Abstract
Since connective tissue pulleys implement Listing's law by systematically changing rectus extraocular muscle (EOM) pulling directions, non-Listing's law gaze-dependence of the vestibulo-ocular reflex is currently inexplicable. Differential activation of compartments within rectus EOMs may endow the ocular motor system with more behavioral diversity than previously supposed. Innervation to horizontal, but not vertical, rectus EOMs of mammals is segregated into superior and inferior compartments. Magnetic resonance imaging in normal subjects demonstrates contractile changes in the lateral rectus (LR) inferior, but not superior, compartment during ocular counter-rolling (OCR) induced by head tilt. In human orbits ipsilesional to unilateral superior oblique palsy, neither LR compartment exhibits contractile change during head tilt, although the inferior compartment contracts normally in contralesional orbits. This suggests that differential compartmental LR contraction assists normal OCR. Computational simulation suggests that differential compartmental action in horizontal rectus EOMs could achieve more force than required by vertical fusional vergence.
Keywords: extraocular muscles, magnetic resonance imaging, motor nerve, pulleys, vestibulo-ocular reflex
Introduction
Kinematic Mystery
Listing's law (LL) specifies unique eye torsion in any gaze direction1, and is satisfied if the ocular rotational velocity axis shifts by half the shift in ocular orientation.2 Recent data has compelled rejection of the formerly prevalent belief that the brain explicitly commands the LL.3-7 Inconsistent with that idea is the observation that motor neurons activating cyclovertical EOMs of monkeys do not encode LL torsion during smooth pursuit,8 that electrical stimulation of the abducens nerve evokes saccade-like movements that do conform to LL while the head is upright,9 and that these electrically stimulated movements change vertical direction during head tilt by the amount of ocular counter-rolling (OCR).10 The Active Pulley Hypothesis (APH) alternatively explains absence of such neural torsion signals by positing that systematic changes in rectus EOM pulling directions generate LL torsion, even as offset by oblique EOM action during OCR. 11-14 The APH suggests that pulleys, comprised of connective tissue rings encircling rectus EOMs, are translated by the EOMs' fibers while global layer fibers insert on the eye to rotate it.11-13, 15, 16 Anteroposterior locations of rectus pulleys are thus neurally controlled,15 influencing ocular torsion indirectly via EOM path geometry as set by contractile force in the EOM's orbital layer.
While no controversy remains that LL is the peripheral oculomotor apparatus's kinematic default behavior, a serious paradox has intruded: the vestibulo-ocular reflex (VOR) violates LL such that the VOR's velocity axis changes by one-fourth or less of eye position, not half as required by LL. 17 This means that ocular torsion is different during a VOR eye movement than during an otherwise identical horizontal or vertical eye movement evoked by a visual stimulus with the head stationary. Motor neurons driving cyclovertical EOMs of behaving monkeys have been demonstrated not to command the VOR's violations of LL.8 This means that neural commands for torsion necessary to override half angle APH behavior of the orbital tissues are not executed by cyclovertical EOMs! So where could this non-LL torsion arise? Having long abandoned as unrealistic5, 17 our early suggestion16 that LL violations could be implemented by retraction of the rectus pulleys, we proposed that LL violations are executed by differential compartmental activity in one or both horizontal rectus EOMs.18, 19 The current report contains novel, preliminary functional evidence that differential compartmental activity does indeed occur in humans during the static torsional VOR.
Historical Suggestions of EOM Compartmentalization
There are seemingly more EOM fibers and motor neurons than required for conventionally recognized eye movements20, 21, suggesting potential for compartmental functions. For example, the feline inferior oblique muscle is innervated by distinct medial and lateral branches that generate different contractile responses.22 With a dual headed origin23, 24 arising from dual embryologic precursors,25, 26 the LR seems designed to have two parallel compartments. Longitudinal LR splitting is clearly evident by MRI in congenital cranial dysinnervation disorders, including congenital fibrosis of the EOMs type 1,27 Duane syndrome,28-30 and trochlear,31 and oculomotor palsy.31 Gross dissection demonstrates that LR innervation is often bifid in humans, with frequent abducens nerve spliting into parallel nerves,32-35 that sometimes extends even as far proximally as the pons.35
Histological Investigation
In order to examine the possibility of compartmental control of rectus EOMs, their peripheral intramuscular innervation was studied by serial sectioning of whole, undisturbed orbits at 10 μm thickness. Sectioning was in the quasi-coronal plane perpendicular to the long orbital axis, and thus perpendicular to most nerve and EOM fibers. Typically, this required about 3,000 sections per monkey orbit, 4,000 sections per human orbit, and about 6,000 sections per bovine orbit. In general, every tenth section was stained with Masson trichrome, which stains EOM fibers bright red in the global layer and dark red in the orbital layer, collagenous connective tissues blue, and nerves purple. The distance between sections was sufficiently small that the transverse profiles of both types of fibers retain such substantial similarity between stained sections as to be individually identifiable from section to section. In the occasional event of ambiguities in fiber branching between sections, we stained and examined as required the unstained serial sections between the regular staining intervals of 100 μm, reducing the sampling interval to as little as 10 μm. Using these methods, it was possible reliably to trace motor nerves from their proximal trunks in the deep orbit through many levels of bifurcation in which their motor axons coursed anteriorly through the rectus EOM.
Anatomical Potential for Muscle Compartmentalization
Exact counting shows that orbital and global layers of each rectus EOM contain on the order of 10,000 fibers each in humans and monkeys.36 Longitudinal tracing of individual bundles of rectus EOM fibers suggests that there are very few myomyous junctions among adjacent fibers, and certainly not between orbital and global layer fibers.37 Longitudinal tracing of hypertrophic fibers in the superior oblique (SO) global layer following trochlear neurectomy confirms that most such fibers extend nearly the entire length of the EOM belly without myomyous junctions; the few fibers that exhibit myomyous junctions do so only near the myotendonous junction.38 There are few collagenous or other mechanical intercouplings among EOM fiber bundles except near the sites of entries of the major motor nerve trunks. This overall organization suggests the potential for mechanical independence among compartments consisting of groups of EOM fibers.37
Tendons of EOMs also consist of parallel arrangements of thick, highly stiff fibers that are loosely wrapped transversely by much finer fibers. Such architecture appears structurally suited to transmit differing forces in tendon bundles lying in parallel along the transverse tendon widths. For example, during strabismus surgery under topical anesthesia, one observes marked posterior retraction of a partially severed portion of a tendon whose remainder remains attached at the sclera. Thus, individual bundles of EOM fibers are in continuity with their own individual bundles of tendon fibers, and can apparently exert differing forces on the globe.
Anatomical Potential for Motor Nerve Compartmentalization
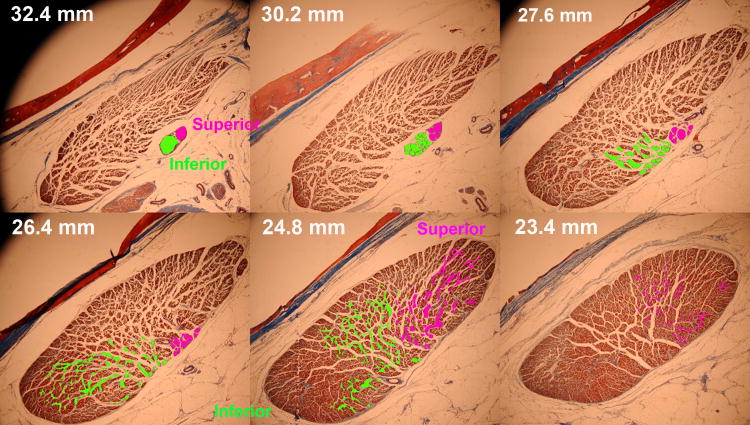
The motor nerves divide into branches on the global surface of each EOM and travel anteriorly, sometimes with further branching, before penetrating into the EOM in an anterior continuation of further bifurcation and arborization. These motor nerve distributions can be traced in serial digital micrographs by creation of color-coded contour overlays that are then reconstructed three-dimensionally using software.18, 19, 39 In rabbit, cow, monkey, and human, the main motor nerves to the LR divide into two divisions posterior to entry into the EOM. This segregated innervation is seen for a sequence of serial sections of the human LR in Fig. 1. It may be seen from Fig. 1 that the inferior division of the human abducens nerve arborized within the LR about 1.5 mm more posteriorly than did the superior division. Examination via similar serial sections of two human and five monkey orbits demonsrated consistency of this finding, with the inferior division entering the LR 0.4 – 2.5 mm more posteriorly than the superior division.18 Moreover, the inferior division arborized into extensive intramuscular branches posterior to the first extensive intramuscular arborization of the superior division (Fig. 1). More anteriorly, the superior division completed its arborization anterior to completion of the inferior arborization, without significant overlap of the two divisions.
Fig. 1.
Planar histological views from serial quasi-coronal sections of human lateral rectus muscle showing non-overlapping arborization of the superior and inferior divisions of the abducens nerve within the respective compartments of the muscle. Masson trichrome stain. Section distances are indicated posterior to the corneal surface.
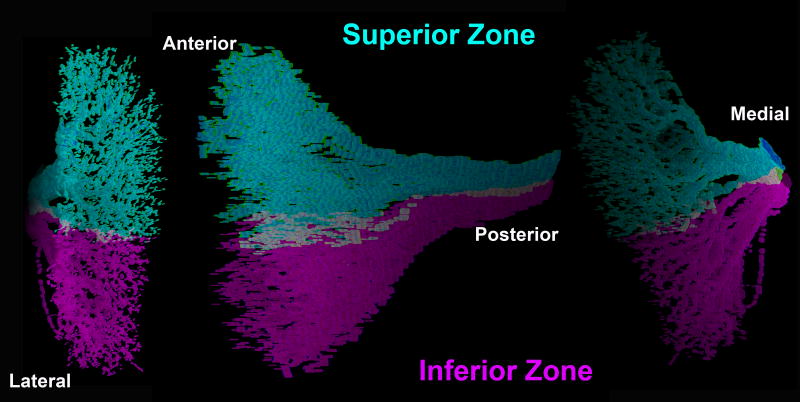
Three dimensional reconstruction of intramuscular abducens nerve arborization vividly confirms the anatomy suggested by serial histological sections. Such reconstruction necessarily includes only the larger nerve branches, and stops short of terminal neuromuscular junctions because the nerves become too small to trace reliably. Figure 2 illustrates three perspectives of a three-dimensional reconstruction of the human LR showing segregation of the terminal arborization of the superior and inferior division of the abducens nerve. The intramuscular course of subsequent abducens nerve branches followed almost parallel, separate courses, remaining within superior or inferior LR compartment with no turns and at most rare anastomoses, even between branches of the same main trunk. Note absence of any recurrent motor nerve branches; all branches coursed from posterior to anterior, without turning back. These findings were consistent in all species examined.
Fig. 2.
Three dimensional reconstruction of intramuscular arborization of superior and inferior divisions of the abducens nerve within the respective compartments of the human lateral rectus muscle. This arborization measured 6.6 mm vertically, 12 mm anteroposteriorly, and 1.2 mm mediolaterally.
Although the medial rectus (MR) did not show as clearly segregated a bizonal innervational pattern as found in the abducens nerve, the intramuscular branches of the oculomotor nerve innervating the MR of humans and monkeys divided external to the EOM and subsequently distributed within it in an organized pattern maintained along the MR length. Anastomoses between branches either from the same or from different main trunks of intramuscular oculomotor nerve occur occasionally. Occasional motor nerve branches in the MR took a recurrent course from anterior to posterior, mainly in the initial intramuscular divisions of the nerve.
Quantitative comparison of nerve segregation within superior and inferior compartments of the LR and MR was obtained by determining, along the length of each EOM, the percentage of transverse EOM width occupied by exclusively segregated nerve projections, and the proportion containing overlapping projections. In both human and monkey, 1.7 – 4.1% of LR width contains nerve branches from both the superior and inferior divisions of the abducens nerve, while the remaining LR contained nerve arborizations exclusively from either the superior or inferior division. Among individual specimens, the ratio of superior to inferior compartment transverse LR width ranged from 60:40 to 40:40, averaging approximately equal proportions. The situation differs slightly for the MR, where in both human and monkey, 2 - 41% of EOM width contains mixed branches of both the superior and inferior divisions of the motor nerve; the remaining 20 – 40% of MR width receives exclusive innervation from either superior or inferior division.19
These general findings have been confirmed for the LR in rabbit, cow, monkey, and human orbits, and for the MR in human and monkey orbits. One implication of this anatomy is that it would be experimentally feasible to perform selective electrical stimulation, tracer injection, or surgical section of the motor nerve branch to either nerve division, with identification based upon the anteroposterior location of the nerve branch's entry into the EOM.
Identical tracing methods have failed to demonstrate topography of intramuscular innervation within the human superior rectus (SR) muscle.19 Motor nerves within the human inferior rectus (IR) muscle are “mixed” in the lateral portion, but the medial division of the motor nerve exclusively innervates the medial portion of the EOM. This finding may indicate existence of a limited potential for differential activation of the lateral portion of the IR.
Anatomically, therefore, there exists an excellent basis for selective compartmental control of the superior and inferior compartments of the LR, and probably also of the MR. While there might be limited potential for selective innervational control of the lateral compartment of the IR, selective compartmental innervation appears highly unlikely for the SR. We therefore first sought functional evidence for selective compartmental control in the LR, during an oculomotor behavior known to violate LL.
Magnetic Resonance Imaging (MRI) of Compartmental LR Function
High resolution MRI with surface coils readily demonstrates large changes in EOM cross-section directly corresponding to contraction during ocular versions40 and convergence.41 Moreover, EOM changes during ocular counter-rolling (OCR) to 90° roll head tilts can also be demonstrated by MRI.42 Static OCR is a VOR that can be evoked during fixation of a centered target that inhibits changes in horizontal and vertical eye position that would create large, potentially confounding changes in rectus EOM contractility. Obviously, OCR violates LL. We therefore chose to examine possible differential compartmental contractility of the LR during OCR.
Normal subjects, recruited by advertisement, underwent complete ophthalmic examinations to verify that they were visually normal, with visual acuity correctable to at least 0.0 logMAR (20/20 Snellen) in each eye, normal ocular motility, and normal contour stereo resolution of at least 40 arcsec. Also studied were 12 subjects, recruited from a clinical strabismus practice, who exhibited the pattern of cyclovertical strabismus typical of SO palsy, and who had unilateral SO palsy manifested by significant SO atrophy of about 50% (P < 0.00001).43 Subjects with SO palsy had normal uncorrected or correct visual acuity in each eye, but an average of 17.4 ± 2.4 (SEM) Δ ipsilateral hypertropia that was 25.1 ± 5.1 Δ greater in ipsilesional than contralesional head tilt. 43
Both groups of subjects were scanned in 90° right side down (decubitus) and left side down head tilt positions while lying on the scanner bed, using surface coils as previously described.42 Details of this technique41, 42 include use of a dual-phased surface coil array (Medical Advances, Milwaukee, WI) to improve signal-to-noise ratio and fixation targets to avoid motion artifacts. Imaging employed T1 or T2 fast spin echo pulse sequences.44 Initially, a triplanar scan was obtained to verify correct head positioning relative to gravitational scanner coordinates, with head repositioning as necessary. Sets of 18 - 20 contiguous, 2-mm-thick quasicoronal images were placed perpendicular to the long axis of each orbit using a 256 × 256 matrix over an 8-cm FOV, yielding a final pixel resolution of 312 μm. During imaging, the scanned eye monocularly fixated a fine optical fiber illuminated from its distal end by a red, light emitting diode in straight ahead gaze 2 cm distant. Repeatability of horizontal and vertical central fixation in both head orientations was confirmed by computation of the area centroid of the globe-optic nerve junction relative to the orbital centroid, a method validated to be effective in demonstrating eye position during convergence.41
Quasi-coronal images of the orbit were analyzed digitally. It was recognized that while the relative sizes of the superior and inferior LR and MR compartments are roughly equal on average, their ratios may vary from 60:40 to 40:60. Since MRI demonstrates no landmarks permitting reliable segregation of compartments, we adopted the conservative approach of rotating each EOM cross section to make its major axis vertical, then arbitrarily bisecting the vertical dimension of the EOM into two equal parts along a horizontal line. Averaged over multiple subjects, the superior half of EOM cross sections would thus be expected to contain predominantly superior compartment fibers, and the inferior half to contain predominantly inferior compartment fibers. The inevitable misallocation of some fibers to the two arbitrary analysis compartments might diminish the potential significance of any differential activity observed, but could not invalidate a differential effect that reached statistical significance. We considered change in maximum EOM cross section to be a good indication of contractility.17, 45
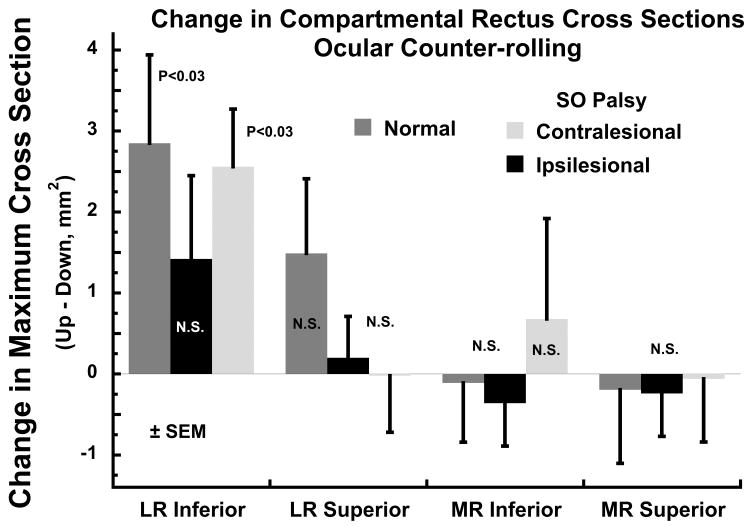
This functional MRI study did provide evidence for differential compartmental activity in the LR during OCR. Twelve orbits of 8 visually normal adult subjects were imaged with adequate repeatability of central fixation in the two head tilt positions; orbits were excluded from analysis when there were significant variations in horizontal or vertical eye position between the two head tilt positions. As demonstrated in Fig. 3, there was a significantly greater change in maximum cross section of the inferior LR compartment in the orbit up vs down head tilt. In 9 subjects with SO palsy, mean horizontal and vertical positions of the contralesional eye were not significantly different in the two head tilts, while a slight abduction in the ipsilesional eye did not differ significantly from eye position changes in the contralesional eye or in normal subjects. As also seen in Fig. 3, there was a significantly greater change in maximum cross section of the inferior LR compartment in the orbit up vs down head tilt in unaffected orbits contralateral to unilateral SO palsy, the the change was reduced to a non-significant level ipsilesional to SO palsy. This contractile effect was not evident in the superior LR compartment, indicating a differential response from the inferior compartment. There was no significant change in maximum LR superior compartment cross section in the orbit up vs down head tilt, in normal subjects or in subjects with SO palsy. There was no effect of head tilt on change in maximum MR cross section in either compartment in normal subjects or in SO palsy (Fig. 3).
Fig. 3.
Change in maximum lateral (LR) and medial rectus (MR) muscle maximum cross section, a reflection of contractile change, between the orbit tilted up vs down positions in normal control subjects, and in the ipsi- and contralesional orbits in superior oblique (SO) palsy. The inferior LR compartment exhibited significant contractility in normal subjects and in orbits contralesional to SO palsy, but did not do so ipsilesional to SO palsy. No significant contractility was observed in the superior compartment of the LR, or in either compartment of the MR. N.S. – not significant at 0.05 level. SEM – standard error of mean.
Since horizontal and vertical eye positions were held constant, the findings of contractile change in the LR inferior compartment suggests that it actively participates in normal ocular torsion during OCR. Absence of a corresponding contractile change in the normal LR superior compartment, or in either MR compartment, suggests that the observed change in the LR inferior compartment is not a passive or nonspecific effect of the ocular torsion that occurs during OCR. Moreover, ablation of the contractile effect in the LR inferior compartment ipsilesional to SO palsy suggests that there may be a complex interplay between oblique EOM and compartmental LR function during OCR.
Potential Kinematic Effects of Compartmentalization
Strabismus surgeons have long recognized that rectus EOMs can be made to have torsional or vertical effects; transverse surgical transposition of rectus EOM insertions is known to impart torsional actions.46, 47 Prior mechanical models of LR action have assumed that the LR tendon acts on the globe as if at a single point corresponding to the force centroid of its insertion; the LR is thus modeled as a line of thin string.48 The simplest model of a dual compartment LR would be a pair of such strings, one for each compartment, and acting at their force centroids. The LR tendon is less than 1 mm thick, but 10 – 12 mm in vertical dimension. It may be reasonably assumed that the superior and inferior LR compartments insert on tendon portions of equal width totaling about 12 mm. The force centroid of each equal compartment would act on the sclera at the midpoint of each compartment's width, or about ± 3 mm from the overall LR midpoint. In the extreme case, total relaxation to zero of one compartment, with non-zero tension in the other, would shift the net LR force centroid toward the active compartment by 3 mm. However, the mechanical effect of such a shift depends also upon the location of each compartmental centroid farther posteriorly at its effective origin, the LR pulley. If the LR is as wide at the pulley as at the tendon insertion, and if both compartments remain mechanically independent there, then the mechanical effect of differential tension would be to generate vertical without torsional force. If there is substantial mechanical coupling between compartments at the pulley, or in the unlikely event the LR narrows significantly at the pulley, then more of the differential force would be directed towards torsion.
Another analytical method suggests similar magnitude of effect. The Orbit 1.8® simulation treats each EOM as a line passing through a pulley of infinitesimal size.48 Computational simulation using this model's formulation suggests that the consequent vertical net LR force centroid shift, without change in net pulley location, would impart vertical action ± 13-15% of total LR tendon force, and torsional action ± 16 - 22% of total LR force.48 If pulley location is also assumed to shift as described above, resulting torsion would be reduced to to 0 – 4% of total LR force, but vertical action would be increased to 22 - 24% of total LR force. While the present data have not indicated that it occurs, simulation using Orbit 1.8® of shift in effective MR insertion and MR centroid at the pulley yields 5 – 7% torsional, and 20 – 21% vertical components of total MR force. Taking this analysis to the extreme, simulation of simultaneous activation of the superior compartments of both the MR and LR in one eye, with inhibition of the inferior compartments, predicts a 6° supraduction relative to a central target, with torsion less than 0.5°; this vertical rotation markedly exceeds the 1 – 1.5° amplitude of normal vertical fusional vergence. 49, 50
Summary of Overall Implications
Additional anatomical and biomechanical data are required to predict the precise kinematic effects of differential compartmental activity in the LR. Nevertheless, reasonable assumptions suggest that differential compartmental LR activation might, without additional latency, alter LR pulling direction without shifting its pulley or changing oblique EOM activity.12 Differential compartmental LR activation could permit the LR pulling direction to vary from that required by LL, for example generating the torsional LL violations typical of the VOR. Such violations of LL would be implemented by differential compartmental LR activation commanded by innervation patterns limited to regional variations in activity within the abducens nerve that would have been inapparent in single unit recordings in the abducens nucleus. Experimental demonstration of this phenomenon at the single unit level would require simultaneous recording from at least two motor neurons projecting to different LR compartments.
The preliminary functional data did not demonstrate differential compartmental activation in the MR. Failure to demonstrate this finding might be because differential MR activity actually does not occur during OCR, or might be the result the conservative analysis assumption employed here of equal cross sectional areas in the MR's superior and inferior compartments. This simple assuption may have resulted in erroneous mixing of observed MR activity in the two compartments, reducing or eliminating the apparent significance of compartmental differences. Of course, it may alternatively be that differential compartmental activity does not occur in the MRI during the VOR.
Additional functional studies of potential compartmental specialization in both LR and MR are warranted for behaviors in which LL is violated, including convergence, where there is non-LL excycloduction in infraversion, and incycloduction in supraversion.51-56 It might be that differential compartmental contraction in the horizontal rectus EOMs occurs during vertical fusional vergence, and perhaps sometimes during conjugate versions. Differential compartmental activation of horizontal rectus EOMs might have confounded prior interpretations of motor mechanisms of vergence based on classically assumed mechanical actions of the cyclovertical EOMs.57
Acknowledgments
Grant Support: Supported by National Institutes of Health EY08313. JLD was recipient of a grant from Research to Prevent Blindness, and is Leonard Apt Professor of Ophthalmology.
References
- 1.Ruete CGT. Ocular physiology. Strabismus. 1999;7:43–60. doi: 10.1076/stra.7.1.43.654. [DOI] [PubMed] [Google Scholar]
- 2.Tweed D, Vilis T. Geometric relations of eye position and velocity vectors during saccades. Vision Res. 1990;30:111–127. doi: 10.1016/0042-6989(90)90131-4. [DOI] [PubMed] [Google Scholar]
- 3.Crawford JD, Vilis T. Symmetry of oculomotor burst neuron coordinates about Listing's plane. J Neurophysiol. 1992;68:432–448. doi: 10.1152/jn.1992.68.2.432. [DOI] [PubMed] [Google Scholar]
- 4.Tweed D. Visual-motor optimization in binocular control. Vis Res. 1997;37:1939–1951. doi: 10.1016/s0042-6989(97)00002-3. [DOI] [PubMed] [Google Scholar]
- 5.Misslisch H, Tweed D. Neural and mechanical factors in eye control. J Neurophysiol. 2001;86:1877–1883. doi: 10.1152/jn.2001.86.4.1877. [DOI] [PubMed] [Google Scholar]
- 6.Angelaki DE, Hess BJ. Control of eye orientation: where does the brain's role end and the muscle's begin? Eur J Neurosci. 2004;19:1–10. doi: 10.1111/j.1460-9568.2004.03068.x. [DOI] [PubMed] [Google Scholar]
- 7.Angelaki DE. Three-dimensional ocular kinematics during eccentric rotations: Evidence for functional rather than mechanical constraints. J Neurophysiol. 2003;89:2685–2696. doi: 10.1152/jn.01137.2002. [DOI] [PubMed] [Google Scholar]
- 8.Ghasia FF, Angelaki DE. Do motoneurons encode the noncommutativity of ocular rotations? Neuron. 2005;47:281–293. doi: 10.1016/j.neuron.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 9.Klier EM, Meng H, Angelaki DE. Three-dimensional kinematics at the level of the oculomotor plant. J Neurosci. 2006;26:2732–2737. doi: 10.1523/JNEUROSCI.3610-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Klier EM, Meng H, Angelaki D. Revealing the kinematics of the oculomotor plant with tertiary eye positions and ocular counterroll. J Neurophysiol. 2011;105:640–649. doi: 10.1152/jn.00737.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demer JL. Mechanics of the orbita. Dev Ophthalmol. 2007;40:132–157. doi: 10.1159/0000100353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demer JL. Current concepts of mechanical and neural factors in ocular motility. Cur Opin Neurol. 2006;19:4–13. doi: 10.1097/01.wco.0000198100.87670.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demer JL. Pivotal role of orbital connective tissues in binocular alignment and strabismus. The Friedenwald lecture. Invest Ophthalmol Vis Sci. 2004;45:729–738. doi: 10.1167/iovs.03-0464. [DOI] [PubMed] [Google Scholar]
- 14.Demer JL. The orbital pulley system: A revolution in concepts of orbital anatomy. Ann NY Acad Sci. 2002;956:17–32. doi: 10.1111/j.1749-6632.2002.tb02805.x. [DOI] [PubMed] [Google Scholar]
- 15.Kono R, Clark RA, Demer JL. Active pulleys: Magnetic resonance imaging of rectus muscle paths in tertiary gazes. Invest Ophthalmol Vis Sci. 2002;43:2179–2188. [PubMed] [Google Scholar]
- 16.Demer JL, Oh SY, Poukens V. Evidence for active control of rectus extraocular muscle pulleys. Invest Ophthalmol Vis Sci. 2000;41:1280–1290. [PubMed] [Google Scholar]
- 17.Crane BT, Tian J, Demer JL. Kinematics of vertical saccades during the yaw vestibulo-ocular reflex in humans. Invest Ophthalmol Vis Sci. 2005;46:2800–2809. doi: 10.1167/iovs.05-0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peng M, et al. Compartmentalized innervation of primate lateral rectus muscle. Invest Ophthalmol Vis Sci. 2010;51:4612–4617. doi: 10.1167/iovs.10-5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.da Silva Costa RM, et al. Intramuscular innervation of primate extraocular muscles: Unique compartmentalization in horizontal recti. Inv Ophtalmol Vis Sci. 2011 doi: 10.1167/iovs.10-6651. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldberg SJ, Wilson KE, Shall MS. Summation of extraocular motor unit tensions in the lateral rectus muscle of the cat. Muscle Nerve. 1997;20:1229–1235. doi: 10.1002/(sici)1097-4598(199710)20:10<1229::aid-mus4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 21.Goldberg SJ, Meredith MA, Shall MS. Extraocular motor unit and whole-muscle responses in the lateral rectus muscle of the squirrel monkey. J Neurosci. 1998;18:10629–10639. doi: 10.1523/JNEUROSCI.18-24-10629.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shall MS, et al. Relationship of the mechanical properties of the cat inferior oblique muscle to the anatomy of its motoneurons and nerve branches. Acta Anat (Basel) 1995;153:151–160. doi: 10.1159/000313649. [DOI] [PubMed] [Google Scholar]
- 23.Govsa F, et al. The superior orbital fissure and its contents. J Surg Radiol Anat. 1999;21:181–185. doi: 10.1007/BF01630898. [DOI] [PubMed] [Google Scholar]
- 24.Spencer RF, Porter JD. Biological organization of the extraocular muscles. Prog Brain Res. 2006;151:43–80. doi: 10.1016/S0079-6123(05)51002-1. [DOI] [PubMed] [Google Scholar]
- 25.Gilbert PW. The origin and development of the extrinsic ocular muscles in the domestic cat. Contrib Embryol. 1957;36:61–78. [PubMed] [Google Scholar]
- 26.Neal HV. Ths history of the eye muscles. J Morphol. 1918;30:433–453. [Google Scholar]
- 27.Demer JL, Clark RA, Engle EC. Magnetic resonance imaging evidence for widespread orbital dysinnervation in congenital fibrosis of extraocular muscles due to mutations in KIF21A. Invest Ophthalmol Vis Sci. 2005;46:530–539. doi: 10.1167/iovs.04-1125. [DOI] [PubMed] [Google Scholar]
- 28.Demer JL, et al. Magnetic resonance imaging evidence for widespread orbital dysinnervation in dominant Duane's retraction syndrome linked to the DURS2 locus. Invest Ophthalmol Vis Sci. 2007;48:194–202. doi: 10.1167/iovs.06-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Demer JL, et al. Magnetic resonance imaging of innervational and extraocular muscle abnormalities in Duane-radial ray syndrome. Invest Ophthalmol Vis Sci. 2007;48:5505–5511. doi: 10.1167/iovs.07-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okanobu H, et al. Magnetic resonance imaging findings in Duane's retraction syndrome type III. Rinsho Ganka (Jpn Clin Ophthalmol) 2008;62:65–69. [Google Scholar]
- 31.Okanobu H, et al. Splitting of the extraocular horizontal rectus muscle in congenital cranial dysinnervation disorders. Am J Ophthalmol. 2009;147:550–556. doi: 10.1016/j.ajo.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 32.Nathan H, Ouaknine G, Kosary IZ. The abducens nerve: Anatomical variations in its course. J Neurosurg. 1974;42:561–566. doi: 10.3171/jns.1974.41.5.0561. [DOI] [PubMed] [Google Scholar]
- 33.Jain KK. Aberrant roots of the abducens nerve. J Neurosurg. 1964;21:349–351. doi: 10.3171/jns.1964.21.5.0349. [DOI] [PubMed] [Google Scholar]
- 34.Iaconetta G, Tessitore E, Samii M. Duplicated abducent nerve and its course: Microanatomical study and surgery-related considerations. J Neurosurg. 2001;95:853–858. doi: 10.3171/jns.2001.95.5.0853. [DOI] [PubMed] [Google Scholar]
- 35.Ozeren MF, et al. Duplication of the abducens nerve at the petroclival region: An anatomic study. Neurosurgery. 2003;52:645–651. doi: 10.1227/01.neu.0000048186.18741.3c. [DOI] [PubMed] [Google Scholar]
- 36.Oh SY, Poukens V, Demer JL. Quantitative analysis of rectus extraocular muscle layers in monkey and humans. Invest Ophthalmol Vis Sci. 2001;42:10–16. [PubMed] [Google Scholar]
- 37.Lim KH, Poukens V, Demer JL. Fascicular specialization in human and monkey rectus muscles: evidence for anatomic independence of global and orbital layers. Invest Ophthalmol Vis Sci. 2007;48:3089–3097. doi: 10.1167/iovs.06-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Demer JL, et al. Effects of intracranial trochlear neurectomy on the structure of the primate superior oblique muscle. Invest Ophthalmol Vis Sci. 2010;51:3485–3493. doi: 10.1167/iovs.09-5120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson BC, et al. Increased extraocular muscle strength with direct injection of insulin-like growth factor-I. Invest Ophthalmol Vis Sci. 2006;47:2461–2467. doi: 10.1167/iovs.05-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Demer JL, Miller JM. Orbital imaging in strabismus surgery. In: Rosenbaum AL, Santiago AP, editors. Clinical Strabismus Management: Principles and Techniques. WB Saunders; Philadelphia: 1999. pp. 84–98. [Google Scholar]
- 41.Demer JL, Kono R, Wright W. Magnetic resonance imaging of human extraocular muscles in convergence. J Neurophysiol. 2003;89:2072–2085. doi: 10.1152/jn.00636.2002. [DOI] [PubMed] [Google Scholar]
- 42.Demer JL, Clark RA. Magnetic resonance imaging of human extraocular muscles during static ocular counter-rolling. J Neurophysiol. 2005;94:3292–3302. doi: 10.1152/jn.01157.2004. [DOI] [PubMed] [Google Scholar]
- 43.Demer JL, Clark RA, Kung J. Functional imaging of human extraocular muscles In head tilt dependent hypertropia. Inv Ophtalmol Vis Sci. 2011 doi: 10.1167/iovs.10-6596. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Demer JL, Dusyanth A. T2 fast spin echo magnetic resonance imaging of extraocular muscles. J AAPOS. 2011;15:17–23. doi: 10.1016/j.jaapos.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Demer JL, Miller JM. Magnetic resonance imaging of the functional anatomy of the superior oblique muscle. Invest Ophthalmol Vis Sci. 1995;36:906–913. [PubMed] [Google Scholar]
- 46.Kono R, et al. Displacement of rectus muscle pulleys by torsional muscle surgery for treatment of full macular translocation-induced incyclotropia. Am J Ophthalmol. 2005;140:144–146. doi: 10.1016/j.ajo.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 47.Iwata EA, et al. Magnetic resonance imaging of the extraocular muscle path before and after strabismus surgery for a large degree of cyclotorsion induced by macular translocation surgery. Jap J Ophthalmol. 2008;53:131–137. doi: 10.1007/s10384-008-0632-9. [DOI] [PubMed] [Google Scholar]
- 48.Miller JM, Pavlovski DS, Shaemeva I. Eidactics. San Francisco: 1999. Orbit 1.8 Gaze Mechanics Simulation. [Google Scholar]
- 49.Mottier ME, B MM. Vertical fusional vergences in patients with superior oblique muscle palsies. Am Orthoptic J. 1990;40:88–93. [Google Scholar]
- 50.von Noorden GK. Binocular vision and ocular motility: Theory and management of strabismus. Mosby; St Louis: 1990. [Google Scholar]
- 51.Allen MJ, Carter JH. The torsional component of the near reflex. Am J Optom. 1967;44:343–349. [PubMed] [Google Scholar]
- 52.Bruno P, van den Berg AV. Relative orientation of primary positions of the two eyes. Vision Res. 1997;37:935–947. doi: 10.1016/s0042-6989(96)00219-2. [DOI] [PubMed] [Google Scholar]
- 53.Mikhael S, Nicolle D, Vilis T. Rotation of Listing's plane by horizontal, vertical and oblique prism-induced vergence. Vision Res. 1995;35:3243–3254. doi: 10.1016/0042-6989(95)00033-b. [DOI] [PubMed] [Google Scholar]
- 54.Somani RAB, et al. Visual test of Listing's law during vergence. Vision Res. 1998;38:911–923. doi: 10.1016/s0042-6989(97)00228-9. [DOI] [PubMed] [Google Scholar]
- 55.Mok D, et al. Rotation of Listing's plane during vergence. Vision Res. 1992;32:2055–2064. doi: 10.1016/0042-6989(92)90067-s. [DOI] [PubMed] [Google Scholar]
- 56.Misslisch H, Tweed D, Hess BJM. Stereopsis outweighs gravity in the control of the eyes. J Neurosci. 2001;21:RC126. doi: 10.1523/JNEUROSCI.21-03-j0004.2001. online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mudgil AV, et al. Motor mechanisms of vertical fusion in individuals with superior oblique paresis. J AAPOS. 2002;6:145–153. doi: 10.1067/mpa.2002.122521. [DOI] [PubMed] [Google Scholar]