 6-8 weeks) were anesthetized using sodium pentobarbital 120 mg/kg by IP injection as described elsewhere18 with some modifications. A midline incision was made in the neck, and the left carotid artery was surgically exposed by blunt dissection. A 1 × 2 mm patch of no. 1 Whatman filter paper, soaked in 5% FeCl3, was applied under and above the exposed artery for 2 minutes. After removal of the filter paper, the artery was washed with PBS and an imaging ultrasound gel (MS400-0090; VisualSonics) was placed in the surgical wound to allow Doppler monitoring. The artery was identified using a small animal blood flow transducer (MS400, 18-38 MHz; VisualSonics) and the color Doppler mode of the VisualSonics Vevo model 2100 flowmeter.19,20 The transducer was fixed in place using the Vevo Imaging Station (Integrated Rail System), a bench mounted adjustable rail system custom designed for small animal positioning and imaging. Mouse temperature was maintained at 37°C. Baseline systolic and diastolic blood velocity (mm/s) was recorded using the two-dimensional (2D) imaging Doppler mode. The occlusion time was defined as the time from the initiation of arterial injury to complete occlusion of blood flow. An occlusion was determined to be stable when the flow completely stopped for at least 5 minutes. Four hundred fifty micrograms of the anti-ERp57 Ab or control IgG2a were injected before the FeCl3 injury through the right common iliac vein as described elsewhere.21 The operator was blinded to the Ab infused while performing all experiments.
Flow cytometric studies on mouse platelets after infusion of ERp57
Mice were anesthetized and recombinant mutant ERp57 (oo-oo; 100 μg/mouse) in 100 μL of PBS or PBS only was injected using an insulin syringe into the iliac vein. After 5 minutes, 180 μL of blood was drawn into 20 μL of acid-citrate dextrose solution through the inferior vena cava as described elsewhere22 using a 21-gauge needle. Platelet-rich plasma (PRP) was made by centrifugation of whole blood at 80g for 10 minutes as described elsewhere.23 The PRP obtained was diluted into 2 volumes of Tyrode buffer. Convulxin (500 ng/mL) was added for 5 minutes and 1 μL of the PE-labeled JON/A Ab that recognizes activated mouse αIIbβ3 (Emfret) or PE-conjugated anti–P-selectin (eBioscience) was then incubated with 10 μL of platelets for 30 minutes in the dark. The platelets were fixed and analyzed as with human platelets.
Statistics
For bleeding time experiments, data were statistically analyzed by ANOVA (nonparametric) using GraphPad Prism software. If ANOVA conveyed a P < .05, a Dunn test was performed to further assess significance. The Student t test was used to calculate P values for differences in PAC1 binding and aggregation results. Differences were considered significant at P ≤ .05.
Results
Characterization of ERp57, PDI, and ERp5
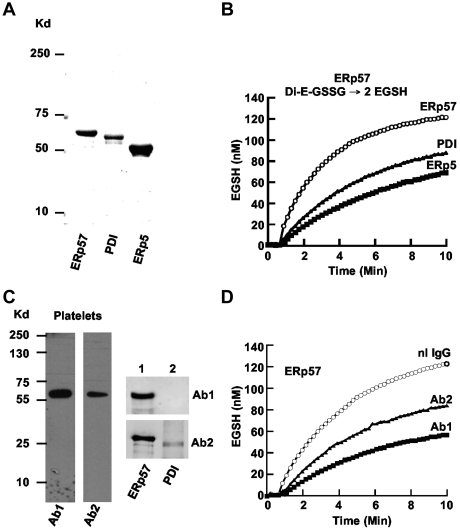
The disulfide isomerase enzymes PDI and ERp5 have a role in platelet aggregation.3,7,24 ERp57, the closest homologue to PDI,25 is recruited to the platelet surface and secreted with platelet activation. Using the cDNAs for human ERp57, PDI, and ERp5 subcloned into a vector, we expressed the 3 proteins and purified them using a nickel column. ERp57, PDI, and ERp5 of appropriate sizes were seen on a gel (Figure 1A). ERp57 had greater disulfide reductase activity in the GSSG assay than PDI or ERp5 (Figure 1B) with initial velocities of 40, 20, and 16nM EGSH/min, respectively.
Figure 1.
Comparison of purified ERp57, PDI, and ERp5 and characterization of rabbit anti-ERp57 Abs. (A) The relative sizes of the 3 enzymes on a gel under reducing conditions. The enzymes were expressed in E coli and purified by the His tag using a nickel column. (B) The enzymatic activities in the GSSG assay. A total of 150nM Di-E-GSSG was incubated in the presence of ERp57, PDI, or ERp5. The amount of EGSH formed over time is shown. (C) The reactivity of rabbit Ab 1 (Ab1) and Ab 2 (Ab2) on Western blot of platelet lysate (4 × 108 platelets/lane) and purified ERp57 (lane 1) or PDI (lane 2; 200 ng protein/lane). (D) The inhibitory effect of Ab1 and Ab2 against ERp57 in the Di-E-GSSG assay compared with a normal rabbit IgG control (nL IgG); 30 μg/mL of each Ab was used in these studies. For the curves in panels B and D, the enzyme was added at ∼ 60 seconds.
Characterization of rabbit anti-ERp57 Abs by Western blot
The anti-ERp57 Abs raised from the 2 rabbits (Ab 1 and Ab 2) gave single bands against platelets by Western blot (Figure 1C). There was no cross-reactivity of Ab 1 to PDI (or ERp5-not shown) by Western blot (Figure 1C lane 2). Ab 2 had cross-reactivity to PDI by Western blot. Ab 1 provided the greatest inhibition (∼ 84% of the initial velocity) of ERp57 activity in the Di-E-GSSG assay (Figure 1D).
Characterization of rabbit anti-ERp57 Abs in the Di-E-GSSG assay
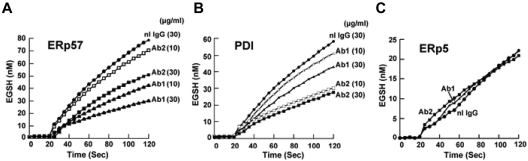
Dose response studies of the Abs against ERp57 showed that 30 μg/mL of either Ab provided greater inhibition than 10 μg/mL (Figure 2A). Because immunoreactivity to an epitope may be lost under reduced denaturing gel conditions but the Ab may still be inhibitory in a functional assay, we also tested the Abs for inhibition of PDI. There was minimal inhibition at 10 μg/mL by Ab 1 and a relatively small amount of inhibition of PDI activity at 30 μg/mL (23% of the initial velocity). Ab 2 inhibited more of the PDI activity (50% of the initial velocity using 30 μg/mL; Figure 2B). Therefore, Abs 1 and 2 have differential inhibitory activities against ERp57 and PDI. Ab 1 has substantially more inhibitory activity against ERp57 and substantially less inhibitory activity against PDI than Ab 2. ERp5 is another member of the PDI family that also has a role in platelet aggregation,7 but has much less homology to PDI than ERp57. There was no inhibition of ERp5 in the functional assay with the anti-ERp57 Abs (Figure 2C).
Figure 2.
Differential inhibition of purified ERp57 and PDI by rabbit anti-ERp57 Ab 1 and Ab 2. (A-C) The activity of ERp57, PDI, or ERp5 in the presence of Ab 1 (Ab1) and Ab 2 (Ab2) at 10 μg/mL or 30 μg/mL, or normal rabbit IgG (nL IgG) at 30 μg/mL. The amount of EGSH (nM) formed from Di-E-GSSG in the presence of enzyme over 120 seconds is shown. Each point is the composite of at least 3 samples (nL IgG, ●; Ab1 10 μg/mL, ▵; Ab1 30 μg/mL, ▴; Ab2 10 μg/mL, □; Ab2 30 μg/mL, ■). Inhibition was not increased with 60 μg/mL of either Ab (not shown). For these curves, the enzyme was added at ∼ 20 seconds.
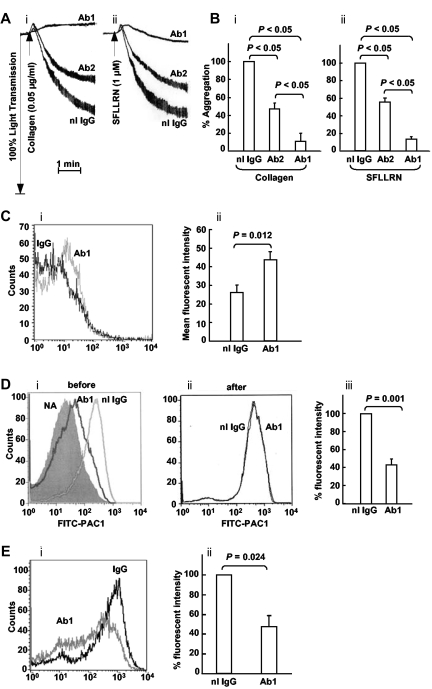
Anti-ERp57 Abs inhibit platelet aggregation
The differential inhibition of the 2 Abs against ERp57 and PDI provides a useful tool to test the role of ERp57 in platelet function. The Ab that strongly inhibits ERp57 (Ab 1) also strongly inhibits aggregation to both collagen and SFRLLN (Figure 3A-B). The Ab that more weakly inhibits ERp57 (Ab 2) also weakly inhibits platelet aggregation. This is the Ab that cross-reacts with PDI by Western blot (Figure 1C lane 2) and has the greatest inhibition of PDI (Figure 2B). Although PDI has a role in platelet aggregation,24,26,27 the anti-PDI effect of Ab 2 is evidently insufficient to substantially inhibit platelet aggregation (Figure 3A-B). Therefore, the inhibition of platelet aggregation by the Ab that strongly inhibits ERp57 is likely primarily by inhibition of ERp57.
Figure 3.
The rabbit anti-ERP57 Ab 1 preferentially inhibits platelet aggregation. (A) The effect of Ab 1 and 2 are seen in representative tracings of platelet aggregation induced with (i) collagen and (ii) SFLLRN. (B) The combined data ± SE of at least 3 independent experiments is shown for collagen and SFLLRN. (C) The binding of Ab1 to resting platelets relative to normal IgG (i) with the cumulative results ± SE of a gated population of platelets also shown (ii; n = 8). (D) Anti-ERp57 (Ab1) inhibits SFLLRN (10μM)–induced PAC1 binding when added before (i), but not after (ii), activation. (i) PAC1 binding to nonactivated platelets (NA) is seen on the left. (iii) Inhibition of PAC1 binding by Ab1 as the percentage of fluorescent intensity relative to the normal rabbit IgG control, ± SE (n = 4). (E) A representative histogram (i) and combined data (ii) show that Ab1 inhibits P-selectin binding to convulxin (10 ng/mL)–activated platelets relative to normal IgG ± SE (n = 5). In these experiments, the Abs (30 μg/mL) were incubated with the platelets for 10 minutes before the addition of the agonist.
Anti-ERp57 Abs inhibit activation of αIIbβ3
Flow cytometric analysis showed binding of the polyclonal Ab 1 to resting platelets (Figure 3C). The anti-ERp57 Ab 1 substantially inhibited activation of the αIIbβ3 fibrinogen receptor measured by binding of an activation-specific Ab, PAC-1 (Figure 3D). This was true only if the anti-ERp57 Ab was added before the SFLLRN-agonist (Figure 3Di) but not if it was added after the SFLLRN agonist (Figure 3Dii). This indicates that inhibition of activation of αIIbβ3 by anti-ERp57 is not by steric effects on PAC-1 binding but by inhibition of ERp57. The fluorescent intensity of PAC-1 binding was inhibited by over 50% by anti-ERp57 compared with the IgG control (Figure 3Diii). Ab 1 also had an inhibitory effect on P-selectin expression (Figure 3E) a measure of α-granule secretion.
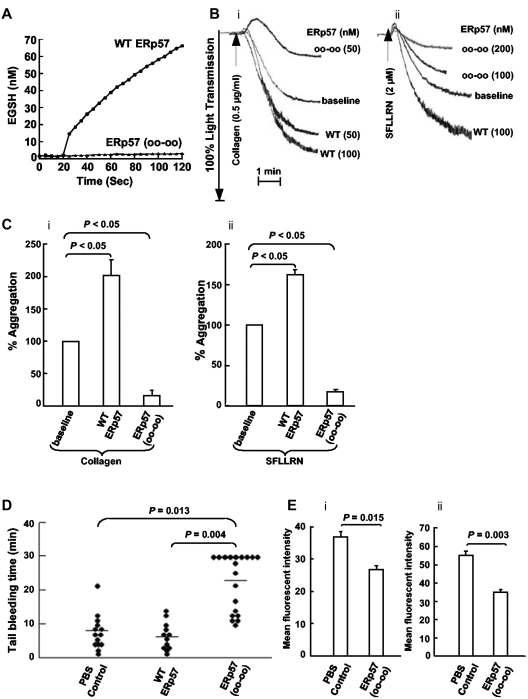
Wild-type ERp57 potentiates platelet aggregation while the catalytically inactive ERp57 inhibits platelet aggregation and prolongs the tail bleeding time in mice
As expected, the catalytically inactive mutant ERp57 had no activity in the Di-E-GSSG assay (Figure 4A). We tested the effect of adding wild-type ERp57 or the catalytically inactive ERp57 to human platelets on platelet aggregation. Using concentrations of collagen and the SFLLRN peptide that induced submaximal aggregation, wild-type ERp57 potentiated platelet aggregation while the inactive mutant ERp57 inhibited aggregation (Figure 4B-C). The mean tail bleeding times of mice infused with the mutant ERp57 were prolonged by > 2-fold compared with mice infused with the buffer or wild-type controls (Figure 4D). Mouse platelets prepared from blood collected 5 minutes after infusing the inactive ERp57 showed inhibition of αIIbβ3 activation and P-selectin expression (Figure 4Ei-ii).
Figure 4.
Wild-type ERp57 potentiates platelet aggregation and an inactive mutant ERp57 inhibits aggregation, prolongs the tail bleeding time in mice, and inhibits activation of αIIbβ3 and P-selectin expression ex vivo. (A) ERp57 with the 4 active site cysteines mutated to serine (oo-oo) is catalytically inactive. (B) Submaximal aggregation (baseline) was stimulated with collagen or the SFLLRN peptide. The potentiating effect of preincubating the platelets with purified wild-type ERp57 (WT ERp57) in the concentrations indicated is seen. The inhibitory (i) effect of adding the inactive (ii) mutant ERp57 (oo-oo) in the concentrations indicated is also seen. (C) The cumulative data ± SE of at least 3 different experiments, showing potentiation of aggregation by WT ERp57 and inhibition by the mutant ERp57 (oo-oo) relative to the baseline aggregation (i, collagen; ii, SFLLRN). The concentration of WT or mutant ERp57 that provided maximal potentiation or inhibition of responses varied a little between experiments. Fifty to 100nM WT or mutant ERp57 generally gave maximal potentiation and inhibition responses with collagen; 100 to 200nM of WT or mutant ERp57 provided maximal responses with the SFLLRN peptide. In these studies the enzymes were added for 10 minutes before the addition of the agonist. (D) The PBS control, 100 μg of WT ERp57, or 100 μg of the mutant ERp57 (oo-oo) were infused into mice, and the tail bleeding times were recorded up to 30 minutes. Horizontal bars represent mean bleeding times. (E) Results of ex vivo studies of platelets prepared from mice infused with PBS (control) or inactive ERp57 (oo-oo). The platelets were activated with convulxin (500 ng/mL) and activation of (i) αIIbβ3 (measured by binding of the JON/A Ab) and (ii) P-selectin expression were determined (n = 3).
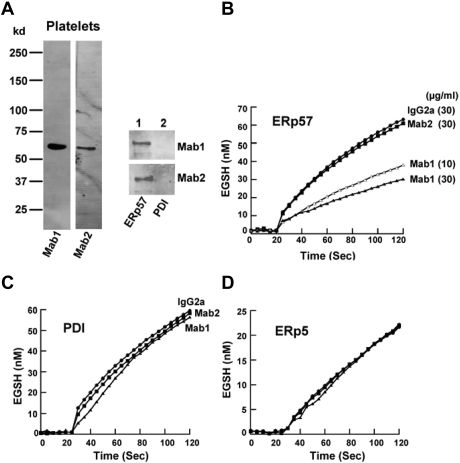
Characterization of 2 monoclonal anti-ERp57 Abs by Western blot and the GSSG assay
We raised mAbs to ERp57 and characterized 2 IgG2a Abs. Both Abs reacted by Western blot with ERp57 in platelet lysate and with purified ERp57 but not with purified PDI (Figure 5A). However, only one of the 2 Abs inhibited ERp57-dependent conversion of Di-E-GSSG to EGSH in a dose-dependent manner (inhibiting ∼ 64% of the initial velocity at 30 μg/mL; Figure 5B). This Ab did not inhibit PDI or ERp5 in the GSSG assay (Figure 5C-D).
Figure 5.
Characterization of 2 monoclonal IgG2a Abs raised to ERp57. (A) The Western blots of the Abs called Mab1 and Mab2 against human platelet lysate (4 × 108 platelets/lane) and purified ERp57 or PDI (200 μg protein/lane). Differential effect of the 2 IgG2a monoclonal anti-ERp57 Abs on (B) ERp57 activity and (C) lack of cross-reactivity to PDI or (D) ERp5 in the GSSG assay. For these studies, the activity of ERp57, PDI, and ERp5 alone (not shown in curves) was identical to enzyme with 30 μg/mL of the control IgG2a. A total of 30 μg/mL of each Ab was used in these studies except for ERp57 (B) where 10 μg/mL of the inhibitory Mab1 Ab was also tested. A total of 60 μg/mL of Mab 1 did not increase the inhibition (not shown). IgG2a control, ●; Mab1 10μg/mL, ▵; Mab1 30 μg/mL, ▴; Mab2 30 μg/mL, ■). (D) The curves for ERp5 with IgG2a, Mab1, or Mab2 (30 μg/mL of each Ab was used) completely overlapped. Each point is the composite of at least 3 samples using the Di-E-GSSG assay. (B-D) The enzyme was added at ∼ 20 seconds.
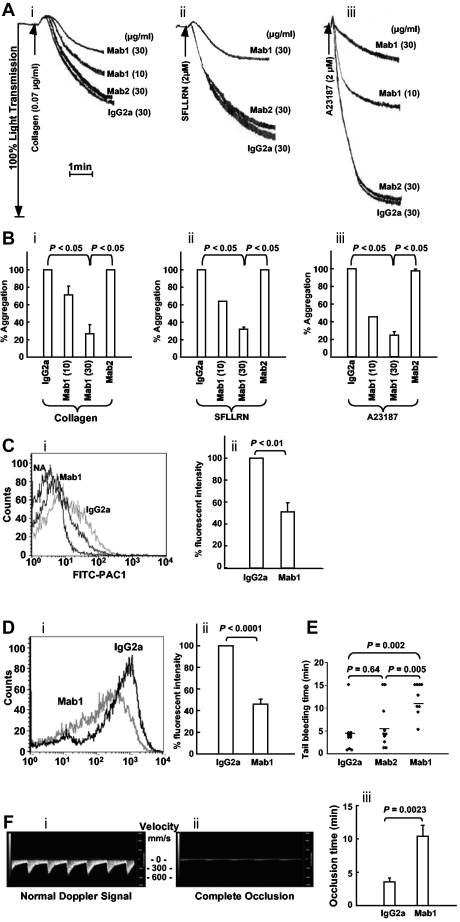
Differential effect of the inhibitory anti-ERp57 Ab (Mab1) compared with the noninhibitory Ab (Mab2) on platelet aggregation and tail bleeding times in mice
When we studied the 2 anti-ERp57 Abs in platelet aggregation, the inhibitory anti-ERp57 Ab (Mab1) inhibited aggregation to collagen, SFLLRN, and calcium ionophore (Figure 6A-B). The P2Y12 ADP receptor contains 2 thiols that are potentially redox reactive28 making it a possible target of ERp57. In the calcium ionophore studies, we excluded this possibility by removing the P2Y12 pathway (using apyrase and 2-MeSAMP as described previously).27 The noninhibitory anti-ERp57 Ab (Mab2) did not inhibit platelet aggregation. Activation of αIIbβ3 and P-selectin expression were inhibited ∼ 50% by the inhibitory Ab (Mab1; Figure 6C-D). The mean tail bleeding times in mice infused with the inhibitory anti-ERp57 Ab (Mab1) were prolonged compared with the tail bleeding times in mice infused with the control mouse IgG2a or the noninhibitory anti-ERp57 Ab (Mab2; Figure 6E).
Figure 6.
The inhibitory monoclonal anti-ERp57 Ab inhibits platelet aggregation, activation of αIIbβ3, P-selectin expression, and prolongs the tail bleeding times and the time to thrombosis in mice. (A) Representative tracings show Mab1 but not Mab2 inhibits aggregation of human platelets stimulated with collagen (i), the peptide SFLLRN (ii), or calcium ionophore (ii; A23187). (B) The combined data ± SE for at least 3 separate experiments (for collagen (i) and SFLLRN (ii), Mab1 at 10 μg/mL also inhibited aggregation to a P < .05 compared with either the IgG2a or Mab2 controls.) Calcium ionophore (iii) was used with platelets preincubated with apyrase (10 U/mL) and MeSAMP (100μM). (Ci) Mab1 inhibits PAC1 binding to SFLLRN (1μM)–activated platelets. PAC1 binding to nonactivated platelets (NA) is seen on the left. (ii) The results of inhibition of PAC1 binding as the percentage of fluorescent intensity relative to the normal IgG2a control ± SE (n = 3). (Di) A representative histogram and (ii) the combined data show the inhibitory monoclonal anti-ERp57 Ab Mab1 inhibits P-selectin binding to convulxin (10 ng/mL)–activated platelets relative to normal IgG2a ± SE (n = 7). (A-D) The platelets were preincubated with the inhibitory mAb (Mab1), noninhibitory Ab (Mab2), or control mouse IgG2a at 30 μg/mL for 10 minutes before the addition of the agonist. (E) A total of 200 μg of each Ab was infused into mice and the tail bleeding time recorded up to 15 minutes. Horizontal bars represent mean bleeding times. (Fi) The systolic and diastolic mouse carotid artery blood velocity monitored in mm of blood per second (mm/s) using the small animal Doppler probe with the Visual Sonics Vevo2100 flowmeter. (ii) Complete occlusion of blood flow after treatment with FeCl3. (iii) Mab1 inhibits FeCl3-induced occlusion relative to the normal mouse IgG2a control (n = 8). In these experiments, the carotid artery was treated with 5% FeCl3 for 2 minutes as described in “Studies using FaCl2-induced thrombosis of the carotid artery. A total of 450 μg of the control IgG2a or Mab1 were infused immediately before the filter paper soaked in 5% FeCl3 was applied to the carotid artery.”
The inhibitory anti-ERp57 Ab Mab1 inhibits thrombosis
To study in vivo thrombosis, we used an FeCl3-induced injury model with a sensitive doppler flow probe that detects systolic and diastolic blood flow through the mouse carotid artery (Figure 6Fi). Blood flow ceases when complete occlusion occurs (Figure 6Fii). The time to complete occlusion after the FeCl3 injury was prolonged by infusion of Mab1 relative to normal mouse IgG2a (Figure 6Fiii).
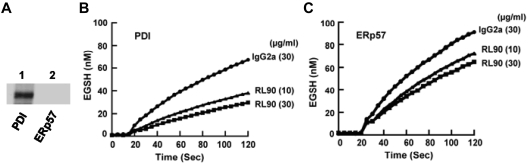
The monoclonal anti-PDI Ab RL90 cross-reacts with ERp57 in the Di-E-GSSG assay
We have developed a mAb that inhibits ERp57 and does not cross-react with PDI. A commercially available mAb called RL90 has been used to demonstrate the role of PDI in platelet aggregation24,27 and thrombosis and hemostasis.5,6 Because of the high homology of ERp57 with PDI, we tested this Ab to PDI for cross-reactivity with ERp57. RL90 did not cross-react with ERp57 by Western blot (Figure 7A). As expected, RL90 inhibited PDI activity (Figure 7B) in a dose-dependent manner giving ∼ 61% inhibition of initial velocity at 30 μg/mL. These findings are in line with previous studies where RL90 was reported to provide up to 50% to 60% inhibition of PDI.5,29 However, RL90 also inhibited ERp57 activity (Figure 7C) giving ∼ 39% inhibition of initial velocity at 30 μg/mL. Thus, a commonly used mAb to PDI has some cross-reactivity with ERp57 in a functional assay.
Figure 7.
The monoclonal anti-PDI Ab RL90 cross-reacts with ERp57 in the Di-E-GSSG assay. (A) Lack of cross- reactivity by Western blot to ERp57. (B) The inhibition of PDI. (C) The inhibition of ERp57 by RL90, at 10 μg/mL and 30 μg/mL relative to an isotype specific monoclonal mouse IgG2a Ab (30 μg/mL); 60 μg/mL did not provide more inhibition of PDI or ERp57 with the curves overlapping with the curves for 30 μg/mL. The amount of EGSH formed over 120 seconds from Di-E-GSSG is shown with the enzyme added at ∼ 20 seconds.
Discussion
Although discovered relatively recently, ERp57 has quickly become one of the better-studied members of the PDI family.8 ERp57 catalyzes isomerization of disulfide bonds in glycoproteins and we hypothesized that extracellular ERp57 had a role in platelet aggregation, a process that is dependent on the cysteine-rich αIIbβ3 glycoprotein. PDI has a role in platelet function4 and has homology and structural similarity with ERp57.30 We therefore needed to document that inhibition of platelet function by the Abs against ERp57 was not because of inhibition of PDI. Using 2 rabbit Abs raised to ERp57 we found that the best inhibitory Ab to ERp57 (Ab 1) only weakly inhibited PDI. It is possible that a small amount of the inhibition of platelet aggregation by Ab 1 in Figure 3A and B is because of inhibition of PDI. However, it is apparent that only Ab 2 reacts with PDI by Western blot (Figure 1C) and more strongly inhibits PDI (Figure 2B) yet only weakly inhibits aggregation. Because Ab 1 inhibits PDI only weakly, we conclude that it primarily inhibits platelet aggregation by inhibiting ERp57.
Using a polyclonal rabbit Ab to ERp57, we found a low but statistically significant level of ERp57 on the surface of resting platelets. Resting platelets display a relatively low level of active PDI on the platelet surface.27,31 Similarly, only low levels of ERp5 are expressed on resting platelets.7 A limited surface expression of the PDI enzymes has been proposed as a way of “setting the gain” or threshold for platelet activation.7 In principle, a small amount of enzyme could activate a large number of receptors. The surface amount of active PDI increases dramatically with thrombin stimulation of the platelets31; collagen-induced platelet activation also increases the active form of PDI on the platelet surface.27 In preliminary studies (using polyclonal Abs and mAbs), we have found that the level of ERp57 on the surface increases substantially with activation as previously reported.9 In vivo, other sources of ERp57 (such as ERp57 secreted from endothelial cells) at the site of vascular injury could increase the local concentration of ERp57 at the platelet surface further enhancing receptor activation.
To further document a role for extracellular ERp57 in platelet function, we examined the effect of wild-type ERp57 and a mutant ERp57 rendered catalytically inactive on platelet aggregation. The wild-type enzyme potentiated aggregation while the inactive enzyme had the opposite effect, further suggesting that ERp57 on the platelet surface has a role in platelet aggregation. These findings imply that the added ERp57 interacts with a platelet surface substrate of ERp57 to potentiate platelet aggregation. On the other hand, inactive ERp57 appears to compete with normal platelet surface ERp57 resulting in inhibition of aggregation. The prolongation of tail bleeding times in mice by infusion of catalytically inactive ERp57 suggests a role for circulating or intravascular ERp57 in hemostasis. Platelets collected after infusion of inactive ERp57 show inhibition of activation of αIIbβ3 and P-selectin expression. This suggests that some infused ERp57 binds to the platelet surface in vivo and continues to inhibit the platelets ex vivo. This may suggest a role for platelet ERp57 in the hemostatic mechanisms of tail bleeding times.
Most reports on the role of PDI in platelet aggregation and hemostasis were performed using only one inhibitory Ab3,5,6,24,27 although the role of PDI in regulation of the α2β1 collagen receptor was studied using both a polyclonal and mAb.32 Cross-reactivity of the anti-PDI Abs with ERp57 was not tested. Bacitracin has also been used to inhibit PDI5,24; however, this is not a specific inhibitor of PDI.33 We wanted to confirm a role for ERp57 using a third inhibitor specific for ERp57 and therefore generated 2 IgG2a mAbs to ERp57. Although both Abs reacted with ERp57 by Western blot, only one Ab inhibited ERp57 activity. This allowed us to use the noninhibitory Ab as a control. The inhibitory Ab did not react with PDI or inhibit PDI or ERp5 activity implying that there is no functional cross-reactivity with these enzymes. This is especially important in the case of PDI because there is 50% identity in the amino acid sequence of the active site containing a and a′ domains of the 2 enzymes.14
Using these Abs, we found that the inhibitory anti-ERp57 Ab inhibited platelet aggregation while the noninhibitory control anti-ERp57 Ab did not. This confirms a role for platelet surface ERp57 in platelet aggregation. The inhibitory Ab also prolonged the tail bleeding times in mice confirming a role for intravascular ERp57 in hemostasis. Furthermore, the inhibitory Ab prolonged the time to occlusion in a FeCl3-induced thrombosis model. This implies that intravascular ERp57 has a role in thrombosis.
The high homology between ERp57 and PDI raises the possibility that some of the inhibition observed by Abs against PDI may actually represent inhibition of ERp57. The original rabbit anti-PDI Ab we used to document a role for PDI in platelet aggregation reacted strongly and apparently specifically with PDI,3,34 suggesting the major part of the inhibition effect was on PDI. However, we did not at the time test this Ab for cross-reactivity to ERp57. In subsequent inhibition studies the monoclonal anti-PDI Ab RL90 was also found to inhibit platelet aggregation5,24,27 indicating a role for the traditional PDI in platelet aggregation. However, we found some cross-reactivity of monoclonal anti-PDI Ab RL90 with ERp57 in our functional assay. It is therefore possible that previously reported effects of RL90 are due in part to inhibition of ERp57.
The fact that a specific inhibitory monoclonal anti-ERp57 Ab inhibits platelet aggregation does not exclude a role for the traditional PDI (or other PDIs recruited to the platelet surface)9 in platelet aggregation and hemostasis. It may be that both PDI and ERp57 have important roles in platelet aggregation. For example, numerous proteins interact with the cytoplasmic tails of αIIbβ3 and inhibition of the loss of the interaction of some of the proteins within the αIIbβ3 tail on an individual basis results in complete inhibition of platelet aggregation or thrombus formation.35–38 The selective loss of the Kindlin3 interaction with the cytoplasmic tail of β3 results in the complete loss of the aggregation response in mouse or human platelets.35,36 Similarly, loss of the interaction of talin with the cytoplasmic tail of β3 also results in the complete loss of aggregation and thrombus formation.37,38 These findings imply that both Kindlin3 and talin are important. Our findings with respect to inhibition by Abs to PDI and ERp57 also suggest that both are important and they work by different or separate mechanisms.
The substrate or target for ERp57 is not yet known. Because anti-ERp57 inhibits aggregation induced by several different agonists including calcium ionophore, a surface agonist receptor is unlikely the target. We performed some platelet aggregation experiments in which the dithiol containing P2Y12 receptor28 was excluded as the mechanism. Other possible targets of ERp57 in platelet aggregation include αIIbβ3 or the fibrinogen added to support platelet aggregation. Because fibrinogen was not added to the flow cytometric assay, the inhibition of PAC-1 binding by the anti-ERp57 Abs implies that fibrinogen is not the target of ERp57. Functional and physical associations of PDI and ERp5 with αIIbβ37,27 have been noted, raising the possibility that αIIbβ3 is a target of ERp57. This would be consistent with the proposed role for disulfide exchange in αIIbβ3 and disulfide isomerases in the final steps of physiologic agonist-induced activation of integrins.4,39 Extracellular ERp57 may have a role in conformational changes that occur in αIIbβ3 as this receptor transitions from a low-affinity conformation to a high-affinity ligand-binding conformation.40,41
Conceivable mechanisms by which both ERp57 and PDI could be required for aggregation include the sequential catalysis of different reactions. For example, one enzyme could catalyze a required disulfide reduction or cleavage reaction thus allowing the other enzyme to catalyze a thiol disulfide exchange reaction. Alternatively, PDI and ERp57 could catalyze simultaneous reactions at different important sites of αIIbβ. Interestingly, a recent report found that a “PDI family network” was required for the unfolding of a polyomavirus protein.42 Specifically, PDI and ERp72 reduced the viral protein and ERp57 isomerized the protein in a process that required free thiols. Thus, in platelets reactions catalyzed by both ERp57 and PDI could be essential with inhibition of either enzyme leading to loss of activation of αIIbβ3. With platelet activation or aggregation an increase in thiols in PDI is found27,31 suggesting that the thiol form of the active site is generated. It is this form that catalyzes disulfide reduction and isomerization.4 Although ERp57 catalyzes similar reactions as PDI, substantially more work is needed to determine the status of the active sites of ERp57 and the reactions it catalyzes in platelets. These interactions need to be defined, as little is currently understood.
We found that inhibition of ERp57 by rabbit and monoclonal anti-ERp57 Abs decreased convulxin-induced P-selectin expression by ∼ 50% (Figures 3Eii, 6Dii) indicating a role for ERp57 in α-granule release. Jordan et al similarly found that a rabbit Ab to the disulfide isomerase ERp5 substantially decreased convulxin-induced P-selectin expression on platelets.7 This suggests that both ERp5 and ERp57 have a role in P-selectin exposure. Jordan et al also found a role for PDI in P-selectin expression7 although Lahav et al documented a lesser role for PDI.24 The interrelationships of PDI, ERp57, and ERp5 in P-selectin expression will need further characterization.
The role of platelet ERp57 in vivo will require further study. In regard to PDI, it is known that PDI is found on platelet microparticles from patients43 and that platelets release PDI in vivo at the site of vascular injury.5,6 A laser-induced injury model found that endothelial cells could provide the extracellular PDI needed for thrombosis, although platelet microparticles were also a potential source of PDI contributing to thrombosis.44 Although this injury model provides evidence of a role for endothelial cell PDI in thrombosis, the issue of a contribution from platelet PDI was not addressed. The relative contribution of ERp57 from endothelial cells and platelets or other sources of ERp57 needs to be studied. Eventually, targeted knockouts of PDI and ERp57 in megakaryocytes/platelets will help define the platelet contribution of these PDIs in hemostasis and thrombosis.
In summary, using 3 different reagents we have found that ERp57 on the platelet surface mediates platelet aggregation. ERp57 is also required for hemostasis and thrombosis. Although the degree to which the different members of the PDI family have unique versus overlapping roles in platelet function remains to be further elucidated, in these studies the inhibitory Abs to ERp57 do not significantly inhibit PDI. Importantly, the newly generated inhibitory mAb to ERp57 does not show antigenic or functional cross-reactivity with PDI. Therefore, ERp57 appears to have a role in activation of the αIIbβ3 integrin and platelet aggregation that is distinct from the role of PDI.
Note added in proof: It has come to our attention after our manuscript was accepted for publication that similar findings with respect to the role of platelet ERp57 have been obtained by Holbrook et al, whose work is being published in the Journal of Thrombosis and Haemostasis.45
Acknowledgments
This work was supported by startup funds from Temple University School of Medicine, a VA Merit grant (Audie L. Murphy VA Hospital, San Antonio, TX; D.W.E.), a National Institutes of Health grant (T32 HL007777) for training in hemostasis and vascular biology (M.P.C.), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: Y.W. helped with the design and supervised experiments; Y.W. and S.S.A. collected, analyzed, and interpreted data, and helped revise parts of the manuscript; Y.W., S.S.A., J.Z., L.W., and M.P.C. performed research; and D.W.E. designed the project, analyzed the data, supervised the research, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David W. Essex, MD, Temple University School of Medicine, Rm 300C OMS, 3400 North Broad St, Philadelphia, PA 19140; e-mail: david.essex@temple.edu.
References
- 1.Goldberger RF, Epstein CJ, Anfinsen CB. Acceleration of reactivation of reduced bovine pancreatic ribonuclease by a microsomal system from rat liver. J Biol Chem. 1963;238:628–635. [PubMed] [Google Scholar]
- 2.Venetianer P, Straub FB. The enzymic reactivation of reduced ribonuclease. Biochim Biophys Acta. 1963;67:166–168. doi: 10.1016/0006-3002(63)91812-2. [DOI] [PubMed] [Google Scholar]
- 3.Essex DW, Li M. Protein disulphide isomerase mediates platelet aggregation and secretion. Br J Haematol. 1999;104(3):448–454. doi: 10.1046/j.1365-2141.1999.01197.x. [DOI] [PubMed] [Google Scholar]
- 4.Essex DW. Redox control of platelet function. Antioxid Redox Signal. 2009;11(5):1191–1225. doi: 10.1089/ars.2008.2322. [DOI] [PubMed] [Google Scholar]
- 5.Cho J, Furie BC, Coughlin SR, Furie B. A critical role for extracellular protein disulfide isomerase during thrombus formation in mice. J Clin Invest. 2008;118(3):1123–1131. doi: 10.1172/JCI34134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reinhardt C, von Bruhl ML, Manukyan D, et al. Protein disulfide isomerase acts as an injury response signal that enhances fibrin generation via tissue factor activation. J Clin Invest. 2008;118(3):1110–1122. doi: 10.1172/JCI32376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jordan PA, Stevens JM, Hubbard GP, et al. A role for the thiol isomerase protein ERP5 in platelet function. Blood. 2005;105(4):1500–1507. doi: 10.1182/blood-2004-02-0608. [DOI] [PubMed] [Google Scholar]
- 8.Hatahet F, Ruddock LW. Protein disulfide isomerase: a critical evaluation of its function in disulfide bond formation. Antioxid Redox Signal. 2009;11(11):2807–2850. doi: 10.1089/ars.2009.2466. [DOI] [PubMed] [Google Scholar]
- 9.Holbrook LM, Watkins NA, Simmonds AD, Jones CI, Ouwehand WH, Gibbins JM. Platelets release novel thiol isomerase enzymes which are recruited to the cell surface following activation. Br J Haematol. 2010;148(4):627–637. doi: 10.1111/j.1365-2141.2009.07994.x. [DOI] [PubMed] [Google Scholar]
- 10.Schulz C, Leuschen NV, Frohlich T, et al. Identification of novel downstream targets of platelet glycoprotein VI activation by differential proteome analysis: implications for thrombus formation. Blood. 2010;115(20):4102–4110. doi: 10.1182/blood-2009-07-230268. [DOI] [PubMed] [Google Scholar]
- 11.Jessop CE, Chakravarthi S, Garbi N, Hammerling GJ, Lovell S, Bulleid NJ. ERp57 is essential for efficient folding of glycoproteins sharing common structural domains. EMBO J. 2007;26(1):28–40. doi: 10.1038/sj.emboj.7601505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wearsch PA, Cresswell P. The quality control of MHC class I peptide loading. Curr Opin Cell Biol. 2008;20(6):624–631. doi: 10.1016/j.ceb.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Kozlov G, Pocanschi CL, et al. ERp57 does not require interactions with calnexin and calreticulin to promote assembly of class I histocompatibility molecules, and it enhances peptide loading independently of its redox activity. J Biol Chem. 2009;284(15):10160–10173. doi: 10.1074/jbc.M808356200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coe H, Michalak M. ERp57, a multifunctional endoplasmic reticulum resident oxidoreductase. Int J Biochem Cell Biol. 2010;42(6):796–799. doi: 10.1016/j.biocel.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Scandura JM, Ahmad SS, Walsh PN. A binding site expressed on the surface of activated human platelets is shared by factor X and prothrombin. Biochemistry. 1996;35(27):8890–8902. doi: 10.1021/bi9525029. [DOI] [PubMed] [Google Scholar]
- 16.Raturi A, Mutus B. Characterization of redox state and reductase activity of protein disulfide isomerase under different redox environments using a sensitive fluorescent assay. Free Radic Biol Med. 2007;43(1):62–70. doi: 10.1016/j.freeradbiomed.2007.03.025. [DOI] [PubMed] [Google Scholar]
- 17.Essex DW, Li M, Miller A, Feinman RD. Protein disulfide isomerase and sulfhydryl-dependent pathways in platelet activation. Biochemistry. 2001;40(20):6070–6075. doi: 10.1021/bi002454e. [DOI] [PubMed] [Google Scholar]
- 18.Lian L, Wang Y, Draznin J, et al. The relative role of PLCbeta and PI3Kgamma in platelet activation. Blood. 2005;106(1):110–117. doi: 10.1182/blood-2004-05-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu W, Jin R, Zhang J, et al. The critical roles of platelet activation and reduced NO bioavailability in fatal pulmonary arterial hypertension in a murine hemolysis model. Blood. 2010;116(9):1613–1622. doi: 10.1182/blood-2010-01-267112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mutlu GM, Green D, Bellmeyer A, et al. Ambient particulate matter accelerates coagulation via an IL-6-dependent pathway. J Clin Invest. 2007;117(10):2952–2961. doi: 10.1172/JCI30639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanoh K, Shimura T, Suzuki H, Nomoto K, Asao T, Kuwano H. Antitumor effect of a splenic injection of 5-fluorouracil on metastatic liver cancer in mice. J Pharmacol Exp Ther. 2004;308(1):168–174. doi: 10.1124/jpet.103.057547. [DOI] [PubMed] [Google Scholar]
- 22.Fuentes R, Wang Y, Hirsch J, et al. Infusion of mature megakaryocytes into mice yields functional platelets. J Clin Invest. 2010;120(11):3917–3922. doi: 10.1172/JCI43326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Y, Suzuki-Inoue K, Satoh K, et al. Role of Fc receptor gamma-chain in platelet glycoprotein Ib-mediated signaling. Blood. 2001;97(12):3836–3845. doi: 10.1182/blood.v97.12.3836. [DOI] [PubMed] [Google Scholar]
- 24.Lahav J, Jurk K, Hess O, et al. Sustained integrin ligation involves extracellular free sulfhydryls and enzymatically catalyzed disulfide exchange. Blood. 2002;100(7):2472–2478. doi: 10.1182/blood-2001-12-0339. [DOI] [PubMed] [Google Scholar]
- 25.Frickel EM, Frei P, Bouvier M, et al. ERp57 is a multifunctional thiol-disulfide oxidoreductase. J Biol Chem. 2004;279(18):18277–18287. doi: 10.1074/jbc.M314089200. [DOI] [PubMed] [Google Scholar]
- 26.Essex DW, Chen K, Swiatkowska M. Localization of protein disulfide isomerase to the external surface of the platelet plasma membrane. Blood. 1995;86(6):2168–2173. [PubMed] [Google Scholar]
- 27.Manickam N, Sun X, Li M, Gazitt Y, Essex DW. Protein disulphide isomerase in platelet function. Br J Haematol. 2008;140(2):223–229. doi: 10.1111/j.1365-2141.2007.06898.x. [DOI] [PubMed] [Google Scholar]
- 28.Ding Z, Kim S, Dorsam RT, Jin J, Kunapuli SP. Inactivation of the human P2Y12 receptor by thiol reagents requires interaction with both extracellular cysteine residues, Cys17 and Cys270. Blood. 2003;101(10):3908–3914. doi: 10.1182/blood-2002-10-3027. [DOI] [PubMed] [Google Scholar]
- 29.Kaetzel CS, Rao CK, Lamm ME. Protein disulphide-isomerase from human placenta and rat liver. Purification and immunological characterization with monoclonal antibodies. Biochem J. 1987;241(1):39–47. doi: 10.1042/bj2410039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maattanen P, Kozlov G, Gehring K, Thomas DY. ERp57 and PDI: multifunctional protein disulfide isomerases with similar domain architectures but differing substrate-partner associations. Biochem Cell Biol. 2006;84(6):881–889. doi: 10.1139/o06-186. [DOI] [PubMed] [Google Scholar]
- 31.Burgess JK, Hotchkiss KA, Suter C, et al. Physical proximity and functional association of glycoprotein 1balpha and protein-disulfide isomerase on the platelet plasma membrane. J Biol Chem. 2000;275(13):9758–9766. doi: 10.1074/jbc.275.13.9758. [DOI] [PubMed] [Google Scholar]
- 32.Lahav J, Wijnen EM, Hess O, et al. Enzymatically catalyzed disulfide exchange is required for platelet adhesion to collagen via integrin alpha2beta1. Blood. 2003;102(6):2085–2092. doi: 10.1182/blood-2002-06-1646. [DOI] [PubMed] [Google Scholar]
- 33.Karala AR, Ruddock LW. Bacitracin is not a specific inhibitor of protein disulfide isomerase. FEBS J. 2010;277(11):2454–2462. doi: 10.1111/j.1742-4658.2010.07660.x. [DOI] [PubMed] [Google Scholar]
- 34.Chen K, Detwiler TC, Essex DW. Characterization of protein disulphide isomerase released from activated platelets. Br J Haematol. 1995;90(2):425–431. doi: 10.1111/j.1365-2141.1995.tb05169.x. [DOI] [PubMed] [Google Scholar]
- 35.Moser M, Nieswandt B, Ussar S, Pozgajova M, Fassler R. Kindlin-3 is essential for integrin activation and platelet aggregation. Nat Med. 2008;14(3):325–330. doi: 10.1038/nm1722. [DOI] [PubMed] [Google Scholar]
- 36.Malinin NL, Zhang L, Choi J, et al. A point mutation in KINDLIN3 ablates activation of three integrin subfamilies in humans. Nat Med. 2009;15(3):313–318. doi: 10.1038/nm.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petrich BG, Fogelstrand P, Partridge AW, et al. The antithrombotic potential of selective blockade of talin-dependent integrin alpha IIb beta 3 (platelet GPIIb-IIIa) activation. J Clin Invest. 2007;117(8):2250–2259. doi: 10.1172/JCI31024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petrich BG, Marchese P, Ruggeri ZM, et al. Talin is required for integrin-mediated platelet function in hemostasis and thrombosis. J Exp Med. 2007;204(13):3103–3111. doi: 10.1084/jem.20071800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shattil SJ, Kim C, Ginsberg MH. The final steps of integrin activation: the end game. Nat Rev Mol Cell Biol. 2010;11(4):288–300. doi: 10.1038/nrm2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coller BS, Shattil SJ. The GPIIb/IIIa (integrin alphaIIbbeta3) odyssey: a technology-driven saga of a receptor with twists, turns, and even a bend. Blood. 2008;112(8):3011–3025. doi: 10.1182/blood-2008-06-077891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bennett JS, Berger BW, Billings PC. The structure and function of platelet integrins. J Thromb Haemost. 2009;1(7 suppl):200–205. doi: 10.1111/j.1538-7836.2009.03378.x. [DOI] [PubMed] [Google Scholar]
- 42.Walczak CP, Tsai B. A PDI family network acts distinctly and coordinately with ERp29 to facilitate polyomavirus infection. J Virol. 2011;85(5):2386–2396. doi: 10.1128/JVI.01855-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raturi A, Miersch S, Hudson JW, Mutus B. Platelet microparticle-associated protein disulfide isomerase promotes platelet aggregation and inactivates insulin. Biochim Biophys Acta. 2008;1778(12):2790–2796. doi: 10.1016/j.bbamem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 44.Jasuja R, Furie B, Furie BC. Endothelium-derived but not platelet-derived protein disulfide isomerase is required for thrombus formation in vivo. Blood. 2010;116(22):4665–4674. doi: 10.1182/blood-2010-04-278184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Holbrook LM, Sasikumar P, Stanley RG, Simmonds AD, Bicknell AB, Gibbins JM. The platelet-surface thiol isomerase enzyme ERp57 modulates platelet function [published online ahead of print December 13, 2011]. J Thromb Haemost. doi: 10.1111/j.1538-7836.2011.04593.x. doi: 10.1111/j.1538-7836.2011.04593.x. [DOI] [PMC free article] [PubMed] [Google Scholar]